INTRODUCTION
In early-stage breast cancer (BC), molecular subtyping and gene expression profiling have improved our ability to estimate the risk of distant recurrence above and beyond clinicopathologic factors and commonly used biomarkers.1-6 Several gene expression signatures have been validated to predict risk of distant recurrence both in patients who have received no adjuvant systemic therapy and in those treated with adjuvant hormonal therapy with or without adjuvant chemotherapy.1,3-13
CONTEXT
Key Objective
To review evidence on the association between breast cancer subtypes/genomic classifiers and locoregional recurrence in early-stage breast cancer.
Knowledge Generated
Several studies have demonstrated an association between breast cancer subtypes and risk of locoregional recurrence. In addition, multiple studies have also demonstrated a strong association between commercially available and noncommercially available genomic classifiers and risk of locoregional recurrence.
Relevance
Although currently available evidence provides great insight on tumor biology and its locoregional behavior, such evidence has not yet translated into meaningful changes in locoregional treatment approaches. The surgical management of early-stage breast cancer is still based on the anatomic extent of the disease in the breast and axilla. However, it is hoped that by individualizing risk of locoregional recurrence and radiosensitivity with molecular subtyping/genomic classifiers, use and/or extent of adjuvant radiotherapy could be tailored. Prospective clinical trials are currently underway to test the validity of this approach.
Despite the recent progress in refining risk of distant recurrence with genomic classifiers, assessment of risk for locoregional recurrence (LRR) still primarily relies on traditional clinicopathologic factors such as patient age, tumor size, grade, pathologic nodal status, presence of lymphovascular space invasion (LVSI), and margin width.14,15 In addition, several studies have shown that tumor subtype alone is strongly predictive of LRR.16-23 Given the strong correlation between the risk of LRR and distant recurrence,24-26 recent studies have investigated whether genomic assays that predict risk of distant recurrence can also predict risk of local recurrence.27-35 Increasingly, new genomic classifiers are being developed specific to LRR in patients with node-negative and node-positive invasive BC.36,37 There is also increasing interest in the development of gene expression assays to predict response to radiation (XRT) therapy.37-46
Local recurrence is also strongly influenced by the surgical approach; however, to date, molecular subtyping and genomic profiling have little to no influence on the extent of surgical therapy, which is traditionally based on the anatomic extent of the tumor and not on underlying tumor biology. Anatomic extent of the tumor in the breast and axilla can be reduced by neoadjuvant therapy, with resulting tailoring of the surgical approach.
MOLECULAR AND RECEPTOR-BASED SURROGATE SUBTYPES FOR PREDICTION OF RISK OF LRR
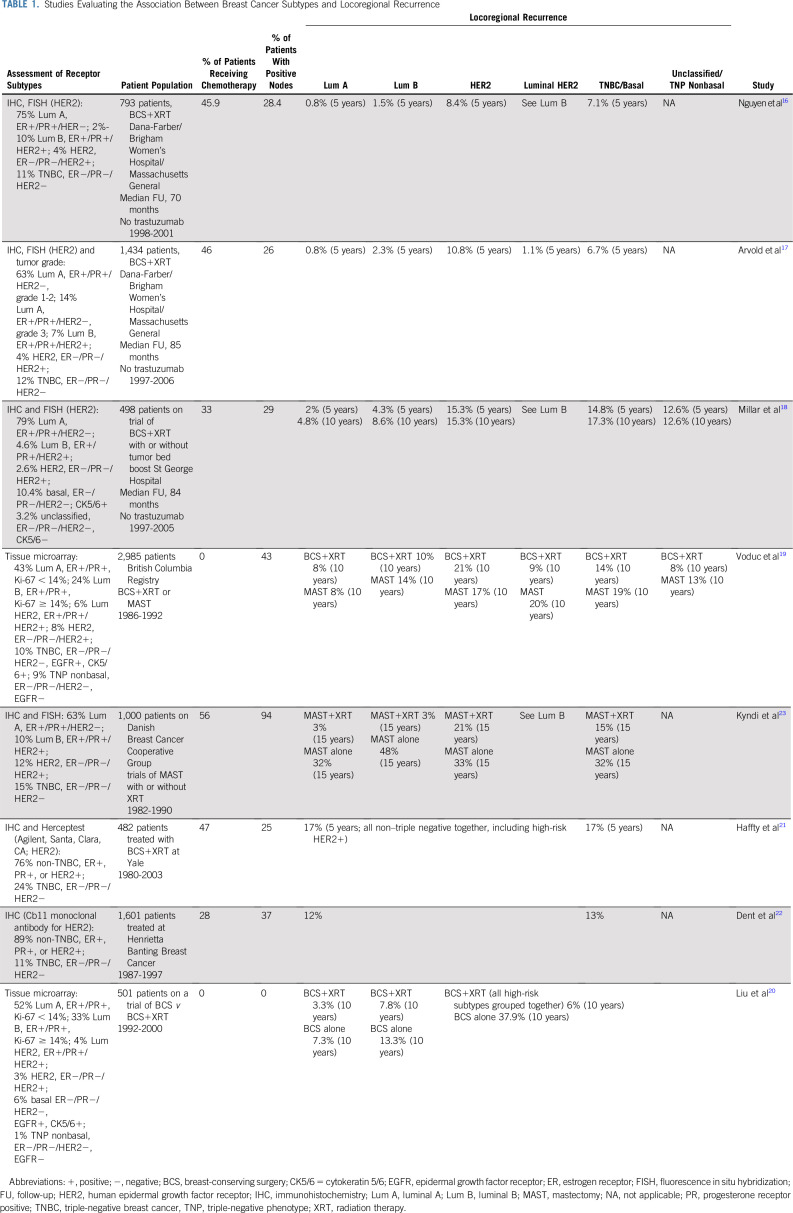
Several studies have examined the association between BC subtypes based on receptor surrogates for gene expression and LRR, and the results are summarized in Table 1. Nguyen et al16 were among the first to report the association between LRR and BC subtype as determined by immunohistochemistry in patients treated with breast-conserving surgery (BCS) plus breast XRT. They used receptor status to approximate subtype as follows: luminal A—estrogen receptor (ER) and progesterone receptor (PR) positive and human epidermal growth factor receptor 2 (HER2) negative; luminal B—ER, PR, and HER2 positive; HER2—ER and PR negative and HER2 positive; and basal—ER, PR, and HER2 negative. The 5-year cumulative incidence of LRR was 0.8% for luminal A, 1.5% for luminal B, 8.4% for HER2, and 7.1% for basal/triple-negative subtype. On multivariable analysis with luminal A as baseline, HER2 (hazard ratio [HR], 9.2; P = .012) and basal (HR, 7.1; P = .009) subtypes were associated with increased risk of LRR. On multivariable analysis, luminal B (HR, 2.9; P = .007) and basal (HR, 2.3; P = .035) subtypes were associated with increased risk of distant metastases. These findings suggest a potentially increased XRT benefit in the luminal B subtype and potential XRT resistance in HER2 and basal subtypes. Of note, these patients were treated in the pretrastuzumab era. A more recent report from the same group on an expanded cohort of patients17 showed similar findings, with a 5-year cumulative incidence of LRR of 0.8% for luminal A, 2.3% for luminal B, 1.1% for luminal HER2, 10.8% for HER2, and 6.7% for triple negative.
TABLE 1.
Studies Evaluating the Association Between Breast Cancer Subtypes and Locoregional Recurrence
Several other investigators have reported similar findings. Retrospective assessment of molecular phenotypes in an Australian randomized trial of BCS and whole-breast XRT with or without boost reported 10-year LRR rates of 4.8% for luminal A, 8.6% for luminal B, 17.3% for basal, and 15.3% for HER2-positive tumors (P = .012).18 Investigators from the British Columbia Cancer Agency reported 10-year LRR rates with BCS plus XRT of 8% for luminal A and 10% for luminal B tumors, compared with 21% for HER2-enriched and 14% for basal subtypes, which was statistically significant on multivariable analysis.19 Interestingly, for mastectomy patients, who generally did not receive XRT, luminal B tumors were also associated with an increased risk of LRR on multivariable analysis. The higher LRR for luminal B tumors in patients undergoing mastectomy but not in those undergoing BCS plus XRT suggests that luminal B tumors are sensitive to XRT. The same group recently published data from a randomized trial of BCS with or without XRT.20 They identified a low-risk subgroup of patients to be considered for XRT omission (age > 60 years, T1 disease, luminal A, with 1.3% 10-year risk of LRR without XRT). The LUMINA trial described later was designed to validate these findings prospectively.
The aforementioned studies suggest that the LRR rate for the basal/triple-negative subtype is higher than that of the luminal A and B subtypes and similar to that of the HER2 subtype. However, 2 studies have shown no differences in the risk of LRR between triple-negative and non–triple-negative subtypes.21,22 The results of these 2 studies are not necessarily contradictory to the ones presented previously because HER2-positive patients were included in the non–triple-negative cohort and all patients were treated in the pretrastuzumab era, which likely inflated the LRR risk for the non–triple-negative cohorts.
Additional support for the hypothesis that luminal tumors are more radiosensitive than triple-negative and HER2-positive tumors (in the pretrastuzumab era) is provided by Kyndi et al,23 who evaluated tumor subtypes as predictors of LRR in 1,000 of the 3,083 patients with high-risk BC randomly assigned to postmastectomy radiation therapy (PMRT) in the Danish Breast Cancer Cooperative Group (DBCG) protocol 82b and 82c trials. In general, PMRT is defined as radiation to the chest wall and draining lymphatics. Median follow-up for patients who were alive was 17 years. Significantly smaller improvements in local control from PMRT were observed in the triple-negative (HR, 0.33; P = .001) and hormone receptor–negative/HER2-positive subtypes (HR, 0.53; P = .2) compared with the hormone receptor–positive/HER2-negative subtype (HR, 0.09; P < .01).
Furthermore, significantly improved overall survival after PMRT was seen only among patients characterized by good prognostic markers, including hormone receptor–positive/HER2-negative disease. These findings are in agreement with the observations from studies of molecular subtypes and LRR in patients treated with BCS plus XRT and indicate potentially higher sensitivity of the high-risk ER-positive tumors to XRT.
GENOMIC CLASSIFIERS FOR PREDICTION OF RISK OF LRR IN PATIENTS WITH INVASIVE BC
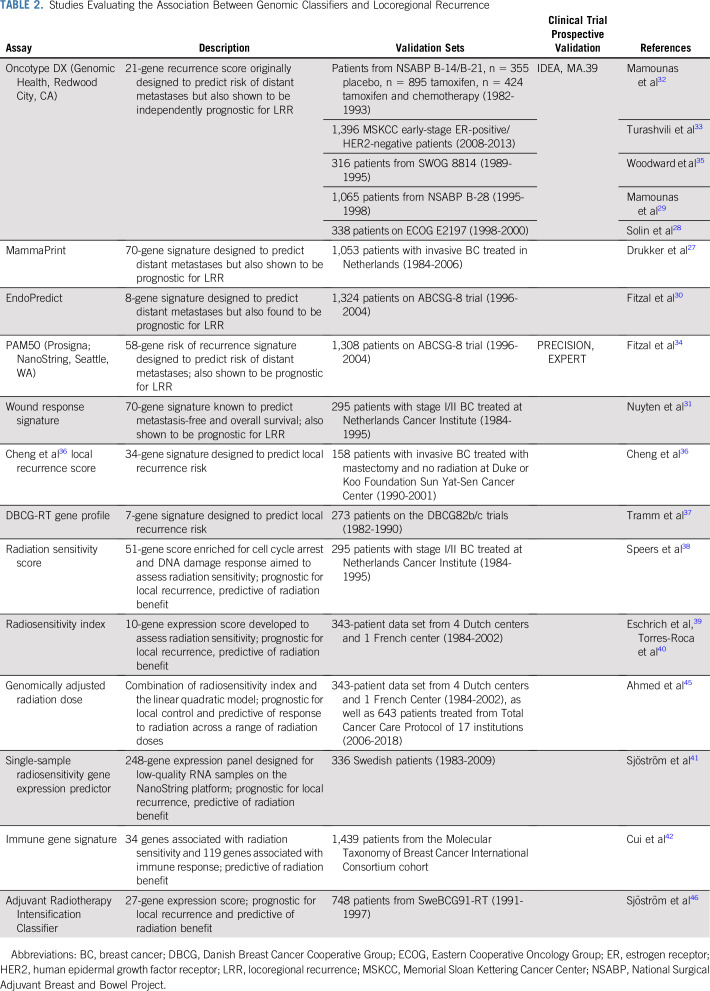
The demonstration of BC intrinsic subtypes based on microarray gene expression profiling1,47 signaled the beginning of the genomic era in BC that has led to the development of several commercially (and noncommercially) available genomic classifiers developed to predict distant recurrence.1,3-13 A logical next step was to evaluate these classifiers for prediction of LRR (Table 2).
TABLE 2.
Studies Evaluating the Association Between Genomic Classifiers and Locoregional Recurrence
Mamounas et al32 evaluated the association between the 21-gene recurrence score (RS) in patients with node-negative, ER-positive BC from 2 National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials (B-14 and B-20) in patients treated with no adjuvant therapy, tamoxifen, or tamoxifen plus chemotherapy. RS was a significant predictor of LRR in all 3 groups. In tamoxifen-treated patients, the 10-year risk of LRR was 4.3% with an RS of 0-17, 7.2% with an RS of 18-30, and 15.8% with an RS ≥ 31 (P < .001). This association provided biologic insights into LRR but had limited clinical implications regarding tailoring XRT use because, in the modern era, patients with high RS would receive chemotherapy. When the association between RS and risk of LRR was examined in patients treated with chemotherapy plus tamoxifen, the 10-year risk of LRR was 1.6% for RS of 0-17, 2.7% for RS of 18-30, and 7.8% for RS ≥ 31 (P = .028).
The low LRR risk in patients with low RS (< 18) was recently confirmed in a large contemporary cohort of node-negative, ER-positive/HER2-negative patients from the Memorial Sloan Kettering Cancer Center.33 Most patients were treated with BCS plus XRT (66.6%) or total mastectomy alone (29.7%). Most patients (84.8%) received endocrine therapy alone, whereas 12.1% of patients were treated with chemotherapy plus endocrine therapy. With a median follow-up of 52 months, LRR rate was 0.9% overall and 0.7% in patients treated with adjuvant endocrine therapy only.
Similar findings were reported by Drukker et al,27 who evaluated the 70-gene signature (MammaPrint; Agendia, Irvine, CA) in patients with T1-3, N0-1 disease. MammaPrint was significantly associated with LRR risk, with 10-year LRR rates of 6.1% for low-risk scores and 12.6% for high-risk scores. Adjusting the 70-gene signature in a competing risk model for clinicopathologic factors (age, tumor size, grade, hormone receptor status, LVSI, nodal stage, surgical treatment, endocrine treatment, and chemotherapy) resulted in a multivariable HR of 1.73 (95% CI, 1.02 to 2.93; P = .042).
Fitzal et al30,34 evaluated both the PAM50 assay and the 8-gene assay EndoPredict (EP; Myriad Genetics, Salt Lake City, UT) in the ABCSG-8 trial of postmenopausal, ER-positive/HER2-negative patients treated with endocrine therapy. The majority of the patients (79%) were treated with BCS. With a median follow-up of 11 years, 10-year local recurrence–free survival (LRFS) risk was significantly associated with PAM50 risk of recurrence (ROR; 98.4% in the low/intermediate ROR group v 94.4% in the high ROR group). ROR score was the only significant independent predictor of LRR in multivariable analysis. For EP, the 10-year LRFS was significantly worse among patients with high-risk lesions (91%) versus those with low-risk lesions (97.5%; HR, 1.31; 95% CI, 1.16 to 1.48; P < .005). The groups that received BCS and mastectomy had similar LRR rates (P = .879). In a subgroup of BCS patients randomly assigned to breast XRT or no XRT,30,48 EP was a significant predictor of LRR but not predictive of benefit from breast XRT. Breast XRT significantly improved LRFS both in the EP low-risk cohort (10-year LRR decreased from 11.1% to 0.2%; P < .005) and in the EP high-risk cohort (10-year LRR decreased from 12.0% to 2.5%; P < .005).
Noncommercially available predictors of LRR include a 34-gene expression profile developed using DNA microarray analysis by Cheng et al36 that was significant for predicting LRR risk in patients subdivided by nodal stage. In addition, Nuyten et al31 showed that a microarray-based 70-gene expression profile developed as a wound-response signature independently predicted BCS patients at high (29%) versus low (5%) risk of LRR at 10 years.
Currently, there are no commercially available genomic classifiers that identify subgroups of patients with BC who can be spared breast XRT after BCS. Several prospective trials (single-arm or randomized) are currently underway to address this question in patients with early-stage disease treated with BCS. The LUMINA trial (ClinicalTrials.gov identifier: NCT01791829) is enrolling patients ≥ 55 years old with T1N0 luminal A tumors in a single-arm prospective observation trial, with a primary end point to measure the 5-year ipsilateral breast recurrence rate. The IDEA trial (ClinicalTrials.gov identifier: NCT02400190) completed enrollment of postmenopausal patients with T1N0 ER-positive BC and low RS (< 18) onto a single-arm prospective observation trial, with a primary end point of 5-year LRR. The PRECISION trial (ClinicalTrials.gov identifier: NCT02653755) is enrolling women > 50 years old with T1N0 ER-positive BC and low PAM50 ROR score onto a single-arm prospective observation trial, also with a primary end point of 5-year LRR. The EXPERT trial (ClinicalTrials.gov identifier: NCT02889874) is a randomized study of BCS with or without XRT in T1N0 ER-positive BC in women older than 50 years with a PAM50 ROR of < 60. Until results from these trials become available, BCS plus XRT remains the standard of care for the majority of patients with invasive BC.
GENOMIC PROFILING AND RISK OF LRR IN PATIENTS WITH NODE-POSITIVE BC
The significant association between LRR and the 21-gene RS in node-negative patients treated with chemotherapy and endocrine therapy provided rationale for evaluating RS in node-positive patients, hoping that improved LRR stratification could help to tailor PMRT and/or regional nodal XRT use. Three studies have reported such an association based on retrospective assessment of RS in exclusively node-positive patients included in randomized clinical trials of adjuvant chemotherapy plus endocrine therapy (NSABP B-28, Eastern Cooperative Oncology Group [ECOG] E2197, and SWOG 8814).28,29,35
In the NSABP B-28 trial, patients with positive nodes were randomly assigned to doxorubicin plus cyclophosphamide with or without paclitaxel. Patients ≥ 50 years old and those < 50 years old with ER-positive and/or PR-positive tumors also received tamoxifen. Per protocol, BCS patients received breast XRT, but mastectomy patients did not receive XRT. RS was obtained in 1,065 ER-positive patients.29 With median follow-up of 11.2 years, the 10-year cumulative incidence of LRR was 3.3%, 7.2%, and 12.3% for patients with low RS (0-17), intermediate RS (18-30), and high RS (≥ 31), respectively (P < .001). In multivariable regression analysis, RS remained an independent predictor of LRR (HR, 2.61 for a 50-point difference in RS; P = .008), along with pathologic nodal status (HR, 1.91 for ≥ 4 v 1-3 positive nodes; P = .007) and tumor size (HR, 1.28 for a 1-cm difference; P = .015). These findings suggest that RS can be used along with clinicopathologic factors to better stratify risk of LRR in node-positive, ER-positive patients treated with chemotherapy plus endocrine therapy to better select appropriate candidates for PMRT and/or regional nodal irradiation after BCS and breast XRT.
Solin et al28 evaluated RS for prediction of LRR risk in patients treated with BCS plus XRT with 1-3 positive nodes in the ECOG E2197 study comparing 2 chemotherapy regimens. In their study population, 10-year rates of local recurrence were 3.2%, 2.0%, and 10.1% for low, intermediate, and high RS, respectively, which was not statistically significant (P = .17). However, as a continuous variable, RS was a significant predictor of LRR (HR, 2.66; P = .03).
Woodward et al35 recently reported on the retrospective assessment of RS in patients treated on SWOG 8814, comparing chemotherapy plus endocrine therapy versus endocrine therapy alone in node-positive, hormone receptor–positive BC. Estimated 10-year cumulative LRR rates were 9.7% and 16.5% for low and intermediate or high RS, respectively (log-rank P = .018). The RS remained significantly associated with LRR in an analysis of mastectomy patients alone (low RS, 7.7%; intermediate or high RS, 16.8%; P = .025). In a subset analysis of patients with a mastectomy and 1-3 involved nodes who did not receive XRT, patients with RS < 18 had a low LRR rate (1.5%), suggesting this may be a population for omission of PMRT.
These findings suggest that genomic profiling can significantly predict risk of LRR in node-positive patients, and this association could have clinical implications regarding tailoring regional nodal XRT or PMRT. However, before such an approach becomes accepted clinical practice, validation in a prospective clinical trial is needed. Such a trial is NCIC MA.39, which is currently accruing patients through the National Cancer Trials Network (ClinicalTrials.gov identifier: NCT03488693). MA.39 includes patients with ER-positive/HER2-negative, node-positive BC and a 21-gene RS of < 18. Mastectomy-treated patients are randomly assigned to chest wall plus regional nodal XRT versus no XRT, whereas patients treated with BCS are randomly assigned to breast plus regional nodal XRT versus breast XRT only. Patients are eligible if they have 1-3 positive nodes after axillary lymph node dissection, 1-2 positive nodes after BCS plus sentinel lymph node biopsy, or only 1 positive node after mastectomy plus sentinel lymph node biopsy. The primary objective of the trial is to determine whether omitting regional nodal XRT after BCS or PMRT in mastectomy patients is noninferior to its use in women with ER-positive BC, 1-3 positive axillary nodes, and an RS of < 18 treated with endocrine therapy. A total of 2,140 patients will be included in the study. Mastectomy-treated patients are randomly assigned to PMRT versus no PMRT, whereas BCS patients are randomly assigned to breast XRT with or without regional nodal XRT.
GENOMIC PROFILING FOR PREDICTION OF XRT BENEFIT
The aforementioned trials were designed to identify patient populations with a low risk of LRR, in whom the addition of chest or breast XRT or regional nodal XRT would not add substantial absolute benefit in local control. Another approach to tailor use of XRT is development of genomic classifiers predictive of XRT benefit, rather than LRR.
Tramm et al37 attempted to develop a genomic predictor of XRT benefit in patients with high-risk BC treated with systemic therapy and randomly assigned to PMRT or no PMRT. Seven genes were identified, and the derived DBCG-RT profile divided the patients into high LRR risk and low LRR risk groups. PMRT significantly reduced risk of LRR in high LRR risk patients but not in low LRR risk patients.
Speers et al38 developed a new genomic predictor for XRT sensitivity using clonogenic survival assays for BC cell lines exposed to XRT. Their 51-gene radiation sensitivity score (RSS) is enriched for genes involved in cell cycle arrest and DNA damage response. In a data set of patients undergoing BCS plus XRT, RSS was more strongly predictive of LRR than any clinicopathologic factors.
Eschrich et al49 developed a generalized genetic assay for radiosensitivity by performing clonogenic survival studies in 48 different human cancer cell lines exposed to XRT. This radiosensitivity index (RSI) was validated in established databases of patients treated with BCS plus XRT or mastectomy without XRT.39 BCS plus XRT patients predicted to be radiosensitive by RSI had improved 5-year relapse-free survival (RFS) compared with radioresistant patients (95% v 75%, respectively; P = .0212). In patients treated with mastectomy alone, there was no difference in 5-year RFS between radiosensitive and radioresistant patients (71% v 77%, respectively; P = .67). When combining RSI with molecular subtype,40 patients at greatest risk of LRR had triple-negative and radioresistant disease (HR, 0.37; P = .02).
Sjöström et al41 used fresh frozen tissue from 336 patients undergoing BCS with or without XRT to develop a new radiosensitivity assay using the NanoString (Seattle, WA) platform suitable for low-quality RNA. In BCS patients with high risk of LRR, they identified 3 groups. In the low LRR risk/low-radiosensitivity group, XRT would not reduce risk of LRR. In the second group of patients, XRT was recommended as a result of high radiosensitivity. In the third group, escalated treatment was recommended, because their tumors had a high risk of LRR that was not significantly reduced by XRT.
More recently, Sjöström et al46 identified a new 27-gene Adjuvant Radiotherapy Intensification Classifier (ARCTIC) using publicly available gene databases with known outcomes. This included a database of 336 patients with early-stage BC treated in Sweden with surgery first from 1983 to 2009,41 a database of 295 patients with early-stage BC treated in the Netherlands from 1984 to 1995 treated with BCS or mastectomy,2 and a database of 343 patients with early-stage BC treated with BCS treated in the Netherlands or France between 1984 and 2002.50 It should be noted that these databases provided long-term follow-up; however, unlike the modern treatment regimens, no patients in these databases received neoadjuvant chemotherapy or trastuzumab as a part of their care.
ARCTIC scores were calculated for patients treated with BCS with or without XRT on the SweBCG91 trial. Paraffin-fixed tissue samples were available for 922 patients on the SweBCG91 trial, of which 748 had sufficient RNA for analysis.46 Using these samples, they compared the results of ARCTIC to 8 different genomic signature scores from the aforementioned studies.27,29,32,38,39,41,42,44 ARCTIC outperformed the other gene expression scores for predicting elevated risk of LRR as well as benefit from XRT. Patients with low ARCTIC score had a large XRT benefit with 10-year LRR risk reduced from 21% to 6% (HR, 0.33; P < .001). Patients with high ARCTIC score had a higher LRR risk and less XRT benefit, with 10-year LRR risk reduced from 32% to 25% (HR, 0.73; P = .23).
Several other groups have used publicly available BC gene data sets to develop and test radiosensitivity signatures. Cui et al42 combined radiosensitivity genes with an immune signature, including genes involved in antigen processing and presentation. In the Molecular Taxonomy of Breast Cancer International Consortium cohort, patients predicted as XRT sensitive and immune effective had significantly improved survival with XRT (HR, 0.43; P = .022). In patients predicted as XRT resistant and immune defective, survival was worse with XRT (HR, 1.69; P = .045). Jang and Kim43 used The Cancer Genome Atlas data set to develop and test a radiosensitivity signature. In radiosensitive patients who received XRT, there was improvement in recurrence-free survival (HR, 0.45; P = .008). The signature was not predictive of outcome in patients without XRT as a component of their care. Zhang et al44 developed a centromere and kinetochore gene expression score (CES) signature that correlated with genomic instability. Patients with a high CES score treated with XRT had a significant improvement in overall survival (HR, 0.279; P = .008) and disease-free survival (HR, 0.254; P = .016). In patients with low CES score, there was no difference with XRT addition to the treatment course for overall survival (HR, 1.309; P = .58) or disease-free survival (HR, 0.95; P = .98).
The aforementioned studies identify gene expression assays that are predictive of XRT sensitivity but not correlated to XRT dose. Ahmed et al45 integrated the RSI into the linear quadratic model used in radiobiology to provide the relationship between cell survival and dose. The resultant genomically adjusted XRT dose (GARD) score was calculated for patients with triple-negative BC in 2 independent data sets. Using the median GARD score as the cutoff between high and low score, GARD as a dichotomous variable was significant for LRR. In a model of GARD score versus XRT dose, optimal XRT was achieved in 78% of patients with a dose of 60 Gy. At 70 Gy, 91% of patients would receive optimal XRT.
DISCUSSION AND FUTURE DIRECTIONS
Although currently available evidence with molecular subtyping and genomic profiling provides great insight on tumor biology and its locoregional behavior, such evidence has not yet translated into meaningful changes in locoregional therapy approaches. In the setting of ER-positive/HER2-negative disease, multiple secondary analyses of randomized studies independently show that RS correlates to LRR similar to known prognostic variables, such as LVSI or stage. As such, RS may be incorporated into estimations of LRR risk. However, care must be taken when omitting XRT off protocol among women with low RS given the benefit from breast XRT in reducing in-breast recurrence after BCS and the benefit from PMRT and regional nodal XRT on disease-free survival.
Minimal data are available on the utility of genomic assays for LRR prognosis in the setting of neoadjuvant chemotherapy or with use of trastuzumab, and this remains a significant gap in knowledge. Development of predictive assays are promising but yet unproven. Studies are needed to prospectively correlate pathologic response to XRT with the existing genetic assays for radiosensitivity. Enrollment on prospective studies such as MA.39 is strongly encouraged, and development of new trials to test the hypothesis that genomic assays can predict XRT benefit is greatly needed.
By individualizing risk of LRR and radiosensitivity with molecular subtyping/genomic classifiers, it is hoped that use and/or extent of adjuvant XRT could be tailored. Studies such as MA.39 will provide information on whether these assays can guide decision making for PMRT in patients with 1-3 lymph nodes. Use of LRR and radiosensitivity assays in patients undergoing BCS may help to identify patients at sufficiently low risk of LRR to omit breast XRT. Tailoring of XRT dose or adding radiosensitizers could be considered to improve outcome in patients with high LRR risk and radioresistant tumors. Another provocative, yet unaddressed, question is whether use of genomic assays could identify a population of early-stage, hormone receptor–positive patients with primarily local recurrence risk rather than distant recurrence risk, who could be adequately treated with surgery plus XRT alone, without the need for 5 years of adjuvant endocrine therapy.
AUTHOR CONTRIBUTIONS
Conception and design: Eleftherios P. Mamounas, Wendy A. Woodward
Administrative support: Wendy A. Woodward
Collection and assembly of data: All authors
Data analysis and interpretation: Eleftherios P. Mamounas, Wendy A. Woodward
Manuscript writing: All authors
Final approvalof manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHOR’S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Molecular Predictive and Prognostic Markers in Locoregional Management
The following represents disclosure information provided by the author of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eleftherios P. Mamounas
Honoraria: Genentech, Genomic Health
Consulting or Advisory Role: Genomic Health, bioTheranostics, Genentech, Merck, Daiichi Sankyo
Speakers' Bureau: Genomic Health, Genentech
Travel, Accommodations, Expenses: Genomic Health, Genentech
Wendy A. Woodward
Consulting or Advisory Role: Genomic Health, Epic Sciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications Proc Natl Acad Sci USA 9810869–108742001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer N Engl J Med 3471999–20092002 [DOI] [PubMed] [Google Scholar]
- 3.van ’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer Nature 415530–5362002 [DOI] [PubMed] [Google Scholar]
- 4.Foekens JA, Atkins D, Zhang Y, et al. Multicenter validation of a gene expression-based prognostic signature in lymph node-negative primary breast cancer J Clin Oncol 241665–16712006 [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer N Engl J Med 3512817–28262004 [DOI] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study J Clin Oncol 281829–18342010 [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M, Cuzick J, Wales C, et al. Risk of distant recurrence using oncotype DX in postmenopausal primary breast cancer patients treated with anastrozole or tamoxifen: A TransATAC study . Cancer Res. 2009;69:75s. (suppl; abstr 53) [Google Scholar]
- 8.Zhang Y, Schnabel CA, Schroeder BE, et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence Clin Cancer Res 194196–42052013 [DOI] [PubMed] [Google Scholar]
- 9.Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: A prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population Lancet Oncol 141067–10762013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubsky P, Filipits M, Jakesz R, et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer Ann Oncol 24640–6472013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin M, Brase JC, Calvo L, et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: Results from the GEICAM 9906 trial. Breast Cancer Res. 2014;16:R38. doi: 10.1186/bcr3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016;108:djw149. doi: 10.1093/jnci/djw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer Clin Cancer Res 165222–52322010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials Lancet 3781707–17162011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaker NG, Hoffman KE, Stauder MC, et al. The 21-gene recurrence score complements IBTR! Estimates in early-stage, hormone receptor-positive, HER2-normal, lymph node-negative breast cancer. Springerplus. 2015;4:36. doi: 10.1186/s40064-015-0840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy J Clin Oncol 262373–23782008 [DOI] [PubMed] [Google Scholar]
- 17.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy J Clin Oncol 293885–38912011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millar EK, Graham PH, O’Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel J Clin Oncol 274701–47082009 [DOI] [PubMed] [Google Scholar]
- 19.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse J Clin Oncol 281684–16912010 [DOI] [PubMed] [Google Scholar]
- 20.Liu FF, Shi W, Done SJ, et al. Identification of a low-risk luminal A breast cancer cohort that may not benefit from breast radiotherapy J Clin Oncol 332035–20402015 [DOI] [PubMed] [Google Scholar]
- 21.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer J Clin Oncol 245652–56572006 [DOI] [PubMed] [Google Scholar]
- 22.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence Clin Cancer Res 134429–44342007 [DOI] [PubMed] [Google Scholar]
- 23.Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group J Clin Oncol 261419–14262008 [DOI] [PubMed] [Google Scholar]
- 24.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials J Clin Oncol 242028–20372006 [DOI] [PubMed] [Google Scholar]
- 25.Taghian A, Jeong JH, Mamounas E, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials J Clin Oncol 224247–42542004 [DOI] [PubMed] [Google Scholar]
- 26.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer J Clin Oncol 272466–24732009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drukker CA, Elias SG, Nijenhuis MV, et al. Gene expression profiling to predict the risk of locoregional recurrence in breast cancer: A pooled analysis Breast Cancer Res Treat 148599–6132014 [DOI] [PubMed] [Google Scholar]
- 28.Solin LJ, Gray R, Goldstein LJ, et al. Prognostic value of biologic subtype and the 21-gene recurrence score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: Results from the Eastern Cooperative Oncology Group E2197 study Breast Cancer Res Treat 134683–6922012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamounas EP, Liu Q, Paik S, et al. 21-gene recurrence score and locoregional recurrence in node-positive/ER-positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017;109:djw259. doi: 10.1093/jnci/djw259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzal F, Filipits M, Rudas M, et al. The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial Br J Cancer 1121405–14102015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuyten DS, Kreike B, Hart AA, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8:R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSABP B-14 and NSABP B-20 J Clin Oncol 281677–16832010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turashvili G, Brogi E, Morrow M, et al. Breast carcinoma with 21-gene recurrence score lower than 18: Rate of locoregional recurrence in a large series with clinical follow-up. BMC Cancer. 2018;18:42. doi: 10.1186/s12885-017-3985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzal F, Filipits M, Fesl C, et al. Predicting local recurrence using PAM50 in postmenopausal endocrine responsive breast cancer patients. J Clin Oncol. 2014;32(suppl 15; abstr 1008) [Google Scholar]
- 35.Woodward WA, Barlow WE, Jagsi R, et al. The 21-gene recurrence score and locoregional recurrence rates in patients with node-positive breast cancer treated on SWOG S8814. JAMA Oncol. doi: 10.1001/jamaoncol.2019.55592020. [epub ahead of print on January 9, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng SH, Horng CF, West M, et al. Genomic prediction of locoregional recurrence after mastectomy in breast cancer J Clin Oncol 244594–46022006 [DOI] [PubMed] [Google Scholar]
- 37.Tramm T, Mohammed H, Myhre S, et al. Development and validation of a gene profile predicting benefit of postmastectomy radiotherapy in patients with high-risk breast cancer: A study of gene expression in the DBCG82bc cohort Clin Cancer Res 205272–52802014 [DOI] [PubMed] [Google Scholar]
- 38.Speers C, Zhao S, Liu M, et al. Development and validation of a novel radiosensitivity signature in human breast cancer Clin Cancer Res 213667–36772015 [DOI] [PubMed] [Google Scholar]
- 39.Eschrich SA, Fulp WJ, Pawitan Y, et al. Validation of a radiosensitivity molecular signature in breast cancer Clin Cancer Res 185134–51432012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres-Roca JF, Fulp WJ, Caudell JJ, et al. Integration of a radiosensitivity molecular signature into the assessment of local recurrence risk in breast cancer Int J Radiat Oncol Biol Phys 93631–6382015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjöström M, Staaf J, Edén P, et al. Identification and validation of single-sample breast cancer radiosensitivity gene expression predictors. Breast Cancer Res. 2018;20:64. doi: 10.1186/s13058-018-0978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui Y, Li B, Pollom EL, et al. Integrating radiosensitivity and immune gene signatures for predicting benefit of radiotherapy in breast cancer Clin Cancer Res 244754–47622018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang BS, Kim IA.A radiosensitivity gene signature and PD-L1 status predict clinical outcome of patients with invasive breast carcinoma in The Cancer Genome Atlas (TCGA) dataset Radiother Oncol 124403–4102017 [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Mao JH, Zhu W, et al. Centromere and kinetochore gene misexpression predicts cancer patient survival and response to radiotherapy and chemotherapy. Nat Commun. 2016;7:12619. doi: 10.1038/ncomms12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed KA, Liveringhouse CL, Mills MN, et al. Utilizing the genomically adjusted radiation dose (GARD) to personalize adjuvant radiotherapy in triple negative breast cancer management EBioMedicine 47163–1692019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sjöström M, Chang SL, Fishbane N, et al. Clinicogenomic radiotherapy classifier predicting the need for intensified locoregional treatment after breast-conserving surgery for early-stage breast cancer J Clin Oncol 373340–33492019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours Nature 406747–7522000 [DOI] [PubMed] [Google Scholar]
- 48.Pötter R, Gnant M, Kwasny W, et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer Int J Radiat Oncol Biol Phys 68334–3402007 [DOI] [PubMed] [Google Scholar]
- 49.Eschrich S, Zhang H, Zhao H, et al. Systems biology modeling of the radiation sensitivity network: A biomarker discovery platform Int J Radiat Oncol Biol Phys 75497–5052009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Servant N, Bollet MA, Halfwerk H, et al. Search for a gene expression signature of breast cancer local recurrence in young women Clin Cancer Res 181704–17152012 [DOI] [PubMed] [Google Scholar]