Abstract
It is known that the microbiome affects human physiology, emotion, disease, growth, and development. Most humans exhibit reduced appetites under high temperature and high humidity (HTHH) conditions, and HTHH environments favor fungal growth. Therefore, we hypothesized that the colonic mycobiota may affect the host’s appetite under HTHH conditions. Changes in humidity are also associated with autoimmune diseases. In the current study mice were fed in an HTHH environment (32°C ± 2°C, relative humidity 95%) maintained via an artificial climate box for 8 hours per day for 21 days. Food intake, the colonic fungal microbiome, the feces metabolome, and appetite regulators were monitored. Components of the interleukin 17 pathway were also examined. In the experimental groups food intake and body weight were reduced, and the colonic mycobiota and fecal metabolome were substantially altered compared to control groups maintained at 25°C ± 2°C and relative humidity 65%. The appetite-related proteins LEPT and POMC were upregulated in the hypothalamus (p < 0.05), and NYP gene expression was downregulated (p < 0.05). The expression levels of PYY and O-linked β-N-acetylglucosamine were altered in colonic tissues (p < 0.05), and interleukin 17 expression was upregulated in the colon. There was a strong correlation between colonic fungus and sugar metabolism. In fimo some metabolites of cholesterol, tromethamine, and cadaverine were significantly increased. There was significant elevation of the characteristic fungi Solicoccozyma aeria, and associated appetite suppression and interleukin 17 receptor signaling activation in some susceptible hosts, and disturbance of gut bacteria and fungi. The results indicate that the gut mycobiota plays an important role in the hypothalamus endocrine system with respect to appetite regulation via the gut-brain axis, and also plays an indispensable role in the stability of the gut microbiome and immunity. The mechanisms involved in these associations require extensive further studies.
Keywords: colonic mycobiota, host appetite, gut-brain axis, climate change, immunity, IL-17R signaling
Introduction
Our understanding of when and how abruptly this climate-driven disruption of biodiversity will occur is limited because biodiversity forecasts typically focus on individual snapshots of the future. It is certain that biodiversity is threatened by climate change. Climate change can have adverse effects on biodiversity, by shifting species distributions (Barnosky et al., 2012; Wernberg et al., 2016; Provost et al., 2017), increasing extinction rates (Hultman et al., 2015), altering breeding times (Miller et al., 2018; Lv et al., 2020), and changing plant growth periods (Piao et al., 2019).
An increasing number of reports indicate that climate change effects the microbial diversity in soil, forests, and oceans (Sen and Samanta, 2015; Ladau et al., 2018; Malard and Pearce, 2018; Praeg et al., 2019). Recent research suggests that gut microbes are involved in nearly every aspect of our lives, from nutrition (Gentile and Weir, 2018), to behavior (O’Toole and Jeffery, 2015; Torres-Fuentes et al., 2017; Strandwitz, 2018), to diseases and mental health (Jiang et al., 2017; Quigley, 2017; Brial et al., 2018; Strandwitz, 2018). Studies on gut microflora have generally focused on the characteristics of bacteria, and ignored the potential effects of fungi on metabolic health.
Although fungi are only a small subset of microbes, they are very important in homeostatic balance (Li et al., 2019; Richard and Sokol, 2019). Many fungi may serve as a pathogen reservoir, and also play a key role in maintaining the functioning of the gut microbiota (Leonardi et al., 2018; Jones et al., 2019; Zhang et al., 2020). Specifically, Malassezia spp. was enriched markedly in both mice and humans. Ablation of the mycobiome was protective against tumor growth in slowly progressive and invasive models of pancreatic ductal adenocarcinoma, and repopulation with a Malassezia species accelerated oncogenesis (Aykut et al., 2019; Wolf et al., 2020). Thus, it is very important to determine the composition and function of intestinal fungi that influence human health.
Temperature and humidity have been linked to almost all human diseases, such as influenza (Sooryanarain and Elankumaran, 2015), cardiovascular mortality (Zeng et al., 2017), chronic obstructive pulmonary disease (Mu et al., 2017), allergic rhinitis (Duan et al., 2019), and arthritis (Bai et al., 2012; Bossema et al., 2013; Beukenhorst et al., 2020), among others (Onozuka and Hashizume, 2011; Balato et al., 2013; Yang et al., 2017). Infectious diseases such as coronavirus disease 2019 and H7N9 are evidently particularly affected by temperature and humidity (Zhang et al., 2015; Yao et al., 2020). A study using a guinea pig model provided direct experimental evidence supporting the role of weather conditions in the dynamics of influenza, thereby addressing a long-standing question fundamental to the understanding of influenza epidemiology and evolution (Lowen et al., 2007; Sooryanarain and Elankumaran, 2015). Potential links between Candida auris and climate change (Hofer, 2019) and many skin diseases (Balato et al., 2013; Balato et al., 2014) have been suggested. In traditional Chinese medicine the climatic factors of season, weather, wind, temperature, and humidity are comprehensively considered in the diagnosis of human diseases. Climatic temperature and humidity can substantially affect pathogenic microorganisms and the occurrence and development of diseases related to them. In the present study temperature and humidity were investigated with the aim of generating experimental data on the effects of climatic factors on microorganisms, particularly fungi, and host health.
Methods
Animals and Treatments
Male C57BL/6 mice weighing 14–16 g and aged 28–35 days were purchased from the Center for Laboratory Animals, Guangdong Province (Certification number SCXK[Yue]2018-0002, Table 1). They were maintained in a temperature-controlled (25°C) and humidity-controlled (55% ± 10%) room at a 12-h light–dark cycle in an ordinary clean environment within the Guangdong Institute of Microbiology. Food and water were available to the mice. The Animal Ethics Committee of the Guangdong Institute of Microbiology approved all experimental protocols. All efforts were made to minimize the number of mice used, and their suffering.
Table 1.
Experimental Model: Organisms/Strains.
| Reagent or Resource | Source | Identifier | Official website |
|---|---|---|---|
| Mouse:C57BL/6 | the Center of Laboratory Animals of Guangdong Province (SCXK [Yue] 2008-0020, SYXK [Yue] 2008-0085 | N/A | http://www.gdmlac.com.cn/ |
Model 1
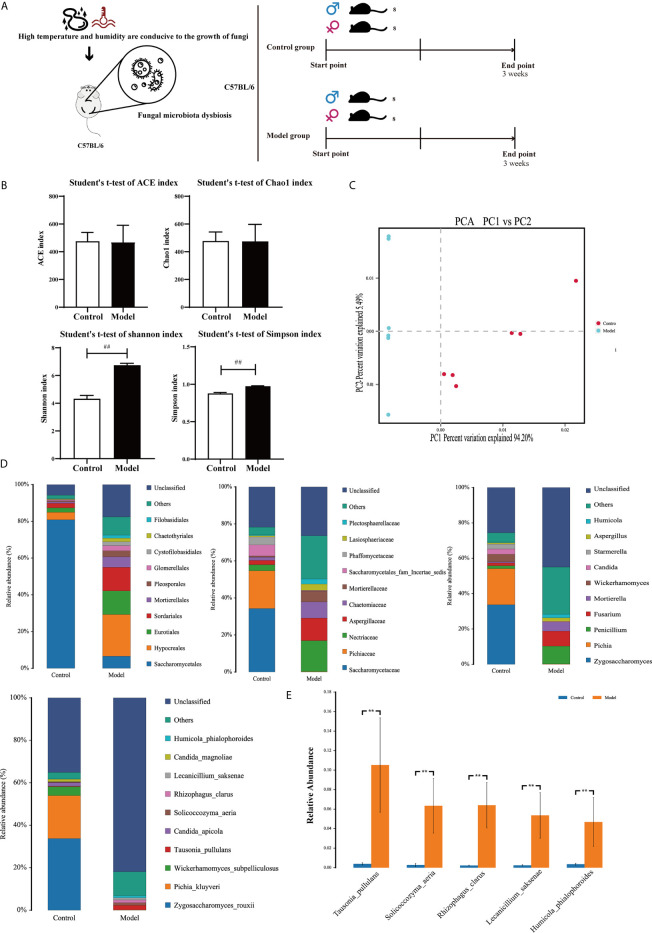
Mice were randomly divided into two groups of 16; an experimental group and a control group. The control group was not treated. The experimental group was exposed to high temperature and high humidity (HTHH) conditions (temperature 32°C ± 2°C, relative humidity 95%) via an artificial climate box (Figure 1A).
Figure 1.
Colonic mycobiota dysbiosis under HTHH conditions (A) Schematic diagram of the HTHH model. (B) Alpha diversity analysis of colonic fungal microbiota, and the beta diversity analysis effects ACE, the Chao1 estimator, Simpson’s diversity index, and the Shannon diversity index. (C) Principal component analysis. (D) Histogram of microbe distributions at the order, family, genus, and species levels. Only the top ten most abundant species are individually shown, and the additional microbes are combined as “Others”. “Unclassified” represents species that have not been taxonomically annotated. (E) Analysis of variance between groups at the species level, selecting five with the greatest changes in abundance.
Model 2
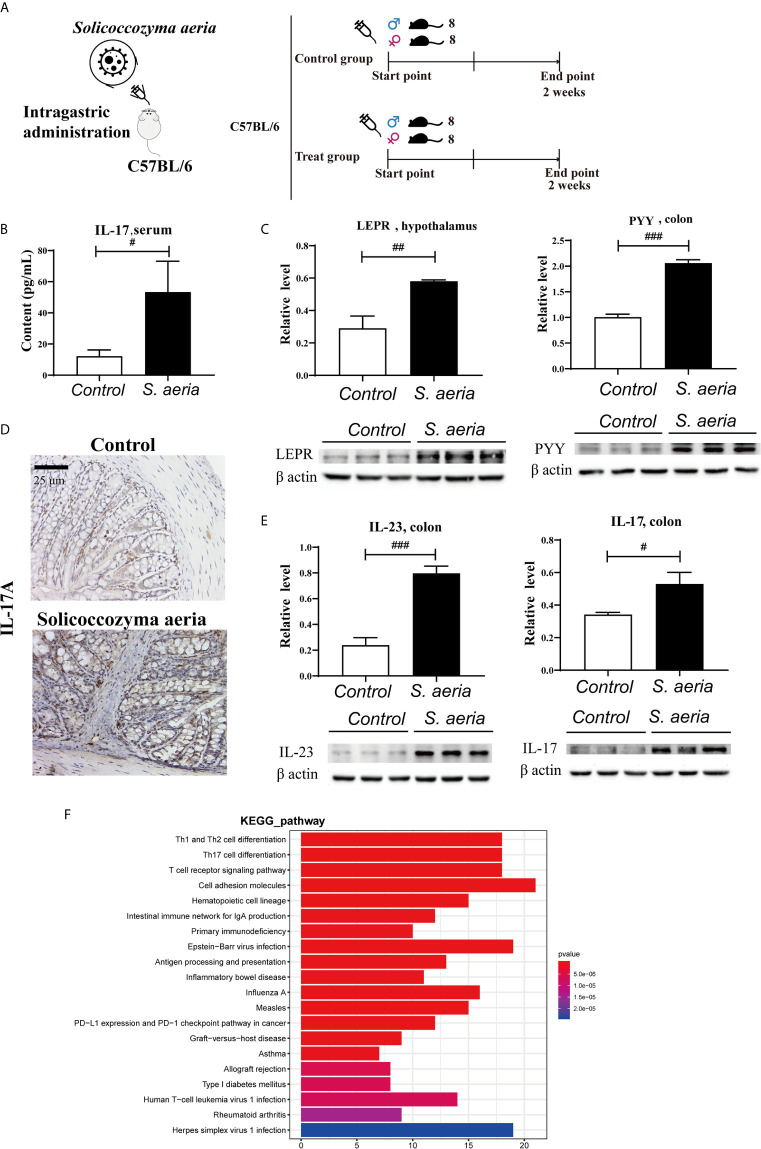
Mice were randomly divided into two groups of 16, the treatment group, and the control group. The treatment groups were generated by gavage (ig) of 0.4 mL Solicoccozyma aeria (7.0 × 108 colony-forming units [CFU]/mL) once a day for 2 weeks. The control group was generated by gavage (ig) of 0.4 mL distilled water once a day for 2 weeks (Figure 4A).
Figure 4.
Appetite suppression and IL17RA pathway activation by S. aeria gavage (A) Schematic diagram of the S. aeria gavage experiment. (B) Changes in serum IL-17 determined using an enzyme-linked immunosorbent assay kit (n = 6, two-tailed Student’s t-test). (C) PYY expression levels in colon tissues, and LEPT expression levels in the hypothalamus determined via western blotting (n = 3, two-tailed Student’s t-test). (D) IL-17A expression levels in colon tissues determined via immunohistochemistry. (E) IL-23 and IL-17A expression levels in colon tissues determined via western blotting (n = 3, two-tailed Student’s t-test). # p < 0.05, ## p < 0.01, ### p < 0.001 (F) Enrichment analysis of colonic differentially expressed genes in KEGG pathway Note: The abscisate is the number of genes of interest annotated in this entry, and the ordinate is each pathway entry. The color of the column represents the P value of the hypergeometric test.
Saliva Collection From Humans During Warm and Humid Conditions
From May to June in Guangdong in 2020, volunteers with a lack of appetite and a greasy tongue coating due to warm and humid conditions were recruited. According to our long-term observations, people living in humid conditions or warm and humid conditions tend to exhibit a thick and greasy tongue coating.
Microbiome ITS and 16S rDNA Analysis
Colonic digesta were collected after mice were killed, then frozen in liquid nitrogen. Saliva was collected in a tube with a DNA-protective fluid. Total DNA was extracted from 250–500mg of sample.
Construction and Sequencing
Primers were designed based on the conservative regions of the genes of interest, and a sequencing connector was added to the ends of the primers. Microbial 16S rDNA genes were amplified using the forward primer 338F 5’-ACTCCTACGGGAGGCAGCA-3’ and the reverse primer 806R 5’-GGACTACHVGGGTWTCTAAT-3’. Microbial ITS genes were amplified using the forward primer ITS1F 5’-CTTGGTCATTTAGAGGAAGTAA-3’ and the reverse primer ITS2 5’-GCTGCGTTCTTCATCGATGC-3’. PCR amplification was performed and the products were purified, quantified, and homogenized to form a sequencing library. The library was sequenced via an Illumina HiSeq 2500. The original image data files obtained via high-throughput sequencing (Illumina HiSeq and other sequencing platforms) were analyzed and converted into original sequencing reads by Base Calling, and the results were stored in FASTQ (fq) file format, which contained the sequence information and the corresponding sequencing quality information. Data is stored in SRA and the access can be found in Table 2.
Table 2.
Deposited Data.
| Resource | Description | Identifier |
|---|---|---|
| PRJNA693676 | Changes of colonic fungal microbiome in C57BL/6 mice under warm and humid environment (T=32 ± 2 °C, RH=95%) | SRA |
| PRJNA694096 | Fungal microbiome in colon of C57BL/6J mice by gavage Solicoccozyma aeria | SRA |
| PRJNA694055 | Bacterial microbiome in colon of C57BL/6J mice by gavage Solicoccozyma aeria | SRA |
| PRJNA690659 | Changes of human saliva fungal microbiomes in high temperature and high humidity environment | SRA |
GC-MS Fecal Metabolomics Analysis
Each 40-mg feces sample was homogenized in 400 μL deionized water containing 10 μg/mL L-norvaline as an internal standard. After centrifugation at 14,000 g and 4°C for 15 min, 300 μL of supernatant was transferred. The extraction was repeated by adding 600 μL of ice-cold methanol to the residue. The supernatants from two extractions were combined. A 400-μL sample of combined supernatants and 10 μL of internal standard solution (50 μg/mL of L-norleucine) were combined and evaporated to dryness under a nitrogen stream. The residue was reconstituted in 30 μL of 20 mg/mL methoxyamine hydrochloride in pyridine, and the resulting mixture was incubated at 37°C for 90 min. A 30-μL aliquot of BSTFA with 1% TMCS was added to the mixture and derivatized at 70°C for 60 min prior to GC-MS metabolomics analysis.
Metabolomics instrumental analysis was performed using an Agilent 7890A gas chromatography system coupled with an Agilent 5975C inert MSD system (Agilent Technologies Inc., CA, USA). An Optima® 5 MS Accent fused-silica capillary column (30 m × 0.25 mm × 0.25 μm; Macherey-Nagel, Düren, Germany) was utilized to separate the derivatives. Helium (> 99.999% pure) was used as a carrier gas at a constant flow rate of 1 mL/min through the column. The injection volume was 1 μL in split mode (2:1), and the solvent delay time was 6 min. The initial oven temperature was 70°C for 2 min, then it was increased to 160°C at a rate of 6°C per min, then to 240°C at a rate of 10°C per min, then to 300°C at a rate of 20°C per min, and lastly it was held at 300°C for 6 min. The temperatures of the injector, transfer line, and electron impact ion source were set to 250°C, 260°C, and 230°C, respectively. The electron ionization energy was 70 eV, and data were collected in full scan mode (m/z 50–600).
Correlational Analysis of Microbiome Metabolomics
Spearman’s correlation conjoint analysis of different colonic fungal genera and different fecal metabolites was performed. R was used to generate a heat map. Red represents positive correlations and blue represents negative correlations. Based on the results of the correlational analysis, correlation networks were constructed by selecting p values < 0.01 and correlation coefficients > 0.7 using Cytoscape software.
Histopathology and Immunostaining
Colonic tissues were removed and fixed in 4% paraformaldehyde at pH 7.4 for pathological observation. The samples were then washed, dehydrated, transparency, dipped in paraffin wax, and embedded, then 3-μm sections were generated. Immunostaining and a two-step peroxidase conjugated polymer technique (DAKO Envision kit, DAKO, Carpinteria, CA, USA) were applied, then the slides were observed via light microscopy.
Western Blotting
Briefly, colon and hypothalamus tissue were dissected from mice and proteins were extracted with radioimmunoprecipitation assay lysis buffer. The proteins were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After blocking with 5% skim milk in Tris-buffered saline (20 mM Tris-HCl, 500 mM NaCl, pH 7.4) with 0.2% Tween-20 (Aladdin, T104863) the membranes were probed with antibodies overnight at 4°C, followed by incubation with a horseradish peroxidase-conjugated goat anti-mouse (Servicebio, G2211-1-A) or goat anti-rabbit (Servicebio, G2210-2-A) secondary IgG antibody (1:2000). The primary antibodies and the reagents used were found in Tables 3 and 4.
Table 3.
Antibodies.
| Reagent or Resource | Source | Identifier | Working concentration | Official website |
|---|---|---|---|---|
| Leptin Receptor Antibody - C-terminal | Affinity | DF7139 | 1:2000 | http://www.affbiotech.com/ |
| POMC Antibody - C-terminal | Affinity | DF7154 | 1:1500 | http://www.affbiotech.com/ |
| IL17A Antibody - Internal | Affinity | DF6127 | 1:1000 | http://www.affbiotech.com/ |
| Peptide YY Polyclonal Antibody | proteintech | 24294-1-AP | 1:500 | http://www.ptgcn.com/ |
| Anti-IL-23 antibody | Abcam | ab45420 | 1:1000 | https://www.abcam.com/ |
| Anti-O-GlcNAc mouse mAb | PTM BIO | PTM-952 | 1:1000 | http://www.ptm-biolab.com.cn/ |
Table 4.
Chemicals, Reagent kit, Peptides, and Recombinant Proteins.
| Reagent or Resource | Source | Identifier | Official website |
|---|---|---|---|
| RNAiso Plus | Takara | 9109 | http://www.takarabiomed.com.cn/ |
| DNAiso Reagent | Takara | 9770Q | http://www.takarabiomed.com.cn/ |
| PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) | Takara | RR047A | http://www.takarabiomed.com.cn/ |
| TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) | Takara | RR820A | http://www.takarabiomed.com.cn/ |
| Glycogen Periodic Acid Schiff (PAS/Hematoxylin) Stain Kit | Solarbio | G1281 | http://www.solarbio.com/index.php |
| Mouse IL-17(Interleukin 17) ELISA Kit E-EL-M0047c | Elabscience | E-EL-M0047c | https://www.elabscience.cn/ |
| Saliva DNA Storage Tube | cwbio | CW2667M | https://www.cwbiosciences.com/home |
| T-PER™ Tissue Protein Extraction Reagent | Thermo Scientific™ | 78510 | https://www.thermofisher.com/cn/zh/home.html |
Transcriptome Analysis
RNA concentration and purity was measured using NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). A total amount of 1 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext UltraTM RNA Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations and index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5X). First strand cDNA was synthesized using random hexamer primer and M-MuLV Reverse Transcriptase. Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities. After adenylation of 3’ ends of DNA fragments, NEBNext Adaptor with hairpin loop structure were ligated to prepare for hybridization. In order to select cDNA fragments of preferentially 240 bp in length, the library fragments were purified with AMPure XP system (Beckman Coulter, Beverly, USA). Then 3 μl USER Enzyme (NEB, USA) was used with size-selected, adaptor-ligated cDNA at 37°C for 15 min followed by 5 min at 95°C before PCR. Then PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. At last, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v4-cBot-HS (Illumia) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina platform and paired-end reads were generated. KOBAS software was used to test the statistical enrichment of differential expression genes in KEGG pathways (Mao et al., 2005).
Description of S. aeria
S. aeria were obtained from the feces of a patient under HTHH conditions. The results of 16S rDNA identification are shown in the Supplementary Materials.
S. aeria Preparation
S. aeria were cultured in Yeast Mold Broth (YM broth) at 37°C for 18 hours, then fungal cells were concentrated via centrifugation at 14,000 rpm for 15 min and washed twice with sterilized phosphate-buffered saline (Ph 7.2). OD values were measured in triplicate at 600 nm, and the average value was used. The fungal cells were diluted to a final dose of 7.0 × 108 CFU/mL (OD 600 = 1 was approximately equal to a bacterial concentration of 1.0 × 108 CFU/mL).
Statistical Analysis
Data are described as means ± the standard deviation of at least three independent experiments. Datasets that involved more than two groups were analyzed via one-way analysis of variance using Statistical Package for the Social Sciences software (SPSS version 17.0, Abacus Concepts, Berkeley, CA, USA) or Prism8 software (GraphPad, San Diego, CA, USA, Table 5).
Table 5.
Software and Algorithms.
| Reagent or Resource | Source | Identifier |
|---|---|---|
| GraphPad Prism 8.0.1 | GraphPad Software | https://www.graphpad.com/ |
| R version 4.0.1 | Bell Laboratories (formerly AT&T, now Lucent Technologies) | https://www.r-project.org/ |
Results
Colonic Mycobiota Dysbiosis Under HTHH Conditions
In alpha diversity analysis of colonic mycobiota the indexes of Shannon differed significantly (Figure 1B, Table 6), and in principal component analysis there were differences between the control group and the experimental group (Figure 1C). Colonic mycobiota changes were assessed via the ITS1 technique. A histogram of species distribution at the levels of order, family, genus, and species are shown in Figure 1D. The relative abundances of Zygosaccharomyces, Wickerhamomyces, Starmerella, Stagonospora, Pichia, Sporobolomyces, Rhodotorula, Rhizophagus, Purpureocillium, Plectosphaerella, Penicillium, Mortierella, Kodamaea, Kazachstania, Hanseniaspora, Fusarium, Filobasidium, Candida, and Botryotrichum were increased (Figure 1D, Table 7, and Figure S1). The species that changed most significantly are shown in Figure 1E. Histograms of species distribution indicate that HTHH conditions altered the colonic mycobiota extensively.
Table 6.
Microbial diversity index .
| Group | ACE | Chao1 | Simpson | Shannon |
|---|---|---|---|---|
| Control | 475.2 ± 26.3 | 477.2 ± 26.5 | 0.8765 ± 0.0055 | 4.3248 ± 0.097 |
| Model | 468.1 ± 50.2 | 474.5 ± 50.0 | 0.9738 ± 0.0023 | 6.7435 ± 0.0575 |
Table 7.
71 fungus at genus level in normal group were changed by the condition of the high temperature and high humidity (p value <0.01).
| Genus | Changes in abundance | Modify | Genus | Changes in abundance | Modify |
|---|---|---|---|---|---|
| Penicillium | 7.8869% | up | Pseudogymnoascus | 0.0958% | up |
| Fusarium | 6.3765% | up | Paraglomus | 0.0804% | up |
| Mortierella | 4.1440% | up | Hannaella | 0.0763% | up |
| Plectosphaerella | 1.7074% | up | Malassezia | 0.0751% | up |
| Tausonia | 1.6460% | up | Microdochium | 0.0738% | up |
| Humicola | 1.4352% | up | Exophiala | 0.0735% | up |
| Rhizophagus | 1.0118% | up | Mycosphaerella | 0.0722% | up |
| Solicoccozyma | 0.9925% | up | Stropharia | 0.0720% | up |
| Trichoderma | 0.9365% | up | Septoria | 0.0691% | up |
| Lecanicillium | 0.8303% | up | Botrytis | 0.0687% | up |
| Alternaria | 0.8222% | up | Nigrospora | 0.0651% | up |
| Fusicolla | 0.7223% | up | Phialophora | 0.0620% | up |
| Acrocalymma | 0.7166% | up | Conlarium | 0.0563% | up |
| Purpureocillium | 0.6670% | up | Leptosphaeria | 0.0527% | up |
| Botryotrichum | 0.6629% | up | Thelonectria | 0.0380% | up |
| Cladosporium | 0.5244% | up | Conocybe | 0.0373% | up |
| Clonostachys | 0.4180% | up | Knufia | 0.0317% | up |
| Leucoagaricus | 0.4045% | up | Achroiostachys | 0.0296% | up |
| Coniochaeta | 0.3746% | up | Paraphoma | 0.0293% | up |
| Thielavia | 0.3625% | up | Clitopilus | 0.0288% | up |
| Trichosporon | 0.2555% | up | Cyphellophora | 0.0269% | up |
| Chaetomium | 0.2544% | up | Didymella | 0.0207% | up |
| Staphylotrichum | 0.2478% | up | Peziza | 0.0148% | up |
| Corynascella | 0.2403% | up | Saccharomyces | 0.0107% | up |
| Microascus | 0.2362% | up | Sterigmatomyces | -0.0113% | down |
| Marasmius | 0.1931% | up | Stagonospora | -0.0130% | down |
| Codinaea | 0.1916% | up | Kodamaea | -0.0145% | down |
| Filobasidium | 0.1845% | up | Hyphopichia | -0.0165% | down |
| Panaeolus | 0.1692% | up | Sporobolomyces | -0.0309% | down |
| Stachybotrys | 0.1491% | up | Rhodotorula | -0.0368% | down |
| Wallemia | 0.1239% | up | Hanseniaspora | -0.2018% | down |
| Papiliotrema | 0.1174% | up | Kazachstania | -0.5368% | down |
| Polyschema | 0.1145% | up | Starmerella | -2.8127% | down |
| Tetracladium | 0.1102% | up | Wickerhamomyces | -4.2380% | down |
| Pichia | -20.1428% | down | Zygosaccharomyces | -33.6231% | down |
| Candida | -2.5646% | down |
HTHH Conditions and Appetite Regulation via the Gut-Brain Axis
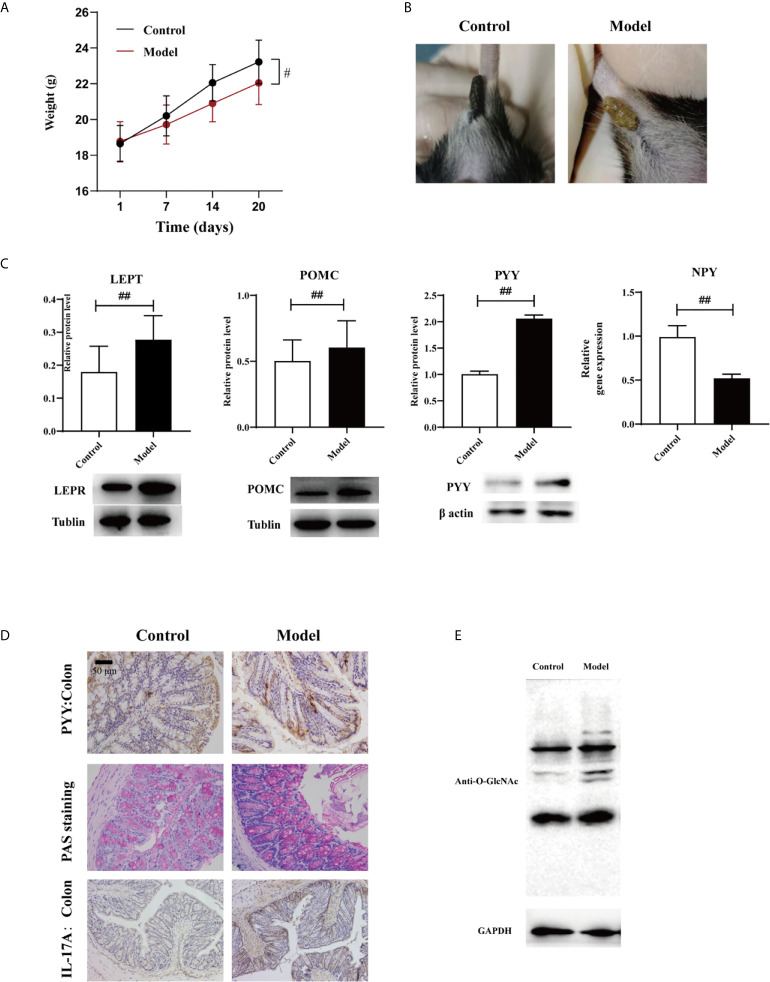
Body weight was lost and fecal traits were altered in the experimental group (Figures 2A, B). Expression levels of LEPT and POMC proteins in the hypothalamus determined via western blotting were altered in the experimental group, as was the expression of NPY determined via qPCR (Table 8), and the levels of all three were associated with leptin (Figure 2C, p < 0.01). This suggests that HTHH conditions may influence the central nervous system and negative feedback regulation of appetite and metabolism, and immunohistochemistry results exhibited the same trends (Figure 2D). PYY was activated in the colon (Figures 2C, D). GlcNAcylation was increased in the colon in the experimental group (Figure 2E, p < 0.05). Periodic acid–Schiff staining showed that glycogen and mucin were increased in colon tissues in the experimental group (Figure 2D).
Figure 2.
Effects of HTHH conditions on appetite in mice exerted via the gut-brain axis (A) Body weight in the experimental group was significantly lower than that in the control group. (B) The appearance and morphology of feces differed in the control group and the experimental group. (C) Western blotting results of LEPT and POMC expression in the hypothalamus, and PYY expression in the colon. NPY expression in the hypothalamus was detected via qPCR (n = 3, two-tailed Student’s t-test. #p < 0.05, ##p < 0.01. (D) PYY and IL-17A expression detected via immunohistochemistry. Periodic acid–Schiff staining was used to detect glycogen and mucin in colon tissue. (E) GlcNAcylation in the colon was detected using a pan anti-O-GlcNAc monoclonal antibody (n = 3).
Table 8.
Sequence-Based Reagents.
| Gene | Primer | Sequence (5’→3’) | Amplicon Size |
|---|---|---|---|
| NPY | Mus-NPY-F161 | 5’-ATGCTAGGTAACAAGCGAATGG-3’ | 161 |
| Mus-NPY-R161 | 5’-TGTCGCAGAGCGGAGTAGTAT-3’ | ||
| GAPDH | Mus-GAPDH-F117 | 5’-TCTCCTGCGACTTCAACA-3’ | 117 |
| Mus-GAPDH-R117 | 5’-TGTAGCCGTATTCATTGTCA-3’ |
Fecal Content Metabolomics and HTHH Conditions
In metabolic analysis of fecal contents in the experimental group 17 different metabolites were detected via GC/MS. Of these 17, D-glucose and maltose were downregulated whereas the other 15 were upregulated (Figure S2A, Table 9), including oleanitrile, stearic acid, octadecadienoic acid, aspartic acid, tromethamine, norleucine, alanine, glycine, cadaverine, oxoproline, hydroxy-L-norleucine, phosphonic acid, cholesterol, and fucose (Figure S2A, Table 9).
Table 9.
17 fecal metabolites which in normal group changed by the condition of the high temperature and high humidity (p value < 0.01).
| Metabolite (M vs N) | Modify | Metabolite (M vs N) | Modify |
|---|---|---|---|
| Phosphonic acid | up | Cholesterol | up |
| Stearic acid | up | L-Aspartic acid | up |
| L-5-Oxoproline | up | Glycine | up |
| 9,12-Octadecadienoic acid (Z,Z)- | up | Tromethamine | up |
| Oleanitrile | up | (-)-Fucose | up |
| L-Alanine | up | Cadaverine | up |
| 2,6-Bis (tert-butyl)phenol | up | Maltose | down |
| 6-Hydroxy-L-norleucine | up | D-glucose | down |
| L-Norleucine | up |
Conjoint Correlational Analysis of Mycobiota Genus-Fecal Metabolites
Based on Spearman’s correlational analysis, correlation networks were generated using a probability threshold of p < 0.01 (the top 20 of changes in abundance) and a correlation coefficient threshold of > 0.7 (Figures S2B, C). The mycobiota genera Candida, Starmerella, Wickerhamomyces, Pichia, and Zygosaccharomyces were significantly associated with the metabolites phosphonic acid, D-glucose, stearic acid, L-5-oxoproline, 9,12-(Z, Z)-octadecadienoic acid, oleanitrile, L-alanine, 2,6-bis(tert-butyl)phenol, 6-hydroxy-L-norleucine, L-norleucine, cholesterol, L-aspartic acid, glycine, tromethamine, (-)-fucose, cadaverine, and maltose.
Some harmful metabolites were positively correlated with the abundance of fungus. Metabolites of cholesterol were positively correlated with Penicillium, Fusarium, Mortierella, Plectosphaerella, Purpureocillium, Botryotrichum, and Candida (Figures S2B, C). Metabolites of stearic acid were positively correlated with Penicillium, Fusarium, Mortierella, Plectosphaerella, Tausonia, Trichoderma, Solicoccozyma, Rhizophagus, Lecanicillium, Purpureocillium, Botryotrichum, and Candida (Figures S2B, C). Metabolites of tromethamine were positively correlated with Penicillium, Fusarium, Mortierella, Humicola, Plectosphaerella, Tausonia, Trichoderma, Solicoccozyma, Rhizophagus, Lecanicillium, Pichia, Candida, Acrocalymma, Wickerhamomyces, Starmerella, Zygosaccharomyces, Botryotrichum, and Purpureocillium (Figures S2B, C). Metabolites of D-glucose were negatively correlated with Penicillium, Fusarium, Mortierella, Plectosphaerella, Tausonia, Humicola, Rhizophagus, Solicoccozyma, Trichoderma, Lecanicillium, Alternaria, Fusicolla, Acrocalymma, Purpureocillium, and Botryotrichum (Figures S2B, C). Metabolites of maltose were negatively correlated with Penicillium, Fusarium, Mortierella, Plectosphaerella, Rhizophagus, Purpureocillium, and Botryotrichum (Figures S2B, C).
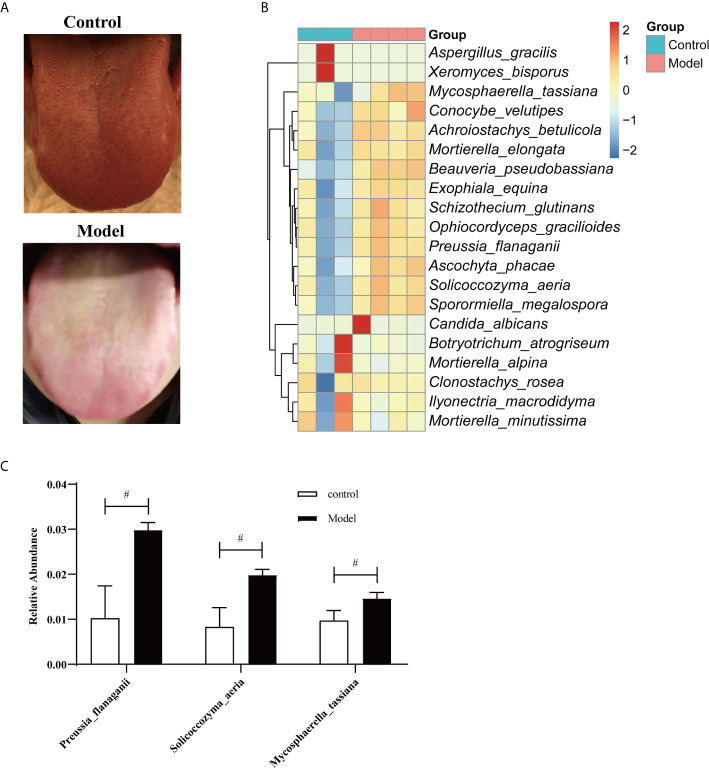
S. aeria Was Higher in the Saliva of Humans Under HTHH Conditions
Tongue coatings were thick and greasy during HTHH conditions (Figure 3A). A species-level heatmap is shown in Figure 3B. Mycosphaerella tassiana, Conocybe velutipes, Achroiostachys betulicola, Morietella elongate, Beauveria pseudobassiana, Exophiala equina, Schizothecium glutinans, Ophiocordyceps gracilioides, Preussia flanaganii, Ascochyta phacae, Solicoccozyma aeria, and Sporormiella megalospora were increased, indicating that the saliva mycobiota was significantly altered. The three most significantly affected species are shown in Figure 3C. S. aeria was significantly altered in humans and mice.
Figure 3.
Fungal microbiome in saliva from humans during HTHH conditions (A) The appearance and morphology of the tongue coatings are different. (B) Heatmap of the fungal microbiome at the species level. The x-coordinate represents species, and the 20 species with the lowest p values are shown. The ordinate represents the relative abundance of species. Columns of different colors represent each sample. (C) The three with the greatest changes in abundance at the species level (t-test). #p < 0.05.
S. aeria-Associated Appetite Suppression and IL-17R Pathway Activation in Mice
In western blotting analyses LEPT and PYY protein levels were upregulated in the treatment group (Figure 4C, p < 0.05). This suggests that S. aeria may influence the central nervous system, and negative feedback regulation of appetite. Serum levels of IL-17 (Figure 4B, p < 0.05), and colon tissue expression levels of IL-23 and IL-17 were significantly upregulated (Figures 4D, E, p < 0.05). The IL-17RA pathway may be activated by gut microbiota (Figure 4F), and is related to most autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis (AS), and inflammatory bowel disease (Atarashi et al., 2015; Kim et al., 2016). IL-23 may induce pathogenic Th17 cells and promote inflammatory disease (Lee et al., 2020).
S. aeria and Gut Microbiota in C57BL/6 Mice
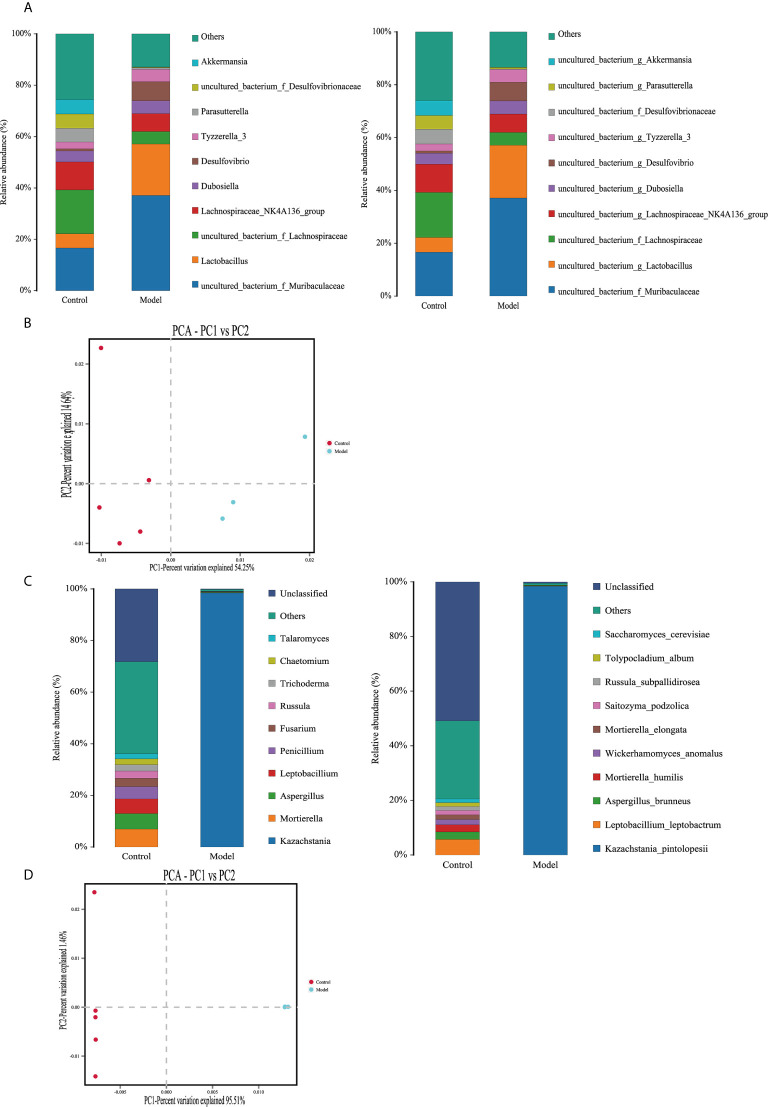
In 16S rDNA sequencing analyses at the genus level the relative abundances of Bacteroidales, Lactobacillales, Desulfovibrionales, Saccharimonadales, and Erysipelotrichales were increased in the S. aeria-treated mice, whereas those of Clostridiales, Verrucomicrobiales, Betaproteobacteriales, Coriobacteriales, and Campylobacterales were reduced (Figure 5A, left). At the species level the relative abundances of Muribaculaceae, Lactobacillus, Desulfovibrio, Tyzzerella 3, and Dubosiella were increased, whereas those of Lachnospiraceae, Akkermansia, Desulfovibrionaceae, Parasutterella, and Lachnospiraceae NK4A136 group were reduced (Figure 5A, right). In principal component analysis there were differences between the control group and the treated group (Figure 5B).
Figure 5.
Changes in the bacterial microbiomes and fungal microbiomes in the treatment group and the control group (A) Histogram of species distribution in the bacterial microbiome (left, genus level; right, species level). (B) Principal component analysis. (C) Histogram of species distribution in the fungal microbiome (left, genus level; right, species level). (D) Left, principal component analysis.
In ITS sequencing analyses, at the genus level in the S. aeria-treated group the relative abundance of Kazachstania was increased, whereas those of Talaromyces, Chaetomium, Trichoderma, Russula, Fusarium, Penicillium, Leptobacillium, Aspergillus, and Mortierella were reduced (Figure 5C, left). At the species level Kazachstania pintolopesii was significantly increased, whereas Leptobacillium leptobactrum, Mortierella humilis, Aspergillus brunneus, Wickerhamomyces anomalus, Mortierella elongata, Saitozyma podzolica, Russula subpallidirosea, Tolypocladium album, and Saccharomyces cerevisiae were reduced (Figure 5C, right). In principal component analysis there were differences between the control group and the treated group (Figure 5D).
In previous studies enrichment of Escherichia-Shigella (Proteobacteria), Veillonella (Firmicutes), Faecalibacterium (Firmicutes), Eubacterium rectale group (Firmicutes), Streptococcus (Firmicutes), Lachnospiraceae NK4A136 group (Firmicutes), and reduced Prevotella strain 9 (Bacteroidetes), Megamonas (Firmicutes), and Fusobacterium (Fusobacteria) were detected in AS patients (Li et al., 2019). AS-enriched species including Bacteroides coprophilus, Parabacteroides distasonis, Eubacterium siraeum, Acidaminococcus fermentans and Prevotella copri were identified in AS patients via metagenomic analyses (Zhou et al., 2020). Desulfovibrionales is the main producer of hydrogen sulfide in the intestinal tract. High concentrations of hydrogen sulfide are associated with intestinal inflammation, and Desulfovibrionales plays an important role in the occurrence and development of inflammatory bowel disease (Dordević et al., 2021; Kushkevych et al., 2021).
Lachnospiraceae NK4A136 group, Desulfovibrionales, and Bacteroidales were increased in treatment group. Desulfovibrionales could produce toxic H2S and have a strong association with IBD (Inness et al., 2007), and that S. aeria is a powerful trigger in some susceptible hosts. S. aeria significantly altered the abundance of K. pintolopesii. It’s reported that C.albians is not a natural colonizer of mucosal surfaces in these-the rodent equivalent of normal flora yeast is K.pintolopesii (Naglik et al., 2008), thus we speculate that K. pintolopesii may be a conditional pathogen which activate the IL17A pathway.
Discussion
Illness-associated behaviors such as loss of appetite, fatigue, low-grade fever, drowsiness, and/or chills usually indicate pathogenic infection and/or low immunity. In the current study food intake and body weight were reduced, and in the hypothalamus the appetite-related proteins LEPT and POMC were upregulated (p < 0.05) and NPY gene expression was downregulated (Figure 2, p < 0.05). In colonic tissues the expression levels of PYY and O-GlaNAc were altered, as was periodic acid–Schiff staining (Figure 2, p < 0.05). IL-17 expression was upregulated in the colon, indicating that in mice appetite and immunity were altered under HTHH conditions. Other colonic mycobiota and fecal metabolome parameters were also significantly altered under HTHH conditions (Figures 1 and 2), indicating that HTHH can change the mycobiota and metabolome, then influence host behaviors.
Little attention has been paid to autoimmune diseases triggered by weather or high humidity. Few studies have investigated relationships between humidity-induced microbial alterations and autoimmune diseases, and no causative characteristic microbe has been reported. In the present study environmental temperature and humidity could trigger homeostasis changes in human microbes, especially fungi, activate IL17A pathway and elevate the level of IL17A in serum. There was also significant elevation of the characteristic fungi S. aeria, and that species was present at high relative abundance in the HTHH mice and humans. Appetite suppression and IL-17R signaling activation were detected in mice, as were disturbances of the gut microbiota and mycobiota. S. aeria may trigger K. pintolopesii proliferation in some susceptible hosts, but this requires extensive further investigation.
In previous studies germ-free conditions or limited microbiota content attenuated arthritis severity in the ZAP70W163C BALB/c (SKG) mouse model (Rehaume et al., 2014), and spondylitis and colitis disappear when breeding in the GF state in the HLA-B27 transgenic mouse model (Taurog et al., 1994). These observations indicate that microbes play an important role in autoimmune diseases. Th17 cells and IL-17 are involved in host defenses by way of immunothrombosis, and promote the removal of pathogenic microorganisms (Amezcua Vesely et al., 2019; Kitamoto et al., 2020), but too much IL-17A is fatal for the host, and can lead to a variety of inflammatory or autoimmune diseases (Gravallese and Schett, 2018; Jo et al., 2018).
It is not clear what substances cause the activation of autoimmune disease and activate the IL-17RA pathway, or whether S. aeria is significantly increased during the onset of inflammatory bowel disease, rheumatoid arthritis, AS and other autoimmune diseases. HTHH conditions can lead to imbalanced gut and oral microbiomes, particularly significant changes in fungi, which provides a new perspective with respect to the study of microbe-related diseases induced by climate changes. The microbial diversity hosted by specific pathogen-free mice is relatively simple, and the microbial diversity hosted by wild mice is reportedly completely different, which imposes substantial limitations on the study of interactions and functions of the relevant microbes in the natural environment (Rosshart et al., 2019). In the future, germ-free and ZAP70W163C BALB/c (SKG) mice will be used to test the effects of S. aeria and K. pintolopesii.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Guangdong Second Provincial General Hospital, Guangzhou 510000, Guangdong, China. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. The animal protocols used in this study were approved by the Institutional Animal Care and Use committee of the Center of Laboratory Animals of the Guangdong Institute of Microbiology.
Author Contributions
DC and JW designed this study. YG wrote the manuscript. YG and HG collected animal physiological data and fecal samples, and did the physiological and biochemical indexes measurement. YG and XZ did the western blotting analysis. YG did the metabolomic analysis. YG collected data regarding the microbial metabolic networks analysis. DC and JW helped to design the study and to develop the multi-omics analysis methods, reviewed this manuscript and offer all the necessary research start-up fund, experimental platform. All authors contributed to the article and approved the submitted version.
Funding
The present work was supported by the financial support from the National Natural Science Foundation of China (81941010 and 8187151662), Guangzhou Science and Technology Plan Projects (2017A070701003, 2017B020231001, and 2019A030317009), GDAS’ Project of Science and Technology Development (2019GDASYL-0104007), and Project of Science and Technology Development of Guangdong Second Provincial General Hospital (YY2019-006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to the Research Center of Basic Integrative Medicine of Guangzhou University of Chinese Medicine for supplying experiment platform.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2021.657807/full#supplementary-material
Species-level heatmap Species-level heatmap generated using sample abundance similarity for clustering. The difference between two samples is represented visually by the change in color gradient.
(A) Heatmap of significant metabolites depicting differences between the control group and the experimental group. (B) Conjoint analysis of 71 significantly different fungal genera and 17 significantly different fecal metabolites (+p < 0.1, ++p < 0.05, +++ p < 0.01). (C) Spearman’s correlational coefficients for different fungal genera (top 20 with respect to changes in abundance) and 17 significantly different fecal metabolites. In the correlational analysis the probability threshold used to construct correlation networks was p < 0.01, and the correlation coefficient threshold used was > 0.7. Blue nodes represent metabolites, and yellow nodes represent fungal genera. The red line indicates a positive correlation and the black line indicates a negative correlation. More nodal connections indicates more associations with others, and the sizes of nodes correspond with the change in abundance.
Fecal content metabolomics were altered under HTHH conditions (A) Principal component analysis, PLS-DA analysis, and OPLS-DA analysis of fecal metabolites. (B) Pathway analysis of fecal metabolites in the control group and the experimental group.
References
- Amezcua Vesely M. C., Pallis P., Bielecki P., Low J. S., Zhao J., Harman C. C. D., et al. (2019). Effector TH17 Cells Give Rise to Long-Lived Trm Cells That Are Essential for an Immediate Response Against Bacterial Infection. Cell 178 No. 5, 1176–1188.e15. doi: 10.1016/j.cell.2019.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K., Tanoue T., Ando M., Kamada N., Nagano Y., Narushima S., et al. (2015). Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 163 No. 2, 367–380. doi: 10.1016/j.cell.2015.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aykut B., Pushalkar S., Chen R., Li Q., Abengozar R., Kim J. I., et al. (2019). The Fungal Mycobiome Promotes Pancreatic Oncogenesis Via Activation of MBL. Nature 574 No. 7777, 264–267. doi: 10.1038/s41586-019-1608-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y. J., Jiang D. X., An N., Shen H. B., Hu Y. Q. (2012). Effects of Cold-Damp and Hot-Damp Environment on VEGF and IL-1 Expression in Joint Cartilage Cells in Adjuvant Arthritis in Rats. J. Tradit Chin. Med. 32 No. 2, 256–260. doi: 10.1016/S0254-6272(13)60021-7 [DOI] [PubMed] [Google Scholar]
- Balato N., Ayala F., Megna M., Balato A., Patruno C. (2013). Climate Change and Skin. G Ital Dermatol. Venereol 148 No. 1, 135–146. [PubMed] [Google Scholar]
- Balato N., Megna M., Ayala F., Balato A., Napolitano M., Patruno C. (2014). Effects of Climate Changes on Skin Diseases. Expert Rev. Anti Infect. Ther. 12 No. 2, 171–181. doi: 10.1586/14787210.2014.875855 [DOI] [PubMed] [Google Scholar]
- Barnosky A. D., Hadly E. A., Bascompte J., Berlow E. L., Brown J. H., Fortelius M., et al. (2012). Approaching a State Shift in Earth’s Biosphere. Nature 486 No. 7401, 52–58. doi: 10.1038/nature11018 [DOI] [PubMed] [Google Scholar]
- Beukenhorst A. L., Schultz D. M., Mcbeth J., Sergeant J. C., Dixon W. G. (2020). Are Weather Conditions Associated With Chronic Musculoskeletal Pain? Rev. results Methodol. Pain 161 No. 4, 668–683. doi: 10.1097/j.pain.0000000000001776 [DOI] [PubMed] [Google Scholar]
- Bossema E. R., Van Middendorp H., Jacobs J. W., Bijlsma J. W., Geenen R. (2013). Influence of Weather on Daily Symptoms of Pain and Fatigue in Female Patients With Fibromyalgia: A Multilevel Regression Analysis. Arthritis Care Res. (Hoboken) 65 No. 7, 1019–1025. doi: 10.1002/acr.22008 [DOI] [PubMed] [Google Scholar]
- Brial F., Le Lay A., Dumas M. E., Gauguier D. (2018). Implication of Gut Microbiota Metabolites in Cardiovascular and Metabolic Diseases. Cell Mol. Life Sci. 75 No. 21, 3977–3990. doi: 10.1007/s00018-018-2901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordević D., Jančíková S., Vítězová M., Kushkevych I. (2021). Hydrogen Sulfide Toxicity in the Gut Environment: Meta-analysis of Sulfate-Reducing and Lactic Acid Bacteria in Inflammatory Processes. J. Advanced Res. 27, 55–69. doi: 10.1016/j.jare.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J., Wang X., Zhao D., Wang S., Bai L., Cheng Q., et al. (2019). Risk Effects of High and Low Relative Humidity on Allergic Rhinitis: Time Series Study. Environ. Res. 173, 373–78. doi: 10.1016/j.envres.2019.03.040 [DOI] [PubMed] [Google Scholar]
- Gentile C. L., Weir T. L. (2018). The Gut Microbiota At the Intersection of Diet and Human Health. Science 362 No. 6416, 776–780. doi: 10.1126/science.aau5812 [DOI] [PubMed] [Google Scholar]
- Gravallese E. M., Schett G. (2018). Effects of the IL-23-IL-17 Pathway on Bone in Spondyloarthritis. Nat. Rev. Rheumatol 14 No. 11, 631–640. doi: 10.1038/s41584-018-0091-8 [DOI] [PubMed] [Google Scholar]
- Hofer U. (2019). Candida Auris’ Potential Link to Climate Change. Nat. Rev. Microbiol. 17 No. 10, 588. doi: 10.1038/s41579-019-0254-x [DOI] [PubMed] [Google Scholar]
- Hultman J., Waldrop M. P., Mackelprang R., David M. M., Mcfarland J., Blazewicz S. J., et al. (2015). Multi-Omics of Permafrost, Active Layer and Thermokarst Bog Soil Microbiomes. Nature 521 No. 7551, 208–212. doi: 10.1038/nature14238 [DOI] [PubMed] [Google Scholar]
- Inness V. L., Mccartney A. L., Khoo C., Gross K. L., Gibson G. R. (2007). Molecular Characterisation of the Gut Microflora of Healthy and Inflammatory Bowel Disease Cats Using Fluorescence in Situ Hybridisation With Special Reference to Desulfovibrio Spp. J. Anim. Physiol. Anim. Nutr. (Berl) 91 No. 1-2, 48–53. doi: 10.1111/j.1439-0396.2006.00640.x [DOI] [PubMed] [Google Scholar]
- Jiang C., Li G., Huang P., Liu Z., Zhao B. (2017). The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 58 No. 1, 1–15. doi: 10.3233/JAD-161141 [DOI] [PubMed] [Google Scholar]
- Jones L., Kumar J., Mistry A., Sankar C. M. T., Perry G., Reddy V. P., et al. (2019). The Transformative Possibilities of the Microbiota and Mycobiota for Health, Disease, Aging, and Technological Innovation. Biomedicines 7 No. 2, 24. doi: 10.3390/biomedicines7020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S., Wang S. E., Lee Y. L., Kang S., Lee B., Han J., et al. (2018). Il-17a Induces Osteoblast Differentiation by Activating JAK2/STAT3 in Ankylosing Spondylitis. Arthritis Res. Ther. 20 No. 1, 115. doi: 10.1186/s13075-018-1582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Yoo S., Kim W. (2016). Gut Microbiota in Autoimmunity: Potential for Clinical Applications. Arch. Pharmacal Res. 39 No. 11, 1565–1576. doi: 10.1007/s12272-016-0796-7 [DOI] [PubMed] [Google Scholar]
- Kitamoto S., Nagao-Kitamoto H., Jiao Y., Gillilland M. R., Hayashi A., Imai J., et al. (2020). The Intermucosal Connection Between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 182 No. 2, 447–462.e14. doi: 10.1016/j.cell.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushkevych I., Dordević D., Vítězová M. (2021). Possible Synergy Effect of Hydrogen Sulfide and Acetate Produced by Sulfate-Reducing Bacteria on Inflammatory Bowel Disease Development. J. Advanced Res. 27, 71–78. doi: 10.1016/j.jare.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladau J., Shi Y., Jing X., He J. S., Chen L., Lin X., et al. (2018). Existing Climate Change Will Lead to Pronounced Shifts in the Diversity of Soil Prokaryotes. mSystems 3 No. 5, e00167–18. doi: 10.1128/mSystems.00167-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hall J. A., Kroehling L., Wu L., Najar T., Nguyen H. H., et al. (2020). Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell 180 No. 1, 79–91.e16. doi: 10.1016/j.cell.2019.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi I., Li X., Semon A., Li D., Doron I., Putzel G., et al. (2018). Cx3cr1(+) Mononuclear Phagocytes Control Immunity to Intestinal Fungi. Science 359 No. 6372, 232–236. doi: 10.1126/science.aao1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Dai B., Tang Y., Lei L., Li N., Liu C., et al. (2019). Altered Bacterial-Fungal Interkingdom Networks in the Guts of Ankylosing Spondylitis Patients. mSystems 4 No. 2, e00176–18. doi: 10.1128/mSystems.00176-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. V., Leonardi I., Iliev I. D. (2019). Gut Mycobiota in Immunity and Inflammatory Disease. Immunity 50 No. 6, 1365–1379. doi: 10.1016/j.immuni.2019.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A. C., Mubareka S., Steel J., Palese P. (2007). Influenza Virus Transmission is Dependent on Relative Humidity and Temperature. PloS Pathog. 3 No. 10, 1470–1476. doi: 10.1371/journal.ppat.0030151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L., Liu Y., Osmond H. L., Cockburn A., Kruuk L. (2020). When to Start and When to Stop: Effects of Climate on Breeding in a Multi-Brooded Songbird. Glob Chang Biol. 26 No. 2, 443–457. doi: 10.1111/gcb.14831 [DOI] [PubMed] [Google Scholar]
- Malard L. A., Pearce D. A. (2018). Microbial Diversity and Biogeography in Arctic Soils. Environ. Microbiol. Rep. 10 No. 6, 611–625. doi: 10.1111/1758-2229.12680 [DOI] [PubMed] [Google Scholar]
- Mao X., Cai T., Olyarchuk J. G., Wei L. (2005). Automated Genome Annotation and Pathway Identification Using the KEGG Orthology (KO) as a Controlled Vocabulary. Bioinformatics 21 No. 19, 3787–3793. doi: 10.1093/bioinformatics/bti430 [DOI] [PubMed] [Google Scholar]
- Miller D., Grant E., Muths E., Amburgey S. M., Adams M. J., Joseph M. B., et al. (2018). Quantifying Climate Sensitivity and Climate-Driven Change in North American Amphibian Communities. Nat. Commun. 9 No. 1, 3926. doi: 10.1038/s41467-018-06157-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z., Chen P. L., Geng F. H., Ren L., Gu W. C., Ma J. Y., et al. (2017). Synergistic Effects of Temperature and Humidity on the Symptoms of COPD Patients. Int. J. Biometeorol 61 No. 11, 1919–1925. doi: 10.1007/s00484-017-1379-0 [DOI] [PubMed] [Google Scholar]
- Naglik J. R., Fidel P. J., Odds F. C. (2008). Animal Models of Mucosal Candida Infection. FEMS Microbiol. Lett. 283 No. 2, 129–139. doi: 10.1111/j.1574-6968.2008.01160.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozuka D., Hashizume M. (2011). The Influence of Temperature and Humidity on the Incidence of Hand, Foot, and Mouth Disease in Japan. Sci. Total Environ. 410-411, 119–125. doi: 10.1016/j.scitotenv.2011.09.055 [DOI] [PubMed] [Google Scholar]
- O’Toole P. W., Jeffery I. B. (2015). Gut Microbiota and Aging. Science 350 No. 6265, 1214–1215. doi: 10.1126/science.aac8469 [DOI] [PubMed] [Google Scholar]
- Piao S., Liu Q., Chen A., Janssens I. A., Fu Y., Dai J., et al. (2019). Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob Chang Biol. 25 No. 6, 1922–1940. doi: 10.1111/gcb.14619 [DOI] [PubMed] [Google Scholar]
- Praeg N., Pauli H., Illmer P. (2019). Microbial Diversity in Bulk and Rhizosphere Soil of Ranunculus Glacialis Along a High-Alpine Altitudinal Gradient. Front. Microbiol. 10, 1429. doi: 10.3389/fmicb.2019.01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost E. J., Kelaher B. P., Dworjanyn S. A., Russell B. D., Connell S. D., Ghedini G., et al. (2017). Climate-Driven Disparities Among Ecological Interactions Threaten Kelp Forest Persistence. Glob Chang Biol. 23 No. 1, 353–361. doi: 10.1111/gcb.13414 [DOI] [PubMed] [Google Scholar]
- Quigley E. (2017). Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 17 No. 12, 94. doi: 10.1007/s11910-017-0802-6 [DOI] [PubMed] [Google Scholar]
- Rehaume L. M., Mondot S., Aguirre De Cárcer D., Velasco J., Benham H., Hasnain S. Z., et al. (2014). Zap-70 Genotype Disrupts the Relationship Between Microbiota and Host, Leading to Spondyloarthritis and Ileitis in SKG Mice. Arthritis Rheumatol. 66 No. 10, 2780–2792. doi: 10.1002/art.38773 [DOI] [PubMed] [Google Scholar]
- Richard M. L., Sokol H. (2019). The Gut Mycobiota: Insights Into Analysis, Environmental Interactions and Role in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol 16 No. 6, 331–345. doi: 10.1038/s41575-019-0121-2 [DOI] [PubMed] [Google Scholar]
- Rosshart S. P., Herz J., Vassallo B. G., Hunter A., Wall M. K., Badger J. H., et al. (2019). Laboratory Mice Born to Wild Mice Have Natural Microbiota and Model Human Immune Responses. Science 365 No. 6452, eaaw4361. doi: 10.1126/science.aaw4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen T., Samanta S. K. (2015). Medicinal Plants, Human Health and Biodiversity: A Broad Review. Adv. Biochem. Eng. Biotechnol. 147, 59–110. doi: 10.1007/10_2014_273 [DOI] [PubMed] [Google Scholar]
- Sooryanarain H., Elankumaran S. (2015). Environmental Role in Influenza Virus Outbreaks. Annu. Rev. Anim. Biosci. 3, 347–73. doi: 10.1146/annurev-animal-022114-111017 [DOI] [PubMed] [Google Scholar]
- Strandwitz P. (2018). Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 1693 No. Pt B, 128–133. doi: 10.1016/j.brainres.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog J. D., Richardson J. A., Croft J. T., Simmons W. A., Zhou M., Fernandez-Sueiro J. L., et al. (1994). The Germfree State Prevents Development of Gut and Joint Inflammatory Disease in HLA-B27 Transgenic Rats. J. Exp. Med. 180 No. 6, 2359–2364. doi: 10.1084/jem.180.6.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Fuentes C., Schellekens H., Dinan T. G., Cryan J. F. (2017). The Microbiota-Gut-Brain Axis in Obesity. Lancet Gastroenterol. Hepatol 2 No. 10, 747–756. doi: 10.1016/S2468-1253(17)30147-4 [DOI] [PubMed] [Google Scholar]
- Wernberg T., Bennett S., Babcock R. C., De Bettignies T., Cure K., Depczynski M., et al. (2016). Climate-Driven Regime Shift of a Temperate Marine Ecosystem. Science 353 No. 6295, 169–172. doi: 10.1126/science.aad8745 [DOI] [PubMed] [Google Scholar]
- Wolf A. J., Limon J. J., Nguyen C., Prince A., Castro A., Underhill D. M. (2020). Malassezia Spp. Induce Inflammatory Cytokines and Activate Nlrp3 Inflammasomes in Phagocytes. J. Leukoc. Biol. 109 (1), 161–172. doi: 10.1002/JLB.2MA0820-259R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Wu J., Cheng J., Wang X., Wen L., Li K., et al. (2017). Is High Relative Humidity Associated With Childhood Hand, Foot, and Mouth Disease in Rural and Urban Areas? Public Health 142, 201–207. doi: 10.1016/j.puhe.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Yao M., Zhang L., Ma J., Zhou L. (2020). On Airborne Transmission and Control of SARS-Cov-2. Sci. Total Environ. 73, 139178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Zhang X., Yang J., Bao J., Xiang H., Dear K., et al. (2017). Humidity May Modify the Relationship Between Temperature and Cardiovascular Mortality in Zhejiang Province, China. Int. J. Environ. Res. Public Health 14 No. 11, 1383. doi: 10.3390/ijerph14111383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Feng C., Ma C., Yang P., Tang S., Lau A., et al. (2015). The Impact of Temperature and Humidity Measures on Influenza A (H7n9) Outbreaks-Evidence From China. Int. J. Infect. Dis. 30, 122–124. doi: 10.1016/j.ijid.2014.11.010 [DOI] [PubMed] [Google Scholar]
- Zhang X., Pan L. Y., Zhang Z., Zhou Y. Y., Jiang H. Y., Ruan B. (2020). Analysis of Gut Mycobiota in First-Episode, Drug-Naive Chinese Patients With Schizophrenia: A Pilot Study. Behav. Brain Res. 39, 11234. doi: 10.1016/j.bbr.2019.112374 [DOI] [PubMed] [Google Scholar]
- Zhou C., Zhao H., Xiao X., Chen B., Guo R., Wang Q., et al. (2020). Metagenomic Profiling of the Pro-Inflammatory Gut Microbiota in Ankylosing Spondylitis. J. Autoimmun. 107, 102360. doi: 10.1016/j.jaut.2019.102360 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Species-level heatmap Species-level heatmap generated using sample abundance similarity for clustering. The difference between two samples is represented visually by the change in color gradient.
(A) Heatmap of significant metabolites depicting differences between the control group and the experimental group. (B) Conjoint analysis of 71 significantly different fungal genera and 17 significantly different fecal metabolites (+p < 0.1, ++p < 0.05, +++ p < 0.01). (C) Spearman’s correlational coefficients for different fungal genera (top 20 with respect to changes in abundance) and 17 significantly different fecal metabolites. In the correlational analysis the probability threshold used to construct correlation networks was p < 0.01, and the correlation coefficient threshold used was > 0.7. Blue nodes represent metabolites, and yellow nodes represent fungal genera. The red line indicates a positive correlation and the black line indicates a negative correlation. More nodal connections indicates more associations with others, and the sizes of nodes correspond with the change in abundance.
Fecal content metabolomics were altered under HTHH conditions (A) Principal component analysis, PLS-DA analysis, and OPLS-DA analysis of fecal metabolites. (B) Pathway analysis of fecal metabolites in the control group and the experimental group.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.