Supplemental Digital Content is available in the text.
Keywords: clinical trial, colchicine, heart injuries, inflammation, myocardial infarction, thrombosis, ventricular remodeling
Abstract
Background:
Inflammation is a key factor of myocardial damage in reperfused ST-segment–elevation myocardial infarction. We hypothesized that colchicine, a potent anti-inflammatory agent, may reduce infarct size (IS) and left ventricular (LV) remodeling at the acute phase of ST-segment–elevation myocardial infarction.
Methods:
In this double-blind multicenter trial, we randomly assigned patients admitted for a first episode of ST-segment–elevation myocardial infarction referred for primary percutaneous coronary intervention to receive oral colchicine (2-mg loading dose followed by 0.5 mg twice a day) or matching placebo from admission to day 5. The primary efficacy outcome was IS determined by cardiac magnetic resonance imaging at 5 days. The relative LV end-diastolic volume change at 3 months and IS at 3 months assessed by cardiac magnetic resonance imaging were among the secondary outcomes.
Results:
We enrolled 192 patients, 101 in the colchicine group and 91 in the control group. At 5 days, the gadolinium enhancement–defined IS did not differ between the colchicine and placebo groups with a mean of 26 interquartile range (IQR) [16–44] versus 28.4 IQR [14–40] g of LV mass, respectively (P=0.87). At 3 months follow-up, there was no significant difference in LV remodeling between the colchicine and placebo groups with a +2.4% (IQR, –8.3% to 11.1%) versus –1.1% (IQR, –8.0% to 9.9%) change in LV end-diastolic volume (P=0.49). Infarct size at 3 months was also not significantly different between the colchicine and placebo groups (17 IQR [10–28] versus 18 IQR [10–27] g of LV mass, respectively; P=0.92). The incidence of gastrointestinal adverse events during the treatment period was greater with colchicine than with placebo (34% versus 11%, respectively; P=0.0002).
Conclusions:
In this randomized, placebo-controlled trial, oral administration of high-dose colchicine at the time of reperfusion and for 5 days did not reduce IS assessed by cardiac magnetic resonance imaging.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03156816.
Clinical Perspective.
What Is New?
Colchicine has been shown to reduce ischemic events in chronic coronary syndromes.
This double-blinded, randomized, controlled trial tested the effect of colchicine on the injury after acute myocardial infarction assessed by cardiac magnetic resonance.
Colchicine did not reduce infarct size and myocardial injury at 5 days and 3 months.
An unexpected increase of left ventricular thrombus incidence in the colchicine group warrants further research.
What Are the Clinical Implications?
Colchicine does not reduce myocardial injury in the acute phase of myocardial infarction.
Other studies exploring the timing, pharmacokinetics, and dose response of colchicine and other anti-inflammatory agents are needed to identify an effective method to reduce infarct size or limit remodeling.
After acute myocardial infarction, an important inflammatory response starts in the minutes after reperfusion and peaks in the first days after reperfusion.1–3 Inflammatory cells such as neutrophils, followed by monocytes and macrophages, rapidly infiltrate the injured myocardium with abundant proinflammatory cytokine secretions that may cause additional damage to the myocardium.4 These inflammatory processes have been identified as key mediators of reperfusion injury in ST-segment–elevation myocardial infarction (STEMI).3 Until now, the various anti-inflammatory approaches tested to reduce this acute inflammatory injury have yielded disappointing results.5
Colchicine is a well-known major alkaloid from Colchicum autumnale with potent anti-inflammatory properties. It accumulates in white blood cells and decreases their motility, mobilization (especially chemotaxis), and adhesion to the endothelium, thereby reducing the proinflammatory cytokine release and potential ensuing myocardial damage.6–8
Long-term colchicine treatment in patients with chronic coronary syndrome or the month after an acute coronary syndrome has recently been found to reduce the risk of major adverse cardiovascular events compared with placebo.9–11 However, it is unclear whether short-term colchicine therapy given at the time of reperfusion reduces myocardial injury compared with placebo in patients with acute STEMI.
We designed the COVERT-MI trial (Colchicine for Left Ventricular Infarct Size Treatment in Acute Myocardial Infarction) to investigate whether colchicine reduces myocardial injury in comparison with placebo in patients who have acute STEMI referred for percutaneous coronary intervention (PCI).
Methods
The data that support the findings of this study are available from the corresponding author and the sponsor of this trial, the Hospices Civils de Lyon, on reasonable request.
Trial Design and Oversight
From July 20, 2018, to July 28, 2020, we conducted this investigator-initiated, randomized, double-blinded, placebo-controlled, multicenter trial at 10 tertiary-referral sites in France.12 The trial protocol (available in the Data Supplement) was approved by the Comité de Protection Personnes Sud-Est IV and by the French competent authority Agence Nationale de Sécurité du Médicament in December 2017. A data- and safety-monitoring committee provided oversight and assessed the safety profile of the trial. The Clinical Investigation Center of the Hospices Civils de Lyon (INSERM 1407) conducted and coordinated the trial and collected all trial data. Independent clinical research associates monitored the sites and verified the data.
The study was approved by an institutional review committee and all patients gave written informed consent.
Colchicine 0.5 mg and matching placebo capsules were provided, in the same appearance (color, size, and packaging), by the sponsor. Production, blinded labeling, packaging, and delivery of the study drugs in every site of the trial was performed by a provider following the European Union’s Good Manufacturing Practice.
The study drugs and placebo were prepared by the Pharmacy of the Edouard Herriot Hospital (FRIPHARM, Hospices Civils Lyon, France) according to good preparation practices from the tablets of a commercial specialty of Colchicine (Opocalcium 1 mg, Laboratory Mayoly Spindler).
The trial was supported by a grant from the French Ministry of Health (PHRCN-16-0357). The trial was sponsored by the Hospices Civils de Lyon. The study was registered in https://www.clinicaltrials.gov (Unique identifier: NCT03156816) and EudraCT 2017-004090-13, before the enrollment of the first patient.
Patients
All adult patients (>18 and <80 years of age) with a first-time STEMI referred for primary or rescue PCI admitted to the participating centers were screened against eligibility criteria. The infarct-related artery had to be occluded at the time of initial angiography (defined as having a Thrombosis in Myocardial Infarction score ≤1). The additional key eligibility criterium was presentation within 12 hours of chest pain onset.
Key exclusion criteria were hemodynamic instability (ie, cardiogenic shock), any obvious contraindication to cardiac magnetic resonance (CMR) imaging (claustrophobia, pacemaker, defibrillator, history of hypersensitivity to gadoteric acid or gadolinium contrast agents or meglumine), severe liver or known renal dysfunction as defined by a glomerular filtration rate ≤30 mL/min and chronic treatment with colchicine. A full list of the inclusion and exclusion criteria is provided in the Data Supplement.
Consent and Randomization
Information and informed consent were obtained before inclusion. After consent, eligible patients were randomly assigned to receive oral colchicine or placebo. Randomization was performed in a 1:1 ratio using permuted blocks and was stratified by center and by culprit coronary artery status (to ensure equivalent proportions of left anterior descending versus non–left anterior descending coronary artery culprit artery myocardial infarctions in both groups). A centralized randomization process was performed by internet using the ClinSight software (Ennov Clinical Software, Paris, France).
Interventions
After randomization, each patient received colchicine or matching placebo, as close as possible to PCI. Patients were given a 2-mg oral loading dose, followed by 0.5 mg twice a day by oral route for 5 days. The loading dose was recommended to be given before PCI and, if not possible, immediately after PCI.12
Dose reduction to 0.5 mg every day or early discontinuation was permitted in case of any gastrointestinal side effects (diarrhea, nausea, abdominal pain) or in case of an acute, unexpected decline in the glomerular filtration rate or red and white blood cell counts monitored throughout the first 48 hours.
Except for the study drug administration, patients received standard care in terms of revascularization procedures and medications according to international guidelines.13
Assessments during initial follow-up were made at inclusion, and on the first day, second day, and at hospital discharge. Patients remained in the hospital as long as clinically indicated.
At 5 days follow-up, patients underwent an initial CMR study with intravenous gadolinium injection for primary end point assessment. Then, a follow-up clinical visit was done at 3 months with a CMR follow-up study. The full trial design and procedures have been reported previously.12
Trial Outcomes
The primary outcome was a comparison of infarct size (IS) in grams of left ventricular (LV) mass assessed by late gadolinium enhancement CMR at 5 days between groups. Secondary outcomes were considered in hierarchical order as follows: LV ejection fraction at 5 days by CMR, microvascular obstruction mass at 5 days by CMR; absolute adverse LV remodeling between 5 days and 3 months by CMR, relative LV remodeling between 5 days and 3 months defined as a relative increase in LV end-diastolic volume >12% by CMR,14 IS at 3 months, LV ejection fraction at 3 months, LV end-diastolic volume at 3 months, LV end-systolic volume at 3 months, and LV thrombus frequency at the acute phase or during follow-up.
The incidence of major adverse cardiovascular events at discharge (all-cause death, nonfatal myocardial infarction, nonfatal stroke, or heart failure events) at 3 months and 1 year follow-up, quality of life measured at 12 months, and markers of inflammation during the acute phase were secondary exploratory outcomes.
CMR Protocol and Postprocessing
All CMR studies were performed on 1.5 or 3 Tesla scanners (multivendor Siemens, Philips) at 5±2 days and 3 months±15 days after admission.
All sequences were performed by using vectocardiogram monitoring and 12-element phased-array cardiac receiver coils. After localization, rest LV function was assessed with retrospective ECG-gated steady-state free precession pulse cine sequences in long- and short-axis views in the true heart axis. The short-axis scans covered the whole left ventricle.
Late gadolinium enhancement was evaluated in short-axis orientation covering the whole ventricle 10 minutes after contrast injection of gadoteric acid (0.2 mmol/kg body weight; Dotarem, Guerbet) using 3-dimensional gradient spoiled inversion recovery TurboFLASH sequence covering the left ventricle in short axis. Additional 2-chamber and 4-chamber long-axis phase-sensitive inversion recovery sequences were also performed for better spatial assessment of late gadolinium enhancement–enhanced areas.
All CMR and coronary angiogram images were transferred from the trial sites to a central image database by using a software platform as described previously.12
Centralized, off-line image analysis of the CMR images was performed by an experienced observer on a dedicated workstation for all CMR studies using the Circle imaging software (CVI42, Circle Cardiovascular Imaging Inc). This single observer was blinded to all other clinical characteristics or study status.
LV volumes and function were assessed. The infarct zone was defined semiautomatically on late gadolinium enhancement imaging by using the full-width half-maximum technique.15 Microvascular obstruction was defined as areas of hypoenhancement on the late gadolinium enhancement images within the hyperenhanced myocardium.
The extent of myocardial infarcted myocardium and microvascular obstruction was expressed in grams of tissue according to the following formula: ∑ (hypoenhanced and hyperenhanced area [in cm2])×slice thickness (in cm)×myocardial specific density).
The area at risk was assessed by a single expert reader from the initial angiograms, using angiographic scoring of the area at risk with the APPROACH angiographic score (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease),16 with angiograms before reperfusion and immediately after. This centralized reading was also blinded to any other clinical or imaging data from the study.
Statistical Analysis
Assuming an infarct size at 5 days in the control group equal to 34 g (SD 22 g),2,17–19 we calculated that a sample size of 194 patients would provide the trial with a power of at least 80% to detect an expected reduction of 30% in the experimental group using a Mann-Whitney rank-sum test at a 2-sided α of 5%, considering an anticipated dropout rate of 15%.
All analyses were performed according to the intention-to-treat principle and were prespecified before database lock and described in the statistical analysis plan available in the Data Supplement.
Analysis of the primary outcome was performed by using a linear regression model with adjustment for the randomization stratification factor (center and culprit artery), providing an estimated treatment effect with a 95% CI, and a Wald test to reject the null hypothesis of no difference between groups.
Secondary efficacy outcomes were classified in a prespecified hierarchical order and tested, in turn, using a Wilcoxon rank-sum test according to a closed-test procedure to maintain an overall α-level of 5%.20 We further analyzed the secondary end points by using linear or logistic regression models with adjustment for the randomization stratification factor (center and culprit artery).
Nonparametric Wilcoxon rank-sum tests or Fisher exact tests were used according to the nature of the secondary outcomes.
To assess the relationship between the area at risk and IS, we performed a prespecified analysis of regression plots of IS at 5 days on angiographically estimated area at risk and compared the 2 regression plots with covariance analysis.
Planned subgroup analyses of the primary outcome were performed according to age (<70 years versus.≥70 years), sex, diabetes, multivessel disease status, time from symptom onset to hospital admission, type of culprit coronary artery, glomerular filtration rate, and myocardial area at risk size.
A 2-sided P value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using SAS Software version 9.4 in a Windows environment.
Results
Patients
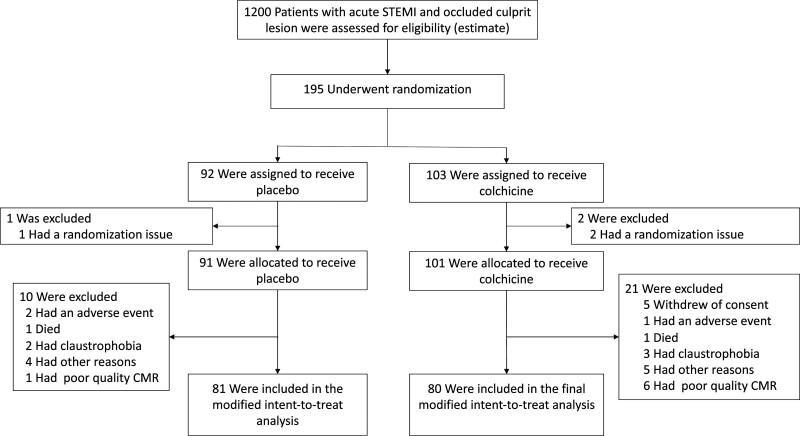
A total of 195 patients with STEMI were enrolled and underwent randomization (103 to the colchicine group and 92 to the placebo group). Screening logs were not maintained for the trial at each participating site, but an estimate of the STEMI population admitted at these centers during the inclusion period is presented in Figure 1. Three randomizations were randomization errors, 10 patients were excluded from the modified intent-to-treat analysis in the placebo group, and 23 in the colchicine group were excluded from the primary outcome analysis (Figure 1).
Figure 1.
Enrollment and follow-up of the patients. CMR indicates cardiac magnetic resonance; and STEMI, ST-segment–elevation myocardial infarction.
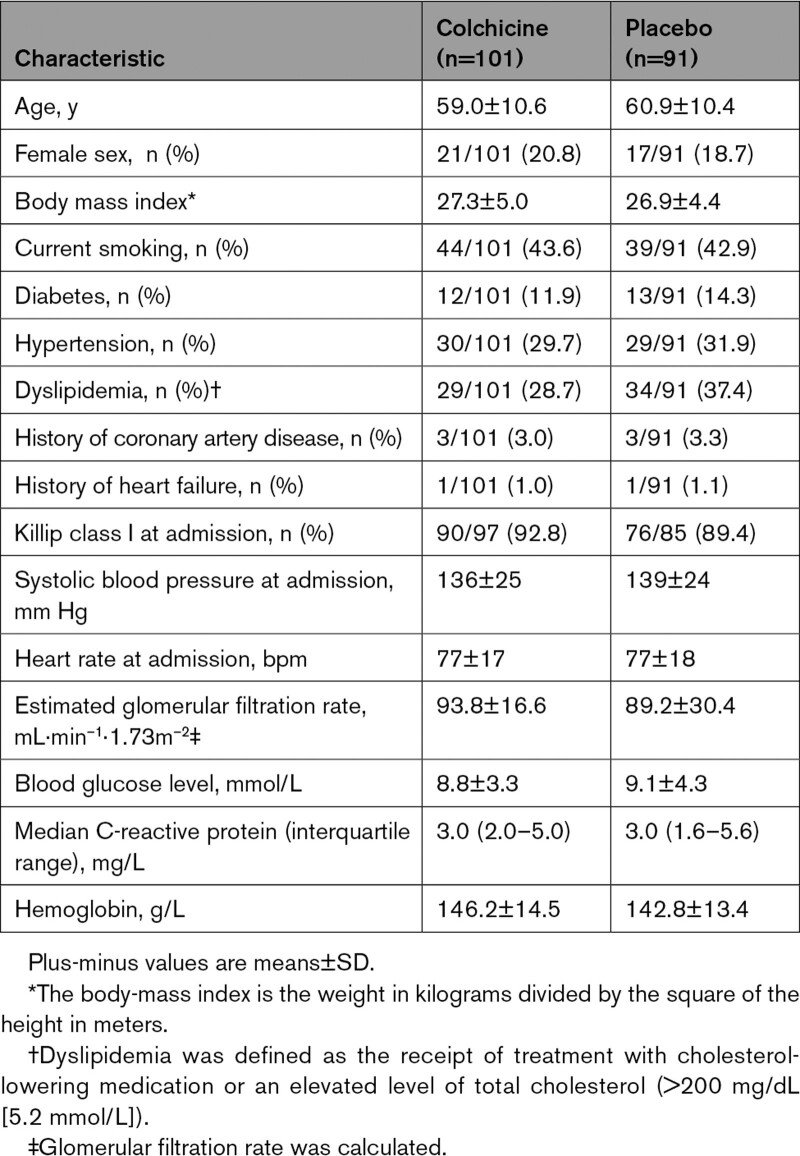
Baseline patients’ characteristics were similar in the 2 groups, with a mean age of 60.0±10.5 years and 19.5% female sex (Table 1). Infarct location as determined by angiography and CMR was similar between anterior and nonanterior territories. Baseline angiographic, intervention, and pharmacological treatment at discharge were also well balanced between groups (Table 2).
Table 1.
Characteristics of the Patients at Baseline
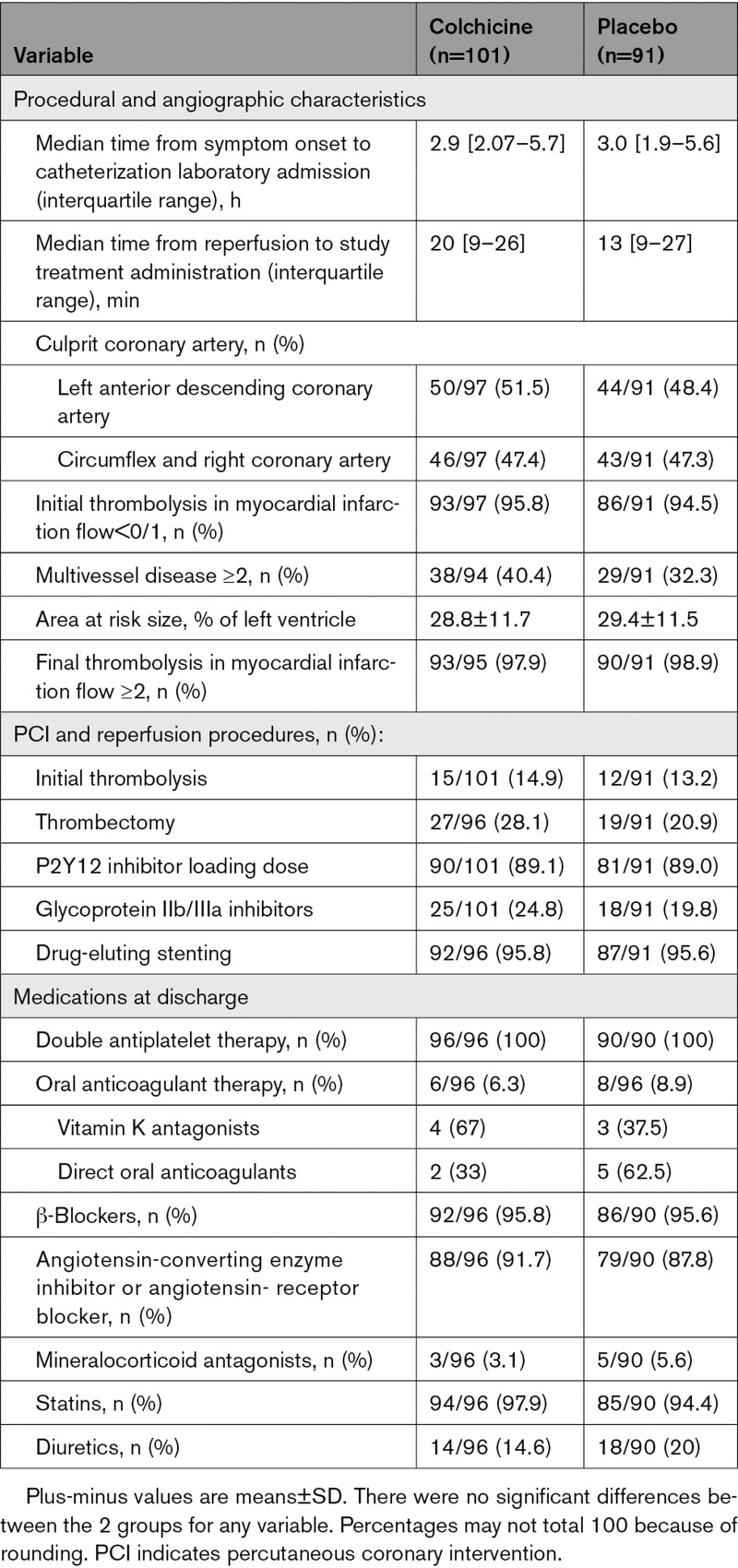
Table 2.
Coronary Angiography, Interventions, and Medications at Discharge
The 2 study groups were similar concerning time from symptom onset to catheterization laboratory admission and the size of the angiographic area at risk. Rescue PCI after thrombolysis was performed in 27 patients (15 in the colchicine group and 12 in the placebo group). Stenting with drug-eluting stents of the culprit lesion was performed in 95.7% of patients (Table 2). Thrombosis in Myocardial Infarction 2 flow was not achieved after PCI in 1 patient from the placebo group.
Study Treatment Delivery and Side Effects
Median delays of study treatment administration were 20 minutes (IQR, 9–26) in the colchicine group and 13 minutes (IQR, 9–27) in the placebo group. Colchicine had significantly more gastrointestinal side effects (mostly diarrhea) than placebo (33 [34.4%] versus 9 [10.1%], respectively; P=0.0001), and 1 patient in the colchicine group presented with anemia.
CMR Assessment
Of 192 patients, 168 (87.5%) underwent a CMR study at a median of 5 days (IQR, 4–7 days). Of these CMR studies, 161 (95.8%) were analyzable by the centralized core laboratory. For the primary end point assessment, 80 of 101 patients in the colchicine group and 81 of 91 patients in the placebo group had an accurate end point assessment at 5 days. Of 192 patients, 154 (80.2%) underwent a follow-up CMR study at a median of 95 days (IQR, 88–102 days). Of these CMR studies, 151 (98.1%) were analyzable. Reasons for noncompletion of CMR are shown in Figure 1. The delays within each group at baseline and follow-up are reported in Table I in the Data Supplement.
Primary Outcome
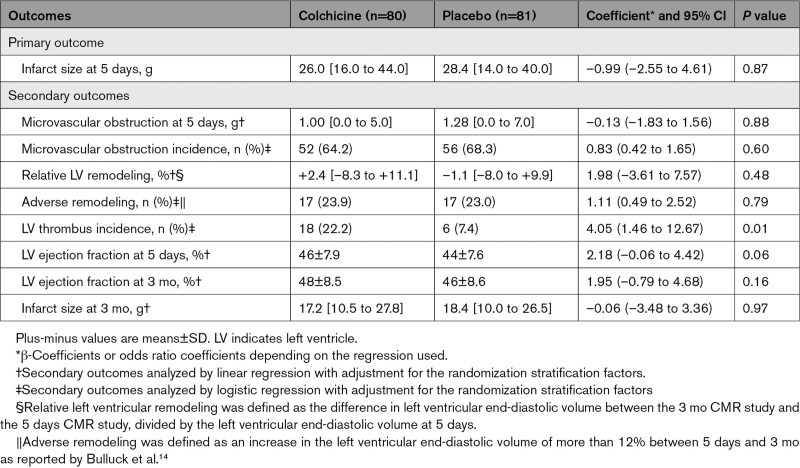
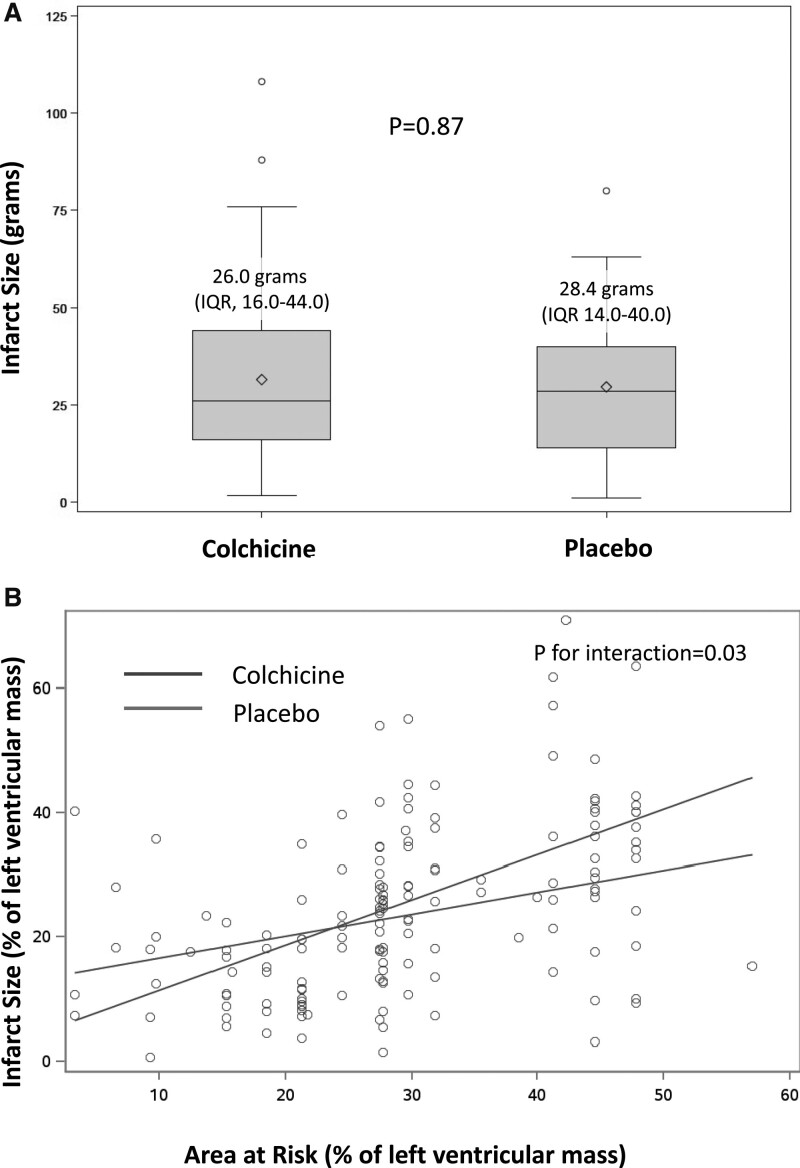
All prespecified study outcomes are summarized in Table 3. The IS after reperfusion was not significantly reduced in the colchicine group compared with the placebo group, with a mean of 26.0 g (IQR, 16.0–44.0) of LV mass in the colchicine group versus 28.4 g (IQR, 14.0–40.0) in the placebo group (P=0.87 for the difference; Figure 2A). As shown in Figure 2B, there was a significant positive relationship between the IS and the angiographic area at risk (as defined by the angiographic APPROACH score on initial angiography16), larger in the colchicine group than in the placebo group (P for interaction=0.03; Figure 2B).
Table 3.
Prespecified Primary and Secondary Outcomes
Figure 2.
Assessment of infarct size by late gadolinium enhancement cardiac magnetic resonance and as a function of the area at risk (A). Infarct size (IS) was measured in a centralized core laboratory by quantification of the area of late gadolinium enhancement (LGE) by cardiac magnetic resonance at 5 days. IS Colchicine administration did not result in a significant reduction in IS in comparison with placebo (estimate: 0.99 [95% CI, –2.64 to 4.61]; P=0.59). The IS measured by LGE was expressed as a function of the APPROACH angiographic score,16 an estimate of the area at risk, as shown in B. To assess the relationship between the area at risk and IS, we performed a prespecified analysis of regression plots of IS by LGE at 5 days on angiographically estimated area at risk. There was a significant association between the 2 variables in the colchicine group (β=0.73; P<0.001) and the placebo group (β=0.35; P=0.003). There was a significant positive relationship between the 2 variables in both groups, and significantly larger in the colchicine group (P interaction=0.03). These data suggest that, for the largest areas at risk, colchicine administration was associated with an increase in the resulting infarct size as measured by LGE. This difference was confirmed to be significant by analysis of covariance (P for interaction= 0.03). APPROACH indicates Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease; and IQR, interquartile range.
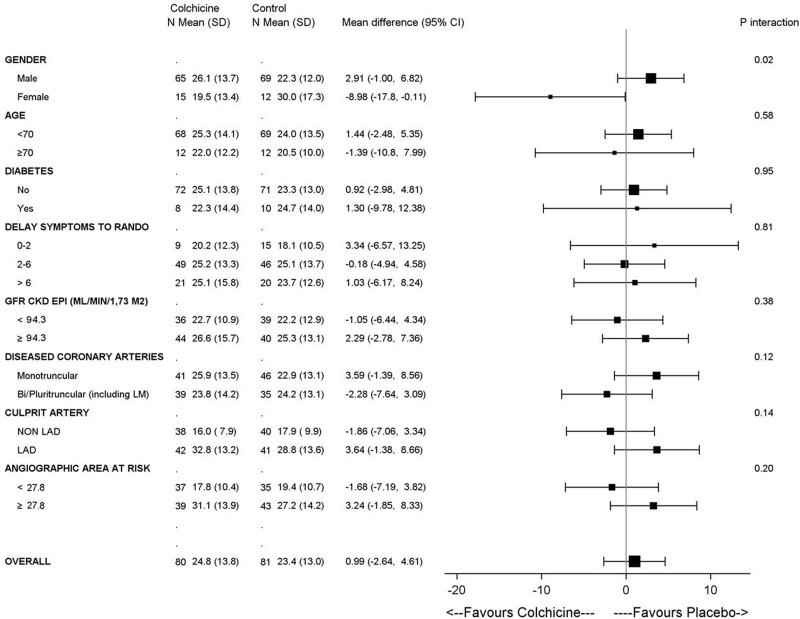
Results according to prespecified subgroups are presented in Figure 3. There were no significant differences across prespecified subgroups except for sex (P for interaction=0.02), with a suggestion of smaller infarct size in women treated with colchicine. Caution is needed in interpreting this subgroup given the small number of women enrolled.
Figure 3.
Subgroup analyses for the primary outcome at 5 days. CKD indicates chronic kidney disease; CKD-EPI, chronic kidney disease epidemiology collaboration; GFR, glomerular filtration rate; LAD, left anterior descending coronary artery; LM, left main coronary artery; and RANDO, randomization.
Secondary Outcomes
There were no significant differences in other prespecified secondary outcomes in terms of microvascular obstruction extent, LV remodeling, and LV ejection fraction between the colchicine and placebo groups, as reported in Table 3.
There was no difference between groups in IS at 3 months (17.2 IQR [10.5–27.8] versus 18.4 IQR [10.0–26.5] g of LV mass, respectively; P=0.92).
There was an unexpected significant increase in the rate of LV thrombus at 5 days, however, with a greater proportion of LV thrombus in the colchicine group 18 (22.2%) compared with the placebo group 6 (7.4%; P=0.01). This difference was confirmed by the adverse event rates reporting of LV thrombi with 23 (22.8%) cases in the colchicine group and 8 (8.7%) cases in the placebo group (P=0.032) over the whole study follow-up at 3 months. On the CMR follow-up studies at 3 months, this difference in the proportion of LV thrombus was no longer significant with 4 (5.3%) cases in the colchicine group compared with 2 (2.6%) cases in the placebo group (P=0.68). At 3 months of follow-up, 2 (1.9%) ischemic strokes were reported in the colchicine group and 1 (1.1%) was reported in the placebo group (P=1.0). None of the ischemic stroke events occurred in patients in whom a LV thrombus had been identified.
In an exploratory analysis comparing patients with and without LV thrombus, the only 2 baseline variables associated with a subsequent LV thrombus were a culprit coronary artery identified as the left anterior descending or left main and the use of thrombus aspiration during PCI.
Major Adverse Cardiovascular Outcomes at 3 Months and Serious Adverse Events
There were no significant differences in major cardiovascular events reported at 3 months between groups (Table II in the Data Supplement). The number of serious adverse events was 40 (38.8%) in the colchicine group and 32 (34.8%) in the placebo group at 3 months, with no significant difference (P=0.66; Table III in the Data Supplement).
Biomarkers
There were no differences in total creatine kinase release at admission, 6 hours, 24 hours, and 48 hours between groups.
We observed no significant differences between groups regarding inflammatory biomarkers such as white blood cell count, neutrophil count, and fibrinogen at admission, 24 hours, and 48 hours. C-reactive protein levels were not significantly lower in the colchicine group than in the placebo group at 48 hours (15.7 mg/L [IQR, 6.3–37.5] versus 23.6 mg/L [IQR, 10.9–46.0]; P=0.06).
Discussion
Our findings show that short-term oral colchicine treatment at high doses given at the time of reperfusion in patients who have acute STEMI did not reduce infarct size as determined by CMR in comparison with placebo. There was also no significant change in other indices of myocardial damage such as microvascular obstruction and LV remodeling. However, there was an unexpected 3-fold increase in the incidence of LV thrombus in patients receiving colchicine compared with those receiving placebo, without evidence of subsequent adverse clinical outcomes.
There has been recent interest in treating the chronic inflammation associated with atherosclerotic disease with low-dose colchicine. The Colcot (Colchicine Cardiovascular Outcome Trial) and LoDoCo2 (Low-Dose Colchicine 2) trials10,11 showed benefit of low-dose colchicine (0.5 mg every day) started within a month after myocardial infarction or in patients with chronic coronary disease after at least 6 months of a clinically stable condition. In another recent trial, colchicine given earlier and at a higher dose of 0.5 mg twice a day for 1 month followed by 0.5 mg every day for 11 months after an acute coronary syndrome failed to demonstrate any benefit, and there was increased mortality in the colchicine group.21 This discrepancy has generated debate.22 The present phase II randomized trial was designed to explore a different hypothesis. We specifically targeted the inflammatory response that occurs in the acutely injured myocardium immediately after reperfusion.3 There was no reduction of myocardial infarct size in patients receiving colchicine. This finding is in keeping with previous reports assessing the effects of colchicine on myocardial injury and inflammation,23–26 but it does not confirm previous results suggesting up to a 50% reduction of IS with a similar regimen.27 In this latter study, IS reduction was assessed by using myocardial biomarker release, and IS reduction on CMR imaging was only reported in a subgroup of patients. Another explanation for this difference may be related to a difference between study populations. In this former study, patients’ Thrombosis in Myocardial Infarction flow was not reported, suggesting potential confounding factors.27 The present trial used an accepted primary end point with core laboratory measurement of CMR infarct size.
One may hypothesize that the discrepancy between our study results and recent phase III colchicine trials could be related to a dose effect. Colchicine has dose-related effects with a narrow therapeutic margin.7 Gastrointestinal effects are the most common adverse effect and the first signs of toxicity, as well.28 Our dose regimen was established on a previous report by Deftereos et al,27 and we found a similar frequency of gastrointestinal side effects, mostly diarrhea. These side effects were significantly increased compared with placebo, and their frequency was also significantly greater than that reported in trials with low-dose colchicine of 0.5 mg every day.10,11
Other factors influencing IS may contribute to the absence of the efficacy of colchicine in reducing final IS. Total ischemic time, area at risk size, thrombolysis, or factors associated with reperfusion could have interfered with the effect of colchicine. Although our protocol strictly followed the protocol of the initial report by Deftereos et al,27 the timing, dose, and duration of colchicine administration remain poorly explored. Regarding the oral administration used in our study, it was also reported and used in the study by Deftereos et al.27 In addition, previous pharmacokinetic reports indicate that, after an oral ingestion of colchicine, the plasma levels peak at ≈1 hour with a mean half-life of 26 hours. The bioavailability of colchicine ranges from 24% to 88% (mean 45%).29 There are no data regarding the impact of myocardial infarction on colchicine gastrointestinal absorption. It may be delayed, as previously seen for instance, with ticagrelor with a 34% reduction of ticagrelor bioavailability in patients with STEMI versus patients with non–ST-segment–elevation myocardial infarction.30 This could affect its efficacy, although it was not the case in the report by Deftereos et al.27
The present knowledge regarding the inflammatory response after myocardial ischemia reperfusion shows a proinflammatory phase that lasts for 3 to 7 days with marked immune cell recruitment, tissue digestion, and reactive oxygen species production. The peak of this inflammatory phase is between 24 and 72 hours, and its duration is ≈5 to 7 days. The proinflammatory phase switches toward a reparative and proliferative phase with inflammation resolution, scar formation, and wound healing.31 Thus, the timing of an early administration of colchicine seemed appropriate.
Regarding the increased incidence of early LV thrombus in the colchicine group, there is no evidence in the literature of any prothrombotic effect associated with colchicine,28 but the effect of colchicine on platelet function appears to be complex.26,32 Several hypotheses can be raised to explain this finding. The first is a chance finding attributable to the small sample size. Nonetheless, the magnitude of the difference and the consistency between the core laboratory assessment and the investigators’ adverse event notifications suggest otherwise. Our exploratory analysis plotting IS against angiographic area at risk showed significantly larger IS in the colchicine group. This may be related to the short duration of high-dose colchicine therapy in our study. Colchicine’s antithrombotic effect is mostly related to its anti-inflammatory properties through inhibition of neutrophil degranulation33; it is conceivable that a proinflammatory rebound at the early discontinuation of therapy (as described with other anti-inflammatory drugs34) may have fostered increased LV damage and subsequent thrombus formation.35–37 Of note, an in vitro study on human umbilical vein endothelial cell line investigating colchicine anti-inflammatory mechanisms suggested that, in addition to its interaction with microtubules, colchicine also impacts cell response at the transcriptional level. The effects of colchicine are complex and activate different pathways at different doses and duration regimens.38 These observations regarding early thrombus formation and IS in patients with a larger area at risk are purely exploratory and should interpreted with caution. We suggest that thrombosis should be prospectively examined in future trials investigating anti-inflammatory interventions in acute myocardial infarction.
A recent study39 found a promising effect of interleukin 6 selective inhibition with tocilizumab in patients with acute myocardial infarction. Colchicine features an original anti-inflammatory mechanism of action combining tubulin disruption, inhibition of NLRP3 inflammasome, and stimulation of dendritic cell maturation and antigen presentation.7 We hypothesized that these mechanisms were more likely to induce a more favorable modulation of the immunoinflammatory response than a targeted cytokine inhibition. Together with the unexpected results of our study, this demonstrates the complex nature of this inflammatory response and the numerous remaining unexplored areas that need to be tackled in further studies to better understand and treat it to limit the final myocardial damage.
The present trial has several limitations. First, it is a phase II trial with a limited sample size. However, adherence to the protocol and the overall good quality in data acquisition and processing with centralized reading make the findings robust. Second, there was a higher-than-expected number of missing CMR studies. We had anticipated 15% of missing data but the actual proportion was eventually 20%. However, the absence of difference between groups regarding the primary outcome makes it unlikely that 5 more CMR studies in each group would have changed the conclusion. Third, the primary end point assessment by CMR at 5 days may have led to overestimation of final IS as suggested by previous reports.40 However, IS was also assessed at 3 months follow-up, with consistent results.
In patients with a first episode of STEMI and occluded culprit coronary artery, high-dose colchicine given orally at the time of reperfusion for a short period did not reduce myocardial damage induced by ischemia-reperfusion and the resulting inflammation compared with placebo. Further studies exploring the timing, pharmacokinetics, and dose response of colchicine and other anti-inflammatory agents are needed to identify an effective therapy to reduce infarct size or limit remodeling.
Acknowledgments
The authors thank the Hospices Civils de Lyon sponsor authorities and specifically A. Pachot, V. Plattner, J. Bricout, and all clinical research associates including A. Veré, N. Sbaghdi, P. Biesuz, R. Euphrosine, and L. Laugier. We also thank C. Amaz for her rapid and extensive statistical assistance during the revision of this manuscript.
Sources of Funding
The trial was supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique National PHRCN-16-0357). This work was also supported in the Recherche Hospitalo-Universitaire MARVELOUS (ANR-16-RHUS-0009) of Université de Lyon, within the program “Investissements d’Avenir” operated by the French National Research Agency (ANR).
Disclosures
None.
Supplemental Materials
Expanded Methods
Data Supplement Tables I–III
Protocol Versions and Statistical Analysis Plan
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CMR
- cardiac magnetic resonance
- IS
- infarct size
- LV
- left ventricular
- PCI
- percutaneous coronary intervention
- STEMI
- ST segment elevation myocardial infarction
A complete list of the investigators in the COVERT-MI Study is provided in the Data Supplement.
This work was presented as an abstract at the European Society of Cardiology Congress, August 27 through 30, 2021.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.056177.
For Sources of Funding and Disclosures, see page 868.
Contributor Information
François Roubille, Email: francois.derimay@chu-lyon.fr.
Didier Bresson, Email: didier.bresson@ghrmsa.fr.
Cyril Prieur, Email: cyril.prieur@chu-lyon.fr.
Claire Bouleti, Email: claire.bouleti@gmail.com.
Thomas Bochaton, Email: thomas.bochaton@gmail.com.
Fabrice Ivanes, Email: faprunier@chu-angers.fr.
Olivier Dubreuil, Email: odubs6@free.fr.
Loïc Biere, Email: loic.biere@gmail.com.
Ahmad Hayek, Email: ahmad.hayek0@gmail.com.
François Derimay, Email: francois.derimay@chu-lyon.fr.
Mariama Akodad, Email: akodadmyriam@gmail.com.
Benjamin Alos, Email: benjamin.alos@chu-poitiers.fr.
Lamis Haider, Email: Lamishaidar91@gmail.com.
Naoual El Jonhy, Email: naoual.el-jonhy@chu-lyon.fr.
Rachel Daw, Email: rachel.daw@chu-lyon.fr.
Charles De Bourguignon, Email: charles.de-bourguignon@chu-lyon.fr.
Carole Dhelens, Email: carole.dhelens@chu-lyon.fr.
Gérard Finet, Email: gerard.finet@univ-lyon1.fr.
Eric Bonnefoy-Cudraz, Email: eric.bonnefoy-cudraz@univ-lyon1.fr.
Gabriel Bidaux, Email: gabriel.bidaux@univ-lyon1.fr.
Florent Boutitie, Email: florent.boutitie@chu-lyon.fr.
Delphine Maucort-Boulch, Email: delphine.maucort-boulch@chu-lyon.fr.
Pierre Croisille, Email: croisille@creatis.insa-lyon.fr.
Gilles Rioufol, Email: gilles.rioufol@univ-lyon1.fr.
Fabrice Prunier, Email: faprunier@chu-angers.fr.
Denis Angoulvant, Email: denis.angoulvant@univ-tours.fr.
References
- 1.Anzai T. Post-infarction inflammation and left ventricular remodeling: a double-edged sword. Circ J. 2013;77:580–587. doi: 10.1253/circj.cj-13-0013 [DOI] [PubMed] [Google Scholar]
- 2.Bochaton T, Lassus J, Paccalet A, Derimay F, Rioufol G, Prieur C, Bonnefoy-Cudraz E, Crola Da Silva C, Bernelin H, Amaz C, et al. Association of myocardial hemorrhage and persistent microvascular obstruction with circulating inflammatory biomarkers in STEMI patients. PLoS One. 2021;16:e0245684. doi: 10.1371/journal.pone.0245684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167:152–166. doi: 10.1016/j.trsl.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. doi: 10.1016/j.jacc.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 6.Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther. 2014;36:1465–1479. doi: 10.1016/j.clinthera.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 7.Leung YY, Yao Hui LL, Kraus VB. Colchicine–update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Opstal TSJ, Hoogeveen RM, Fiolet ATL, Silvis MJM, The SHK, Bax WA, de Kleijn DPV, Mosterd A, Stroes ESG, Cornel JH. Colchicine attenuates inflammation beyond the inflammasome in chronic coronary artery disease: a LoDoCo2 Proteomic Substudy. Circulation. 2020;142:1996–1998. doi: 10.1161/CIRCULATIONAHA.120.050560 [DOI] [PubMed] [Google Scholar]
- 9.Bouabdallaoui N, Tardif JC, Waters DD, Pinto FJ, Maggioni AP, Diaz R, Berry C, Koenig W, Lopez-Sendon J, Gamra H, et al. Time-to-treatment initiation of colchicine and cardiovascular outcomes after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J. 2020;41:4092–4099. doi: 10.1093/eurheartj/ehaa659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, et al. ; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. doi: 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 11.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 12.Bresson D, Roubille F, Prieur C, Biere L, Ivanes F, Bouleti C, Dubreuil O, Rioufol G, Boutitie F, Sideris G, et al. Colchicine for left ventricular infarct size reduction in acute myocardial infarction: a phase II, multicenter, randomized, double-blinded, placebo-controlled study protocol – the COVERT-MI study. Cardiology. 2021;146:151–160. doi: 10.1159/000512772 [DOI] [PubMed] [Google Scholar]
- 13.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. ; ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 14.Bulluck H, Go YY, Crimi G, Ludman AJ, Rosmini S, Abdel-Gadir A, Bhuva AN, Treibel TA, Fontana M, Pica S, et al. Defining left ventricular remodeling following acute ST-segment elevation myocardial infarction using cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19:26. doi: 10.1186/s12968-017-0343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004;44:2383–2389. doi: 10.1016/j.jacc.2004.09.020 [DOI] [PubMed] [Google Scholar]
- 16.Vauchot F, Ben Bouallègue F, Hedon C, Piot C, Roubille F, Mariano-Goulart D. Assessment of the area at risk after acute myocardial infarction using 123I-MIBG SPECT: comparison with the angiographic APPROACH-score. J Nucl Cardiol. 2018;25:572–580. doi: 10.1007/s12350-016-0644-7 [DOI] [PubMed] [Google Scholar]
- 17.Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G, et al. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–1205. doi: 10.1016/j.jacc.2009.10.052 [DOI] [PubMed] [Google Scholar]
- 18.Mewton N, Thibault H, Roubille F, Lairez O, Rioufol G, Sportouch C, Sanchez I, Bergerot C, Cung TT, Finet G, et al. Postconditioning attenuates no-reflow in STEMI patients. Basic Res Cardiol. 2013;108:383. doi: 10.1007/s00395-013-0383-8 [DOI] [PubMed] [Google Scholar]
- 19.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142 [DOI] [PubMed] [Google Scholar]
- 20.Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655–660. [Google Scholar]
- 21.Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, Sriamareswaran R, Htun NM, Wilson W, Stub D, et al. Colchicine in patients with acute coronary syndrome: the Australian COPS randomized clinical trial. Circulation. 2020;142:1890–1900. doi: 10.1161/CIRCULATIONAHA.120.050771 [DOI] [PubMed] [Google Scholar]
- 22.Roubille F, Tardif JC. Colchicine for secondary cardiovascular prevention in coronary disease. Circulation. 2020;142:1901–1904. doi: 10.1161/CIRCULATIONAHA.120.051240 [DOI] [PubMed] [Google Scholar]
- 23.Akodad M, Lattuca B, Nagot N, Georgescu V, Buisson M, Cristol JP, Leclercq F, Macia JC, Gervasoni R, Cung TT, et al. COLIN trial: value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis. 2017;110:395–402. doi: 10.1016/j.acvd.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 24.Forrat R, Sebbag L, Ferrera R, Hadour G, Canet E, Tabib A, de Lorgeril M. Effect of colchicine on circulating and myocardial neutrophils and on infarct size in a canine model of ischemia and reperfusion. J Cardiovasc Pharmacol. 1996;27:876–883. doi: 10.1097/00005344-199606000-00016 [DOI] [PubMed] [Google Scholar]
- 25.Hennessy T, Soh L, Bowman M, Kurup R, Schultz C, Patel S, Hillis GS. The Low Dose Colchicine after Myocardial Infarction (LoDoCo-MI) study: a pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am Heart J. 2019;215:62–69. doi: 10.1016/j.ahj.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 26.Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: a pilot randomized controlled trial. J Thromb Thrombolysis. 2012;33:88–94. doi: 10.1007/s11239-011-0637-y [DOI] [PubMed] [Google Scholar]
- 27.Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, Sianos G, Goudevenos J, Alexopoulos D, Pyrgakis V, et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation. 2015;132:1395–1403. doi: 10.1161/CIRCULATIONAHA.115.017611 [DOI] [PubMed] [Google Scholar]
- 28.Stewart S, Yang KCK, Atkins K, Dalbeth N, Robinson PC. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020;22:28. doi: 10.1186/s13075-020-2120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girre C, Thomas G, Scherrmann JM, Crouzette J, Fournier PE. Model-independent pharmacokinetics of colchicine after oral administration to healthy volunteers. Fundam Clin Pharmacol. 1989;3:537–543. doi: 10.1111/j.1472-8206.1989.tb00688.x [DOI] [PubMed] [Google Scholar]
- 30.Adamski P, Sikora J, Laskowska E, Buszko K, Ostrowska M, Umińska JM, Sikora A, Skibińska N, Sobczak P, Adamska U, et al. Comparison of bioavailability and antiplatelet action of ticagrelor in patients with ST-elevation myocardial infarction and non-ST-elevation myocardial infarction: a prospective, observational, single-centre study. PLoS One. 2017;12:e0186013. doi: 10.1371/journal.pone.0186013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cirillo P, Taglialatela V, Pellegrino G, Morello A, Conte S, Di Serafino L, Cimmino G. Effects of colchicine on platelet aggregation in patients on dual antiplatelet therapy with aspirin and clopidogrel. J Thromb Thrombolysis. 2020;50:468–472. doi: 10.1007/s11239-020-02121-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah B, Allen N, Harchandani B, Pillinger M, Katz S, Sedlis SP, Echagarruga C, Samuels SK, Morina P, Singh P, et al. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects. Inflammation. 2016;39:182–189. doi: 10.1007/s10753-015-0237-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer LM, Schlienger RG, Matter CM, Jick H, Meier CR. Discontinuation of nonsteroidal anti-inflammatory drug therapy and risk of acute myocardial infarction. Arch Intern Med. 2004;164:2472–2476. doi: 10.1001/archinte.164.22.2472 [DOI] [PubMed] [Google Scholar]
- 35.McCarthy CP, Vaduganathan M, McCarthy KJ, Januzzi JL, Jr, Bhatt DL, McEvoy JW. Left ventricular thrombus after acute myocardial infarction: screening, prevention, and treatment. JAMA Cardiol. 2018;3:642–649. doi: 10.1001/jamacardio.2018.1086 [DOI] [PubMed] [Google Scholar]
- 36.Serebruany VL, Malinin AI, Bhatt DL. Paradoxical rebound platelet activation after painkillers cessation: missing risk for vascular events? Am J Med. 2006;119:707.e11–707.e16. doi: 10.1016/j.amjmed.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Sposito AC, Carvalho LS, Cintra RM, Araújo AL, Ono AH, Andrade JM, Coelho OR, Quinaglia e Silva JCBrasilia Heart Study Group. Rebound inflammatory response during the acute phase of myocardial infarction after simvastatin withdrawal. Atherosclerosis. 2009;207:191–194. doi: 10.1016/j.atherosclerosis.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 38.Ben-Chetrit E, Bergmann S, Sood R. Mechanism of the anti-inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology (Oxford). 2006;45:274–282. doi: 10.1093/rheumatology/kei140 [DOI] [PubMed] [Google Scholar]
- 39.Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damås JK, et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77:1845–1855. doi: 10.1016/j.jacc.2021.02.049 [DOI] [PubMed] [Google Scholar]
- 40.Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Timing of cardiovascular MR imaging after acute myocardial infarction: effect on estimates of infarct characteristics and prediction of late ventricular remodeling. Radiology. 2011;261:116–126. doi: 10.1148/radiol.11110228 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.