ABSTRACT
Sjögren-Larsson syndrome (SLS) is an inherited neurocutaneous disorder whose causative gene encodes the fatty aldehyde dehydrogenase ALDH3A2. To date, the detailed molecular mechanism of the skin pathology of SLS has remained largely unclear. We generated double-knockout (DKO) mice for Aldh3a2 and its homolog Aldh3b2 (a pseudogene in humans). These mice showed hyperkeratosis and reduced fatty aldehyde dehydrogenase activity and skin barrier function. The levels of ω-O-acylceramides (acylceramides), which are specialized ceramides essential for skin barrier function, in the epidermis of DKO mice were about 60% of those in wild-type mice. In the DKO mice, levels of acylceramide precursors (ω-hydroxy ceramides and triglycerides) were increased, suggesting that the final step of acylceramide production was inhibited. A decrease in acylceramide levels was also observed in human immortalized keratinocytes lacking ALDH3A2. Differentiated keratinocytes prepared from the DKO mice exhibited impaired long-chain base metabolism. Based on these results, we propose that the long-chain-base-derived fatty aldehydes that accumulate in DKO mice and SLS patients attack and inhibit the enzyme involved in the final step of acylceramide production. Our findings provide insight into the pathogenesis of the skin symptoms of SLS, i.e., decreased acylceramide production, and its molecular mechanism.
KEYWORDS: aldehyde dehydrogenase, ceramide, ichthyosis, lipid, long-chain base, Sjögren-Larsson syndrome, skin, sphingolipid
INTRODUCTION
Since aldehydes are highly electrophilic, they are reactive and thus toxic to cells and tissues in general. Therefore, aldehydes generated in living organisms must be promptly converted to other molecules with low reactivity, such as carboxylic acids. Aldehyde dehydrogenases (ALDHs) are enzymes that convert aldehydes to carboxylic acids. Humans and mice contain 19 and 21 ALDH isozymes, respectively (1, 2). There are several known inherited diseases caused by mutations in the ALDH genes (1, 3). One of these, Sjögren-Larsson syndrome (SLS; Mendelian Inheritance in Man [MIM] no. 270200), is caused by mutations in the gene encoding the fatty aldehyde dehydrogenase (FALDH) ALDH3A2, which is highly active toward fatty aldehydes (4, 5).
SLS is an autosomal recessive inherited neurocutaneous disorder whose major symptoms are ichthyosis in the skin and spastic paraplegia and mental retardation in the nervous system (6, 7). Ichthyosis is a type of keratosis, characterized by thickening, dryness, and desquamation of the skin. So far, over 100 different mutations in ALDH3A2 have been reported in SLS patients, and the prevalence of SLS is estimated to be 0.4 in 100,000 (7, 8). Accumulated fatty aldehydes in SLS patients are thought to cause the SLS pathology (5). However, the detailed molecular mechanism of SLS pathogenesis, including which fatty aldehydes are involved and which enzymes are inhibited by the accumulated fatty aldehydes, is largely unknown.
We previously analyzed Aldh3a2 knockout (KO) mice and found that they exhibited some behavioral abnormalities, including impaired motor function (9). This phenotype appeared to be related to the neurological symptoms of SLS. In the Aldh3a2 KO brain, levels of the 2-hydroxy galactosylceramides important for myelin function and maintenance were reduced. However, these Aldh3a2 KO mice did not show an SLS-like skin phenotype (10). FALDH activity was reduced to ∼30% of the wild-type (WT) levels in the brains of Aldh3a2 KO mice, but was similar to WT levels in the epidermis. ALDH3A2 belongs to the ALDH3 subfamily. Humans have three ALDH3 subfamily members (ALDH3A1, ALDH3A2, and ALDH3B1), whereas mice have five. In addition to the three orthologs (Aldh3a1, Aldh3a2, and Aldh3b1), mice also express Aldh3b2 (ALDH3B2 is a pseudogene in humans) and the rodent-specific Aldh3b3 (1, 2, 11). Of these ALDH3 subfamily members, only ALDH3A1/Aldh3a1 shows high activity toward medium-chain (C5 to C10) aldehydes; the others are highly active toward long-chain (C11 to C20) aldehydes (11–13). Since Aldh3b2 is the most abundantly expressed of the Aldh3 subfamily members in the mouse epidermis (10), it may compensate for the loss of Aldh3a2 in the Aldh3a2 KO mouse epidermis, resulting in the absence of an SLS-like skin phenotype.
Skin has a permeability barrier function (skin barrier) that prevents the invasion of foreign substances such as pathogens and allergens into the body and the excessive evaporation of water from the body. Abnormalities in the skin barrier cause several skin disorders, such as increased vulnerability to infectious diseases, ichthyosis, and atopic dermatitis (14, 15). Skin is composed of the epidermis, dermis, and subcutaneous tissue, and the epidermis is further divided into the following four layers (in order from outermost to innermost): stratum corneum (SC), stratum granulosum (SG), stratum spinosum (SS), and stratum basale (SB) (16, 17). In the intercellular spaces of the SC, there is a multilayered lipid structure called the lipid lamellae, which plays a pivotal role in the formation of the skin barrier (16, 17). The major lipid components of the lipid lamellae are ceramides, cholesterol, and fatty acids (FAs) (17–19). Ceramides are the hydrophobic backbone of sphingolipids and are composed of a long-chain base (LCB) and an FA linked by an amide bond (19). The epidermis contains a variety of ceramide classes, among which the epidermis-specific ω-O-acylceramides (acylceramides) are essential for skin barrier function (17, 19). Acylceramides have a characteristic structure, namely, three hydrophobic chains consisting of linoleic acid attached to the ω-position of the FA portion of normal ceramides. All of the human genes involved in acylceramide synthesis cause ichthyosis when mutated (17, 19, 20). Furthermore, mice in which any of those genes have been knocked out exhibit neonatal lethality due to markedly impaired skin barrier function (17, 19, 21).
Aldh3a2 KO mice do not show a skin barrier defect under normal conditions (10), probably due to the functional overlap with Aldh3b2, and are therefore unsuitable as an SLS model for skin pathology. In the present study, we generated Aldh3a2 Aldh3b2 double-knockout (DKO) mice as a faithful SLS model and analyzed the resulting skin phenotype. The DKO mice exhibited abnormalities in the skin barrier and decreased acylceramide levels. Furthermore, our results suggest that the transacylation reaction, the final step in the acylceramide synthesis pathway, was inhibited in these mice. These findings provide a novel insight into the molecular mechanism of SLS pathogenesis.
RESULTS
Reduced FALDH activity in the epidermis of Aldh3a2 Aldh3b2 DKO mice.
To generate Aldh3a2 Aldh3b2 DKO mice to use as an SLS skin model, we first created Aldh3b2 KO mice using the CRISPR/Cas9 system. We obtained Aldh3b2 KO mice with an 8-bp deletion in exon 2 of Aldh3b2 (Fig. 1A); these mice developed normally to adulthood. Next, Aldh3b2 KO mice were crossed with previously generated Aldh3a2+/− mice (10) to obtain Aldh3a2+/− Aldh3b2−/− mice. Finally, DKO (Aldh3a2−/− Aldh3b2−/−) mice were generated by crossing male and female Aldh3a2+/− Aldh3b2−/− mice. Since we observed no phenotypic differences between WT and Aldh3b2 KO mice in any of the experiments we conducted, we used littermate Aldh3b2 KO (Aldh3a2+/+ Aldh3b2−/−) mice as a control for the DKO mice in the following experiments. However, in some experiments, as an additional control, we present data from WT (C57BL/6J) mice born on the same day as the DKO mice.
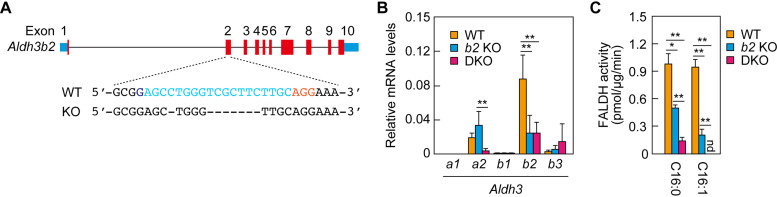
FIG 1.
Creation of Aldh3a2 Aldh3b2 double-knockout (DKO) mice and analysis of fatty aldehyde dehydrogenase (FALDH) activity. (A) Aldh3b2 knockout (KO) mice were generated using the CRISPR/Cas9 system. The exon structure (red, coding sequence; blue, untranslated regions) of Aldh3b2 and the nucleotide sequences of wild-type (WT) and Aldh3b2 KO mice around the guide RNA target sequence (light blue) and the protospacer flanking motif (PAM) sequence (magenta) in exon 2 are shown. (B) Total RNA prepared from the epidermis of WT, Aldh3b2 KO, and DKO mice was subjected to SYBR green-based quantitative real-time reverse transcription-PCR (RT-PCR) using specific primers for Aldh3a1, Aldh3a2, Aldh3b1, Aldh3b2, and Aldh3b3. Values are expressed relative to Gapdh and are the means ± standard deviation (SD) of three independent experiments. Statistically significant differences are indicated (**, P < 0.01; Tukey’s test). (C) Total lysates (15 μg) prepared from the epidermis of WT, Aldh3b2 KO, and DKO mice were incubated with 1.5 mM NAD+ and 100 μM hexadecanal (C16:0) or trans-2-hexadecenal (C16:1) at 37°C for 30 min. The quantity of NADH product was determined by measuring the fluorescence of NADH (461.5 nm). Values presented are the means ± SD of three independent experiments. Statistically significant differences are indicated (*, P < 0.05; **, P < 0.01; Tukey’s test). b2 KO, Aldh3b2 KO; nd, not detected.
Based on genotyping at 3 weeks of age, crosses between male and female Aldh3a2+/− Aldh3b2−/− mice generated 26 Aldh3b2 KO mice (Aldh3a2+/+ Aldh3b2−/−) and 48 Aldh3a2+/− Aldh3b2−/− mice. No living Aldh3a2 Aldh3b2 DKO (Aldh3a2−/− Aldh3b2−/−) mice were observed. Genotyping during the brief postnatal period revealed that DKO mice were viable at birth and 1 day after birth but not beyond 2 days after birth. The numbers of Aldh3b2 KO, Aldh3a2+/− Aldh3b2−/−, and DKO mice on postnatal day 0 (P0) were 13, 35, and 16, respectively. All of the DKO mice had no milk in their stomachs and were left outside the nest, implying that they had been abandoned by their mothers.
To examine whether the expression of other Aldh3 family members increased in a complementary manner in the Aldh3b2 KO and DKO mice, we conducted real-time quantitative reverse transcription-PCR (RT-PCR) on the epidermis of P0 mice. In agreement with the results of our previous study (10), Aldh3b2 was the most abundantly expressed Aldh3 gene in WT mice, followed by Aldh3a2 (Fig. 1B). In the Aldh3b2 KO and DKO mice, expression levels of Aldh3 family members other than the disrupted gene(s) were similar to those in WT mice, indicating no complementary increase in their expression. Expression levels of the disrupted genes were lower than those in WT mice (Aldh3a2, ∼20% in DKO mice; Aldh3b2, ∼30% in Aldh3b2 KO and DKO mice), probably due to nonsense-mediated mRNA decay.
Next, we measured the FALDH activity toward C16:0 (hexadecanal) and C16:1 (trans-2-hexadecenal) aldehydes in the epidermis of WT, Aldh3b2 KO, and DKO mice. In the Aldh3b2 KO mice, activity toward C16:0 and C16:1 aldehydes was reduced to ∼50% and ∼20% of that in WT mice, respectively (Fig. 1C). In the DKO mice, activity toward C16:0 aldehyde was reduced to ∼15% of that in WT mice, and activity toward C16:1 aldehyde disappeared completely. Considering our previous finding that FALDH activity in the epidermis of Aldh3a2 KO mice was almost identical to that in WT mice (10), we conclude that Aldh3b2 and Aldh3a2 have overlapping FALDH activity, with Aldh3b2 being the predominant form in the mouse epidermis.
Decreased skin barrier function in DKO mice.
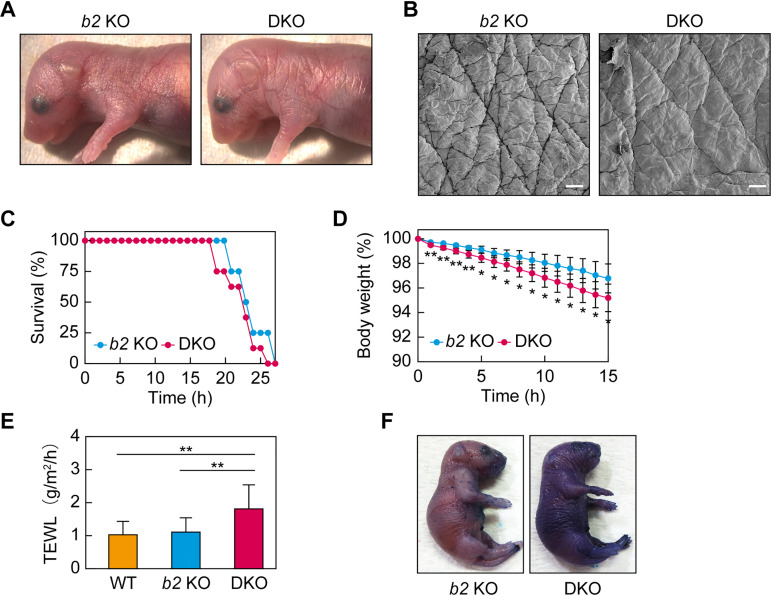
At P0, the skin of the DKO mice looked rougher in texture than that of the Aldh3b2 KO mice (Fig. 2A), and scanning electron microscopy revealed that their sulcus cutis was shallower than that of the Aldh3b2 KO mice (Fig. 2B). We then investigated the neonatal lethality of DKO mice in detail. DKO and Aldh3b2 KO mice were prepared by Caesarean section at embryonic day 18.5 (E18.5), and their survival rate over time was measured. The control Aldh3b2 KO mice died after 20 to 26 h (mean, 23.8 h) when they were kept away from their mother and unable to drink milk (Fig. 2C). This result was similar to our previous findings for WT mice (22, 23). The DKO mice died on average 1.5 h earlier than Aldh3b2 KO mice, but this difference was not large enough to explain the neonatal lethality observed following natural birth. We also measured the time course of body weight. The DKO mice showed a slightly but significantly greater weight loss than that of Aldh3b2 KO mice (Fig. 2D).
FIG 2.
Impaired skin permeability barrier formation in Aldh3a2 Aldh3b2 DKO mice. (A and B) Aldh3b2 KO and DKO mice were photographed on postnatal day 0 (P0) (A) and subjected to scanning electron microscopy (B). Bars, 100 μm. (C and D) Time courses of survival rates (C) and body weights (D) of Aldh3b2 KO (n = 4) and DKO (n = 8) mice after Caesarean section, examined at embryonic day 18.5 (E18.5). Values presented in panel D are means ± SD, and statistically significant differences from Aldh3b2 KO mice are indicated (*, P < 0.05; **, P < 0.01; Student’s t test). (E) Transepidermal water loss (TEWL) from WT (n = 8), Aldh3b2 KO (n = 13), and DKO mice (n = 16) were measured at P0. Values presented are means ± SD, and statistically significant differences are indicated (**, P < 0.01; Tukey’s test). (F) Aldh3b2 KO and DKO mice at P0 were stained with 0.1% toluidine blue for 40 h and photographed. b2 KO, Aldh3b2 KO.
To determine whether this weight loss was due to greater water loss from the body, we measured transepidermal water loss (TEWL). We observed no difference between the WT and Aldh3b2 KO mice, but the DKO mice exhibited approximately double the TEWL of these lines, suggesting reduced inside-to-outside skin barrier function (Fig. 2E). Next, we investigated the outside-to-inside skin barrier function using toluidine blue staining. Under long-term (40-h) staining, darker staining was observed in the DKO mice than that in the Aldh3b2 KO mice (Fig. 2F). These results indicate that the DKO mice exhibited weak skin barrier abnormalities.
Hyperkeratosis in DKO mice.
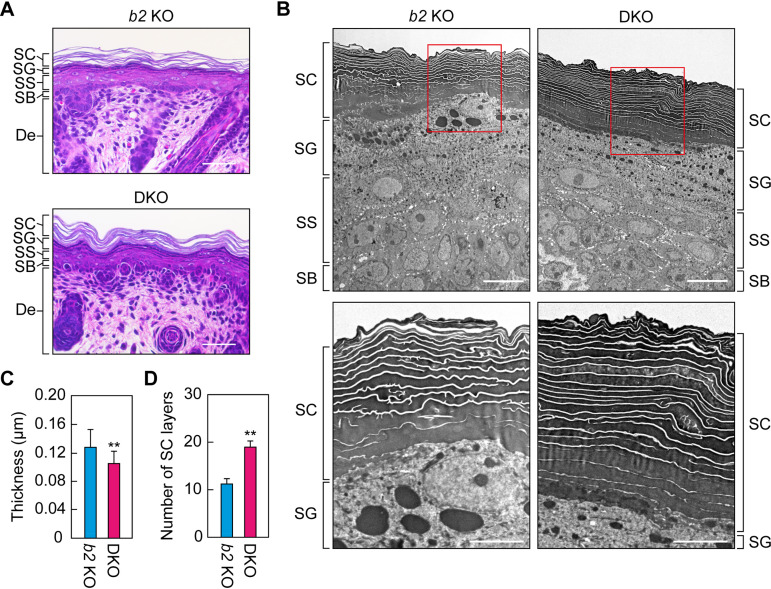
To investigate the cause of the skin barrier abnormalities in DKO mice, we performed a histological analysis of the epidermis via hematoxylin and eosin staining. In the epidermis of the control Aldh3b2 KO mice, we observed gaps in the SC (Fig. 3A). These gaps represent the areas where lipid lamellae were originally present and are generated by the lipid elution that occurs during the alcohol dehydration step of the staining procedure. It has been reported that such gaps are absent in mice with defective lipid lamella formation (22–24). In the epidermis of the DKO mice, we did observe gaps in the SC, although they appeared to be slightly narrower than those in the Aldh3b2 KO mice (Fig. 3A).
FIG 3.
Hyperkeratosis and narrowed lipid lamellae in the Aldh3a2 Aldh3b2 DKO mouse epidermis. (A) Paraffin sections (4 μm) of skin prepared from Aldh3b2 KO and DKO mice at P0 were subjected to hematoxylin and eosin staining, and bright-field images were obtained. Bars, 50 μm. (B to D) Ultrathin sections (80 nm) of skin prepared from Aldh3b2 KO and DKO mice at P0 were subjected to transmission electron microscopy. The lower images are enlarged views of the red rectangles in the upper images. Bars, 10 μm (upper panels) or 5 μm (lower panels). Lipid lamella thickness (C) and number of stratum corneum (SC) layers (D) were quantified from five different images. Values presented are means ± SD, and statistically significant differences are indicated (**, P < 0.01; Student’s t test). SG, stratum granulosum; SS, stratum spinosum; SB, stratum basale; b2 KO, Aldh3b2 KO.
We used transmission electron microscopy analysis to obtain more detailed views of the epidermis. Similar to the results described above (Fig. 3A), the lipid lamellae were slightly but significantly narrower in the DKO epidermis than in the Aldh3b2 KO epidermis (Fig. 3B and C). Furthermore, the number of cell layers in the SC of the DKO mice was approximately twice that in the Aldh3b2 KO mice (Fig. 3D). Such hyperkeratosis in the SC and abnormalities in the formation of the lipid lamellae have also been observed in SLS patients (25). The DKO mice therefore exhibited an SLS-like skin phenotype and are thus useful as a model for SLS skin pathology.
Decrease in acylceramide levels in the epidermis of DKO mice.
Since the formation of the lipid lamellae was impaired in the DKO mice, we analyzed the lipid composition of the epidermis. Lipids were prepared from WT, Aldh3b2 KO, and DKO mice at P0 and were separated via thin-layer chromatography (TLC), followed by detection using copper phosphate reagent. There were no obvious differences in lipid composition between the WT and Aldh3b2 KO mice (Fig. 4A). However, in the epidermis of the DKO mice, we observed lower quantities of acylceramides and (O-acyl)-ω-hydroxy FAs (OAHFAs), which may be degradation products of acylceramides, and higher quantities of ω-hydroxy (ω-OH) ceramides, which are precursors of acylceramides. The quantities of other lipids were similar to those in the WT and Aldh3b2 KO mice.
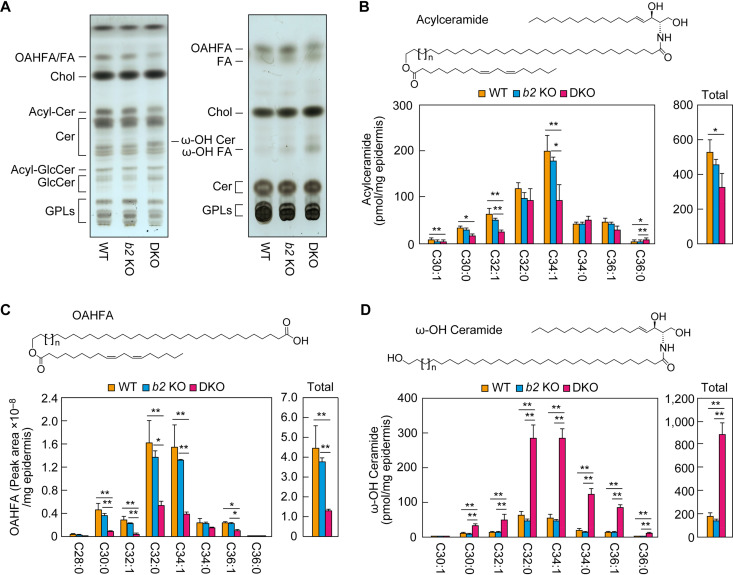
FIG 4.
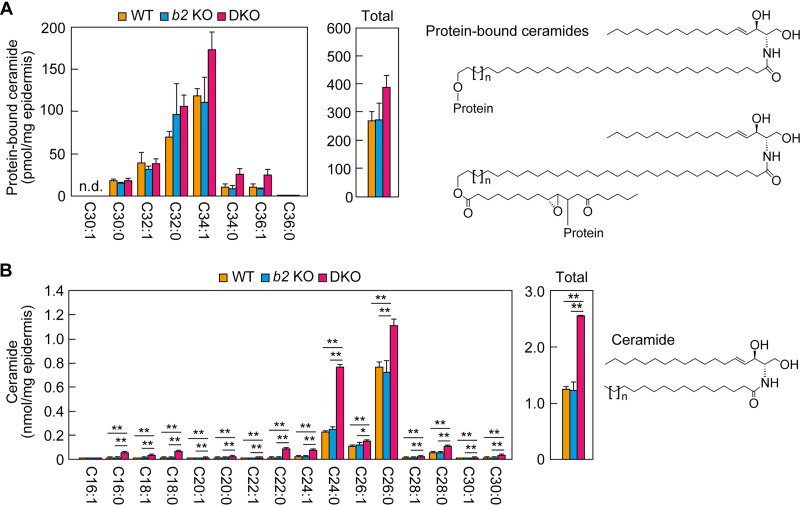
Reduced acylceramide levels in the Aldh3a2 Aldh3b2 DKO mouse epidermis. (A) Lipids extracted from the epidermis of WT, Aldh3b2 KO, and DKO mice at P0 were separated via thin-layer chromatography (TLC) using two different solvent systems suitable for the separation of ceramides (left) and less polar lipids (right). Lipids were stained with copper phosphate reagent. Acyl-Cer, acylceramide; Acyl-GlcCer, acyl-glucosylceramide; Cer, ceramide; Chol, cholesterol; GlcCer, glucosylceramide; GPL, glycerophospholipid. (B to D) Lipids were extracted from the epidermis of WT (n = 3), Aldh3b2 KO (n = 3), and DKO (n = 3) mice at P0, and acylceramides (B), OAHFAs (C), and ω-OH ceramides (D) were analyzed via liquid chromatography-tandem mass spectrometry (LC-MS/MS). The quantities of each lipid species (according to chain length and saturation) and of total lipids are shown in the left and right panels, respectively. Values presented are means ± SD (*, P < 0.05, **, P < 0.01; Tukey’s test). The structure of each type of lipid is shown above the graph. b2 KO, Aldh3b2 KO.
We next performed detailed quantitative analyses of the lipids, especially those that differed between mouse lines in the above TLC analyses, using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Although total acylceramide levels were almost identical in the WT and Aldh3b2 KO mice, those in the DKO mice were lower (∼60% those of WT mice) (Fig. 4B), consistent with the TLC results (Fig. 4A). Acylceramides include multiple species that differ in the chain length and degree of saturation of the ω-OH FA moiety. The quantities of C32:1 and C34:1 acylceramides in particular were greatly reduced, to approximately half of WT levels (Fig. 4B).
The quantities of many molecular species of linoleic acid-containing OAHFAs—the putative degradation products of acylceramides—were also lower in the epidermis of the DKO mice than in the WT and Aldh3b2 KO mice, with the total being approximately ∼25% of that in the WT mice (Fig. 4C). In contrast, the quantities of almost all molecular species of ω-OH ceramides—the precursors of acylceramides—were greater in the epidermis of the DKO mice than in those of the WT and Aldh3b2 KO mice, and the total quantity of ω-OH ceramides was approximately five times that in the WT mice (Fig. 4D). In the final step of acylceramide production, acylceramides are produced from ω-OH ceramides by a transacylation reaction (26). Our results—increased precursors (ω-OH ceramides) and decreased products (acylceramides)—suggest that this transacylation reaction is inhibited in DKO mice. Acylceramides are essential for skin barrier function, and all of the genes involved in their synthesis can cause ichthyosis when mutated (17, 19, 21). Therefore, it is highly likely that the abnormalities we observed in skin barrier formation in the DKO mice were caused by these reduced acylceramide levels.
Normal levels of protein-bound ceramides in DKO mouse epidermis.
Some of the acylceramides are converted to protein-bound ceramides, which bind covalently to the surface proteins of corneocytes (fully differentiated keratinocytes) (27, 28). Protein-bound ceramides are a component of a cell surface structure, the corneocyte lipid envelope (CLE), and are important for the formation of the skin barrier (29). We next quantified protein-bound ceramides using LC-MS/MS. Although acylceramide levels were reduced in the epidermis of the DKO mice (Fig. 4B), there were no significant differences in the quantities of protein-bound ceramides among the WT, Aldh3b2 KO, and DKO mice (Fig. 5A). This result is consistent with the ultrastructure of the epidermis of SLS patients, in which the CLE structure is normal (25). Therefore, the reduced skin barrier function in DKO mice can be attributed only to the reduced acylceramide levels.
FIG 5.
Levels of protein-bound and nonacylated ceramides in the Aldh3a2 Aldh3b2 DKO mouse epidermis. (A and B) Lipids were extracted from the epidermis of WT (n = 3), Aldh3b2 KO (n = 3), and DKO (n = 3) mice at P0, and protein-bound ceramides (A) and ceramides (B) were analyzed via LC-MS/MS. The quantities of each lipid species (according to chain length and saturation) and of total lipids are shown in the left and right panels, respectively. Values presented are means ± SD (*, P < 0.05; **, P < 0.01; Tukey’s test). The structure of each type of lipid is shown to the right of the graph. The structure of a conventional protein-bound ceramide and that of a newly proposed one (28) are shown (upper and lower structures in panel A, respectively). b2 KO, Aldh3b2 KO; n.d., not detected.
Next, we quantified normal (nonacylated) ceramides using LC-MS/MS. Again, we found no difference in quantities or composition between the WT and Aldh3b2 KO mice, but in the DKO mice, the quantities of almost all molecular species were higher than those in the other two lines, and the total ceramide quantity was approximately doubled (Fig. 5B). This is likely to be a compensatory response to the reduced acylceramide levels.
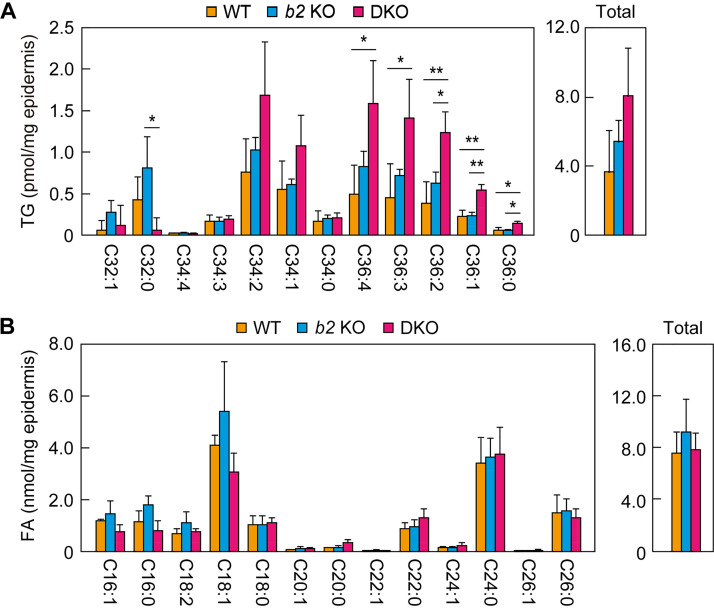
Higher triglyceride levels in the epidermis of DKO mice.
In the final step of acylceramide production, linoleic acid is transferred from triglycerides (TGs) to ω-OH ceramides via transacylation (26). In the DKO mouse epidermis, levels of ω-OH ceramides (one of the substrates of the transacylation reaction) were higher than those in the other two mouse lines (Fig. 4D). We next examined the quantities of linoleic acid-containing TGs (another substrate of the transacylation reaction) using LC-MS/MS. The WT and the Aldh3b2 KO mice exhibited similar quantities and compositions of TGs (Fig. 6A). However, in the epidermis of the DKO mice, the quantities of many TG species were greater than those in the WT and Aldh3b2 KO mice. The total quantity of linoleic acid-containing TGs in the DKO mice was about double that in the WT mice, although this difference was not statistically significant. We also quantified free FAs in the epidermis, but there were no differences in their quantities, including that of linoleic acid, among the WT, Aldh3b2 KO, and DKO mice (Fig. 6B). These results indicate that the reduced acylceramide levels in the epidermis of the DKO mice were not caused by a decrease in the levels of the substrates (ω-OH ceramides or TGs).
FIG 6.
Levels of triglycerides (TGs) and fatty acids (FAs) in the Aldh3a2 Aldh3b2 DKO mouse epidermis. (A and B) Lipids were extracted from the epidermis of WT (n = 3), Aldh3b2 KO (n = 3), and DKO (n = 3) mice at P0, and linoleic acid (C18:2)-containing TGs (A) and FAs (B) were analyzed via LC-MS/MS. The quantities of each lipid species (according to chain length and saturation) and total lipids are shown in the left and right panels, respectively. The chain lengths in panel A represent the sum of the two FAs other than linoleic acid. Values presented are means ± SD (*, P < 0.05; **, P < 0.01; Tukey’s test). b2 KO, Aldh3b2 KO.
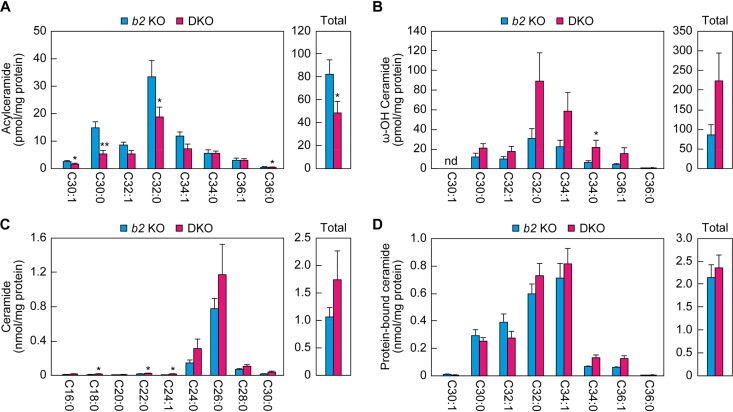
Decreased acylceramide levels in the SC of DKO mice.
In the above-described analyses, we measured lipids prepared from the whole epidermis. Next, we focused on the lipids in the SC, which plays the most important role in skin barrier formation in the epidermis. The SC was isolated from the backs of Aldh3b2 KO and DKO mice at P0 by tape stripping, and the lipids were extracted and subjected to LC-MS/MS to measure the acylceramides, ω-OH ceramides, ceramides, and protein-bound ceramides. As in the epidermis, acylceramide levels in the SC of the DKO mice were ∼60% of those of the Aldh3b2 KO mice (Fig. 7A). The quantities of both ω-OH ceramides and ceramides were again higher in the DKO mice (Fig. 7B and C), but at 2.6- and 1.6-fold, respectively, these differences were less pronounced in the SC than in the epidermis. The quantities of protein-bound ceramides were similar in the Aldh3b2 KO and DKO mice (Fig. 7D). The results obtained for the epidermis and the SC were thus similar with respect to ceramide composition.
FIG 7.
Reduced acylceramide levels in the SC of Aldh3a2 Aldh3b2 DKO mice. (A to D) The SC was prepared via tape stripping from Aldh3b2 KO (n = 3) and DKO (n = 3) mice at P0. Lipids were extracted, and acylceramides (A), ω-OH ceramides (B), ceramides (C), and protein-bound ceramides (D) were analyzed via LC-MS/MS. The quantities of each lipid species (according to chain length and saturation) and of total lipids are shown in the left and right panels, respectively. Values presented are means ± SD (*, P < 0.05; **, P < 0.01; Student’s t test). b2 KO, Aldh3b2 KO; nd, not detected.
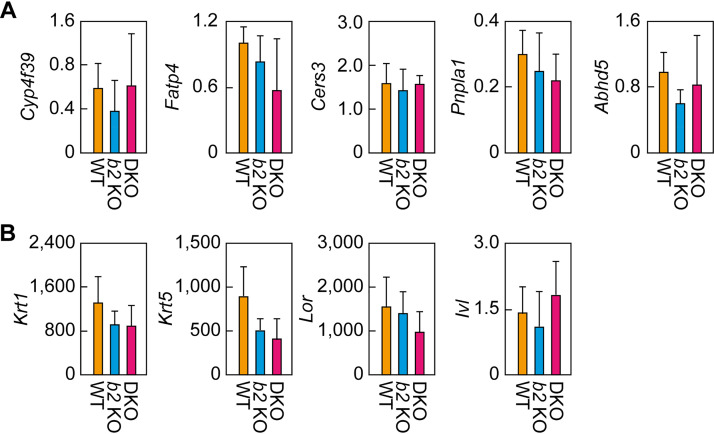
Normal expression levels of acylceramide synthesis-related genes in the DKO mouse epidermis.
The final reaction in the acylceramide synthesis pathway is transacylation, which transfers the linoleic acid in TGs to ω-OH ceramides. The decrease in acylceramides and the increase in the substrates (ω-OH ceramides and TGs) in the epidermis/SC of DKO mice suggest that the activity of the enzyme(s) catalyzing this transacylation reaction was reduced. This reaction is catalyzed by the transacylase PNPLA1 (26), and the α/β hydrolase family protein ABHD5 enhances the PNPLA1-catalyzed reaction, probably by stimulating the utilization of TG by PNPLA1 (30). There are two possible causes for this postulated decrease in enzyme activity, namely, a reduced quantity of enzyme(s) or inhibition of the enzyme(s). We investigated the former possibility—that the lower production of acylceramides in the DKO mouse epidermis was due to the lower expression levels of Pnpla1 or Abhd5 mRNA. Real-time quantitative RT-PCR revealed that both Pnpla1 and Abhd5 mRNA levels were comparable in the WT, Aldh3b2 KO, and DKO mice (Fig. 8A). Similarly, the expression levels of other genes involved in acylceramide synthesis (those encoding FA ω-hydroxylase [Cyp4f39], acyl-CoA synthetase [Fatp4], and ceramide synthase [Cers3]), as well as those of keratinocyte differentiation markers (the basal layer marker keratin 5 [Krt5] and the spinous layer/granular layer markers keratin 1 [Krt1], loricrin [Lor], and involucrin [Ivl]), were similar in the three mouse lines (Fig. 8A and B). These results indicate that the reduced acylceramide levels in the DKO mouse epidermis were not the result of lower expression levels of genes involved in acylceramide synthesis.
FIG 8.
Expression levels of acylceramide synthesis-related genes and keratinocyte differentiation markers. (A and B) Total RNA prepared from the epidermis of WT (n = 3), Aldh3b2 KO (n = 3), and Aldh3a2 Aldh3b2 DKO (n = 3) mice at P0 was subjected to SYBR green-based quantitative real-time RT-PCR using specific primers for acylceramide synthesis-related genes (Cyp4f39, Fatp4, Cers3, Pnpla1, and Abhd5) (A) and keratinocyte differentiation markers (Krt1, Krt5, Lor, and Ivl) (B). Values are expressed relative to Hprt1 and are means ± SD. b2 KO, Aldh3b2 KO.
Altered LCB metabolism in differentiated keratinocytes from DKO mice.
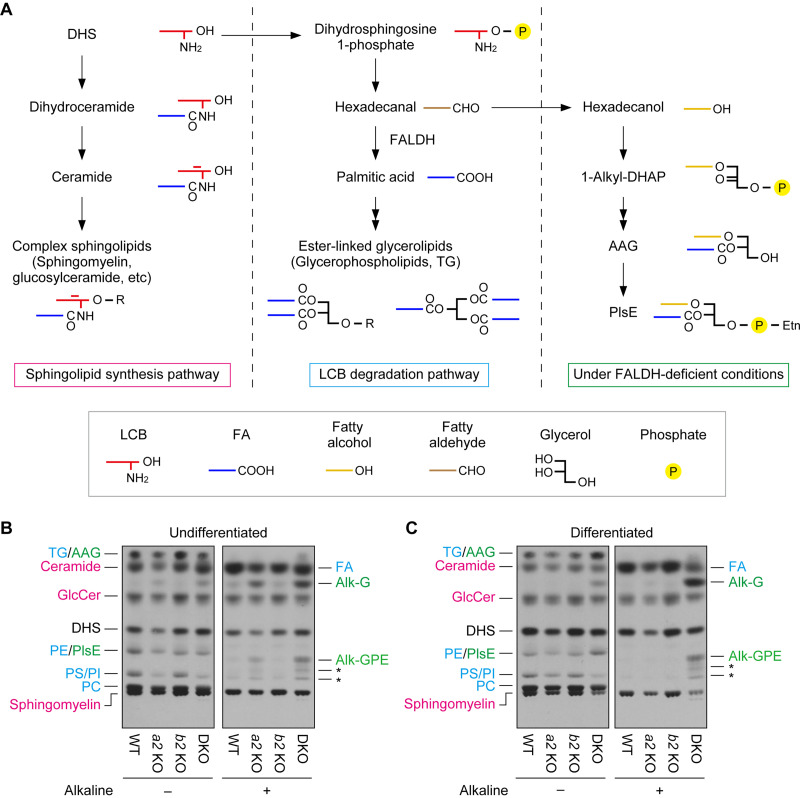
One of the major species of fatty aldehydes in keratinocytes is long-chain aldehydes derived from LCBs, such as trans-2-hexadecenal, derived from sphingosine, and hexadecanal, derived from dihydrosphingosine (DHS) (10). LCBs produced during the de novo sphingolipid synthesis pathway or the degradation pathway of sphingolipids/ceramides are metabolized to complex sphingolipids (such as sphingomyelin or glycosphingolipids) or glycerolipids (ester-linked types) (Fig. 9A) (19, 31). In the LCB-to-glycerolipid metabolic pathway, LCBs are converted to LCB 1-phosphates, then to long-chain aldehydes by sphingosine 1-phosphate lyase, and further to long-chain FAs by FALDH. We have previously shown that the conversion of long-chain aldehydes to long-chain FAs in Aldh3a2-deficient CHO-K1 cells, neurons, and undifferentiated keratinocytes is impaired (9, 10, 31). In these cells, long-chain aldehydes are instead metabolized to long-chain alcohols, followed by conversion to ether-linked glycerolipids (such as plasmanyl/plasmenyl ethanolamine).
FIG 9.
Impaired long-chain base (LCB) metabolism in Aldh3a2 Aldh3b2 DKO mouse keratinocytes. (A) Metabolism of dihydrosphingosine (DHS) is illustrated. Under normal conditions, DHS is metabolized to complex sphingolipids in the sphingolipid synthesis pathway (left) or to ester-linked glycerolipids in the degradation pathway (middle). However, under FALDH-deficient conditions, such as ALDH3A2 mutation in humans and Aldh3a2 Aldh3b2 DKO in mice (right), the conversion of DHS to ester-linked glycerolipids is impaired, and DHS is instead metabolized to ether-linked glycerolipids. The simplified structure of each lipid is also shown. AAG, 1-alkyl/alkenyl-2-acyl-glycerol; DHAP, dihydroxyacetone phosphate; PlsE, plasmanyl/plasmenyl phosphatidylethanolamine; R, polar head group. (B and C) Primary keratinocytes were prepared from WT, Aldh3a2 KO, Aldh3b2 KO, and DKO mice, and undifferentiated (B) and differentiated (C) keratinocytes were labeled with [3H]DHS at 37°C for 4 h. Lipids were extracted, given alkaline treatment or left untreated, separated via TLC, and detected using autoradiography. Asterisks indicate unidentified products of PlsE resulting from alkaline treatment. The colors represent lipids generated via the sphingolipid synthesis pathway (magenta) or the LCB degradation pathway under normal conditions (blue) or FALDH-deficient conditions (green). Alk-G, 1-alkyl/alkenyl-glycerol; Alk-GPE, 1-alkyl/alkenyl-glycerophosphoethanolamine; GlcCer, glucosylceramide; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; a2 KO, Aldh3a2 KO; b2 KO, Aldh3b2 KO.
We performed a [3H]DHS labeling assay to investigate LCB metabolism in keratinocytes prepared from the DKO mice. In undifferentiated keratinocytes from WT mice, DHS was metabolized to sphingolipids (ceramide, glucosylceramide, and sphingomyelin) and ester-linked glycerolipids (phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, phosphatidylcholine, and TG) (Fig. 9B). As previously reported (10), in undifferentiated keratinocytes from Aldh3a2 KO mice, the metabolism of DHS to ester-linked glycerolipids was reduced, and instead ether-linked glycerolipids (plasmanyl/plasmenyl ethanolamine and 1-alkyl/alkenyl-2-acyl-glycerol) were produced. Through alkaline treatment, which hydrolyzes ester bonds, FAs were released from the ester-linked glycerolipids, whereas 1-alkyl/alkenyl-glycerol and its phosphoethanolamine adduct were released from the ether-linked glycerolipids. The metabolism of DHS was normal in undifferentiated keratinocytes from the Aldh3b2 KO mice. However, undifferentiated keratinocytes prepared from the DKO mice also exhibited the abnormal metabolism of DHS to ether-linked glycerolipids that was observed in Aldh3a2 KO keratinocytes, but at greater levels than those in the KO keratinocytes.
We then performed the [3H]DHS labeling assay on differentiated keratinocytes. The DHS metabolism in these cells prepared from the Aldh3a2 KO and Aldh3b2 KO mice was similar to that in WT mice (Fig. 9C), but in cells from the DKO mice, we observed metabolism of DHS to ether-linked glycerolipids. Thus, the keratinocytes from the DKO mice exhibited impaired LCB metabolism regardless of whether they were differentiated or not, implying that abnormalities in LCB metabolism are responsible for the pathogenesis of skin symptoms in SLS.
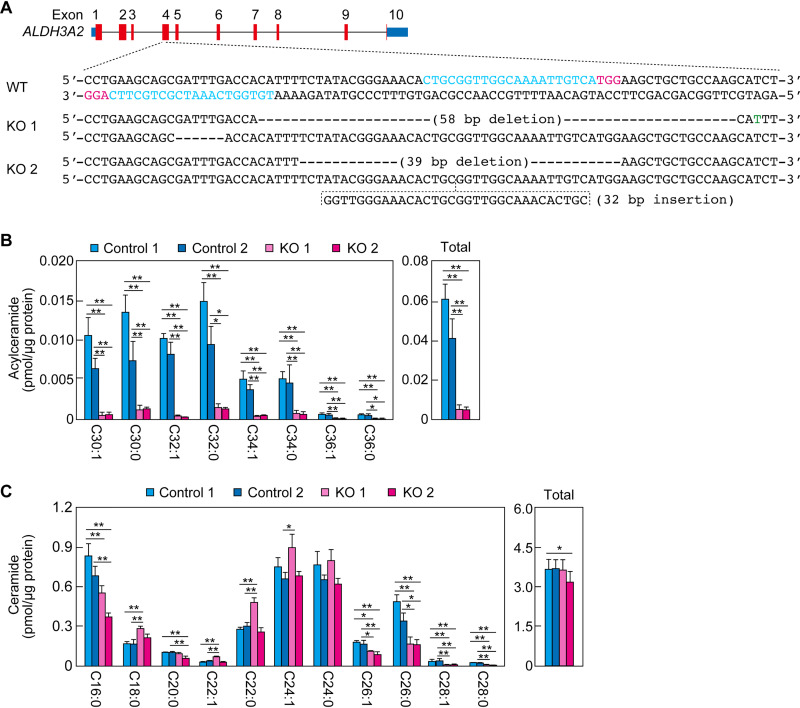
Acylceramide levels in human keratinocytes reduced by ALDH3A2 KO.
To investigate whether FALDH deficiency also causes a decrease in acylceramide levels in humans, we disrupted ALDH3A2 in human immortalized keratinocytes using CRISPR/Cas9. We obtained two ALDH3A2 KO clones (KO 1 and KO 2) (Fig. 10A). These KO keratinocytes and two controls (controls 1 and 2) were differentiated, and the levels of acylceramides and ceramides were measured via LC-MS/MS. The quantities of acylceramides of all chain lengths were greatly reduced in both types of KO keratinocyte compared to those in the control keratinocytes (Fig. 10B). The total quantities in the KO keratinocytes were approximately 10% of those in the controls. With regard to the ceramides, the quantities of some species (as identified by chain length) were higher in the KO keratinocytes than in the controls, and those of others were lower, but the total quantities were similar (Fig. 10C). These results indicate that FALDH deficiency causes a decrease in acylceramide levels in both mice and humans.
FIG 10.
Reduced acylceramide levels in ALDH3A2 KO human immortalized keratinocytes. (A) ALDH3A2 KO keratinocytes were generated using the CRISPR/Cas9 system. The exon structure (red, coding sequence; blue, untranslated regions) of human ALDH3A2 and the nucleotide sequences of WT and ALDH3A2 KO cells (KO clones 1 and 2) around the guide RNA target sequences (light blue) and the PAM sequences (magenta) in exon 4 are shown. The green nucleotide indicates a missense mutation. (B and C) Lipids were extracted from two control cells and two ALDH3A2 KO cells on day 14 of differentiation, and acylceramides (B) and ceramides (C) were analyzed via LC-MS/MS. The quantities of each lipid species (according to chain length and saturation) and of total lipids are shown in the left and right panels, respectively. Values presented are means ± SD (*, P < 0.05; **, P < 0.01; Tukey’s test) from three independent experiments.
DISCUSSION
ALDH3A2 is the causative gene of SLS, and Aldh3a2 KO mice have been analyzed previously as a model of SLS (9, 10). In the brain, FALDH activity toward C16:0 aldehyde in Aldh3a2 KO mice is reduced to ∼30% of that in WT mice, and Aldh3a2 KO mice exhibit neuronal phenotypes, such as motor dysfunction (9). However, in the epidermis, FALDH activity is not reduced in Aldh3a2 KO mice, due to functional overlap with Aldh3b2, and the skin barrier function under nonstressed conditions is normal (10). In the present study, we generated Aldh3a2 Aldh3b2 DKO mice and found that they exhibited phenotypes similar to the skin symptoms observed in SLS patients (decreased skin barrier function and hyperkeratosis) (Fig. 2 and 3), confirming that these mice are useful as a faithful SLS pathological model.
The abundance of acylceramides in the epidermis and SC of these DKO mice was ∼60% of that in WT mice (Fig. 4 and 7). Reduced acylceramide levels have also been reported in SLS patients (32) and observed in ALDH3A2 KO human keratinocytes (Fig. 10). Analyses of KO mice for the genes involved in acylceramide synthesis or other ichthyosis-causing genes have shown that there is a good correlation between the degree of reduction in acylceramide levels and the degree of skin barrier abnormality (22, 24, 33). In the epidermis of our DKO mice, in line with the partial reduction of acylceramide levels (∼60% of those of WT mice) (Fig. 4 and 7), the degree of abnormality in the skin barrier function was mild (Fig. 2). In the epidermis of the DKO mice, the only lipids that were reduced were acylceramides and their putative degradation products, OAHFAs (Fig. 4 to 7). We therefore speculate that this reduced abundance of acylceramides is the primary cause of the impaired skin barrier function seen in these DKO mice.
None of the DKO mice survived beyond 2 days after birth under natural delivery conditions. They had no milk in their stomachs, and many of them were abandoned outside the nest. Among the mice born by Caesarean section, the DKO mice died 1.5 h earlier, on average, than the control mice (Fig. 2C). However, this slight difference was not sufficient to explain the neonatal lethality of the naturally born DKO mice. Therefore, it is likely that this neonatal lethality is not caused by water loss from the body due to the abnormal skin barrier formation but is mainly due to abandonment by the mother. The appearance (different skin morphology) or odor of the DKO mice may lead to this behavior by the mother. Such abandonment of their babies by mother mice has also been reported in ceramide synthase CerS3 KO mice (34), and it appears to be common in mice with defective acylceramide production.
The pathology of SLS is thought to be caused by the toxicity of accumulated fatty aldehydes, especially long-chain aldehydes, which are the substrates of ALDH3A2 (11, 12). Aldehydes generally form Schiff bases with primary amines, such as those in Lys and Arg residues. Furthermore, α,β-unsaturated aldehydes are more toxic than normal aldehydes, since the double bond that is conjugated with the carbonyl group can react with amino acid residues that have general nucleophiles, such as His, Cys, or Trp residues, via a Michael addition reaction (35, 36). In the brain and epidermis of SLS patients, certain proteins with important functions in these tissues are thought to be attacked by the accumulated long-chain aldehydes, resulting in the inhibition of their activities. What proteins are actually inhibited in SLS patients? Since ALDH3A2 is an endoplasmic reticulum (ER) protein (37), ALDH3A2 mutations may cause the accumulation of long-chain aldehydes in the ER membrane. Therefore, it is highly likely that the target proteins of the aldehydes are ER membrane proteins. Among these, candidates are proteins that allow long-chain aldehydes to enter the active site, i.e., enzymes whose substrates are long-chain lipids, and those that have nucleophilic active-site residues that can be attacked by aldehydes. We previously found fatty acid 2-hydroxylase (FA2H) to be such a candidate target in the nervous system, via analyses of Aldh3a2 KO mice (9). FA2H is an ER membrane protein and has His residues in its active site (38, 39). It catalyzes the production of 2-OH FAs using long-chain FAs as the substrates (40). 2-OH FAs are precursors of 2-OH galactosylceramides, which play an important role in myelin (41, 42).
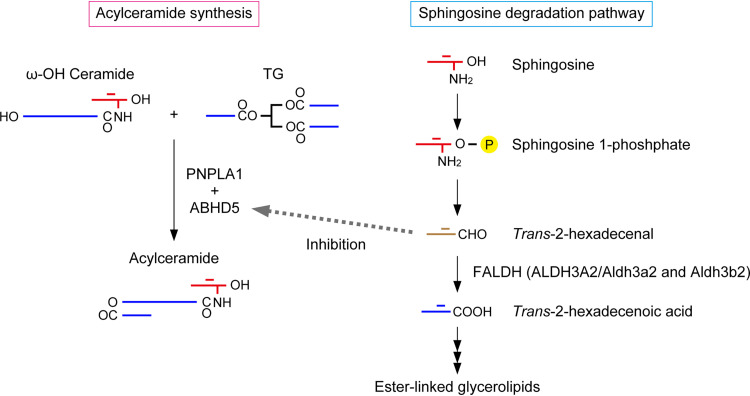
In the epidermis of the DKO mice, acylceramide levels were reduced, but levels of the substrates ω-OH ceramides and TGs were enhanced (Fig. 4 and 6). These results suggest that the transacylation reaction that produces acylceramides from ω-OH ceramides and TGs was inhibited in these mice (Fig. 11). This reaction is catalyzed by the transacylase PNPLA1, and the α/β hydrolase family protein ABHD5 seems to enhance TG utilization of PNPLA1 (26, 30). It is likely that ABHD5 also promotes TG utilization of the TG lipases ATGL/PNPLA2 and PNPLA3 (43, 44). PNPLA1 and ABHD5 are the causative genes of autosomal recessive congenital ichthyosis and the syndromic form of ichthyosis Dorfman-Chanarin syndrome, respectively (17, 20, 45, 46). Since the expression levels of PNPLA1 and ABHD5 were unchanged in the epidermis of the DKO mice (Fig. 8A), it is likely that the activity of one of the proteins they encode, not the quantity, was reduced. Analyses of KO mice for these genes revealed that TG levels are normal in the Pnpla1 KO mouse epidermis, whereas they are increased in the Abhd5 KO mouse epidermis (47). Considering that the levels of many TG species were increased in our DKO mice (Fig. 6A), we speculate that ABHD5 is inhibited by the accumulated long-chain aldehydes due to impaired FALDH activity. ABHD5 belongs to the α/β hydrolase family, and members of this family generally have three catalytic residues: a nucleophile (Ser, Cys, or Asp), an acidic amino acid (Asp or Glu), and His (45). However, in ABHD5, the nucleophilic residue is replaced by Asn, resulting in no hydrolytic activity. Instead, ABHD5 may retain the ability to bind TGs and present them to the PNPLA proteins. ABHD5 is localized at the interface between lipid droplets and the ER (30) and has a His residue conserved in the α/β hydrolase family. Therefore, ABHD5 meets the above-mentioned criteria for the target protein of the long-chain aldehydes that accumulate in DKO mice and SLS patients.
FIG 11.
A model for reduced acylceramide production in SLS. Acylceramides are produced from ω-OH ceramide and TG via a transacylation reaction (left). The LCB sphingosine is converted to trans-2-hexadecenal in the degradation pathway and further metabolized to ester-linked glycerolipids under normal conditions (right). Under FALDH-deficient conditions, such as ALDH3A2 mutation in humans (SLS) and Aldh3a2 Aldh3b2 DKO in mice, the accumulated trans-2-hexadecenal attacks and inhibits ABHD5, resulting in reduced acylceramide production.
Fatty aldehydes, substrates of ALDH3A2, are produced via various metabolic pathways and reactions, including the LCB degradation pathway, oxidation of fatty alcohols, degradation of ether phospholipids, lipid peroxidation, and the metabolism of leukotriene B4 (5, 19). Of these, to date, only the LCB degradation pathway has been shown to be impaired by reduced FALDH activity levels in keratinocytes (Fig. 9) (10). Some of the long-chain aldehydes derived from the LCBs that accumulate in FALDH-deficient cells may cause SLS by attacking nearby proteins before they are metabolized to nontoxic ether-linked glycerolipids. In the LCB degradation pathway, long-chain aldehydes are produced in the ER, since sphingosine 1-phosphate lyase, which produces long-chain aldehydes, is localized there (48). Ceramides are abundant in the epidermis (dozens of times more so than in other tissues) (49), suggesting that the quantities of long-chain aldehydes produced by ceramide and LCB degradation are also much higher than in other tissues. Sphingosine is the major LCB that constitutes ceramides, and in its degradation pathway it is converted to α,β-unsaturated aldehyde trans-2-hexadecenal (Fig. 11), which can undergo a Michael addition reaction. In vitro experiments have shown that of all the nucleophilic amino acids, His forms the most stable adduct with trans-2-hexadecenal (36). Based on these considerations, we speculate that of the fatty aldehydes, the long-chain aldehydes produced in the LCB degradation pathway, especially trans-2-hexadecenal, play an important role in SLS pathogenesis.
In the present study, we established a faithful mouse model of SLS and found that a reduction in acylceramide levels is involved in SLS skin pathology. We also proposed the following model for the molecular mechanism of acylceramide reduction: long-chain aldehydes produced via the LCB degradation pathway inhibit the final step of acylceramide production, perhaps by inhibiting the activity of ABHD5 (Fig. 11). We were unfortunately not able to detect the formation of adducts between ABHD5 and trans-2-hexadecenal due to technical issues. Future technological developments, especially those providing increased detection sensitivity for these adducts, are needed to prove this. Finally, our findings suggest the possibility of inhibiting the LCB degradation pathway in SLS patients as a potential therapeutic application.
MATERIALS AND METHODS
Mice.
The Aldh3a2 KO mice used were described previously (10). Aldh3b2 KO mice were created using the CRISPR/Cas9 system as follows. The guide RNA targeted the 24 bases adjacent to the protospacer flanking motif (PAM) sequence present in exon 2 of Aldh3b2. The oligonucleotide pair (primers Aldh3b2 KO-1 and Aldh3b2 KO-2) (Table 1) containing the guide RNA sequence were annealed and then cloned into the BbsI site of the CRISPR/Cas9 vector pX330 (Addgene, Watertown, MA). The resulting plasmid was injected into fertilized eggs of C57BL/6J mice. Genomic DNA was prepared from the tails of the pups born and then subjected to PCR using the primers Aldh3b2-1 and Aldh3b2-2 (Table 1) to amplify exon 2 of Aldh3b2. Subsequent sequencing analysis revealed that one of the mice contained an 8-bp deletion in exon 2. This mouse was crossed with a C57BL/6J mouse to generate Aldh3b2+/− mice. The Aldh3b2+/− mice were twice back-crossed with C57BL/6J mice. Aldh3b2−/− mice were created by crossing male and female Aldh3b2+/− mice. The Aldh3b2−/− mice obtained were crossed with Aldh3a2+/− mice, producing Aldh3a2+/− Aldh3b2+/− mice. Aldh3a2+/− Aldh3b2−/− mice were then obtained by crossing male and female Aldh3a2+/− Aldh3b2+/− mice. These Aldh3a2+/− Aldh3b2+/− mice were maintained by back-crossing with Aldh3b2−/− mice. Aldh3a2−/− Aldh3b2−/− (Aldh3a2 Aldh3b2 DKO) mice were generated by mating Aldh3a2+/− Aldh3b2−/− mice with each other. Littermate Aldh3a2+/+ Aldh3b2−/− (Aldh3b2 KO) and DKO mice were used in the experiments. C57BL/6J mice born on the same day were used as WT controls for some experiments. Genotyping of the Aldh3a2 gene was conducted as described previously (10). Genotyping of the Aldh3b2 gene was performed by PCR using genomic DNA prepared from mouse tails and the primers Aldh3b2-1 and Aldh3b2-2 (Table 1). The amplified DNA fragments of Aldh3b2 were digested using the restriction enzyme MwoI, whose restriction enzyme recognition site is lost in Aldh3b2 KO mice, and separated and identified using agarose gel electrophoresis.
TABLE 1.
DNA oligonucleotides and primers used in this study
| Oligonucleotide DNA or primer | Sequence |
|---|---|
| Aldh3b2 KO-1 | 5′-CACCGAGCCTGGGTCGCTTCTTGC-3′ |
| Aldh3b2 KO-2 | 5′-TTTGGCAAGAAGCGACCCAGGCTC-3′ |
| Aldh3b2-1 | 5′-CGGACTCGGCCTACTGAGTTTCGGA-3′ |
| Aldh3b2-2 | 5′-CAGTCTGTGCATAGAGCAAGGG-3′ |
| Aldh3a1-F | 5′-CGGTGATGCCCATTGTGTGTGTTCG-3′ |
| Aldh3a1-R | 5′-TTCTTCATTCCGCAGAGACCTCACC-3′ |
| Aldh3a2-F | 5′-TTCTCGTAACAATAAGCTCATCAAACG-3′ |
| Aldh3a2-R | 5′-CAGCATCCCCAGCCTTCCTTTGTTG-3′ |
| Aldh3b1-F | 5′-GCTGTATGCCTTCTCCAAGAGAAGC-3′ |
| Aldh3b1-R | 5′-GCAGCTGCAGCACCTCTCCTCCATGG-3′ |
| Aldh3b2-F | 5′-TGAGTTCATCAACCGGCGGGAGAAGC-3′ |
| Aldh3b2-R | 5′-GTTGTTGGTTCCAGGGACCATAAGG-3′ |
| Aldh3b3-F | 5′-CTTTATGCCTATTCCAACAACGCAG-3′ |
| Aldh3b3-R | 5′-GGGTGCAGCTCTCAGAGCCGATAGC-3′ |
| Gapdh-F | 5′-GAACGGGAAGCTCACTGGCATGGCC-3′ |
| Gapdh-R | 5′-TGTCATACCAGGAAATGAGCTTGAC-3′ |
| Cyp4f39-F | 5′-AGCATCTACGGGACCCACCACAACC-3′ |
| Cyp4f39-R | 5′-TGAGGGTAGAGGCTCTACATTGAGC-3′ |
| Fatp4-F | 5′-AATGGCCTCAGCCATCTGTGAG-3′ |
| Fatp4-R | 5′-AGAGGGTCCAGGTGTTCTGTGC-3′ |
| Cers3-F | 5′-CTGGCTTCCTCCAACAATAAAGTGG-3′ |
| Cers3-R | 5′-TCAAGTTACACTTCTTTGCCAGTCC-3′ |
| Pnpla1-F | 5′-CCCCACAAGCCTCTGCTGGTGGAGG-3′ |
| Pnpla1-R | 5′-TGGCCACTCACTCCCTCGGGGTAGC-3′ |
| Abhd5-F | 5′-ATCACACCTTAAAGAAGCTGAAGAG-3′ |
| Abhd5-R | 5′-AATGGATTCCACAAACTGATTCTCC-3′ |
| Krt1-F | 5′-TGAGCTGAAGAACATGCAAGA-3′ |
| Krt1-R | 5′-CATGTAAGCTGAATCCACATCC-3′ |
| Krt5-F | 5′-CAGAGCTGAGGAACATGCAG-3′ |
| Krt5-F | 5′-CATTCTCAGCCGTGGTACG-3′ |
| Lor-F | 5′-GGTTGCAACGGAGACAACA-3′ |
| Lor-R | 5′-CATGAGAAAGTTAAGCCCATCG-3′ |
| Ivl-F | 5′-ACACACTGCCAGTGACTGTTCCAGC-3′ |
| Ivl-R | 5′-CTTCTCCAGATGCAGTTCCTGTTCC-3′ |
| Hprt-F | 5′-GCTGACCTGCTGGATTACATTAAAG-3′ |
| Hprt-R | 5′-CTTAACCATTTTGGGGCTGTACTGC-3′ |
| hALDH3A2 KO-1 | 5′-TGTGGTCAAATCGCTGCTTCGTTTT-3′ |
| hALDH3A2 KO-2 | 5′-GAAGCAGCGATTTGACCACACGGTG-3′ |
| hALDH3A2 KO-3 | 5′-TGCGGTTGGCAAAATTGTCAGTTTT-3′ |
| hALDH3A2 KO-4 | 5′-TGACAATTTTGCCAACCGCACGGTG-3′ |
| hALDH3A2-1 | 5′-GGCAGTGCAAGAGTTTGTGTTTCTC-3′ |
| hALDH3A2-2 | 5′-AGGTATGGGGTTCAAGTACAAAGAG-3′ |
Mice were housed under specific-pathogen-free conditions at a room temperature of 23°C ± 1°C, humidity of 50% ± 5%, and a light-dark cycle of 12 h:12 h, with food and water available ad libitum. All animal experiments were approved by the institutional animal care and use committee of Hokkaido University (permit 17-0017).
Cells and transfection.
Human immortalized keratinocytes (NHEK/SVTERT3-5) were purchased from Evercyte (Vienna, Austria). Cells were cultured in CnT-Prime epithelial culture medium (CELLnTEC, Bern, Switzerland) in dishes coated with 0.3% collagen (Nitta Gelatin, Osaka, Japan) at 37°C in the presence of 5% CO2. Transfection was performed using ViaFect transfection reagent (Promega, Madison, WI), according to the manufacturer’s manual. For differentiation, cells were grown in 12-well dishes to almost 100% confluence, and then the medium was replaced with CnT-Prime epithelial 3D barrier medium (CnT-3D; CELLnTEC) supplemented with linoleic acid (10 μM). The cells were cultured for 14 days, with the medium refreshed every 3 days.
Generation of ALDH3A2 KO cells.
To generate ALDH3A2 KO human immortalized keratinocytes, we used an all-in-one CRISPR/Cas9 vector, pYU417 (50), which consisted of a Cas9 D10A mutant nuclease (Cas9 nickase) gene, a guide RNA cloning cassette, EGFP, and a puromycin N-acetyltransferase gene. Two targets, each of which consisted of 20 bases adjacent to the PAM sequence in exon 4 of ALDH3A2 (Fig. 10A), were selected for the guide RNAs. Each of a pair of oligonucleotides (hALDH3A2 KO-1/2 or hALDH3A2 KO-3/4) (Table 1) corresponding to the respective guide RNA sequence was annealed and cloned into the BaeI site of pYU417. Human immortalized keratinocytes were transfected simultaneously with both of the resulting plasmids. At 24 h after transfection, the cells were cultured for 48 h in KGM-2 keratinocyte growth medium (Lonza, Basel, Switzerland) containing 2 μg/ml puromycin to select the cells containing the plasmids. The medium was then replaced with puromycin-free CnT-Prime epithelial culture medium, and the cells were cultured for another 2 days. Cells were detached from the dishes by treatment with 0.05% trypsin-EDTA (Thermo Fisher Scientific, Waltham, MA), diluted, seeded into 10-cm dishes, and cultured for 7 days. Several clones were selected and cultured in 12-well plates. Genomic DNA was prepared from the cells, and DNA fragments containing the target sequence of ALDH3A2 were amplified via PCR using the primers hALDH3A2-1 and hALDH3A2-2 (Table 1), followed by DNA sequencing. Two clones with mutations in ALDH3A2 (KO clones 1 and 2) were used for the subsequent experiments. As control cells, two clones (control clones 1 and 2) were generated using the same method but with an empty pYU417 vector.
Skin permeability barrier assays.
TEWL was measured from the backs of P0 mice using an AS-VT100RS evaporimeter (Asch Japan, Tokyo, Japan). Toluidine blue staining was performed as previously described (22), except for the staining time. Briefly, mice were soaked in methanol for 5 min, washed with phosphate-buffered saline (PBS), incubated with 0.1% toluidine blue solution at 4°C for 40 h, washed with PBS, and photographed.
FALDH assay.
FALDH activity was measured as described previously (10).
Histological analyses.
The skin of the P0 mice was prepared from their backs and analyzed via hematoxylin and eosin staining (10) or transmission electron microscopy (33), as described previously. Scanning electron microscopy was performed as follows. Skin from the heads of the P0 mice was fixed with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) at 4°C overnight and further fixed with 0.1% tannic acid in 0.1 M cacodylate buffer (pH 7.4) at 4°C overnight. After washing with 0.1 M cacodylate buffer (pH 7.4) four times for 30 min each, samples were postfixed with 2% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.4) at 4°C overnight. The samples were dehydrated in graded ethanol solutions as follows: 50% and 70% ethanol, at 4°C for 1 h each; 90% ethanol at room temperature for 1 h; 100% ethanol four times at room temperature for 1 h each; and 100% ethanol at room temperature overnight. To freeze-dry the samples, we treated them with a 1:1 mixture of ethanol and tert-butyl alcohol at room temperature for 2 h and then with 100% tert-butyl alcohol three times at room temperature for 1 h each, froze them at 4°C, and vacuum dried. The samples were coated with a thin layer (50 nm) of osmium using an osmium plasma coater (NL-OPC80A; Nippon Laser and Electronics Laboratory, Nagoya, Japan) and observed using a scanning electron microscope (JSM-6340F; JEOL, Tokyo, Japan) at an acceleration voltage of 5.0 kV.
Lipid analyses.
Lipids were extracted from the epidermis as described previously (23) and subjected to LC-MS/MS analyses and TLC separation. For TLC separation suitable for ceramides, silica gel 60 TLC plates (Merck Millipore, Darmstadt, Germany) and a previously described solvent system (24) were used. For TLC separation suitable for the less polar lipids, silica gel 60 high-performance TLC plates (Merck Millipore) and the resolving buffer (hexane-diethyl ether-acetic acid, 65:35:1 [vol/vol/vol]) were used, and lipids were developed to the top of the TLC plates, dried, and developed to the top again. They were stained with copper phosphate reagent as described previously (23).
Lipid analyses via LC-MS/MS were performed using a triple-quadrupole mass spectrometer (Xevo TQ-S; Waters, Milford, MA) in multiple-reaction-monitoring mode. Ionization was performed using electrospray ionization. The conditions of LC separation and electrospray ionization were as described previously (51). Prior to LC-MS/MS, FAs and OAHFAs were derivatized to N-(4-aminomethylphenyl)pyridinium (AMPP) amides using the AMP+ mass spectrometry kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s manual. The m/z values of the precursor ion (Q1) and product ion (Q3) and the collision energies used were as previously reported for the ceramides, acylceramides, ω-OH ceramides, protein-bound ceramides, TGs, and FAs (23) and as reported elsewhere for the OAHFAs (52). For quantitation of the ceramides, ω-OH ceramides, protein-bound ceramides, acylceramides, and TGs, N-palmitoyl(d9)-d-erythro-sphingosine (C16:0 ceramide; Avanti Polar Lipids, Alabaster, AL), N-ω-hydroxytriacontanoyl-d-erythro-sphingosine (C30:0 ω-OH ceramide; Cayman Chemical), N-(30-linoleoyloxy-triacontanoyl)-sphingosine (C30:0 acylceramide; Cayman Chemical), and 1,3-dipentadecanoyl-2-oleoyl(d7)-glycerol (Avanti Polar Lipids) were used as external standards. To quantitate the FAs, palmitic acid-d31 (Cayman Chemical) was used as an internal standard. Absolute quantification was not performed for OAHFAs, since no standard is commercially available.
Lipid analyses for the mouse SC samples were performed essentially as described previously (51), except that the quantities of tape, internal standards, and solvent used were double those in that report. The lipid analyses for the differentiated human keratinocytes were as follows. Cells grown in 12-well dishes were washed twice with 500 μl of PBS, suspended in 300 μl of PBS, detached from the dishes using a scraper, and transferred to microcentrifuge tubes. After centrifugation (400 × g, room temperature, 3 min), the cells were suspended in 150 μl of water and lysed by sonication. Samples were divided into 50 μl and 100 μl; the former was used for protein quantification and the latter for lipid extraction. Lipids were extracted via the successive addition and mixing of 375 μl of chloroform-methanol (1:2 [vol/vol]), 125 μl of chloroform, and 125 μl of water. Phases were separated by centrifugation (20,000 × g, room temperature, 3 min), and the lower phase (organic phase) was collected and dried. The dried lipids were dissolved in chloroform-methanol (1:2 [vol/vol]) to a concentration of 0.3 μg protein/μl. LC-MS/MS analyses and the internal standards used were as described previously (51).
Quantitative real-time RT-PCR.
P0 mouse skin was suspended in 600 μl PBS and incubated at 55°C for 5 min to separate the epidermis and dermis. Total epidermal RNA was prepared using a NucleoSpin RNA II kit (Macherey-Nagel, Dueren, Germany) according to the manufacturer’s protocol. Quantitative real-time RT-PCR was performed using a One-Step SYBR PrimeScript RT-PCR kit II (TaKaRa Bio, Shiga, Japan) and forward (F) and reverse (R) primer pairs (Table 1) as described previously (24).
[3H]DHS labeling assay.
Primary keratinocytes were prepared from the mice, cultured, and differentiated as described previously (10). The [3H]DHS labeling assay was conducted as described previously (31).
ACKNOWLEDGMENTS
We thank Tatsuro Naganuma and Chifumi Yagi for technical support and discussions.
This work was supported by the Advanced Research and Development Programs for Medical Innovation (AMED-CREST) (grant JP20gm0910002h0006 to A.K.) from the Japan Agency for Medical Research and Development (AMED) and by KAKENHI (grant JP18H03976 to A.K.) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Marchitti SA, Brocker C, Stagos D, Vasiliou V. 2008. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol 4:697–720. 10.1517/17425255.4.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson B, Brocker C, Thompson DC, Black W, Vasiliou K, Nebert DW, Vasiliou V. 2011. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics 5:283–303. 10.1186/1479-7364-5-4-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasiliou V, Pappa A. 2000. Polymorphisms of human aldehyde dehydrogenases: consequences for drug metabolism and disease. Pharmacology 61:192–198. 10.1159/000028400. [DOI] [PubMed] [Google Scholar]
- 4.Sjögren T, Larsson T. 1957. Oligophrenia in combination with congenital ichthyosis and spastic disorders; a clinical and genetic study. Acta Psychiatr Neurol Scand Suppl 113:1–112. [PubMed] [Google Scholar]
- 5.Rizzo WB. 2014. Fatty aldehyde and fatty alcohol metabolism: review and importance for epidermal structure and function. Biochim Biophys Acta 1841:377–389. 10.1016/j.bbalip.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizzo WB. 2007. Sjögren-Larsson syndrome: molecular genetics and biochemical pathogenesis of fatty aldehyde dehydrogenase deficiency. Mol Genet Metab 90:1–9. 10.1016/j.ymgme.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho KH, Shim SH, Kim M. 2018. Clinical, biochemical, and genetic aspects of Sjögren-Larsson syndrome. Clin Genet 93:721–730. 10.1111/cge.13058. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo WB, Carney G. 2005. Sjögren-Larsson syndrome: diversity of mutations and polymorphisms in the fatty aldehyde dehydrogenase gene (ALDH3A2). Hum Mutat 26:1–10. 10.1002/humu.20181. [DOI] [PubMed] [Google Scholar]
- 9.Kanetake T, Sassa T, Nojiri K, Sawai M, Hattori S, Miyakawa T, Kitamura T, Kihara A. 2019. Neural symptoms in a gene knockout mouse model of Sjögren-Larsson syndrome are associated with a decrease in 2-hydroxygalactosylceramide. FASEB J 33:928–941. 10.1096/fj.201800291R. [DOI] [PubMed] [Google Scholar]
- 10.Naganuma T, Takagi S, Kanetake T, Kitamura T, Hattori S, Miyakawa T, Sassa T, Kihara A. 2016. Disruption of the Sjögren-Larsson syndrome gene Aldh3a2 in mice increases keratinocyte growth and retards skin barrier recovery. J Biol Chem 291:11676–11688. 10.1074/jbc.M116.714030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitamura T, Takagi S, Naganuma T, Kihara A. 2015. Mouse aldehyde dehydrogenase ALDH3B2 is localized to lipid droplets via two C-terminal tryptophan residues and lipid modification. Biochem J 465:79–87. 10.1042/BJ20140624. [DOI] [PubMed] [Google Scholar]
- 12.Kelson TL, Secor McVoy JR, Rizzo WB. 1997. Human liver fatty aldehyde dehydrogenase: microsomal localization, purification, and biochemical characterization. Biochim Biophys Acta 1335:99–110. 10.1016/s0304-4165(96)00126-2. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura T, Naganuma T, Abe K, Nakahara K, Ohno Y, Kihara A. 2013. Substrate specificity, plasma membrane localization, and lipid modification of the aldehyde dehydrogenase ALDH3B1. Biochim Biophys Acta 1831:1395–1401. 10.1016/j.bbalip.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Traupe H, Fischer J, Oji V. 2014. Nonsyndromic types of ichthyoses—an update. J Dtsch Dermatol Ges 12:109–121. 10.1111/ddg.12229. [DOI] [PubMed] [Google Scholar]
- 15.Goleva E, Berdyshev E, Leung DY. 2019. Epithelial barrier repair and prevention of allergy. J Clin Invest 129:1463–1474. 10.1172/JCI124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouwstra JA, Ponec M. 2006. The skin barrier in healthy and diseased state. Biochim Biophys Acta 1758:2080–2095. 10.1016/j.bbamem.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Hirabayashi T, Murakami M, Kihara A. 2019. The role of PNPLA1 in ω-O-acylceramide synthesis and skin barrier function. Biochim Biophys Acta Mol Cell Biol Lipids 1864:869–879. 10.1016/j.bbalip.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Feingold KR, Elias PM. 2014. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta 1841:280–294. 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Kihara A. 2016. Synthesis and degradation pathways, functions, and pathology of ceramides and epidermal acylceramides. Prog Lipid Res 63:50–69. 10.1016/j.plipres.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Takeichi T, Akiyama M. 2016. Inherited ichthyosis: non-syndromic forms. J Dermatol 43:242–251. 10.1111/1346-8138.13243. [DOI] [PubMed] [Google Scholar]
- 21.Breiden B, Sandhoff K. 2014. The role of sphingolipid metabolism in cutaneous permeability barrier formation. Biochim Biophys Acta 1841:441–452. 10.1016/j.bbalip.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Sassa T, Ohno Y, Suzuki S, Nomura T, Nishioka C, Kashiwagi T, Hirayama T, Akiyama M, Taguchi R, Shimizu H, Itohara S, Kihara A. 2013. Impaired epidermal permeability barrier in mice lacking Elovl1, the gene responsible for very-long-chain fatty acid production. Mol Cell Biol 33:2787–2796. 10.1128/MCB.00192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto H, Hattori M, Chamulitrat W, Ohno Y, Kihara A. 2020. Skin permeability barrier formation by the ichthyosis-causative gene FATP4 through formation of the barrier lipid ω-O-acylceramide. Proc Natl Acad Sci USA 117:2914–2922. 10.1073/pnas.1917525117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto M, Itoh N, Sawai M, Sassa T, Kihara A. 2020. Severe skin permeability barrier dysfunction in knockout mice deficient in a fatty acid ω-hydroxylase crucial to acylceramide production. J Invest Dermatol 140:319–326. 10.1016/j.jid.2019.07.689. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo WB. 2011. The role of fatty aldehyde dehydrogenase in epidermal structure and function. Dermatoendocrinology 3:91–99. 10.4161/derm.3.2.14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno Y, Kamiyama N, Nakamichi S, Kihara A. 2017. PNPLA1 is a transacylase essential for the generation of the skin barrier lipid ω-O-acylceramide. Nat Commun 8:14610. 10.1038/ncomms14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz-Garcia A, Thomas CP, Keeney DS, Zheng Y, Brash AR. 2014. The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim Biophys Acta 1841:401–408. 10.1016/j.bbalip.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeichi T, Hirabayashi T, Miyasaka Y, Kawamoto A, Okuno Y, Taguchi S, Tanahashi K, Murase C, Takama H, Tanaka K, Boeglin WE, Calcutt MW, Watanabe D, Kono M, Muro Y, Ishikawa J, Ohno T, Brash AR, Akiyama M. 2020. SDR9C7 catalyzes critical dehydrogenation of acylceramides for skin barrier formation. J Clin Invest 130:890–903. 10.1172/JCI130675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elias PM, Gruber R, Crumrine D, Menon G, Williams ML, Wakefield JS, Holleran WM, Uchida Y. 2014. Formation and functions of the corneocyte lipid envelope (CLE). Biochim Biophys Acta 1841:314–318. 10.1016/j.bbalip.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohno Y, Nara A, Nakamichi S, Kihara A. 2018. Molecular mechanism of the ichthyosis pathology of Chanarin-Dorfman syndrome: stimulation of PNPLA1-catalyzed ω-O-acylceramide production by ABHD5. J Dermatol Sci 92:245–253. 10.1016/j.jdermsci.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Nakahara K, Ohkuni A, Kitamura T, Abe K, Naganuma T, Ohno Y, Zoeller RA, Kihara A. 2012. The Sjögren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol Cell 46:461–471. 10.1016/j.molcel.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima K, Sano S, Uchida Y, Akiyama M, Morita Y, Shimizu H. 2011. Altered lipid profiles in the stratum corneum of Sjögren-Larsson syndrome. J Dermatol Sci 63:64–66. 10.1016/j.jdermsci.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Honda Y, Kitamura T, Naganuma T, Abe T, Ohno Y, Sassa T, Kihara A. 2018. Decreased skin barrier lipid acylceramide and differentiation-dependent gene expression in ichthyosis gene Nipal4-knockout mice. J Invest Dermatol 138:741–749. 10.1016/j.jid.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, Bayerle A, van der Hoeven F, Imgrund S, Kirsch J, Nickel W, Willecke K, Riezman H, Grone HJ, Sandhoff R. 2012. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet 21:586–608. 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- 35.Catalá A. 2009. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids 157:1–11. 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher F, Neuber C, Finke H, Nieschalke K, Baesler J, Gulbins E, Kleuser B. 2017. The sphingosine 1-phosphate breakdown product, (2E)-hexadecenal, forms protein adducts and glutathione conjugates in vitro. J Lipid Res 58:1648–1660. 10.1194/jlr.M076562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashibe B, Hirai T, Higashi K, Sekimizu K, Motojima K. 2007. Dual subcellular localization in the endoplasmic reticulum and peroxisomes and a vital role in protecting against oxidative stress of fatty aldehyde dehydrogenase are achieved by alternative splicing. J Biol Chem 282:20763–20773. 10.1074/jbc.M611853200. [DOI] [PubMed] [Google Scholar]
- 38.Eckhardt M, Yaghootfam A, Fewou SN, Zöller I, Gieselmann V. 2005. A mammalian fatty acid hydroxylase responsible for the formation of α-hydroxylated galactosylceramide in myelin. Biochem J 388:245–254. 10.1042/BJ20041451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu G, Koszelak-Rosenblum M, Connelly SM, Dumont ME, Malkowski MG. 2015. The crystal structure of an integral membrane fatty acid α-hydroxylase. J Biol Chem 290:29820–29833. 10.1074/jbc.M115.680124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hama H. 2010. Fatty acid 2-hydroxylation in mammalian sphingolipid biology. Biochim Biophys Acta 1801:405–414. 10.1016/j.bbalip.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zöller I, Meixner M, Hartmann D, Büssow H, Meyer R, Gieselmann V, Eckhardt M. 2008. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J Neurosci 28:9741–9754. 10.1523/JNEUROSCI.0458-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potter KA, Kern MJ, Fullbright G, Bielawski J, Scherer SS, Yum SW, Li JJ, Cheng H, Han X, Venkata JK, Khan PA, Rohrer B, Hama H. 2011. Central nervous system dysfunction in a mouse model of FA2H deficiency. Glia 59:1009–1021. 10.1002/glia.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman syndrome. Cell Metab 3:309–319. 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Yang A, Mottillo EP, Mladenovic-Lucas L, Zhou L, Granneman JG. 2019. Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes. Nat Metab 1:560–569. 10.1038/s42255-019-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lord CC, Thomas G, Brown JM. 2013. Mammalian alpha beta hydrolase domain (ABHD) proteins: lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim Biophys Acta 1831:792–802. 10.1016/j.bbalip.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown AL, Mark Brown J. 2017. Critical roles for α/β hydrolase domain 5 (ABHD5)/comparative gene identification-58 (CGI-58) at the lipid droplet interface and beyond. Biochim Biophys Acta Mol Cell Biol Lipids 1862:1233–1241. 10.1016/j.bbalip.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirabayashi T, Anjo T, Kaneko A, Senoo Y, Shibata A, Takama H, Yokoyama K, Nishito Y, Ono T, Taya C, Muramatsu K, Fukami K, Munoz-Garcia A, Brash AR, Ikeda K, Arita M, Akiyama M, Murakami M. 2017. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat Commun 8:14609. 10.1038/ncomms14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikeda M, Kihara A, Igarashi Y. 2004. Sphingosine-1-phosphate lyase SPL is an endoplasmic reticulum-resident, integral membrane protein with the pyridoxal 5′-phosphate binding domain exposed to the cytosol. Biochem Biophys Res Commun 325:338–343. 10.1016/j.bbrc.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 49.Edagawa M, Sawai M, Ohno Y, Kihara A. 2018. Widespread tissue distribution and synthetic pathway of polyunsaturated C24:2 sphingolipids in mammals. Biochim Biophys Acta Mol Cell Biol Lipids 1863:1441–1448. 10.1016/j.bbalip.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Sawai M, Uchida Y, Ohno Y, Miyamoto M, Nishioka C, Itohara S, Sassa T, Kihara A. 2017. The 3-hydroxyacyl-CoA dehydratases HACD1 and HACD2 exhibit functional redundancy and are active in a wide range of fatty acid elongation pathways. J Biol Chem 292:15538–15551. 10.1074/jbc.M117.803171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawana M, Miyamoto M, Ohno Y, Kihara A. 2020. Comparative profiling and comprehensive quantification of stratum corneum ceramides in humans and mice by LC/MS/MS. J Lipid Res 61:884–895. 10.1194/jlr.RA120000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyamoto M, Sassa T, Sawai M, Kihara A. 2020. Lipid polarity gradient formed by ω-hydroxy lipids in tear film prevents dry eye disease. Elife 9:e53582. 10.7554/eLife.53582. [DOI] [PMC free article] [PubMed] [Google Scholar]