Abstract
PURPOSE
Preclinical studies report that trastuzumab (T) can boost radiotherapy (RT) effectiveness. The primary aim of the B-43 trial was to assess the efficacy of RT alone vs concurrent RT plus T in preventing recurrence of ipsilateral breast cancer (IBTR) in women with ductal carcinoma in situ (DCIS).
PATIENTS AND METHODS
Eligibility: Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, DCIS resected by lumpectomy, known estrogen receptor (ER) and/or progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) status by centralized testing. Whole-breast RT was given concurrently with T. Stratification was by menopausal status, adjuvant endocrine therapy plan, and nuclear grade. Definitive intent-to-treat primary analysis was to be conducted when either 163 IBTR events occurred or all accrued patients were on study ≥ 5 years.
RESULTS
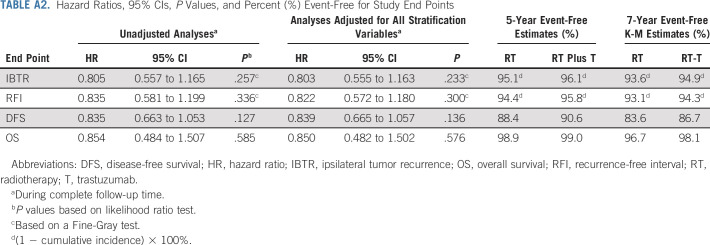
There were 2,014 participants who were randomly assigned. Median follow-up time as of December 31, 2019, was 79.2 months. At primary definitive analysis, 114 IBTR events occurred: RT arm, 63 and RT plus T arm, 51 (hazard ratio [HR], 0.81; 95% CI, 0.56 to 1.17; P value = .26). There were 34 who were invasive: RT arm, 18 and RT plus T arm, 20 (HR, 1.11; 95% CI, 0.59 to 2.10; P value = .71). Seventy-six were DCIS: RT arm, 45 and RT plus T arm, 31 (HR, 0.68; 95% CI, 0.43 to 1.08; P value = .11). Annual IBTR event rates were: RT arm, 0.99%/y and RT plus T arm, 0.79%/y. The study did not reach the 163 protocol-specified events, so the definitive analysis was triggered by all patients having been on study for ≥ 5 years.
CONCLUSION
Addition of T to RT did not achieve the objective of 36% reduction in IBTR rate but did achieve a modest but statistically nonsignificant reduction of 19%. Nonetheless, this trial had negative results. Further exploration of RT plus T is needed in HER2-positive DCIS before its routine delivery in patients with DCIS resected by lumpectomy.
INTRODUCTION
The American Cancer Society predicts that 276,480 new invasive breast cancers (IBC) will be detected in the United States in the year 2020. In addition, about 48,530 ductal carcinoma in situ (DCIS) cases will be newly diagnosed.1
CONTEXT
Key Objective
To explore the impact of trastuzumab (T), when combined with radiation therapy (RT), on preventing ipsilateral breast cancer recurrence (IBTR) in women with human epidermal growth factor receptor 2 (HER2)-positive ductal carcinoma in situ resected by lumpectomy.
Knowledge Generated
Among 2,014 enrolled participants, of whom 1,998 had follow-up information, the addition of T to RT reduced the relative risk of IBTR, the primary end point, by 19% (hazard ratio, 0.81; 95% CI, 0.56 to 1.17; P value = .26), which was not statistically significant.
Relevance
The combination of T plus RT did not statistically reduce rates of IBTR compared with RT alone in this prospective, randomized controlled clinical trial and did not achieve its primary aim.
DCIS gives rise to the majority (approximately 85%) of invasive breast cancers2 and is treated to prevent progression to IBC. Yet, most lesions will never progress, implying that overtreatment exists. For this reason, it is important to identify factors that distinguish harmless from potentially hazardous DCIS. van de Vijver3 was one of the first to note that all DCIS showing human epidermal growth factor receptor 2 (HER2) overexpression had a large-cell, comedo-type histologic appearance, whereas none of the small-cell papillary or cribriform-type cases did. Since then, others have noted that poorly differentiated DCIS frequently overexpresses HER2/neu and p53.4–8 An overview of the four randomized trials of radiotherapy (RT) in DCIS showed that higher nuclear grade was associated with a higher risk of IBTR in both control and experimental groups.9 In the EORTC randomized controlled clinical trial of lumpectomy with or without radiation, analysis of recurrences showed a high correlation between HER2 expression in the initial DCIS and the recurrence.10 Nested case-control studies in population-based cohorts with DCIS treated with breast-conserving surgery alone have shown that HER2 expression in the primary tumor predicts the progression of DCIS to IBC.11
DCIS with microinvasion is considered an interim stage in the progression from DCIS to IBC. Analysis of the differences between DCIS and DCIS with microinvasion may aid in understanding the characteristics of DCIS with microinvasion and in identifying biologic factors that determine the progression of DCIS to invasive disease. Retrospective analysis of DCIS and DCIS with microinvasion has revealed that cases with microinvasion had statistically significantly higher nuclear grade, sentinel lymph node involvement, lower estrogen receptor (ER) and progesterone receptor (PR) expression, and higher HER2 expression than did cases with pure DCIS. Furthermore, DCIS with microinvasion was statistically significantly more likely to be triple-negative or HER2-enriched.12
Thus far, the treatment of DCIS has been limited to mastectomy or lumpectomy plus RT with or without endocrine therapy in hormone receptor (HR)-positive cases. A proportion of DCIS overexpresses HER2, and targeting this receptor is standard of care for HER2-positive IBC, but this has not been studied in phase III trials that include HER2-overexpressing DCIS. There is evidence from one clinical trial13 that targeting HER2 may improve outcomes in DCIS using an HER2 peptide-pulsed dendritic cell (DC1) vaccine engineered to induce anti-HER2 immune response. The vaccine proved to be immunogenic, safe, and effective in a six-week neoadjuvant trial, resulting in pCR in 12/30 patients with pure DCIS.
Trastuzumab (T), a monoclonal antibody that specifically targets the HER2 protein,14 was approved by the US Food and Drug Administration (FDA) in 1998 and is now widely prescribed for patients with HER2-positive breast cancer, often in combination with chemotherapy, in the metastatic, adjuvant, or neoadjuvant settings.
Preclinical studies have shown that T and/or tyrosine kinase inhibitors of HER2 boost the effectiveness of RT in xenograft models and in cell lines without producing a detrimental effect on irradiated HER2-normal cells. Mechanisms include the inhibition of RT-induced activation of HER1 and HER2 as well as the inhibition of downstream survival signaling through Akt and MEK1/2.15,16 T activates natural killer cells.17 Finally, treatment with T inhibits NF-κB activation and radiosensitizes HER2-overexpressing cells.18
Information in humans that correlates clinical response with molecular markers in T-treated patients showed that apoptosis occurs quickly (within 1 week of beginning single agent T).19 This suggests that shorter treatment durations with T, rather than indefinite or prolonged therapy, should be investigated. In this trial, we hypothesized that two doses of T given concurrently with RT would improve IBTR rates compared with RT alone.
PATIENTS AND METHODS
The B-43 trial was carried out after approval from local Human Investigations Committees in accordance with assurances filed with and approved by the Department of Health and Human Services.
Enrollment was a two-step consent process. In step 1, participants with hormone-positive or hormone-negative DCIS removed by lumpectomy were consented for submission of a representative tumor block to Rush University Medical Center for centralized HER2 testing by immunohistochemistry or fluorescent in situ hybridization analysis before random assignment (enrolling sites did not perform HER2 testing). Testing followed ASCO/CAP guidelines in place at the time. In step 2, following central HER2 testing, premenopausal and postmenopausal women with DCIS or mixed DCIS plus lobular carcinoma in situ (LCIS; regardless of ER or PgR status), plus HER2-positive disease with no invasive component, were eligible for the trial. Blocks from enrolled participants were stored centrally for future study.
Consent to use an effective nonhormonal method of contraception during therapy and for at least 6 months after completion of therapy for women of reproductive potential was required. Patients had to have had a lumpectomy with negative surgical margins (no ink on tumor) and to have negative nodes if axillary staging was performed (pN0, pN0(i−), pN0(i+) pN0(mol−), or pN0(mol+)).
Patients who had had a mastectomy or those who had a history of IBC, DCIS, or a cancer not of the breast within 5 years before random assignment were ineligible except for those who had had carcinoma in situ of the cervix, carcinoma in situ of the colon, melanoma in situ, or basal or squamous cell carcinoma of the skin. Participants could not have major cardiac risk factors (see the Protocol, online only). Functional cardiac imaging was not required for enrollment.
Treatment Regimen
Eligible participants were randomly assigned to receive either RT alone or two doses of T given concurrently with RT. All participants underwent postlumpectomy whole-breast irradiation, which was to begin following random assignment. RT could be delivered with conventional fractionation (25+ fractions) or hypofractionation (16-17 fractions). RT boost, including brachytherapy, was administered at the radiation oncologist's discretion. Partial breast irradiation and regional nodal irradiation were prohibited. A normal dose of T was administered: dose 1, 8 mg/kg IV during 90 minutes, within 1 week before RT began or within the first 5 days of RT (on or before day 5) for those who received conventional fractionation of 25+ fractions. No T dose modifications were allowed. For those receiving hypofractionated RT, administration occurred within 1 week before RT began or within the first 2 days of RT (on or before day 2). The second dose of T, 6 mg/kg IV during 30 minutes, was given 3 weeks after dose 1 for all patients.
Participants with ER-positive and/or PgR-positive DCIS were recommended to receive hormonal therapy, which could begin before, during, or after RT, and was recommended for 5 years.
Statistical Considerations
The primary end point for analysis was time from random assignment to an ipsilateral IBC, ipsilateral skin cancer recurrence, or DCIS (IIBCR-DCIS, shortened here to ipsilateral breast tumor recurrence [IBTR]). In the determination of time to an IBTR, no statistical censoring was performed with respect to any previous local, regional, or distant recurrences or second primary cancer.
Among the secondary end points were (1) invasive disease-free or DCIS-free survival (shortened to DFS), (2) invasive or DCIS recurrence-free interval (RFI), and (3) overall survival (OS). Events for calculation of DFS were any recurrence, whether invasive or DCIS, second primary cancer, and death from any cause. LCIS, basal cell carcinoma, squamous cell carcinoma, melanoma in situ, carcinoma in situ of the colon, and carcinoma in situ of the cervix were not included as recurrences or second primaries. For calculation of OS, any death (cancer-related or not) was considered an event.
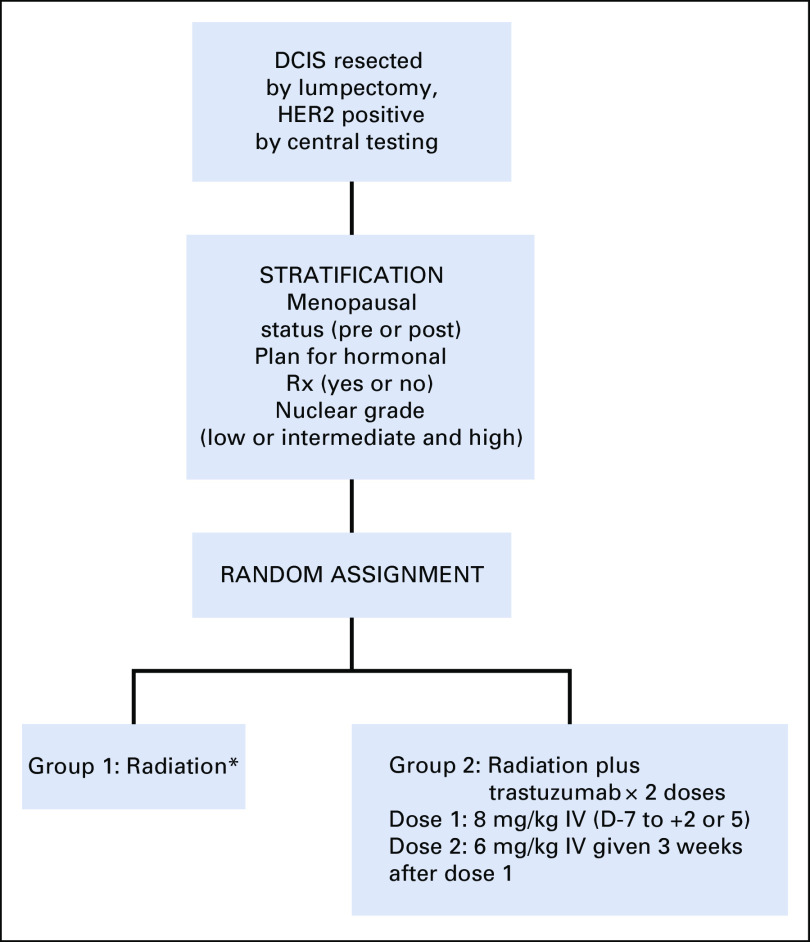
The assignment of treatment to participants was balanced with respect to menopausal status (postmenopausal or not) hormone therapy planned (yes or no), and nuclear grade (low or intermediate and high). Participants were randomly assigned to group 1 RT alone or group 2 RT plus T (Fig 1). Upon verification of inclusion and exclusion criteria, the NRG Oncology Statistical and Data Management Center stratified and randomly assigned the participants to either group 1 or group 2 using a biased-coin-minimization approach.
FIG 1.
Schema for protocol NSABP B-43. *Whole-breast only. Fractionation: conventional fractionation (25+ fractions) or hypofractionation (16-17 fractions). Boost at discretion of physician. DCIS, ductal carcinoma in situ; HER2, human epidermal growth factor receptor 2.
The study design called for the accrual of 2,000 participants during a period of roughly 8 years. A definitive analysis of the primary end point was to be performed when 163 IBTR events were observed, which was expected to be achieved between 10 and 10.5 years after the start of the protocol. This number of events afforded 80% power to detect a hazard reduction of 36%, from 1.73 IBC events per 100 patient-years to 1.11 events per 100 patient-years. The 36% observed reduction in the hazard of IBTR on the RT plus T arm is based on a projection of 40% hazard reduction if the compliance were perfect, with a 10% noncompliance rate. In those 10% of patients, regardless of when and why they stopped study medication, we assumed that no effect would be observed, thus attenuating the hazard reduction to 0.9 × 40% = 36%. The power calculations also took into account a 1% loss to follow-up in each arm of the study.
Three interim analyses were originally planned. Only two interim analyses were conducted: the first when 42 IBTR events (RT group, 26; RT plus T group, 16; hazard ratio [HR], 0.61; P = .12) were reported, and the second when 87 IBTR events (RT group, 49; RT plus T group, 38; HR, 0.77; P = .22) were reported. In both cases, the P values were larger than the preset O'Brien-Fleming P values of .00164 and .00194, respectively. A protocol amendment, approved by the study Data Monitoring Committee and implemented in 2014, indicated that if 163 IBTR events had not occurred by 5 years after the last patient was enrolled (ie, as of December 2019), a definitive analysis would be performed, using whatever events had occurred at that time. This definitive analysis was triggered by the amendment when 114 IBTR events occurred. With that number of events, the power to detect the protocol-specified 36% hazard rate reduction was reduced from 80% to 64.9%.
Times to IBTR and recurrences (IBTR and RFI, respectively) were compared across treatment arms using cumulative incidence curves,20 Cox proportional hazard models, and the Kaplan-Meier method. For formal comparison of cumulative incidence curves, the method by Fine and Gray21 was used and treatment P values were based on score tests with 1 df. In secondary analyses, Cox proportional hazards models were used to evaluate the effect of treatment on time to IBTR, RFI, OS, and DFS. Analyses were done both univariately and multivariately, adjusting for the stratification factors. Because the results were similar, inferences for treatment differences were based on univariate (unstratified) tests. Finally, formal statistical tests for treatment by covariate interactions were also performed. All P values were 2-sided, and P < .05 was considered to be statistically significant.
RESULTS
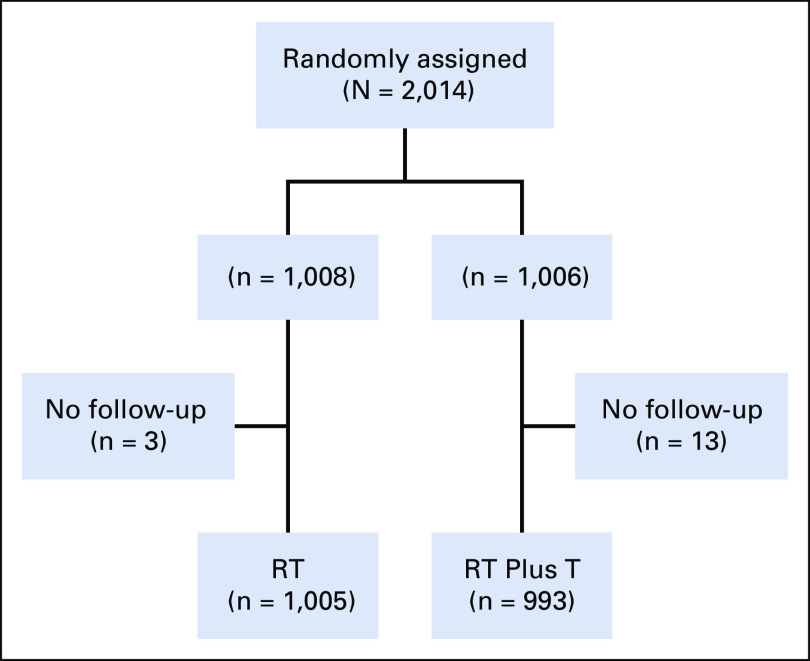
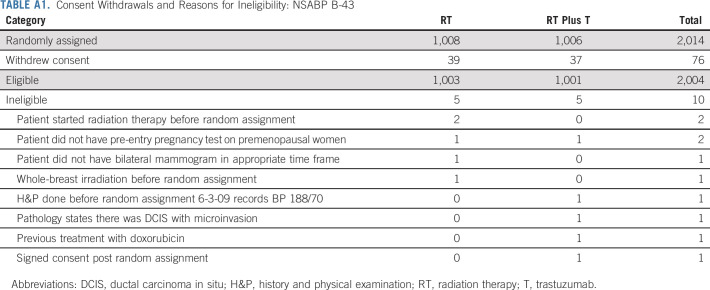
In this study, 7,888 specimens from patients were submitted for central review. There were 2,751 (35%) patients with adequate tissue samples who were found to be HER2-positive. Two thousand fourteen participants were entered and randomly assigned between December 10, 2008, and December 8, 2014 (Fig 2). Ten randomly assigned participants were ineligible, five in the RT group and five in the RT plus T group. Seventy-six participants withdrew their consent to be followed, 39 in the RT group and 37 in the RT plus T group (Appendix Table A1, online only). Following the intent-to-treat principle, all patients with follow-up information (1998) were included in the analysis (1,005 in RT and 993 in RT plus T) regardless of their eligibility status (Appendix Table A2, online only).
FIG 2.
CONSORT diagram: NSABP B-43. RT, radiation therapy; T, trastuzumab.
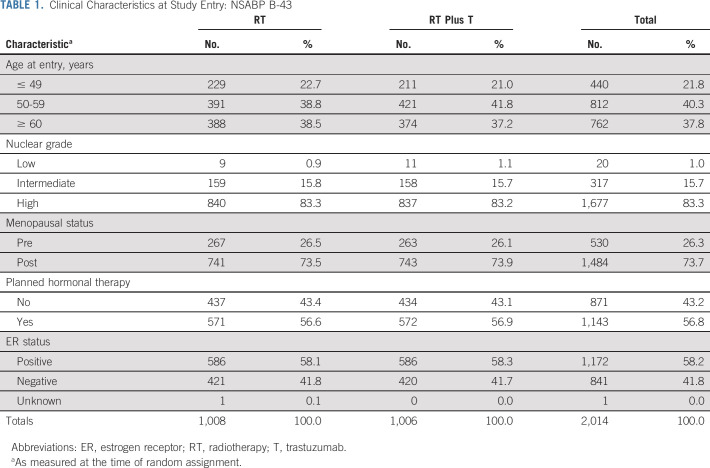
As of December 31, 2019, the median follow-up time for participants with follow-up data was 79.2 months. Patient characteristics were well balanced (Table 1). The majority were postmenopausal, and endocrine therapy was planned for slightly over half. High nuclear grade was common, as would be expected in a HER2-positive patient cohort. Of 2,001 participants with RT information, 1,679 (83.9%) had an RT boost: 846/1,001 (84.5%) in the RT group and 833/1,000 (83.3%) in the RT plus T group. Of 1,988 patients with fractionation information, 284 (14.3%) had hypofractionation (147/998 [14.7%] in the RT group and 137/990 [13.8%] in the RT plus T group).
TABLE 1.
Clinical Characteristics at Study Entry: NSABP B-43
Compliance was excellent. Of those with RT information completed, 1,965/2,001 participants (98.2%) completed RT per protocol (98.3% in the RT group and 98.1% in the RT plus T group). In the RT plus T group, 96.8% received at least one dose and 94.3% received both doses of T.
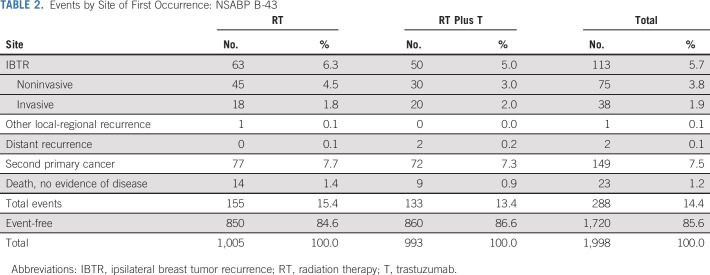
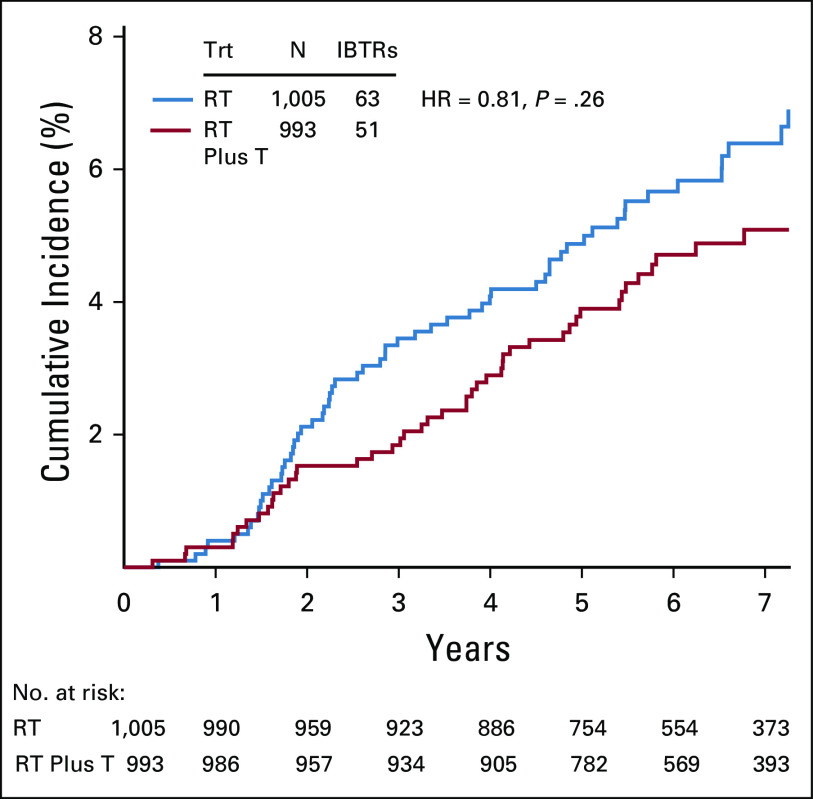
There were 63 (6.3%) confirmed IBTR events in the RT group and 51 (5.1%; 50 were first events) in the RT plus T group (HR, 0.81; 95% CI, 0.56 to 1.17; P value = .26). Events by site of first occurrence are shown in Table 2. Note that 113/114 of IBTR events occurred as a first event. Figure 3 shows the cumulative incidence curves by treatment group for IBTR. Among the 114 IBTR events, 38 (33.3%) were invasive (RT group, 18; RT plus T group, 20 [HR, 1.11; 95% CI, 0.59 to 2.10; P value = .71]) and 76 (66.7%) (RT group, 45 and RT plus T group, 31 [HR, 0.68; 95% CI, 0.43 to 1.08; P value = .11]) were noninvasive (Fig A1, online only).
TABLE 2.
Events by Site of First Occurrence: NSABP B-43
FIG 3.
Cumulative incidence of IBTR by treatment group: NSABP B-43. IBTR, ipsilateral breast tumor recurrence; RT, radiotherapy; T, trastuzumab.
The number of confirmed recurrences, both invasive and noninvasive, was 118 (11.7%): 64 (6.4%) in the RT group and 54 (5.4%) in the RT plus T group (HR, 0.83; 95% CI, 0.58 to 1.20; P value = .34). One other local event was recorded (in the RT treatment group) but as not being an IBTR. Altogether, there were 288 (14.4%) confirmed protocol events of any kind (iDFS-DCIS; designated also as DFS events): 155 (15.5%) in the RT group and 133 (13.4%) in the RT plus T group (HR, 0.84; 95% CI, 0.66 to 1.05; P value = .13).
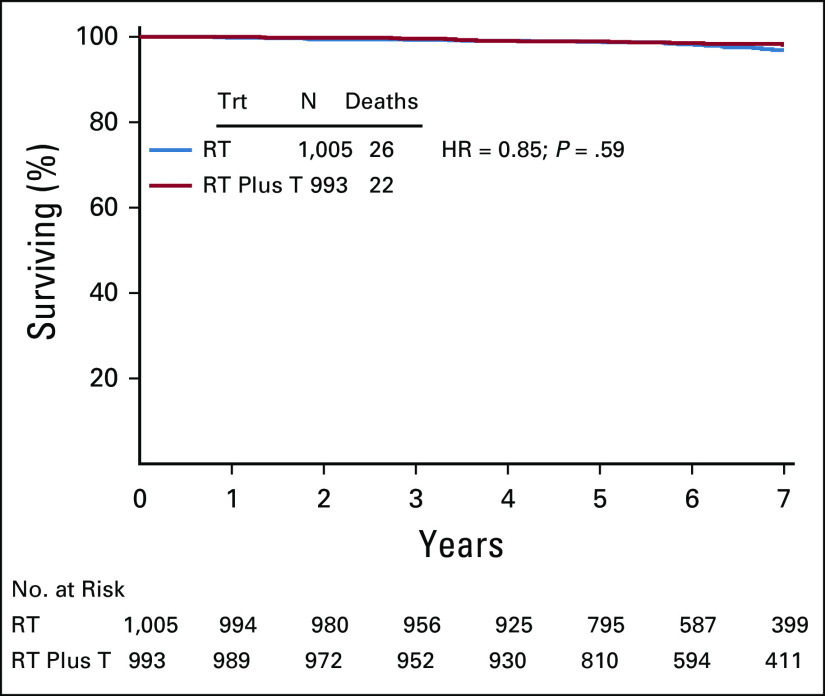
Forty-eight deaths occurred: RT group, 26, and RT plus T group, 22 (HR = 0.85; 95% CI, 0.48 to 1.51; P = .59; Fig A2, online only). There were also 150 (7.5%) confirmed second primary cancers: 78 (7.9%) in the RT group and 72 (7.3%) in the RT plus T group (HR, 0.91; 95% CI, 0.66 to 1.25; P = .57). Finally, four distant recurrences were reported: one in the RT and three in the RT plus T group. Two of these distant recurrences were first events (both in the RT plus T arm; Table 2).
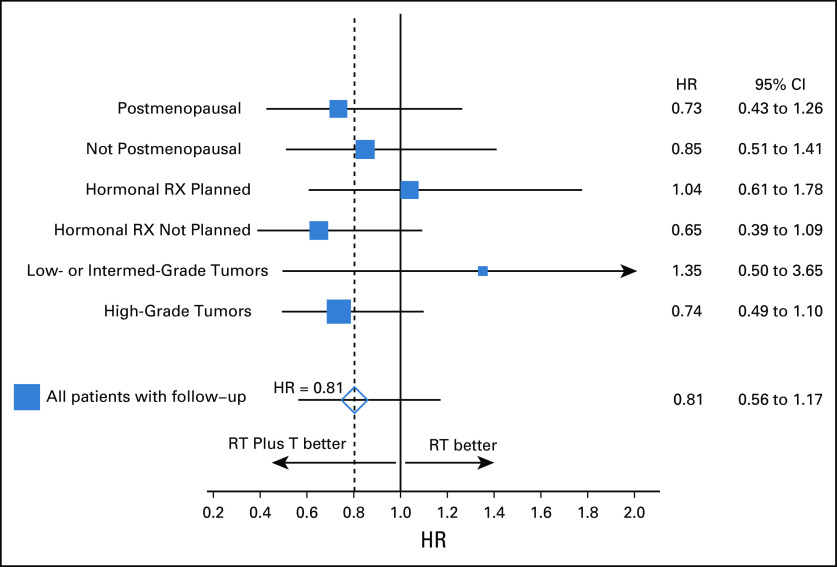
A forest plot for time to IBTR is displayed in Figure 4. There were no statistically significant heterogeneities of treatment effects among the stratification variables for IBTR. Formal tests of treatment by stratification variable interactions did not reveal any statistically significant treatment by stratification variable heterogeneities for any of the secondary end points.
FIG 4.
Forest plot of HRs of time to IBTR between groups by stratification variables. HR, hazard ratio; IBTR, ipsilateral breast tumor recurrence; RT, radiotherapy; RX, therapy; T, trastuzumab.
Acute toxicity was low in both arms (Table 3). No acute toxic deaths occurred. Two patients (both in the RT plus T arm) experienced grade 4 adverse events (AEs). One was because of an injury and the other because of a metabolic disorder, both deemed unrelated to therapy. Thirty-nine (3.9%) in the RT arm and 49 (4.9%) in the RT plus T arm experienced grade 3 AEs. Two patients in each group had G3 cardiac AEs. Long-term AEs were also rare. One patient in the RT group developed invasive HER2-positive IBTR, received adjuvant anthracycline-based HER2-directed adjuvant therapy, and later succumbed to acute myelogenous leukemia.
TABLE 3.
Distribution of Patients by Highest-Grade Adverse Event: NSABP B-43
DISCUSSION
To our knowledge, this is the first prospective, randomized controlled clinical trial to evaluate the effectiveness of T when added to RT in a HER2-positive patient cohort with DCIS. The addition of T to RT did not achieve the 36% reduction in the IBTR hazard ratio that drove the power calculations in the protocol. The observed reduction was 19% (20% if adjusted for stratification variables). The number of confirmed IBTR events (114) reported in the actual study was far lower than originally projected. Had there actually been 163 IBTR events in this cohort, the rate would have been roughly 1.24 IBTR events per 100 person-years in both groups combined. Instead, roughly 0.87 IBTR events per 100 person-years were reported, representing about a 30% IBTR event rate reduction from what was originally projected.
Annual IBTR event rates have fallen consistently throughout the period during which the four randomized controlled clinical trials conducted by NSABP (B-17, B-24, B-35, and B-43) were performed (NRG Oncology SDMC Pittsburgh Biostatistical office).22,23 This is likely because of improved imaging and treatment. Furthermore, clear margins were not required in B-24.
The presence of the HER2 protein appears to be a negative prognostic indicator in a modern treatment era. The annual IBTR rates in B-35, a population of HR-positive patients unselected for HER2, were 0.48 and 0.39 for the control and experimental groups, respectively (R.S. Checchini, personal communication, August 2020).24 In B-43, a population of HER2-positive patients unselected for HR expression, those rates were 0.99 and 0.79, respectively.
An intriguing observation from B-43 was the marked reduction (although not statistically significant) in noninvasive IBTRs with no effect on invasive IBTRs. Possible explanations may include a chance finding related to unplanned subset analysis, a true prevention effect somehow blocking progression of in situ to IBC, or an unknown mechanism. In the NSABP B-17 study, the addition of RT to lumpectomy alone significantly reduced the risk of both DCIS and IIBTRs.22 The same was true in the EORTC 10853, SweDCIS, and UK/ANZ DCIS trials.24–26 DCIS is considered a nonobligate precursor of IBC. But other possibilities include that DCIS is a marker of risk rather than a precursor or that a focus of IBC existing in conjunction with DCIS might remain after lumpectomy. If there were a true treatment effect of T in this trial, it would support the idea that T or T plus RT prevents DCIS from progressing to IBC but has no effect on pre-existing IBC.
DCIS recurrences were nearly twice as high as the invasive recurrences in B-43. This may reflect more hormone-receptor-low or negative cases, known to be associated with HER2 overexpression and which would not benefit from endocrine therapy. It may also reflect the HER2-positive nature of this cohort. Long-term follow-up of the B-17 and B-24 studies revealed that the hazard for DCIS IBTR with comedo necrosis (a surrogate for HER2 overexpression) was 2.21 compared with noncomedo DCIS (P = .001). There was no difference in IIBTR according to comedo status.
In summary, although NSABP B-43 did not meet its primary end point and had negative results, the trial did show that the addition of T to RT was safe and, although not statistically significant, results were associated with a 19% reduction in IBTRs (the rate of which has decreased over time), a 17% reduction in recurrences, and a 16% reduction in all DFS events. It should be noted that this trial did not address the efficacy of T in patients with HER2-positive DCIS so extensive that mastectomy was required but, rather, a population of HER2-positive patients whose disease could be resected by lumpectomy with clear margins. Further exploration of RT plus T in lumpectomy candidates is needed in HER2-positive DCIS before this therapy is offered routinely.
ACKNOWLEDGMENT
The authors thank Elaina Harper and Marlon Jones for data management, and acknowledge the contributions of Barbara C. Good, PhD, Director of Scientific Publications; Christine I. Rudock, Publications and Graphics Specialist; and Wendy L. Rea, BA, Editorial Associate, all of whom are employees of NSABP. They were not compensated beyond their normal salaries for this work.
APPENDIX
FIG A1.
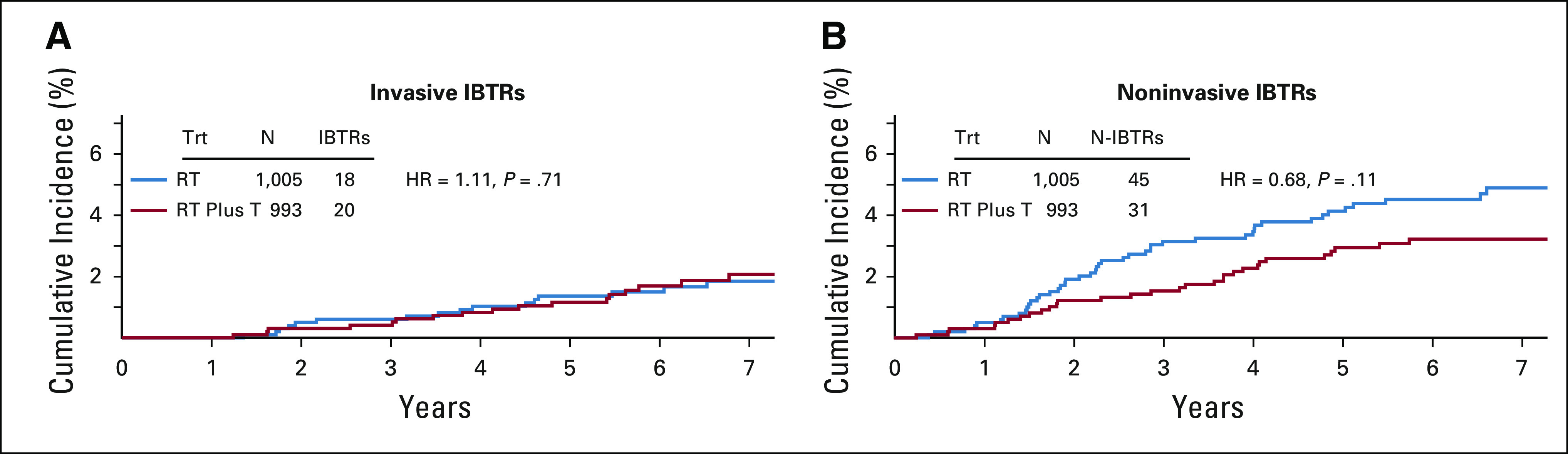
Cumulative incidence curves for invasive and noninvasive IBTRs: NSABP B-43. HR, hazard ratio; IBTR, ipsilateral breast cancer recurrence; RT, radiation therapy; T, trastuzumab; Trt, treatment.
FIG A2.
Kaplan-Meier plots of overall survival by treatment group: NSABP B-43. HR, hazard ratio; RT, radiation therapy; RT plus T, radiation therapy plus trastuzumab; Trt, treatment.
TABLE A1.
Consent Withdrawals and Reasons for Ineligibility: NSABP B-43
TABLE A2.
Hazard Ratios, 95% CIs, P Values, and Percent (%) Event-Free for Study End Points
Melody A. Cobleigh
Consulting or Advisory Role: Roche/Genentech, Immunomedics, Genomic Health, Puma Biotechnology, Seattle Genetics
Research Funding: Macrogenics, Radius Health, Genentech/Roche, Seattle Genetics, Zymeworks, Synthon
Patents, Royalties, Other Intellectual Property: Genomic Health
Travel, Accommodations, Expenses: Genentech, Immunomedics, Puma Biotechnology, Seattle Genetics
Stewart J. Anderson
Consulting or Advisory Role: Jazz Pharmaceuticals
Kalliopi P. Siziopikou
Honoraria: Ventana Medical Systems, Lilly, Merck
Douglas W. Arthur
Stock and Other Ownership Interests: Advanced Radiation Therapy
Rachel Rabinovitch
Stock and Other Ownership Interests: Abbott Laboratories, Bristol-Myers Squibb, Intuitive Surgical, IDEXX Laboratories
Research Funding: Prelude Therapeutics
Dennis L. Carter
Employment: Rocky Mountain Cancer Centers
Melissa S. Dillmon
Stock and Other Ownership Interests: Johnson & Johnson
Consulting or Advisory Role: Puma Biotechnology
Vivek S. Kavadi
Employment: US Oncology Network
Matthew L. Hill
Stock and Other Ownership Interests: AstraZeneca, Newlink Genetics, Kazia Therapeutics, Leap Therapeutics, OncoSec, MEI Pharma, PLx Pharma, Radius Health, Crispr Therapeutics, Cassava Sciences
Sushil Beriwal
Honoraria: Varian Medical Systems, XOFT
Eleftherios P. Mamouna
Honoraria: Genentech/Roche, Genomic Health, Precisca
Consulting or Advisory Role: Genomic Health, BioTheranostics, Roche/Genentech, Merck, Daiichi Sankyo, Puma Biotechnology, Precisca
Speakers' Bureau: Genomic Health, Genentech/Roche
Travel, Accommodations, Expenses: Genomic Health, Genentech/Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported by Grants No. U10-180868, U10-180822, and UG1-189867; and by Genentech.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Melody A. Cobleigh, Stewart J. Anderson, Kalliopi P. Siziopikou, Douglas W. Arthur, Thomas B. Julian, David S. Parda, Eleftherios P. Mamounas, Norman Wolmark
Administrative support: Norman Wolmark
Provision of study materials or patients: Melody A. Cobleigh, Thomas B. Julian, David S. Parda, Samantha A. Seaward, Dennis L. Carter, Gustav C. Magrinat, Vivek S. Kavadi, Allison M. Zibelli, Lavanya Tiriveedhi, Marianne K. Melnik, Eleftherios P. Mamounas
Collection and assembly of data: Stewart J. Anderson, Kalliopi P. Siziopikou, Samantha A. Seaward, Dennis L. Carter, Melissa S. Dillmon, Gustav C. Magrinat, Allison M. Zibelli, Lavanya Tiriveedhi, Matthew L. Hill, Marianne K. Melnik, Eleftherios P. Mamounas
Data analysis and interpretation: Melody A. Cobleigh, Stewart J. Anderson, Douglas W. Arthur, Rachel Rabinovitch, Thomas B. Julian, David S. Parda, Janice A. Lyons, Vivek S. Kavadi, Sushil Beriwal, Eleftherios P. Mamounas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comparison of Radiation With or Without Concurrent Trastuzumab for HER2-Positive Ductal Carcinoma In Situ Resected by Lumpectomy: A Phase III Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Melody A. Cobleigh
Consulting or Advisory Role: Roche/Genentech, Immunomedics, Genomic Health, Puma Biotechnology, Seattle Genetics
Research Funding: Macrogenics, Radius Health, Genentech/Roche, Seattle Genetics, Zymeworks, Synthon
Patents, Royalties, Other Intellectual Property: Genomic Health
Travel, Accommodations, Expenses: Genentech, Immunomedics, Puma Biotechnology, Seattle Genetics
Stewart J. Anderson
Consulting or Advisory Role: Jazz Pharmaceuticals
Kalliopi P. Siziopikou
Honoraria: Ventana Medical Systems, Lilly, Merck
Douglas W. Arthur
Stock and Other Ownership Interests: Advanced Radiation Therapy
Rachel Rabinovitch
Stock and Other Ownership Interests: Abbott Laboratories, Bristol-Myers Squibb, Intuitive Surgical, IDEXX Laboratories
Research Funding: Prelude Therapeutics
Dennis L. Carter
Employment: Rocky Mountain Cancer Centers
Melissa S. Dillmon
Stock and Other Ownership Interests: Johnson & Johnson
Consulting or Advisory Role: Puma Biotechnology
Vivek S. Kavadi
Employment: US Oncology Network
Matthew L. Hill
Stock and Other Ownership Interests: AstraZeneca, Newlink Genetics, Kazia Therapeutics, Leap Therapeutics, OncoSec, MEI Pharma, PLx Pharma, Radius Health, Crispr Therapeutics, Cassava Sciences
Sushil Beriwal
Honoraria: Varian Medical Systems, XOFT
Eleftherios P. Mamouna
Honoraria: Genentech/Roche, Genomic Health, Precisca
Consulting or Advisory Role: Genomic Health, BioTheranostics, Roche/Genentech, Merck, Daiichi Sankyo, Puma Biotechnology, Precisca
Speakers' Bureau: Genomic Health, Genentech/Roche
Travel, Accommodations, Expenses: Genomic Health, Genentech/Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.American Cancer Society . Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carraro DM, Elias EV, Andrade VP. Ductal carcinoma in situ of the breast: Morphological and molecular features implicated in progression. Biosci Rep. 2014;34:e00090. doi: 10.1042/BSR20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Vijver MJ, Peterse JL, Mooi WJ, et al. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer N Engl J Med 3191239–12451994 [DOI] [PubMed] [Google Scholar]
- 4.Bobrow LG, Happerfield LC, Gregory WM, et al. The classification of ductal carcinoma in situ and its association with biological markers Semin Diagn Pathol 11199–2071994 [PubMed] [Google Scholar]
- 5.Zafrani B, Leroyer A, Fourquet A, et al. Mammographically-detected ductal in situ carcinoma of the breast analyzed with a new classification. A study of 127 cases: Correlation with estrogen and progesterone receptors, p53 and c-erbB-2 proteins, and proliferative activity Semin Diagn Pathol 11208–2141994 [PubMed] [Google Scholar]
- 6.Leal CB, Schmitt FC, Bento MJ, et al. Ductal carcinoma in situ of the breast. Histologic categorization and its relationship to ploidy and immunohistochemical expression of hormone receptors, p53, and c-erbB-2 protein Cancer 752123–21311995 [DOI] [PubMed] [Google Scholar]
- 7.Perin T, Canzonieri V, Massarut S, et al. Immunohistochemical evaluation of multiple biological markers in ductal carcinoma in situ of the breast Eur J Cancer 32A1148–11551996 [DOI] [PubMed] [Google Scholar]
- 8.Mack L, Kerkvliet N, Doig G, et al. Relationship of a new histological categorization of ductal carcinoma in situ of the breast with size and the immunohistochemical expression of p53, c-erb B2, bcl-2, and ki-67 Hum Pathol 28974–9791997 [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast J Natl Cancer Inst Monogr 2010162–1772010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bijker N, Peterse JL, Duchateau L, et al. Histological type and marker expression of the primary tumour compared with its local recurrence after breast-conserving therapy for ductal carcinoma in situ Br J Cancer 84539–5442001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visser LL, Elshof LE, Schaapveld M, et al. Clinicopathological risk factors for an invasive breast cancer recurrence after ductal carcinoma in situ—A nested case-control study Clin Cancer Res 243593–36012018 [DOI] [PubMed] [Google Scholar]
- 12.Wan ZB, Gao HY, Wei L, et al. Expression of estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, and Ki-67 in ductal carcinoma in situ (DCIS) and DCIS with microinvasion. Medicine (Baltimore) 2018;97:e13055. doi: 10.1097/MD.0000000000013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowenfeld L, Mick R, Datta J, et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2pos DCIS independent of route: Results of randomized selection design trial Clin Cancer Res 232961–29712017 [DOI] [PubMed] [Google Scholar]
- 14.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease J Clin Oncol 172639–26482003 [DOI] [PubMed] [Google Scholar]
- 15.Fukutome M, Maebayashi M, Nasu S, et al. Enhancement of radiosensitivity by dual inhibtion of the HER family with ZD1839 (Iressa) and trastuzumab (Herceptin) Int J Radiat Oncol Biol Phys 66528–5362006 [DOI] [PubMed] [Google Scholar]
- 16.Liang K, Lu Y, Jin W, et al. Sensitization of breast cancer cells to radiation by trastuzumab Mol Cancer Ther 21113–11202003 [PubMed] [Google Scholar]
- 17.Chi CH, Wang YS, Yang CH, et al. Neoadjuvant immunotherapy enhances radiosensitivity through natural killer cell activation Cancer Biother Radiopharm 2539–452010 [DOI] [PubMed] [Google Scholar]
- 18.Guo G, Wang T, Gao Q, et al. Expression of ErbB2 enhances radiation-induced NF-kappa B activation Oncogene 23535–5452004 [DOI] [PubMed] [Google Scholar]
- 19.Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers J Clin Oncol 232460–24682005 [DOI] [PubMed] [Google Scholar]
- 20.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data J Am Stat Assoc 88400–4091993 [Google Scholar]
- 21.Fine JP, Gray RJ.A proportional hazards model for the subdistribution of a competing risk J Am Stat Assoc 94496–5091999 [Google Scholar]
- 22.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS J Natl Cancer Inst 103478–4882011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): A randomised, double-blind, phase 3 clinical trial Lancet 387849–8562016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donker M, Litière S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial J Clin Oncol 314054–40592013 [DOI] [PubMed] [Google Scholar]
- 25.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast J Clin Oncol 261247–12522008 [DOI] [PubMed] [Google Scholar]
- 26.Cuzick J, Sestak I, Pinder SE.Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial Lancet Oncol 1221–292011 [DOI] [PMC free article] [PubMed] [Google Scholar]