Abstract
Purpose
Several open-label randomized studies have suggested that in vivo T-cell depletion with anti–T-lymphocyte globulin (ATLG; formerly antithymocyte globulin-Fresenius) reduces chronic graft-versus-host disease (cGVHD) without compromising survival. We report a prospective, double-blind phase III trial to investigate the effect of ATLG (Neovii Biotech, Lexington, MA) on cGVHD-free survival.
Patients and Methods
Two hundred fifty-four patients 18 to 65 years of age with acute leukemia or myelodysplastic syndrome who underwent myeloablative HLA-matched unrelated hematopoietic cell transplantation (HCT) were randomly assigned one to one to placebo (n =128 placebo) or ATLG (n = 126) treatment at 27 sites. Patients received either ATLG or placebo 20 mg/kg per day on days −3, −2, −1 in addition to tacrolimus and methotrexate as GVHD prophylaxis. The primary study end point was moderate-severe cGVHD-free survival.
Results
Despite a reduction in grade 2 to 4 acute GVHD (23% v 40%; P = .004) and moderate-severe cGVHD (12% v 33%; P < .001) in ATLG recipients, no difference in moderate-severe cGVHD-free survival between ATLG and placebo was found (2-year estimate: 48% v 44%, respectively; P = .47). Both progression-free survival (PFS) and overall survival (OS) were lower with ATLG (2-year estimate: 47% v 65% [P = .04] and 59% v 74% [P = .034], respectively). Multivariable analysis confirmed that ATLG was associated with inferior PFS (hazard ratio, 1.55; 95% CI, 1.05 to 2.28; P = .026) and OS (hazard ratio, 1.74; 95% CI, 1.12 to 2.71; P = .01).
Conclusion
In this prospective, randomized, double-blind trial of ATLG in unrelated myeloablative HCT, the incorporation of ATLG did not improve moderate-severe cGVHD-free survival. Moderate-severe cGVHD was significantly lower with ATLG, but PFS and OS also were lower. Additional analyses are needed to understand the appropriate role for ATLG in HCT.
INTRODUCTION
Chronic graft-versus-host disease (cGVHD) is the leading cause of morbidity in long-term survivors after hematopoietic cell transplantation (HCT).1-4 The use of in vivo T-cell depletion with anti–T-cell or antithymocyte globulin (ATG) products has been associated with decreased cGVHD.5-7 However, some studies have suggested increased relapse and infectious complications.8,9 Distinct ATG products differ in their manufacturing process, including the cell line used for immunization as well as the animal source for production. Anti–T-lymphocyte globulin (ATLG; Grafalon; Neovii, Lexington, MA [formerly termed ATG-Fresenius]) is produced by immunizing rabbits with the Jurkat T-lymphoblastoid cell line followed by extraction of immunoglobulin G from sera.
Two open-label randomized trials have reported that ATLG reduces cGVHD without increasing transplantation-related mortality or disease relapse.10,11 We report the results of the first double-blind randomized study to our knowledge designed to investigate whether the addition of ATLG to standard GVHD prophylaxis for patients who undergo myeloablative HLA-matched unrelated donor HCT would increase moderate-severe cGVHD-free survival.
PATIENTS AND METHODS
Recipients and Donors
Eligible recipients were 18 to 65 years of age with acute lymphoblastic leukemia, acute myeloid leukemia in complete morphologic remission, or myelodysplastic syndrome with < 10% bone marrow blasts. Donors were unrelated and allele-level matched at HLA-A, -B, -C, and -DRΒ1. Either peripheral blood progenitor cells (PBPCs) or bone marrow was acceptable as a graft source. Cytogenetic risk for each disease was determined per European Leukemia Net consensus guidelines.
Conditioning Regimens
Recipients were treated with one of three myeloablative regimens: Cy-TBI, cyclophosphamide 120 mg/kg total intravenously (IV) and fractionated total body irradiation (≥ 12 Gy); Bu-Cy, busulfan 16 mg/kg orally or 12.8 mg/kg IV and cyclophosphamide 120 mg/kg IV; or Bu/Flu, busulfan 16 mg/kg orally or 12.8 mg/kg IV and fludarabine 120 mg/m2 IV. Choice of regimen was determined by the treating physician but assigned before random assignment.
GVHD Prophylaxis
All recipients received GVHD prophylaxis that was comprised of tacrolimus (with a target serum trough level of 5 to 15 ng/mL) and IV methotrexate (15 mg/m2 on day 1, then 10 mg/m2 on days 3, 6, and 11). In the absence of clinical GVHD, tacrolimus was tapered starting on day 50 or later over a minimum of 26 weeks and then discontinued.
Random Assignment, Masking, and Study Design
This prospective, randomized, double-blind, placebo-controlled, multicenter, phase III study was conducted in North America and Australia. The study was registered as a clinical trial (ClinicalTrials.gov identifier: NCT01295710). Patients were randomly assigned one to one in a double-blinded fashion between ATLG given at 20 mg/kg/day IV versus placebo given in 250 mL of normal saline on days −3, −2, and −1 before HCT. All patients received antihistamine and IV methylprednisolone 2 mg/kg on day −3 and 1 mg/kg on days −2 and −1. A blocked randomization schedule was generated by Neovii Biotech (the study sponsor), with records preallocated to each of the following strata: age (≤ 40 v > 40 years), source of stem cells (bone marrow v PBPCs), and status of disease (early v intermediate).
This event-driven study had a projected sample size of 250 and 124 events with one planned interim analysis at 50% information time. The sample size was calculated on the basis of the assumption of a hazard ratio (HR) of ATLG to placebo of 0.5 from an exponential distribution of moderate-severe cGVHD-free survival. The assumed 2-year event rate (moderate-severe cGVHD or death) in the placebo arm was 68%. Under the alternative hypothesis, the study would have 90% power at a two-sided significance level of .01. Although the sample size was calculated by using a .01 significance level to achieve sufficient evidence, the primary end point would be analyzed by using a two-sided .05 level. Per the prespecified interim analysis procedure, the interim analysis was performed when 225 patients were enrolled, and the sample size was re-estimated to 1,315. The study team then consulted with the data safety and monitoring board, which recommended completion of the study as initially planned.
Study End Points
The primary end point was moderate-severe cGVHD-free survival. cGVHD was diagnosed by treating physicians and graded per National Institutes of Health consensus critiera.12 The diagnosis and grading of cGVHD was then confirmed or overturned after evaluation by an independent end point adjudication committee. The primary end point was analyzed according to the modified intention-to-treat (mITT) principle. Secondary end points were overall survival (OS), progression-free survival (PFS), moderate-severe cGVHD-free and relapse-free survival (cGRFS), cumulative incidence of relapse and nonrelapse mortality (NRM), acute GVHD, and engraftment. Acute GVHD was diagnosed by treating physicians and graded per previously published consensus criteria.13 Neutrophil engraftment was defined as the first of 3 consecutive days of absolute neutrophil count ≥ 500/μL. Platelet engraftment was defined as the first of 3 consecutive days with self-supported platelet counts ≥ 20,000/μL. OS was defined as from the first day of study drug administration to death as a result of any cause. Follow-up for survival was censored when the patient was last verified to be alive. PFS was defined from the first day of study drug administration to relapse, disease progression, or death, whichever occurred first. Patients who withdrew consent after the start of study drug but before HCT (n = 9) were included per the mITT principle as censored observations at the time of consent withdrawal for all time-to-event end points except engraftment.
Immune Monitoring
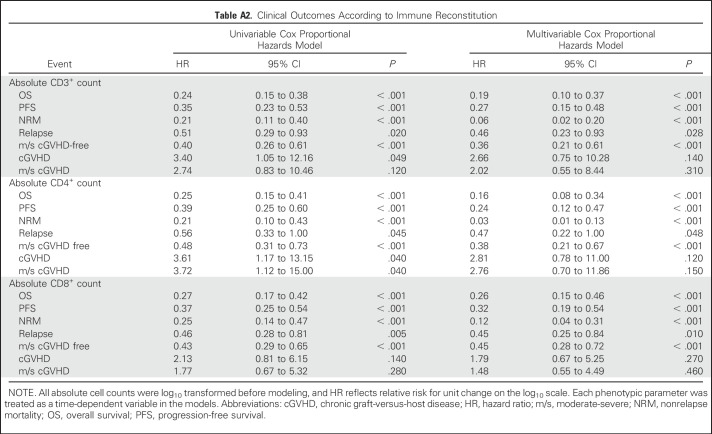
Immune reconstitution was studied in a subset of patients who consented to have peripheral blood samples collected at 30, 100, 180, and 360 days after HCT. Multiparameter flow cytometry was performed in a blinded fashion (J.R. laboratory, Dana-Farber Cancer Institute). After unblinding, the impact of treatment arm on immune recovery at each time point was determined (Wilcoxon rank sum test). Multivariable Cox proportional hazards regression models that treated each phenotypic parameter as a time-dependent variable were constructed to study the impact of reconstitution on clinical outcomes.
Statistical Considerations
The mITT analysis included patients who were randomly assigned and who started the study drug. Baseline characteristics were compared by using the Fisher’s exact test, χ2 test, or Wilcoxon rank sum test as appropriate. Moderate-severe cGVHD-free survival, cGRFS, OS, and PFS were estimated by using the Kaplan-Meier method. The log-rank test was used for group comparisons of survival distributions. Cumulative incidences of NRM, relapse, and GVHD were estimated in the competing risks framework that considered relapse, NRM, and death or relapse without developing GVHD, respectively, as competing events. The group difference in cumulative incidence in the presence of a competing risk was tested by using the Gray method.14 Of note, for ease of presentation, point estimates at a particular time point for time-to-event end points are presented, although P values were calculated by comparing distributions with the use of the log-rank or Gray test, as appropriate. Multivariable regression analysis was performed by using the Cox proportional hazards regression model for moderate-severe cGVHD-free survival, OS, and PFS. Center effect was tested by using a frailty model and was not significant (P = .22). Potential prognostic factors considered in the regression analyses were age, recipient and donor sex, disease, disease risk, Karnofsky performance score, conditioning regimen, cytogenetic risk, cytomegalovirus (CMV) serostatus of recipient and donor at HCT, and graft source. Before modeling, the proportional hazards assumption and significance of two-way interaction terms were examined. In the exploratory analysis of absolute lymphocyte count (ALC) on day −3, the ALC level was dichotomized (> 0.1 v ≤ 0.1 × 109 lymphocytes/L) by using restricted cubic spline estimates of the relationship between ALC and log-relative hazard of death15 and the method of recursive partitioning for survival trees.16 Per protocol, the threshold for statistical significance was set at the .05 level. All tests were two-sided, and all analyses were performed with SAS 9.3 statistical software (SAS Institute, Cary, NC) and R version 3.2.2 (www.cran.r-project.org). A heat map was generated for immune reconstitution parameters by using GENE-E (www.broadinstitute.org/cancer/software/GENE-E), and samples were clustered by using the unsupervised hierarchical clustering method.
RESULTS
Donor and Recipient Characteristics
Two hundred sixty patients were enrolled between October 2011 and October 2014, of whom 132 were randomly assigned to placebo and 128 to ATLG (Fig 1). Six patients were never treated in the study (four in the placebo group, two in the ATLG group), so the analysis included 128 patients in the placebo group and 126 patients in the ATLG group. Nine patients (all in the ATLG group) withdrew consent after receiving ATLG but before HCT because of infusion reactions. Per the mITT principle, these nine patients were censored at the time of withdrawal. Clinical and transplantation characteristics are listed in Table 1. There were no statistically significant between-group differences other than the ATLG group comprised more females (52.4% v 38.3%; P = .03). Median follow-up for survivors was 24 months.
Fig 1.
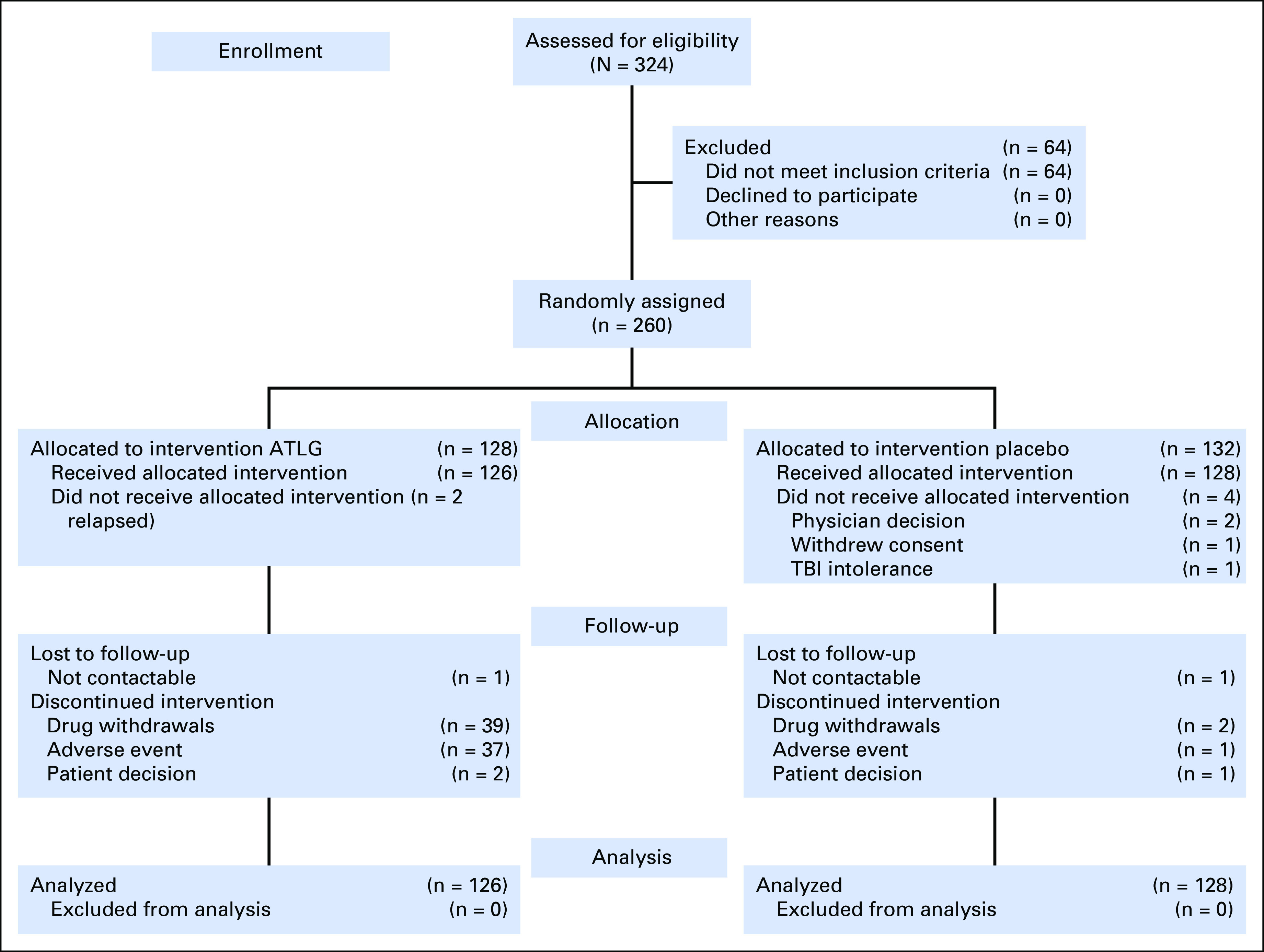
CONSORT diagram. ATLG, anti–T-lymphocyte globulin; TBI, total body irradiation.
Table 1.
Clinical Characteristics of Patients Who Received ATLG and Placebo
Engraftment and Graft Failure
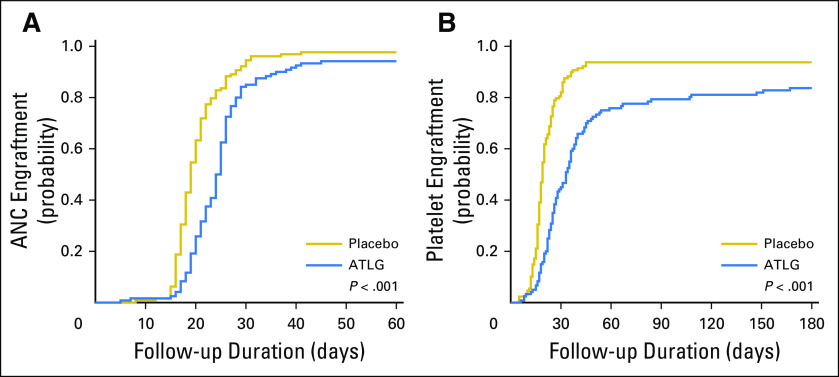
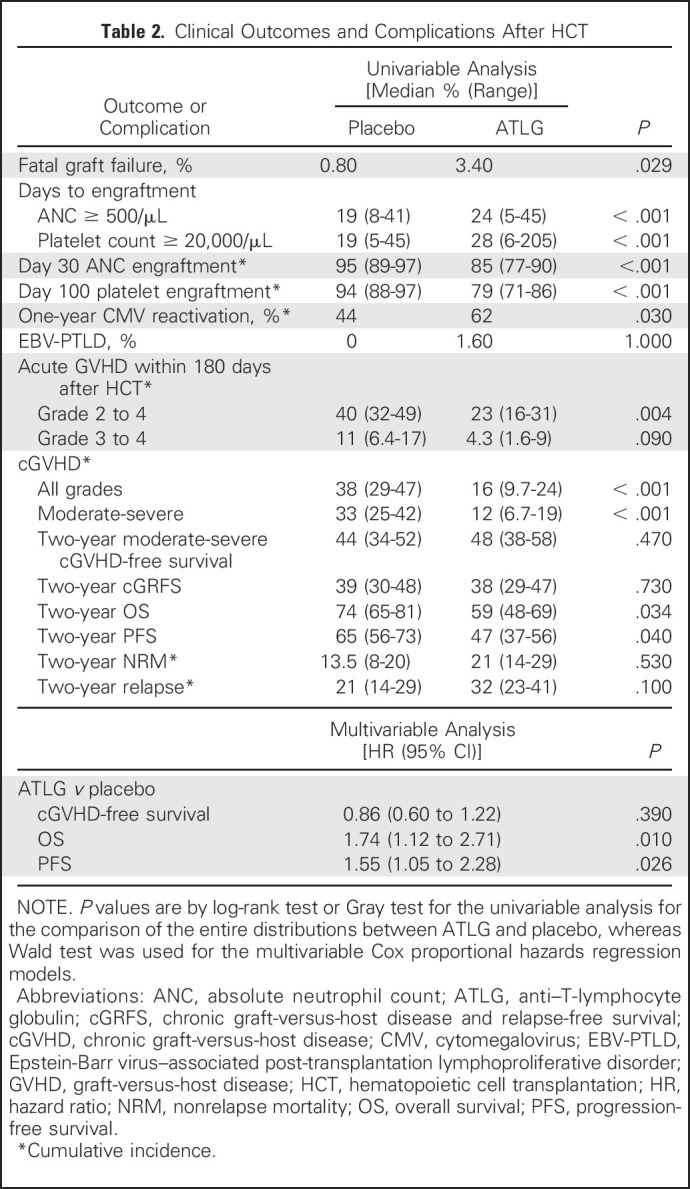
The median times to neutrophil and platelet engraftment were significantly longer in the ATLG recipients (24 [range, 5 to 45] v 19 [range, 8 to 41] days, respectively; P < .001) than in the placebo group (28 [range, 6 to 205] v 19 [range, 5 to 45] days, respectively; P < .001; Table 2; Appendix Fig A1, online only). The day 30 cumulative incidence of neutrophil engraftment was 85% in ATLG and 95% in placebo (P < .001). The day 100 cumulative incidence of platelet engraftment was 79% and 94% in ATLG and placebo, respectively (P < .001). Five ATLG recipients and one placebo recipient died without engraftment before day 28 (3.4% v 0.8%; P = .029).
Table 2.
Clinical Outcomes and Complications After HCT
Acute GVHD and cGVHD
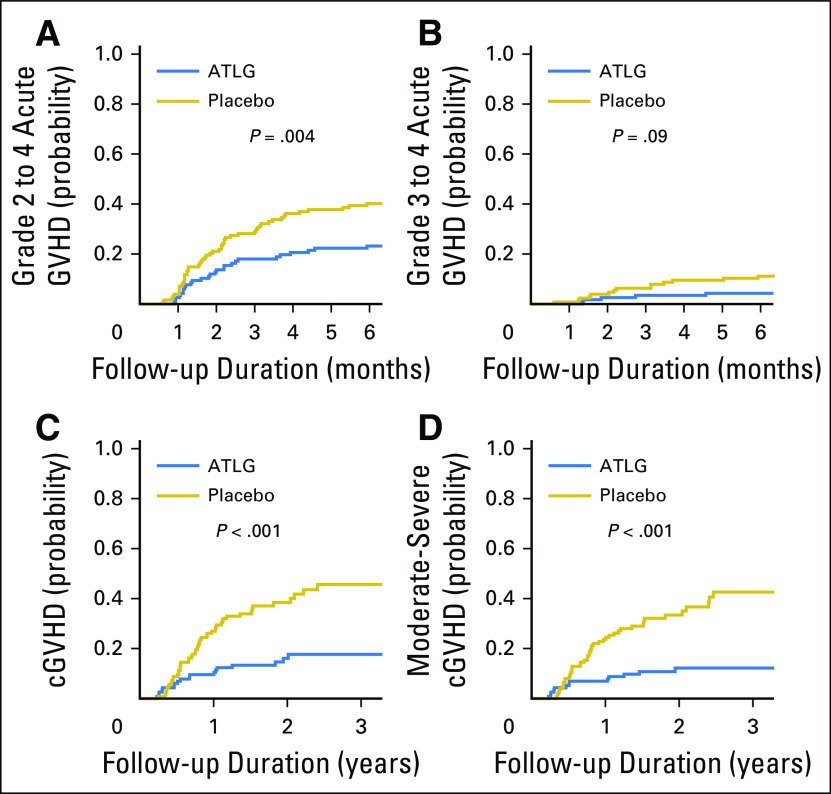
The day 180 cumulative incidence of grade 2 to 4 acute GVHD was 23% (95% CI, 16% to 31%) in ATLG recipients and 40% (95% CI, 32% to 49%) in placebo recipients (P = .004; Table 2; Fig 2A). Cumulative incidence of grade 3 to 4 acute GVHD was 4.3% (95% CI, 1.6% to 9%) in ATLG v 11% (95% CI, 6.4% to 17%) in placebo (P = .09; Fig 2B). Two-year cumulative incidence of all grades of cGVHD was 16% (95% CI, 9.7% to 24%) in ATLG and 38% (95% CI, 29% to 47%) in placebo (P < .001; Fig 2C), and the 2-year cumulative incidence of National Institutes of Health consensus criteria moderate-severe cGVHD was 12% (95% CI, 6.7% to 19%) and 33% (95% CI, 25% to 42%), respectively (P < .001; Fig 2D).
Fig 2.
Cumulative incidence of (A) grade 2 to 4 acute graft-versus-host disease (GVHD), (B) grades 3 to 4 acute GVHD, (C) chronic GVHD (cGVHD), and (D) moderate-severe cGVHD. ATLG, anti–T-lymphocyte globulin.
Moderate-Severe cGVHD-Free Survival and OS
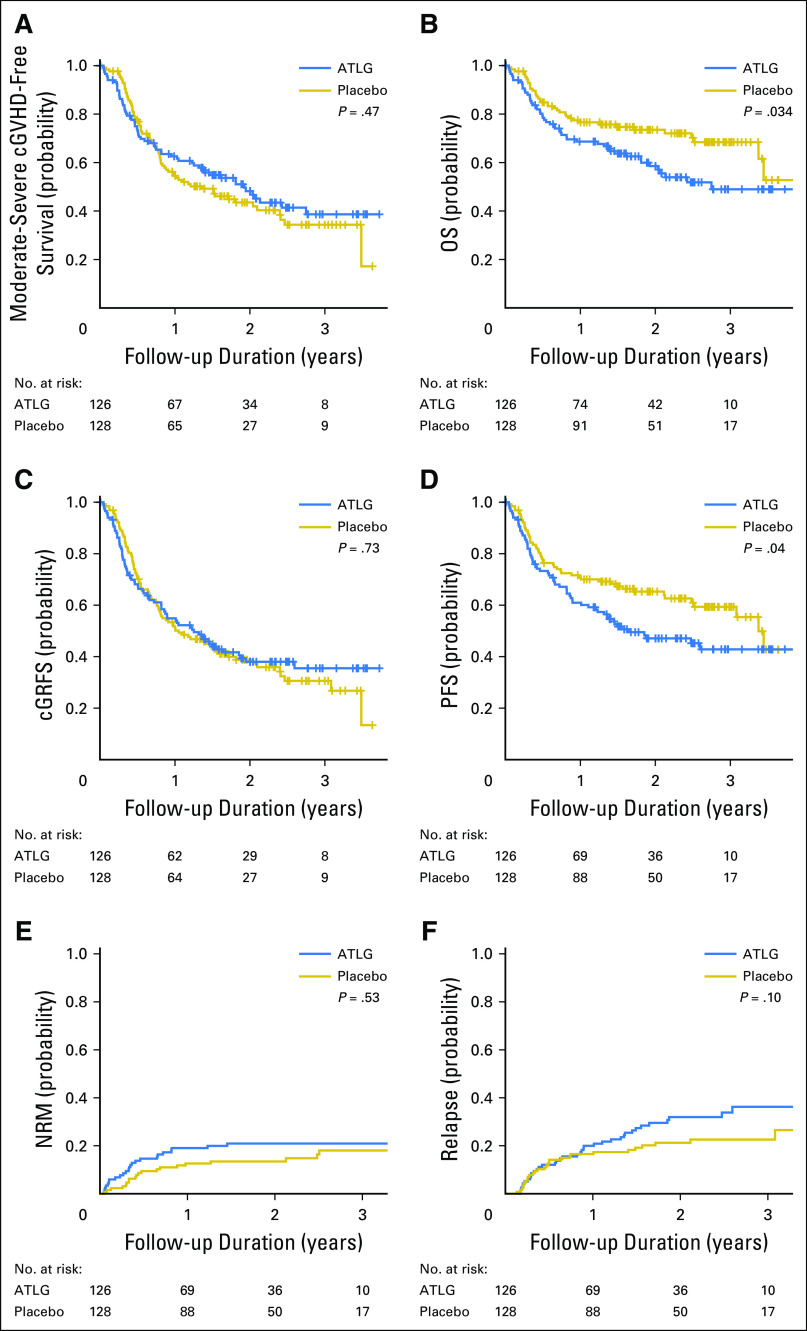
Moderate-severe cGVHD-free survival at 2 years was 48% (95% CI, 38% to 58%) in ATLG recipients and 44% (95% CI, 34% to 52%) in placebo recipients (P = .47; Table 2; Fig 3A). The lower rate of moderate-severe cGVHD in ATLG did not translate into improved moderate-severe cGVHD-free survival because of inferior OS in this group. Two-year OS was 59% (95% CI, 48% to 69%) in ATLG v 74% (95% CI, 65% to 81%) in placebo (P = .034; Fig 3B). The 2-year moderate-severe cGRFS was 38% (95% CI, 29% to 47%) in ATLG and 38% (95% CI, 30% to 48%) in placebo (P = .73; Fig 3C). Two-year PFS was 47% (95% CI, 37% to 56%) in ATLG and 65% (95% CI, 56% to 73%) in placebo (P = .04; Fig 3D).
Fig 3.
(A) Moderate-severe chronic graft-versus-host disease (cGVHD)–free survival. (B) Overall survival (OS). (C) cGVHD and relapse-free survival (cGRFS). (D) Progression-free survival (PFS). (E) Nonrelapse mortality (NRM). (F) Disease relapse. Log-rank test was used for group comparison for (A) to (D) and Gray test for (E) and (F). ATLG, anti–T-lymphocyte globulin.
Multivariable analysis that adjusted for age, recipient and donor sex, disease, disease risk, Karnofsky performance score, conditioning regimen, cytogenetic risk, CMV serostatus, and graft source confirmed that ATLG was associated with inferior PFS (HR, 1.55; 95% CI, 1.05 to 2.28; P = .0026) and OS (HR, 1.74; 95% CI, 1.12 to 2.71; P = .01) and comparable moderate-severe cGVHD-free survival (HR, 0.86; 95% CI, 0.6 to 1.22; P = 0.39; Table 2).
In multivariable analysis, older age (P = .0027), diagnosis of acute myeloid leukemia (P = .03), second complete remission (P = .007), Cy-TBI conditioning (P = .012), and high-risk cytogenetics (P = .009) were significantly associated with inferior OS. Older age (P = .0029), second complete remission (P = .019), Cy-TBI conditioning (P = .02), and high-risk cytogenetics (P = .029) were associated with inferior PFS. Older age (P = .012) and peripheral blood graft (P = .014) were associated with inferior moderate-severe cGVHD-free survival.
NRM and Relapse
The 2-year cumulative incidence of NRM was 21% (95% CI, 14% to 29%) in ATLG recipients and 13.5% (95% CI, 8% to 20%) in placebo recipients (P = .53; Table 2; Fig 3E). The 2-year cumulative incidence of disease relapse was 32% (95% CI, 23% to 41%) in ATLG and 21% (95% CI, 14% to 29%) in placebo (P = .1; Table 2; Fig 3F). Relapse was the leading cause of death, which comprised 42% of deaths in the ATLG group and 24% of deaths in the placebo group.
Toxicity and Viral Reactivation
Overall, 39 ATLG recipients (30.9%) did not complete all 3 days of infusion compared with three placebo recipients (2.3%) largely because of infusion reaction. Nine grade 3 to 4 infusion-related severe adverse events (7.1%) were reported in ATLG compared with none in placebo. No difference was found in the incidence of hepatic veno-occlusive disease, hemorrhagic cystitis, or thrombotic microangiopathy. In patients who were CMV seropositive, 1-year cumulative incidence of CMV reactivation was 62% v 44% for ATLG and placebo, respectively (P = .03). Two patients had post-transplantation Epstein-Barr virus–associated lymphoproliferative disease in the ATLG group, and none experienced this in the placebo group.
Impact of ALC at the Time of ATLG or Placebo Administration
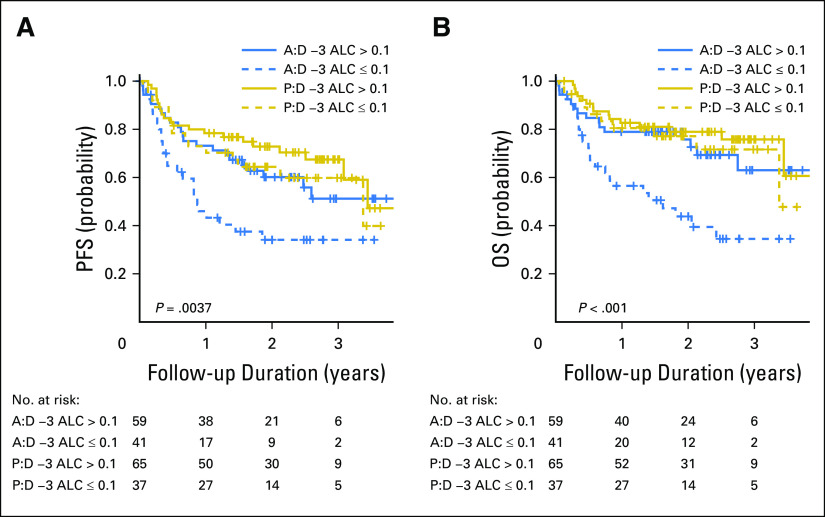
On the basis of evidence that links ALC at the time of ATG product exposure to post-transplantation globulin pharmacokinetics, immune reconstitution, and transplantation outcomes,17,18 we conducted an unplanned exploratory analysis that investigated the interaction of ALC on day −3 (at the time of drug initiation) with the effect of ATLG. In patients with day −3 ALC ≥ 0.1 × 109/L, ATLG did not compromise PFS (Fig 4A) or OS (Fig 4B). Only in patients with ALC < 0.1 were PFS and OS negatively affected.
Fig 4.
Outcomes according to day −3 absolute lymphocyte count (ALC) level and treatment arm. (A) Progression-free survival (PFS). (B) Overall survival (OS). Log-rank test was used for four group comparisons. A:D −3, anti–T-lymphocyte globulin day −3; P:D −3, placebo day −3.
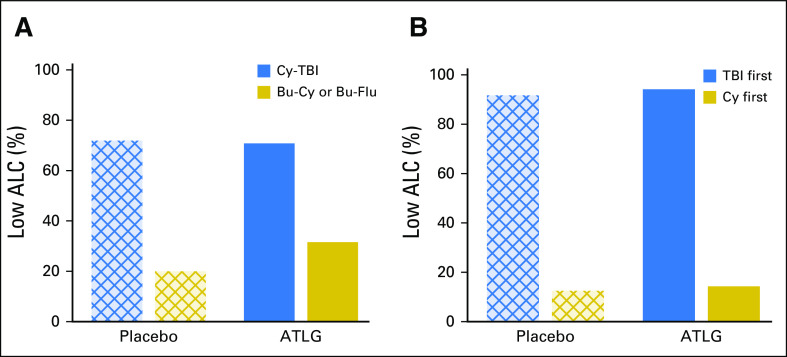
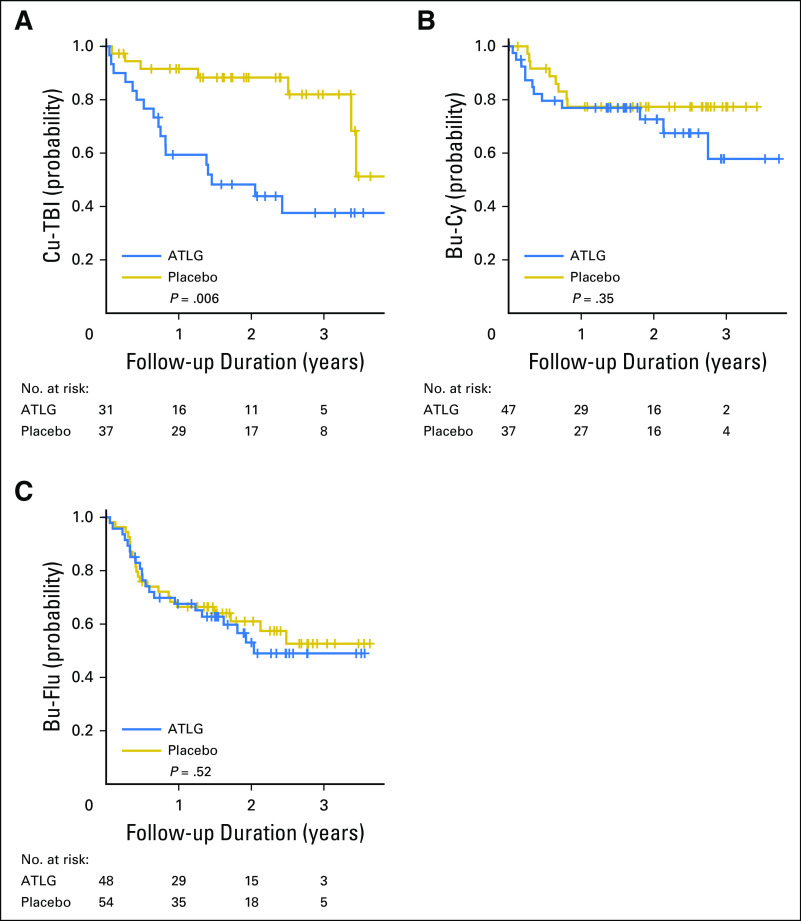
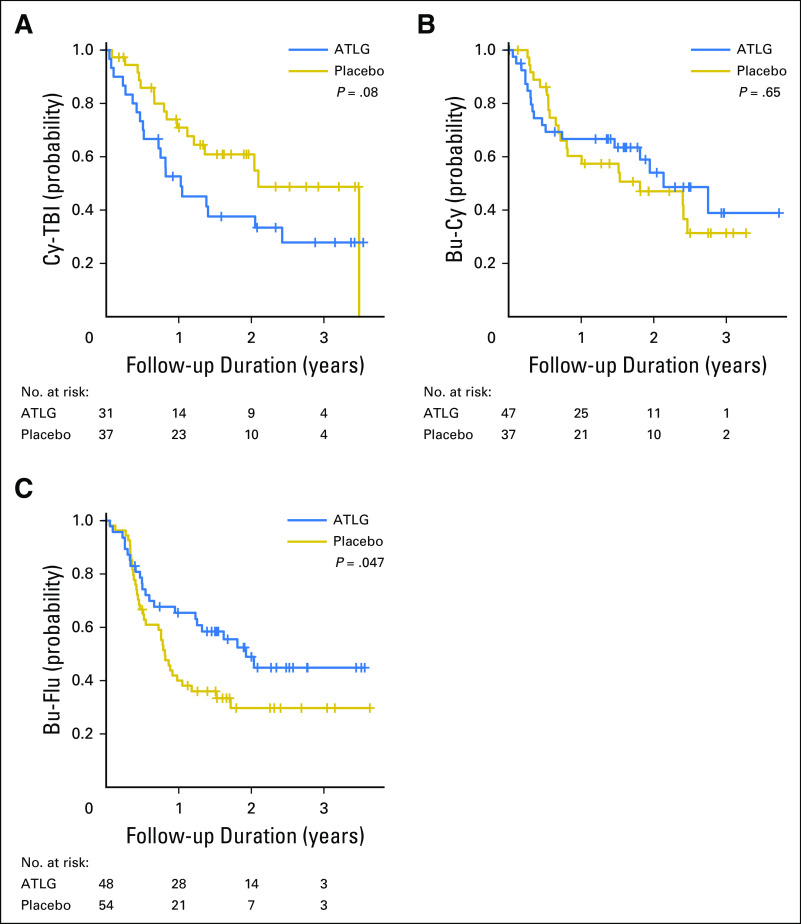
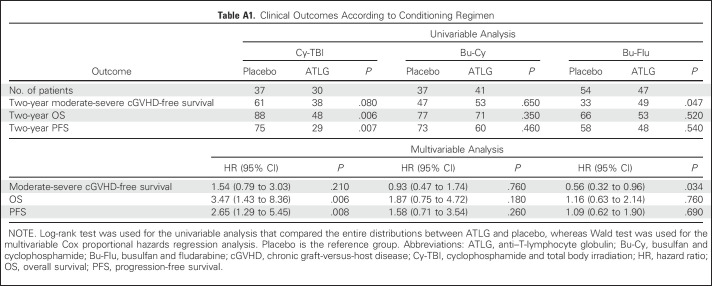
The choice of conditioning regimen had a profound effect on day −3 ALC, with > 70% of patients who received TBI having an ALC < 0.1 × 109/L compared with < 35% of patients who received Bu conditioning (Appendix Fig A2, online only). Low day −3 ALC was noted specifically in patients who received TBI before Cy. Correspondingly, among TBI recipients, ATLG was associated with inferior PFS (HR, 2.65; 95% CI, 1.29 to 5.45; P = .008) and OS (HR, 3.47; 95% CI, 1.43 to 8.36; P = .006), whereas no difference was noted in patients who received Bu-based conditioning (Appendix Fig A3, online only). Moderate-severe cGVHD-free survival seemed superior for ATLG recipients who received Bu-Flu conditioning (HR, 0.56; 95% CI, 0.32 to 0.96; P = .034; Appendix Fig A4, online only), whereas OS and PFS were similar (HR, 1.16 [P = .64] v 1.09 [P = 0.76], respectively; Appendix Table A1, online only).
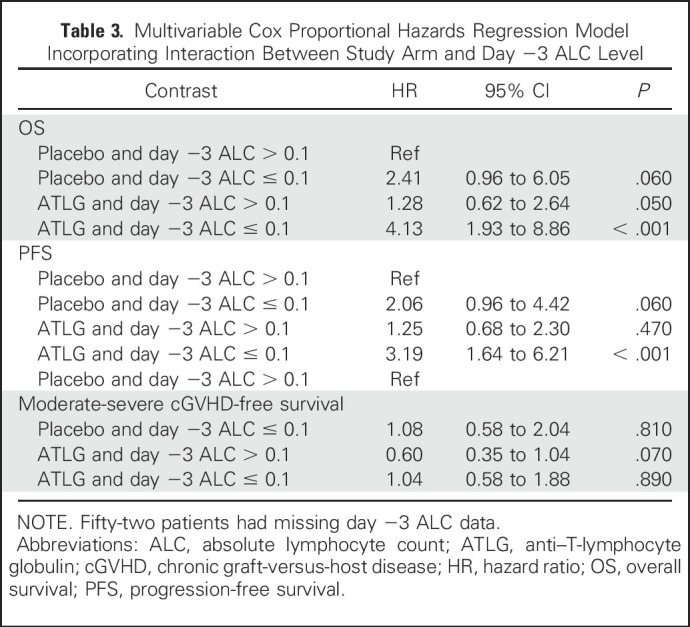
We then examined the interaction between ALC and each treatment arm. By using a contrast statement, we assessed the relative risk of low ALC in ATLG compared with placebo with ALC ≥ 0.1 (reference group) and found that ATLG recipients with low ALC had significantly worse OS (HR, 4.13; P < .001) and PFS (HR, 3.19; P < .001) relative to the reference group (Table 3). In addition, OS and PFS were not significantly compromised in ATLG recipients when ALC ≥ 0.1, indicating a synergistic effect between low ALC and ATLG.
Table 3.
Multivariable Cox Proportional Hazards Regression Model Incorporating Interaction Between Study Arm and Day −3 ALC Level
Immune Reconstitution
In 91 patients (44 ATLG and 47 placebo) enrolled in an ancillary immune reconstitution companion study, ATLG treatment was associated with lower absolute CD3+ and CD4+ counts at days 30, 100, 180, and 360. Absolute CD8 counts were significantly lower in ATLG recipients at day 30, but not subsequently. Detailed heat maps comparing the impact of ATLG on immune reconstitution are shown in Appendix Fig A5 (online only). Cox proportional hazards regression models suggest that delayed CD3+, CD4+, and CD8+ cell count recovery was associated with inferior NRM, which led to lower PFS and OS (Appendix Table A2, online only). Delayed CD3+, CD4+, or CD8+ cell recovery was not associated with disease relapse or development of moderate-severe cGVHD.
DISCUSSION
We conducted a prospective, randomized, double-blind trial of ATLG added to tacrolimus and methotrexate in recipients of myeloablative HLA-matched unrelated donor HCT. Although the incidence of both grade 2 to 4 acute GVHD and moderate-severe cGVHD were lower with ATLG, no significant difference was found in 2-year moderate-severe cGVHD-free survival or cGRFS because OS and PFS were lower in the ATLG arm.
Several open-label randomized trials have reported a benefit of ATLG for the prevention of cGVHD. Finke et al10 conducted a randomized phase III open-label trial that added ATLG to cyclosporine and methotrexate in 202 patients with hematologic malignancies who underwent myeloablative unrelated donor HCT. Extended follow-up of this trial showed that ATLG lowered the incidence of extensive cGVHD and significantly increased the probability of survival free of systemic immunosuppression (52.9% v 16.9%) at 3 years19 and 8 years (47% v 11%) without any increase in underlying disease relapse.20 Kröger et al11 conducted a prospective, randomized, open-label study of ATLG added to cyclosporine and methotrexate in 168 patients who underwent matched related donor HCT with PBPCs. Patients who had received ATLG had a higher 2-year cGRFS (36.6% v 16.8%; P = .005). By using another ATG product (Thymoglobulin; Genzyme, Cambridge, MA), Walker et al21 reported results of a randomized, open-label study in which the 12-month incidence of cGVHD was lower in the ATG group (13% v 29%, P = .0083), with no significant differences in relapse, NRM, PFS, or OS. Why the current results did not recapitulate outcomes from prior studies is unclear, although some important differences exist among them. Finke et al allowed mismatches at HLA-C, used cyclosporine, and had more heterogeneity in underlying disease. The study by Kröger et al was conducted with related donors, used cyclosporine, and administered ATLG at 50% of the dose used in the current study (30 v 60 mg/kg total). Finally, although more patients discontinued ATLG compared with placebo in our study, this is a highly unlikely reason for the inferior survival observed with ATLG use.
Another potential explanation for the difference in outcomes may be related to the impact of the conditioning regimen on ALC and an interaction between ALC and ATLG. We found a striking relationship between low ALC at the time of ATLG administration with inferior PFS and OS. Although we did not measure ATLG levels, low ALC before HCT likely resulted in less binding with subsequent delayed clearance and higher concentrations of ATLG after HCT.17,18,22 Other investigators have correlated high ATG (thymoglobulin) levels after bone marrow transplantation with increased infectious complications.17,18,22-24
Routine use of lower dosing schedules of ATLG as in the study by Kröger et al11 could potentially maintain protective effects against cGVHD but not increase mortality.25-27 As well, individualized dosing that is based on real-time ALC measurements could optimize outcomes.22 Patients who receive Bu-based conditioning or other regimens that do not precipitously drop the ALC pretransplantation might be more suited to receive ATLG. Given the long-term of effects of cGVHD, extended follow-up might yield different results, and specifically, quality-of-life measures and other patient-reported outcomes will be important end points to analyze at these later times.
In conclusion, this study confirms that the incorporation of ATLG results in significantly less cGVHD after HCT. However, in contrast to other trials in myeloablative related and unrelated donor HCT, ATLG use was associated with decreased OS and PFS. Continued investigation is needed to determine the appropriate role for ATLG in GVHD prevention.
ACKNOWLEDGMENT
We thank all the patients and their families for participating in this trial as well as the clinical research teams of each participating center.
Appendix
Fig A1.
Cumulative incidence of neutrophil and platelet engraftment. (A) Absolute neutrophil count (ANC) ≥ 500/μL. (B) Platelets ≥ 20,000/μL. Gray test was used for group comparisons. ATLG, anti–T-lymphocyte globulin.
Fig A2.
(A) Percent low absolute lymphocyte count (ALC) according to treatment arm and conditioning regimen. (B) Percent low ALC according to treatment arm in patients administered cyclophosphamide and total body irradiation (Cy-TBI) on the basis of order of TBI. ATLG, anti–T-lymphocyte globulin; Bu-Cy, busulfan and cyclophosphamide; Bu-Flu, busulfan and fludarabine.
Fig A3.
Overall survival by arm in each conditioning regimen. (A) Cyclophosphamide and total body irradiation (Cy-TBI). (B) Busulfan and cyclophosphamide (Bu-Cy). (C) Busulfan and fludarabine (Bu-Flu). Log-rank test was used for group comparisons. ATLG, anti–T-lymphocyte globulin.
Fig A4.
Moderate-severe chronic graft-versus-host disease–free survival by arm in each conditioning regimen. (A) Cyclophosphamide and total body irradiation (Cy-TBI). (B) Busulfan and cyclophosphamide (Bu-Cy). (C) Busulfan and fludarabine (Bu-Flu). Log-rank test was used for group comparisons. ATLG, anti–T-lymphocyte globulin.
Fig A5.
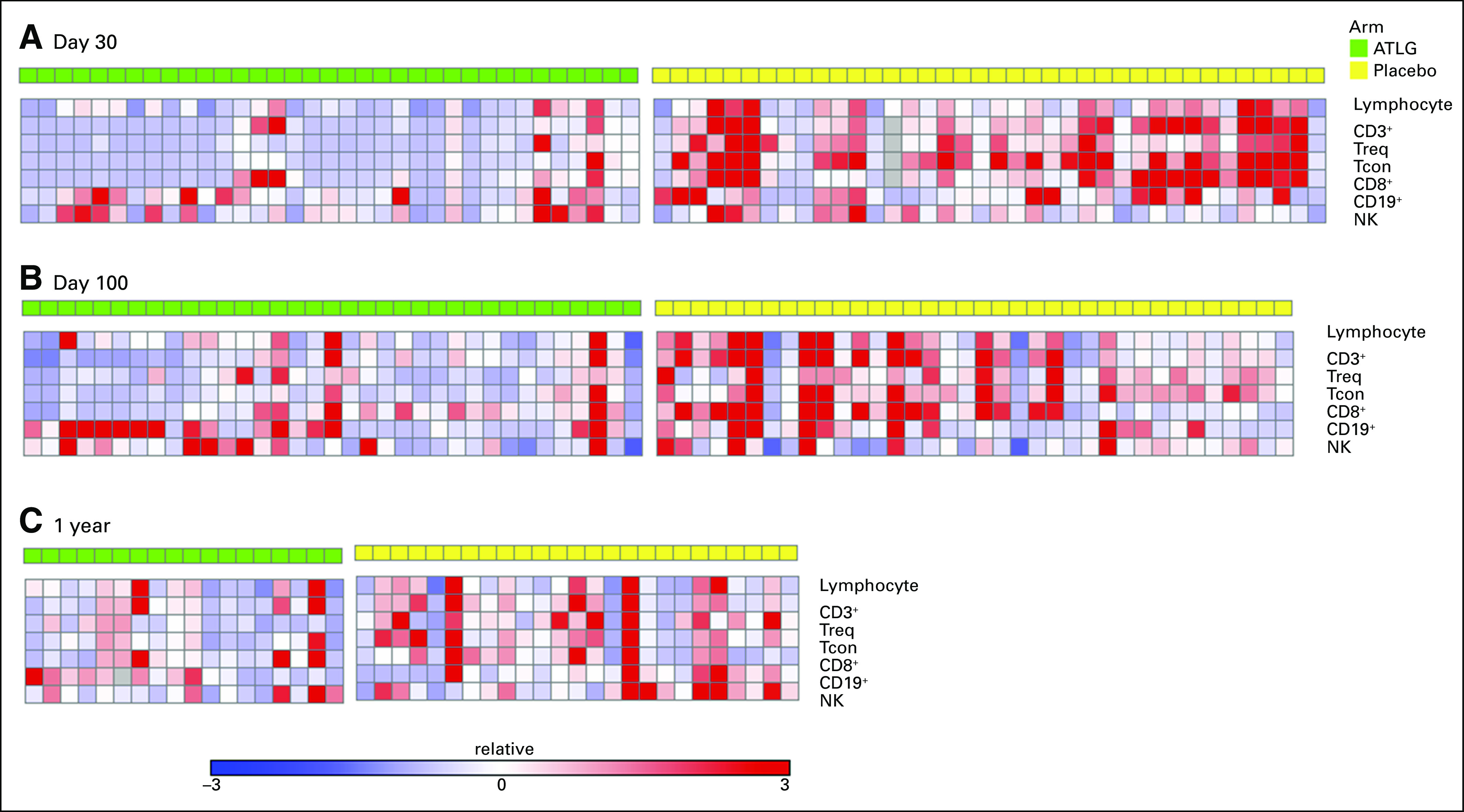
Heat map of T-cell reconstitution. (A) Day 30. (B) Day 100. (C) One year. All values are absolute counts and normalized by subtracting by row median and divided by row median absolute deviation. Each column represents a single patient sample. Red indicates higher absolute counts. ATLG, anti–T-lymphocyte globulin.
Table A1.
Clinical Outcomes According to Conditioning Regimen
Table A2.
Clinical Outcomes According to Immune Reconstitution
Footnotes
Supported by Neovii Biotech.
Processed as a Rapid Communication manuscript.
Clinical trial information: NCT01295710.
See accompanying Editorial on page 3993
AUTHOR CONTRIBUTIONS
Conception and design: Frank Glavin
Financial support: Frank Glavin
Administrative support: Robert J. Soiffer, Frank Glavin, Yi-Bin Chen
Provision of study materials or patients: Jerome Ritz
Collection and assembly of data: Robert J. Soiffer, Joseph McGuirk, Mitchell E. Horwitz, Laura Johnston, Mrinal M. Patnaik, Witold Rybka, Andrew Artz, David L. Porter, Thomas C. Shea, Michael W. Boyer, Paul J. Shaughnessy, Usama Gergis, Hana Safah, Ran Reshef, John F. DiPersio, Patrick J. Stiff, Madhuri Vusirikala, Jeff Szer, Jennifer Holter, James D. Levine, Paul J. Martin, Joseph A. Pidala, Ian D. Lewis, Vincent T. Ho, Edwin P. Alyea, Jerome Ritz, Peter Westervelt, Madan H. Jagasia, Yi-Bin Chen
Data analysis and interpretation: Robert J. Soiffer, Haesook T. Kim, Joseph McGuirk, Mitchell E. Horwitz, Laura Johnston, Mrinal M. Patnaik, Witold Rybka, Andrew Artz, David L. Porter, Thomas C. Shea, Michael W. Boyer, Richard T. Maziarz, Paul J. Shaughnessy, Usama Gergis, Hana Safah, Ran Reshef, John F. DiPersio, Patrick J. Stiff, Madhuri Vusirikala, Jeff Szer, Jennifer Holter, James D. Levine, Paul J. Martin, Joseph A. Pidala, Ian D. Lewis, Vincent T. Ho, Edwin P. Alyea, Jerome Ritz, Peter Westervelt, Madan H. Jagasia, Yi-Bin Chen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti–T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease–Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Robert J. Soiffer
Leadership: Kiadis Pharma
Consulting or Advisory Role: Sandoz, Juno Therapeutics, Gilead Sciences, MedImmune, GlaxoSmithKline
Haesook T. Kim
Consulting or Advisory Role: Neovii Biotech
Joseph McGuirk
Honoraria: Kite Pharma
Speakers’ Bureau: Kite Pharma
Research Funding: Novartis, Bellicum Pharmaceuticals, Fresenius Biotech SE & Co. KGaA, Astellas Pharma
Mitchell E. Horwitz
No relationship to disclose
Laura Johnston
No relationship to disclose
Mrinal M. Patnaik
No relationship to disclose
Witold Rybka
Consulting or Advisory Role: Merck Sharp & Dohme
Research Funding: Merck Sharp & Dohme, Seattle Genetics, Pfizer
Andrew Artz
Research Funding: Miltenyi Biotec, Neovii Biotech
David L. Porter
No relationship to disclose
Thomas C. Shea
Consulting or Advisory Role: Novartis, Merck
Michael W. Boyer
Consulting or Advisory Role: Novartis
Richard T. Maziarz
Consulting or Advisory Role: Novartis, Incyte, Juno Therapeutics, Kite Pharma
Paul J. Shaughnessy
Employment: HCA Healthcare
Honoraria: Sanofi, Millennium Pharmaceuticals
Consulting or Advisory Role: Sanofi, Millennium Pharmaceuticals
Speakers’ Bureau: Sanofi, Millennium Pharmaceuticals
Usama Gergis
Consulting or Advisory Role: Jazz Pharmaceuticals
Speakers’ Bureau: Merck, Incyte, Alexion Pharmaceuticals, Astellas Pharma, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Merck, Incyte, Astellas Pharma, Jazz Pharmaceuticals
Hana Safah
Speakers’ Bureau: Incyte, Alexion Pharmaceuticals, Celgene, Astellas Pharma, Jazz Pharmaceuticals, Sanofi
Ran Reshef
Consulting or Advisory Role: Pfizer, Concert Pharmaceuticals, Incyte, Atara Biotherapeutics, TEVA Pharmaceuticals Industries, Takeda Pharmaceuticals, Bristol-Myers Squibb, Kite Pharma
John F. DiPersio
Stock or Other Ownership: Magenta
Honoraria: Celgene, Incyte, Zymeworks, RiverVest, Cellworks, MacroGenics
Patrick J. Stiff
No relationship to disclose
Madhuri Vusirikala
Consulting or Advisory Role: Amgen
Jeff Szer
Consulting or Advisory Role: Alexion Pharmaceuticals, Sanofi, Shire, Pfizer
Travel, Accommodations, Expenses: Sanofi, Shire, Pfizer, Alexion Pharmaceuticals
Jennifer Holter
No relationship to disclose
James D. Levine
Stock or Other Ownership: Pfizer (I), Amgen
Paul J. Martin
Consulting or Advisory Role: Pfizer, Incyte
Research Funding: Neovii Biotech (Inst)
Joseph A. Pidala
No relationship to disclose
Ian D. Lewis
Consulting or Advisory Role: Neovii Biotech, Amgen, Roche, Pfizer
Vincent T. Ho
Consulting or Advisory Role: Jazz Pharmaceuticals
Edwin P. Alyea
No relationship to disclose
Jerome Ritz
Consulting or Advisory Role: Biothera, Celgene, Juno Therapeutics, Amgen, Draper Laboratory, ZIOPHARM Oncology
Research Funding: Roche
Frank Glavin
Employment: Neovii Biotech, Orphan Technologies
Leadership: Orphan Technologies
Peter Westervelt
No relationship to disclose
Madan H. Jagasia
No relationship to disclose
Yi-Bin Chen
Consulting or Advisory Role: Jazz Pharmaceuticals, REGiMMUNE, Takeda Pharmaceuticals, INSYS Therapeutics, Magenta Therapeutics, EnGeneIC
Research Funding: Seattle Genetics, Novartis, Celgene
REFERENCES
- 1.Anasetti C, Logan BR, Lee SJ, et al. : Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med 367:1487-1496, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingard JR, Majhail NS, Brazauskas R, et al. : Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 29:2230-2239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holtan SG, DeFor TE, Lazaryan A, et al. : Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 125:1333-1338, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ, Kim HT, Ho VT, et al. : Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant 38:305-310, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bacigalupo A, Lamparelli T, Barisione G, et al. : Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: Long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant 12:560-565, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Mohty M, Labopin M, Balère ML, et al. : Antithymocyte globulins and chronic graft-vs-host disease after myeloablative allogeneic stem cell transplantation from HLA-matched unrelated donors: A report from the Sociéte Française de Greffe de Moelle et de Thérapie Cellulaire. Leukemia 24:1867-1874, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Storek J, Mohty M, Boelens JJ: Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 21:959-970, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Baron F, Labopin M, Blaise D, et al. : Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: A report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 49:389-396, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Soiffer RJ, Lerademacher J, Ho V, et al. : Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 117:6963-6970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finke J, Bethge WA, Schmoor C, et al. : Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10:855-864, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Kröger N, Solano C, Wolschke C, et al. : Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med 374:43-53, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Filipovich AH, Weisdorf D, Pavletic S, et al. : National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 11:945-956, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Weisdorf D, Martin P, et al. : 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15:825-828, 1995 [PubMed] [Google Scholar]
- 14.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1140-1154, 1988 [Google Scholar]
- 15. Harrell F. Regression Modeling Strategies. New York, NY, Springer-Verlag, 2001. [Google Scholar]
- 16.Therneau TM, Grambsch PM, Fleming TR: Martingale-based residuals for survival models. Biometrika 77:147-160, 1990 [Google Scholar]
- 17.Admiraal R, Nierkens S, de Witte MA, et al. : Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: A multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol 4:e183-e191, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. : Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: A multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol 2:e194-e203, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Socié G, Schmoor C, Bethge WA, et al. : Chronic graft-versus-host disease: Long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood 117:6375-6382, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Finke J, Schmoor C, Bethge WA, et al. : Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: Final results of a randomised controlled trial. Lancet Haematol 4:e293-e301, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Walker I, Panzarella T, Couban S, et al. : Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: A randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol 17:164-173, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy VE, Chen H, Savani BN, et al. : Optimizing anti-thymocyte globulin dosing based on recipient absolute lymphocyte count after unrelated allogeneic hematopoietic cell transplant. Blood 126:4410, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Podgorny PJ, Ugarte-Torres A, Liu Y, et al. : High rabbit-antihuman thymocyte globulin levels are associated with low likelihood of graft-vs-host disease and high likelihood of posttransplant lymphoproliferative disorder. Biol Blood Marrow Transplant 16:915-926, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Remberger M, Persson M, Mattsson J, et al. : Effects of different serum-levels of ATG after unrelated donor umbilical cord blood transplantation. Transpl Immunol 27:59-62, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Binkert L, Medinger M, Halter JP, et al. : Lower dose anti-thymocyte globulin for GvHD prophylaxis results in improved survival after allogeneic stem cell transplantation. Bone Marrow Transplant 50:1331-1336, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Salem G, Ruppert AS, Elder P, et al. : Lower dose of antithymocyte globulin does not increase graft-versus-host disease in patients undergoing reduced-intensity conditioning allogeneic hematopoietic stem cell transplant. Leuk Lymphoma 56:1058-1065, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayuk F, Diyachenko G, Zabelina T, et al. : Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant 14:913-919, 2008 [DOI] [PubMed] [Google Scholar]