Abstract
Background:
The concept of chemical agents having properties that confer potential hazard called key characteristics (KCs) was first developed to identify carcinogenic hazards. Identification of KCs of cardiovascular (CV) toxicants could facilitate the systematic assessment of CV hazards and understanding of assay and data gaps associated with current approaches.
Objectives:
We sought to develop a consensus-based synthesis of scientific evidence on the KCs of chemical and nonchemical agents known to cause CV toxicity along with methods to measure them.
Methods:
An expert working group was convened to discuss mechanisms associated with CV toxicity.
Results:
The group identified 12 KCs of CV toxicants, defined as exogenous agents that adversely interfere with function of the CV system. The KCs were organized into those primarily affecting cardiac tissue (numbers 1–4 below), the vascular system (5–7), or both (8–12), as follows: 1) impairs regulation of cardiac excitability, 2) impairs cardiac contractility and relaxation, 3) induces cardiomyocyte injury and death, 4) induces proliferation of valve stroma, 5) impacts endothelial and vascular function, 6) alters hemostasis, 7) causes dyslipidemia, 8) impairs mitochondrial function, 9) modifies autonomic nervous system activity, 10) induces oxidative stress, 11) causes inflammation, and 12) alters hormone signaling.
Discussion:
These 12 KCs can be used to help identify pharmaceuticals and environmental pollutants as CV toxicants, as well as to better understand the mechanistic underpinnings of their toxicity. For example, evidence exists that fine particulate matter [PM in aerodynamic diameter ()] air pollution, arsenic, anthracycline drugs, and other exogenous chemicals possess one or more of the described KCs. In conclusion, the KCs could be used to identify potential CV toxicants and to define a set of test methods to evaluate CV toxicity in a more comprehensive and standardized manner than current approaches. https://doi.org/10.1289/EHP9321
Introduction
According to the World Health Organization, cardiovascular disease (CVD) is the leading cause of death worldwide, taking lives annually (WHO 2017). Four of five of those deaths are due to myocardial infarction or stroke. Certain environmental pollutants, such as fine particulate matter [PM in aerodynamic diameter ()] (Brook et al. 2010, 2016), arsenic (States et al. 2009) and tobacco smoke (Gallucci et al. 2020), are well known to be associated with CVD, but other environmental contaminants, as well as natural toxins, viruses, and other agents, may also be cardiovascular (CV) toxicants.
A systematic approach to identifying chemical hazards was recently developed for carcinogens (Smith et al. 2016), endocrine-disrupting chemicals (La Merrill et al. 2020), and reproductive toxicants (Arzuaga et al. 2019; Luderer et al. 2019) based on the established properties of chemicals known to cause cancer, endocrine disruption, and reproductive toxicity, respectively. These properties, called key characteristics (KCs), have quickly proved useful for the systematic evaluation of the literature on mechanisms by which chemicals induce these toxic effects (Guyton et al. 2018a, 2018b). The KCs are now widely used by various authoritative bodies and regulatory agencies and form the basis for the evaluation of mechanistic data at the International Agency for Research on Cancer (IARC 2019; Samet et al. 2020). Scientists in the pharmaceutical industry have also recognized that the KCs are likely to be useful in the design of a comprehensive set of tests to evaluate the potential hazards of novel drug candidates (Fielden et al. 2018; Smith et al. 2020).
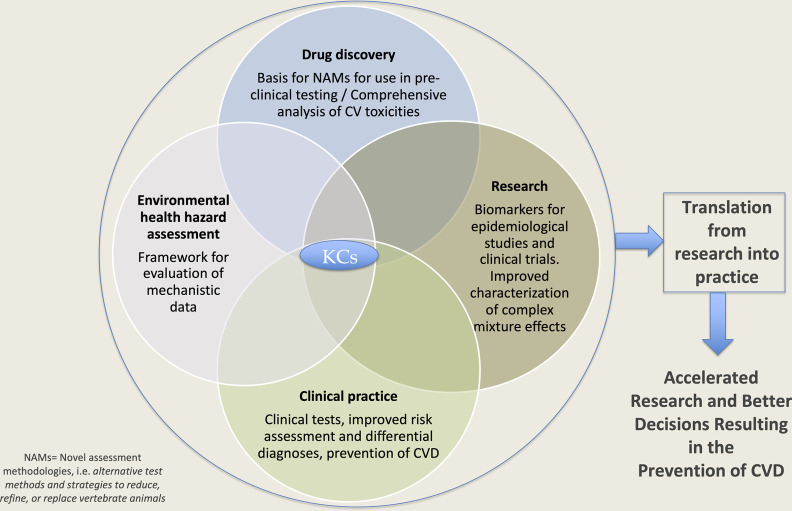
Our goal was to develop a consensus on the KCs of chemical and nonchemical agents known to cause CV toxicity and to provide a comprehensive list of tests to be used to evaluate chemicals and other environmental pollutants for CV toxicity. Given that many pharmaceutical drugs have adverse effects on the CV system and because those mechanisms are generally better understood than those of environmental pollutants, we included data from pharmaceuticals in the development of the KCs of CV toxicants. As outlined in Figure 1, we believe there are multiple ways in which these KCs of CV toxicants could be used to enhance current approaches in the clinic and in pharmaceutical development, environmental research, and hazard assessment.
Figure 1.
Utility of the key characteristics (KCs) of cardiovascular toxicants in research, drug discovery, hazard assessment, and clinical practice. An illustration of how the KCs could be used in different areas and how translation of the resulting information could lead to accelerated research, inform better regulatory decisions, improve clinical practice, and ultimately prevent CVD. Note: CV, cardiovascular; CVD, cardiovascular disease; NAM, novel assessment methodologies.
Descriptions of the KCs of CV Toxicants
Experts from various fields related to CV toxicity and chemical regulation convened and identified 12 KCs of CV toxicants using current scientific evidence, such as the earlier work of Laverty et al. (2011), expert knowledge, known examples of CV toxicants, and extensive debate. We acknowledge that these will likely evolve with new scientific discoveries. In considering the differences between acute and chronic effects, as well as between high- and low-dose effects, we concluded that these KCs cover temporal and dose-dependent cardiotoxic mechanisms. We did, however, restrict our task to adult CVD, excluding possible teratogenic effects of environmental pollutants on the developing CV system.
We also identified representative biomarkers, assays, and end points that are most useful for testing each KC using experimental in vitro/ex vivo studies and in vivo animal models, as well as clinical or epidemiological findings in humans (Table 1). Further, we identified classic examples of CV toxicants for each KC (Table 1) and illustrated how some CV toxicants exhibit multiple KCs, whereas other toxicants may exhibit only one (Tables 2 and 3). We have divided the KCs into those primarily affecting cardiac tissue (numbered 1–4), vascular tissues (5–7), and those which could affect both the heart and vasculature (8–12).
Table 1.
Key characteristics (KCs) of cardiovascular (CV) toxicants: relevant assays and biomarkers and representative agents.
| KC | In vitro/ex vivo | Relevant assays and biomarkers | Representative chemical and other agents | ||
|---|---|---|---|---|---|
| Animal | Human | Pharmaceutical | Environmental | ||
| Mainly cardiac | |||||
| 1. Impairs regulation of cardiac excitability | Patch-clamp recordings in heterologous expression systems, isolated myocytes, or human induced pluripotent stem cell-derived cardiomyocytes (blockade of Na or K ion currents, enhancement of late Na ion current); microelectrode array recordings or optical mapping in ex vivo heart preparation or monolayers of stem cell-derived cardiomyocytes (action potential duration and heterogeneity, conduction velocity), intracellular calcium imaging/measurements. | ECG recordings (QRS duration, QTc intervals), electrophysiologic studies (HV intervals, effective refractory period, and cardiac arrhythmia inducibility), ambulatory ECG recordings (occurrences of torsade de pointes ventricular arrhythmias and sudden cardiac death). | ECG recordings (QRS duration, QTc intervals), electrophysiologic studies (HV intervals, effective refractory period, and cardiac arrhythmia inducibility), cardiac implantable electronic device interrogation (occurrences of ventricular arrhythmias), development of torsade de pointes ventricular arrhythmias, and sudden cardiac death. | Anti-arrhythmic drugs (sotalol, dofetilide, ibutilide, quinidine, procainamide, disopyramide); anti-malarial drug (chloroquine); antibiotics (clarithromycin, erythromycin, azithromycin); tyrosine kinase inhibitors (nilotinib, dasatinib, and sunitinib); antipsychotics (thioridazine, haloperidol); antidepressants (amitriptyline, imiprmaine, fluoxetine, desipramine, paroxetine); anticonvulsants (felbamate and fosphenytoin); gastric motility drug (cisapride). | Tetrodotoxin, saxitoxin, batrachotoxin, and conotoxin (naturally occurring toxins); lead, alcohol, BPA. |
| 2. Impairs cardiac contractility and relaxation | Contractile measurements via edge detection or sarcomere detection, impedance-based contractility, force transducer, pressure–volume catheter or balloon catheter. Measure the above in isolated cardiomyocytes, stem cell-derived cardiomyocytes, isolated muscle fibers, intact heart preparations. |
Pressure–volume catheter; ejection fraction on echocardiography. | Ejection fraction on echocardiography, cardiac CT and MRI; blood pressure and cardiac catheterization. | Glycosides (e.g., digoxin); beta-adrenergic antagonists (e.g., metoprolol, atenolol, carvedilol); calcium sensitizer (e.g., levosimendan); adrenergic agonists (e.g., dobutamine, isoproterenol); haloanesthetics (e.g., halothane, isoflurane); chemotherapeutics (e.g., arsenic trioxide). | Metals (e.g., barium, cadmium, cobalt, lead, nickel); ethanol; BPA. |
| 3. Induces cardiomyocyte injury and death | Cytotoxicity (troponin release, ATP production, nuclear integrity, mitochondrial integrity) in isolated or induced pluripotent stem cell-derived cardiomyocytes; cytochrome complex release, loss of mitochondrial membrane potential. | Cardiac biomarkers (e.g., troponin). Histopathological evaluation (hypertrophy, hyalinization, necrosis, vacuolation, fibrosis). |
Cardiac biomarkers (e.g., troponin). Histopathological evaluation (hypertrophy, hyalinization, necrosis, vacuolation, fibrosis). |
Anthracyclines (e.g., doxorubicin); sympathomimetics (e.g., isoproterenol); cardiac calcitropes (e.g., milrinone); imatinib mesylate; trastuzumab. | Ethanol, air pollution; diethanolamine; ephedrine; methyl bromide; monochloroacetic acid; 3,3′,4′,4′,5 pentachlorobiphenyl (PCB 126); 2,3′,7,8-tetrachlorodibenzo--dioxin; urethane, cadmium. |
| 4. Induces proliferation of valve stroma | In vitro activation of receptors; DNA synthesis induction in cultured interstitial cells from human cardiac values via activation; receptor activation and/or in silico screening incorporated to de-select compounds during drug development. | Valve leaflet fibroplasia and thickening in mice and rats; increased 5-HT levels in whole blood; echocardiogram assessment showing cardiac valve regurgitation. | Valve leaflet fibroplasia and thickening; echocardiogram assessment showing cardiac valve regurgitation. | Fenfluramine, pergolide, cabergoline, ergotamines, MDMA. | None identified. |
| Mainly vascular | |||||
| 5. Impacts endothelial and vascular function | Measurement of binding affinity, functional potency, or expression of vascular receptors and enzymes. Functional effects in isolated vascular tissue preparations segments (human and animal); enzymatic or biochemical effects in endothelial cell culture (e.g., nitric oxide synthase activity, endothelin). Intracellular calcium imaging/measurements. |
Blood pressure; regional blood flow measurement (Doppler, ultrasonic transit time, microspheres); vascular resistance determinations. | Blood pressure; cutaneous blood flow assessment (laser Doppler); brachial flow-mediated dilation; arterial stiffness (pulse wave velocity). | Phenylephrine, sunitinib, sodium nitroprusside, prazosin, minoxidil; calcium channel blockers (e.g., verapamil, nifedipine, diltiazem). | PCBs, BPA, malathion, DDT, air pollution, cigarette smoke, arsenic, cadmium, lead. |
| 6. Alters hemostasis | Platelet aggregation; platelet activation and function (e.g., surface and cytoplasmic markers and EVs by flow cytometry). Altered coagulation and fibrinolysis (e.g., ACT, PT, APTT; assays of global coagulation; levels of coagulation factors). Endothelial cell anti-aggregation and coagulation function. |
Blood cell and platelet counts, MPV; platelet aggregation; platelet activation and function, tail vein bleeding time. Serum antibodies (e.g., anti-PF4 in HIT, lupus anticoagulants). Altered coagulation and fibrinolysis (e.g., ACT, PT, APTT; coagulation component levels; thrombus formation in blood vessels, tissue ischemia). |
Blood cell and platelet count, MPV; platelet activation and function. Serum antibodies (e.g., anti-PF4 in HIT, lupus anticoagulants). Altered coagulation and fibrinolysis (e.g., ACT, PT, APTT; assays of global coagulation). Vitamin K and vitamin K epoxide levels in serum or plasma (warfarin). |
Ibuprofen, quinine, oxaliplatin (immune-mediated thrombocytopenia); heparin (HIT); warfarin (interferes with fibrin clot formation by vitamin K deficiency); procainamide, chlorpromazine, and hydralazine (may induce lupus anticoagulants. | Air pollution (), arsenic, cadmium. |
| 7. Causes dyslipidemia | Altered gene expression of lipid-related genes and altered synthesis and secretion of VLDL in cultured hepatocytes. | Altered plasma levels of lipids in rodents; altered gene expression of lipid- related genes in liver specimens. | Altered plasma levels of lipids in occupational and epidemiological studies. | Human immunodeficiency virus protease inhibitors; antipsychotic drugs. | PCBs, PFAS, BPA, phthalates, cadmium and lead. |
| Both cardiac and vascular | |||||
| 8. Impairs mitochondrial function | Mitochondrial oxygen consumption determination; mitochondrial ROS measurement; mitochondrial imaging; mitochondrial biogenesis, and mitochondrial content determination; mitochondrial membrane polarization measurements; mitochondrial DNA oxidation measurements; ultrastructure imaging. Measurement of the above in isolated cardiomyocytes; submitochondrial preparations; intact heart preparations; human induced pluripotent stem cell-derived cardiomyocytes. |
8-OHdG adducts of mitochondrial DNA; mitochondrial oxidative damage (e.g., protein carbonyls and malondialdehyde); histopathological, immunohistochemical, and mitochondrial ultrastructure examination; cardiac contractility–ejection fraction, diastolic relaxation, instrumented LV pressures, QA interval. | Blood mitochondrial DNA methylation; cardiac magnetic resonance. | Chemotherapeutics (e.g., anthracyclines, cisplatin, arsenic trioxide); antiviral compounds (e.g., azidothymidine); anti-diabetics (e.g., rosiglitazone). | Air pollution; metals (e.g., arsenic, mercury, cadmium and lead); diphenylmethane derivatives (e.g., BPA); ethanol, chlorinated hydrocarbons (e.g., PCBs). |
| 9. Modifies autonomic nervous system activity | Measurement of binding affinity or functional potency at autonomic receptors (e.g., alpha and beta-adrenergic; muscarinic subtypes) and transporters (e.g., norepinephrine). Assessment of sympathetic/parasympathetic receptor-mediated function (e.g., cAMP levels, protein phosphorylation) in isolated tissues (heart or vascular tissues). Assess membrane currents/action potentials in isolated neurons/nerves that control CV function. Measure electrical and mechanical activity in co-cultures of cardiomyocytes with para/sympathetic neurons. |
Direct measures of sympathetic nerve activity using electrodes or implantable telemetry (membrane currents, action potentials); heart rate variability, baroreflex sensitivity chemoreceptor sensitivity, with linkage to functional and biochemical measures of CV function (e.g., echocardiography, blood pressure and ECG telemetry, pressure–volume catheter, plasma and urinary catecholamines) in rodents and/or dogs. | Heart rate variability, baroreflex sensitivity, chemoreceptor sensitivity, Valsalva maneuver, isometric handgrip test, deep breathing test, cold pressor test, mental arithmetic, orthostatic test, head-up tilt test, plasma and urinary catecholamines, noradrenaline spillover rate, microneurography (e.g., muscle sympathetic nerve activity), sudomotor function (responses of sweat glands to stimuli), and linkage to measures of CV function (e.g., echocardiography, ECG, blood pressure, and plasma and urinary catecholamines). | Beta-adrenergic agonists (e.g., dobutamine), beta-adrenergic antagonists (atenolol and esmolol), alpha-adrenergic agonists (e.g., clonidine), alpha-adrenergic antagonists (prazosin), muscarinic antagonists (atropine). | Ambient particulate matter air pollution, heavy metals (lead, mercury), cigarette smoke, BPA. |
| 10. Induces oxidative stress | Increased ROS generation in macrophages, endothelial cells, cardiomyocytes, fibroblasts, human induced pluripotent stem cell-derived cardiomyocytes; increased lipid peroxidation in liposomes. | Increased lipid peroxidation in rats and mice (e.g., malondialdehyde, 8-isoprostanes, hydroxyeicosatetraenoates, hydroxyoctadecadienoates); decreased paraoxonase 1 activity in mice and glutathione peroxidase in rats; oxidative changes in plasma lipoproteins of hyperlipidemic mice resulting in proatherogenic LDL and dysfunctional pro-inflammatory high-density lipoprotein. | Increased lipid peroxidation and decreased paraoxonase 1 activity in plasma; decreased glutathione peroxidase, decreased superoxide dismutase in blood; Increased NOX in blood. | Anthracyclines. | Air pollution, (, ultrafine particles); diesel exhaust; gasoline exhaust; PAHs; arsenic; cadmium; lead; mercury; pesticides (organophosphates); insecticides (carbamates, fenitrothion), tobacco cigarette. |
| 11. Causes inflammation | Analysis of pro-inflammatory gene expression; measurement of cytokine secretion by immune cells (e.g., macrophages); flow cytometry analysis of immune cells; immunofluorescent staining of inflammatory markers; characterization of macrophage polarization; analysis of endothelial cell function. | Analysis of circulating cytokine levels (e.g., , IL-6); flow cytometry analysis of immune cell population in blood and tissues; analysis of inflammatory gene expression in various tissues including aorta; immunostaining of key inflammatory markers in tissues; characterization of macrophage phenotypes within atherosclerotic plaques; calculation of atherosclerotic plaque stability. | Measurement of circulation inflammatory markers (e.g., IL-6, CRP); analysis of immune cells by flow cytometry or other standard methods. | Procainamide (antiarrhythmic); hydralazine (vasodilator); doxorubicin (anthracycline). | PCBs, BPA, arsenic, cadmium, lead, and air pollution (). |
| 12. Alters hormone signaling | Altered contractility in isolated cardiomyocytes or intact heart preparations; whole-heart ECG; modifications of intracellular calcium imaging; changes in vascular contractility; changes in SR protein expression, or posttranslational modifications, signal transduction; pharmacological agonist/antagonist studies; adrenal-derived cell lines, increased expression of vascular endothelial growth factor and endothelial nitric oxide synthase in human primary endothelial cells. | Multiple end points in numerous experimental species (including rodents, canine, porcine, primates): ECG recordings, heart rate variability, baroreflex sensitivity, increased blood pressure, in hormone-receptor knockout rodent models; altered responses to ischemia, cardiac transcriptome; changes in fibrosis and extracellular matrix composition. | Multiple end points in epidemiological studies: modifications in blood pressure, hemostasis and vascular resistance; sex-specific lipid profiles, arrhythmia risk, increased hypertrophy, heart failure and dilated cardiomyopathy; altered ECG; increased risk for coronary and peripheral artery disease and atherosclerosis, atrial fibrillation, disturbances in cardiac output and contractility; atherogenic lipid profiles. | Amiodarone; rosiglitazone; testosterone; androgens and anabolic steroids; adrenergic agonists and antagonists; selective estrogen receptor modulators and anti-estrogens; glucocorticoids. | BPA, PCBs, arsenic, cadmium, and lead. |
Note: 5-HT, 5-hydroxytryptamine (serotonin); , 5-HT subtype 2B; ACT, activated clotting time; APTT, activated partial thromboplastin time; ATP, adenosine triphosphate; BPA, bisphenol A; cAMP, cyclic adenosine monophosphate; CRP, C-reactive protein; CT, computed tomography; DDT, dichlorodiphenyltrichloroethane; ECG, electrocardiogram; EV, extracellular vesicle; HIT, heparin-induced thrombocytopenia; HV interval, conduction time through the distal His- Purkinje tissue measured from the onset of the His-bundle deflection to the earliest ventricular activation; , potassium ion; LDL, low-density lipoprotein; LV, left ventricular; MDMA, 3,4-methylenedioxymethamphetamine; MPV, mean platelet volume; MRI, magnetic resonance imaging; , sodium ion; NOX, nicotinamide adenine dinucleotide phosphate oxidase; PAH, polycyclic aromatic hydrocarbon; PCBs, polychlorinated biphenyls; PF4, platelet factor 4; PFAS, per- and poly-fluorinated substances; , particulate matter in aerodynamic diameter (fine particulate matter); PT, prothrombin time; QTc, corrected QT interval; ROS, reactive oxygen species; SR, sarcoplasmic reticulum; VLDL, very-low-density lipoprotein.
Table 2.
Key characteristics (KCs) of cardiovascular toxicants applied to three established environmental contaminants that are cardiotoxic.
| KC | Evidence for each KC for air pollution (human–animal–in vitro) |
Evidence for each KC for PCBs (human–animal–in vitro) |
Evidence for each KC for BPA (human–animal–in vitro) |
|---|---|---|---|
| Mainly cardiac | |||
| 1. Impairs regulation of cardiac excitability | — | — | Disrupts intracellular calcium ion homeostasis in excised rat hearts and ventricular myocytes (Posnack et al. 2015; Ramadan et al. 2018; Yan et al. 2011). Directly inhibits multiple voltage-gated calcium channels human cells in vitro and ex vivo in rat aorta (Deutschmann et al. 2013; Feiteiro et al. 2018; Michaela et al. 2014), which are important for nodal cell depolarization, atrioventricular conduction, and the plateau phase of the cardiac action potential. Sinus bradycardia and slowed cardiac electrical conduction observed in experimental models in ex vivo and in vivo studies (Belcher et al. 2015; Patel et al. 2015; Posnack et al. 2014). |
| 2. Impairs cardiac contractility and relaxation | — | — | — |
| 3. Induces cardiomyocyte injury and death | — | — | — |
| 4. Induces proliferation of valve stroma | — | — | — |
| Mainly vascular | |||
| 5. Impacts endothelial and vascular function | Altered vasomotor tone in epidemiological (Dales et al. 2007; Krishnan et al. 2012; Zanobetti et al. 2014) and experimental in vivo (Hansen et al. 2007) and ex vivo (Hansen et al. 2007) studies. | — | — |
| 6. Alters hemostasis | Altered hemostasis in epidemiological (Hajat et al. 2015; Riediker et al. 2004; Viehmann et al. 2015; Zhang et al. 2018), and experimental in vivo studies (Liang et al. 2019; Sun et al. 2008). | — | — |
| 7. Causes dyslipidemia | Induced dyslipidemia in epidemiological (Mathew et al. 2018; McGuinn et al. 2019), and experimental in vivo (Li et al. 2020; Xu et al. 2019b) studies. | Dyslipidemia in humans resulting in increased serum levels of cholesterol and triglycerides (Chase et al. 1982; Penell et al. 2014; Tokunaga and Kataoka 2003). In rodents and zebrafish, PCBs most likely cause dyslipidemia in vivo by altering the regulation of genes related to lipogenesis and lipid catabolism in liver cells (Chapados and Boucher 2017; Li et al. 2019; Wahlang et al. 2013). In vitro, human and mouse hepatocytes exposed to PCBs in vitro have increased triglyceride and total cholesterol concentrations (Boucher et al. 2015; Chen et al. 2020a; Wu et al. 2017). |
— |
| Both cardiac and vascular | |||
| 8. Impairs mitochondrial function | — | — | — |
| 9. Modifies autonomic nervous system activity | Altered autonomic nervous system activity in multiple epidemiological (Kirrane et al. 2019; Lee et al. 2014; Mordukhovich et al. 2015; Park et al. 2010; Peters et al. 2015; Pieters et al. 2012), experimental in vivo (Anselme et al. 2007; Bessac and Jordt 2008; Carll et al. 2013; Hazari et al. 2011; Widdicombe and Lee 2001), and in vitro (Deering-Rice et al. 2011) studies. | — | Differences in beta-adrenergic receptor expression have been observed in animal models (Belcher et al. 2015) and alterations in heart rate variability have been reported in human subjects (Bae et al. 2012). |
| 10. Induces oxidative stress | Induced oxidative stress in epidemiological (Lee et al. 2014; Li et al. 2016; Weichenthal et al. 2016), experimental in vivo (Xu et al. 2019b; Yue et al. 2019), and in vitro lung epithelial (Niu et al. 2020) and dual lung and cardiomyocyte (Gorr et al. 2015) studies. | Altered glutathione metabolism and lipid peroxidation in humans, and in vivo in rats, mice, and crabs (Deng et al. 2019; Feng et al. 2019; Kumar et al. 2014b; Shan et al. 2020; Tremblay-Laganière et al. 2019). Increased ROS production in vitro in human ECs and neutrophil granulocytes (Berntsen et al. 2016; Long et al. 2017; Tang et al. 2017), and in various tissues in pig, mice, hamster, and fish (scup) (Green et al. 2008; Han et al. 2012; Hennig et al. 2002; Long et al. 2020; Majkova et al. 2011; Murati et al. 2017; Schlezinger et al. 2006). | Population-based epidemiological studies have noted associations between BPA exposure, inflammation, and oxidative stress (Kataria et al. 2017; Steffensen et al. 2020; Wang et al. 2019b; Yang et al. 2009). |
| 11. Causes inflammation | Induced inflammation in epidemiological (Altuwayjiri et al. 2021; Liu et al. 2019; Pope et al. 2016; Riediker et al. 2004; Zhang et al. 2020a), experimental in vivo (Bai and Sun 2016; Hadei and Naddafi 2020; Tong 2016), and in vitro lung epithelial (Schwarze et al. 2007) macrophage (Zhao et al. 2016), and dual lung and cardiomyocyte (Gorr et al. 2015) studies. | Increased biomarkers of inflammation, such as ICAM-1 and VCAM-1, in humans (Kumar et al. 2014a). Elevated blood levels and hepatic expression of IL-6 and in rats and mice after exposure to a PCB mixture (Wahlang et al. 2014; Xu et al. 2019a). In vitro exposure of human monocytes and vascular ECs to PCB 126 induced expression of inflammatory cytokines, including , monocyte/macrophage chemokine 1f and in the monocytes and up-regulated inflammatory genes, such as IL-6, CRP, ICAM-1, and VCAM-1 in the EC (Milner 1989). |
BPA exposure is associated with increased inflammatory makers (Song et al. 2017) and atherosclerosis or coronary artery disease in epidemiological studies (Lind and Lind 2011; Melzer et al. 2012a, 2012b). In utero BPA exposure increases cardiac fibrosis and inflammation in offspring (Belcher et al. 2015; Gear et al. 2017; Rasdi et al. 2020) and impedes recovery after myocardial infarction in adult animals (Patel et al. 2015; Shang et al. 2019). Notably, the effects of BPA on cardiac remodeling and inflammation are significantly attenuated in estrogen knockout mice, which further suggests a mechanistic link to BPA’s estrogenic activity (Kasneci et al. 2017). |
| 12. Alters hormone signaling | Altered hypothalamus–pituitary–adrenal axis-related stress hormones (Niu et al. 2018), altered thyroid hormone levels during pregnancy (Zhao et al. 2019), and altered insulin and glucose homeostasis (Brook et al. 2016; Zheng et al. 2013), in epidemiological studies. Altered hypothalamus–pituitary–adrenal axis-related stress hormones (Liu et al. 2020), altered renin–angiotensin system signaling (Ghelfi et al. 2010), altered insulin and glucose homeostasis (Xu et al. 2011), altered testosterone synthesis (Yang et al. 2019), and altered signaling (Zheng et al. 2013), in experimental in vivo studies. |
Altered circulating thyroid hormone levels in humans, rats, and fish (sea bass) (Collins et al. 1977; Meeker et al. 2007; Schnitzler et al. 2011; Takser et al. 2005). In young non-pregnant women, a clinical indicator of ovarian responsiveness, the FSH:LH ratio, was associated with PCBs (Gallo et al. 2018). In rats, the pituitary content of FSH and LH was increased by PCB-126 exposure (Desaulniers et al. 1999), and rats exposed to a PCB mixture showed increases in uterine weights and uterine -thymidine labeling (Jansen et al. 1993). In vitro effects on the estrogen receptor have also been observed (Gjernes et al. 2012; Tavolari et al. 2006). |
Disruption of intracellular calcium homeostasis is likely mediated through estrogenic effects of BPA, which results in posttranslational modifications of key calcium-handling proteins (Belcher et al. 2012; Gao et al. 2013; Liang et al. 2014). |
Note: Details are provided for those KCs that we for cancer treatments and cardiovascular toxicity of the European Society of Cardiology considered to have the strongest evidence for each agent (e.g., a combination of data from human epidemiological/clinical studies and in vivo animal studies, as well as in vitro studies). —, Other KCs; BPA, Bisphenol A; CRP, C-reactive protein; ECs, endothelial cells; FSH, follicle-stimulating hormone; ICAM-1, intracellular adhesion molecule 1; , interleukin 1 beta; IL-6, interleukin 6; LH, luteinizing hormone; PCBs, polychlorinated biphenyls; , particulate matter in aerodynamic diameter (fine particulate matter); , peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; , tumor necrosis factor alpha; VCAM-1, vascular cell adhesion molecule 1.
Table 3.
Key characteristics (KCs) of cardiovascular toxicants applied to two classic cardiotoxic drugs and the chemotherapeutic agent arsenic trioxide.
| KC | Evidence for each KC for doxorubicin (human–animal–in vitro) | Evidence for each KC for fenfluramine (human–animal–in vitro) | Evidence for each KC for arsenic trioxide (human–animal–in vitro) |
|---|---|---|---|
| Mainly cardiac | |||
| 1. Impairs regulation of cardiac excitability | QTc prolongation in humans and monkeys unrelated to potassium channel (hERG) inhibition (Engwall et al. 2021; Nousiainen et al. 1999). | — | QTc prolongation in humans with animal and in vitro evidence of potassium channel (hERG) inhibition (Alexandre et al. 2018; Dennis et al. 2007). |
| 2. Impairs cardiac contractility and relaxation | Alters calcium homeostasis by inducing calcium leakage from the sarcoplasmic reticulum (Nebigil and Désaubry 2018). | — | Clinically relevant concentrations of arsenic trioxide causes intracellular calcium overload from damaged mitochondria (Varga et al. 2015). |
| 3. Induces cardiomyocyte injury and death | Induces cardiomyocyte apoptosis, necrosis, necroptosis, and autophagy in cardiac cells and mice, which lead to injury and cell death (Ma et al. 2020). | — | Induces cardiomyocyte apoptosis and death in animal and cell culture models (Varga et al. 2015). |
| 4. Induces proliferation of valve stroma | — | In vitro activation of receptors; dose-dependent valve leaflet fibroplasia and thickening in mice, rats, and humans; development of valvular cardiac disease in clinical studies (Elangbam et al. 2008; Elangbam 2010; Fitzgerald et al. 2000; Reid et al. 2013; Roth 2007; Rothman et al. 2000; Taylor et al. 2007). | — |
| Mainly vascular | |||
| 5. Impacts endothelial and vascular function | — | — | — |
| 6. Alters hemostasis | — | — | — |
| 7. Causes dyslipidemia | — | — | — |
| Both cardiac and vascular | |||
| 8. Impairs mitochondrial function | Promotes mitochondrial fission, inhibits mitochondrial fusion, and impairs mitochondrial function in several ways, including decreasing the oxygen consumption rate and altering mitochondrial membrane potential (Osataphan et al. 2020). | — | Pro-apoptotic effect of arsenic trioxide in ventricular cardiomyocytes shown to be associated with Parkin-dependent ubiquitin proteasome activation and loss of mitochondrial membrane potential (Varga et al. 2015). |
| 9. Modifies autonomic nervous system activity | — | — | — |
| 10. Induces oxidative stress | Induces ROS and decreases superoxide dismutase‐2 in cardiac tissues (Osataphan et al. 2020). | — | — |
| 11. Causes inflammation | Induces markers of inflammation in vivo, as reviewed by Prathumsap et al. (2020). | — | Chronic environmental exposures are associated with elevated circulating inflammatory markers in humans (Cosselman et al. 2015; Wu et al. 2014). Leads to vascular inflammation, endothelial dysfunction and atherosclerosis development in animals (Cosselman et al. 2015; States et al. 2009). |
| 12. Alters hormone signaling | — | — | — |
Note: Details are provided for those KCs that we for cancer treatments and cardiovascular toxicity of the European Society of Cardiology considered to have the strongest evidence for each agent (e.g., a combination of data from human epidemiological/clinical studies, in vivo animal studies and in vitro studies). —, Other KCs; , 5-HT subtype 2B; hERG, ether-à-go-go-related gene; QTc, corrected QT interval; ROS, reactive oxygen species; RyR2, ryanodine receptors.
Primarily Cardiac
KC1: impairs regulation of cardiac excitability.
Cardiac ion channels play critical roles in generating action potentials (APs) given that the cardiac AP is shaped by a balance of inward and outward currents. In ventricular myocytes, depolarization is initiated by sodium ion () channel opening during the AP upstroke, followed by calcium ion () channel opening during the plateau phase. Subsequently, ventricular repolarization is mediated by multiple potassium ion () channels (Chiamvimonvat et al. 2017; Grandi et al. 2017). Coordinated channel activity is critical to cardiac excitation–contraction coupling, and therefore a disturbance of ion concentrations can lead to cardiac arrhythmias and sudden cardiac death. Classic examples include antiarrhythmic drugs, non-CV drugs that cause QT prolongation (Vlachos et al. 2016), drugs that interfere with [ether-à-go-go-related gene product (hERG)] potassium channel trafficking (Cubeddu 2016), drugs that cause QRS widening, and tyrosine kinase inhibitors that cause QT prolongation by enhancing inward late current during the plateau phase, leading to AP prolongation (Roden 2019). Finally, toxins from diverse organisms have evolved to disrupt the activities of ion channels (Morales-Lázaro et al. 2015): For example, tetrodotoxin and saxitoxin block channels, whereas batrachotoxin induces persistent activation of channels (Restrepo-Angulo et al. 2010).
ions play critical roles in cardiac automaticity, electrical conduction, excitation–transcription coupling and maintenance of vascular tone. Agents that depress current can decrease the AP upstroke of the sinoatrial node and slow heart rate and atrioventricular conduction, for example, beta-adrenergic antagonists and L-type channel (LTCC) blockers (Abernethy and Schwartz 1999; Olson et al. 2005). Conversely, beta-adrenergic agonists increase the AP upstroke and heart rate (Movsesian 1999).
Alterations in ion homeostasis can promote triggered activity including delayed after depolarizations (DADs), under conditions of high intracellular and sarcoplasmic reticulum (SR) concentration, as previously reviewed (Bers 2002; Eisner et al. 2017). DADs are observed with excessive catecholamine or digitalis toxicity; digitalis blocks the , which elevates intracellular concentration and increases influx through the sarcolemmal exchanger (NCX) (Rehman and Hai 2021). Beta-adrenergic agonists increase the probability of DADs by stimulating current and SR uptake. Environmental exposures can also promote arrhythmias and include alcohol consumption (Yan et al. 2018) and bisphenol A (BPA) exposure (Gao et al. 2013; Yan et al. 2011). Arsenic trioxide can increase currents and precipitate QT prolongation, torsade de pointes, and sudden cardiac death (Ficker et al. 2004).
KC2: impairs cardiac contractility and relaxation.
The opening of LTCCs allows entry, which triggers SR release via ryanodine receptors (RyR2), leading to crossbridge formation between actin and myosin molecules. Cardiac relaxation requires a decline in intracellular concentration through the SR adenosine triphosphate (ATP)ase (SERCA) and the NCX. Drugs or xenobiotics that alter the LTCC, RyR2, SERCA, or NCX can significantly affect cardiac contractility. Beta-adrenergic agonists increase cAMP-dependent protein kinase A, leading to the phosphorylation of the LTCC and phospholamban (PLB). Phosphorylation of PLB releases the inhibition on SERCA and increases SR uptake and SR load. Therefore, beta-adrenergic agonist stimulation of LTCCs and SR uptake significantly increases cardiac contractility; the opposite effects occur with beta-adrenergic blockers (Movsesian 1999). channel blockers can significantly decrease cardiac contractility and may precipitate heart failure in patients with reduced left ventricular function. For example, diltiazem and verapamil exhibit negative inotropic effects that can worsen heart failure to a greater extent than the dihydropyridine channel blockers (e.g., nifedipine) because the negative inotropic effects are not offset by vasodilation (Elliott and Ram 2011). Drugs that may cause or exacerbate heart failure have been summarized in a recent scientific statement from the American Heart Association (Page et al. 2016). Exposure to cadmium may modulate intracellular concentration (Thévenod and Lee 2013), and high levels are associated with future heart failure (Borné et al. 2015).
In contrast to our current knowledge regarding agents or drugs that directly affect cardiac inotropy, there is a significant paucity in our understanding for drugs or xenobiotics that may alter cardiac lusitropy, the rate of cardiac relaxation. Experimental studies demonstrate that several drugs may improve left ventricular diastolic function; for example, JTV519 reduces SR leak and SES0400 inhibits the NCX entry mode. However, clinical data are not currently available for these drugs (Tschöpe et al. 2017).
KC3: induces cardiomyocyte injury and death.
Cardiomyocytes, although critical for both myocardial contraction and electrical conduction, are thought to have little, if any, regenerative capacity. Hence, the injury or death of cardiomyocytes can have progressive, debilitating, and lethal consequences. Morphological changes to cardiomyocytes include hypertrophy, hyalinization, or vacuolation (Berridge et al. 2016). Hypertrophy is often a response to increased work and may be generalized or regional. Hyalinization generally represents hypercontraction of the cell, condensation of the cytoplasm, and fragmentation of the myofibrillar contractile apparatus. Vacuolation may represent swelling of cellular organelles (e.g., mitochondria), dilation of the SR, or lipid accumulation. Cardiomyocyte cell death is usually a lytic event, with the release of cellular contents prompting a mixed inflammatory cell response and repair by fibrosis (Clements et al. 2010; Kong et al. 2014). Although apoptosis may be observed in vitro, it is difficult to demonstrate in vivo.
Anthracyclines, such as doxorubicin, are well-established CV toxicants, as are many other cancer chemotherapeutics (Bhagat and Kleinerman 2020; Herrmann 2020; Jain et al. 2017; Octavia et al. 2012; Shan et al. 1996). Clinical presentations for these toxicities include arrhythmias and decreased contractile function. Increased serum troponins are often important precedent biomarkers that indicate cardiomyocyte injury (Taggart et al. 2021). Endogenous and synthetic catecholamines are also well recognized CV toxicants at high levels of exposure, and direct and indirect cardiomyocyte injury are important mediators of systolic dysfunction, such as in methamphetamine-associated cardiomyopathy (Reddy et al. 2020). Intraperitoneal administration of the short-acting sympathomimetic drug, isoproterenol, to rodents induces a dose-related cardiomyocyte necrosis (Clements et al. 2010). Myocardial degeneration, characterized as diffuse cardiomyocyte degeneration and necrosis, with varying levels of inflammatory cell infiltrate and fibrosis, has been observed with a number of industrial chemicals in rodent studies conducted by the National Toxicology Program (Jokinen et al. 2005). Cardiomyocyte injury was generally dose progressive and occurred in studies from 6–13 wk in length. Tested chemicals have included monochloroacetic acid (used in the synthesis of herbicides and other organic compounds), 3,3′-4,4′-tetrachloroazoxybenzene (a dioxin-like compound), diethanolamine (used in the synthesis of a variety of chemicals), and urethane (used in a variety of industrial processes). Exposure to cadmium has also been associated with apoptosis and cell death in a mouse fibroblast cell line in vitro (Biagioli et al. 2008).
KC4: induces proliferation of valve stroma.
A novel mechanism of cardiotoxicity was discovered with fenfluramine, a 5-hydroxytryptamine (5-HT; serotonin) agonist used to treat obesity, which inadvertently targets 5-HT subtype 2B () receptors on heart valves (Fitzgerald et al. 2000) (Table 3). An activation can induce pathological proliferation of valve leaflet stroma myofibroblasts, leading to abnormalities in leaflet structure and subsequent valvular heart disease (Elangbam 2010; Reid et al. 2013; Taylor et al. 2007), with plaques containing proliferative myofibroblasts in an abundant extracellular matrix (ECM) and lymphocytic infiltrations (Connolly et al. 2009; Steffee et al. 1999; Volmar and Hutchins 2001). This mechanism of cardiotoxicity was recapitulated in mice and rats following administration of 5-HT (Elangbam et al. 2008).
Pharmaceutical drugs associated with similar valvular pathology related to activation of receptors include ergot alkaloids (e.g., methysergide, ergotamine), designer drugs [e.g., 3,4-methylenedioxymethamphetamine (MDMA, or Ecstasy)], anorexigens (e.g., fenfluramine, dexfluramine), and dopamine agonists (e.g., pergolide, cabergoline) [reviewed by Elangbam (2010)]. Fenfluramine was withdrawn from the market because of a high incidence of drug-induced valvular heart disease (Roth 2007; Rothman et al. 2000), and candidate drugs are now routinely screened for agonist activity before progressing to clinical trials (Reid et al. 2013). We currently are not aware of any environmental agents that possess this KC.
Primarily Vascular
KC5: impacts endothelial and vascular function.
Blood vessels are composed of endothelial cells (ECs), smooth muscle cells (SMCs), and adventitia (fibroblasts and connective tissue). The ECs have surface receptors and intracellular pathways [e.g., nitric oxide (NO), endothelin] that maintain critical functions, such as organ blood flow and the transcellular transport of nutrients and lipids (Alexander et al. 2021). Endothelial dysfunction—characterized by its hallmark feature of impaired vasodilation, among other changes (e.g., loss of vascular integrity, increased expression of adhesion molecules, pro-atherogenic phenotype, up-regulation of cytokine production)—is linked to inflammation, increased vascular permeability, a pro-thrombotic phenotype, and atherosclerotic disease (Mundi et al. 2018). The ECM, a structural scaffold for ECs that regulates ECs and SMCs through integrins, undergoes increased or abnormal ECM protein expression and collagen deposition in vascular disease or injury, which in turn may modify normal EC and SMC function (González-Santiago et al. 2002). Vascular SMCs contain a variety of extracellular receptors and intracellular pathways that regulate the contraction of the vascular wall and modulate the diameter of the blood vessel lumen (Brozovich et al. 2016; Tykocki et al. 2017). Vascular SMC activity is modified by extrinsic (e.g., neural, endocrine) and intrinsic factors (e.g., endothelial and muscle cell metabolites, myogenic mechanisms). Vascular contraction is linked tightly to intracellular calcium fluctuations that activate the actin–myosin complex and drive SMC shortening (Fan et al. 2019). The vasculature is an important target for amlodipine and other voltage-sensitive LTCC blockers used for the treatment of hypertension (Luo et al. 2019). Vascular dysfunction contributes to various CV disease processes, including hypertension, atherosclerosis, coronary vasospasm, and myocardial infarction (Thygesen et al. 2018).
Chronic exposure to arsenic is associated with hypertension due to vascular injury and endothelial dysfunction (Ellinsworth 2015; Stea et al. 2014). Exposure to polychlorinated biphenyls (PCBs) has been linked to increased blood pressure (Goncharov et al. 2011), and may occur via increased aldosterone biosynthesis (Goncharov et al. 2011; Perkins et al. 2016). NO, which is physiologically produced in ECs, plays a critical role in CV function and the deregulation of NO contributes to many CVDs (Vanhoutte et al. 2017). PCBs have been shown to impair endothelium-mediated vasodilation (EVD) of rat aortic rings ex vivo (Helyar et al. 2009), suggesting reduced NO synthase (NOS) activity. The organophosphate pesticide malathion impairs EVD in rats (de Carvalho et al. 2014), and other pesticides, such as dichlorodiphenyltrichloroethane, have been linked to hypertension in humans (Lind et al. 2014). Arsenic and cadmium exposures have been linked to reduced NOS activity in ECs in vitro (Majumder et al. 2008; Pi et al. 2000), impaired endothelial function and hypertension in experimental animals (Pinheiro Júnior et al. 2020), and depressed NO production in humans (Pi et al. 2000). Chemicals and metals, such as arsenic, that increase endothelial and smooth muscle nicotinamide adenine dinucleotide phosphate (i.e., NADPH) oxidase catalyze superoxide generation and decrease NO availability as a result of the formation of vasotoxic peroxynitrite (Al Ghouleh et al. 2011; Barchowsky et al. 1999).
KC6: alters hemostasis.
Hemostasis is mainly primed to prevent blood loss and involves circulating platelets, coagulation proteins, and vascular ECs. Loss of, or interference with, any of these components can cause either anticoagulant or procoagulant actions that lead to either blood loss or thrombosis, microangiopathies, and organ ischemia. Immune responses to xenobiotics can target platelet or protein activation or clearance, as well as endothelial antithrombotic activity.
Chemotherapy-induced thrombocytopenia is a frequent side-effect of myelosuppressive cancer therapy (Weycker et al. 2019). Multiple drugs are implicated in immune-mediated thrombocytopenia (Curtis 2014) (Table 1). In heparin-induced thrombocytopenia (HIT), antibodies are formed against a heparin/protein (usually platelet factor 4) complex that activates platelets and aggregation (Evans and Gomes 2017; Greinacher et al. 2017). Drug-induced thrombotic thrombocytopenic purpura, a rare and life-threatening thrombotic microangiopathy (Joly et al. 2017), is caused by quinine, cyclosporine, and tacrolimus (Al-Nouri et al. 2015) through antibody generation and direct EC toxicity (Lian 2005; Veyradier and Meyer 2005). In addition, certain classes of drugs modulate platelet procoagulant and endothelial anticoagulant function through mechanisms that include prostaglandin synthesis inhibition, interference with platelet agonist–receptors interactions, and interference with calcium translocation (Abrams 2006). Exogenous chemicals could interfere with fibrin clot formation in several ways. For example, warfarin promotes vitamin K deficiency (Berry et al. 2000; Chua and Friedenberg 1998). Recently, a class of new oral anticoagulants has emerged to treat thromboembolic diseases. They are selective for one specific coagulation factor, either thrombin (e.g., dabigatran) or factor Xa (e.g., rivaroxaban, apixaban, edoxaban) (Almarshad et al. 2018). Procainamide, chlorpromazine, and hydralazine may induce lupus anticoagulants, which are antibodies that interfere with the protein C system regulating thrombosis (Bertolaccini et al. 2004). Thrombosis associated with exposure to air pollution may involve platelet activation and the promotion of circulating toxic microvesicles (Robertson and Miller 2018). Cadmium exposure has been reported to increase plasminogen activation inhibitor-1 generation in a human vascular EC line (Hara et al. 2021).
KC7: causes dyslipidemia.
Low-density lipoprotein (LDL)-cholesterol is necessary for atherosclerosis development, where deposits of LDL-cholesterol in plaque accumulate in the intima layer of blood vessels and trigger chronic vascular inflammation. LDL-cholesterol is increased either by dietary overfeeding, increased synthesis and output from the liver, or by an increased uptake from the intestine/change in bile acids and enterohepatic circulation (Lorenzatti and Toth 2020). A number of drugs reduce LDL-cholesterol and include statins and cholestyramine (López-Miranda and Pedro-Botet 2021), but other drugs might increase cholesterol as an adverse effect, such as some antiretroviral drugs (e.g., human immunodeficiency virus protease inhibitors) (Distler et al. 2001) and some antipsychotic drugs (Meyer and Koro 2004; Rummel-Kluge et al. 2010). A number of environmental contaminants, such as PCBs and pesticides (Aminov et al. 2014; Goncharov et al. 2008; Lind et al. 2004; Penell et al. 2014) and phthalates (Olsén et al. 2012) have also been associated with increased levels of LDL-cholesterol and triglycerides. In addition, some metals, such as cadmium (Zhou et al. 2016) and lead (Xu et al. 2017), have also been linked to dyslipidemia. Proposed mechanisms leading to dyslipidemia are reduced and increased lipid biosynthesis in the liver (Li et al. 2019; Wahlang et al. 2013; Wan et al. 2012), altered synthesis and secretion of very-low-density lipoprotein (Boucher et al. 2015), increased intestinal lipid absorption and chylomicron secretion (Abumrad and Davidson 2012), and increased activity of fatty acid translocase (FAT/CD36) and lipoprotein lipase (Wan et al. 2012). Furthermore, dioxins, PCBs, BPA, and per- and poly-fluorinated substances have been associated with atherosclerosis in humans (Lind et al. 2017; Melzer et al. 2012a) and in mice (Kim et al. 2014) and with increased prevalence of CVD (Huang et al. 2018; Lang et al. 2008).
Both Cardiac and Vascular
KC8: impairs mitochondrial function.
Mitochondria generate energy in the form of ATP and also play vital roles in homeostasis, apoptosis regulation, intracellular redox potential regulation, and heat production, among other roles (Westermann 2010). In cardiac cells, mitochondria are highly abundant and needed for the synthesis of ATP as well as to synthesize different metabolites such as succinyl-coenzyme A, an essential signaling molecule in protein lysine succinylation, and malate, which plays a significant role in energy homeostasis (Frezza 2017).
Impairment of cardiac mitochondrial function—as demonstrated by lower energy metabolism, increased reactive oxygen species (ROS) generation, altered handling, and apoptosis—can be induced by environmental chemical exposure or by commonly prescribed drugs. Arsenic exposure can induce mitochondrial DNA damage, decrease the activity of mitochondrial complexes I–IV, decrease ATP levels, alter membrane permeability, increase ROS levels, and induce apoptosis (Pace et al. 2017). The increased ROS production triggered by arsenic is most likely via the inhibition of mitochondrial complexes I and III (Pace et al. 2017). Similarly, the environmental pollutant methylmercury may impair mitochondrial function by inhibiting mitochondrial complexes, resulting in increased ROS production and inhibiting the citric acid cycle in the mitochondria (Jia et al. 2015). Several prescribed drugs induce mitochondrial dysfunction that is linked to their CV toxicity (Varga et al. 2015). Anthracyclines can exert significant damage to the heart by impairing mitochondrial biogenesis and cause mitochondrial dysfunction by increasing iron accumulation, resulting in increased ROS production (Henriksen 2018). Rosiglitazone impairs mitochondrial biogenesis by inhibiting peroxisome proliferator-activated receptor (PPAR)-coactivator-1 and azidothymidine inhibits the enzyme needed for mitochondrial DNA replication, mitochondrial DNA (Varga et al. 2015). Nitrogen dioxide, a component in diesel exhaust, has been shown in rats to produce impairment in endothelial function by means of mitochondrial dysfunction (Karoui et al. 2020), and exposure to air pollution has been shown to induce vascular fibrosis in rats by mitochondrial down-regulation (Ning et al. 2020). Cadmium has been linked to mitochondrial dysfunction in a human cell line (Xu et al. 2021).
KC9: modifies autonomic nervous system activity.
The autonomic nervous system (ANS) consists of counter-balancing sympathetic (SNS) and parasympathetic (PNS) nervous systems (Chen et al. 2014) that maintain homeostatic control of CV function. Activation of the SNS by endogenous chemicals could promote arrhythmia by increasing AP firing in pacemaker cells, leading to increased heart rate and atrioventricular conduction velocity and by modulating atrial and ventricular repolarization (Lederer 2017; Shen and Zipes 2014). By contrast, agents that activate the PNS decrease AP firing, reducing heart rate and atrioventricular conduction velocity, and reduce the effective refractory period, mainly in the atria (Lederer 2017; Shen and Zipes 2014). Agents that block SNS activity may also impair cardiac systolic and diastolic function and disrupt vascular smooth muscle tone by altering intracellular levels (Boulpaep 2017).
Sympathomimetic drugs mimic increased sympathetic activity by activating beta-adrenergic receptors in the heart and are often used to treat acute heart failure (Tariq and Aronow 2015). Sympatholytic drugs, on the other hand, block sympathetic neurotransmission at the peripheral organ level or in the central nervous system and decrease blood pressure (Becker 2012). Anticholinergics (i.e., muscarinic antagonists) block PNS transmission and cause tachycardia (Andersson et al. 2011). Importantly, a shift toward increased SNS tone, via sympathetic activation or parasympathetic withdrawal, increases CV morbidity and mortality (Brook et al. 2010). Environmental exposure to air pollution has been linked with increased cardiac sympathetic tone, decreased heart rate variability, and the attendant increased risk of ischemic heart disease and heart failure (Brook et al. 2010). These effects of air pollution likely involve ANS reflexes, including the activation of respiratory sensory mechanisms and altered baroreceptor responsiveness (Perez et al. 2015).
KC10: induces oxidative stress.
In atherosclerosis, the interplay between pro- and anti-oxidant factors in the blood vessels may determine the degree of ROS generation and plaque formation (Dubois-Deruy et al. 2020). These oxidative effects can derive from direct redox chemistry given that some CV toxicants (e.g., ) have a high content of redox-active chemicals, or from the exacerbation of endogenous sources of ROS. ROS originate from all the main cell types present in atherosclerotic lesions, including ECs, macrophages, SMCs, and cardiac cells. Enhanced ROS production could be due to the activation of nicotinamide adenine dinucleotide phosphate oxidase (NOX), which has been shown to be an important source of vascular ROS (Ying et al. 2009). Enhanced ROS could also be due to activation of endogenous pro-oxidative pathways, such as the lipoxygenase pathways (Lin et al. 2019; Yin et al. 2013), which enable enzymatically mediated oxidation of polyunsaturated fatty acids and amplification of lipid peroxidation cascades, through xanthine oxidase, through the induction of mitochondrial dysfunction (Yin et al. 2019), or through the inactivation of antioxidant extracellular and intracellular defense pathways, such as enzymatic inhibition of antioxidant enzyme paraoxonase 1 (PON1) (Lin et al. 2019; Yin et al. 2013), glutathione peroxidase, and superoxide dismutase (SOD) (Delfino et al. 2009).
In vitro and in vivo animal evidence support the ROS-inducing actions of CV toxicants, including various metals such as lead and arsenic (Balali-Mood et al. 2021) and polycyclic aromatic hydrocarbons (PAHs) (Låg et al. 2020). Some commonly used drugs, such as anthracyclines, are known CV toxicants and have been shown to induce ROS production in cardiac, endothelial, and fibroblast cells (Nebigil and Désaubry 2018). Studies have shown that arsenic exposure induces NOX2 expression/activity, which increases ROS in several CV cell types and depletes cardiac glutathione and increases oxidized glutathione levels (Alamolhodaei et al. 2015; Waghe et al. 2015). Interaction with glutathione and the depletion of SOD, catalase, and glutathione peroxidase—all enzymes involved in the regulation of oxidative stress—have been shown to contribute to cadmium-induced endothelial dysfunction (Almenara et al. 2020). Acrolein depletes glutathione and causes ROS-mediated suppression of glutathione S-transferase activity in CV tissues (Henning et al. 2017). Human studies also support the pro-oxidative effects of CV toxicants (Delfino et al. 2009; Lin et al. 2019) and tobacco cigarette smoke (Zhou et al. 2000) via the inhibition of the antioxidant enzymes PON1, glutathione peroxidase, SOD, and catalase.
KC11: causes inflammation.
Exposure to many chemicals, including PCBs and BPA, has been associated with elevated levels of inflammatory markers and the increased risk of atherosclerosis or CV disease in humans (Table 1). Unresolved acute inflammation can lead to the development of chronic inflammation, which is a well-known mediator of some common and important CVDs, such as atherosclerosis (Hansson 2017; Libby 2002). Chronic inflammation has also been associated with cardiac arrhythmia (Lewek et al. 2014). Animal studies have confirmed that exposure to dioxin-like PCBs increases systemic inflammation and accelerates atherosclerosis in mouse models, probably by activation of aryl hydrocarbon receptor (AhR) or nuclear factor kappa-light-chain-enhancer of activated B cells (i.e., ) signaling (Petriello et al. 2018; Wang et al. 2019a). BPA exposure can increase macrophage foam cell formation and atherosclerosis in hyperlipidemic mouse models (Sui et al. 2014, 2018). In addition to activating estrogen receptors (ERs), BPA is also a ligand for human pregnane X receptor that may contribute its atherogenic effects (Sui et al. 2012, 2014). Epidemiological studies have associated arsenic (States et al. 2009) and certain heavy metals, including lead (Boskabady et al. 2018), with elevated levels of inflammatory markers and CV disease. Chronic exposure to arsenic can also lead to vascular inflammation, endothelial dysfunction, and increased atherosclerosis in animal models (States et al. 2009).
in air has been consistently associated with increased atherosclerotic risk and inflammation and is considered one of their main mechanisms (Bai and Sun 2016). exposure can dysregulate inflammatory pathways in the lung and induce secretion of inflammatory factors into the circulation, leading to enhanced EC activation and vascular inflammation. exposure can also affect macrophage polarization, resulting in increased pro-inflammatory M1 macrophages, but decreased anti-inflammatory M2 macrophages (Zhao et al. 2016). In addition, cadmium exposure has been associated with a pro-inflammatory state in rats (Kumar et al. 2021).
KC12: alters hormone signaling.
Membrane and intracellular hormone receptors (e.g., G-protein coupled receptors, receptor tyrosine kinases, and nuclear receptors) are expressed throughout the CV system and regulate numerous critical CV functions. PPAR-gamma () agonists have been linked to increased risks for heart failure and ischemic heart disease in diabetic patients (Kaul et al. 2010; Nissen and Wolski 2007; Wallach et al. 2020). The mechanisms of the adverse CV effects of rosiglitazone are related to its dependent-induction of atherogenic lipid profiles and renal-mediated changes in fluid balance that increases intravascular blood volume (Wallach et al. 2020). Excessive thyroid hormone receptor activation activity is associated with atrial fibrillation and disturbances in cardiac output, contractility, blood pressure, hemostasis, and vascular resistance (Osuna et al. 2017). Amiodarone, an iodine-rich Class 3 antiarrhythmic drug, can cause hyperthyroidism, which causes hemodynamic changes that lead to heart failure and dilated cardiomyopathy (Macchia and Feingold 2000).
Increased exposure to PCBs is associated with CV diseases, including hypertension (Donat-Vargas et al. 2015; Lind and Lind 2012). Potential mechanisms of PCB action include increased activation of the AhR and dysregulation of the renin–angiotensin–aldosterone system (RAAS) (Kraugerud et al. 2010; Li and Lin 2007; Lin et al. 2006; Perkins et al. 2016). CV toxicity associated with BPA, such as hypertension (Han and Hong 2016; Rancière et al. 2015), is mediated by signaling via nuclear receptors , , and membrane associated ERs (La Merrill et al. 2020). Exposure to BPA has also been associated with increased risk for coronary and peripheral artery disease in humans (Lind and Lind 2011; Melzer et al. 2012b; Shankar et al. 2012) and a mouse model (Sui et al. 2014). Experimental animal studies have also identified sex-specific mechanisms of BPA actions in the heart that cause pro-arrhythmic changes in excitation–contraction coupling (Gao and Wang 2014; Zhang et al. 2020b).
Measuring the KCs of CV Toxicants
Table 1, which is based on the expertise of the authors, summarizes established assays and other methods to evaluate the 12 different KCs in vitro, in animal models, and in humans; see also a summary of evaluation methods by Berridge et al.(2013). From these summaries, it is evident that a) although some assays used in vitro (e.g., cytotoxicity assays, quantitative polymerase chain reaction) are high-throughput and, therefore, suited to serve as screening for multiple chemicals, such high-throughput assays are not available for most KCs (Villeneuve et al. 2019); b) there are usually fewer end points that can be measured in humans, and those are most often not high-throughput; and c) in some cases, the output of the assays and end points used in vitro and in animals are not easily translated into the output of the biomarkers used in humans.
Regarding KC1 (cardiac excitability), robust in vitro functional assays and end points (e.g., half maximal inhibitory concentration values) are available to determine altered or channel function (Mathie et al. 2021; Sigg et al. 2010); in nonrodent animal models and humans, the fast sodium and hERG channels are linked directly to ventricular depolarization (e.g., QRS interval) and repolarization (e.g., QT interval), respectively (Baldrick 2021; Edwards and Louch 2017; Strauss et al. 2021). The evaluation of intracellular concentrations in humans is not currently feasible in clinical practice (Bruton et al. 2020). For KC2 (contractility and relaxation), ultrasound of the heart is a very commonly used technique both in animal models and in humans (Lindsey et al. 2018; Wang et al. 2018). Cardiomyocyte injury and death for KC3 can be evaluated in vitro and in animals in several ways. Cardiac troponins are a very useful and rather high-throughput biomarker in humans regarding myocardial infarction (Taggart et al. 2021), but a good assay to evaluate apoptosis is needed in the clinical setting (Mohamad Kamal et al. 2020). Echocardiography, cardiac magnetic resonance imaging, and myocardial perfusion scan can, however, provide evaluation of overall cardiac function and areas of myocardial injury (Makavos et al. 2021; Sivapackiam et al. 2020; Sreenivasan et al. 2021). Regarding the proliferation of valve stroma for KC4, a high-throughput assay is available for in vitro use (Reid et al. 2013), and echocardiographic imaging in humans is routinely performed to evaluate valvular heart disease (Jain et al. 2021). Endothelial and vascular function for KC5 can be assessed with isolated blood vessels obtained from animals [medium-throughput (Knox et al. 2019)] and human donors [low-throughput (Virdis and Taddei 2016)]. It could be studied in humans in vivo by flow-mediated vasodilation (Tremblay and Pyke 2018), but this technique is only reliable in younger subjects (Lind 2006). Mitochondrial function for KC8 can be measured well in vitro (Koklesova et al. 2021), but is difficult to directly evaluate in humans (Pelletier-Galarneau et al. 2021). There are several techniques (e.g., heart rate variability) to measure ANS activity (Nolte et al. 2017) and understand sympathetic and parasympathetic involvement for KC9 that are relatively easy to apply in humans (Cygankiewicz and Zareba 2013) but more challenging in animal models. Measurements of biomarkers related to hemostasis (KC6) (Adelborg et al. 2021; Lind et al. 2011), dyslipidemia (KC7) (Lind 2019; van Wijk et al. 2009), oxidative stress (KC10) (Kumar et al. 2014b; Tejchman et al. 2021), inflammation (KC11) (Friedman and Shorey 2019; Kumar et al. 2014a; Libby 2002), and hormone signaling (KC12) (Penell et al. 2021; Svobodová and Cajthaml 2010) are readily achievable in both experimental models and humans.
We therefore conclude that there is an unmet need for a systematic improvement of the nonclinical assays, biomarkers, and physical tests used to evaluate all of the KCs. There is also a need to standardize nonclinical tests to assure data quality and reproducibility, as well as their value for translation to human investigations. Hence, the systematic and comprehensive identification of the KCs and the available end points presented herein will help to prioritize the development of improved methods to evaluate potential CV toxicants both experimentally and in humans. Ideally, qualified biomarkers could be used to advance public health by assisting regulatory decision-making (FDA 2019).
Examples of How the KCs May Produce CV Dysfunction and Disease
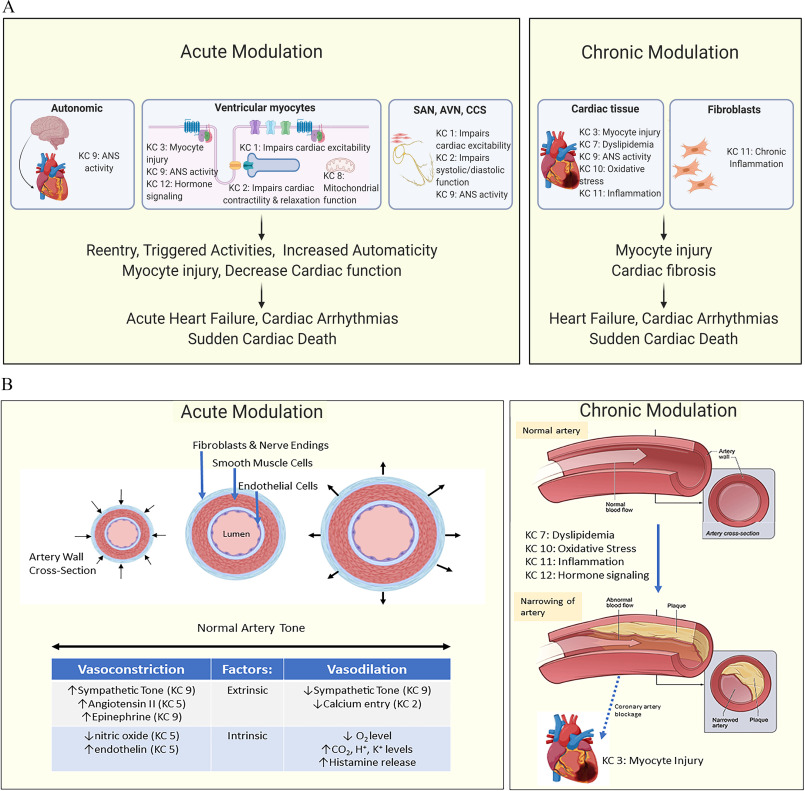
Figure 2 illustrates how the KCs may contribute to the pathogenesis of acute and chronic injury to the heart (Figure 2A) and blood vessels (Figure 2B). Note that multiple KCs may contribute at different locations in the CV system to produce short- or long-term injury and eventually disease. Below and in Tables 2 and 3 we detail how the KCs can be used to generate a holistic picture of how environmental pollutants and drugs that are established CV toxicants can cause CV toxicity. We also describe how the KCs can contribute to understanding the effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These examples further illustrate how evidence for each KC can be organized and evaluated using the published literature.
Figure 2.
Key characteristics (KCs) associated with cardiac and vascular dysfunction. A summary of how different KCs of cardiovascular toxicant could affect (A) the heart and (B) the vasculature in both the acute and chronic setting. Some of the detailed mechanisms are given, as well as some clinical end points. Note: ANS, autonomic nervous system; AVN, avascular necrosis; CCS, cardiac conduction system; , carbon dioxide; , hydrogen ion; , potassium ion; , oxygen; SAN, sinoatrial node.
Fine PM air pollution
Exposure to ambient PM in air pollution increases CVD risk. Although exposures to coarse ( in aerodynamic diameter) and ultrafine ( in aerodynamic diameter) PM have both been linked to adverse effects, the evidence is strongest for regarding incident CVD (Brook et al. 2010; Newby et al. 2015). Because the lung is the initial organ of contact upon inhalation, most CV effects ascribed to are likely secondary to the interaction of PM with lung tissue, with less evidence for direct effects of PM components on CV tissue (Brook et al. 2010). These early effects and initiating KCs include 1) oxidative stress (KC10) and 2) inflammation (KC11) that may originate from lung injury and 3) modulation of cardiac autonomic tone (KC9), potentially stemming from activation of lung sensory afferents (Thompson et al. 2019). also demonstrates well-documented effects on at least four other KCs (5, 6, 7, and 12), see Table 2. Figure 3 shows how these KCs are interconnected and may work in concert to produce CV toxicity from air pollution.
Figure 3.
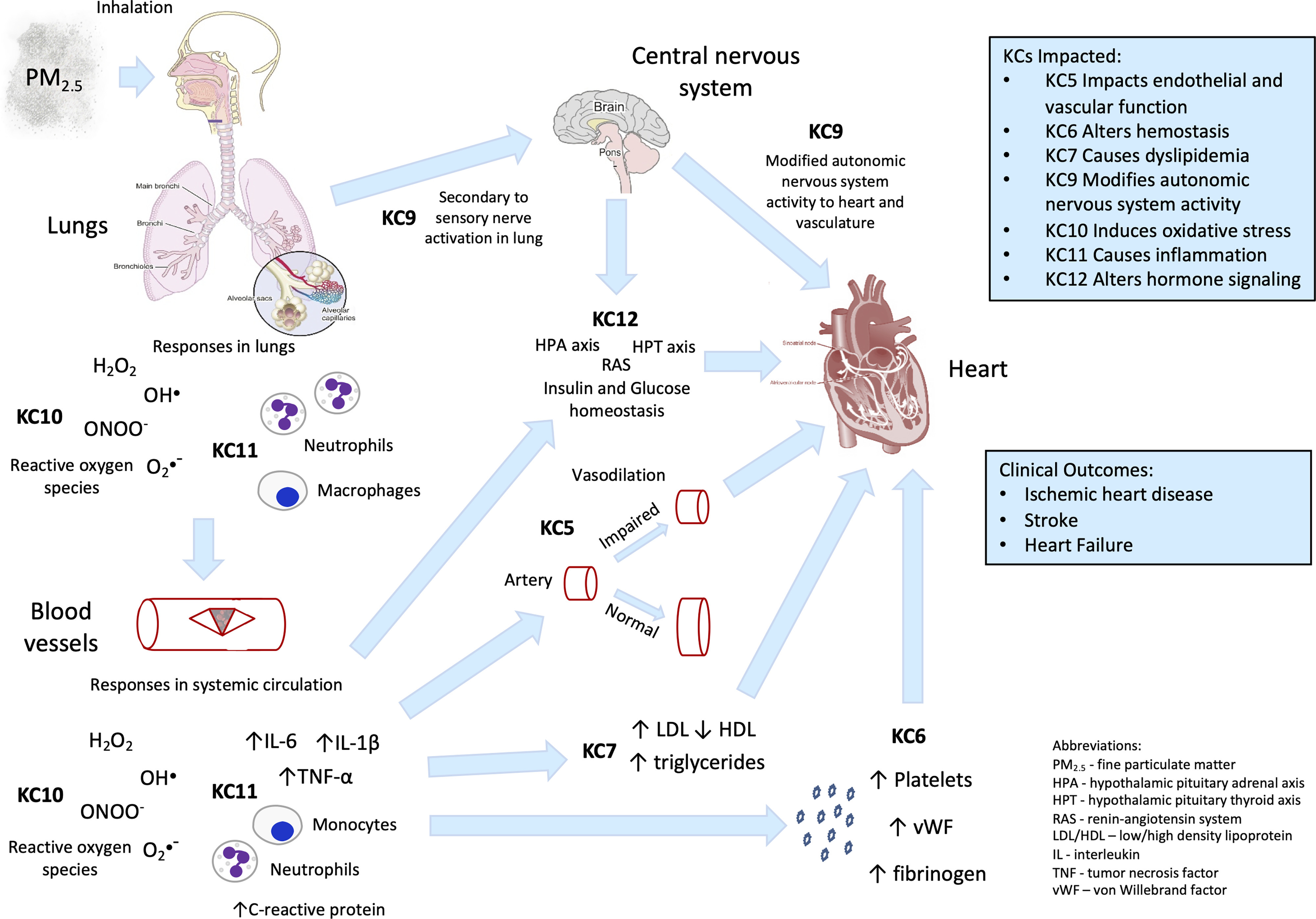
Key characteristics (KCs) associated with toxicity. A summary of how different KCs of fine particulate air pollution () could affect the heart and the vasculature. Some of the detailed mechanisms are given, as well as some clinical end points. Note: , hydrogen peroxide; , hydroxide; •−, reactive oxygen species; , peroxynitrite; , particulate matter in aerodynamic diameter (fine particulate matter).
Polychlorinated biphenyls (PCBs)
There are 209 different PCBs congeners of varying biological activity. Some of these are found in the circulation of almost all humans (Salihovic et al. 2012). The majority of experimental studies use dioxin-like PCBs or a PCB mixture that induces biological effects by binding to the AhR. In humans, high background exposure to PCBs has been linked to CV disease processes (Ha et al. 2007) that may increase CV-related mortality (Li et al. 2015), including carotid and coronary atherosclerosis (Lind et al. 2012) and systolic dysfunction (Sjöberg Lind et al. 2013) leading to stroke (Lee et al. 2012), myocardial infarctions (Bergkvist et al. 2015, 2016), and clinical heart failure (Akahane et al. 2018; Åkesson et al. 2019). There is strong evidence for at least four KCs (7, 10, 11, and 12) being involved in these CV effects of PCBs (Table 2).
Bisphenol A
The ER agonist BPA is ubiquitous in both the environment and clinical setting, and human exposure is nearly continuous, with biomonitoring studies detecting BPA in of the population (Calafat et al. 2005, 2008, 2009; Vandenberg et al. 2010). Population-based epidemiological studies have noted associations between BPA exposure, inflammation, and oxidative stress markers (Kataria et al. 2017; Steffensen et al. 2020; Wang et al. 2019b; Yang et al. 2009), which can contribute to endothelial dysfunction and the development of hypertension in adults and children (Bae et al. 2017; Han and Hong 2016; Ramadan et al. 2020; Warembourg et al. 2019). In a randomized trial, the consumption of canned beverages with a BPA-liner resulted in higher urinary BPA concentrations and an acute increase in blood pressure (Bae and Hong 2015). Given its estrogenic properties (Khan et al. 2021), some biological effects of BPA on the CV system are likely mediated by endocrine disruption (KC12), but BPA may also exert its biological effects through multiple other KCs (e.g., KCs 1, 9, 10, and 11), see Table 2.
Doxorubicin, an anthracycline
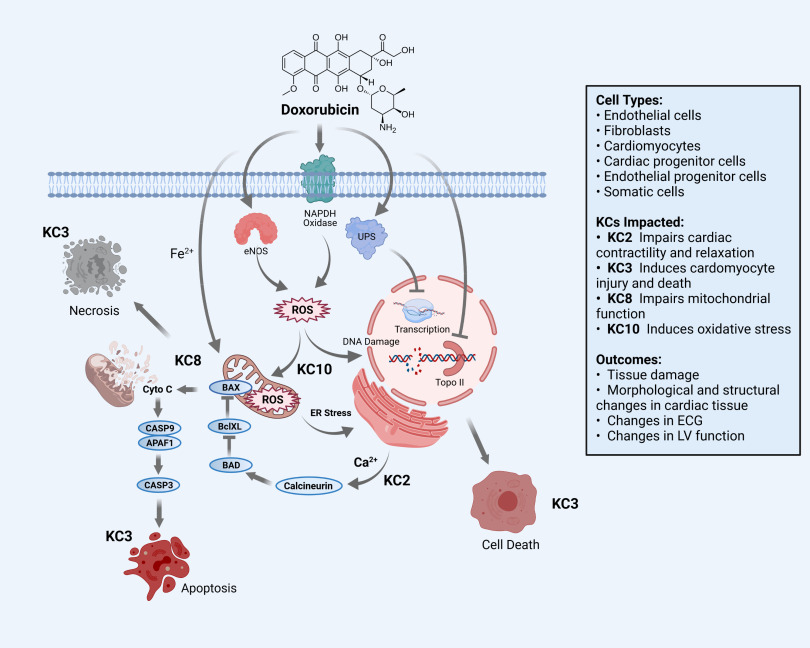
Anthracycline chemotherapy regimens are widely used to treat breast cancer, lymphomas, and childhood solid tumors (McGowan et al. 2017; Nebigil and Désaubry 2018). Doxorubicin was one of the first anthracyclines to be used in clinical practice, but other analogs are also used (McGowan et al. 2017). A significant clinical safety issue associated with doxorubicin and other anthracyclines is the development of dilated cardiomyopathy and heart failure, which increase the mortality of cancer survivors (Gilchrist et al. 2019). The incidence of heart failure is dose dependent and can occur early after initiation of treatment (within 1 y) or emerge decades after cumulative exposure (Zamorano et al. 2016). As illustrated in Figure 4, there is strong evidence, documented in Table 3, that multiple KCs (2, 3, 8, 10, and 11) contribute either directly or act together to cause cardiac dysfunction or failure (Mele et al. 2016; Minotti et al. 2004).
Figure 4.
Key characteristics (KCs) associated with doxorubicin cardiotoxicity. A summary of how different KCs of doxorubicin could affect the heart and the vasculature. Some detailed mechanisms are given, as well as some clinical outcomes. Note: APAF1, apoptotic protease activating factor 1; Bad, Bcl-2-associated agonist of cell death; Bax, Bcl-associated X; BclXL, B-cell lymphoma-extra large; calcium ion; CASP3, caspase 3; CASP9, caspase 9; CytoC, cytochrome complex; ECG, electrocardiogram; eNOS, endothelial nitric oxide synthase; ER, estrogen receptor; , iron ion; LV, left ventricular; NADPH, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; Topo II, topoisomerase II; UPS, ubiquitin-proteasome system.
Arsenic
Arsenic is a unique example of a CV toxicant that is both an approved human therapeutic and an environmental contaminant. Arsenic exhibits multiple KCs, depending on dose and type of exposure. Acute lethality results from mitochondrial collapse in many tissues, including blood vessels and the myocardium (KC8). Arsenic trioxide is also used to treat leukemia and as an adjuvant in treating some solid tumors, but it is considered among the most hazardous anticancer drugs for increasing cardiac QTc prolongation and risk of torsade de pointes arrhythmias, potentially through direct inhibition of hERG current (Drolet et al. 2004) and altered channel expression (KC1) (Alexandre et al. 2018; Dennis et al. 2007). Arsenic trioxide also exhibits KCs 2, 8, and 10 (Varga et al. 2015). In contrast to the toxicities from arsenic therapies, chronic environmental arsenic exposure is closely associated with increased risk of coronary heart disease at exposures of in drinking water (Moon et al. 2018; Wu et al. 2014) and occlusive peripheral vascular disease at higher exposure levels (Newman et al. 2016). Chronic exposure from contaminated drinking water was linked to ventricular wall thickness and hypertrophy in young adults (Pichler et al. 2019). There is well-documented evidence that chronic environmental arsenic exposure exhibits KCs 5, 6, 7, 10, and 11 (Cosselman et al. 2015; Moon et al. 2018; Straub et al. 2008, 2009; Wu et al. 2014).
Lead
Epidemiological studies have linked lead exposure with CVD mortality and persistent hypertension, as reviewed by Lamas et al.(2021) and Navas-Acien(2021). There is evidence that lead exhibits KCs 1, 2, 5, 7, 8, 10, 11, and 12. Occupational exposure modulated cardiac conduction (KC1) (Kiełtucki et al. 2017) and acute exposure altered cardiac excitability in isolated guinea pig hearts (Ferreira de Mattos et al. 2017). Exposure of rats to low concentrations exerted direct positive inotropic and lusitropic effects on contractile function (KC2) and increased ventricular systolic pressure (Silva et al. 2015). Occupational exposure induced electrocardiogram disturbances, possibly related to decreased RyR1 expression (Xie et al. 2019). Lead replaces calcium in cellular signaling and may cause hypertension by inhibiting the calmodulin-dependent synthesis of NO (KC5) (Vaziri 2008). Lead exposures have also been linked to dyslipidemia (KC7) (Dudka et al. 2014; Xu et al. 2017). Altered cardiac mitochondrial activity (KC8), including increased oxidant and malondialdehyde generation, was associated with lead exposure in animals (Basha et al. 2012; Davuljigari and Gottipolu 2020; Roshan et al. 2011). Lead-exposed male workers had dysfunctional ANS activity (KC9), manifest as a significant decrease of R-R interval variation during deep breathing (Teruya et al. 1991) and chronic exposure in rats caused sympathovagal imbalance and reduced baroreflex sensitivity (Shvachiy et al. 2020; Simões et al. 2017). Lead can increase oxidative stress (KC10) by altering cardiac mitochondrial activity (KC8) (Basha et al. 2012; Davuljigari and Gottipolu 2020; Roshan et al. 2011) and inhibiting glutathione synthesis and SOD (Navas-Acien 2021). The resulting increase in oxidants can increase lipid peroxidation and reduce NO (KC5) levels, leading to endothelial dysfunction and atherosclerosis (Navas-Acien 2021). Epidemiological studies have associated lead with elevated inflammatory markers (KC11) (Boskabady et al. 2018). Finally, lead-induced blood pressure elevation may be mediated by stimulation of the renin–angiotensin system (KC12) (Fiorim et al. 2011; Simões et al. 2011).
SARS-CoV-2
The KC approach for CV toxicants above was developed based on data from chemical agents, but this approach can also be applied to nonchemical agents such as SARS-CoV-2, the infectious agent responsible for the current pandemic of coronavirus disease starting in 2019 (COVID-19). Indeed, CV toxicity has emerged as a serious complication of SARS-CoV-2 infection, presenting with acute myocardial injury in 10–15% of patients (defined by elevated troponin levels) (Cheng and Leedy 2020). Numerous hypotheses as to how SARS-CoV-2 might cause or mediate CV toxicity have emerged, and the KCs can serve as a useful organizing framework for systematically mapping the mechanistic evidence. At present, data in humans suggest that SARS-CoV-2 exhibits multiple KCs given that it has been reported to induce inflammation (KC11), induce vasodilation and hypotension through alterations in the RAAS (KC12) (Chen et al. 2020b; Garvin et al. 2020), increase SNS activity (KC9), alter hemostasis giving rise to thrombosis (KC6), and induce myocyte injury (KC3) that can result in lethal cardiac arrhythmias (Cheng and Leedy 2020; Xiong et al. 2020; Zheng et al. 2020). Moreover, the KCs, along with the biomarkers and assays listed in Table 1, provide a systematic roadmap for ongoing and future experimental studies to evaluate SARS-CoV-2 with respect to end points of known relevance to established mechanisms of toxicity to the heart and vasculature.
Discussion
Regulatory agencies consider a broad range of health end points when determining if a drug or an exogenous chemical poses a hazard. Given the importance of CVD as a major heath burden on society, it is critical to identify potential environmental CVD hazards and reduce exposure to them. Like the KCs for other organ systems, the 12 KCs described here will help these agencies better evaluate hazards and risks to human health by facilitating the systematic assessment of the mechanistic data (Figure 1). In the area of clinical practice, the KCs can help to target improvements in assays, biomarkers, and physiological tests used for risk assessment and differential diagnoses. For toxicologists, the KCs provide a potential framework to facilitate a holistic approach to studies of the potential effects of both pharmaceutical drugs and environmental chemicals on CV toxicity through in vitro screening, in vivo characterization, and human data. Further, the identification of KCs and knowledge of the methods to evaluate them will inform the development of high-throughput assays and in silico screens that could be used to expedite acquisition of information regarding potential CV toxicity (Blanchette et al. 2019; Burnett et al. 2021; Sirenko et al. 2017). The KC framework also enables study of the CV effects of mixtures comprising chemicals that exhibit different KCs, as was recently described for studies of the carcinogenic effects of mixtures (Rider et al. 2021).
Development of the 12 KCs described herein benefited substantially from experience with pharmaceutical drugs, by taking advantage of knowledge collected over decades of drug development and intensive research on adverse effects of pharmaceuticals. We have applied this list of KCs to both pharmaceutical agents and environmental pollutants to illustrate the utility of this approach for both classes of chemicals. However, not all possible mechanisms whereby a chemical could cause CV toxicity may be covered by these 12 KCs, and it is likely that as knowledge in the field of CV toxicity advances, more KCs could be added in the future. For example, emerging evidence suggests that exposure to PM in air pollution impacts the human gut microbiome, resulting in an altered intestinal redox lipidome that may contribute to CV and gastrointestinal diseases (Feng et al. 2020). However, further elucidation is needed to determine whether this represents a characteristic of CV toxicants, as well as whether it is sufficiently distinct from other KCs, such as KC7.
The examples of SARS-CoV-2 and air pollution show that the KCs also could be used for nonchemical agents and mixtures, respectively, and not just individual chemicals. Other such examples include food items, such as foods high in saturated fat (Saluja et al. 2021) and sweetened beverages that have been linked to CVD (Kim et al. 2020), radiation therapy that could give rise to a great number of myocardial pathologies ranging from fibrosis to ischemic disease (Burke et al. 2020), and the well-known endocarditis induced by Streptococcal infections (Chamat-Hedemand et al. 2020). Because food items are a mixture of naturally occurring and synthetic chemicals, the KC approach could be used to investigate which components of the food may possess CV toxicant properties.
It also should be emphasized that a KC is akin to an umbrella under which many different detailed pathophysiological events take place. These events and their underlying molecular mechanisms are understood to varying degrees. For some KCs, such as effects on the ion channels and the valvular stroma, the critical mechanisms are known at the molecular and cellular levels, but for KCs such as inflammation and oxidative stress, much knowledge on basic pathophysiology is yet to be uncovered in detail. The KC approach represents a systematic way to not only define the pathophysiological events involved in CV toxicity but also to identify and address gaps in the mechanistic understanding of how exogenous chemicals could harm the heart or vasculature.
Some environmental pollutants, such as PCBs, arsenic, and dioxins, have been linked to both CVD (Li et al. 2015; Lind et al. 2019) and cancer (Lauby-Secretan et al. 2016). The identification of KCs for the major organ systems, or health conditions, could facilitate identification of important mechanisms whereby chemicals could affect multiple different organ systems. Thus, if a KC were found to be linked to impairments in several organ systems or pathological processes, such as cancer development, the need to develop high-throughput testing assays for this KC would be of higher priority. In a similar manner, although our focus was on CV toxicity in adults in this commentary, KCs relevant to the developing organ systems, including the CV system, and associated health conditions could be developed in the future.
In conclusion, the identification of KCs for CV toxicants facilitates, in an objective and systematic fashion, the further understanding of CV effects of exogenous chemicals, chemical mixtures, and other agents, both in drug development as well as for environmental pollutants. When coupled to the development of high-throughput assays, new candidate drugs, novel marketplace chemicals, as well as existing agents to which the population is exposed, could be screened for specific unwanted characteristics. Thus, the systematic use of KCs may serve as a powerful tool to prevent and mitigate CV toxicity.
Acknowledgments
This project was supported by contracts 17-E0023 and 18-E0034 from the Office of Environmental Health Hazard Assessment of the California Environmental Protection Agency (EPA) and the Research Translation Core of the University of California (UC), Berkeley National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program (SRP) under National Institutes of Health (NIH) grant P42ES004705 (M.T.S.); Project 4 of the UC Davis NIEHS SRP under NIH grant P42 ES004699 (A.V.G. and N.C.); the Texas A&M SRP under NIH grant P42 ES027704, Texas A&M NIEHS Center grant P30 ES029067, and a cooperative agreement with the U.S. EPA, STAR RD83580201 (W.A.C.); NIH grant 5R01HL139472 (N.G.P.); NIH grant R01ES023470 (C.Z.), and NIEHS grant R01 ES029395 and R01ES032806 (J.A.A.). The opinions are those of the authors and do not reflect official views of their respective agencies.
The views expressed in this publication are those of the authors and do not necessarily represent the decisions, policy or views of their respective institutions. Reference to commercial products or services does not constitute endorsement or recommendation for use. This manuscript has been reviewed by the Center for Public Health and Environmental Assessment, U.S. EPA, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Abernethy DR, Schwartz JB. 1999. Calcium-antagonist drugs. N Engl J Med 341(19):1447–1457, PMID: 10547409, 10.1056/NEJM199911043411907. [DOI] [PubMed] [Google Scholar]
- Abrams CS.2006. Acquired qualitative platelet disorders. In: Williams Hematology. 7th ed. Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, eds. New York, NY: McGraw-Hill, 1833–1855. [Google Scholar]
- Abumrad NA, Davidson NO. 2012. Role of the gut in lipid homeostasis. Physiol Rev 92(3):1061–1085, PMID: 22811425, 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelborg K, Larsen JB, Hvas A-M. 2021. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol 192(5):803–818, PMID: 33555051, 10.1111/bjh.17172. [DOI] [PubMed] [Google Scholar]
- Akahane M, Matsumoto S, Kanagawa Y, Mitoma C, Uchi H, Yoshimura T, et al. . 2018. Long-term health effects of PCBs and related compounds: a comparative analysis of patients suffering from Yusho and the general population. Arch Environ Contam Toxicol 74(2):203–217, PMID: 29256109, 10.1007/s00244-017-0486-6. [DOI] [PubMed] [Google Scholar]
- Åkesson A, Donat-Vargas C, Berglund M, Glynn A, Wolk A, Kippler M. 2019. Dietary exposure to polychlorinated biphenyls and risk of heart failure—a population-based prospective cohort study. Environ Int 126:1–6, PMID: 30776745, 10.1016/j.envint.2019.01.069. [DOI] [PubMed] [Google Scholar]
- Al Ghouleh I, Khoo NKH, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, et al. . 2011. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51(7):1271–1288, PMID: 21722728, 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamolhodaei NS, Shirani K, Karimi G. 2015. Arsenic cardiotoxicity: an overview. Environ Toxicol Pharmacol 40(3):1005–1014, PMID: 26606645, 10.1016/j.etap.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Alexander Y, Osto E, Schmidt-Trucksäss A, Shechter M, Trifunovic D, Duncker DJ, et al. . 2021. Endothelial function in cardiovascular medicine: a consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc Res 117:29–42, PMID: 32282914, 10.1093/cvr/cvaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre J, Moslehi JJ, Bersell KR, Funck-Brentano C, Roden DM, Salem J-E. 2018. Anticancer drug-induced cardiac rhythm disorders: current knowledge and basic underlying mechanisms. Pharmacol Ther 189:89–103, PMID: 29698683, 10.1016/j.pharmthera.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Almarshad F, Alaklabi A, Bakhsh E, Pathan A, Almegren M. 2018. Use of direct oral anticoagulants in daily practice. Am J Blood Res 8(4):57–72, PMID: 30697449. [PMC free article] [PubMed] [Google Scholar]
- Almenara CCP, Oliveira TF, Padilha AS. 2020. The role of antioxidants in the prevention of cadmium-induced endothelial dysfunction. Curr Pharm Des 26(30):3667–3675, PMID: 32294029, 10.2174/1381612826666200415172338. [DOI] [PubMed] [Google Scholar]
- Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN. 2015. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood 125(4):616–618, PMID: 25414441, 10.1182/blood-2014-11-611335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuwayjiri A, Taghvaee S, Mousavi A, Sowlat MH, Hassanvand MS, Kashani H, et al. . 2021. Association of systemic inflammation and coagulation biomarkers with source-specific PM2.5 mass concentrations among young and elderly subjects in central Tehran. J Air Waste Manag Assoc 71(2):191–208, PMID: 32758070, 10.1080/10962247.2020.1806140. [DOI] [PubMed] [Google Scholar]
- Aminov Z, Haase R, Olson JR, Pavuk M, Carpenter DO, Anniston Environmental Health Research Consortium. 2014. Racial differences in levels of serum lipids and effects of exposure to persistent organic pollutants on lipid levels in residents of Anniston, Alabama. Environ Int 73:216–223, PMID: 25160080, 10.1016/j.envint.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Andersson K-E, Campeau L, Olshansky B. 2011. Cardiac effects of muscarinic receptor antagonists used for voiding dysfunction. Br J Clin Pharmacol 72(2):186–196, PMID: 21595741, 10.1111/j.1365-2125.2010.03813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselme F, Loriot S, Henry J-P, Dionnet F, Napoleoni J-G, Thuillez C, et al. . 2007. Inhalation of diluted diesel engine emission impacts heart rate variability and arrhythmia occurrence in a rat model of chronic ischemic heart failure. Arch Toxicol 81(4):299–307, PMID: 17024498, 10.1007/s00204-006-0147-4. [DOI] [PubMed] [Google Scholar]
- Arzuaga X, Smith MT, Gibbons CF, Skakkebæk NE, Yost EE, Beverly BEJ, et al. . 2019. Proposed key characteristics of male reproductive toxicants as an approach for organizing and evaluating mechanistic evidence in human health hazard assessments. Environ Health Perspect 127(6):65001, PMID: 31199676, 10.1289/EHP5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S, Hong Y-C. 2015. Exposure to bisphenol A from drinking canned beverages increases blood pressure: randomized crossover trial. Hypertension 65(2):313–319, PMID: 25489056, 10.1161/HYPERTENSIONAHA.114.04261. [DOI] [PubMed] [Google Scholar]
- Bae S, Kim JH, Lim Y-H, Park HY, Hong Y-C. 2012. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 60(3):786–793, PMID: 22851732, 10.1161/HYPERTENSIONAHA.112.197715. [DOI] [PubMed] [Google Scholar]
- Bae S, Lim Y-H, Lee YA, Shin CH, Oh S-Y, Hong Y-C. 2017. Maternal urinary bisphenol A concentration during midterm pregnancy and children’s blood pressure at age 4. Hypertension 69(2):367–374, PMID: 27920131, 10.1161/HYPERTENSIONAHA.116.08281. [DOI] [PubMed] [Google Scholar]
- Bai Y, Sun Q. 2016. Fine particulate matter air pollution and atherosclerosis: mechanistic insights. Biochim Biophys Acta 1860(12):2863–2868, PMID: 27156486, 10.1016/j.bbagen.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. 2021. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol 12:643972, PMID: 33927623, 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrick P. 2021. Core battery safety pharmacology testing—an assessment of its utility in early drug development. J Pharmacol Toxicol Methods 109:107055, PMID: 33813006, 10.1016/j.vascn.2021.107055. [DOI] [PubMed] [Google Scholar]
- Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. 1999. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med 27(11–12):1405–1412, PMID: 10641735, 10.1016/S0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Basha DC, Basha SS, Reddy GR. 2012. Lead-induced cardiac and hematological alterations in aging Wistar male rats: alleviating effects of nutrient metal mixture. Biogerontology 13(4):359–368, PMID: 22534743, 10.1007/s10522-012-9380-9. [DOI] [PubMed] [Google Scholar]
- Becker DE. 2012. Basic and clinical pharmacology of autonomic drugs. Anesth Prog 59(4):159–168, PMID: 23241039, 10.2344/0003-3006-59.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Chen Y, Yan S, Wang H-S. 2012. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17β-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology 153(2):712–720, PMID: 22166976, 10.1210/en.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher SM, Gear RB, Kendig EL. 2015. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology 156(3):882–895, PMID: 25594700, 10.1210/en.2014-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergkvist C, Berglund M, Glynn A, Julin B, Wolk A, Åkesson A. 2016. Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction in men—a population-based prospective cohort study. Environ Int 88:9–14, PMID: 26690540, 10.1016/j.envint.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Bergkvist C, Berglund M, Glynn A, Wolk A, Åkesson A. 2015. Dietary exposure to polychlorinated biphenyls and risk of myocardial infarction—a population-based prospective cohort study. Int J Cardiol 183:242–248, PMID: 25679993, 10.1016/j.ijcard.2015.01.055. [DOI] [PubMed] [Google Scholar]
- Berntsen HF, Fonnum F, Walaas SI, Bogen IL. 2016. Low-chlorinated non-dioxin-like polychlorinated biphenyls present in blood and breast milk induce higher levels of reactive oxygen species in neutrophil granulocytes than high-chlorinated congeners. Basic Clin Pharmacol Toxicol 119(6):588–597, PMID: 27194217, 10.1111/bcpt.12620. [DOI] [PubMed] [Google Scholar]
- Berridge BR, Mowat V, Nagai H, Nyska A, Okazaki Y, Clements PJ, et al. . 2016. Non-proliferative and proliferative lesions of the cardiovascular system of the rat and mouse. J Toxicol Pathol 29(suppl 3):1S–47S, PMID: 27621537, 10.1293/tox.29.3S-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge BR, Van Fleet JF, Herman E.2013. Cardiac, vascular, and skeletal muscle systems. In: Handbook of Toxicologic Pathology. 3rd ed. Haschek WM, Rousseau CG, Wallig MA, eds. Orlando, FL: Academic Press, 1567–1665. [Google Scholar]
- Berry RG, Morrison JA, Watts JW, Anagnost JW, Gonzalez JJ. 2000. Surreptitious superwarfarin ingestion with brodifacoum. South Med J 93(1):74–75, PMID: 10653073, 10.1097/00007611-200001000-00016. [DOI] [PubMed] [Google Scholar]
- Bers DM. 2002. Cardiac excitation–contraction coupling. Nature 415(6868):198–205, PMID: 11805843, 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bertolaccini ML, Hughes GR, Khamashta MA. 2004. Revisiting antiphospholipid antibodies: from targeting phospholipids to phospholipid binding proteins. Clin Lab 50(11–12):653–665, PMID: 15575307. [PubMed] [Google Scholar]
- Bessac BF, Jordt S-E. 2008. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23:360–370, PMID: 19074743, 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat A, Kleinerman ES. 2020. Anthracycline-induced cardiotoxicity: causes, mechanisms, and prevention. Adv Exp Med Biol 1257:181–192, PMID: 32483740, 10.1007/978-3-030-43032-0_15. [DOI] [PubMed] [Google Scholar]
- Biagioli M, Pifferi S, Ragghianti M, Bucci S, Rizzuto R, Pinton P. 2008. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium 43(2):184–195, PMID: 17588656, 10.1016/j.ceca.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Blanchette AD, Grimm FA, Dalaijamts C, Hsieh N-H, Ferguson K, Luo Y-S, et al. . 2019. Thorough QT/QTc in a dish: an in vitro human model that accurately predicts clinical concentration–QTc relationships. Clin Pharmacol Ther 105(5):1175–1186, PMID: 30346629, 10.1002/cpt.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borné Y, Barregard L, Persson M, Hedblad B, Fagerberg B, Engström G. 2015. Cadmium exposure and incidence of heart failure and atrial fibrillation: a population-based prospective cohort study. BMJ Open 5(6):e007366, PMID: 26078311, 10.1136/bmjopen-2014-007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskabady M, Marefati N, Farkhondeh T, Shakeri F, Farshbaf A, Boskabady MH. 2018. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ Int 120:404–420, PMID: 30125858, 10.1016/j.envint.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Boucher M-P, Lefebvre C, Chapados NA. 2015. The effects of PCB126 on intra-hepatic mechanisms associated with non alcoholic fatty liver disease. J Diabetes Metab Disord 14(1):88, PMID: 26693162, 10.1186/s40200-015-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulpaep EL.2017. Regulation of arterial pressure and cardiac output. In: Medical Physiology. 3rd ed. Boron WF, Boulpaep EL, eds. Philadelphia, PA: Elsevier, 533–555. [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. . 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brook RD, Sun Z, Brook JR, Zhao X, Ruan Y, Yan J, et al. . 2016. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: the Air Pollution and Cardiometabolic Disease study. Hypertension 67(1):77–85, PMID: 26573709, 10.1161/HYPERTENSIONAHA.115.06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG. 2016. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol Rev 68(2):476–532, PMID: 27037223, 10.1124/pr.115.010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton J, Cheng AJ, Westerblad H. 2020. Measuring Ca2+ in living cells. Adv Exp Med Biol 1131:7–26, PMID: 31646505, 10.1007/978-3-030-12457-1_2. [DOI] [PubMed] [Google Scholar]
- Burke AM, Yeh C, Kim S, Bergquist P, Krishnan P, Barac A, et al. . 2020. A prospective study of early radiation associated cardiac toxicity following neoadjuvant chemoradiation for distal esophageal cancer. Front Oncol 10:1169, PMID: 32903617, 10.3389/fonc.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett SD, Blanchette AD, Chiu WA, Rusyn I. 2021. Human induced pluripotent stem cell (iPSC)-derived cardiomyocytes as an in vitro model in toxicology: strengths and weaknesses for hazard identification and risk characterization. Expert Opin Drug Metab Toxicol 17(8):887–902, PMID: 33612039, 10.1080/17425255.2021.1894122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. 2005. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113(4):391–395, PMID: 15811827, 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. . 2009. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 117(4):639–644, PMID: 19440505, 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116(1):39–44, PMID: 18197297, 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carll AP, Hazari MS, Perez CM, Krantz QT, King CJ, Haykal-Coates N, et al. . 2013. An autonomic link between inhaled diesel exhaust and impaired cardiac performance: insight from treadmill and dobutamine challenges in heart failure–prone rats. Toxicol Sci 135(2):425–436, PMID: 23872579, 10.1093/toxsci/kft155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamat-Hedemand S, Dahl A, Østergaard L, Arpi M, Fosbøl E, Boel J, et al. . 2020. Prevalence of infective endocarditis in streptococcal bloodstream infections is dependent on streptococcal species. Circulation 142(8):720–730, PMID: 32580572, 10.1161/CIRCULATIONAHA.120.046723. [DOI] [PubMed] [Google Scholar]
- Chapados NA, Boucher M-P. 2017. Liver metabolic disruption induced after a single exposure to PCB126 in rats. Environ Sci Pollut Res Int 24(2):1854–1861, PMID: 27796995, 10.1007/s11356-016-7939-8. [DOI] [PubMed] [Google Scholar]
- Chase KH, Wong O, Thomas D, Berney BW, Simon RK. 1982. Clinical and metabolic abnormalities associated with occupational exposure to polychlorinated biphenyls (PCBs). J Occup Med 24(2):109–114, PMID: 6799628. [PubMed] [Google Scholar]
- Chen N, Shan Q, Qi Y, Liu W, Tan X, Gu J. 2020a. Transcriptome analysis in normal human liver cells exposed to 2, 3, 3′, 4, 4′, 5-hexachlorobiphenyl (PCB 156). Chemosphere 239:124747, PMID: 31514003, 10.1016/j.chemosphere.2019.124747. [DOI] [PubMed] [Google Scholar]
- Chen P-S, Chen LS, Fishbein MC, Lin S-F, Nattel S. 2014. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res 114(9):1500–1515, PMID: 24763467, 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Xu L, Dai Y, Ling Y, Mao J, Qian J, et al. . 2020b. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin Cardiol 43(7):796–802, PMID: 32562427, 10.1002/clc.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R, Leedy D. 2020. COVID-19 and acute myocardial injury: the heart of the matter or an innocent bystander? Heart 106(15):1122–1124, PMID: 32354799, 10.1136/heartjnl-2020-317025. [DOI] [PubMed] [Google Scholar]
- Chiamvimonvat N, Chen-Izu Y, Clancy CE, Deschenes I, Dobrev D, Heijman J, et al. . 2017. Potassium currents in the heart: functional roles in repolarization, arrhythmia and therapeutics. J Physiol 595(7):2229–2252, PMID: 27808412, 10.1113/JP272883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua JD, Friedenberg WR. 1998. Superwarfarin poisoning. Arch Intern Med 158(17):1929–1932, PMID: 9759690, 10.1001/archinte.158.17.1929. [DOI] [PubMed] [Google Scholar]
- Clements P, Brady S, York M, Berridge B, Mikaelian I, Nicklaus R, et al. . 2010. Time course characterization of serum cardiac troponins, heart fatty acid-binding protein, and morphologic findings with isoproterenol-induced myocardial injury in the rat. Toxicol Pathol 38(5):703–714, PMID: 20585145, 10.1177/0192623310374969. [DOI] [PubMed] [Google Scholar]
- Collins WT Jr, Capen CC, Kasza L, Carter C, Dailey RE. 1977. Effect of polychlorinated biphenyl (PCB) on the thyroid gland of rats. Ultrastructural and biochemical investigations. Am J Pathol 89(1):119–136, PMID: 199066. [PMC free article] [PubMed] [Google Scholar]
- Connolly JM, Bakay MA, Fulmer JT, Gorman RC, Gorman JH III, Oyama MA, et al. . 2009. Fenfluramine disrupts the mitral valve interstitial cell response to serotonin. Am J Pathol 175(3):988–997, PMID: 19679875, 10.2353/ajpath.2009.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosselman KE, Navas-Acien A, Kaufman JD. 2015. Environmental factors in cardiovascular disease. Nat Rev Cardiol 12(11):627–642, PMID: 26461967, 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX. 2016. Drug-induced inhibition and trafficking disruption of ion channels: pathogenesis of QT abnormalities and drug-induced fatal arrhythmias. Curr Cardiol Rev 12(2):141–154, PMID: 26926294, 10.2174/1573403x12666160301120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis BR. 2014. Drug-induced immune thrombocytopenia: incidence, clinical features, laboratory testing, and pathogenic mechanisms. Immunohematology 30(2):55–65, PMID: 25247620, 10.21307/immunohematology-2019-099. [DOI] [PubMed] [Google Scholar]
- Cygankiewicz I, Zareba W. 2013. Heart rate variability. Handb Clin Neurol 117:379–393, PMID: 24095141, 10.1016/B978-0-444-53491-0.00031-6. [DOI] [PubMed] [Google Scholar]
- Dales R, Liu L, Szyszkowicz M, Dalipaj M, Willey J, Kulka R, et al. . 2007. Particulate air pollution and vascular reactivity: the bus stop study. Int Arch Occup Environ Health 81(2):159–164, PMID: 17492462, 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- Davuljigari CB, Gottipolu RR. 2020. Late-life cardiac injury in rats following early life exposure to lead: reversal effect of nutrient metal mixture. Cardiovasc Toxicol 20(3):249–260, PMID: 31541351, 10.1007/s12012-019-09549-2. [DOI] [PubMed] [Google Scholar]
- de Carvalho MTM, Celotto AC, Albuquerque AAS, Ferreira LG, Capellini VK, Silveira APC, et al. . 2014. In vitro effects of the organophosphorus pesticide malathion on the reactivity of rat aorta. Pharmacology 94(3–4):157–162, PMID: 25301379, 10.1159/000367897. [DOI] [PubMed] [Google Scholar]
- Deering-Rice CE, Romero EG, Shapiro D, Hughen RW, Light AR, Yost GS, et al. . 2011. Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem Res Toxicol 24(6):950–959, PMID: 21591660, 10.1021/tx200123z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, et al. . 2009. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect 117(8):1232–1238, PMID: 19672402, 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Barney J, Petriello MC, Morris AJ, Wahlang B, Hennig B. 2019. Hepatic metabolomics reveals that liver injury increases PCB 126-induced oxidative stress and metabolic dysfunction. Chemosphere 217:140–149, PMID: 30415113, 10.1016/j.chemosphere.2018.10.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis A, Wang L, Wan X, Ficker E. 2007. hERG channel trafficking: novel targets in drug-induced long QT syndrome. Biochem Soc Trans 35(pt 5):1060–1063, PMID: 17956279, 10.1042/BST0351060. [DOI] [PubMed] [Google Scholar]
- Desaulniers D, Leingartner K, Wade M, Fintelman E, Yagminas A, Foster WG. 1999. Effects of acute exposure to PCBs 126 and 153 on anterior pituitary and thyroid hormones and FSH isoforms in adult Sprague Dawley male rats. Toxicol Sci 47(2):158–169, PMID: 10220852, 10.1093/toxsci/47.2.158. [DOI] [PubMed] [Google Scholar]
- Deutschmann A, Hans M, Meyer R, Häberlein H, Swandulla D. 2013. Bisphenol A inhibits voltage-activated Ca2+ channels in vitro: mechanisms and structural requirements. Mol Pharmacol 83(2):501–511, PMID: 23197648, 10.1124/mol.112.081372. [DOI] [PubMed] [Google Scholar]
- Distler O, Cooper DA, Deckelbaum RJ, Sturley SL. 2001. Hyperlipidemia and inhibitors of HIV protease. Curr Opin Clin Nutr Metab Care 4(2):99–103, PMID: 11224652, 10.1097/00075197-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Donat-Vargas C, Gea A, Sayon-Orea C, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Bes-Rastrollo M. 2015. Association between dietary intake of polychlorinated biphenyls and the incidence of hypertension in a Spanish cohort: the Seguimiento Universidad de Navarra project. Hypertension 65(4):714–721, PMID: 25646299, 10.1161/HYPERTENSIONAHA.114.04435. [DOI] [PubMed] [Google Scholar]
- Drolet B, Simard C, Roden DM. 2004. Unusual effects of a QT-prolonging drug, arsenic trioxide, on cardiac potassium currents. Circulation 109(1):26–29, PMID: 14691044, 10.1161/01.CIR.0000109484.00668.CE. [DOI] [PubMed] [Google Scholar]
- Dubois-Deruy E, Peugnet V, Turkieh A, Pinet F. 2020. Oxidative stress in cardiovascular diseases. Antioxidants (Basel) 9(9):864, PMID: 32937950, 10.3390/antiox9090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudka I, Kossowska B, Senhadri H, Latajka R, Hajek J, Andrzejak R, et al. . 2014. Metabonomic analysis of serum of workers occupationally exposed to arsenic, cadmium and lead for biomarker research: a preliminary study. Environ Int 68:71–81, PMID: 24713610, 10.1016/j.envint.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Edwards AG, Louch WE. 2017. Species-dependent mechanisms of cardiac arrhythmia: a cellular focus. Clin Med Insights Cardiol 11:117954681668606, PMID: 28469490, 10.1177/1179546816686061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Caldwell JL, Kistamás K, Trafford AW. 2017. Calcium and excitation-contraction coupling in the heart. Circ Res 121(2):181–195, PMID: 28684623, 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangbam CS. 2010. Drug-induced valvulopathy: an update. Toxicol Pathol 38(6):837–848, PMID: 20716786, 10.1177/0192623310378027. [DOI] [PubMed] [Google Scholar]
- Elangbam CS, Job LE, Zadrozny LM, Barton JC, Yoon LW, Gates LD, et al. . 2008. 5-Hydroxytryptamine (5HT)-induced valvulopathy: compositional valvular alterations are associated with 5HT2b receptor and 5HT transporter transcript changes in Sprague-Dawley rats. Exp Toxicol Pathol 60(4–5):253–262, PMID: 18511249, 10.1016/j.etp.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Ellinsworth DC. 2015. Arsenic, reactive oxygen, and endothelial dysfunction. J Pharmacol Exp Ther 353(3):458–464, PMID: 25788710, 10.1124/jpet.115.223289. [DOI] [PubMed] [Google Scholar]
- Elliott WJ, Ram CVS. 2011. Calcium channel blockers. J Clin Hypertens (Greenwich) 13(9):687–689, PMID: 21896151, 10.1111/j.1751-7176.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwall MJ, Everds N, Turk JR, Vargas HM. 2021. The effects of repeat-dose doxorubicin on cardiovascular functional endpoints and biomarkers in the telemetry-equipped cynomolgus monkey. Front Cardiovasc Med 8:587149, PMID: 33708802, 10.3389/fcvm.2021.587149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans NS, Gomes M. 2017. Heparin-induced thrombocytopenia (HIT). Vasc Med 22(4):353–355, PMID: 28606001, 10.1177/1358863X17714340. [DOI] [PubMed] [Google Scholar]
- Fan G, Cui Y, Gollasch M, Kassmann M. 2019. Elementary calcium signaling in arterial smooth muscle. Channels (Austin) 13(1):505–519, PMID: 31797713, 10.1080/19336950.2019.1688910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration). 2019. Biomarker qualification program: about biomarkers and qualification. https://www.fda.gov/drugs/biomarker-qualification-program/about-biomarkers-and-qualification [accessed 9 September 2021].
- Feiteiro J, Mariana M, Glória S, Cairrao E. 2018. Inhibition of L-type calcium channels by bisphenol A in rat aorta smooth muscle. J Toxicol Sci 43(10):579–586, PMID: 30298846, 10.2131/jts.43.579. [DOI] [PubMed] [Google Scholar]
- Feng D, Wang X, Li E, Bu X, Qiao F, Qin J, et al. . 2019. Dietary Aroclor 1254-induced toxicity on antioxidant capacity, immunity and energy metabolism in Chinese mitten crab Eriocheir sinensis: amelioration by vitamin A. Front Physiol 10:722, PMID: 31244681, 10.3389/fphys.2019.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Cavallero S, Hsiai T, Li R. 2020. Impact of air pollution on intestinal redox lipidome and microbiome. Free Radic Biol Med 151:99–110, PMID: 31904545, 10.1016/j.freeradbiomed.2019.12.044. [DOI] [PubMed] [Google Scholar]
- Ferreira de Mattos G, Costa C, Savio F, Alonso M, Nicolson GL. 2017. Lead poisoning: acute exposure of the heart to lead ions promotes changes in cardiac function and Cav1.2 ion channels. Biophys Rev 9(5):807–825, PMID: 28836190, 10.1007/s12551-017-0303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficker E, Kuryshev YA, Dennis AT, Obejero-Paz C, Wang L, Hawryluk P, et al. . 2004. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol 66(1):33–44, PMID: 15213294, 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- Fielden MR, Ward LD, Minocherhomji S, Nioi P, Lebrec H, Jacobson-Kram D. 2018. Modernizing human cancer risk assessment of therapeutics. Trends Pharmacol Sci 39(3):232–247, PMID: 29242029, 10.1016/j.tips.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Fiorim J, Ribeiro Júnior RF, Silveira EA, Padilha AS, Vescovi MVA, de Jesus HC, et al. . 2011. Low-level lead exposure increases systolic arterial pressure and endothelium-derived vasodilator factors in rat aortas. PLoS One 6(2):e17117, PMID: 21364929, 10.1371/journal.pone.0017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, et al. . 2000. Possible role of valvular serotonin 5-HT2B receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 57(1):75–81, PMID: 10617681. [PubMed] [Google Scholar]
- Frezza C. 2017. Mitochondrial metabolites: undercover signalling molecules. Interface Focus 7(2):20160100, PMID: 28382199, 10.1098/rsfs.2016.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E, Shorey C. 2019. Inflammation in multimorbidity and disability: an integrative review. Health Psychol 38(9):791–801, PMID: 31436464, 10.1037/hea0000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo MV, Ravenscroft J, Carpenter DO, Schell LM, Akwesasne Task Force on the Environment. 2018. Persistent organic pollutants as predictors of increased FSH:LH ratio in naturally cycling, reproductive age women. Environ Res 164:556–564, PMID: 29621723, 10.1016/j.envres.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci G, Tartarone A, Lerose R, Lalinga AV, Capobianco AM. 2020. Cardiovascular risk of smoking and benefits of smoking cessation. J Thorac Dis 12(7):3866–3876, PMID: 32802468, 10.21037/jtd.2020.02.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Liang Q, Chen Y, Wang H-S. 2013. Molecular mechanisms underlying the rapid arrhythmogenic action of bisphenol A in female rat hearts. Endocrinology 154(12):4607–4617, PMID: 24140712, 10.1210/en.2013-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wang H-S. 2014. Impact of bisphenol A on the cardiovascular system—epidemiological and experimental evidence and molecular mechanisms. Int J Environ Res Public Health 11(8):8399–8413, PMID: 25153468, 10.3390/ijerph110808399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin MR, Alvarez C, Miller JI, Prates ET, Walker AM, Amos BK, et al. . 2020. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. elife 9:e59177, PMID: 32633718, 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear R, Kendziorski JA, Belcher SM. 2017. Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: a CLARITY-BPA study. Toxicol Lett 275:123–135, PMID: 28499613, 10.1016/j.toxlet.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelfi E, Wellenius GA, Lawrence J, Millet E, Gonzalez-Flecha B. 2010. Cardiac oxidative stress and dysfunction by fine concentrated ambient particles (CAPs) are mediated by angiotensin-II. Inhal Toxicol 22(11):963–972, PMID: 20718632, 10.3109/08958378.2010.503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al. . 2019. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation 139(21):e997–e1012, PMID: 30955352, 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjernes MH, Schlenk D, Arukwe A. 2012. Estrogen receptor-hijacking by dioxin-like 3,3′4,4′,5-pentachlorobiphenyl (PCB126) in salmon hepatocytes involves both receptor activation and receptor protein stability. Aquat Toxicol 124–125:197–208, PMID: 22982498, 10.1016/j.aquatox.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Haase RF, Santiago-Rivera A, Morse G, Akwesasne Task Force on the Environment; McCaffrey RJ, et al. . 2008. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ Res 106(2):226–239, PMID: 18054906, 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov A, Pavuk M, Foushee HR, Carpenter DO, Anniston Environmental Health Research Consortium. 2011. Blood pressure in relation to concentrations of PCB congeners and chlorinated pesticides. Environ Health Perspect 119(3):319–325, PMID: 21362590, 10.1289/ehp.1002830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Santiago L, López-Ongil S, Griera M, Rodríguez-Puyol M, Rodríguez-Puyol D. 2002. Regulation of endothelin synthesis by extracellular matrix in human endothelial cells. Kidney Int 62(2):537–543, PMID: 12110015, 10.1046/j.1523-1755.2002.00466.x. [DOI] [PubMed] [Google Scholar]
- Gorr MW, Youtz DJ, Eichenseer CM, Smith KE, Nelin TD, Cormet-Boyaka E, et al. . 2015. In vitro particulate matter exposure causes direct and lung-mediated indirect effects on cardiomyocyte function. Am J Physiol Heart Circ Physiol 309(1):H53–H62, PMID: 25957217, 10.1152/ajpheart.00162.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi E, Sanguinetti MC, Bartos DC, Bers DM, Chen-Izu Y, Chiamvimonvat N, et al. . 2017. Potassium channels in the heart: structure, function and regulation. J Physiol 595(7):2209–2228, PMID: 27861921, 10.1113/JP272864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RM, Hodges NJ, Chipman JK, O’Donovan MR, Graham M. 2008. Reactive oxygen species from the uncoupling of human cytochrome P450 1B1 may contribute to the carcinogenicity of dioxin-like polychlorinated biphenyls. Mutagenesis 23(6):457–463, PMID: 18583386, 10.1093/mutage/gen035. [DOI] [PubMed] [Google Scholar]
- Greinacher A, Selleng K, Warkentin TE. 2017. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost 15(11):2099–2114, PMID: 28846826, 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Rieswijk L, Wang A, Chiu WA, Smith MT. 2018a. Key characteristics approach to carcinogenic hazard identification. Chem Res Toxicol 31(12):1290–1292, PMID: 30521319, 10.1021/acs.chemrestox.8b00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton KZ, Rusyn I, Chiu WA, Corpet DE, van den Berg M, Ross MK, et al. . 2018b. Application of the key characteristics of carcinogens in cancer hazard identification. Carcinogenesis 39(4):614–622, PMID: 29562322, 10.1093/carcin/bgy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M-H, Lee D-H, Jacobs DR. 2007. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environ Health Perspect 115(8):1204–1209, PMID: 17687448, 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadei M, Naddafi K. 2020. Cardiovascular effects of airborne particulate matter: a review of rodent model studies. Chemosphere 242:125204, PMID: 31675579, 10.1016/j.chemosphere.2019.125204. [DOI] [PubMed] [Google Scholar]
- Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, et al. . 2015. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA). Epidemiology 26(3):310–320, PMID: 25710246, 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Hong Y-C. 2016. Bisphenol A, hypertension, and cardiovascular diseases: epidemiological, laboratory, and clinical trial evidence. Curr Hypertens Rep 18(2):11, PMID: 26781251, 10.1007/s11906-015-0617-2. [DOI] [PubMed] [Google Scholar]
- Han SG, Han S-S, Toborek M, Hennig B. 2012. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol 261(2):181–188, PMID: 22521609, 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CS, Sheykhzade M, Møller P, Folkmann JK, Amtorp O, Jonassen T, et al. . 2007. Diesel exhaust particles induce endothelial dysfunction in apoE-/- mice. Toxicol Appl Pharmacol 219(1):24–32, PMID: 17234226, 10.1016/j.taap.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Hansson GK. 2017. Inflammation and atherosclerosis: the end of a controversy. Circulation 136(20):1875–1877, PMID: 28916641, 10.1161/CIRCULATIONAHA.117.030484. [DOI] [PubMed] [Google Scholar]
- Hara T, Sakuma M, Fujie T, Kaji T, Yamamoto C. 2021. Cadmium induces plasminogen activator inhibitor-1 via Smad2/3 signaling pathway in human endothelial EA.hy926 cells. J Toxicol Sci 46(5):249–253, PMID: 33952801, 10.2131/jts.46.249. [DOI] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. . 2011. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect 119(7):951–957, PMID: 21377951, 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helyar SG, Patel B, Headington K, El Assal M, Chatterjee PK, Pacher P, et al. . 2009. PCB-induced endothelial cell dysfunction: role of poly(ADP-ribose) polymerase. Biochem Pharmacol 78(8):959–965, PMID: 19549508, 10.1016/j.bcp.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, et al. . 2002. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol 181(3):174–183, PMID: 12079426, 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Henning RJ, Johnson GT, Coyle JP, Harbison RD. 2017. Acrolein can cause cardiovascular disease: a review. Cardiovasc Toxicol 17(3):227–236, PMID: 28084565, 10.1007/s12012-016-9396-5. [DOI] [PubMed] [Google Scholar]
- Henriksen PA. 2018. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart 104(12):971–977, PMID: 29217634, 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- Herrmann J. 2020. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 17(8):474–502, PMID: 32231332, 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Jiao J, Zhuang P, Chen X, Wang J, Zhang Y. 2018. Serum polyfluoroalkyl chemicals are associated with risk of cardiovascular diseases in national US population. Environ Int 119:37–46, PMID: 29933236, 10.1016/j.envint.2018.05.051. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2019. Preamble. IARC monographs on the evaluation of carcinogenic risks to humans. Lyons, France. https://monographs.iarc.who.int/wp-content/uploads/2019/07/Preamble-2019.pdf [accessed 9 September 2021]. [Google Scholar]
- Jain D, Russell RR, Schwartz RG, Panjrath GS, Aronow W. 2017. Cardiac complications of cancer therapy: pathophysiology, identification, prevention, treatment, and future directions. Curr Cardiol Rep 19(5):36, PMID: 28374177, 10.1007/s11886-017-0846-x. [DOI] [PubMed] [Google Scholar]
- Jain V, Ghosh R, Gupta M, Saijo Y, Bansal A, Farwati M, et al. . 2021. Contemporary narrative review on left atrial strain mechanics in echocardiography: cardiomyopathy, valvular heart disease and beyond. Cardiovasc Diagn Ther 11(3):924–938, PMID: 34295714, 10.21037/cdt-20-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen HT, Cooke PS, Porcelli J, Liu TC, Hansen LG. 1993. Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reprod Toxicol 7(3):237–248, PMID: 8318755, 10.1016/0890-6238(93)90230-5. [DOI] [PubMed] [Google Scholar]
- Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. 2015. Mitochondrial functional impairment in response to environmental toxins in the cardiorenal metabolic syndrome. Arch Toxicol 89(2):147–153, PMID: 25559775, 10.1007/s00204-014-1431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen MP, Lieuallen WG, Johnson CL, Dunnick J, Nyska A. 2005. Characterization of spontaneous and chemically induced cardiac lesions in rodent model systems: the National Toxicology Program experience. Cardiovasc Toxicol 5(2):227–244, PMID: 16046796, 10.1385/ct:5:2:227. [DOI] [PubMed] [Google Scholar]
- Joly BS, Coppo P, Veyradier A. 2017. Thrombotic thrombocytopenic purpura. Blood 129(21):2836–2846, PMID: 28416507, 10.1182/blood-2016-10-709857. [DOI] [PubMed] [Google Scholar]
- Karoui A, Crochemore C, Harouki N, Corbière C, Preterre D, Vendeville C, et al. . 2020. Nitrogen dioxide inhalation exposures induce cardiac mitochondrial reactive oxygen species production, impair mitochondrial function and promote coronary endothelial dysfunction. Int J Environ Res Public Health 17(15):5526, PMID: 32751709, 10.3390/ijerph17155526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasneci A, Lee JS, Yun TJ, Shang J, Lampen S, Gomolin T, et al. . 2017. From the cover: lifelong exposure of C57bl/6n male mice to bisphenol A or bisphenol S reduces recovery from a myocardial infarction. Toxicol Sci 159(1):189–202, PMID: 28903498, 10.1093/toxsci/kfx133. [DOI] [PubMed] [Google Scholar]
- Kataria A, Levine D, Wertenteil S, Vento S, Xue J, Rajendiran K, et al. . 2017. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr Res 81(6):857–864, PMID: 28099427, 10.1038/pr.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Bolger AF, Herrington D, Giugliano RP, Eckel RH. 2010. Thiazolidinedione drugs and cardiovascular risks: a science advisory from the American Heart Association and American College of Cardiology Foundation. Circulation 121(16):1868–1877, PMID: 20179252, 10.1161/CIR.0b013e3181d34114. [DOI] [PubMed] [Google Scholar]
- Khan NG, Correia J, Adiga D, Rai PS, Dsouza HS, Chakrabarty S, et al. . 2021. A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence. Environ Sci Pollut Res Int 28(16):19643–19663, PMID: 33666848, 10.1007/s11356-021-13071-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiełtucki J, Dobrakowski M, Pawlas N, Średniawa B, Boroń M, Kasperczyk S. 2017. The analysis of QT interval and repolarization morphology of the heart in chronic exposure to lead. Hum Exp Toxicol 36(10):1081–1086, PMID: 27903879, 10.1177/0960327116680277. [DOI] [PubMed] [Google Scholar]
- Kim J, Hoang T, Bu SY, Kim J-M, Choi J-H, Park E, et al. . 2020. Associations of dietary intake with cardiovascular disease, blood pressure, and lipid profile in the Korean population: a systematic review and meta-analysis. J Lipid Atheroscler 9(1):205–229, PMID: 32821732, 10.12997/jla.2020.9.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Moon MK, Kang GH, Lee KJ, Choi SH, Lim S, et al. . 2014. Chronic exposure to bisphenol A can accelerate atherosclerosis in high-fat-fed apolipoprotein E knockout mice. Cardiovasc Toxicol 14(2):120–128, PMID: 24234673, 10.1007/s12012-013-9235-x. [DOI] [PubMed] [Google Scholar]
- Kirrane EF, Luben TJ, Benson A, Owens EO, Sacks JD, Dutton SJ, et al. . 2019. A systematic review of cardiovascular responses associated with ambient black carbon and fine particulate matter. Environ Int 127:305–316, PMID: 30953813, 10.1016/j.envint.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox M, Vinet R, Fuentes L, Morales B, Martínez JL. 2019. A review of endothelium-dependent and -independent vasodilation induced by phytochemicals in isolated rat aorta. Animals (Basel) 9(9):623, PMID: 31470540, 10.3390/ani9090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koklesova L, Samec M, Liskova A, Zhai K, Büsselberg D, Giordano FA, et al. . 2021. Mitochondrial impairments in aetiopathology of multifactorial diseases: common origin but individual outcomes in context of 3P medicine. EPMA J 12(1):1–14, PMID: 33686350, 10.1007/s13167-021-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong P, Christia P, Frangogiannis NG. 2014. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71(4):549–574, PMID: 23649149, 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraugerud M, Zimmer KE, Dahl E, Berg V, Olsaker I, Farstad W, et al. . 2010. Three structurally different polychlorinated biphenyl congeners (PCB 118, 153, and 126) affect hormone production and gene expression in the human H295R in vitro model. J Toxicol Environ Health A 73(16):1122–1132, PMID: 20574914, 10.1080/15287394.2010.484338. [DOI] [PubMed] [Google Scholar]
- Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, et al. . 2012. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution). J Am Coll Cardiol 60(21):2158–2166, PMID: 23103035, 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Siddiqi NJ, Alrashood ST, Khan HA, Dubey A, Sharma B. 2021. Protective effect of eugenol on hepatic inflammation and oxidative stress induced by cadmium in male rats. Biomed Pharmacother 139:111588, PMID: 33862491, 10.1016/j.biopha.2021.111588. [DOI] [PubMed] [Google Scholar]
- Kumar J, Lind PM, Salihovic S, van Bavel B, Ingelsson E, Lind L. 2014a. Persistent organic pollutants and inflammatory markers in a cross-sectional study of elderly Swedish people: the PIVUS cohort. Environ Health Perspect 122(9):977–983, PMID: 24911359, 10.1289/ehp.1307613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Lind PM, Salihovic S, van Bavel B, Lind L, Ingelsson E. 2014b. Influence of persistent organic pollutants on oxidative stress in population-based samples. Chemosphere 114:303–309, PMID: 25113216, 10.1016/j.chemosphere.2014.05.013. [DOI] [PubMed] [Google Scholar]
- La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, et al. . 2020. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol 16(1):45–57, PMID: 31719706, 10.1038/s41574-019-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Låg M, Øvrevik J, Refsnes M, Holme JA. 2020. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Respir Res 21(1):299, PMID: 33187512, 10.1186/s12931-020-01563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas GA, Ujueta F, Navas-Acien A. 2021. Lead and cadmium as cardiovascular risk factors: the burden of proof has been met. J Am Heart Assoc 10(10):e018692, PMID: 33942628, 10.1161/JAHA.120.018692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. . 2008. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 300(11):1303–1310, PMID: 18799442, 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Baan R, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. . 2016. Use of mechanistic data in the IARC evaluations of the carcinogenicity of polychlorinated biphenyls and related compounds. Environ Sci Pollut Res Int 23(3):2220–2229, PMID: 26077316, 10.1007/s11356-015-4829-4. [DOI] [PubMed] [Google Scholar]
- Laverty HG, Benson C, Cartwright EJ, Cross M, Garland C, Hammond T, et al. . 2011. How can we improve our understanding of cardiovascular safety liabilities to develop safer medicines? Br J Pharmacol 163(4):675–693, PMID: 21306581, 10.1111/j.1476-5381.2011.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer WJ.2017. Cardiac electrophysiology and the electrocardiogram. In: Medical Physiology. 3rd ed. Boron WF, Boulpaep EL, eds. Philadelphia, PA: Elsevier, 483–506. [Google Scholar]
- Lee D-H, Lind PM, Jacobs DR Jr, Salihovic S, van Bavel B, Lind L. 2012. Background exposure to persistent organic pollutants predicts stroke in the elderly. Environ Int 47:115–120, PMID: 22809777, 10.1016/j.envint.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Lee M-S, Eum K-D, Fang SC, Rodrigues EG, Modest GA, Christiani DC. 2014. Oxidative stress and systemic inflammation as modifiers of cardiac autonomic responses to particulate air pollution. Int J Cardiol 176(1):166–170, PMID: 25074558, 10.1016/j.ijcard.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewek J, Kaczmarek K, Cygankiewicz I, Wranicz JK, Ptaszynski P. 2014. Inflammation and arrhythmias: potential mechanisms and clinical implications. Expert Rev Cardiovasc Ther 12(9):1077–1085, PMID: 25060800, 10.1586/14779072.2014.942286. [DOI] [PubMed] [Google Scholar]
- Li D-L, Huang Y-J, Gao S, Chen L-Q, Zhang M-L, Du Z-Y. 2019. Sex-specific alterations of lipid metabolism in zebrafish exposed to polychlorinated biphenyls. Chemosphere 221:768–777, PMID: 30684774, 10.1016/j.chemosphere.2019.01.094. [DOI] [PubMed] [Google Scholar]
- Li L-A, Lin T-CE. 2007. Interacting influence of potassium and polychlorinated biphenyl on cortisol and aldosterone biosynthesis. Toxicol Appl Pharmacol 220(3):252–261, PMID: 17350062, 10.1016/j.taap.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Li M-C, Chen P-C, Tsai P-C, Furue M, Onozuka D, Hagihara A, et al. . 2015. Mortality after exposure to polychlorinated biphenyls and polychlorinated dibenzofurans: a meta-analysis of two highly exposed cohorts. Int J Cancer 137(6):1427–1432, PMID: 25754105, 10.1002/ijc.29504. [DOI] [PubMed] [Google Scholar]
- Li R, Wang Y, Chen R, Gu W, Zhang L, Gu J, et al. . 2020. Ambient fine particulate matter disrupts hepatic circadian oscillation and lipid metabolism in a mouse model. Environ Pollut 262:114179, PMID: 32145476, 10.1016/j.envpol.2020.114179. [DOI] [PubMed] [Google Scholar]
- Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, et al. . 2016. Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham Heart Study. J Am Heart Assoc 5(5):e002742, PMID: 27126478, 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian EC-Y. 2005. Pathogenesis of thrombotic thrombocytopenic purpura: ADAMTS13 deficiency and beyond. Semin Thromb Hemost 31(6):625–632, PMID: 16388413, 10.1055/s-2005-925468. [DOI] [PubMed] [Google Scholar]
- Liang Q, Gao X, Chen Y, Hong K, Wang H-S. 2014. Cellular mechanism of the nonmonotonic dose response of bisphenol A in rat cardiac myocytes. Environ Health Perspect 122(6):601–608, PMID: 24569941, 10.1289/ehp.1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Zhao T, Hu H, Shi Y, Xu Q, Miller MR, et al. . 2019. Repeat dose exposure of PM2.5 triggers the disseminated intravascular coagulation (DIC) in SD rats. Sci Total Environ 663:245–253, PMID: 30711591, 10.1016/j.scitotenv.2019.01.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. 2002. Inflammation in atherosclerosis. Nature 420(6917):868–874, PMID: 12490960, 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Lin T-C, Chien S-C, Hsu P-C, Li L-A. 2006. Mechanistic study of polychlorinated biphenyl 126-induced CYP11B1 and CYP11B2 up-regulation. Endocrinology 147(3):1536–1544, PMID: 16396990, 10.1210/en.2005-0823. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ramanathan G, Zhu Y, Yin F, Rea ND, Lu X, et al. . 2019. Pro-oxidative and proinflammatory effects after traveling from Los Angeles to Beijing: a biomarker-based natural experiment. Circulation 140(24):1995–2004, PMID: 31744317, 10.1161/CIRCULATIONAHA.119.042054. [DOI] [PubMed] [Google Scholar]
- Lind L. 2006. Impact of ageing on the measurement of endothelium-dependent vasodilation. Pharmacol Rep 58 (suppl):41–46, PMID: 17332670. [PubMed] [Google Scholar]
- Lind L. 2019. A detailed lipoprotein profile in relation to intima-media thickness and echogenicity of three major arteries. Clin Physiol Funct Imaging 39(6):415–421, PMID: 31529768, 10.1111/cpf.12594. [DOI] [PubMed] [Google Scholar]
- Lind L, Lind PM. 2012. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? J Intern Med 271(6):537–553, PMID: 22372998, 10.1111/j.1365-2796.2012.02536.x. [DOI] [PubMed] [Google Scholar]
- Lind PM, Lind L. 2011. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 218(1):207–213, PMID: 21621210, 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Lind PM, Orberg J, Edlund U-B, Sjöblom L, Lind L. 2004. The dioxin-like pollutant PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol Lett 150(3):293–299, PMID: 15110081, 10.1016/j.toxlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Lind PM, Penell J, Salihovic S, van Bavel B, Lind L. 2014. Circulating levels of p,p′-DDE are related to prevalent hypertension in the elderly. Environ Res 129:27–31, PMID: 24528999, 10.1016/j.envres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Lind PM, Salihovic S, Stubleski J, Kärrman A, Lind L. 2019. Association of exposure to persistent organic pollutants with mortality risk: an analysis of data from the Prospective Investigation of Vasculature in Uppsala Seniors (PIVUS) study. JAMA Netw Open 2(4):e193070, PMID: 31026035, 10.1001/jamanetworkopen.2019.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Salihovic S, van Bavel B, Lind L. 2017. Circulating levels of perfluoroalkyl substances (PFASs) and carotid artery atherosclerosis. Environ Res 152:157–164, PMID: 27771570, 10.1016/j.envres.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Lind L, Siegbahn A, Ingelsson E, Sundström J, Arnlöv J. 2011. A detailed cardiovascular characterization of obesity without the metabolic syndrome. Arterioscler Thromb Vasc Biol 31:e27–34, PMID: 21546604, 10.1161/ATVBAHA.110.221572. [DOI] [PubMed] [Google Scholar]
- Lind PM, van Bavel B, Salihovic S, Lind L. 2012. Circulating levels of persistent organic pollutants (POPs) and carotid atherosclerosis in the elderly. Environ Health Perspect 120(1):38–43, PMID: 22222676, 10.1289/ehp.1103563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. 2018. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314(4):H733–H752, PMID: 29351456, 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang J, Guan L, Zhu Y, Geng X. 2020. Filtered air intervention reduces inflammation and hypothalamus-pituitary-adrenal axis activation in adult male and female rats after PM 2.5 exposure. Environ Sci Pollut Res 27(28):35341–35348, PMID: 32592061, 10.1007/s11356-020-09564-9. [DOI] [PubMed] [Google Scholar]
- Liu Q, Gu X, Deng F, Mu L, Baccarelli AA, Guo X, et al. . 2019. Ambient particulate air pollution and circulating C-reactive protein level: a systematic review and meta-analysis. Int J Hyg Environ Health 222(5):756–764, PMID: 31103472, 10.1016/j.ijheh.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Long Y, Huang C, Wu J, Cheng J-N, Liang G-N, Jiang C-X, et al. . 2017. 2,3’,4,4’,5-Pentachlorobiphenyl impairs insulin-induced NO production partly through excessive ROS production in endothelial cells. Toxicol Mech Methods 27(8):592–597, PMID: 28592194, 10.1080/15376516.2017.1337259. [DOI] [PubMed] [Google Scholar]
- Long Y, Liu X, Tan X-Z, Jiang C-X, Chen S-W, Liang G-N, et al. . 2020. ROS-induced NLRP3 inflammasome priming and activation mediate PCB 118-induced pyroptosis in endothelial cells. Ecotoxicol Environ Saf 189:109937, PMID: 31785945, 10.1016/j.ecoenv.2019.109937. [DOI] [PubMed] [Google Scholar]
- López-Miranda J, Pedro-Botet J. 2021. Therapeutic targets in the treatment of dyslipidaemias: from statins to PCSK9 inhibitors. Unmet needs. Clin Investig Arterioscler 33 (suppl 1):46–52, PMID: 33966813, 10.1016/j.arteri.2020.12.005. [DOI] [PubMed] [Google Scholar]
- Lorenzatti AJ, Toth PP. 2020. New perspectives on atherogenic dyslipidaemia and cardiovascular disease. Eur Cardiol 15:1–9, PMID: 32180834, 10.15420/ecr.2019.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderer U, Eskenazi B, Hauser R, Korach KS, McHale CM, Moran F, et al. . 2019. Proposed key characteristics of female reproductive toxicants as an approach for organizing and evaluating mechanistic data in hazard assessment. Environ Health Perspect 127(7):75001, PMID: 31322437, 10.1289/EHP4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Ren L, Jiang M, Chu Y. 2019. Anti-hypertensive efficacy of amlodipine dosing during morning versus evening: a meta-analysis. Rev Cardiovasc Med 20:91–98, PMID: 31345001, 10.31083/j.rcm.2019.02.31814. [DOI] [PubMed] [Google Scholar]
- Ma W, Wei S, Zhang B, Li W. 2020. Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Front Cell Dev Biol 8:434, PMID: 32582710, 10.3389/fcell.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchia PE, Feingold KR, et al. 2000. Amiodarone induced thyrotoxicosis. In: Endotext. Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., eds. South Dartmouth, MA: MDText.com, Inc. [Google Scholar]
- Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. 2011. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol Appl Pharmacol 251(1):41–49, PMID: 21130106, 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder S, Muley A, Kolluru GK, Saurabh S, Tamilarasan KP, Chandrasekhar S, et al. . 2008. Cadmium reduces nitric oxide production by impairing phosphorylation of endothelial nitric oxide synthase. Biochem Cell Biol 86(1):1–10, PMID: 18364740, 10.1139/o07-146. [DOI] [PubMed] [Google Scholar]
- Makavos G, Ikonomidis I, Palios J, Rigopoulos A, Katogiannis K, Parissis J, et al. . 2021. Cardiac imaging in cardiotoxicity: a focus on clinical practice. Heart Fail Rev 26(5):1175–1187, PMID: 32306221, 10.1007/s10741-020-09952-w. [DOI] [PubMed] [Google Scholar]
- Mathew AV, Yu J, Guo Y, Byun J, Chen YE, Wang L, et al. . 2018. Effect of ambient fine particulate matter air pollution and colder outdoor temperatures on high-density lipoprotein function. Am J Cardiol 122(4):565–570, PMID: 30005891, 10.1016/j.amjcard.2018.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Veale EL, Golluscio A, Holden RG, Walsh Y. 2021. Pharmacological approaches to studying potassium channels. Handb Exp Pharmacol Preprint posted online 1 July 2021, PMID: 34195873, 10.1007/164_2021_502. [DOI] [PubMed] [Google Scholar]
- McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. 2017. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther 31(1):63–75, PMID: 28185035, 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinn LA, Schneider A, McGarrah RW, Ward-Caviness C, Neas LM, Di Q, et al. . 2019. Association of long-term PM2.5 exposure with traditional and novel lipid measures related to cardiovascular disease risk. Environ Int 122:193–200, PMID: 30446244, 10.1016/j.envint.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Altshul L, Hauser R. 2007. Serum PCBs, p,p′-DDE and HCB predict thyroid hormone levels in men. Environ Res 104(2):296–304, PMID: 17189629, 10.1016/j.envres.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele D, Nardozza M, Spallarossa P, Frassoldati A, Tocchetti CG, Cadeddu C, et al. . 2016. Current views on anthracycline cardiotoxicity. Heart Fail Rev 21(5):621–634, PMID: 27230651, 10.1007/s10741-016-9564-5. [DOI] [PubMed] [Google Scholar]
- Melzer D, Gates P, Osborne NJ, Henley WE, Cipelli R, Young A, et al. . 2012a. Urinary bisphenol A concentration and angiography-defined coronary artery stenosis. PLoS One 7(8):e43378, PMID: 22916252, 10.1371/journal.pone.0043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, et al. . 2012b. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125(12):1482–1490, PMID: 22354940, 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Koro CE. 2004. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res 70(1):1–17, PMID: 15246458, 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Michaela P, Mária K, Silvia H, Ľubica L. 2014. Bisphenol A differently inhibits CaV3.1, CaV3.2 and CaV3.3 calcium channels. Naunyn-Schmiedebergs Arch Pharmacol 387(2):153–163, PMID: 24170242, 10.1007/s00210-013-0932-6. [DOI] [PubMed] [Google Scholar]
- Milner B. 1989. Obituary. Professor O. L. Zangwill 1913–1987. Neuropsychologia 27(1):v–vi, PMID: 2651961, 10.1016/0028-3932(89)90083-3. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. 2004. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229, PMID: 15169927, 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Mohamad Kamal NS, Safuan S, Shamsuddin S, Foroozandeh P. 2020. Aging of the cells: insight into cellular senescence and detection methods. Eur J Cell Biol 99(6):151108, PMID: 32800277, 10.1016/j.ejcb.2020.151108. [DOI] [PubMed] [Google Scholar]
- Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, et al. . 2018. A dose–response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol 47(3):1013, PMID: 29697784, 10.1093/ije/dyy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Lázaro SL, Hernández-García E, Serrano-Flores B, Rosenbaum T. 2015. Organic toxins as tools to understand ion channel mechanisms and structure. Curr Top Med Chem 15(7):581–603, PMID: 25686735, 10.2174/1568026615666150217110710. [DOI] [PubMed] [Google Scholar]
- Mordukhovich I, Coull B, Kloog I, Koutrakis P, Vokonas P, Schwartz J. 2015. Exposure to sub-chronic and long-term particulate air pollution and heart rate variability in an elderly cohort: the Normative Aging Study. Environ Health 14(1):87, PMID: 26546332, 10.1186/s12940-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesian MA. 1999. Beta-adrenergic receptor agonists and cyclic nucleotide phosphodiesterase inhibitors: shifting the focus from inotropy to cyclic adenosine monophosphate. J Am Coll Cardiol 34(2):318–324, PMID: 10440139, 10.1016/s0735-1097(99)00220-x. [DOI] [PubMed] [Google Scholar]
- Mundi S, Massaro M, Scoditti E, Carluccio MA, van Hinsbergh VWM, Iruela-Arispe ML, et al. . 2018. Endothelial permeability, LDL deposition, and cardiovascular risk factors—a review. Cardiovasc Res 114(1):35–52, PMID: 29228169, 10.1093/cvr/cvx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murati T, Šimić B, Pleadin J, Vukmirović M, Miletić M, Durgo K, et al. . 2017. Reduced cytotoxicity in PCB-exposed Chinese Hamster Ovary (CHO) cells pretreated with vitamin E. Food Chem Toxicol 99:17–23, PMID: 27865896, 10.1016/j.fct.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A. 2021. Lead and cardiovascular mortality: Evidence supports lead as an independent cardiovascular risk factor. National Center for Environmental Economics (NCEE) working paper 21-03. Washington, DC: U.S. Environmental Protection Agency, NCEE. https://www.epa.gov/environmental-economics/lead-and-cardiovascular-mortality-evidence-supports-lead-independent [accessed 9 September 2021]. [Google Scholar]
- Nebigil CG, Désaubry L. 2018. Updates in anthracycline-mediated cardiotoxicity. Front Pharmacol 9:1262, PMID: 30483123, 10.3389/fphar.2018.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, et al. . 2015. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 36(2):83–93, PMID: 25492627, 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JD, Navas-Acien A, Kuo C-C, Guallar E, Howard BV, Fabsitz RR, et al. . 2016. Peripheral arterial disease and its association with arsenic exposure and metabolism in the Strong Heart Study. Am J Epidemiol 184(11):806–817, PMID: 27810857, 10.1093/aje/kww002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning R, Shi Y, Jiang J, Liang S, Xu Q, Duan J, et al. . 2020. Mitochondrial dysfunction drives persistent vascular fibrosis in rats after short-term exposure of PM2.5. Sci Total Environ 733:139135, PMID: 32438194, 10.1016/j.scitotenv.2020.139135. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. 2007. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356(24):2457–2471, PMID: 17517853, 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Niu B-Y, Li W-K, Li J-S, Hong Q-H, Khodahemmati S, Gao J-F, et al. . 2020. Effects of DNA damage and oxidative stress in human bronchial epithelial cells exposed to PM2.5 from Beijing, China, in winter. Int J Environ Res Public Health 17(13):4874, PMID: 32640694, 10.3390/ijerph17134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Chen R, Xia Y, Cai J, Ying Z, Lin Z, et al. . 2018. Fine particulate matter constituents and stress hormones in the hypothalamus–pituitary–adrenal axis. Environ Int 119:186–192, PMID: 29960262, 10.1016/j.envint.2018.06.027. [DOI] [PubMed] [Google Scholar]
- Nolte IM, Munoz ML, Tragante V, Amare AT, Jansen R, Vaez A, et al. . 2017. Genetic loci associated with heart rate variability and their effects on cardiac disease risk. Nat Commun 8(1):15805, PMID: 28613276, 10.1038/ncomms15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousiainen T, Vanninen E, Rantala A, Jantunen E, Hartikainen J. 1999. QT dispersion and late potentials during doxorubicin therapy for non-Hodgkin’s lymphoma. J Intern Med 245(4):359–364, PMID: 10356598, 10.1046/j.1365-2796.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. 2012. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52(6):1213–1225, PMID: 22465037, 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Olsén L, Lind L, Lind PM. 2012. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol Environ Saf 80:179–183, PMID: 22421452, 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Olson KR, Erdman AR, Woolf AD, Scharman EJ, Christianson G, Caravati EM, et al. . 2005. Calcium channel blocker ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 43(7):797–822, PMID: 16440509, 10.1080/15563650500357404. [DOI] [PubMed] [Google Scholar]
- Osataphan N, Phrommintikul A, Chattipakorn SC, Chattipakorn N. 2020. Effects of doxorubicin-induced cardiotoxicity on cardiac mitochondrial dynamics and mitochondrial function: insights for future interventions. J Cell Mol Med 24(12):6534–6557, PMID: 32336039, 10.1111/jcmm.15305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna PM, Udovcic M, Sharma MD. 2017. Hyperthyroidism and the heart. Methodist Debakey Cardiovasc J 13(2):60–63, PMID: 28740583, 10.14797/mdcj-13-2-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C, Dagda R, Angermann J. 2017. Antioxidants protect against arsenic induced mitochondrial cardio-toxicity. Toxics 5(4):38, PMID: 29206204, 10.3390/toxics5040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RL II, O’Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, et al. . 2016. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation 134(6):e32–e69, PMID: 27400984, 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- Park SK, Auchincloss AH, O’Neill MS, Prineas R, Correa JC, Keeler J, et al. . 2010. Particulate air pollution, metabolic syndrome, and heart rate variability: the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 118(10):1406–1411, PMID: 20529761, 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel BB, Raad M, Sebag IA, Chalifour LE. 2015. Sex-specific cardiovascular responses to control or high fat diet feeding in C57bl/6 mice chronically exposed to bisphenol A. Toxicol Rep 2:1310–1318, PMID: 28962473, 10.1016/j.toxrep.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier-Galarneau M, Detmer FJ, Petibon Y, Normandin M, Ma C, Alpert NM, et al. . 2021. Quantification of myocardial mitochondrial membrane potential using PET. Curr Cardiol Rep 23(6):70, PMID: 33970353, 10.1007/s11886-021-01500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penell JC, Kushnir MM, Lind L, Bergquist J, Bergquist J, Lind PM, et al. . 2021. Concentrations of nine endogenous steroid hormones in 70-year-old men and women. Endocr Connect 10(5):511–520, PMID: 33878730, 10.1530/EC-21-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penell J, Lind L, Salihovic S, van Bavel B, Lind PM. 2014. Persistent organic pollutants are related to the change in circulating lipid levels during a 5 year follow-up. Environ Res 134:190–197, PMID: 25173051, 10.1016/j.envres.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Perez CM, Hazari MS, Farraj AK. 2015. Role of autonomic reflex arcs in cardiovascular responses to air pollution exposure. Cardiovasc Toxicol 15(1):69–78, PMID: 25123706, 10.1007/s12012-014-9272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins JT, Petriello MC, Newsome BJ, Hennig B. 2016. Polychlorinated biphenyls and links to cardiovascular disease. Environ Sci Pollut Res Int 23(3):2160–2172, PMID: 25877901, 10.1007/s11356-015-4479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Hampel R, Cyrys J, Breitner S, Geruschkat U, Kraus U, et al. . 2015. Elevated particle number concentrations induce immediate changes in heart rate variability: a panel study in individuals with impaired glucose metabolism or diabetes. Part Fibre Toxicol 12(1):7, PMID: 25888845, 10.1186/s12989-015-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petriello MC, Brandon JA, Hoffman J, Wang C, Tripathi H, Abdel-Latif A, et al. . 2018. Dioxin-like PCB 126 increases systemic inflammation and accelerates atherosclerosis in lean LDL receptor-deficient mice. Toxicol Sci 162(2):548–558, PMID: 29216392, 10.1093/toxsci/kfx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi J, Kumagai Y, Sun G, Yamauchi H, Yoshida T, Iso H, et al. . 2000. Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in Inner Mongolia. Free Radic Biol Med 28(7):1137–1142, PMID: 10832076, 10.1016/S0891-5849(00)00209-4. [DOI] [PubMed] [Google Scholar]
- Pichler G, Grau-Perez M, Tellez-Plaza M, Umans J, Best L, Cole S, et al. . 2019. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults. Circ Cardiovasc Imaging 12(5):e009018, PMID: 31060373, 10.1161/CIRCIMAGING.119.009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters N, Plusquin M, Cox B, Kicinski M, Vangronsveld J, Nawrot TS. 2012. An epidemiological appraisal of the association between heart rate variability and particulate air pollution: a meta-analysis. Heart 98(15):1127–1135, PMID: 22628541, 10.1136/heartjnl-2011-301505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro Júnior JEG, Moraes PZ, Rodriguez MD, Simões MR, Cibin F, Pinton S, et al. . 2020. Cadmium exposure activates NADPH oxidase, renin–angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage. Toxicol Lett 333:80–89, PMID: 32738273, 10.1016/j.toxlet.2020.07.027. [DOI] [PubMed] [Google Scholar]
- Pope CA III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. 2016. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119(11):1204–1214, PMID: 27780829, 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnack NG, Brooks D, Chandra A, Jaimes R, Sarvazyan N, Kay M. 2015. Physiological response of cardiac tissue to bisphenol A: alterations in ventricular pressure and contractility. Am J Physiol Heart Circ Physiol 309(2):H267–H275, PMID: 25980024, 10.1152/ajpheart.00272.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnack NG, Jaimes R III, Asfour H, Swift LM, Wengrowski AM, Sarvazyan N, et al. . 2014. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ Health Perspect 122(4):384–390, PMID: 24487307, 10.1289/ehp.1206157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathumsap N, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. 2020. Effects of doxorubicin on the heart: from molecular mechanisms to intervention strategies. Eur J Pharmacol 866:172818, PMID: 31758940, 10.1016/j.ejphar.2019.172818. [DOI] [PubMed] [Google Scholar]
- Ramadan M, Cooper B, Posnack NG. 2020. Bisphenols and phthalates: plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res 112(17):1362–1385, PMID: 32691967, 10.1002/bdr2.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan M, Sherman M, Jaimes R III, Chaluvadi A, Swift L, Posnack NG. 2018. Disruption of neonatal cardiomyocyte physiology following exposure to bisphenol-A. Sci Rep 8(1):7356, PMID: 29743542, 10.1038/s41598-018-25719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancière F, Lyons JG, Loh VHY, Botton J, Galloway T, Wang T, et al. . 2015. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health 14(1):46, PMID: 26026606, 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasdi Z, Kamaludin R, Ab Rahim S, Syed Ahmad Fuad SB, Othman MHD, Siran R, et al. . 2020. The impacts of intrauterine bisphenol A exposure on pregnancy and expression of miRNAs related to heart development and diseases in animal model. Sci Rep 10(1):5882, PMID: 32246001, 10.1038/s41598-020-62420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PKV, Ng TMH, Oh EE, Moady G, Elkayam U. 2020. Clinical characteristics and management of methamphetamine-associated cardiomyopathy: state-of-the-art review. J Am Heart Assoc 9(11):e016704, PMID: 32468897, 10.1161/JAHA.120.016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman R, Hai O. 2021. Digitalis toxicity. In: StatPearls. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Reid T-E, Kumar K, Wang XS. 2013. Predictive in silico studies of human 5-hydroxytryptamine receptor subtype 2B (5-HT2b) and valvular heart disease. Curr Top Med Chem 13(11):1353–1362, PMID: 23675941, 10.2174/15680266113139990039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Angulo I, De Vizcaya-Ruiz A, Camacho J. 2010. Ion channels in toxicology. J Appl Toxicol 30(6):497–512, PMID: 20583319, 10.1002/jat.1556. [DOI] [PubMed] [Google Scholar]
- Rider CV, McHale CM, Webster TF, Lowe L, Goodson WH III, La Merrill MA, et al. . 2021. Using the key characteristics of carcinogens to develop research on chemical mixtures and cancer. Environ Health Perspect 129(3):35003, PMID: 33784186, 10.1289/EHP8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, et al. . 2004. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med 169(8):934–940, PMID: 14962820, 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Robertson S, Miller MR. 2018. Ambient air pollution and thrombosis. Part Fibre Toxicol 15(1):1, PMID: 29298690, 10.1186/s12989-017-0237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM. 2019. A current understanding of drug-induced QT prolongation and its implications for anticancer therapy. Cardiovasc Res 115(5):895–903, PMID: 30689740, 10.1093/cvr/cvz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan VD, Assali M, Moghaddam AH, Hosseinzadeh M, Myers J. 2011. Exercise training and antioxidants: effects on rat heart tissue exposed to lead acetate. Int J Toxicol 30(2):190–196, PMID: 21378372, 10.1177/1091581810392809. [DOI] [PubMed] [Google Scholar]
- Roth BL. 2007. Drugs and valvular heart disease. N Engl J Med 356(1):6–9, PMID: 17202450, 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. . 2000. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 102(23):2836–2841, PMID: 11104741, 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, et al. . 2010. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 123(2–3):225–233, PMID: 20692814, 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihovic S, Lampa E, Lindström G, Lind L, Lind PM, van Bavel B. 2012. Circulating levels of persistent organic pollutants (POPs) among elderly men and women from Sweden: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Environ Int 44:59–67, PMID: 22361238, 10.1016/j.envint.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Saluja T, Davies A, Oldmeadow C, Boyle A. 2021. Impact of fast food outlet density on incidence of myocardial infarction in the Hunter region. Intern Med J 51(2):243–248, PMID: 31908114, 10.1111/imj.14745. [DOI] [PubMed] [Google Scholar]
- Samet JM, Chiu WA, Cogliano V, Jinot J, Kriebel D, Lunn RM, et al. . 2020. The IARC Monographs: updated procedures for modern and transparent evidence synthesis in cancer hazard identification. J Natl Cancer Inst 112(1):30–37, PMID: 31498409, 10.1093/jnci/djz169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. 2006. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol 77(4):422–432, PMID: 16500718, 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Schnitzler JG, Celis N, Klaren PHM, Blust R, Dirtu AC, Covaci A, et al. . 2011. Thyroid dysfunction in sea bass (Dicentrarchus labrax): underlying mechanisms and effects of polychlorinated biphenyls on thyroid hormone physiology and metabolism. Aquat Toxicol 105(3–4):438–447, PMID: 21872555, 10.1016/j.aquatox.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Schwarze PE, Øvrevik J, Hetland RB, Becher R, Cassee FR, Låg M, et al. . 2007. Importance of size and composition of particles for effects on cells in vitro. Inhal Toxicol 19(suppl 1):17–22, PMID: 17886045, 10.1080/08958370701490445. [DOI] [PubMed] [Google Scholar]
- Shan K, Lincoff AM, Young JB. 1996. Anthracycline-induced cardiotoxicity. Ann Intern Med 125(1):47–58, PMID: 8644988, 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- Shan Q, Chen N, Liu W, Qu F, Chen A. 2020. Exposure to 2,3,3′,4,4′,5-hexachlorobiphenyl promotes nonalcoholic fatty liver disease development in C57BL/6 mice. Environ Pollut 263(pt A):114563, PMID: 32304952, 10.1016/j.envpol.2020.114563. [DOI] [PubMed] [Google Scholar]
- Shang J, Corriveau J, Champoux-Jenane A, Gagnon J, Moss E, Dumas P, et al. . 2019. Recovery from a myocardial infarction is impaired in male C57bl/6 N mice acutely exposed to the bisphenols and phthalates that escape from medical devices used in cardiac surgery. Toxicol Sci 168(1):78–94, PMID: 30398665, 10.1093/toxsci/kfy276. [DOI] [PubMed] [Google Scholar]
- Shankar A, Teppala S, Sabanayagam C. 2012. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect 120(9):1297–1300, PMID: 22645278, 10.1289/ehp.1104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MJ, Zipes DP. 2014. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 114(6):1004–1021, PMID: 24625726, 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- Shvachiy L, Geraldes V, Amaro-Leal A, Rocha I. 2020. Persistent effects on cardiorespiratory and nervous systems induced by long-term lead exposure: results from a longitudinal study. Neurotox Res 37(4):857–870, PMID: 31997153, 10.1007/s12640-020-00162-8. [DOI] [PubMed] [Google Scholar]
- Sigg DC, Iaizzo PA, Xiao Y-F, He B, eds. 2010. Cardiac Electrophysiology Methods and Models. Boston, MA: Springer. [Google Scholar]
- Silva MASC, de Oliveira TF, Almenara CCP, Broseghini-Filho GB, Vassallo DV, Padilha AS, et al. . 2015. Exposure to a low lead concentration impairs contractile machinery in rat cardiac muscle. Biol Trace Elem Res 167(2):280–287, PMID: 25795172, 10.1007/s12011-015-0300-0. [DOI] [PubMed] [Google Scholar]
- Simões MR, Preti SC, Azevedo BF, Fiorim J, Freire DD Jr, Covre EP, et al. . 2017. Low-level chronic lead exposure impairs neural control of blood pressure and heart rate in rats. Cardiovasc Toxicol 17(2):190–199, PMID: 27272938, 10.1007/s12012-016-9374-y. [DOI] [PubMed] [Google Scholar]
- Simões MR, Ribeiro Júnior RF, Vescovi MVA, de Jesus HC, Padilha AS, Stefanon I, et al. . 2011. Acute lead exposure increases arterial pressure: role of the renin-angiotensin system. PLoS One 6(4):e18730, PMID: 21494558, 10.1371/journal.pone.0018730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko O, Grimm FA, Ryan KR, Iwata Y, Chiu WA, Parham F, et al. . 2017. In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell-derived model. Toxicol Appl Pharmacol 322:60–74, PMID: 28259702, 10.1016/j.taap.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivapackiam J, Sharma M, Schindler TH, Sharma V. 2020. PET radiopharmaceuticals for imaging chemotherapy-induced cardiotoxicity. Curr Cardiol Rep 22(8):62, PMID: 32562004, 10.1007/s11886-020-01315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg Lind Y, Lind PM, Salihovic S, van Bavel B, Lind L. 2013. Circulating levels of persistent organic pollutants (POPs) are associated with left ventricular systolic and diastolic dysfunction in the elderly. Environ Res 123:39–45, PMID: 23562393, 10.1016/j.envres.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, et al. . 2016. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect 124(6):713–721, PMID: 26600562, 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, Guyton KZ, Kleinstreuer N, Borrel A, Cardenas A, Chiu WA, et al. . 2020. The key characteristics of carcinogens: relationship to the hallmarks of cancer, relevant biomarkers, and assays to measure them. Cancer Epidemiol Biomarkers Prev 29(10):1887–1903, PMID: 32152214, 10.1158/1055-9965.EPI-19-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Park J, Bui PTC, Choi K, Gye MC, Hong Y-C, et al. . 2017. Bisphenol A induces COX-2 through the mitogen-activated protein kinase pathway and is associated with levels of inflammation-related markers in elderly populations. Environ Res 158:490–498, PMID: 28709031, 10.1016/j.envres.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Sreenivasan J, Hooda U, Ranjan P, Jain D. 2021. Nuclear imaging for the assessment of cardiotoxicity from chemotherapeutic agents in oncologic disease. Curr Cardiol Rep 23(6):65, PMID: 33961140, 10.1007/s11886-021-01493-4. [DOI] [PubMed] [Google Scholar]
- States JC, Srivastava S, Chen Y, Barchowsky A. 2009. Arsenic and cardiovascular disease. Toxicol Sci 107(2):312–323, PMID: 19015167, 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea F, Bianchi F, Cori L, Sicari R. 2014. Cardiovascular effects of arsenic: clinical and epidemiological findings. Environ Sci Pollut Res Int 21(1):244–251, PMID: 24019140, 10.1007/s11356-013-2113-z. [DOI] [PubMed] [Google Scholar]
- Steffee CH, Singh HK, Chitwood WR. 1999. Histologic changes in three explanted native cardiac valves following use of fenfluramines. Cardiovasc Pathol 8(5):245–253, PMID: 10533956, 10.1016/s1054-8807(99)00019-8. [DOI] [PubMed] [Google Scholar]
- Steffensen I-L, Dirven H, Couderq S, David A, D’Cruz SC, Fernández MF, et al. . 2020. Bisphenols and oxidative stress biomarkers—associations found in human studies, evaluation of methods used, and strengths and weaknesses of the biomarkers. Int J Environ Res Public Health 17(10):3609, PMID: 32455625, 10.3390/ijerph17103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Clark KA, Ross MA, Chandra AG, Li S, Gao X, et al. . 2008. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J Clin Invest 118(12):3980–3989, PMID: 19033667, 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Klei LR, Stolz DB, Barchowsky A. 2009. Arsenic requires sphingosine-1-phosphate type 1 receptors to induce angiogenic genes and endothelial cell remodeling. Am J Pathol 174(5):1949–1958, PMID: 19349368, 10.2353/ajpath.2009.081016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss DG, Wu WW, Li Z, Koerner J, Garnett C. 2021. Translational models and tools to reduce clinical trials and improve regulatory decision making for QTc and proarrhythmia risk (ICH E14/S7B updates). Clin Pharmacol Ther 109(2):319–333, PMID: 33332579, 10.1002/cpt.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Ai N, Park S-H, Rios-Pilier J, Perkins JT, Welsh WJ, et al. . 2012. Bisphenol A and its analogues activate human pregnane X receptor. Environ Health Perspect 120(3):399–405, PMID: 22214767, 10.1289/ehp.1104426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Park S-H, Helsley RN, Sunkara M, Gonzalez FJ, Morris AJ, et al. . 2014. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J Am Heart Assoc 3(2):e000492, PMID: 24755147, 10.1161/JAHA.113.000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Park S-H, Wang F, Zhou C. 2018. Perinatal bisphenol A exposure increases atherosclerosis in adult male PXR-humanized mice. Endocrinology 159(4):1595–1608, PMID: 29425287, 10.1210/en.2017-03250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yue P, Kirk RI, Wang A, Moatti D, Jin X, et al. . 2008. Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal Toxicol 20(2):127–137, PMID: 18236227, 10.1080/08958370701821482. [DOI] [PubMed] [Google Scholar]
- Svobodová K, Cajthaml T. 2010. New in vitro reporter gene bioassays for screening of hormonal active compounds in the environment. Appl Microbiol Biotechnol 88(4):839–847, PMID: 20737269, 10.1007/s00253-010-2833-7. [DOI] [PubMed] [Google Scholar]
- Taggart C, Wereski R, Mills NL, Chapman AR. 2021. Diagnosis, investigation and management of patients with acute and chronic myocardial injury. J Clin Med 10:2331, PMID: 34073539, 10.3390/jcm10112331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L, Mergler D, Baldwin M, de Grosbois S, Smargiassi A, Lafond J. 2005. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environ Health Perspect 113(8):1039–1045, PMID: 16079076, 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Cheng J-N, Long Y, He X-M, Liang G-N, Tang X-P, et al. . 2017. PCB 118-induced endothelial cell apoptosis is partially mediated by excessive ROS production. Toxicol Mech Methods 27(5):394–399, PMID: 28399781, 10.1080/15376516.2017.1296050. [DOI] [PubMed] [Google Scholar]
- Tariq S, Aronow WS. 2015. Use of inotropic agents in treatment of systolic heart failure. Int J Mol Sci 16(12):29060–29068, PMID: 26690127, 10.3390/ijms161226147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavolari S, Bucci L, Tomasi V, Guarnieri T. 2006. Selected polychlorobiphenyls congeners bind to estrogen receptor alpha in human umbilical vascular endothelial (HUVE) cells modulating angiogenesis. Toxicology 218(1):67–74, PMID: 16293362, 10.1016/j.tox.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Smith RKW, Clegg PD. 2007. Mesenchymal stem cell therapy in equine musculoskeletal disease: scientific fact or clinical fiction? Equine Vet J 39(2):172–180, PMID: 17378447, 10.2746/042516407X180868. [DOI] [PubMed] [Google Scholar]
- Tejchman K, Kotfis K, Sieńko J. 2021. Biomarkers and mechanisms of oxidative stress—last 20 years of research with an emphasis on kidney damage and renal transplantation. Int J Mol Sci 22(15):8010, PMID: 34360776, 10.3390/ijms22158010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruya K, Sakurai H, Omae K, Higashi T, Muto T, Kaneko Y. 1991. Effect of lead on cardiac parasympathetic function. Int Arch Occup Environ Health 62(8):549–553, PMID: 1856009, 10.1007/BF00381107. [DOI] [PubMed] [Google Scholar]
- Thévenod F, Lee W-K. 2013. Cadmium and cellular signaling cascades: interactions between cell death and survival pathways. Arch Toxicol 87(10):1743–1786, PMID: 23982889, 10.1007/s00204-013-1110-9. [DOI] [PubMed] [Google Scholar]
- Thompson LC, Walsh L, Martin BL, McGee J, Wood C, Kovalcik K, et al. . 2019. Ambient particulate matter and acrolein co-exposure increases myocardial dyssynchrony in mice via TRPA1. Toxicol Sci 167(2):559–572, PMID: 30351402, 10.1093/toxsci/kfy262. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. . 2018. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72(18):2231–2264, PMID: 30153967, 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Kataoka K. 2003. A longitudinal analysis on the association of serum lipids and lipoproteins concentrations with blood polychlorinated biphenyls level in chronic “Yusho” patients. Fukuoka Igaku Zasshi 94(5):110–117, PMID: 12872711, 10.15017/18744. [DOI] [PubMed] [Google Scholar]
- Tong H. 2016. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta 1860(12):2891–2898, PMID: 27189803, 10.1016/j.bbagen.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Tremblay JC, Pyke KE. 2018. Flow-mediated dilation stimulated by sustained increases in shear stress: a useful tool for assessing endothelial function in humans? Am J Physiol Heart Circ Physiol 314(3):H508–H520, PMID: 29167121, 10.1152/ajpheart.00534.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay-Laganière C, Garneau L, Mauger J-F, Peshdary V, Atlas E, Nikolla AS, et al. . 2019. Polychlorinated biphenyl 126 exposure in rats alters skeletal muscle mitochondrial function. Environ Sci Pollut Res Int 26(3):2375–2386, PMID: 30467749, 10.1007/s11356-018-3738-8. [DOI] [PubMed] [Google Scholar]
- Tschöpe C, Van Linthout S, Kherad B. 2017. Heart failure with preserved ejection fraction and future pharmacological strategies: a glance in the crystal ball. Curr Cardiol Rep 19(8):70, PMID: 28656481, 10.1007/s11886-017-0874-6. [DOI] [PubMed] [Google Scholar]
- Tykocki NR, Boerman EM, Jackson WF. 2017. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr Physiol 7(2):485–581, PMID: 28333380, 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk DF, Stroes ESG, Kastelein JJP. 2009. Lipid measures and cardiovascular disease prediction. Dis Markers 26(5–6):209–216, PMID: 19773610, 10.3233/DMA-2009-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118(8):1055–1070, PMID: 20338858, 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Feletou M, Tang EHC. 2017. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol (Oxf) 219(1):22–96, PMID: 26706498, 10.1111/apha.12646. [DOI] [PubMed] [Google Scholar]
- Varga ZV, Ferdinandy P, Liaudet L, Pacher P. 2015. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol 309(9):H1453–H1467, PMID: 26386112, 10.1152/ajpheart.00554.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND. 2008. Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol Heart Circ Physiol 295(2):H454–H465, PMID: 18567711, 10.1152/ajpheart.00158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyradier A, Meyer D. 2005. Thrombotic thrombocytopenic purpura and its diagnosis. J Thromb Haemost 3(11):2420–2427, PMID: 15892859, 10.1111/j.1538-7836.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Möhlenkamp S, et al. . 2015. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med 72(9):656–663, PMID: 26163546, 10.1136/oemed-2014-102800. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Coady K, Escher BI, Mihaich E, Murphy CA, Schlekat T, et al. . 2019. High-throughput screening and environmental risk assessment: state of the science and emerging applications. Environ Toxicol Chem 38(1):12–26, PMID: 30570782, 10.1002/etc.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdis A, Taddei S. 2016. Endothelial dysfunction in resistance arteries of hypertensive humans: old and new conspirators. J Cardiovasc Pharmacol 67(6):451–457, PMID: 26808712, 10.1097/FJC.0000000000000362. [DOI] [PubMed] [Google Scholar]
- Vlachos K, Georgopoulos S, Efremidis M, Sideris A, Letsas KP. 2016. An update on risk factors for drug-induced arrhythmias. Expert Rev Clin Pharmacol 9(1):117–127, PMID: 26460585, 10.1586/17512433.2016.1100073. [DOI] [PubMed] [Google Scholar]
- Volmar KE, Hutchins GM. 2001. Aortic and mitral fenfluramine-phentermine valvulopathy in 64 patients treated with anorectic agents. Arch Pathol Lab Med 125(12):1555–1561, PMID: 11735689, 10.5858/2001-125-1555-AAMFPV. [DOI] [PubMed] [Google Scholar]
- Waghe P, Sarath TS, Gupta P, Kandasamy K, Choudhury S, Kutty HS, et al. . 2015. Arsenic causes aortic dysfunction and systemic hypertension in rats: augmentation of angiotensin II signaling. Chem Biol Interact 237:104–114, PMID: 26079204, 10.1016/j.cbi.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, et al. . 2013. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem 24(9):1587–1595, PMID: 23618531, 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlang B, Song M, Beier JI, Cameron Falkner K, Al-Eryani L, Clair HB, et al. . 2014. Evaluation of Aroclor 1260 exposure in a mouse model of diet-induced obesity and non-alcoholic fatty liver disease. Toxicol Appl Pharmacol 279(3):380–390, PMID: 24998970, 10.1016/j.taap.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach JD, Wang K, Zhang AD, Cheng D, Grossetta Nardini HK, Lin H, et al. . 2020. Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses. BMJ 368:l7078, PMID: 32024657, 10.1136/bmj.l7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CKC. 2012. PFOS-induced hepatic steatosis, the mechanistic actions on β-oxidation and lipid transport. Biochim Biophys Acta 1820(7):1092–1101, PMID: 22484034, 10.1016/j.bbagen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Wang C, Petriello MC, Zhu B, Hennig B. 2019a. PCB 126 induces monocyte/macrophage polarization and inflammation through AhR and NF-κB pathways. Toxicol Appl Pharmacol 367:71–81, PMID: 30768972, 10.1016/j.taap.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Kesteven SH, Huttner IG, Feneley MP, Fatkin D. 2018. High-frequency echocardiography—transformative clinical and research applications in humans, mice, and zebrafish. Circ J 82(3):620–628, PMID: 29415914, 10.1253/circj.CJ-18-0027. [DOI] [PubMed] [Google Scholar]
- Wang Y-X, Liu C, Shen Y, Wang Q, Pan A, Yang P, et al. . 2019b. Urinary levels of bisphenol A, F and S and markers of oxidative stress among healthy adult men: variability and association analysis. Environ Int 123:301–309, PMID: 30553203, 10.1016/j.envint.2018.11.071. [DOI] [PubMed] [Google Scholar]
- Warembourg C, Maitre L, Tamayo-Uria I, Fossati S, Roumeliotaki T, Aasvang GM, et al. . 2019. Early-life environmental exposures and blood pressure in children. J Am Coll Cardiol 74(10):1317–1328, PMID: 31488269, 10.1016/j.jacc.2019.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichenthal S, Crouse DL, Pinault L, Godri-Pollitt K, Lavigne E, Evans G, et al. . 2016. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Res 146:92–99, PMID: 26745732, 10.1016/j.envres.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Westermann B. 2010. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11(12):872–884, PMID: 21102612, 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- Weycker D, Hatfield M, Grossman A, Hanau A, Lonshteyn A, Sharma A, et al. . 2019. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer 19(1):151, PMID: 30764783, 10.1186/s12885-019-5354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2017. Cardiovascular diseases (CVDs). Fact sheet. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [accessed 9 September 2021].
- Widdicombe J, Lee LY. 2001. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect 109 (suppl 4):579–584, PMID: 11544167, 10.1289/ehp.01109s4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Molinaro P, Chen Y. 2014. Arsenic exposure and subclinical endpoints of cardiovascular diseases. Curr Environ Health Rep 1(2):148–162, PMID: 25013752, 10.1007/s40572-014-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Yu W, Meng F, Mi J, Peng J, Liu J, et al. . 2017. Polychlorinated biphenyls-153 induces metabolic dysfunction through activation of ROS/NF-κB signaling via downregulation of HNF1b. Redox Biol 12:300–310, PMID: 28285191, 10.1016/j.redox.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Du G, Zhang Y, Zhou F, Wu J, Jiao H, et al. . 2019. ECG conduction disturbances and ryanodine receptor expression levels in occupational lead exposure workers. Occup Environ Med 76(3):151–156, PMID: 30661027, 10.1136/oemed-2018-105463. [DOI] [PubMed] [Google Scholar]
- Xiong T-Y, Redwood S, Prendergast B, Chen M. 2020. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J 41(19):1798–1800, PMID: 32186331, 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Shu Y, Fu Z, Hu Y, Mo X. 2017. Associations between lead concentrations and cardiovascular risk factors in U.S. adolescents. Sci Rep 7(1):9121, PMID: 28831128, 10.1038/s41598-017-09701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Liu S, Huang M, Jiang X, Yang M. 2021. Cadmium induces apoptosis of human granulosa cell line KGN via mitochondrial dysfunction-mediated pathways. Ecotoxicol Environ Saf 220:112341, PMID: 34020281, 10.1016/j.ecoenv.2021.112341. [DOI] [PubMed] [Google Scholar]
- Xu L, Guo X, Li N, Pan Q, Ma YZ. 2019a. Effects of quercetin on Aroclor 1254-induced expression of CYP450 and cytokines in pregnant rats. J Immunotoxicol 16(1):140–148, PMID: 31290710, 10.1080/1547691X.2019.1604585. [DOI] [PubMed] [Google Scholar]
- Xu M-X, Ge C-X, Qin Y-T, Gu T-T, Lou D-S, Li Q, et al. . 2019b. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic Biol Med 130:542–556, PMID: 30465824, 10.1016/j.freeradbiomed.2018.11.016. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, et al. . 2011. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci 124(1):88–98, PMID: 21873646, 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Thomson JK, Zhao W, Gao X, Huang F, Chen B, et al. . 2018. Role of stress kinase JNK in binge alcohol-evoked atrial arrhythmia. J Am Coll Cardiol 71(13):1459–1470, PMID: 29598867, 10.1016/j.jacc.2018.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang H-S. 2011. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 6(9):e25455, PMID: 21980463, 10.1371/journal.pone.0025455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YJ, Hong Y-C, Oh S-Y, Park M-S, Kim H, Leem J-H, et al. . 2009. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ Res 109(6):797–801, PMID: 19464675, 10.1016/j.envres.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yang T, Liu S, Cao Z, Zhao Y, Su X, et al. . 2019. Concentrated ambient PM2.5 exposure affects mice sperm quality and testosterone biosynthesis. PeerJ 7:e8109, PMID: 31799077, 10.7717/peerj.8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Gupta R, Vergnes L, Driscoll WS, Ricks J, Ramanathan G, et al. . 2019. Diesel exhaust induces mitochondrial dysfunction, hyperlipidemia, and liver steatosis. Arterioscler Thromb Vasc Biol 39(9):1776–1786, PMID: 31340670, 10.1161/ATVBAHA.119.312736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Lawal A, Ricks J, Fox JR, Larson T, Navab M, et al. . 2013. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol 33(6):1153–1161, PMID: 23559632, 10.1161/ATVBAHA.112.300552. [DOI] [PubMed] [Google Scholar]
- Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, et al. . 2009. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci 111(1):80–88, PMID: 19182107, 10.1093/toxsci/kfp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W, Tong L, Liu X, Weng X, Chen X, Wang D, et al. . 2019. Short term PM2.5 exposure caused a robust lung inflammation, vascular remodeling, and exacerbated transition from left ventricular failure to right ventricular hypertrophy. Redox Biol 22:101161, PMID: 30861460, 10.1016/j.redox.2019.101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. . 2016. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37(36):2768–2801, PMID: 27567406, 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Luttmann-Gibson H, Horton ES, Cohen A, Coull BA, Hoffmann B, et al. . 2014. Brachial artery responses to ambient pollution, temperature, and humidity in people with type 2 diabetes: a repeated-measures study. Environ Health Perspect 122(3):242–248, PMID: 24398072, 10.1289/ehp.1206136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Niu Y, Xia Y, Lei X, Wang W, Huo J, et al. . 2020a. The acute effects of fine particulate matter constituents on circulating inflammatory biomarkers in healthy adults. Sci Total Environ 707:135989, PMID: 31874395, 10.1016/j.scitotenv.2019.135989. [DOI] [PubMed] [Google Scholar]
- Zhang Y-F, Shan C, Wang Y, Qian L-L, Jia D-D, Zhang Y-F, et al. . 2020b. Cardiovascular toxicity and mechanism of bisphenol A and emerging risk of bisphenol S. Sci Total Environ 723:137952, PMID: 32213405, 10.1016/j.scitotenv.2020.137952. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chan T-C, Guo C, Chang L-Y, Lin C, Chuang YC, et al. . 2018. Long-term exposure to ambient particulate matter (PM2.5) is associated with platelet counts in adults. Environ Pollut 240:432–439, PMID: 29753251, 10.1016/j.envpol.2018.04.123. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Chen H, Yang T, Rui W, Liu F, Zhang F, et al. . 2016. Direct effects of airborne PM2.5 exposure on macrophage polarizations. Biochim Biophys Acta 1860(12):2835–2843, PMID: 27041089, 10.1016/j.bbagen.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cao Z, Li H, Su X, Yang Y, Liu C, et al. . 2019. Air pollution exposure in association with maternal thyroid function during early pregnancy. J Hazard Mater 367:188–193, PMID: 30594719, 10.1016/j.jhazmat.2018.12.078. [DOI] [PubMed] [Google Scholar]
- Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X. 2020. COVID-19 and the cardiovascular system. Nat Rev Cardiol 17(5):259–260, PMID: 32139904, 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xu X, Zhang X, Wang A, Zhang C, Hüttemann M, et al. . 2013. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol 58(1):148–154, PMID: 22902548, 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JF, Yan XF, Guo FZ, Sun NY, Qian ZJ, Ding DY. 2000. Effects of cigarette smoking and smoking cessation on plasma constituents and enzyme activities related to oxidative stress. Biomed Environ Sci 13:44–55, PMID: 10853840. [PubMed] [Google Scholar]
- Zhou Z, Lu Y-H, Pi H-F, Gao P, Li M, Zhang L, et al. . 2016. Cadmium exposure is associated with the prevalence of dyslipidemia. Cell Physiol Biochem 40(3–4):633–643, PMID: 27898410, 10.1159/000452576. [DOI] [PubMed] [Google Scholar]