Abstract
Purpose
To investigate the risk and outcomes of second hematologic malignancies (SHMs) in a population-based cohort of patients with well-differentiated thyroid cancer (WDTC) treated or not with radioactive iodine (RAI).
Methods
Patients with WDTC were identified from SEER registries. Competing risk regression analysis was performed to calculate the risks of SHMs that occurred after WDTC treatment and outcomes after SHM development were assessed.
Results
Of 148,215 patients with WDTC, 53% received surgery alone and 47% received RAI. In total, 783 patients developed an SHM after a median interval of 6.5 years (interquartile range, 3.3 to 11.2 years) from WDTC diagnosis. In multivariable analysis, compared with those undergoing thyroidectomy alone, RAI treatment was associated with an increased early risk of developing acute myeloid leukemia (AML; hazard ratio, 1.79; 95% CI, 1.13 to 2.82; P = .01) and chronic myeloid leukemia (CML; hazard ratio, 3.44; 95% CI, 1.87 to 6.36; P < .001). This increased risk of AML and CML after RAI treatment was seen even in low-risk and intermediate-risk WDTC tumors. Occurrence of AML but not CML in patients with WDTC was associated with shorter median overall survival compared with matched controls (8.0 years v 31.0 years; P = .001). In addition, AML developing after RAI trended toward inferior survival compared with matched controls with de novo AML (median overall survival, 1.2 years v 2.9 years; P = .06).
Conclusion
Patients with WDTC treated with RAI had an increased early risk of developing AML and CML but no other hematologic malignancies. AML that arises after RAI treatment has a poor prognosis. RAI use in patients with WDTC should be limited to patients with high-risk disease features, and patients with WDTC treated with adjuvant RAI should be monitored for myeloid malignancies as part of cancer surveillance.
INTRODUCTION
Papillary and follicular thyroid carcinomas are well differentiated thyroid cancers (WDTCs) and comprise > 90% of all thyroid cancer cases in the United States.1 Definitive therapy for WDTC is thyroidectomy with adjuvant radioactive iodine (RAI) to ablate residual or unresectable disease.2 In the last three decades, the incidence of WDTC increased four-fold, with the majority of the increase attributed to improved detection of small, low-risk tumors.1,9 Although adjuvant RAI improves overall and disease-free survival in advanced-stage WDTC, most studies report little or no benefit from RAI in low-risk and intermediate-risk tumors,2 where 5-year recurrence-free survival is already > 97% without RAI.4 Because the widespread use of adjuvant RAI has not improved survival,1 its clinical benefit in the treatment of WDTC is controversial.5 Furthermore, several meta-analyses have reported an increase in the incidence of second primary malignancies in patients with WDTC treated with RAI.6,7
Second hematologic malignancies (SHMs) occurring in patients treated for first cancers are a rare and devastating complication. In addition, the determination of whether acute myeloid leukemia (AML) is treatment related or not has significant prognostic and treatment implications.8,9 Although prior studies have shown an increased risk of SHMs in RAI-treated patients with WDTC, these analyses grouped all types of leukemia under one broad category.3,7,8,10-15 This approach oversimplifies risk estimation, considering the biologic heterogeneity among and within SHM entities, their disparate natural histories, and variable prognosis. Acknowledging the differences in pathogenesis and risk factors of different SHMs, we investigated the risk of developing acute and chronic leukemias of both myeloid and lymphoid lineage, lymphomas, and multiple myeloma in patients with WDTC treated with RAI and assessed outcomes.
METHODS
Study Design and Participants
The study cohort was assembled using the April 2017 release of all 18 registries of the SEER program of the National Cancer Institute. SEER provides data from population-based cancer registries, which cover approximately 28% of the US population. Patients were excluded from analysis if their thyroid malignancy was not of follicular or papillary histology (Data Supplement); if they received treatment with chemotherapy or tyrosine kinase inhibitors; if WDTC was not their first cancer; if their hematologic malignancy (HM) was a first, third, or higher order primary cancer; if they received external-beam radiotherapy; and if radiation or survival status was unknown. The primary outcome of interest was the development of SHM, defined as a nonsynchronous HM occurring ≥ 1 year after treatment of WDTC. SHMs included in this study were AML, chronic myeloid leukemia (CML), acute lymphoblastic leukemia, chronic lymphocytic leukemia (CLL), Hodgkin lymphoma, non-Hodgkin lymphoma, and multiple myeloma (MM), as defined by International Classification of Diseases for Oncology (3rd edition) histology codes and International Classification of Diseases (9th and 10th revision) codes (Data Supplement). Myelodysplastic syndromes (MDS) and Philadelphia chromosome-negative myeloproliferative neoplasms were excluded because of SEER-related differences between the reporting of MDS and Philadelphia chromosome-negative myeloproliferative neoplasms ALL, AML, CLL, CML, Hodgkin lymphoma, non-Hodgkin lymphoma, and MM (Data Supplement). SHMs occurring < 1 year after WDTC diagnosis were also excluded.16 Low-/intermediate-risk patients with WDTC were defined per the latest American Thyroid Association guidelines as T1-2N0 tumors ≤ 4 cm in size or T1-3N1 tumors in patients older than 45 years of age.2
Procedures
A previously validated R program, SEERaBomb,17 was used to assess risks of SHM after WDTC treatment in the SEER cohort and a subset of low-/intermediate-risk WDTCs. SEERaBomb was preferred over SEER*Stat MP-SIR (Multiple Primary-Standardized Incidence Ratio), a statistical companion tool developed by the National Cancer Institute, because SEERaBomb captures more patients with second primary cancer (Data Supplement). SHM risk dynamics after diagnosis of WDTC treated with surgery alone or surgery plus RAI were estimated using methodology previously published.17 SEERaBomb was used to calculate relative risk (RR) time courses for developing SHM after WDTC treatment on the basis of the ratio of the observed and expected patients with SHM for each WDTC treatment group. The expected number of patients with SHM was calculated using the background incidence rates of HMs in the US population and the person-years at risk for an SHM after treatment of WDTC as first cancer. RRs were adjusted for age at diagnosis, sex, and year of diagnosis. Additional potential covariables of interest analyzed are described in the Data Supplement. To assess outcomes of patients with WDTC who developed an SHM, we performed survival analyses using two separate case-control designs, in which each patient with WDTC who developed SHM was compared with either five patients with WDTC who did not develop SHM or with five patients whose HM occurred de novo. Cases and controls were matched by histology, type of treatment received, tumor stage, tumor size, age at diagnosis, sex, year of WDTC/HM diagnosis, and race, in that order of priority.
Statistical Analysis
RRs and RAI-attributable RR ratios with 95% CIs and P values were calculated as described in the Data Supplement and explained previously.18 Because of the low event rate of SHMs, Fine-Gray competing risk regression analyses19 were performed with SHM as a time-dependent end point and death from all causes or development of non-SHM malignancy were treated as competing events to calculate hazard ratios (HRs) with 95% CIs of developing an SHM after WDTC. Censoring occurred at follow-up cutoff defined by the April 2017 SEER release (January 1, 2015), death, development of a second primary cancer other than the HM of interest, or when 20 years of follow-up after WDTC treatment were reached, whichever occurred first. Cox regression and standardized incidence ratio (SIR) calculations were performed to compare our results with previous studies that used these procedures to assess hazards of developing an SHM after WDTC treatment. Variables significant at an alpha level of .05 (two-sided) in univariable analyses were included in multivariable analyses. The final multivariable models were built using a backward selection procedure. For regression analyses and SIR calculations, the follow-up period was limited to 20 years to focus on early-onset SHMs because SHMs occurring in relatively young survivors of WDTC have treatment implications. Survival plots were made using the Kaplan-Meier method, and P values for overall survival (OS) comparisons were calculated using the Gehan-Breslow-Wilcoxon test to provide extra weight to early outcomes. All statistical analyses were performed using R software, and all scripts used to produce the results of this study are provided in the Data Supplement.
RESULTS
Patient Characteristics
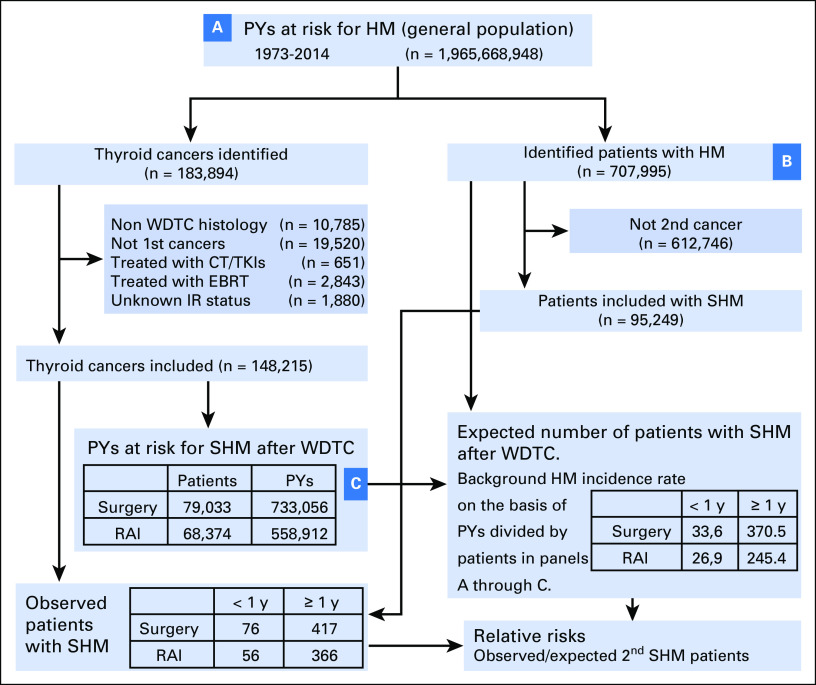
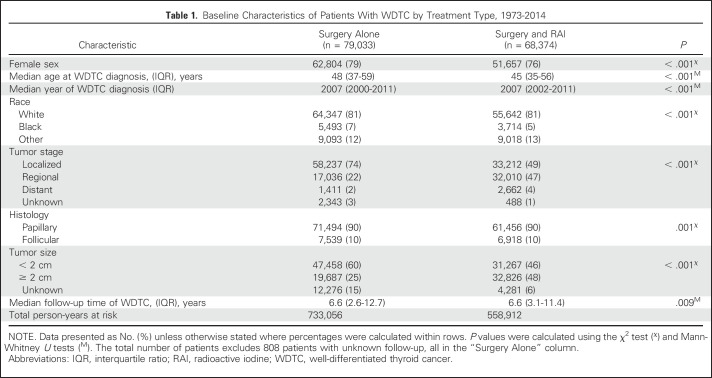
Of the 183,894 patients with thyroid cancer identified from the SEER database, 148,215 patients met the inclusion criteria (Fig 1). Baseline demographic and disease characteristics of patients with WDTC by treatment modality are listed in Table 1. A total of 79,033 patients (53%) received surgery alone, and 68,374 patients (47%) received surgery plus RAI. Among the survivors of WDTC, a total of 783 nonsynchronous SHMs were identified, 417 (53%) after surgery alone and 366 (47%) after surgery plus RAI (Data Supplement). Comparisons of characteristics of patients with WDTC on the basis of RAI treatment status who later developed SHM versus those who did not are shown in the Data Supplement.
Fig 1.
Population-based assessments of second hematologic malignancy (SHM) risks after well-differentiated thyroid cancers (WDTCs). SEER covers an increasing proportion of the US population, 1.97 billion person-years (PYs) since 1973. Shown is a flowchart of the inclusion of patients with WDTC and SHM and their use in calculations of relative risks (RRs) of SHM occurrence after WDTC. RRs are the number of observed patients with SHM after WDTCs divided by the number of expected patients with SHM after WDTCs. The latter is the background incidence rate of SHM per PY, which is calculated by dividing the number of hematologic malignancy (HM) patients by (B) the number of PYs at risk in the general population (A). Separate calculations were performed for each year of age, sex, and year of diagnosis. This was then multiplied by (C), the PYs at risk among WDTC survivors in these demographic cohorts to obtain the expected number of patients with SHMs after WDTCs. In the boxes entitled, “Expected patients with SHM after WDTC. Background on the basis of PYs divided by patients in panels A though C,” the numbers in the boxes represent the expected numbers of patients with SHM diagnosed <1 year or <1 year after WDTC diagnosis, separated by treatment (surgery or surgery+RAI). In box “PYs at risk for SHM after WDTC,” the total number of patients excludes 808 patients with insufficient follow-up. AML, acute myeloid leukemia; CML. chronic myeloid leukemia; CT, chemotherapy; IR, ionizing radiation; EBRT, external beam radiotherapy; int, intermediate; RAI, radioactive iodine; TKI, tyrosine kinase inhibitor; y, year.
Table 1.
Baseline Characteristics of Patients With WDTC by Treatment Type, 1973-2014
Risk of SHMs by Treatment Modality
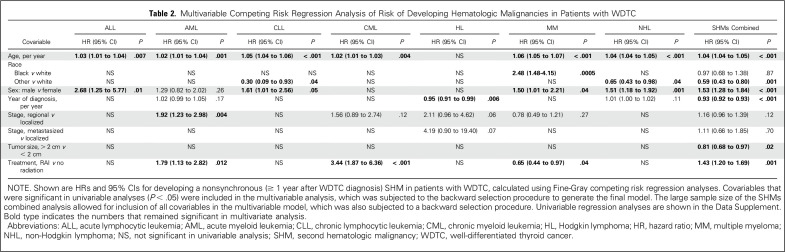
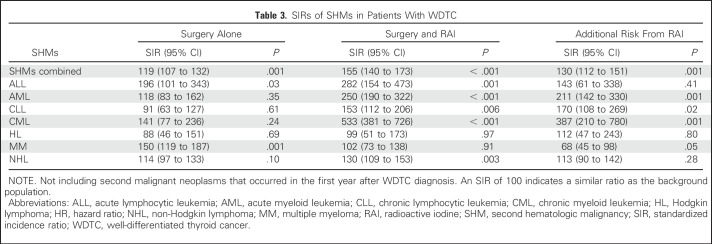
All patient characteristics listed in Table 1 were tested for associations with SHMs as the outcome of interest in univariable (Data Supplement) and multivariable Fine-Gray competing risk regression analysis (Table 2). In multivariable analysis, surgery plus RAI was associated with a significant increase in the risk of developing SHMs (pooled as a group) compared with surgery alone (HR, 1.43; 95% CI, 1.20 to 1.69; P < .001). When analyzed by SHM type, the elevated risk was significant for AML (HR, 1.79; 95% CI, 1.13 to 2.82; P = .01) and CML (HR, 3.44; 95% CI, 1.87 to 6.36; P < .001), but no other SHMs (Table 2). The cumulative risk of any SHM in the first 10 years after WDTC treatment was 0.40% after surgery alone and 0.54% after surgery plus RAI. Cumulative risks of AML and CML during the same time period were 0.08% and 0.01% after surgery alone and 0.12% and 0.06% after surgery plus RAI, respectively. SIR calculations were used to compare the incidence of SHMs among survivors of WDTC with the incidence rates of these HMs in the US population, adjusted for age, sex, and year of WDTC diagnosis. SIRs for the development of all SHMs combined were higher after both surgery alone (SIR, 119; 95% CI, 107 to 132; P = .001) and surgery plus RAI (SIR, 155; 95% CI, 140 to 173; P < .001; Table 3 and Data Supplement). When analyzed by specific SHM, SIRs after surgery plus RAI were significantly higher for ALL, AML, CLL, CML, and non-Hodgkin lymphoma. Excess risk attributable to RAI was observed for all SHMs combined (SIR, 130; 95% CI, 112 to 151; P = .001), and individually for AML (SIR, 211; 95% CI, 142 to 330; P = .001), CLL (SIR, 170; 95% CI, 108 to 269; P = .02), and CML (SIR, 387; 95% CI, 210 to 780; P < .001; Table 3). Conversely, RAI treatment was associated with decreased risk of developing MM (HR, 0.65; 95% CI, 0.44 to 0.97; P = .04; Table 2) and lower SIR (SIR, 68; 95% CI, 45 to 98; P = .05; Table 3).
Table 2.
Multivariable Competing Risk Regression Analysis of Risk of Developing Hematologic Malignancies in Patients with WDTC
Table 3.
SIRs of SHMs in Patients With WDTC
Risk Dynamics of SHMs After WDTC Treatment
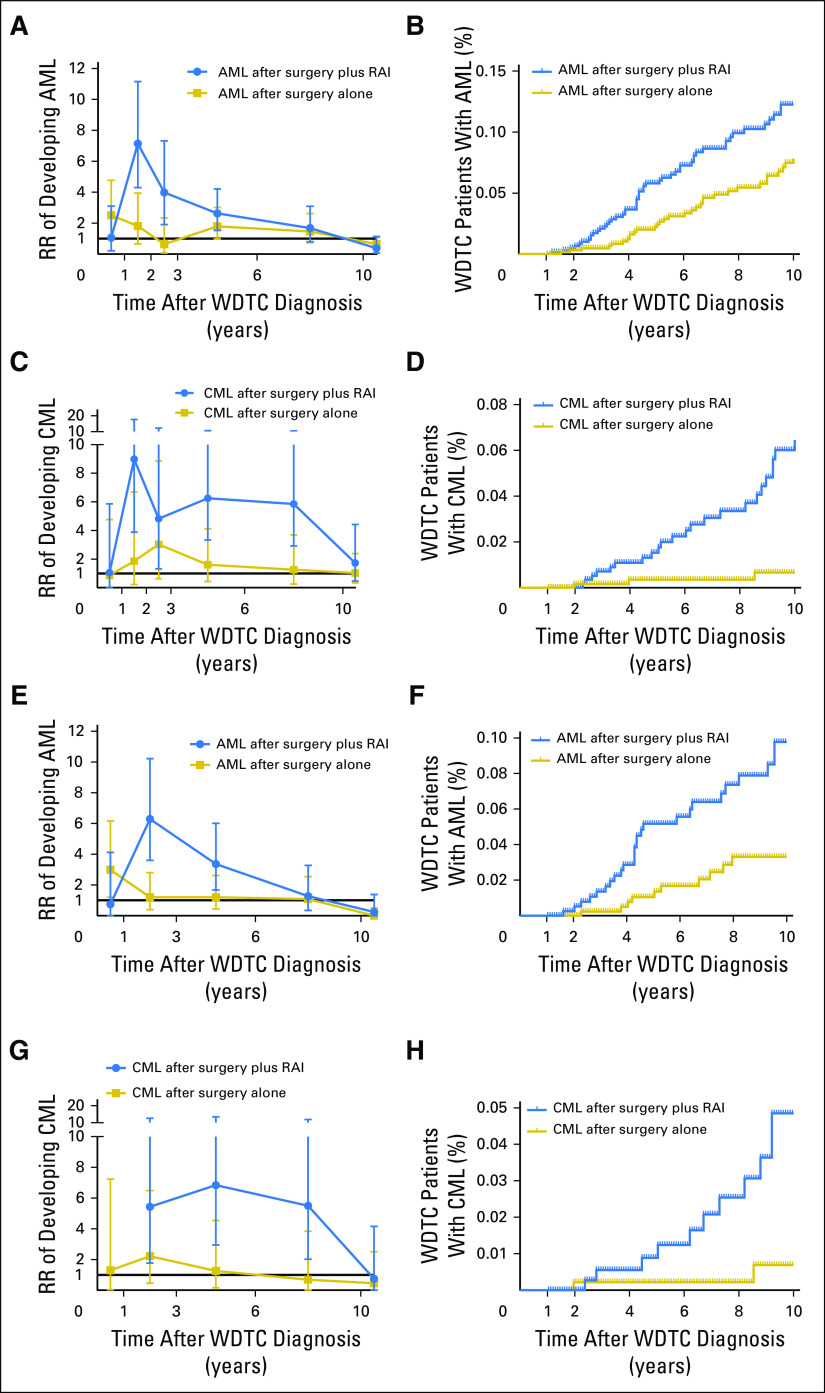
RR time courses and time-to-event courses of developing SHMs after WDTC are shown in Figures 2A, 2B, 2C, and 2D and the Data Supplement. Compared with the background incidence rate of AML, an early increase in the risk of AML was observed in patients with WDTC treated with surgery and RAI that peaked in the second year after treatment (RR, 7.1; 95% CI, 4.3 to 11.2; P < .001; Fig 2A). Beyond 2 years, the risk of AML declined, reaching baseline rates within 6 years after WDTC diagnosis. A similar significant increase in risk of CML was observed in the second year after RAI exposure compared with the background rates; however, this risk remained elevated up to 10 years after WDTC diagnosis (RR for years 2 to 10, 6.3; 95% CI, 4.4 to 8.8; P < .001; Fig 2C). In time-to-event analyses, surgery plus RAI was significantly associated with an increased risk of developing AML compared with surgery alone (HR, 1.6; 95% CI, 1.1 to 2.4; P = .01; Fig 2B) and CML (HR, 2.9; 95% CI, 1.7 to 5.2; P = .001; Fig 2D) but no other SHMs in patients with WDTC treated with adjuvant RAI compared with thyroidectomy alone (Data Supplement). When the RRs of the surgery alone and surgery plus RAI groups were directly compared using radiation-related RR ratios, we observed increased radiation-related RR ratios for AML and CML but no other SHMs (Data Supplement).
Fig 2.
Risk time courses for developing second hematologic malignancy (SHM) after well-differentiated thyroid cancer (WDTC) diagnosis. (A to D) Data for all patients with WDTC; (E to H) data for patients with low-/intermediate-risk WDTC (as defined by the American Tumor Association).2 (A, C, E, G) Plotted are mean relative risks (RRs) ± 95% CIs of developing (A, E) acute myeloid leukemia (AML) or (C, G) chronic myeloid leukemia (CML) as second cancer, on the basis of WDTC treatment type compared with the background US population, which is represented by the black line at y = 1. The number of person-years at risk, expected and observed patients, RRs, and 95% CIs for each RR time course graph are shown in the Data Supplement. Risk-time courses for SHMs other than AML or CML are shown in the Data Supplement. (B, D, F. H) Plotted are the percentage of patients with WDTC diagnosed with (B, F) AML or (D, H) CML as function of the years after WDTC diagnosis. Patients were censored at death, when they were alive at January 1, 2015, or when they developed a non-SHM second cancer. Additional hazard curves are shown in the Data Supplement. P values were calculated using the Gehan-Breslow-Wilcoxon test. RAI, radioactive iodine.
Risk of SHMs in Low-/Intermediate-Risk WDTCs
In a subset analysis among patients with low-risk or intermediate-risk WDTCs, where adjuvant RAI carries no or questionable clinical benefit,2 RAI treatment was the only factor in Fine-Gray competing risk regression analyses that was significantly associated with the development of AML (HR, 2.87; 95% CI, 1.46 to 5.63; P = .002) and CML (HR, 3.94; 95% CI, 1.58 to 9.82; P = .003; Data Supplement). RAI treatment was also associated with increased RRs and decreased SHM-free survival for AML and CML in patients with low-risk or intermediate-risk WDTCs (Figs 2E, 2F, 2G, and 2H and Data Supplement).
Outcomes After Development of AML and CML
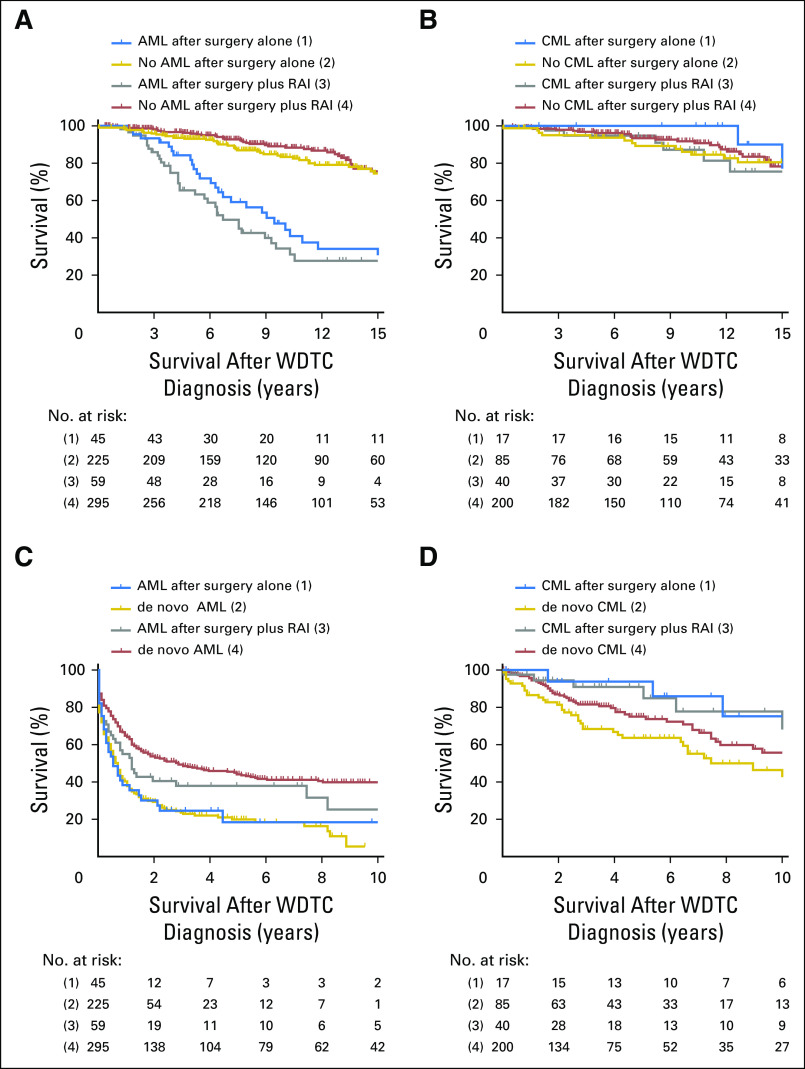
Regardless of the type of treatment received, patients with WDTC who developed AML had shorter OS compared with matched patients with WDTC who did not (median OS, 8.0 years v 31.0 years; P = .001; Fig 3A and Data Supplement). Between the WDTC treatment groups, there was a trend toward truncated OS in those who developed AML after surgery plus RAI compared with patients who developed AML after surgery alone (median OS, 6.7 years v 9.4 years; P = .12). Consistent with a good prognosis of CML, the OS of patients with WDTC who developed CML after surgery alone or surgery plus RAI was not significantly different from matched controls (Fig 3B). Compared with matched population controls with de novo AML, there was no difference in the OS of patients with WDTC who developed AML after surgery, and there was a trend toward decreased OS in patients with AML after RAI treatment (median OS, 1.2 years v 2.9 years; P = .06; Fig 3C and Data Supplement). We observed no differences in OS on the basis of whether CML occurred after WDTC treatment or de novo (Fig 3D).
Fig 3.
Survival curves of patients with well-differentiated thyroid cancer (WDTC) by development of acute myeloid leukemia (AML) or chronic myeloid leukemia (CML) and by treatment type. Shown are Kaplan-Meier plots of case-control studies wherein the following groups were compared: patients with WDTC who developed (A) AML or (B) CML after WDTC treatment (cases) versus those who did not (controls); (C) patients with AML and (D) CML who were diagnosed with these diseases after treatment of WDTC (cases) versus those who developed AML or CML de novo (controls). In all figures, (2) are matched controls for (1), and (4) are matched controls for (2). P values were calculated using the Gehan-Breslow-Wilcoxon test. RAI, radioactive iodine.
DISCUSSION
With rising incidence rates of WDTC20 and a growing population of long-term survivors of WDTC who received prior RAI, there is a clinical concern regarding the risks of adverse effects from this treatment.3,5 This concern is particularly heightened because population-level data show that a majority of patients with WDTC treated with RAI have low-risk tumors, a scenario where patients likely do not derive therapeutic benefit from adjuvant RAI but are exposed to its carcinogenic effects.11 In this population-based study, we performed a comprehensive evaluation of the risk dynamics for development of SHMs in patients with WDTC and the clinical outcome of WDTC patients who developed SHM. The main findings include that (1) patients with WDTC exposed to RAI have a significantly increased risk of AML and CML compared with background incidence rates in the US population; (2) increased risk for AML and CML is seen even in low-/intermediate-risk patients with WDTC treated with RAI; (3) the latency period for AML and CML after RAI therapy is short; (4) although the risk of AML declines quickly to baseline rates by 3 years, the risk of CML remains elevated for up to 10 years after RAI treatment; and (5) development of AML in patients with WDTC predicted for truncated survival compared with de novo AML.
Comparison of SHM risk attributable to RAI across different studies is challenging for several reasons, because of varying definitions of WDTC and SHM nomenclature, including grouping of disparate SHM histologies under broad leukemia and lymphoma categories, differences in methodologic and statistical considerations, and length of follow-up duration—all affecting the interpretation of results.3,6,7,10-15 On one hand, our RR time plots show that the median follow-up of 6.5 years after WDTC diagnosis is adequate for SHM risk assessments; on the other hand, a proportion of SHMs developing in atomic bomb survivors occurred at even later time points21 and these late occurrences may not have been captured by our analysis. We chose to use Fine-Gray competing risk regressions because this approach adequately corrects the risk of developing SHM against the competing risks of occurrence of a nonhematologic second primary malignancy or death, either WDTC-related or WDTC-unrelated. This is a critical consideration because most patients with WDTC are long-term survivors who continue to be at risk for developing solid tumor malignancies and have increased treatment-related cardiovascular mortality.22 Although the occurrence of AML23-26 and CML27 after RAI treatment of WDTC has been previously reported, to our knowledge, this is the first comprehensive report of risk dynamics of individual SHM entities over time after RAI treatment of WDTC. Furthermore, our analyses did not show increased hazards for the development of other SHMs among WDTC survivors previously treated with RAI. Although SIRs yield interesting data on the frequency of SHMs in RAI-treated WDTC survivors compared with the background population (2.5 times higher for AML and 5.3 times higher for CML), SIRs were only corrected for age, sex, and year of diagnosis, but not for other possible confounders. Whereas the Cox regression is inferior for situations where competing risks are at play, they were the preferred approach in previous studies that described second primary cancer risk after RAI treatment of WDTC.10 Therefore, we also performed Cox regression analysis to compare our results with those of previous studies and arrived at the same conclusions resulting from our competing risk regressions (Data Supplement). In conclusion, our findings clearly demonstrate increased hazards of developing myeloid leukemias but no other type of SHMs with adjuvant RAI use. An interesting finding in our study was lower risks of MM after RAI treatment compared with thyroidectomy, the possible mechanism of which needs further investigation.
This study has certain limitations. The decision to use RAI is contingent on several covariables of interest that are not captured in the SEER cohort, such as completeness of resection, tumor multicentricity, and findings from postoperative radiologic scans.2 The SEER database does not record the RAI doses administered to patients; hence, it is not possible to determine the leukemogenic dose-response effect of RAI that some non-SEER studies have shown.10,12 Another drawback is that SEER only captures radiation data during initial treatment and not if patients received delayed radiation or radiation for recurrent disease. Although this can potentially lead to misclassification of patients with RAI-positive disease into the RAI-negative cohort, this is unlikely to affect our conclusions and if at all present, might reflect an underestimation of the elevated risk attributable to RAI. Another limitation of a retrospective study such as ours is that it may be vulnerable to overascertainment bias, a possible explanation of more recorded occurrences of myeloid leukemias after RAI treatment. However, such assumptions are incompatible with the quick rise and fall in AML risk dynamics that we observed. To further address the issue of latency impacting risk estimates, we re-ran the SHM risk analysis using a 2-year cut-off that excluded all SHMs occurring within 24 months from the diagnosis of WDTC. Our repeat analysis showed that the risk of AML and CML following RAI exposure in WDTC patients continued to be significantly elevated even with 2-year cut-off (Data Supplement 2 [http://ascopubs.org/doi/suppl/10.1200/JCO.2017.75.0232/suppl_file/ds_2017.75.0232.pdf]). The strengths of this study include a large population with relatively homogenous treatment exposure; a novel methodology to maximize capture of patients with SHM across all 18 SEER registries, adjusting for competing risk in statistical analysis; and information on post-SHM outcomes.
Development of therapy-related AML is a devastating complication because of its dismal prognosis.8,9 Although patients with WDTC who developed an RAI-related AML had a worse prognosis than matched patients with de novo AML, outcomes for AML that arose after thyroidectomy were comparable to matched de novo controls. This suggests that AML that occurs after RAI treatment of WDTC resembles a treatment-related AML (t-AML) phenotype, which is characterized by inherent refractoriness to conventional chemotherapies.8,9 Our findings corroborate a previous comparison of patients with AML after RAI administration for thyroid cancer or hyperthyroidism and patients with de novo AML.25 A higher proportion of patients with AML with an antecedent history of RAI therapy harbored high-risk cytogenetic abnormalities similar to t-AML/treatment-related MDS arising after other cytotoxic anticancer treatments.25 Unfortunately, SEER does not carry genomic information to facilitate interrogation of molecular and cytogenetic features of SHM arising after RAI treatment.
Our results demonstrate the importance of avoiding treatment with RAI in patients with low-risk or intermediate-risk disease, in whom RAI has shown no or questionable benefit.2 Furthermore, our results support using the least effective dose to treat patients who have high-risk features to avoid excess bone marrow exposure, because the risk of SHM is dose dependent.10,12 These results should also be incorporated in the surveillance strategies for patients who receive high doses of RAI to appropriately monitor blood counts to detect development of myeloid malignancies. It is encouraging to see that after the 2009 release of guidelines from American Thyroid Association, there has been a modest decrease in the use of RAI.28 Strict adherence to these guidelines is essential to decrease the catastrophic consequence of inducing t-AML with RAI in a group of cancers with high cure rates affecting a relatively young patient population.
Supported by American Cancer Society, Institutional research Grant No. IRG-91-022-18 to S.M. and an AMC PhD Scholarship (2013-2017) to R.J.M.
Presented at the 2014 ASCO annual meeting, Chicago, IL, May 30 to June 3, 2014.
R.J.M. and S.S. contributed equally to this study.
AUTHOR CONTRIBUTIONS
Conception and design: Remco J. Molenaar, Surbhi Sidana, Anjali S. Advani, Aaron T. Gerds, Hetty E. Carraway, Sudipto Mukherjee
Financial support: Remco J. Molenaar, Sudipto Mukherjee
Administrative support: Matt Kalaycio, Mikkael A. Sekeres
Collection and assembly of data: Remco J. Molenaar, Surbhi Sidana, Sudipto Mukherjee
Data analysis and interpretation: Remco J. Molenaar, Surbhi Sidana, Tomas Radivoyevitch, Dana Angelini, Matt Kalaycio, Aziz Nazha, David J. Adelstein, Christian Nasr, Jaroslaw P. Maciejewski, Navneet S. Majhail, Mikkael A. Sekeres, Sudipto Mukherjee
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk of Hematologic Malignancies After Radioiodine Treatment of Well-Differentiated Thyroid Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Remco J. Molenaar
No relationship to disclose
Surbhi Sidana
Honoraria: Janssen
Tomas Radivoyevitch
No relationship to disclose
Anjali S. Advani
Honoraria: Sigma-Tau, EUSA Pharma
Consulting or Advisory Role: Seattle Genetics
Research Funding: Pfizer (Inst), Millennium (Inst), KaloBios (Inst), Seattle Genetics (Inst)
Aaron T. Gerds
Consulting or Advisory Role: Incyte, AstraZeneca/MedImmune, CTI
Research Funding: Pfizer, CTI, Incyte, Roche/Genentech, Gilead Sciences, Celgene
Hetty E. Carraway
Honoraria: Celgene, Novartis, Baxalta, Jazz Pharmaceuticals, Agios
Consulting or Advisory Role: Jazz Pharmaceuticals, Celgene, Agios, Astellas Pharma, H3 Biomedicine, Baxalta
Speakers' Bureau: Celgene, Agios, Baxalta
Research Funding: Celgene
Travel, Accommodations, Expenses: Celgene, Agios, Astellas Pharma, Baxalta
Dana Angelini
No relationship to disclose
Matt Kalaycio
No relationship to disclose
Aziz Nazha
No relationship to disclose
David J. Adelstein
No relationship to disclose
Christian Nasr
Honoraria: Sanofi, Nevro, Shire
Speakers' Bureau: Sanofi, Shire
Travel, Accommodations, Expenses: Sanofi, Nevro
Jaroslaw P. Maciejewski
No relationship to disclose
Navneet S. Majhail
Consulting or Advisory Role: Anthem
Travel, Accommodations, Expenses: Sanofi
Mikkael A. Sekeres
Consulting or Advisory Role: Celgene, Millennium
Sudipto Mukherjee
Honoraria: Novartis
Consulting or Advisory Role: Novartis, Takeda Pharmaceuticals, Bristol Myers Squibb
Research Funding: Novartis, Celgene
REFERENCES
- 1. Surveillance E, Results E: (SEER) Program ( www.seer.cancer.gov) Research Data (1973-2014), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2017, based on the November 2016 submission.
- 2.Haugen BR, Alexander EK, Bible KC, et al. : 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1-133, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer NG, Morris LG, Tuttle RM, et al. : Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer 117:4439-4446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nixon IJ, Ganly I, Patel SG, et al. : The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid 23:683-694, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Haymart MR, Banerjee M, Stewart AK, et al. : Use of radioactive iodine for thyroid cancer. JAMA 306:721-728, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian S, Goldstein DP, Parlea L, et al. : Second primary malignancy risk in thyroid cancer survivors: A systematic review and meta-analysis. Thyroid 17:1277-1288, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Sawka AM, Thabane L, Parlea L, et al. : Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: A systematic review and meta-analysis. Thyroid 19:451-457, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Klimek VM, Tray NJ: Therapy-related myeloid neoplasms: What’s in a name? Curr Opin Hematol 23:161-166, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Arber DA, Orazi A, Hasserjian R, et al. : The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127:2391-2405, 2016 [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1093/jnci/djv314. Teng CJ, Hu YW, Chen SC, et al: Use of radioactive iodine for thyroid cancer and risk of second primary malignancy: A nationwide population-based study. J Natl Cancer Inst 108:djv314, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Brown AP, Chen J, Hitchcock YJ, et al. : The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 93:504-515, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Rubino C, de Vathaire F, Dottorini ME, et al. : Second primary malignancies in thyroid cancer patients. Br J Cancer 89:1638-1644, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu CH, Lee KD, Chen PT, et al. : Second primary malignancies following thyroid cancer: A population-based study in Taiwan. Eur J Endocrinol 169:577-585, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Ronckers CM, McCarron P, Ron E: Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer 117:281-288, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Sandeep TC, Strachan MW, Reynolds RM, et al. : Second primary cancers in thyroid cancer patients: A multinational record linkage study. J Clin Endocrinol Metab 91:1819-1825, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (ed 4). Lyon, France, IARC, 2008. [Google Scholar]
- 17.Radivoyevitch T, Sachs RK, Gale RP, et al. : Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation. Leukemia 30:285-294, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Bland JM: How to obtain the confidence interval from a P value. BMJ 343:d2090, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Scrucca L, Santucci A, Aversa F: Regression modeling of competing risk using R: An in depth guide for clinicians. Bone Marrow Transplant 45:1388-1395, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Lim H, Devesa SS, Sosa JA, et al. : Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 317:1338-1348, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu WL, Preston DL, Soda M, et al. : The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res 179:361-382, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein Hesselink EN, Klein Hesselink MS, de Bock GH, et al. : Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: An observational study. J Clin Oncol 31:4046-4053, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Oluwasanjo A, Pathak R, Ukaigwe A, et al. : Therapy-related acute myeloid leukemia following radioactive iodine treatment for thyroid cancer. Cancer Causes Control 27:143-146, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Seidlin SM, Siegal E, Yalow AA, et al. : Acute myeloid leukemia following prolonged iodine-131 therapy for metastatic thyroid carcinoma. Science 123:800-801, 1956 [DOI] [PubMed] [Google Scholar]
- 25.Schroeder T, Kuendgen A, Kayser S, et al. : Therapy-related myeloid neoplasms following treatment with radioiodine. Haematologica 97:206-212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. doi: 10.3324/haematol.2012.067454. Gilabert M, Prebet T: Acute leukemia arising after radioiodine treatment for thyroid cancer. Haematologica 97:e28-e29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimon I, Kneller A, Olchovsky D: Chronic myeloid leukaemia following 131I treatment for thyroid carcinoma: A report of two cases and review of the literature. Clin Endocrinol (Oxf) 43:651-654, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Roman BR, Feingold JH, Patel SG, et al. : The 2009 American Thyroid Association guidelines modestly reduced radioactive iodine use for thyroid cancers less than 1 cm. Thyroid 24:1549-1550, 2014 [DOI] [PubMed] [Google Scholar]