Abstract
PURPOSE
Platinum compounds have activity in triple-negative breast cancer (TNBC) in germline BRCA mutation carriers (BRCA carriers). Limited data exist for estrogen receptor (ER)–positive (+) breast cancer among BRCA carriers. INFORM is a randomized, multicenter, phase II trial comparing pathologic complete response (pCR) rates (ypT0/is, N0) after neoadjuvant single-agent cisplatin (CDDP) versus doxorubicin-cyclophosphamide (AC) in BRCA carriers with stage I-III human epidermal growth factor receptor 2 (HER2)–negative breast cancer. Secondary objectives included residual cancer burden scores (RCB) of 0 or 1 (combined) and toxicity. The goal was to determine whether pCR was ≥ 20% higher with CDDP than AC.
PATIENTS AND METHODS
BRCA carriers with cT1-3 (≥ 1.5 cm), cN0-3 HER2-negative breast cancer were randomly assigned to preoperative CDDP (75 mg/m2 every 3 weeks × 4 doses) or AC (doxorubicin 60 mg/m2; cyclophosphamide 600 mg/m2 every 2-3 weeks × 4 doses) followed by surgery. Pathologic responses were confirmed by central review.
RESULTS
A total of 118 patients were randomly assigned; 117 were included in outcome analyses. Mean age was 42 years (range, 24-73 years); 69% were BRCA1+, 30% were BRCA2+, and 2% had both mutations. Clinical stage was I for 19%, II for 63%, and III for 18%; 45% had nodal involvement at baseline. Seventy percent had TNBC. Clinical and tumor characteristics were well matched between treatment arms. The pCR rate was 18% with CDDP and 26% with AC, yielding a risk ratio (RR) of 0.70 (90% CI, 0.39 to 1.2). The risk of RCB 0 or 1 (RCB 0/1) was 33% with CDDP and 46% with AC (RR, 0.73; 90% CI, 0.50 to 1.1). Both regimens were generally well tolerated without unexpected toxicities.
CONCLUSION
pCR or RCB 0/1 is not significantly higher with CDDP than with AC in BRCA carriers with stage I-III HER2-negative breast cancer for both TNBC and ER+/HER2-negative disease.
INTRODUCTION
Prior studies have shown that platinum chemotherapy agents are active in the treatment of breast cancer that develops in germline BRCA mutation carriers (BRCA carriers).1 In the neoadjuvant setting, a single-arm prospective study using cisplatin monotherapy reported a pathologic complete response (pCR) rate of 61% among BRCA carriers, most of whom had BRCA1 mutations and triple-negative breast cancer (TNBC).2 In contrast, a retrospective analysis from the same group reported a 22% pCR rate among BRCA1 carriers who had been treated with doxorubicin and cyclophosphamide (AC) or the same regimen with fluorouracil.3
The Triple Negative Breast Cancer Trial (TNT) randomly assigned patients with metastatic TNBC to either docetaxel or carboplatin in the first-line treatment setting.4 Results showed that carboplatin was associated with a significantly higher overall response rate and progression-free survival for the 43 BRCA carriers enrolled (32 with TNBC, 11 with estrogen receptor [ER]–positive [+] disease) in contrast to those without a germline BRCA mutation. Of note, the response to docetaxel was similar for BRCA carriers and noncarriers. The TNT trial results confirmed those of smaller studies that demonstrated a higher response to platinum chemotherapy for TNBC in BRCA carriers than in noncarriers.5,6
Although platinum agents have demonstrated activity for the treatment of breast cancer in BRCA carriers, no randomized prospective data exist comparing response to platinum with response to a standard, anthracycline-based regimen in BRCA carriers with newly diagnosed breast cancer. In addition, there are almost no data evaluating the efficacy of platinum agents in ER+ breast cancer in BRCA carriers.
The INFORM trial (Translational Breast Cancer Research Consortium [TBCRC] 031) was an investigator-initiated, prospective, randomized multicenter neoadjuvant trial comparing neoadjuvant single-agent cisplatin (CDDP) with AC for BRCA carriers with stage I-III human epidermal growth factor receptor 2 (HER2)–negative breast cancer. The primary objective was to assess pCR (ie, residual cancer burden [RCB] 0; ypT0/is, N0, and secondary objectives included assessing RCB 0 or 1 (RCB 0/1) responses combined and toxicity. The primary goal was to determine whether pCR was ≥ 20% higher with CDDP than AC.
PATIENTS AND METHODS
The INFORM trial was conducted at 13 academic centers.
Patient Population
Eligible patients had a germline pathogenic or likely pathogenic variant (ie, mutation) in BRCA1 or BRCA2; genetic testing was not performed as part of the trial, but known genetic status was required for eligibility. Patients must have had clinical T1-3, N0-2, biopsy-confirmed, previously untreated, HER2-negative invasive breast cancer, defined by immunohistochemical (IHC) staining 0 to 1+ or fluorescence in situ hybridization ratio < 2.0 if IHC 2+ or IHC was not performed. A tumor size of at least 1.5 cm was required; in December 2017 (after participant No. 97 was enrolled), to increase accrual, an amendment allowed T size ≥ 1.0 cm, consistent with some other ongoing neoadjuvant trials.7 ER and progesterone receptor (PR) status of the tumor was determined locally before registration. Participants with multicentric or bilateral disease were eligible if at least one lesion met stage eligibility criteria and no tumor was HER2+. Patients with metastatic disease were ineligible; in patients with clinical stage III disease, imaging studies were recommended to exclude overt metastatic disease. Previous chemotherapy was not allowed; in December 2017, an amendment allowed patients with prior chemotherapy received for any other cancers to enroll, but patients who had received prior anthracycline or platinum chemotherapy were still ineligible. Adequate hematologic, renal, hepatic, and cardiac (echocardiogram or radionuclide ventriculogram) function; Eastern Cooperative Oncology Group performance status 0-1; and negative pregnancy test in women of childbearing potential were required. Patients were excluded for grade ≥ 2 baseline neuropathy. Imaging of the ipsilateral axilla was required before registration. In patients with clinically positive axillae, histologic confirmation by biopsy or fine-needle aspiration was mandatory before enrollment.
Treatment and Study Procedures
Figure 1 illustrates the treatment schema. Patients were randomly assigned 1:1 to either cisplatin 75 mg/m2 intravenously (IV) × 4 cycles or to AC (doxorubicin 60 mg/m2 IV and cyclophosphamide 600 mg/m2 IV) × 4 cycles every 2 (dose dense [dd]) or 3 weeks. Randomization was stratified by tumor ER status (≥ 1% v < 1%) and by treatment site. A dd schedule was mandatory for patients with TNBC. Growth factor was mandatory for those receiving ddAC but was optional for all other participants. Tumor biopsies for correlative studies—1 to 2 fixed cores in RNA later, Optimal Cutting Temperature (O.C.T.) medium, and formalin—were required before initiating chemotherapy.
FIG 1.
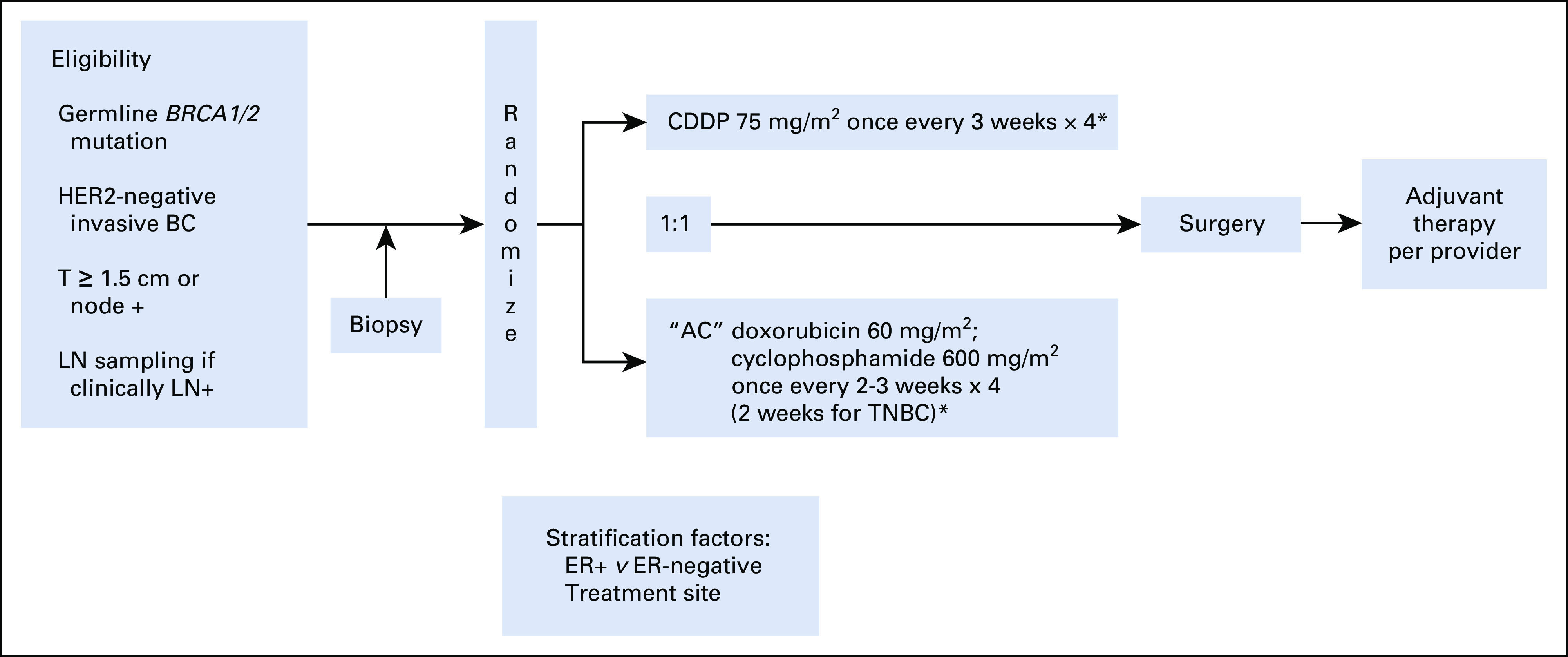
Schema of randomized phase II INFORM trial (TBCRC 031). (*) Granulocyte colony–stimulating factor (G-CSF) mandatory for dose-dense AC (once-every-2-weeks schedule). G-CSF optional for AC once every 3 weeks and cisplatin. AC, doxorubicin plus cyclophosphamide; BC, breast cancer; CDDP, cisplatin; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LN, lymph node; T, tumor size; TNBC, triple-negative breast cancer.
After 4 cycles of protocol-assigned chemotherapy, participants underwent definitive breast surgery (mastectomy or lumpectomy); sentinel lymph node biopsy (SLNB) or axillary dissection was required for all participants. For participants who were determined to have evidence of clinically significant residual disease by physical examination or imaging after completion of protocol-assigned chemotherapy, an image-guided biopsy was required before the initiation of additional therapy. Radiation therapy was mandated for all participants not having a mastectomy. For participants with a clinically negative axilla, an SLNB was performed. Although not mandated by the protocol, it was strongly recommended that participants with pathologically proven positive lymph nodes at baseline undergo a level I and II lymph node dissection at the time of definitive surgery.
Pathologic Evaluation
Baseline tumor features and pathologic responses were centrally determined by the study pathologist (S.J.S.). The prechemotherapy clinical biopsy was assessed for histologic type and grade, lymphovascular invasion, and tumor infiltrating lymphocytes by the International Tumor Infiltrating Lymphocytes (TILs) Working Group criteria.8 Stromal TILs also were scored on a binary scale as moderate/marked for patients with ≥ 25% stromal TILs and absent/scant for patients with < 25% stromal TILs, because when the central pathology review for the trial began, the International TILs Working Group had not yet established criteria or published its guideline for evaluation of TILs. Tumor excision and axillary nodes were assessed for residual cancer. Residual disease in the breast was assessed for overall cancer cellularity (as a percentage), tumor bed area measured in two dimensions, and percentage of cancer that was in situ disease. Residual disease in axillary nodes was assessed for number of involved nodes and diameter of the largest metastasis. pCR in the breast was defined as the absence of residual invasive disease with or without ductal carcinoma in situ (ypT0/is). Participants with pCR in the breast and negative pretreatment SLNB were considered to have achieved pCR in the breast/axilla. The RCB score was determined for each patient using the online calculator available on the MD Anderson Cancer Center website.9
Analysis Plan
The target accrual was 170 participants. Assuming a 2-sided alpha of .1 and allowing for up to 10% dropout, this sample size provided 80% power to detect an improvement in pCR from 30% with AC to 50% with CDDP. This also allowed for an interim analysis for efficacy after 43 participants in each treatment arm completed the trial. The O’Brien-Fleming group sequential design was used to determine stopping boundaries for a 2-sided alpha of the primary outcome for the interim analysis. The data safety monitoring board reviewed the interim analysis. An intent-to-treat approach was used, along with log-binomial regression to calculate risk ratios (RRs; 90% CIs) for the primary outcome (ie, the likelihood of attaining pCR, as well as RCB 0/1, with cisplatin compared with AC). RRs and 95% CIs were calculated for all other analyses. The protocol was approved by the institutional review board of the Dana-Farber/Harvard Cancer Center and of each participating site, as well as by the central committee of the TBCRC.
RESULTS
Patient and Tumor Characteristics
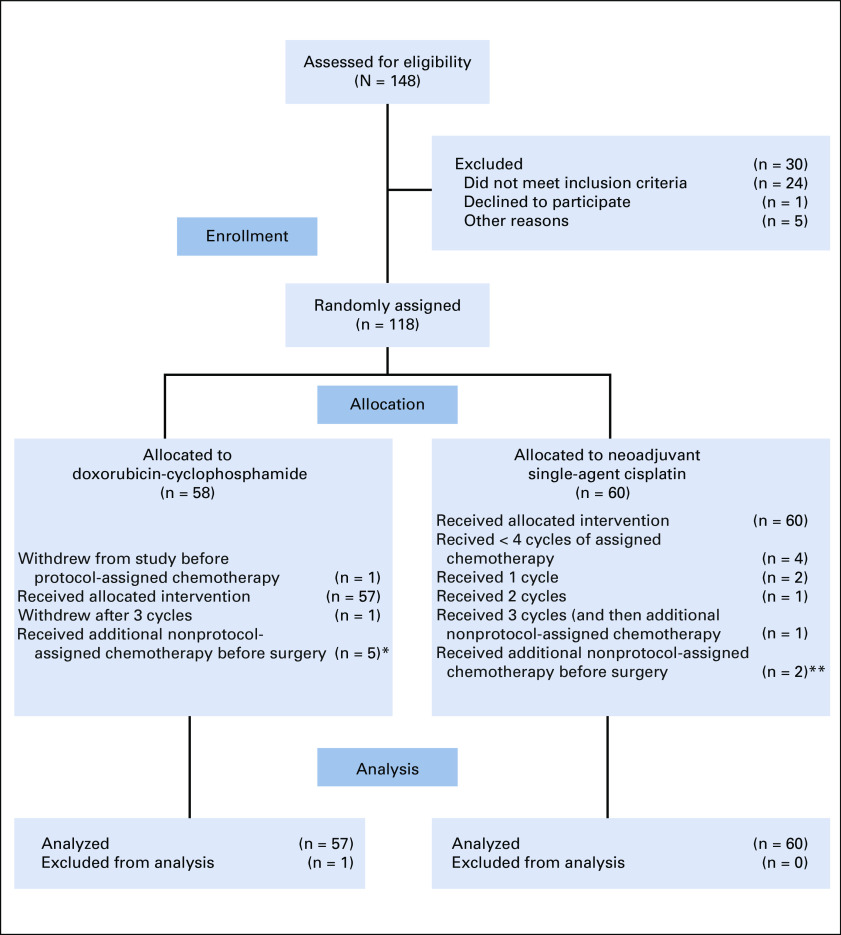
Between January 2012 and January 2019, 118 patients were randomly assigned. The study was closed in February 2019 because of slow accrual. One participant never started protocol treatment (CONSORT diagram shown in Fig 2) and was excluded from outcome analyses.
FIG 2.
CONSORT diagram. (*) Two patients had residual disease at biopsy performed before additional chemotherapy or at definitive tumor resection after additional chemotherapy; for 3 patients, residual cancer was not seen at either biopsy before additional chemotherapy or tumor resection after additional chemotherapy. (**) Both patients had residual disease demonstrated by biopsy before additional nonstudy-assigned chemotherapy.
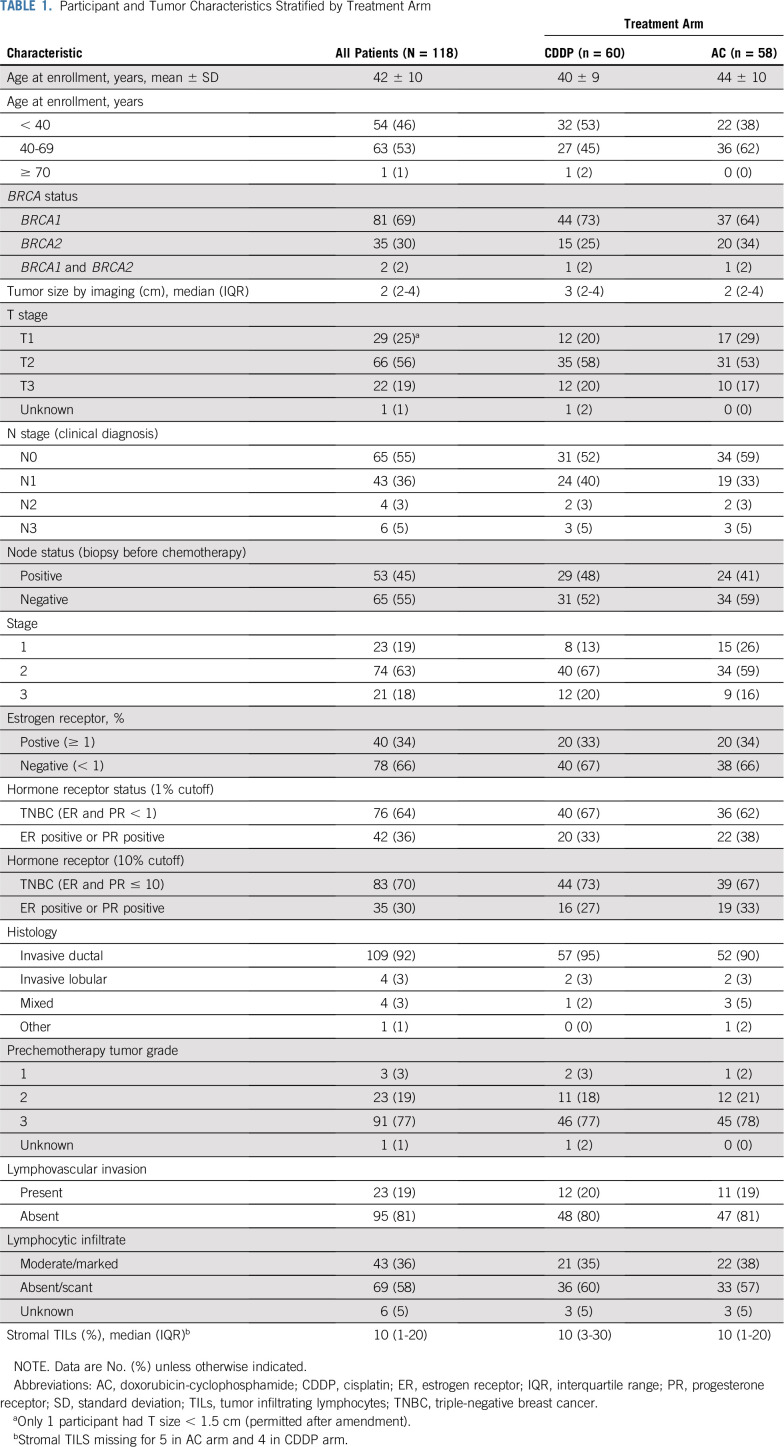
Patient and tumor characteristics are presented in Table 1. The mean age of participants was 42 years (range, 24-73 years). Most participants had a BRCA1 mutation (69%) and T2 or T3 tumors (75%). Nearly half had nodal involvement at baseline, and 81% had clinical stage II or III disease. Although 64% had TNBC defined as < 1% ER and PR IHC staining, 70% had TNBC, defined as ≤ 10% ER and PR IHC staining. Thus, 30%-36% of participants had hormone receptor–positive, HER2-negative breast cancer, depending on the definition of TNBC. Nearly all (92%) of cancers were invasive ductal, and 77% were high grade. In general, clinical and tumor characteristics were similar between treatment arms.
TABLE 1.
Participant and Tumor Characteristics Stratified by Treatment Arm
Pathologic Response
There were 117 participants who were evaluable for response and toxicity; 60 received CDDP, and 57 received AC. The pCR rate (ypT0/is, N0) was 18% for participants randomly assigned to CDDP and 26% for those receiving AC, yielding an RR of 0.70 (90% CI, 0.39 to 1.2) for attaining pCR with CDDP (Fig 3A). Using a 10% cutoff to define TNBC, the RR was 0.79 (90% CI, 0.42 to 1.5) for attaining pCR with CDDP compared with AC among those with TNBC and 0.30 (90% CI, 0.05 to 1.7) among those with ER+/HER2-negative disease.
FIG 3.
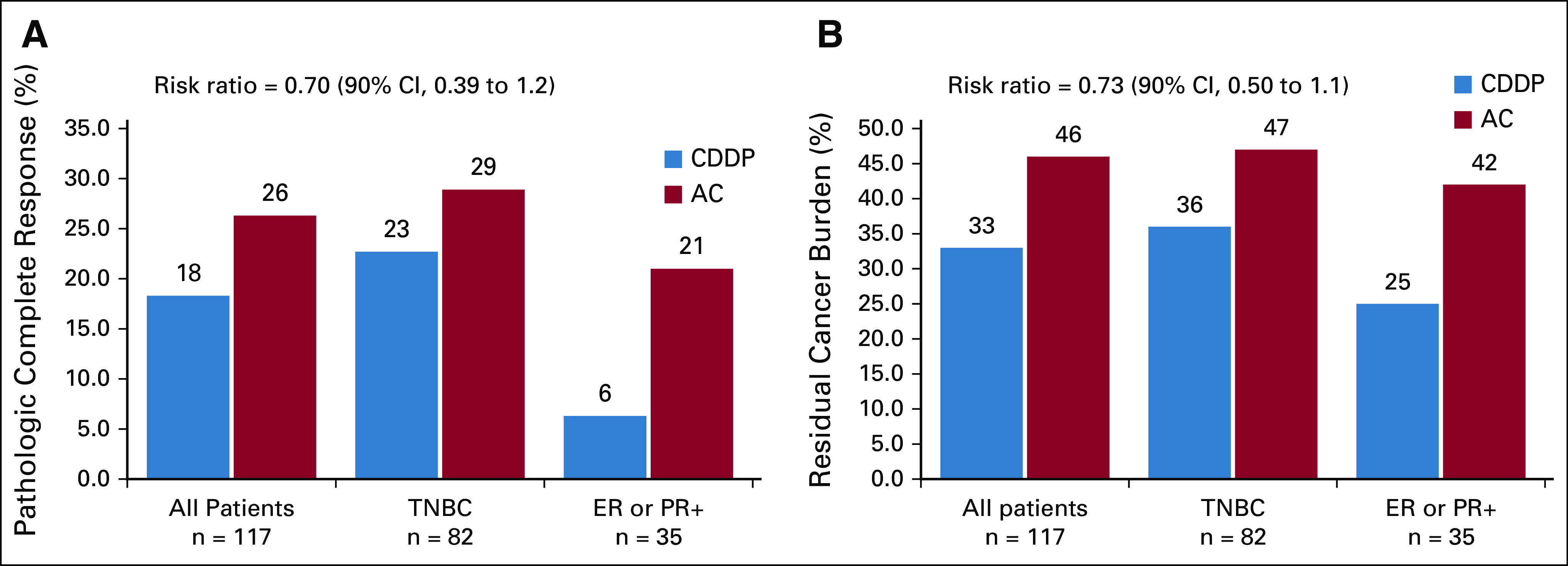
(A) Pathologic complete response (pCR) breast/axilla (ypT0/is, N0); (B) residual cancer burden (RCB) 0 or 1; risk ratio with 90% CI for (A) pCR or for (B) RCB 0/1 with cisplatin (CDDP) compared with doxorubicin-cyclophosphamide (AC) is shown. ER, estrogen receptor; PR, progesterone receptor; TNBC, triple-negative breast cancer.
All 117 patients could be assessed for pCR in both breast and nodes; 3 patients had an SLNB before chemotherapy, but 2 had no tumor in axillary nodes. The one patient with a positive preoperative node at SLNB had significant residual disease in the breast after chemotherapy at the time of definitive surgery (ie, did not have pCR).
The rate of RCB 0/1 was 33% with CDDP and 46% with AC (RR, 0.73; 90% CI, 0.50 to 1.1; Fig 3B). For patients with TNBC (10% cutoff), the rate of RCB 0/1 was 36% with CDDP and 47% with AC. For ER+/HER2-negative disease, the rate of RCB 0/1 was 25% with CDDP and 42% with AC. Eleven patients could not have RCB score assigned because they received fewer than or more than the 4 protocol-assigned chemotherapy cycles. These patients were evaluated as having RCB > 1.
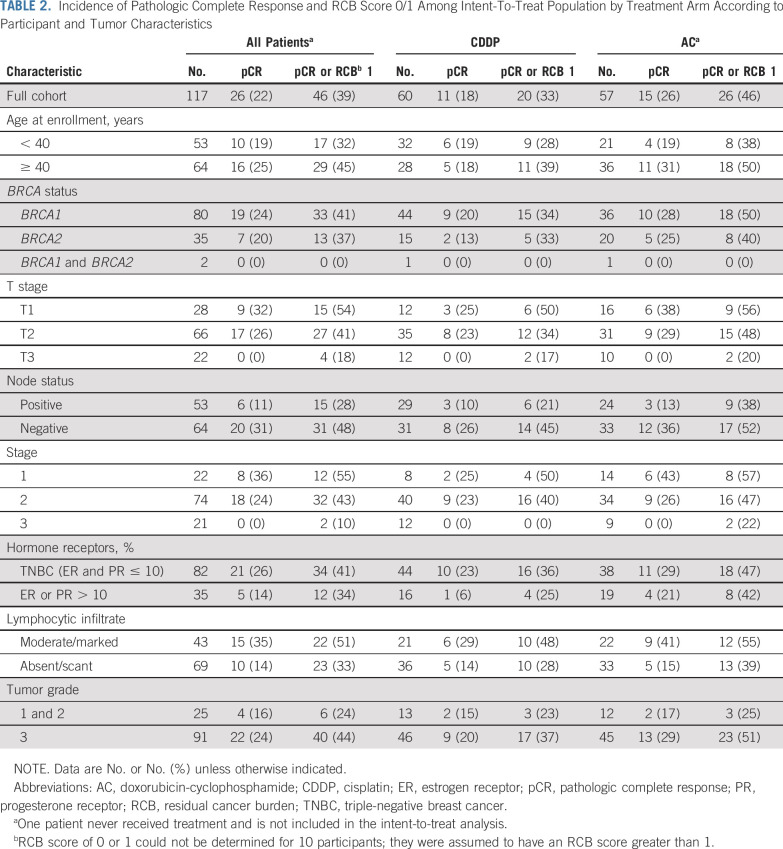
Figure 4 and Table 2 demonstrate the likelihood of pCR with CDDP compared with AC by patient and tumor variables. The pCR rate was lower with CDDP compared with AC in every stratified analysis. Table 2 shows that the pathologic response to neoadjuvant chemotherapy was lower among BRCA carriers diagnosed before the age of 40 years and for those with tumors with larger T size, nodal involvement, ER or PR expression, lower histologic grade, or less lymphocytic infiltration.
FIG 4.
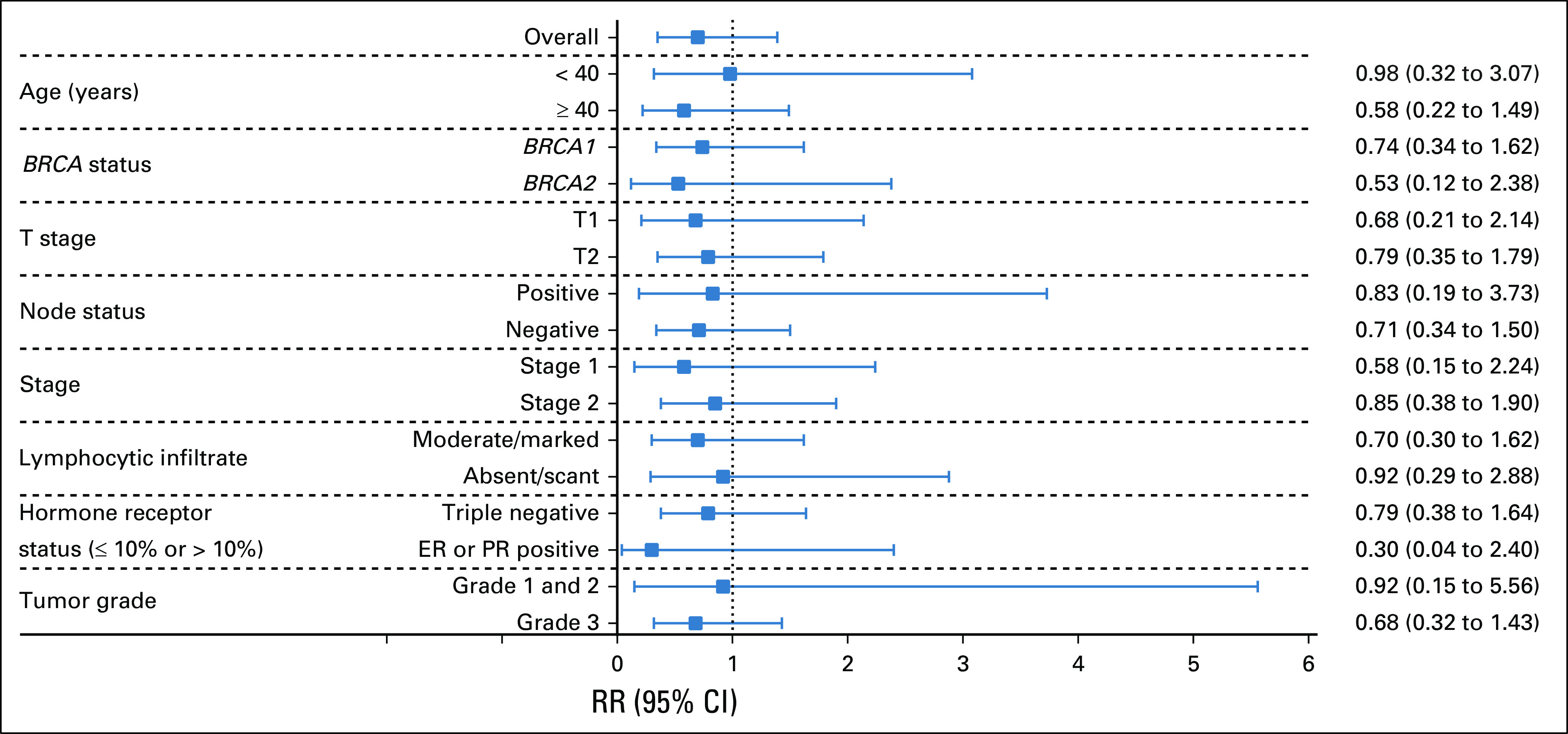
The likelihood (risk ratio [RR]) of pathologic complete response with cisplatin compared with doxorubicin-cyclophosphamide is shown for various patient and tumor characteristics. ER, estrogen receptor; PR, progesterone receptor.
TABLE 2.
Incidence of Pathologic Complete Response and RCB Score 0/1 Among Intent-To-Treat Population by Treatment Arm According to Participant and Tumor Characteristics
Treatment Delivery
At least one dose reduction occurred in 5% of patients in the CDDP arm and 2% in the AC arm. At least one dose delay was experienced among 10% of patients receiving CDDP and 9% receiving AC. Among patients receiving AC, 89% were treated on a 2-week schedule (ie, dd); 93% of patients in the AC arm and 35% in the CDDP arm received growth factor support.
Five patients received fewer than 4 cycles of study-assigned chemotherapy (4 because of toxicity and 1 because of insufficient response) and 7 patients received additional chemotherapy before surgery. One of the 5 patients who received fewer than 4 cycles of protocol-assigned chemotherapy also received additional presurgery chemotherapy and is counted among those 7 patients. The study required a biopsy before additional, nonprotocol-assigned chemotherapy was administered. Residual cancer was demonstrated for 4 of these 7 patients, but for 3, residual cancer was not demonstrated. All 11 patients receiving fewer than or more than 4 cycles of study-assigned chemotherapy were evaluated as not attaining pCR or RCB 1 (CONSORT diagram, Fig 1).
Toxicity
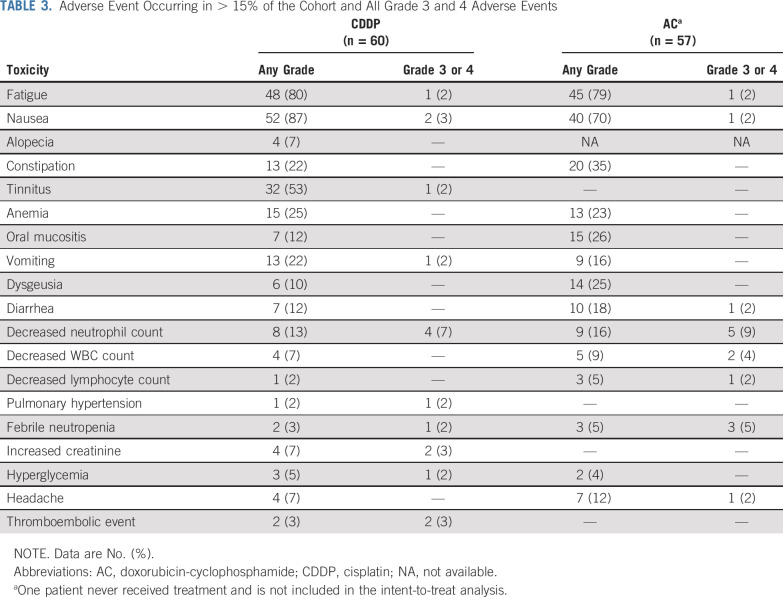
Adverse events are shown in Table 3. In general, toxicities associated with both regimens were as expected, with few grade ≥ 3 toxicities in either arm. There were 11 nonhematologic grade ≥ 3 toxicities in 60 patients receiving CDDP: nausea/vomiting (n = 3), increased creatinine (n = 2), thromboembolic event (n = 2), fatigue (n = 1), tinnitus (n = 1), pulmonary hypertension (n = 1), and hyperglycemia (n = 1). There were 4 nonhematologic grade ≥ 3 toxicities among 57 patients receiving AC: fatigue (n = 1), nausea (n = 1), diarrhea (n = 1), and headache (n = 1). Grade 3 or 4 neutropenia was experienced in 7% of patients receiving CDDP and 5% receiving AC; 3% of patients in the CDDP arm and 10% in the AC arm experienced at least 1 episode of febrile neutropenia. In general, when all grades of toxicity were considered, nausea and vomiting were more frequent with CDDP, and myelosuppression was slightly more frequent with AC (data not shown). Grade 1 alopecia was reported in 7% of patients receiving CDDP; alopecia could not be accurately assessed for patients in the AC arm, because hair loss was anticipated in this study before initiation of scalp-cooling techniques, and many patients shaved their heads in anticipation.
TABLE 3.
Adverse Event Occurring in > 15% of the Cohort and All Grade 3 and 4 Adverse Events
DISCUSSION
To our knowledge, the current trial is the largest prospective randomized study to date of neoadjuvant chemotherapy in BRCA carriers with newly diagnosed breast cancer and the only one to compare the pathologic response of a platinum agent to an anthracycline-based regimen. We demonstrated that the rate of pCR or of RCB 0/1 is not higher with CDDP than with AC in BRCA carriers with stage I-III HER2-negative breast cancer. This observation appears to be true for BRCA carriers with TNBC (whether defined as < 1% or ≤ 10% ER and PR IHC expression) or ER+/HER2-negative breast cancer. Caution must be used when interpreting results for the higher pathologic response observed with AC than with CDDP among those with ER+ disease, given the small sample size of that cohort.
Although these results may be unanticipated given the previously reported significant activity of platinum agents in BRCA-associated breast cancer, these results are consistent with those reported from the Geparsixto10 and BrighTNess11 studies. Both of those trials evaluated the benefit of adding platinum to a neoadjuvant anthracycline- and taxane-based regimen in patients with TNBC, and analyzed results according to BRCA status. Both studies reported that, in contrast to patients without a germline BRCA mutation, among BRCA carriers, the addition of platinum did not significantly improve the pCR rate. In Geparsixto, the addition of platinum led to a significant improvement in disease-free survival only for patients without a BRCA mutation. In both studies, the pCR rate was higher with the nonplatinum-containing regimen among BRCA carriers than among noncarriers. One potential explanation for the lack of additional benefit with platinum for BRCA carriers in these studies is that breast cancer in BRCA carriers is more sensitive to DNA-damaging agents, whether CDDP or anthracyclines plus alkylating agents, than breast cancer in noncarriers, and the superior response to anthracycline and alkylating agent reduces any additional benefit from platinum chemotherapy. BRCA deficiency (ie, homologous recombination deficiency) may simply predict chemosensitivity, at least to DNA-damaging agents, rather than to platinum agents specifically. In the INFORM trial, patients in the AC arm received combination chemotherapy, thereby potentially increasing the likelihood of a pCR compared with single-agent cisplatin.
Consistent with prior reports, we found that a higher level of baseline TILs was associated with a higher pathologic response to both CDDP and AC. Several pooled analyses have reported both a higher pathologic response and improved clinical outcomes among patients with TNBC receiving (neo)adjuvant chemotherapy.12,13 Although this correlation is most prominent in TNBC, a better pathologic response in tumors with higher TIL levels has also been reported among hormone receptor-positive HER2-negative breast cancers.14,15 Of note, a recent pooled analysis of neoadjuvant trials using platinum-based therapy in TNBC found that stromal TIL level and homologous recombination deficiency were independently associated with therapy response.12
One limitation of the trial was that it did not meet target accrual. At the time the study began in 2012, performing genetic testing to make immediate systemic treatment decisions was not often done. Accrual was challenging because many patients had surgery or initiated chemotherapy before BRCA testing. The INFORM trial was one of the first prospective trials to require BRCA results before enrollment, in contrast to studies that analyzed BRCA status post hoc. Nevertheless, there is no realistic scenario by which the pCR rate would have been significantly higher with cisplatin than with AC had target accrual been met. Had the pCR rates continued at the rates observed (ie, 26% with AC and 18% with CDDP), the study would have required 331 patients in each arm to demonstrate a significantly higher pCR rate with AC. However, the study does not definitively rule out the possibility of an advantage of CDDP. A larger sample size would have been desirable to increase precision of the estimated effects.
The pCR rates in the current study are lower than those reported among BRCA carriers in the study from Poland by Byrski et al,2 despite using the same dose and schedule of CDDP. One explanation may be the study population. Patients in the INFORM trial had higher anatomic stage, with only 25% of patients having T1 tumors (v 46% in the Polish trial) and only 55% being clinically node negative at baseline (v 65% in the Polish study). Nevertheless, in the current study, only 25% of BRCA carriers with stage 1 disease attained a pCR with CDDP. Of note, TBCRC 030 was a study that compared neoadjuvant CDDP with paclitaxel in patients with metastatic TNBC. Of the 6 participants enrolled with BRCA-deficient tumors who received single-agent CDDP, only 1 had a pCR (personal communication [N.T. and E.L.M.]). The INFORM trial underscores the importance of confirming the results from single-arm trials with those from randomized multicenter trials.
Recently, a small neoadjuvant study using a single-agent poly (ADP-ribose) polymerase (PARP) inhibitor (talazoparib) reported a pCR rate of 53% among 20 BRCA carriers with breast cancer, 15 of whom had TNBC.7 It will be extremely important to confirm these results in a larger multicenter trial. Ultimately, the pathologic response rates with neoadjuvant chemotherapy and PARP inhibitors may need to be compared in BRCA carriers with breast cancer. The INFORM trial demonstrates that the pathologic response with AC is at least as good as that achieved with CDDP in this population. Both regimens demonstrate activity, and chemotherapy choice may need to incorporate individual comorbidities as well as anticipated toxicities with each regimen.
PRIOR PRESENTATION
Presented at San Antonio Breast Cancer Symposium, San Antonio, TX, December 10-14, 2019.
SUPPORT
Supported by the Breast Cancer Research Foundation, Susan G. Komen, and Myriad Genetics.
AUTHOR CONTRIBUTIONS
Conception and design: Nadine Tung, Michele R. Hacker, Robert D. Legare, Claudine Isaacs, Paul Kelly Marcom, Ian E. Krop, Eric P. Winer, Stuart J. Schnitt, Judy E. Garber
Financial support: Nadine Tung, Judy E. Garber
Administrative support: Nadine Tung, Robert D. Legare, Paulina B. Lange, Judy E. Garber
Provision of study materials or patients: Nadine Tung, Erin Hofstatter, Deborah L. Toppmeyer, Steven J. Isakoff, Virginia Borges, Claudine Isaacs, Erica L. Mayer, Ian E. Krop, Judy E. Garber
Collection and assembly of data: Nadine Tung, Banu Arun, Erin Hofstatter, Deborah L. Toppmeyer, Steven J. Isakoff, Virginia Borges, Robert D. Legare, Antonio C. Wolff, Paulina B. Lange, Andrew J. Goss, Colby Jenkins, Stuart J. Schnitt, Judy E. Garber
Data analysis and interpretation: Nadine Tung, Michele R. Hacker, Erin Hofstatter, Steven J. Isakoff, Virginia Borges, Robert D. Legare, Claudine Isaacs, Antonio C. Wolff, Erica L. Mayer, Stuart J. Schnitt, Judy E. Garber
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
TBCRC 031: Randomized Phase II Study of Neoadjuvant Cisplatin Versus Doxorubicin-Cyclophosphamide in Germline BRCA Carriers With HER2-Negative Breast Cancer (the INFORM trial)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nadine Tung
Research Funding: AstraZeneca, Myriad Genetics (Inst)
Banu Arun
Consulting or Advisory Role: Bright Pink, AbbVie
Research Funding: AbbVie (Inst), PharmaMar (Inst), AstraZeneca (Inst), InVitae (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Erin Hofstatter
Consulting or Advisory Role: Teledoc Health
Other Relationship: UpToDate
Deborah L. Toppmeyer
Employment: Novartis (I), Merck (I)
Stock and Other Ownership Interests: Novartis (I), Merck (I)
Honoraria: Novartis
Consulting or Advisory Role: Merck, Novartis
Steven J. Isakoff
Consulting or Advisory Role: AbbVie, Genentech, Myriad Genetics, Hengrui Therapeutics, Puma Biotechnology, Immunomedics
Research Funding: Genentech (Inst), PharmaMar (Inst), AbbVie (Inst), OncoPep (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst)
Virginia Borges
Research Funding: Abbott/AbbVie (Inst), Seattle Genetics (Inst)
Robert D. Legare
Consulting or Advisory Role: Unum Therapeutics
Claudine Isaacs
Honoraria: Genentech, AstraZeneca, Pfizer
Consulting or Advisory Role: Pfizer, Genentech, Novartis, AstraZeneca, PUMA, Context Therapeutics
Speakers' Bureau: Genentech, Pfizer, AstraZeneca
Research Funding: Novartis (Inst), Pfizer (Inst), Genentech (Inst), Tesaro (Inst)
Patents, Royalties, Other Intellectual Property: McGraw Hill Publishing; UpToDate - Wolters Kluwer, author of chapters; Elsevier, editor of book
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceutical
Research Funding: Biomarin (Inst), Celldex (Inst)
Patents, Royalties, Other Intellectual Property: Antonio C. Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer and has assigned his rights to Johns Hopkins University (JHU) and participates in a royalty sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Paul Kelly Marcom
Consulting or Advisory Role: Genentech, Merck, Celltrion, Immunomedics
Speakers' Bureau: Catamount Medical Education, Clinical Care Options
Research Funding: AbbVie (Inst), Novartis (Inst), Genentech (Inst), Veridex (Inst), Innocrin Pharma (Inst), AstraZeneca (Inst), Verily (Inst)
Erica L. Mayer
Consulting or Advisory Role: Eli Lilly, Eisai, Pfizer, Novartis
Research Funding: Myriad Genetics (Inst), Pfizer (Inst)
Ian E. Krop
Employment: AMAG Pharmaceuticals (I)
Leadership: AMAG Pharmaceuticals (I)
Stock and Other Ownership Interests: AMAG Pharmaceuticals (I)
Honoraria: Genentech
Consulting or Advisory Role: Genentech, Daiichi Sankyo, Context Therapeutics, Macrogenics, Taiho Pharmaceutical
Research Funding: Genentech (Inst), Seattle Genetics (Inst), Pfizer (Inst), Daiichi Sankyo (Inst)
Eric P. Winer
Stock and Other Ownership Interests: Verastem
Honoraria: Genentech, Tesaro, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Eli Lilly, Genomic Health
Research Funding: Genentech (Inst), Novartis (Inst), Merck (Inst)
Judy E. Garber
Consulting or Advisory Role: Novartis (I), GTx (I), Helix BioPharma, Konica Minolta, Aleta BioTherapeutics (I), H3 Biomedicine (I), Kronos Bio (I)
Research Funding: Novartis (I), Ambry Genetics, Invitae Genetics, Myriad Genetics
Other Relationship: Susan G. Komen for the Cure (I), AACR, Diane Helis Henry Medical Foundation (I), James P. Wilmot Foundation (I), Adrienne Helis Malvin Medical Research Foundation (I), Breast Cancer Research Foundation, Facing Our Risk of Cancer Empowered
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.1200/JCO.2009.22.4725. Silver DP, Richardson AL, Eklund AC, et al: Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol 28:1145-1153, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrski T, Huzarski T, Dent R, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients Breast Cancer Res Treat 147401–4052014 [DOI] [PubMed] [Google Scholar]
- 3.Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy J Clin Oncol 28375–3792010 [DOI] [PubMed] [Google Scholar]
- 4.Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial Nat Med 24628–6372018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakoff SJ, Mayer EL, He L, et al. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer J Clin Oncol 331902–19092015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Telli ML, Jensen KC, Vinayak S, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105 J Clin Oncol 331895–19012015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litton JK, Scoggins ME, Hess KR, et al. Neoadjuvant talazoparib for patients with operable breast cancer with a germline BRCA pathogenic variant J Clin Oncol 38388–3942020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014 Ann Oncol 26259–2712015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The University of Texas MD Anderson Cancer Center: Residual Cancer Burden Calculator. http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3.
- 10.Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary analysis of the GeparSixto randomized clinical trial JAMA Oncol 31378–13852017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial Lancet Oncol 19497–5092018 [DOI] [PubMed] [Google Scholar]
- 12. doi: 10.1158/1078-0432.CCR-19-0664. Telli ML, Chu C, Badve SS, et al: Association of tumor infiltrating lymphocytes with homologous recombination deficiency and BRCA1/2 status in patients with early triple-negative breast cancer: A pooled analysis. Clin Cancer Res 10.1158/1078-0432.CCR-19-0664 [epub ahead of print on December 3, 2019] [DOI] [PubMed] [Google Scholar]
- 13.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers J Clin Oncol 37559–5692019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy Lancet Oncol 1940–502018 [DOI] [PubMed] [Google Scholar]
- 15.Issa-Nummer Y, Darb-Esfahani S, Loibl S, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS One. 2013;8:e79775. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]