Abstract
Due to long corticosteroid therapy in patients with COVID‐19, in case of cough, dyspnea, and weight loss, emerging pulmonary aspergillosis and lophomoniasis should be ruled out.
Keywords: corticosteroid, invasive pulmonary aspergillosis, lophomoniasis, post‐COVID‐19
Due to long corticosteroid therapy in patients with COVID‐19, in case of cough, dyspnea, and weight loss, emerging pulmonary aspergillosis and lophomoniasis should be ruled out.
![]()
1. INTRODUCTION
Due to corticosteroid therapy, several fungal and protozoal super infections, such as invasive pulmonary aspergillosis and lophomoniasis, post‐COVID‐19 have been reported. Here, we present a 68‐year‐old man with history of COVID‐19 2 months ago. Monitoring of post‐COVID‐19 patients with prolonged cough and dyspnea is urgently necessary.
COVID‐19, a disease with a wide variety of clinical signs, is caused by SARS‐CoV‐2 infection. While the majority of COVID‐19 patients are asymptomatic or have mild‐to‐moderate symptoms of infection, high‐risk individuals may develop severe illnesses that require hospitalization and support breathing. The effects of being older, as well as underlying comorbidities including hypertension, cardiovascular disease, and diabetes, have been recognized as risk factors for debilitating diseases.1
This infection can cause severe respiratory disease with an important rate of intensive care unit (ICU) admissions. Bacterial and fungal infections are complications of this viral pneumonia due to the severe damage of lung tissue, cytokine storm, and immune‐paralysis caused by viral infection‐induced acute respiratory distress syndrome (ARDS). Invasive pulmonary aspergillosis (IPA) has been reported in other respiratory viruses such as influenza viruses.2
Lophomonas is a neglected and emerging protozoan that infects the lower (primarily) and upper respiratory tracts of humans. It is commonly found as a commensal agent in the hind intestines of cockroaches.2, 3 Lower and upper respiratory infections caused by Lophomonas have been documented in various parts of the world in recent decades, notably in China and Iran. Iran has a reputation for having the largest number of lophomoniasis cases in the world.4, 5, 6, 7, 8, 9
Thus, only a few cases of lophomoniasis co‐infection with other infectious illnesses have been documented throughout the world. There is some evidence that lophomoniasis co‐occurs with HIV and TB.2, 9, 10 Several complications and super infections post‐COVID‐19 have been reported, such as fungal (mostly IPA) and bacterial infections,11 but there is no evidence regarding co‐infection of Lophomonas and IPA before and during the COVID‐19 pandemic worldwide.
2. CASE PRESENTATION
On December 22, 2020, an old man, 68 years old with history of diabetes mellitus (DM), hypertension (HTN), and coronary artery bypass graft (CABG) with symptoms of weakness and lethargy, dyspnea, fever, chills, and cough, was referred to Imam Khomeini hospital in Mazandaran Province, northern Iran. On examination, vital signs of the patient included RR: 28 breaths/min, BP: 140/95 mmHg, PR: 131 beats/min, Spo2: 88%, T: 37/5℃. With suspicion of COVID‐19 pneumonia, a high resolution computed (HRCT) scan and real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) were performed on the patient to rule out COVID‐19 infection. According to the HRCT and RT‐PCR evidence, COVID‐19 pneumonia infection was confirmed. Next, the patient was hospitalized in our infectious ward and, following severe dyspnea and loss of saturation, he was transferred to the ICU and intubated. Finally, after 15 days of hospitalization in the ICU and the current drug treatment, the patient was discharged with reduced symptoms and partial recovery.
Two months later, on February 20, 2021, the patient was referred to Imam Khomeini Hospital with symptoms of weakness and lethargy, weight loss (10–15 kg in 2 months), nausea, dyspnea, and productive coughing during the past week. The history of medications includes Losamix 25 mg, ASA 80 mg, Novomix 30, Metoral 50 mg, Atrovastatin 20 mg and Apixaban 2.5 mg. The results of laboratory tests on admission are shown (Table 1). Subsequently, an HRCT scan was ordered for the patient. The HRCT findings showed bilateral subpleural ground‐glass opacities being highly suggestive of COVID‐19 pneumonia (Figure 1A). Also, a solid mass partially surrounded by a crescent of air (air crescent sign) in the right lower lobe (RLL) characteristic of aspergilloma was remarked (Figure 1B).
TABLE 1.
Baseline laboratory data of the patient
| Laboratory parameter | At admission | Reference range |
|---|---|---|
| Hemoglobin | 11.3 g/dl | 12–16 |
| White blood cells | 19,200/mm3 | 4.5–11 |
| Platelet | 391,000/mm3 | 130–400 |
| Lymph | 4.9% | 20–50 |
| Poly | 91% | 37–72 |
| ESR | 86 mm/h | 0–22 |
| NA | 133 mEq/L | 135–145 |
| K | 4.8 mEq/L | 3.5–5 |
| ALT | 54 IU/L | 10–40 |
| AST | 24 IU/L | 10–40 |
| ALP | 394 IU/L | 20–140 |
| Bilirubin | ||
| Total | 0.7 mg/dl | 0.1–1.2 |
| Direct | 0.3 mg/dl | <0.3 |
| LDH | U/L | 100–500 |
| PT | 18 s | 11–13 |
| PTT | 35 s | 25–35 |
| INR | 1.2 | 1.1 |
| BUN | 68 mg/dl | 8–20 |
| Creatinine | 1.7 mg/dl | 0.5–1 |
FIGURE 1.
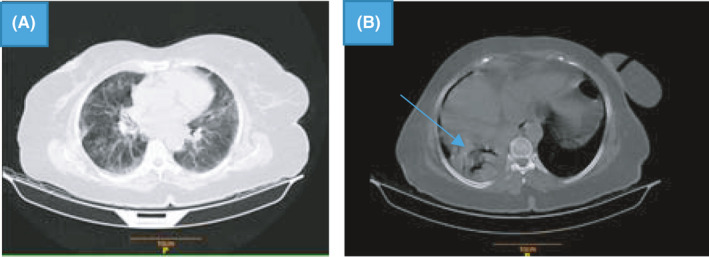
HRCT showing bilateral subpleural ground‐glass opacities (A); a solid mass with air crescent sign in the right lower lobe (RLL) characteristic of aspergilloma (B; arrow ahead)
The patient was nominated for a bronchoscopy due to the recurrence of respiratory symptoms and a cough with sputum. On bronchoscopy, a black purulent discharge was seen up to the end of the trachea. Two BAL samples separately were subjected to the Iranian national registry center for lophomoniasis (INRCL), and tuberculosis reference laboratory, Mazandaran University of Medical Sciences, to rule out (R/O) lophomoniasis, and tuberculosis (smear and PCR) respectively. Furthermore, a serum sample of the patient was tested in the mycological laboratory for diagnosis of IPA using galactomannan (GM) enzyme immunoassay (EIA) test, as an antigen‐based assay.
The obtained laboratory findings were as follows: in a wet smear prepared from the BAL sample, the live and motile protozoan parasite Lophomonas was observed under a light microscope. The infection was also confirmed by a genus‐specific small subunit ribosomal RNA (SSU rRNA) PCR.5 Additionally, GM antigenemia was detected in the patient serum and, consequently, IPA was confirmed. The BAL sample for BK (Bacillus of Koch) was also negative for both smear and PCR.
Based on the above‐mentioned radiological and laboratory evidence, the patient was finally treated with intravenous amphotericin B (50 mg/day; 10 days) and oral voriconazole (200 mg/BD; 14 days) to treat IPA as well as metronidazole (500 mg/TDS; 2 weeks) to treat lophomoniasis. He was discharged in a good general condition from the hospital. This research was carried out in accordance with the principles outlined in the Helsinki Declaration. Also, CARE guidelines and methodology were followed in this study.
3. DISCUSSION
Even though SARS‐CoV‐2 is primarily responsible for severe pneumonia and ARDS, COVID‐19 is associated with a wide variety of extrapulmonary complications, and, hence, it can be considered a systemic disease.11 A small percentage of COVID‐19 patients have fungal or bacterial co‐infections, which are reportedly lower than in the previous influenza pandemic.12 A study showed that among COVID‐19 patients, those who were admitted to the intense care unit (ICU) had a higher probability (57% of ICU cases) of acquiring a fungal or bacterial secondary infection, which was higher than in an earlier study (only 14%).12, 13
All studies of COVID‐19 fungal infections (Aspergillus) reported their occurrence during the COVID‐19 infection, mostly 14 days after the appearance of COVID‐19 symptoms.12 The case in the current study was a 68‐year‐old patient who was previously diagnosed with COVID‐19 for a period of 2 months; after a few weeks of his recovery, the patient developed weakness, nausea, lethargy, weight loss, shortness of breath, and productive coughing.
COVID‐19‐associated pulmonary aspergillosis (CAPA) is difficult to diagnose since harmless replacement of the airways by Aspergillus can spread and cause pulmonary parenchymal injury when immunity is compromised. Patients with CAPA show more frequent positive serum Aspergillus GM EIA results when the fungal infection is invasive beyond the airways into the blood. In immunocompromised patients, IPA is a fatal opportunistic infection. As a result, early diagnosis of IPA is critical for successful therapy, and Aspergillus GM is the only non‐invasive, reliable diagnostic test to monitor the IPA.12, 13
Despite its high specificity, physicians seldom consider direct sampling of the infection site through BAL in COVID‐19 patients due to the risk of increased COVID‐19 transmission. In our patient, diagnostic bronchoscopy was used to further investigation, and Lophomonas and Aspergillus infections were detected. Lophomonas is a neglected parasite reported in a few countries which still remains unknown to many clinicians and laboratory specialists.14 In some parts of Iran, particularly northern and eastern Iran, lophomoniasis is endemic.3, 4, 6, 9 Our patient was from the Mazandaran Province, northern Iran, where lophomoniasis is relatively common. Furthermore, the L. blattarum pathogen was recently isolated from the cockroaches in the province.3
Not only is this disease seen in immunocompromised patients in other countries,15 but it has also been seen in Iran.4, 5, 6, 7, 8, 9 The symptoms of lophomoniasis, such as cough, fever, and shortness of breath, are common in other respiratory infections. As a result, diagnosing and treating this infection are challenging. For the treatment of lophomoniasis, metronidazole is the first line of therapy. Recent studies have revealed that metronidazole reduces the levels of inflammatory factors, such as IL8, IL6, IL1B, TNFα, IL12, and IFNγ, as well as the levels of CRP and neutrophil count, which were increased during COVID‐19 infection. Additionally, metronidazole has the potential to increase the number of circulatory lymphocytes.15 Prescribing this drug, in addition to eliminating the Lophomonas infection, may have accelerated the patient's response to treatment for COVID‐19 infection.
According to several studies, L. blattarum is considered an opportunistic infection in patients with kidney and liver allograft transplantation, under corticosteroid therapy, HIV infection, and tuberculosis.2 SARS‐CoV‐2 infection impairs the immune system, making individuals more susceptible to other infections and reactivating latent infections.16 Patients with chronic pulmonary illness who have underlying lung diseases are at risk of acquiring more severe instances of COVID‐19, according to a meta‐analysis of research. Several problems occur from co‐infection in a pregnant woman with COVID‐19, which may be due to complications of COVID‐19 itself that are caused by another infection. All infections may intensify each other's complications or drug reactions between medications used to treat certain infections.17 The Lophomonas parasite appears to be involved in the severity of the COVID‐19 disease and can be considered as a risk factor. Hence, managing and diagnosing co‐infections are crucial in clinical practice.
4. CONCLUSION
Due to long corticosteroid therapy in patients with COVID‐19, post‐COVID‐19 monitoring of them in the case of symptoms such as cough, shortness of breath and weight loss is urgently necessary. We recommended that diagnostic bronchoscopy, HRCT, and laboratory findings should be worked up for differential diagnosis to R/O IPA and lophomoniasis during COVID‐19 pandemic.
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
AS involved in the interpretation and collecting of data and editing of the manuscript. ZZ and ESB involved in writing, editing, and preparing the final version of the manuscript. MF and MN is involved in critically revising the whole manuscript. MS is responsible for collecting data and submitting the manuscript. All authors reviewed the paper and approved the final version of the manuscript.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This research was reviewed and approved by the research ethics committee of Mazandaran University of Medical Sciences (IR.MAZUMS.REC.1397.2969). Written informed consent was achieved from the patient to include the clinical details.
CONSENT
Informed consent for publication of this case report was taken verbally from the patient.
ACKNOWLEDGMENT
Declared none.
Sharifpour A, Zakariaei Z, Fakhar M, Banimostafavi ES, Nakhaei M, Soleymani M. Post‐COVID‐19 co‐morbidity of emerged Lophomonas infection and invasive pulmonary aspergillosis: First case report. Clin Case Rep. 2021;9:e04822. 10.1002/ccr3.4822
DATA AVAILABILITY STATEMENT
The data are available with the correspondence author and can be achieved on request.
REFERENCES
- 1.Gebrecherkos T, Gessesse Z, Kebede Y, et al. Effect of co‐infection with parasites on severity of COVID‐19. medRxiv. 2021. [Google Scholar]
- 2.Li R, Gao Z‐C. Lophomonas blattarum infection or just the movement of ciliated epithelial cells? Chin Med J. 2016;129(6):739‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motevalli‐Haghi SF, Shemshadian A, Nakhaei M, et al. First report of Lophomonas spp. in German cockroaches (Blattella germanica) trapped in hospitals, northern Iran. J Parasit Dis. 2021;1‐7. 10.1007/s12639-021-01381-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenji F, Fata A, Vakili V, et al. Unexpected high rate of Lophomonas blattarum in resistant upper and lower respiratory infection. Int J Med Res Health Sci. 2016;5(9):74‐80. [Google Scholar]
- 5.Fakhar M, Nakhaei M, Sharifpour A, et al. First molecular diagnosis of Lophomoniasis: the end of a controversial story. Acta Parasitol. 2019;64(2):390‐393. [DOI] [PubMed] [Google Scholar]
- 6.Fakhar M, Nakhaei M, Sharifpour A, et al. Morphological and molecular identification of emerged Lophomonas blattarum infection in Mazandaran Province, Northern Iran: first registry‐based study. Acta Parasitol. 2021:1‐7. 10.1007/s11686-021-00422-3 [DOI] [PubMed] [Google Scholar]
- 7.Ghafarian N, Bakhtiari E, Berenji F, et al. The study of Lophomonas blattarum infection in children with respiratory symptoms: a descriptive clinical study in north east of Iran. Int J Pediatr. 2018;6(6):7797‐7802. [Google Scholar]
- 8.Mirzazadeh F, Berenji F, Amini M, et al. Lophomonas blattarum in asthmatic patients and control group. J Res Med Dent Sci. 2017;5(5):1‐5. [Google Scholar]
- 9.Talebian M, Berenji F, Amini M, et al. A study about clinical symptoms and laboratory signs of adult and pediatric patients with Lophomonas blattarum . J Res Med Dent Sci. 2018;6(1):312‐317. [Google Scholar]
- 10.Verma S, Verma G, Singh DV, et al. Dual infection with pulmonary tuberculosis and Lophomonas blattarum in India. Int J Tuberc Lung Dis. 2015;19(3):368‐369. [DOI] [PubMed] [Google Scholar]
- 11.Zheng KI, Feng G, Liu WY, Targher G, Byrne CD, Zheng MH. Extrapulmonary complications of COVID‐19: a multisystem disease? J Med Virol. 2021;93(1):323‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassetti M, Giacobbe D, Grecchi C, et al. Performance of existing definitions and tests for the diagnosis of invasive aspergillosis in critically ill, adult patients: a systematic review with qualitative evidence synthesis. J Infect. 2020;81(1):131‐146. [DOI] [PubMed] [Google Scholar]
- 13.van de Groep K, Verboom DM, van de Veerdonk FL, et al. Detection of invasive aspergillosis in critically ill patients with influenza: the role of plasma galactomannan. Am J Respir Crit Care Med. 2019;200(5):636‐638. [DOI] [PubMed] [Google Scholar]
- 14.Keighobadi M, Nakhaei M, Sharifpour A, et al. A bibliometric analysis of global research on Lophomonas spp. in Scopus (1933‐2019). Infect Disord Drug Targets. 2021;21(2):230‐237. [DOI] [PubMed] [Google Scholar]
- 15.Gharebaghi R, Heidary F, Moradi M, Parvizi M. Metronidazole; a potential novel addition to the COVID‐19 treatment regimen. Arch Acad Emerg Med. 2020;8(1):e40. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Liao B, Cheng L, et al. The microbial coinfection in COVID‐19. Appl Microbiol Biotechnol. 2020;104:7777‐7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbas AM, Salah S & Fathy SK, et al. Role of co‐infection in the immunopathology of COVID‐19 in pregnancy. Arch Health Sci. 2020;4(1):1‐6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available with the correspondence author and can be achieved on request.


