T-cell acute lymphoblastic leukemia (T-ALL) is often not cured by frontline chemotherapy, and efforts to improve treatment by targeting oncogenes such as NOTCH1 have been hampered by toxicity. Silva and colleagues studied primary patient samples to show that high-level interleukin 7 receptor α (IL7Rα) gene expression correlates with ongoing, oncogenic IL7R-mediated signaling. Using new in vivo models, they characterized the impact of IL7Rα expression on the pathogenesis of T-ALL and its response to various targeted therapies that reduce IL7-related signaling.
Key Points
Mice overexpressing IL-7Rα develop leukemia with features of human T-ALL and sensitivity to ruxolitinib, dactolisib, and venetoclax.
T-ALL patients with high levels of wild-type IL7R present with evidence of ongoing, oncogenic-like IL-7R–mediated activation of signaling.
Visual Abstract
Abstract
Tight regulation of IL-7Rα expression is essential for normal T-cell development. IL-7Rα gain-of-function mutations are known drivers of T-cell acute lymphoblastic leukemia (T-ALL). Although a subset of patients with T-ALL display high IL7R messenger RNA levels and cases with IL7R gains have been reported, the impact of IL-7Rα overexpression, rather than mutational activation, during leukemogenesis remains unclear. In this study, overexpressed IL-7Rα in tetracycline-inducible Il7r transgenic and Rosa26 IL7R knockin mice drove potential thymocyte self-renewal, and thymus hyperplasia related to increased proliferation of T-cell precursors, which subsequently infiltrated lymph nodes, spleen, and bone marrow, ultimately leading to fatal leukemia. The tumors mimicked key features of human T-ALL, including heterogeneity in immunophenotype and genetic subtype between cases, frequent hyperactivation of the PI3K/Akt pathway paralleled by downregulation of p27Kip1 and upregulation of Bcl-2, and gene expression signatures evidencing activation of JAK/STAT, PI3K/Akt/mTOR and Notch signaling. Notably, we also found that established tumors may no longer require high levels of IL-7R expression upon secondary transplantation and progressed in the absence of IL-7, but remain sensitive to inhibitors of IL-7R–mediated signaling ruxolitinib (Jak1), AZD1208 (Pim), dactolisib (PI3K/mTOR), palbociclib (Cdk4/6), and venetoclax (Bcl-2). The relevance of these findings for human disease are highlighted by the fact that samples from patients with T-ALL with high wild-type IL7R expression display a transcriptional signature resembling that of IL-7–stimulated pro-T cells and, critically, of IL7R-mutant cases of T-ALL. Overall, our study demonstrates that high expression of IL-7Rα can promote T-cell tumorigenesis, even in the absence of IL-7Rα mutational activation.
Introduction
Interleukin 7 (IL-7) and its receptor, a heterodimer constituted by IL-7Rα (encoded by IL7R) and γc (encoded by IL2RG) subunits, are essential for normal T-cell development and homeostasis,1-4 with IL7R genetic inactivation leading to severe combined immunodeficiency.5 Contrarily, IL7R gain-of-function mutations, which occur in roughly 10% of cases of T-ALL, are considered drivers of leukemia, being largely mutually exclusive with other mutations that affect downstream IL-7R signaling components, including JAK1/3, STAT5B, PTEN, and Akt.6-13 In agreement with an oncogenic role for excessive IL-7/IL-7R signaling, IL-7 transgenic mice develop lymphomas,14 and xenotransplant models of human T-ALL rely on microenvironmental IL-7 for tumor acceleration.15 Moreover, IL-7 prevents spontaneous apoptosis and promotes proliferation of T-ALL cells in vitro,16-20 in large part by activating JAK/STAT5 and PI3K/Akt/mTOR signaling pathways, consequently promoting glucose uptake, upregulating Bcl-2 and downregulating the cyclin-dependent kinase inhibitor p27kip1.16,21-25 These studies highlight the importance of keeping AQ2 IL-7/IL-7R–mediated signaling levels within strict boundaries and, indeed, IL-7Rα expression at the cell surface is tightly regulated throughout both human and mouse T-cell development.1,4
However, although IL7R gene amplification has been reported in T-ALL,26,27 its functional consequences have not been explored, and it is not known whether high levels of wild-typeIL-7Rα are oncogenic, per se. In this study, forced expression of wild-type IL-7Rα in tetracycline-inducible Il7r transgenic or Rosa26 IL7R knockin mice promoted widespread leukemia/lymphoma with features that resemble human T-ALL, and pediatric T-ALL samples with high levels of wild-type IL7R displayed an IL-7R–mediated gene expression profile similar to that observed in IL-7–activated pro-T cells and, notably, in cases with oncogenic IL7R gain-of-function mutations. These results provide strong evidence, arising from 2 different mouse models and from human T-ALL patient samples, that high expression of IL-7Rα can contribute to T-ALL, even in the absence of mutational activation of the receptor.
Methods
Mouse models
TreIL7R rtTA.C IL7rKO (TetIL-7R) tetracycline-inducible IL-7R transgenic mice have been described.28 T-cell receptor (TCR) transgenes were introduced to the TetIL-7R background by intercrossing with class 1–restricted F5 TCR transgenic and class 2–restricted OTII transgenic lines to generate F5 TetIL-7R, F5 Rag1KO TetIL-7R, and OTII TetIL-7R strains. Mice were fed doxycycline (dox) in food (3 mg/g) to induce IL-7Rα expression. Rosa26 IL7R (Rosa26-hIL-7R.huCD2-Cre) mice were generated as follows. We generated a conditional loxP-STOP-human IL7R knockin mouse line under the control of the ubiquitous Rosa26 promoter on a C57Bl6 background. Homozygous animals were bred with CD2iCre animals,29 to promote expression of human IL7R in T cells. CD2-expressing F1 animals were viable and bred again with homozygous conditional animals, to generate animals carrying 2 alleles of the knocked-in human IL7R. CD2− hIL7R+/+ and CD2− hIL7R+/− animals were used as controls. Experimental mice were weighed weekly and euthanized in a CO2 chamber or via pentobarbital injection when they presented clear disease symptoms, with a defined end point of loss of 20% of body weight, breathing impairment, poor reaction to external stimuli, and appearance of a fur ruff. Disseminated disease was confirmed minimally by analyzing the thymus and spleen. Differences in survival curves were determined by log-rank (Mantel-Cox) test with Prism v6.0. All animals were bred and kept in specific pathogen-free facilities at the National Institutes of Medical Research or Instituto de Medicina Molecular João Lobo Antunes, and experiments were performed according to the University College London Animal Welfare and Ethical Review Body and Home Office regulations, United Kingdom, and Instituto de Medicina Molecular João Lobo Antunes' institutional and Portuguese (Direcção-Geral de Alimentação e Veterinária) regulations.
Organ analysis
The animals were dissected, and the organs were mechanically disintegrated into single-cell suspensions in RPMI/2%(w/v)/bovine serum albumin. Bone marrow was extracted by flushing from or crushing the femurs. Cell counts were determined with an automated cell counter (CASY 1, Scharfe System, Reutlingen, Germany), and the cells were subsequently transferred, immunophenotyped, or lysed for immunoblot analysis.
Adoptive transfer
To assess the malignancy of thymus-recovered cells, 10 × 106 cells per 250 μL Iscove’s modified Dulbecco’s medium/BSA were injected via the tail vein into 6- to 8-week-old Rag1−/− mice. The animals were fed 3 mg/g dox-containing food, monitored daily, and euthanized in a CO2 chamber when moribund or at the scheduled time points. Bones, spleen, and thymus were collected for flow cytometry and histological analysis. Leukemic cells (4 × 105) from the Hu-IL7Rα-expressing model were transferred IV into sex and age-matched Rag2−/−γc−/− and Rag2−/−γc−/− Il7−/− mice. The animals were monitored daily and euthanized via pentobarbital injection when moribund. Bones, spleen, and thymus were collected for flow cytometry and histological analysis.
Immunophenotype
Splenic, thymic, and bone marrow cell suspensions were subjected to immunophenotypic analysis by standard methodology. In brief, 2 × 105 to 5 × 105 cells were stained with specific antibodies for 20 minutes at 4°C in phosphate-buffered saline with 2% BSA. Phycoerythrin-conjugated antibody against human IL-7Rα from R&D or ebioscience and Per-CP, PE-Cy7, APC, APC-Cy7, BV421, BV510, BV605, and BV710 conjugated antibodies against CD4, CD5, CD45, CD8, CD44, CD25, CD3, and TCRβ (H57-597), all from Biolegend, were used in diverse combinations. When lineage+ cells were excluded, biotin coupled anti-Gr-1, -CD11b, -CD19, -Ter119, and -CD11c were used and subsequently stained with BV711 streptavidin. Intracellular staining for Ki67 (APC-conjugated; Biolegend) or Bcl-2 (Phycoerythrin-conjugated; Biolegend) was performed with the Foxp3 staining kit from ebiosciences. Eight- and 10-color analyses were performed on LSR Fortessa II (Becton Dickinson San Jose, CA) flow cytometers. Results were analyzed with FlowJo (Tree Star Inc, Ashland, OR) software.
Immunoblot analysis
Cells were lysed as described elsewhere.21 Equal amounts of protein (50 μg/sample) were analyzed by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membranes, and immunoblotted with the antibodies at 1:1000 dilution: p27Kip1, actin (Santa Cruz Biotechnology, Santa Cruz, CA), PTEN, and p-Akt (S473; Cell Signaling Technology, Danvers, MA). Immunodetection was performed by incubation with horseradish peroxidase–conjugated anti-mouse (1:5c000), anti-rabbit IgG (1:10c000), or anti-goat (1:5c000) (Promega, Madison, WI) and developed by enhanced chemiluminescence (Amersham-Pharmacia, Piscataway, NJ).
Mouse transcriptome data
PolyA+ RNA-seq libraries of mouse tumors and normal samples were sequenced as paired-end 75-bp reads, using the standard Illumina pipeline. Data quality were assessed with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Gene expression levels were determined by mapping RNA-seq reads to the mouse genome (mm10) with Kallisto v.2.8.4.30 Comparative transcriptome analysis between mouse tumors and human T-ALL was performed using previously published microarray data.31 First, the raw microarray data were normalized and summarized by using the robust MultiArray average method, as implemented in the “affy” package32 from R (https://www.r-project.org/). Second, human-mouse orthologues were obtained from Ensembl v95 through the “biomaRt” R package.33 Third, human microarray data and mouse RNA-sequencing expression levels were centered and scaled to remove technical bias. Finally, similarities between human and mouse transcriptome profiles were assessed by Pearson correlation, by using previously defined gene signatures for human T-ALL subgroups.31 The correlation coefficients were graphically represented in an unsupervised clustering heat map. To evaluate signaling pathways affected in tumors, we used the Gene Set Enrichment Analysis (GSEA) tool34 and Kyoto Encyclopedia of Genes and Genomes gene sets from the Molecular Signature Database. This analysis was based on the moderated Student t test values between tumor and normal samples estimated from voom-transformed values, as implemented in the “limma” R package.35
Additional details of the methods used are provided in the supplemental Data, available on the Blood Web site.
Results
Overexpression of IL-7Rα perturbs normal T-cell development in young Il7r transgenic mice
To evaluate whether high levels of IL-7Rα expression are sufficient to promote development of T-cell leukemia, we first used a mouse model in which expression of an Il7r transgene on an Il7r-null background, is induced in T-lineage cells by a tetracycline-responsive promotor (TreIl7r rtTAhuCD2 Il7r−/− mice,28 hereinafter referred to as TetIL-7R). Consistent with earlier studies,28 young adult TetIL-7R animals continuously fed dox from birth (TetIL-7RON) displayed some rescue of peripheral T-cell reconstitution, compared with dox-free mice (TetIL-7ROFF), in line with the requirement of IL-7R signaling for normal thymopoiesis (supplemental Figure 1A). However, thymi showed evidence of perturbed development, with increased frequency of immature TCRlo CD8 single-positive (ISP) thymocytes (supplemental Figure 1B). This finding may reflect a partial differentiation block at the ISP stage, which would be in accordance with exacerbated IL-7R–mediated signaling during β-selection.36 Interestingly, mouse recipients of bone marrow progenitor cell transplants expressing JAK3 mutants display a similar accumulation of ISP CD8+ cells,37 in line with JAK3 being downstream of IL-7–mediated signals. Inducible IL-7Rα expression was particularly elevated on the CD8 ISP population and a subset of double-positive (DP) thymocytes (supplemental Figure 1C). Elevated IL-7Rα levels were associated with increased cell size (supplemental Figure 2A) and increased proliferation, as assessed by both DNA content (supplemental Figure 2B) and Ki67 expression (supplemental Figure 2C). Of significance, DNA content distribution indicated that immature CD8 SP thymocytes in TetIL-7RON mice were highly proliferative (supplemental Figure 2B). Accordingly, the Ki67 profile of TetIL-7RON CD8SP cells resembled that of WT ISP, which are known to have high division rates, and not of mature WT CD8SP, which exhibit much less proliferation (supplemental Figure 2C).
TetIL-7R mice display progressive thymic hyperplasia and eventually develop fatal T-cell leukemia/lymphoma
To assess the consequence of abnormal thymic development in TetIL-7R mice, we analyzed lymphoid compartments in TetIL-7R strains28 over time. Ageing is usually associated with thymic atrophy38,39 as confirmed in the control mice. Instead, TetIL7RON strains exhibited progressive increases in thymic cellularity with age (Figure 1A). Hyperplasia was associated with a broad range of aberrant phenotypes that eventually spread to peripheral lymphoid tissues, as hyperplasia progressed to full-blown leukemia/lymphoma (Figure 1B). Disseminated disease was also associated with hyperproliferation in both thymus and periphery, as assessed by Ki67 expression (Figure 1C). The majority of the mice (90%) eventually died of late-onset fatal leukemia/lymphoma (Figure 1D), with kinetics resembling those of other major T-cell oncogenes such as TAL1 or LMO2.40,41 Importantly, disease progression was dependent on induced TetIL-7R expression, because TetIL-7R mice that were not fed dox, as well as control mice that lacked the rtTA driver transgene, remained healthy. Moreover, disease was transferrable, confirming its malignant nature. Adoptive transfer of thymic cells from TetIL-7R mice with evidence of disseminated leukemia/lymphoma (Figure 1E) to immunodeficient Rag1−/− mice resulted in the rapid onset of disease in all recipients (not shown), which displayed a similar pattern of lymphoid organ infiltration as the host, including bone marrow (Figure 1F). Bone marrow involvement and widespread disease are characteristic features of advanced-stage T-ALL.
Figure 1.
IL-7Rα expression results in progressive thymic hyperplasia and disseminated, fatal T-cell leukemia/lymphoma. (A) Thymus cellularity vs age from wild-type F5 control and F5 TetIL-7RON mice. Numbers indicate slope of line fit and 95% confidence intervals. (B) CD4 vs CD8 expression by thymocytes and splenocytes from TetIL-7RON mice (n = 36). Lymphoma/leukemia present in thymus and spleen, characterized by their expression of CD4 and CD8 into DN, CD8 SP, DP, and CD4 SP. A representative example of each phenotype is shown and the percent incidence of phenotype indicated under the phenotypic label. (C) CD4 vs CD8 expression by thymocytes and splenocytes from TetIL-7RON or control C57Bl6/J mice. Histograms are of Ki67 labeling of thymocytes (top) and splenocytes (bottom) of the indicated subpopulation from either TetIL-7RON or C57Bl6/J control mice. (D) Survival of cohorts of TetIL-7RON (n = 8) vs TetIL-7ROFF (n = 4) and TreIL-7R+ rtTA– Il7r−/− mice (n = 4). Mice were culled when they reached the defined humane end point (see “Methods”). P = .0003. (E) Phenotype in the indicated organs of F5 TetIL-7RON mice identified with clinical signs of disease (tumor), as compared with IL-7RWT F5 control mice (control). Density plots are of CD4 vs CD8 in the thymus, spleen and bone marrow of the indicated conditions. (F) Malignant thymocytes from donor mouse in (E) were transferred into Rag1−/− recipients (n = 8). Four weeks later, thymus, spleen, and bone marrow were analyzed for the presence of donor cells. Shown is pooled data of 2 (D) or 6 (A-B) independent experiments or mean results of 3 (C,E-F) independent experiments.
Given the oncogenic potential displayed by high IL-7R expression, we next addressed whether IL-7R overexpression could engage a self-renewal program before development of leukemia. We discontinued dox in TetIL-7R mice at 8 weeks of age and analyzed their phenotype 12 weeks after dox removal. Of 14 animals, 1 displayed near-normal thymic T-cell distribution, 6 showed signs of preleukemia (aberrant T-cell development with evidence of differentiation blockade), and 2 developed leukemia, altogether suggesting that self-renewal was engaged by Il7r overexpression before 8 weeks in most of the cases (supplemental Figure 3). The remaining Off-dox mice did not display (abnormal) T-cell development or signs of disease, indicating that removal of IL7R early on can, in a minority of the cases, prevent T-cell precursor self-renewal and leukemogenesis. As expected, all mice that continued receiving dox (n = 17) displayed an aberrant phenotype or full-blown leukemia at 12 weeks or earlier (supplemental Figure 3). Altogether, our data suggest that IL-7Rα overexpression may engage a self-renewal program in T-cell precursors, which is established by 8 weeks of age in most cases, eventually leading to development of leukemia.
Leukemia development downstream from IL-7R is influenced by Rag1 expression, but not by TCR signaling
Triggering TCR-dependent signaling in T-ALL using high-affinity self-peptide/major histocompatibility class or anti-CD3 monoclonal antibodies has recently been shown to induce apoptosis of T-ALL cells,42 demonstrating the therapeutic potential of activating TCR signals in this malignancy. The relevance of TCR-mediated signaling for T-ALL development is less clear. Because IL-7R expression in TetIL-7RON mice was maximal in immature SP and DP thymocytes, we asked whether TCR-dependent thymic selection signaling could affect the development of the disease. To assess this notion, we compared disease progression of TetIL-7R mice on a polyclonal Tcr background to strains expressing either class 1– or class 2–restricted TCR transgenes (F5 and OTII, respectively). In polyclonal mice, only a small fraction of DPs received stronger TCR signals compatible with selection, whereas, in TCR transgenic mice, all cells expressed TCRs capable of continuing disease development. Despite this, progression in the 3 strains revealed near identical kinetics of disease development, irrespective of TCR specificity (Figure 2A), arguing against a major role for TCR signaling in modulating IL-7R-dependent leukemogenesis. This result is in line with what has been reported for STAT543 transgenic mice, in which modulation of TCR expression did not affect the development of leukemia/lymphoma.
Figure 2.

Disease development is influenced by Rag1 expression but not TCR signaling. (A) Development of malignant disease was monitored in cohorts of TetIL-7RON mice, whose T cells have a polyclonal TCR repertoire (Poly; n = 8), and TCR transgenic F5 TetIL-7RON (F5; n = 6) and OTII TetIL-7RON (OTII; n = 10) mice. Survival of the different strains over time is shown. (B) Survival of cohorts of F5 TetIL-7RON (F5 Rag1+) and F5 Rag1−/− TetIL-7RON (F5 Rag1−; n = 11) mice was monitored up to 400 days of age. *P = .018; n.s., nonsignificant; log-rank, Mantel-Cox test.
The contribution of Rag activity for T-cell leukemogenesis has long been recognized in the context of TCR recombination-driven chromosomal reciprocal translocations displayed by T-ALL patients and recently has been found to be highly involved in leukemogenesis in ETV6-RUNX1+ B-cell ALL.44 Also, we previously showed that T-ALL–associated PTEN microdeletions resulting in loss of PTEN expression are RAG mediated.45 Because some of the tumors displayed low or absent PTEN protein levels, we analyzed the requirement of RAG activity for IL-7R–mediated tumor development. RAGs are essential for TCR gene rearrangement and subsequent T-cell development past the double-negative (DN) stages. As such, lack of RAG activity could affect T-ALL development, merely because it prevents thymocyte differentiation. To avoid this confounding factor, we analyzed TCR-transgenic F5 mice, which do not require RAG activity for T-cell maturation in the thymus. Comparison of F5 TetIL-7R Rag1−/− and F5 TetIL-7R mice revealed that the absence of Rag1 expression significantly delayed, although it did not fully prevent, tumor development (Figure 2B). This observation suggests that RAG activity contributes to acceleration of leukemia, although it is not absolutely required for leukemia development downstream from IL-7R overexpression in T-cell precursors.
Maintenance of established TetIL-7R tumors may occur in the absence of high IL-7Rα
Making use of our inducible model, we next assessed whether IL-7Rα expression is necessary for maintenance and expansion of established tumors. Malignant cells isolated from 3 independent primary F5 TetIL-7R tumors were transferred into Rag1−/−-recipient mice, either fed doxycycline (On dox), to maintain Il7r expression, or kept dox free, to cease gene induction. Both groups of mice were culled 4 weeks after transplantation because of disease symptoms (≥20% weight loss). As expected, cells recovered from Off-dox recipient mice showed major downregulation of IL-7Rα to levels comparable to DP cells of F5 control mice, which express low-to-undetectable levels of IL-7Rα (Figure 3A). However, the leukemia cells presented a similar immunophenotype (Figure 3B) and were found in numbers similar to those recovered from dox-fed hosts. These results suggest that, at least in some cases, high levels of wild-type IL-7Rα expression may be redundant for the maintenance of fully established leukemias once transformation has occurred.
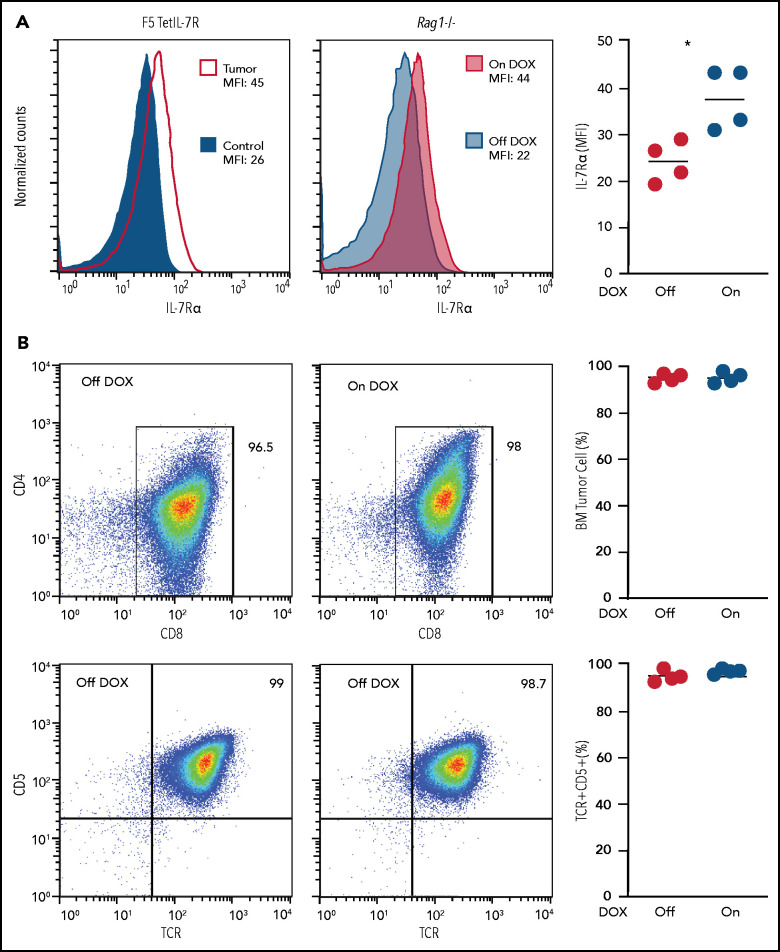
Figure 3.
Maintenance of established TetIL-7R tumors no longer appears to require high IL-7Rα expression. (A) IL-7Rα expression was measured in F5 TetIL-7R primary tumor cells (left), and after adoptive transfer into Rag1−/− mice (middle). Mean fluorescence intensity (MFI) of primary tumor cells collected from the thymus of a sick mouse continuously fed dox (tumor) were compared with the DP (CD4+CD8+) cells of an F5 control mouse (used as the negative control; left). Malignant cells collected from the thymus of the same mouse were transplanted into Rag1−/− recipient mice that were fed with (On dox) or not fed (Off dox) dox-containing food for 4 weeks after transplantation (middle). MFI was compared after 4 weeks between On- and Off-dox groups. IL-7Rα MFI for each transplant-recipient animal (right). (B) Transplanted cells collected from the bone marrow of On- and Off-dox recipient mice 4 weeks after transplantation were compared for their immunophenotype. CD4 vs CD8 (top) and CD5 vs TCRαβ (bottom). Frequency of cells within the gate (left) for each transplant recipient. Results are representative of 3 independent experiments (each from a different primary tumor).
TetIL-7R tumors display hyperactivation of PI3K/Akt pathway and mimic multiple features of human T-ALL
Remarkably, Tet-IL-7R tumors mimicked numerous important features of human T-ALL. First, their immunophenotype varied considerably between animals (from CD4,CD8 DN to DP, to CD4 or CD8 SP cells), reflecting the different stages of maturation block known to occur in the human disease (Figure 1C).46,47 Second, heterogeneity extended to the genetic subset of T-ALL affecting each animal. Transcriptomic analyses showed that tumors resemble different major human T-ALL subsets,31 tending to cluster into 2 main groups (TAL/LMO+proliferative or HOXA/TLX+immature; Figure 4A). Taken together, these results suggest that IL-7R–mediated transformation is not restricted to a particular T-ALL oncogenetic subtype, in agreement with what appears to happen with mutant IL7R in human T-ALL,6,12 or to a single maturation stage, in accordance with the fact that human T-ALL cells respond to IL-7, irrespective of their stage of differentiation.19
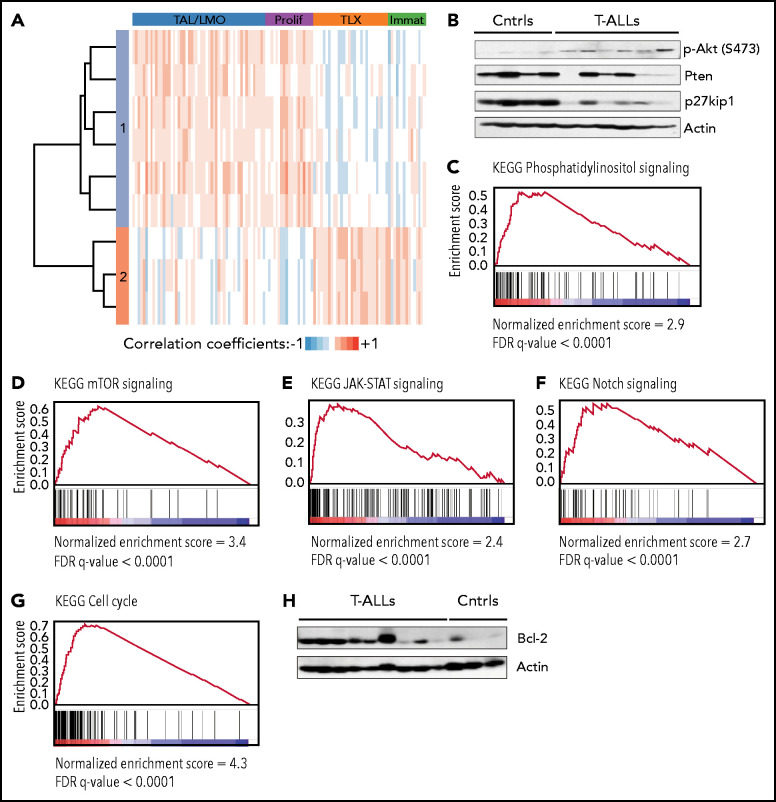
Figure 4.
TetIL-7R tumors display hyperactivation of the PI3K/Akt pathway and mimic multiple features of human T-ALL. (A) Hierarchical clustering analysis of Pearson correlation coefficients between mouse tumors and human T-ALL. Each row corresponds to a mouse leukemia and each lane to a human T-ALL sample. Transcriptomic analyses showed that mouse tumors resemble either TAL/LMO+proliferative T-ALLs (cluster 1) or HOXA/TLX+immature (cluster 2), as defined in Homminga et al.31 Robustness of this analysis is shown by the fact that mouse leukemias that were “classified” as HOXA/TLX+immature–like display features of immature/ETP-ALL, such as higher KIT, CD33, and CD34 than the other tumors (supplemental Figure 4). (B) Akt activation (p-Akt), and PTEN and p27Kip1 expression levels were evaluated by immunoblot in On-dox F5 TetIL-7R thymic tumors (T-ALLs) vs control thymic samples from healthy, F5 mice (Ctrls). (C-G) GSEA of the ranked expression differences between tumors and controls for the KEGG pathways: phosphatidylinositol signaling (C), mTOR signaling (D), JAK-STAT signaling (E), Notch signaling (F), and cell cycle (G). (H) Bcl-2 expression levels were evaluated by immunoblot in tumors and controls. KEGG, Kyoto Encyclopedia of Genes and Genomes.
Third, similar to most primary T-ALL cases, most tumors displayed hyperactivation of the PI3K/Akt pathway,45,46,48 sometimes associated with absence or decreased Pten protein expression (Figure 4B) and Pten mutation (supplemental Table 1). In agreement, GSEA of differentially expressed genes between tumors and controls revealed a strong enrichment for phosphatidylinositol and mTOR (Figure 4C-D) signaling in tumors. Fourth, in accordance with increased IL-7R–mediated signaling, and similar to human T-ALL,6,23,24,49-53 the tumors were enriched in genes upregulated in JAK/STAT pathway signaling (Figure 4E). Fifth, we found evidence of Notch1 mutation (supplemental Table 1) and Notch signaling activation (Figure 4F), a hallmark of both mouse and human T-ALL. Sixth, in accordance with increased proliferation (Figures 1C and 4G), the cell cycle inhibitor p27Kip1 was frequently downregulated (Figure 4B), a molecular characteristic of human T-ALL cells,54 particularly of IL-7–responsive cases.16,21 Finally, the expression of Bcl-2, which is upregulated by and mandatory for IL-7–mediated viability of primary human T-ALL cells,15-17 was also higher in the tumors (Figure 4H).
Human IL-7Rα expression leads to the dose-dependent development of T-cell leukemias that are sensitive to inhibition of different IL-7R downstream effectors
Next, we evaluated whether the human wild-type IL-7Rα had an oncogenic potential similar to that of the mouse and assessed whether higher levels of receptor expression are more efficient in driving T-cell malignancy. To do this, we modified the ubiquitously expressed Rosa26 locus to express human IL7R. LoxP-flanked TpA stop signals prevented constitutive IL7R gene expression. However, introducing an huCD2-Cre transgene generated mice in which human IL-7Rα expression was released in lymphoid precursors (Rosa26-hIL-7R.huCD2-Cre, hereinafter referred to as R26-hIL-7R) developing on an otherwise normal immune background. As expected, homozygous mice (hIL-7R+/+), with 2 copies of hIL7R, displayed higher surface hIL-7Rα levels than heterozygous (hIL-7R+/−) mice (Figure 5A-B), whereas expression of other γc family cytokine receptors was not affected by IL-7R overexpression (supplemental Figure 5). Notably, hIL-7R+/+ animals also developed malignant disease significantly faster than hIL-7R+/− (Figure 5C), indicating an IL-7Rα dose-dependent leukemogenic effect. Analysis of hIL-7R+/+ mice with disease revealed expansion of CD8+CD4− TCRlo thymocytes (Figure 5D) with a proliferative phenotype (Figure 5E) and an increased thymus size (Figure 5F). Malignant T cells spread to the bone marrow (Figure 5D-E) and spleen (Figure 5D-F), which presented with splenomegaly (Figure 5F). Full necropsy showed leukemia spread to the lymph nodes, heart, lung, liver, kidney, and central nervous system (supplemental Figure 6A). Flow cytometry did not reveal any B-cell malignancies, all leukemias being CD19− and displaying only T-cell markers (supplemental Figure 6B). Transplant experiments into Rag1−/− Il2rg−/− vs Rag1−/− Il2rg−/− Il7−/− mice showed that established tumors remained IL-7 responsive, although leukemia/lymphoma was eventually propagated, even in the absence of IL-7 (Figure 5G). These results were in line with those from the dox-inducible model and indicated that full-blown leukemias triggered by high levels of IL-7R expression were no longer necessarily fully reliant on microenvironmental IL-7–mediated signals for their propagation.
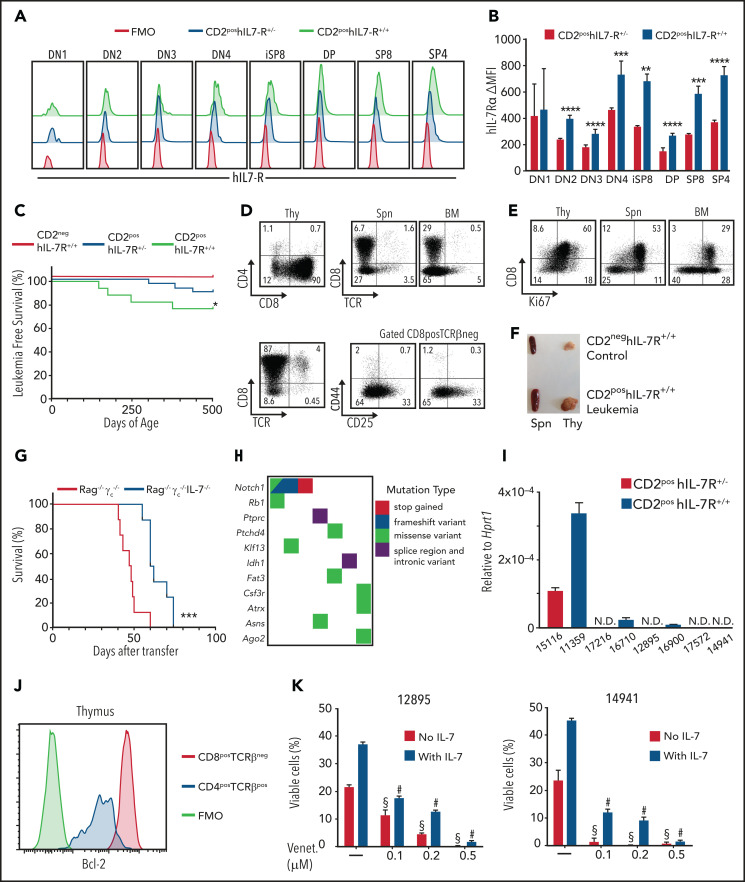
Figure 5.
Human IL-7Rα expression leads to the dose-dependent development of T-cell leukemias that are sensitive to Bcl-2 inhibition. (A) Flow cytometry analysis for hIL-7Rα within Linneg thymocytes from 12-week-old animals of the indicated genotypes. FMO, fluorescence minus 1 negative control. (B) Difference from FMO of MFI of hIL-7Rα within each thymocyte subpopulation in heterozygous or homozygous animals. Standard deviation (SD) is indicated. **P < .01; ***P < .001; ****P < .0001; Student t test. (C) Survival corresponding to the indicated genotypes. No leukemias were observed in the CD2neg hIL-7R+/− cohort (not shown). *P < .05; log-rank, Mantel-Cox test . CD2neg hIL-7R+/+; n = 23; CD2pos hIL-7R+/−; n = 28; and CD2pos hIL7R+/+; n = 18. (D-F) Analysis of a representative leukemic animal euthanized when moribund. (D) Dot plots show CD4/CD8 coreceptor and CD8/TCRβ expression within Linneg thymocytes (Thy) and the presence of the same cells in the spleen (Spn) and bone marrow (BM). (E) CD8/Ki67 expression in thymus (Thy), Spn, and BM. (F) Animal that presented with a very large thymus and enlarged spleen vs control. (G) Survival of Rag−/−γc−/− or Rag−/−γc−/−IL-7−/− recipients of leukemic cells (4 × 105; n = 8 per group). ***P < .001, log-rank, Mantel-Cox. (H) Mutational burden map of single-nucleotide and indel variants with predicted high and moderate impact in functionally relevant genes and drivers of T-ALL or pediatric leukemias in CD2pos hIL-7R leukemias. (I) Il7 messenger RNA expression levels relative to Hprt1 in leukemic cells from CD2pos hIL-7R leukemias were quantified by quantitative real-time reverse transcription polymerase chain reaction. Average of triplicate experiments and SD are shown. (J) Bcl-2 flow cytometry analysis of CD4posTCRβpos normal SP thymocytes and CD8posTCRβneg leukemic cells of the same animal as in panels D-F. (K) Cells from 2 different leukemias (12895 and 14941) were cultured in the presence of the indicated doses of the Bcl-2 inhibitor venetoclax and in the absence (red bars) or presence (purple bars) of IL-7. Data show viability at 48 hours. One-way analysis of variance with Tukey’s correction for multiple comparisons. #P < .0001, venetoclax in the presence of IL-7 vs IL-7 alone; §P < .0001, venetoclax in the absence of IL-7 vs medium alone.
To examine putative mechanisms justifying these observations and identify secondary hits collaborating with high IL-7R expression in driving T-ALL, we performed whole-exome sequencing of leukemia samples from R26-hIL-7R mice. As expected, Notch1, a major T-ALL oncogene, was frequently mutated (Figure 5H; supplemental Table 2). In addition, we found mutations in Rb1, Atrx, Ptchd4, and Idh1, which are known cancer drivers, including in T-ALL. Other affected genes included Fat3 (belonging to the same functional family as the T-ALL driver Fat1), Csfr3 (which is mutated in myeloid leukemias), and Klf13 (involved in B- and T-cell development). Notably, some of the tumors displayed mutations in genes that are directly related to IL-7R downstream signaling, such as Ptprc (CD45), whose loss-of-function mutation in T-ALL potentiates JAK/STAT signaling55; Ago2, which interplays with KRAS signaling56; and Asns, known to be upregulated by IL-7R signaling57 (Figure 5H; supplemental Table 2). In addition, although IL-7 levels were not significantly different between IL-7R–overexpressing mice and controls, as measured by quantitative polymerase chain reaction in lymph nodes and enzyme-linked immunosorbent assay in the blood (supplemental Figure 7), we found that some of the tumors displayed detectable IL-7 transcript levels (Figure 5I), suggesting that, similar to human T-ALL,58 some mouse leukemias may display IL-7 autocrine production.
Irrespective of the mechanism, leukemias arising from hIL-7R overexpression displayed activation of IL-7R signaling, as assessed by Bcl-2 upregulation (Figure 5J). Accordingly, the Bcl-2 inhibitor venetoclax triggered leukemia cell death and prevented IL-7–mediated viability in a dose-dependent manner (Figure 5K). Similar results were obtained with inhibitors of other IL-7R effectors, such as JAK1,24,52,59 PI3K/mTOR,21,22 PIM1,23,60 and Cdk4/6,16 all of which also had cytotoxic effects on T-ALL cells (supplemental Figure 8). Taken together, these observations suggest that acquisition of second hits leading to activation of downstream IL-7R signaling may be the reason that some cases are no longer fully dependent on microenvironmental IL-7 or require high IL-7R surface expression and yet still display clear evidence of IL-7/IL-7R downstream activation.
T-ALL patients with high wild-type IL7R expression display evidence of oncogenic IL-7R–dependent signaling activation
To further confirm the relevance of our findings for human disease, we next analyzed IL7R expression in a cohort of IL7R wild-type T-ALL cases. We found highly heterogeneous expression of IL7R (Figure 6A). We then compared the 20 cases with the highest to the 20 cases with the lowest IL7R expression, and GSEA of differentially expressed genes showed enrichment of genes that are targets of IL-7 stimulation61 (normalized enrichment score [NES], 2.044; P < .001) in the IL7R-high samples. These data demonstrate that human T-ALL cases with high levels of wild-type IL7R displayed evidence of active IL-7 receptor signaling (Figure 6B). Importantly, genes upregulated in IL7R-mutant T-ALL samples were also enriched in wild-type IL7R-high cases (NES, 1.635; P = .01), whereas genes downregulated in IL7R-mutant cases showed negative enrichment (NES, −1.277; P = .0069; Figure 6C). These results indicate that high levels of expression of wild-type IL-7Rα in patients with T-ALL are associated with a gene expression signature that resembles that of IL7R-mutant cases.
Figure 6.
Patients with T-ALL with high wild-type IL7R expression display evidence of oncogenic IL-7R–dependent signaling activation. (A) Normalized IL7R gene expression levels in T-ALL patients with wild-type IL7R (n = 246). Dashed lines mark the 20 cases with the highest expression (above top line) and the 20 cases with lowest IL7R expression (below bottom line), used for comparison in the subsequent analyses. Only IL7R wild-type cases were analyzed. (B-C) Ranked GSEA on differentially expressed genes between IL7R-high and -low cases for the sets of IL-7 target genes in pro-T cells (B), and genes upregulated in IL7R-mutant T-ALL samples (left) and downregulated in IL7R-mutant cases (right) (C).
Discussion
IL7R mutational activation is a known driver of T-ALL.6-13 In addition, several mechanisms can lead to increased expression of wild-type IL-7Rα in T-ALL (eg, Notch activation,62 RPL10 R98S mutation,63 or ZEB2 translocation).64 Mutations in genes such as DNM2,65 which regulate IL-7Rα trafficking and surface availability,66 also potentially contribute to oncogenic IL-7R-mediated signaling. Most notably, there are reported cases of IL7R gene amplification in T-ALL.26,27 However, whether high IL-7Rα levels can drive T-ALL remains unaddressed. Although correlative evidence associates expression of IL-7Rα in AKR/J mice with development of leukemia,67 and a recent study has shown a correlation between high levels of IL-7R expression and increased leukemia stem cell activity in established human T-ALL,68 there is no direct proof of the oncogenic potential of overexpression of IL-7Rα without gain-of-function mutation. This finding is of clinical relevance, because there is a significant fraction of patients with T-ALL who present with very high IL7R levels and, as we demonstrated in this study, gene expression profiling indicates that the leukemia cells display evidence of ongoing IL-7/IL-7R signaling activation that resembles that of oncogenic IL7R-mutant T-ALL cases. Deep characterization of the similarities and differences between mutant and high-level wild-type IL-7R signaling and downstream gene expression changes may expose therapeutic vulnerabilities and merits investigation. Our analyses of patient data suggest that not only mutational activation of IL-7Rα but also high levels of expression are oncogenic. We confirmed this possibility by providing clear evidence, using 2 different in vivo models, that IL-7Rα is oncogenic, even in the absence of mutational activation. Again, this finding is clinically relevant, because it implies that T-ALL cases with high IL-7Rα expression also benefit from treatment with inhibitors of IL7R-mediated signaling, including JAK1/3, PIM1, PI3K, and IL-7R itself.25,69,70 Anti-IL-7Rα antibodies are promising new therapeutic tools against T-ALL,69,70 and their impact, particularly on IL7R high T-ALL cases, warrants investigation. However, our findings indicating that some mouse T-ALLs no longer require high levels of IL-7R expression for leukemia maintenance serve as an alert that targeting of the receptor may not always be effective therapeutically. This lack of efficacy may be caused by genetic lesions on Notch1, Atrx, Ptchd4, or Idh1, which could drive a shift in oncogene addiction, or it may be because of the acquisition of mutations, such as those we found in Ptprc, Ago2, Asns, Pten, or Rb1, which are either regulated by, or interact with, signaling pathways activated by IL-7R and thus can mimic or lead to IL-7R–mediated downstream signaling activation. In agreement with the latter, leukemia samples remain sensitive to pharmacological inhibitors of IL-7R signaling effectors, such as JAK1 (ruxolitinib), PI3K/mTOR (dactolisib), or PIM1 (AZD1208).6,21-24,27,52,59,60
Our studies also suggest that IL-7Rα overexpression promotes thymocyte self-renewal by 8 weeks of age, in most cases, eventually leading to subsequent leukemia development. An alternative (not mutually exclusive) explanation as to why some of the cases display a (pre)leukemic phenotype would be the acquisition of secondary oncogenic hits, which would not necessarily involve previous engagement of a self-renewal program. Further experiments are warranted to determine more exactly how long high expression of IL-7R is needed to consistently engage self-renewal and/or a leukemogenic program.
The mouse tumors that develop downstream from IL-7Rα mimic multiple features of human T-ALL, including interpatient heterogeneity in immunophenotype and oncogenetic subtype, similar to what was found for cases with IL7R mutation, which occur in different T-ALL subtypes.12 Our in vivo models may therefore be instrumental for the thorough characterization of IL-7R–mediated T-ALL, the potential unmasking of molecular targets for therapeutic intervention, and the testing of novel treatment strategies. Obvious candidates for therapeutic intervention include, as mentioned, inhibitors of JAK/STAT/PIM pathway or PI3K/Akt signaling,21-24,27,52,60,71 whose efficacy we also demonstrated in this study by the use of ruxolitinib, AZD1208, and dactolisib in IL-7R–overexpressing mouse leukemias. Given the known positive impact of IL-7/IL-7R–mediated signaling on Bcl-2 expression in T-ALL cells,16,17,21,23,72 we also tested the BH3 mimetic drug venetoclax, which clearly promoted cell death in vitro. These results are in line with other studies providing evidence of the potential of Bcl-2 inhibitors against T-ALL.73-75 In addition, in agreement with IL-7 induction of cell cycle progression in human T-ALL cells,16 we demonstrated the efficacy of the Cdk4/6 inhibitor palbociclib76 in our mouse T-ALLs.
Overall, our study provides the first direct evidence that IL-7Rα can promote T-cell tumorigenesis in a dose-dependent manner, even in the absence of IL7R gain-of-function mutations. Our findings are of particular relevance for the understanding of the biology and the treatment of T-ALL cases with high IL7R levels, including those with IL7R gains.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank S. Tung and the Medical Research Council (MRC) National Institute for Medical Research Biological Services staff for assistance with mouse breeding and typing; the MRC National Institute for Medical Research flow cytometry and high-throughput sequencing core facilities; Pedro Ruivo and iMM’s Comparative Pathology Unit; and Marta Fernandes for preparing the visual abstract.
The work was supported by the MRC under Programme Codes U117573801 and MR/P011225/1, to and by the consolidator grant ERC CoG-648455 from the European Research Council, under the European Union's Horizon 2020 research and innovation programme, and the FAPESP/20015/2014 grant from Fundação para a Ciência e a Tecnologia (FCT) (J.T.B.). A.S. received a fellowship from FCT (SFRH/BD/18388/2004). J.T.B. was an FCT Investigator (consolidator) and A.R.G. was the recipient of an FCT Investigator grant (IF/00510/2014). This work was also supported by a grant from “Fonds Kinderen Kankervrij” (KiKa 2010-082) (Y.L.).
Footnotes
RNA-seq data for mouse tumor and control samples are available at Gene Expression Omnibus (GEO) (accession number GSE128212). WES data for tumor and control samples are available at the Sequence Read Archive (accession number PRJNA716947; under submission).
Original data are available by e-mail request to the corresponding authors.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.S. and A.R.M.A. designed and performed experiments and analyzed and interpreted the data; A.C. and T.H. performed experiments; J.L.N., M.d.M., S.D., and Y.L. conducted bioinformatics analyses; J.M., J.C., and A.R.G. supervised the bioinformatics analyses and provided critical suggestions and feedback; B.S. and J.T.B. designed the research, analyzed and interpreted the data, supervised the experiments and wrote the manuscript; and all authors critically read and contributed to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.S. is Roche Pharma Research and Early Development, Pharmaceutical Sciences, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Correspondence: Benedict Seddon, Division of Infection and Immunity, Institute of Immunity and Transplantation, University College London, Royal Free Hospital, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: benedict.seddon@ucl.ac.uk; and João T. Barata, Instituto de Medicina Molecular João Lobo Antunes, Lisbon University Medical School, Av Prof Egas Moniz, 1649-028 Lisbon, Portugal; e-mail: joao_barata@medicina.ulisboa.pt.
REFERENCES
- 1.Fry TJ, Mackall CL.. Interleukin-7: from bench to clinic. Blood. 2002;99(11):3892-3904. [DOI] [PubMed] [Google Scholar]
- 2.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R.. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181(4):1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barata JT, Durum SK, Seddon B.. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol. 2019;20(12):1584-1593. [DOI] [PubMed] [Google Scholar]
- 5.Puel A, Ziegler SF, Buckley RH, Leonard WJ.. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20(4):394-397. [DOI] [PubMed] [Google Scholar]
- 6.Zenatti PP, Ribeiro D, Li W, et al. Oncogenic IL7R gain-of-function mutations in childhood T-cell acute lymphoblastic leukemia. Nat Genet. 2011;43(10):932-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shochat C, Tal N, Bandapalli OR, et al. Gain-of-function mutations in interleukin-7 receptor-α (IL7R) in childhood acute lymphoblastic leukemias. J Exp Med. 2011;208(5):901-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012; 481(7380):157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treanor LM, Zhou S, Janke L, et al. Interleukin-7 receptor mutants initiate early T cell precursor leukemia in murine thymocyte progenitors with multipotent potential. J Exp Med. 2014;211(4):701-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama K, Yokoyama N, Izawa K, et al. In vivo leukemogenic potential of an interleukin 7 receptor α chain mutant in hematopoietic stem and progenitor cells. Blood. 2013; 122(26):4259-4263. [DOI] [PubMed] [Google Scholar]
- 11.De Keersmaecker K, Atak ZK, Li N, et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat Genet. 2013;45(2):186-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Easton J, Shao Y, et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet. 2017;49(8):1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canté-Barrett K, Spijkers-Hagelstein JA, Buijs-Gladdines JG, et al. MEK and PI3K-AKT inhibitors synergistically block activated IL7 receptor signaling in T-cell acute lymphoblastic leukemia. Leukemia. 2016;30(9):1832-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rich BE, Campos-Torres J, Tepper RI, Moreadith RW, Leder P.. Cutaneous lymphoproliferation and lymphomas in interleukin 7 transgenic mice. J Exp Med. 1993;177(2):305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva A, Laranjeira AB, Martins LR, et al. IL-7 contributes to the progression of human T-cell acute lymphoblastic leukemias. Cancer Res. 2011;71(14):4780-4789. [DOI] [PubMed] [Google Scholar]
- 16.Barata JT, Cardoso AA, Nadler LM, Boussiotis VA.. Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1). Blood. 2001;98(5):1524-1531. [DOI] [PubMed] [Google Scholar]
- 17.Karawajew L, Ruppert V, Wuchter C, et al. Inhibition of in vitro spontaneous apoptosis by IL-7 correlates with bcl-2 up-regulation, cortical/mature immunophenotype, and better early cytoreduction of childhood T-cell acute lymphoblastic leukemia. Blood. 2000;96(1):297-306. [PubMed] [Google Scholar]
- 18.Scupoli MT, Perbellini O, Krampera M, Vinante F, Cioffi F, Pizzolo G.. Interleukin 7 requirement for survival of T-cell acute lymphoblastic leukemia and human thymocytes on bone marrow stroma. Haematologica. 2007;92(2):264-266. [DOI] [PubMed] [Google Scholar]
- 19.Barata JT, Keenan TD, Silva A, Nadler LM, Boussiotis VA, Cardoso AA.. Common gamma chain-signaling cytokines promote proliferation of T-cell acute lymphoblastic leukemia. Haematologica. 2004;89(12):1459-1467. [PubMed] [Google Scholar]
- 20.Dibirdik I, Langlie MC, Ledbetter JA, et al. Engagement of interleukin-7 receptor stimulates tyrosine phosphorylation, phosphoinositide turnover, and clonal proliferation of human T-lineage acute lymphoblastic leukemia cells. Blood. 1991;78(3):564-570. [PubMed] [Google Scholar]
- 21.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA.. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J Exp Med. 2004;200(5):659-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva A, Gírio A, Cebola I, Santos CI, Antunes F, Barata JT.. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia. 2011;25(6):960-967. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro D, Melão A, van Boxtel R, et al. STAT5 is essential for IL-7-mediated viability, growth, and proliferation of T-cell acute lymphoblastic leukemia cells. Blood Adv. 2018;2(17):2199-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado-Martin C, Meyer LK, Huang BJ, et al. JAK/STAT pathway inhibition overcomes IL7-induced glucocorticoid resistance in a subset of human T-cell acute lymphoblastic leukemias. Leukemia. 2017;31(12):2568-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cramer SD, Aplan PD, Durum SK.. Therapeutic targeting of IL-7Rα signaling pathways in ALL treatment. Blood. 2016;128(4):473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vicente C, Schwab C, Broux M, et al. Targeted sequencing identifies associations between IL7R-JAK mutations and epigenetic modulators in T-cell acute lymphoblastic leukemia. Haematologica. 2015;100(10):1301-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Buijs-Gladdines JG, Canté-Barrett K, et al. IL-7 Receptor Mutations and Steroid Resistance in Pediatric T cell Acute Lymphoblastic Leukemia: A Genome Sequencing Study. PLoS Med. 2016;13(12):e1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buentke E, Mathiot A, Tolaini M, Di Santo J, Zamoyska R, Seddon B.. Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood. 2006;108(6):1949-1956. [DOI] [PubMed] [Google Scholar]
- 29.de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33(2):314-325. [DOI] [PubMed] [Google Scholar]
- 30.Grabherr MG, Haas BJ, Yassour M, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homminga I, Pieters R, Langerak AW, et al. Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell. 2011;19(4):484-497. [DOI] [PubMed] [Google Scholar]
- 32.Gautier L, Cope L, Bolstad BM, Irizarry RA.. affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307-315. [DOI] [PubMed] [Google Scholar]
- 33.Durinck S, Spellman PT, Birney E, Huber W.. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc. 2009;4(8):1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545-15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boudil A, Matei IR, Shih HY, et al. IL-7 coordinates proliferation, differentiation and Tcra recombination during thymocyte β-selection. Nat Immunol. 2015;16(4):397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Degryse S, de Bock CE, Cox L, et al. JAK3 mutants transform hematopoietic cells through JAK1 activation, causing T-cell acute lymphoblastic leukemia in a mouse model. Blood. 2014;124(20):3092-3100. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhry MS, Velardi E, Dudakov JA, van den Brink MR.. Thymus: the next (re)generation. Immunol Rev. 2016;271(1):56-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chinn IK, Blackburn CC, Manley NR, Sempowski GD.. Changes in primary lymphoid organs with aging. Semin Immunol. 2012; 24(5):309-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larson RC, Lavenir I, Larson TA, et al. Protein dimerization between Lmo2 (Rbtn2) and Tal1 alters thymocyte development and potentiates T cell tumorigenesis in transgenic mice. EMBO J. 1996;15(5):1021-1027. [PMC free article] [PubMed] [Google Scholar]
- 41.O’Neil J, Shank J, Cusson N, Murre C, Kelliher M.. TAL1/SCL induces leukemia by inhibiting the transcriptional activity of E47/HEB. Cancer Cell. 2004;5(6):587-596. [DOI] [PubMed] [Google Scholar]
- 42.Trinquand A, Dos Santos NR, Tran Quang C, et al. Triggering the TCR Developmental Checkpoint Activates a Therapeutically Targetable Tumor Suppressive Pathway in T-cell Leukemia. Cancer Discov. 2016;6(9):972-985. [DOI] [PubMed] [Google Scholar]
- 43.Bessette K, Lang ML, Fava RA, et al. A Stat5b transgene is capable of inducing CD8+ lymphoblastic lymphoma in the absence of normal TCR/MHC signaling. Blood. 2008; 111(1):344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papaemmanuil E, Rapado I, Li Y, et al. RAG-mediated recombination is the predominant driver of oncogenic rearrangement in ETV6-RUNX1 acute lymphoblastic leukemia. Nat Genet. 2014;46(2):116-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendes RD, Sarmento LM, Canté-Barrett K, et al. PTEN microdeletions in T-cell acute lymphoblastic leukemia are caused by illegitimate RAG-mediated recombination events. Blood. 2014;124(4):567-578. [DOI] [PubMed] [Google Scholar]
- 46.Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118(11):3762-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrando AA, Neuberg DS, Staunton J, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1(1):75-87. [DOI] [PubMed] [Google Scholar]
- 48.Gutierrez A, Sanda T, Grebliunaite R, et al. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114(3):647-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flex E, Petrangeli V, Stella L, et al. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205(4):751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kontro M, Kuusanmäki H, Eldfors S, et al. Novel activating STAT5B mutations as putative drivers of T-cell acute lymphoblastic leukemia. Leukemia. 2014;28(8):1738-1742. [DOI] [PubMed] [Google Scholar]
- 51.Bandapalli OR, Schuessele S, Kunz JB, et al. The activating STAT5B N642H mutation is a common abnormality in pediatric T-cell acute lymphoblastic leukemia and confers a higher risk of relapse. Haematologica. 2014;99(10):e188-e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maude SL, Dolai S, Delgado-Martin C, et al. Efficacy of JAK/STAT pathway inhibition in murine xenograft models of early T-cell precursor (ETP) acute lymphoblastic leukemia. Blood. 2015;125(11):1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Degryse S, Bornschein S, de Bock CE, et al. Mutant JAK3 signaling is increased by loss of wild-type JAK3 or by acquisition of secondary JAK3 mutations in T-ALL. Blood. 2018;131(4):421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfraim LA, Fernandez TM, Mamura M, et al. Loss of Smad3 in acute T-cell lymphoblastic leukemia. N Engl J Med. 2004;351(6):552-559. [DOI] [PubMed] [Google Scholar]
- 55.Porcu M, Kleppe M, Gianfelici V, et al. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood. 2012;119(19):4476-4479. [DOI] [PubMed] [Google Scholar]
- 56.Shankar S, Pitchiaya S, Malik R, et al. KRAS Engages AGO2 to Enhance Cellular Transformation. Cell Rep. 2016;14(6):1448-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng H, Yu M, Tan H, et al. Discrete roles and bifurcation of PTEN signaling and mTORC1-mediated anabolic metabolism underlie IL-7-driven B lymphopoiesis. Sci Adv. 2018;4(1):eaar5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buffière A, Uzan B, Aucagne R, et al. T-cell acute lymphoblastic leukemia displays autocrine production of Interleukin-7. Oncogene. 2019;38(48):7357-7365. [DOI] [PubMed] [Google Scholar]
- 59.Melão A, Spit M, Cardoso BA, Barata JT.. Optimal interleukin-7 receptor-mediated signaling, cell cycle progression and viability of T-cell acute lymphoblastic leukemia cells rely on casein kinase 2 activity. Haematologica. 2016;101(11):1368-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Smedt R, Morscio J, Reunes L, et al. Targeting cytokine- and therapy-induced PIM1 activation in preclinical models of T-cell acute lymphoblastic leukemia and lymphoma. Blood. 2020;135(19):1685-1695. [DOI] [PubMed] [Google Scholar]
- 61.Bornschein S, Demeyer S, Stirparo R, et al. Defining the molecular basis of oncogenic cooperation between TAL1 expression and Pten deletion in T-ALL using a novel pro-T-cell model system. Leukemia. 2018;32(4):941-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.González-García S, García-Peydró M, Martín-Gayo E, et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7Ralpha gene expression in early human thymopoiesis and leukemia. J Exp Med. 2009;206(4):779-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girardi T, Vereecke S, Sulima SO, et al. The T-cell leukemia-associated ribosomal RPL10 R98S mutation enhances JAK-STAT signaling. Leukemia. 2018;32(3):809-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goossens S, Radaelli E, Blanchet O, et al. ZEB2 drives immature T-cell lymphoblastic leukaemia development via enhanced tumour-initiating potential and IL-7 receptor signalling. Nat Commun. 2015;6(1):5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tremblay CS, Brown FC, Collett M, et al. Loss-of-function mutations of Dynamin 2 promote T-ALL by enhancing IL-7 signalling. Leukemia. 2016;30(10):1993-2001. [DOI] [PubMed] [Google Scholar]
- 66.Tremblay CS, Chiu SK, Saw J, et al. Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells [published correction appears in Nat Commun. 2021;12(1): 1288]. Nat Commun. 2020;11(1):6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laouar Y, Crispe IN, Flavell RA.. Overexpression of IL-7R alpha provides a competitive advantage during early T-cell development. Blood. 2004;103(6):1985-1994. [DOI] [PubMed] [Google Scholar]
- 68.González-García S, Mosquera M, Fuentes P, et al. IL-7R is essential for leukemia-initiating cell activity of T-cell acute lymphoblastic leukemia. Blood. 2019;134(24):2171-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akkapeddi P, Fragoso R, Hixon JA, et al. A fully human anti-IL-7Rα antibody promotes antitumor activity against T-cell acute lymphoblastic leukemia. Leukemia. 2019;33(9):2155-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hixon JA, Andrews C, Kashi L, et al. New anti-IL-7Rα monoclonal antibodies show efficacy against T cell acute lymphoblastic leukemia in pre-clinical models. Leukemia. 2020;34(1):35-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ksionda O, Melton AA, Bache J, et al. RasGRP1 overexpression in T-ALL increases basal nucleotide exchange on Ras rendering the Ras/PI3K/Akt pathway responsive to protumorigenic cytokines. Oncogene. 2016; 35(28):3658-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Senkevitch E, Li W, Hixon JA, et al. Inhibiting Janus Kinase 1 and BCL-2 to treat T cell acute lymphoblastic leukemia with IL7-Rα mutations. Oncotarget. 2018;9(32):22605-22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanda T, Tyner JW, Gutierrez A, et al. TYK2-STAT1-BCL2 pathway dependence in T-cell acute lymphoblastic leukemia. Cancer Discov. 2013;3(5):564-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peirs S, Matthijssens F, Goossens S, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124(25):3738-3747. [DOI] [PubMed] [Google Scholar]
- 75.Kampen KR, Sulima SO, Verbelen B, et al. The ribosomal RPL10 R98S mutation drives IRES-dependent BCL-2 translation in T-ALL [published correction appears in Leukemia. 2019;33(4): 1055-1062]. Leukemia. 2019;33(2):319-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gary JM, Simmons JK, Xu J, et al. Hypomorphic mTOR Downregulates CDK6 and Delays Thymic Pre-T LBL Tumorigenesis. Mol Cancer Ther. 2020;19(10):2221-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.