Many animals rely on taste to identify foods that meet their nutritional needs and to avoid the consumption of harmful substances. The taste of macronutrients, as well as of non-caloric micronutrients such as sodium and calcium, contribute to the regulation of ingestive behavior1,2. Whether vitamins also affect feeding behavior through taste is less clear. Here, we show that flies (Drosophila melanogaster) have a strong preference for consuming vitamin-containing diets. Both sexes show preference for folic acid, whereas only females show preference for riboflavin. Female consumption preference is achieved with vitamin concentrations as low as ~10 nM—at least 50,000-fold lower than the concentration needed for sucrose preference. In addition, female vitamin preference requires inputs from external and internal taste organs—suggesting that post-ingestive signals, in the absence of gustatory input, are insufficient to actuate preferential consumption of vitamin-containing diets. Our studies demonstrate that vitamin perception is an important determinant of feeding behavior.
We assessed consumption preference by quantifying accumulated radiolabel—a technique that facilitates increased throughput and power to resolve small feeding differences3—in flies presented with a choice between a chemically defined medium4 with or without the B-group vitamins (Figure 1A). Dietary vitamins did not affect radiolabel absorption efficiency (Figure S1A), which was used to estimate food intake. Females showed a preference over 24 hours for the vitamin-containing diet at all time points tested (Figure 1B), and this preference was maintained for at least 6 days (Figure S1B). Males also showed preference for vitamin-containing food at all time points tested up to 24 hours, except for the earliest (2 hours) (Figure 1C).
Figure 1. Flies show taste-dependent consumption preference for food containing vitamins.
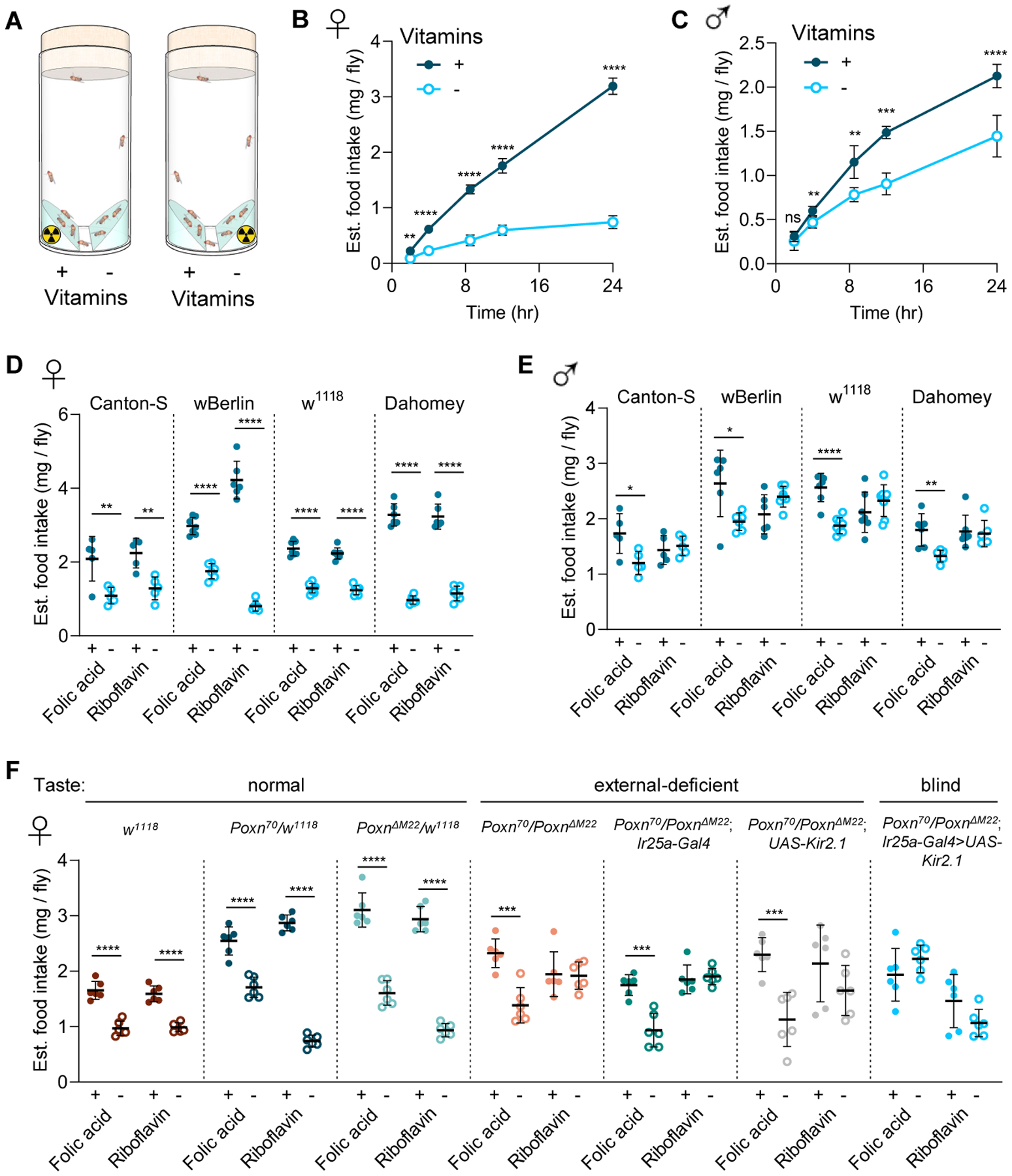
(A) Schematic diagram of the two-choice radiolabeled food intake assay. Flies have access to medium with or without vitamins, and only one of the food patches is radiolabeled in each vial. (B-C) Dahomey females (B) and males (C) consume more of the vitamin-containing food. A time-course for cumulative food intake is shown over 24 hours. Flies had access to two food patches, FLYaa medium with (+) or without (−) vitamins. Each time point is a subsample of 5 replicates of 10 flies each. (D-E) Vitamin consumption preference is sexually dimorphic. Females (D) in all 4 strains tested show preference for both folic acid and riboflavin. Males (E) only show preference for folic acid. (F) Vitamin consumption preference is dependent on taste. Tested lines with “normal” taste include w1118 and the heterozygous Poxn controls. External taste-deficient animals are a heteroallelic combination of the Poxn70 and PoxnΔM22 mutants, with or without one of the two transgenes necessary for silencing Ir25a-expressing neurons. Taste-blind animals (Poxn70/PoxnΔM22; Ir25a-Gal4>UAS-Kir2.1) are the Poxn heteroallelic mutant with pharyngeal GRNs silenced. In all panels, each point represents 1 vial of 10 flies. Panels D-F show consumption preference over 24 hours for FLYaa with (+) or without (−) the indicated vitamin (folic acid: 1.13 μM; riboflavin: 1.36 μM). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 (unpaired t-test). Data are shown as mean ± SD. See also Figures S1 and S2.
We next explored feeding preference over 24 hours for individual vitamins. Among the 7 vitamins tested, females showed preferences for folic acid and riboflavin (Figure S1C). Males only showed a preference for folic acid (Figure S1D). Consumption preference for folic acid and riboflavin was consistent in all four strains tested (Figure 1D–E), and the sexually dimorphic preference for riboflavin was not dependent on mating status (Figure S1E).
To determine the sensitivity to preferred vitamins, we tested a range of folic acid or riboflavin concentrations. Females showed dose-dependent preference for both vitamins at concentrations as low as ~0.01 μM and maximized preference at ~1 μM (Figure S1F-G). In contrast, flies only showed feeding preference for sucrose when the sugar concentration was greater than 500 μM (Figure S1H). Despite the pronounced preference for vitamins, combined total consumption of diets with or without vitamins was consistent over a wide range of vitamin concentrations, showing that vitamin acquisition does not directly affect caloric intake (Figure S1I).
We next asked how vitamin deprivation affects subsequent preference (Figure S2A-B). Depriving flies of folic acid or riboflavin for 5 or 10 days did not affect vitamin consumption preference in either sex ((Figure S2C–F). Preference for lower vitamin concentrations was also not significantly altered following deprivation, suggesting that the lack of change was not due to a limit on daily vitamin intake (Figure S2G-H). Hence, it is unlikely that vitamin consumption preference is driven by homeostatic mechanisms.
The gustatory system plays a critical role in nutrient perception and food choice in flies5. Therefore, we tested whether taste is required for vitamin preference using a minimal taste system. Pox neuro (Poxn) mutants, which lack functional external taste bristles6, showed no preference for riboflavin but maintained preference for folic acid (Figure 1F). These results suggest that external taste contributes to—but is not solely responsible for—mediating vitamin preference in Drosophila. Recent studies have also shown that pharyngeal taste sensation is an important regulator of feeding behavior6. Taste-blind animals—Poxn flies in which all pharyngeal gustatory receptor neurons are silenced6—showed no preference for either the riboflavin or the folic acid diets (Figure 1F), suggesting that folic acid preference requires pharyngeal taste.
The simplest explanation for the taste-dependence of vitamin preference is that the vitamins are highly palatable. However, neither folic acid nor riboflavin substantially altered proboscis extension responses (PER) to 50 mM sucrose (Figure S2I), despite eliciting significant consumption preference in this medium (Figure S2J). Furthermore, there was no observable preference for either vitamin in an agar-only medium (Figure S2J), nor did flies exhibit increased PER to vitamin-only solutions compared to water (Figure S2K). Together, these results suggest that the preferential consumption of vitamin-laced food is not due to increased palatability.
It is unclear why folic acid and riboflavin are unique—all of the vitamins tested except for biotin are required for egg production and viability7. Since vitamins are usually present together in natural food sources, developing mechanisms for identifying only a few may have been evolutionarily sufficient—and more economical given the diversity in vitamin structures2. Although we did not observe compensatory vitamin intake following deprivation, need-based vitamin consumption on natural substrates might be indirectly achieved through homeostatic regulation of macronutrient or caloric intake8.
Our studies show a direct sensory link between feeding behavior and essential micronutrients, extending previously identified associations in mammals between taste preferences and recovery from vitamin deprivation9. Nonetheless, it is unclear whether vitamins are detected directly by flies, or instead modify the taste of food—and whether post-ingestive responses to vitamin consumption are necessary for the preference behavior. Furthermore, responses to non-pheromonal tastants, including sugar, have also been shown to be sex-specific in flies10. Future studies that identify the taste receptors and the effectors that regulate vitamin consumption preference may uncover additional mechanisms underlying sexually dimorphic behavior in Drosophila.
Supplementary Material
Acknowledgments
This work was funded by the NIH (R56AG065986, W.W.J.) and a scholarship from the China Scholarship Council (Q.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
Supplemental Information
Supplemental Information includes experimental procedures and two figures, and can be found with this article online at *bxs.
Supplemental Information
Document S1. Experimental Procedures and Figures S1–S2.
References
- 1.Freeman EG, and Dahanukar A (2015). Molecular neurobiology of Drosophila taste. Curr. Opin. Neurobiol 34, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delompré T, Guichard E, Briand L, and Salles C (2019). Taste perception of nutrients found in nutritional supplements: A review. Nutrients 11, 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SJ, and Ja WW (2020). Absolute ethanol intake predicts ethanol preference in Drosophila melanogaster. J. Exp. Biol 223, jeb224121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piper MD, Soultoukis GA, Blanc E, Mesaros A, Herbert SL, Juricic P, He X, Atanassov I, Salmonowicz H, and Yang M (2017). Matching dietary amino acid balance to the in silico-translated exome optimizes growth and reproduction without cost to lifespan. Cell Metab 25, 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y-CD, and Dahanukar A (2020). Recent advances in the genetic basis of taste detection in Drosophila. Cell. Mol. Life Sci, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y-CD, Park SJ, Joseph RM, Ja WW, and Dahanukar AA (2019). Combinatorial pharyngeal taste coding for feeding avoidance in adult Drosophila. Cell Rep 29, 961–973.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sang JH, and King RC (1961). Nutritional requirements of axenically cultured Drosophila meanogaster adults. J. Exp. Biol 38, 793–809. [Google Scholar]
- 8.Lin S, Senapati B, and Tsao C-H (2019). Neural basis of hunger-driven behaviour in Drosophila. Open Biol 9, 180259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markison S (2001). The role of taste in the recovery from specific nutrient deficiencies in rats. Nutr. Neurosci 4, 1–14. [DOI] [PubMed] [Google Scholar]
- 10.Meunier N, Ferveur JF, and Marion-Poll F (2000). Sex-specific non-pheromonal taste receptors in Drosophila. Curr. Biol 10, 1583–1586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


