Abstract
Fluoropyrimidines (fluorouracil, capecitabine, and other analogs) are highly used anticancer drugs worldwide. However, patients with cancer treated with these drugs might experience severe, life-threatening toxicity because of germline genetic variation in the DPYD gene. This is a genetic predisposition with an established mechanistic basis that links genetic variation in the DPYD gene to an increase in systemic drug exposure, resulting in an increased risk of toxicity. Pharmacology guidelines provide recommendations on avoiding treatment with fluoropyrimidines or reducing their dose in patients carrying DPYD genetic variants conferring an increased risk of toxicity. However, oncology societies in the United States do not recommend systematic testing. Instead, on April 30, 2020, the European Society for Medical Oncology issued a document recommending genetic testing. In this scenario of contradicting information, practicing oncologists struggle with reaching an informed decision on whether genetic testing should be applied before treatment. This is mostly due to uncertainty about the clinical relevance of genetic testing from the perspective of a practicing oncologist. To reach an informed decision, practicing oncologists need access to concise information on the genetic variants to be tested and a practitioner-friendly interpretation of the test results. We believe this information is currently lacking. To our knowledge, for the first time, we provide a single guide for health care professionals to make an evidence-based decision about DPYD testing for patients with cancer. This article provides the essential knowledge base for oncologists to have an informed discussion with their patients about the genetic testing for DPYD. This document assists practitioners in quickly evaluating whether, when, where, and how to order a DPYD genetic test.
INTRODUCTION
Since the discovery of dihydropyrimidine dehydrogenase (DPD) deficiency as an inherited defect and its consequences for patients with cancer treated with fluoropyrimidines,1,2 the genetic risk of toxicity after fluoropyrimidine therapy has been studied for more than 3 decades. Basic and clinical research on heritable DNA variation in the DPD gene (DPYD) has provided a wide range of knowledge, from in vitro function of genetic variants to cost evaluation of genetic testing in the clinic.
Fluoropyrimidines (fluorouracil [FU], capecitabine, and other analogs) are used as core chemotherapies in the treatment of solid tumors, such as colorectal, pancreatic, gastric, breast, and head and neck cancers.3,4 The genetic predisposition to severe toxicity from fluoropyrimidines has a mechanistic basis that links genetic variation in DPYD to an increase in systemic drug exposure, eventually resulting in increased toxicity. Yet, there is a clear disconnection between the collected evidence on the genetic/pharmacologic basis of fluoropyrimidine-related severe toxicity and the limited application of DPYD genetic testing in the clinic. Using FU as an example, this disconnection is described in 10 main points:
1. Patients might experience early-onset, severe FU toxicity due to drug overexposure after standard dosing,5 and an antidote, uridine triacetate, has been approved by the Food and Drug Administration in 2015.6
2. Pharmacokinetic studies have linked the risk of severe toxicity to a reduction in FU clearance (the first observation is dated 1963).7
3. The rate-limiting step of FU clearance is DPD-mediated catabolism of FU in the liver.8
4. Partial and complete DPD deficiency is a well-defined genetic defect.9
5. Genetic variation in DPYD can confer DPD deficiency, resulting in reduced FU clearance, reduced DPD activity in mononuclear cells, and/or increased risk of severe toxicity.10
6. Pharmacology guidelines based on 4 DPYD variants provide information on how to avoid treatment with fluoropyrimidines or reduce their dosage.10
7. The labels of injectable FU and oral capecitabine mention DPD deficiency as one of the risk factors of severe toxicity.3,4
8. The adjustment of FU dosing based on DPYD testing reduced the prevalence of toxicities.11,12
9. In the United States, oncology guidelines do not recommend the use of testing for DPD deficiency in patients treated with FU.
10. In the United States, DPYD screening is not widely adopted.13 A similar picture is observed in Europe.14
Large series and meta-analyses have been recently published,11,12,15-18 providing evidence for clinical validity and, in some cases, clinical utility of DPYD genotyping in patients with cancer. These articles have been often followed by ready-for-prime-time calls for more systematic and routine use of DPYD testing,19,20 which are still left unheard. In this article, we do not enter the debate of adequacy of routine, preemptive testing of DPYD variants in FU- and capecitabine-treated patients. This is because there are good arguments both in favor of and against it, depending on the context of the discussion and the expertise of the parties involved. It is clear that uncertainty exists among health care professionals on the relevance and magnitude of this clinical problem. Specifically, health care professionals face the challenge of understanding and answering questions about whether, when, and how to order a DPYD test, its limitations, which variants are informative in which population, the clinical interpretation of the test, and its impact on dose modification.
Hence, there is a compelling need for improved delivery of information and communication on DPYD genetic testing during the process of delivering care to patients treated with fluoropyrimidines. This document serves as a practical guide for practicing oncologists and health care professionals in general to orient themselves on the appropriateness of testing and the interpretation of its results when an informed, evidence-based decision should be made at the point of care. The basis for this work is an analysis of the facts about the clinical application of DPYD testing in patients with cancer. This analysis avoids confusion and misinterpretations on the existing evidence. This single document is a concise, practitioner-friendly reference for understanding DPYD testing for health care professionals who are not pharmacology experts.
Key Elements to Reach an Informed Decision About DPYD Testing
In this section, we provide a succinct description of key elements of knowledge that can be easily accessed by health care professionals. The most frequent use of fluoropyrimidines is FU treatment of patients with colorectal cancer, and we use this setting as the paradigm for this document. Moreover, because genotyping of DPYD variants is the most clinically accepted (and used) test for DPD deficiency, this practical guide refers to the use of germline DNA testing for DPYD variants. We based our evaluation on information antecedent to June 2020.
Prevalence of grade 3-4 (severe) toxicity attributable to FU and patient characteristics increasing their risk.
Toxicities attributable to FU are usually diarrhea, stomatitis, fatigue, anorexia, nausea/vomiting, dehydration, and pain, combined with myelosuppression. The drugs given in combination with FU have, in part, overlapping toxicities. The risk of severe toxicity of FU is generally unpredictable. Risk factors are older age, female sex, and worse performance status. The FU label indicates additional risk factors, such as high-dose irradiation, previous use of myelosuppressive agents, widespread bone marrow involvement, and impaired liver/kidney function.3 In several studies of adjuvant FU plus oxaliplatin,21,22 severe (grade 3-4) toxicities ranged between 20% and 40%. Similar rates were observed in the metastatic setting.23 Severe toxicity is usually cumulative, occurring at later cycles. In some cases, it develops during cycle 1 within hours or days after the first or second dose of FU. In 398 patients with different tu-mor types treated with fluoropyrimidine-containing regimens, the prevalence of severe toxicity before cycle 1 was 3.0% (18.0% before cycle 2).24 Severe toxicities that have an early onset (within 2-3 cycles of treatment, depending on the regimen), are usually characterized by severe GI symptoms combined with severe neutropenia, often leading to a rapid deterioration of the general conditions.25 The occurrence of cardiotoxicity and central neurotoxicity is rare.
The prevalence of toxicity-related mortality from FU in the United States.
According to the 2019 estimate of the prevalence of colorectal cancer by the American Cancer Society, 147,950 Americans were predicted to receive at least 1 FU-based treatment in 2020. The mortality rate from FU has been estimated at 0.5%-1%.26,27 Based on these numbers, it can be estimated that for every 1,000 patients treated with either FU or capecitabine in the United States, 10 patients will die of treatment-related toxicity, resulting in 700-1,400 patients per year who could die because of FU toxicity in the United States.
DPD deficiency: Symptoms, laboratory alterations, and prevalence in the general population.
DPD deficiency is a condition related to a deficit in the metabolism of thymine and uracil, resulting in excessive amounts of uracil and thymine in the blood, urine, and CSF.28 It can be inherited as an autosomal recessive trait, such as homozygosity for deleterious DPYD variants. Patients with homozygous DPYD variants can manifest with severe DPD deficiency. During infancy, this can result in neurologic and other severe defects; it is a rare condition that has been documented mostly in single case reports. However, patients with homozygous variants also can be asymptomatic. The prevalence of DPD deficiency, based on activity levels measured in blood cells and the uracil breath test, has been reported as 3%-5% (n = 109) in patients of European origin and 8% (n = 149) in patients of African origin.29 In addition, the US National Library of Medicine reports a prevalence of partial deficiency of 2%-8%, based on the aforementioned study and others.28
DPYD variants conferring increased risk of FU toxicity: Nomenclature and population frequency.
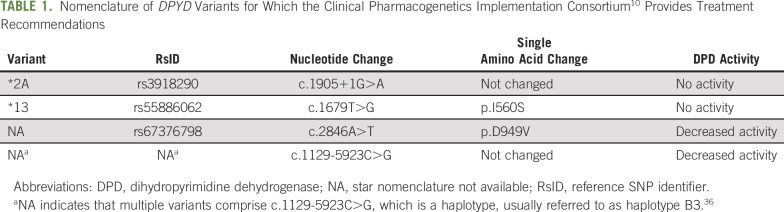
The Clinical Pharmacogenetics Implementation Consortium selected 4 DPYD variants that confer DPD deficiency and for which recommendations exist to aid fluoropyridine treatment and dosing decisions.10 It should be noted that DPYD genetic variants can be expressed as using the star (*) nomenclature for alleles,30 reference SNP identifier, nucleotide base change of the DNA, or amino acid change. We have reported all the nomenclatures for these variants in a single table (Table 1).
TABLE 1.
Nomenclature of DPYD Variants for Which the Clinical Pharmacogenetics Implementation Consortium10 Provides Treatment Recommendations
The global frequency for each of those 4 variants (based on data from > 5,000 individuals from the 1,000 Genomes Project, which is based on dbSNP [Single Nucleotide Polymorphism database] accessed May 2020) ranges from 0.02%-0.96% (Table 1). Under the Hardy-Weinberg equilibrium law of heritance, these frequencies mean that out of 1,000 patients treated with FU, at least 2% of them (n = 20) are expected to be carriers of at least 1 of these 4 variants. In patients of European origin, the frequency is higher (4.8%; n = 48), but this is much lower in patients of African origin (0.16%; n = 2). In patients of South Asian origin (< 0.001%), no patients out of 1,000 are expected to be carriers of any of those variants.
Recently, 23andMe has preliminarily reported DPYD allele frequencies in 6,421,599 genotyped individuals of different ethnic backgrounds, such as European, Hispanic or Latino, African American, East Asian, Ashkenazi Jewish, South Asian, Middle Eastern, and others.31 The allele frequency of *2A was 0.47%, 0.26%, 0.13%, 0.06%, 0.56%, 0.56%, 0.44%, and 0.34%, respectively. The allele frequency of p.D949V was 0.55%, 0.43%, 0.18%, 0.08%, 0.01%, 0.05%, 0.09%, and 0.27%, respectively. On the basis of these 2 DPYD variants (the only ones that the company currently performs genotyping for),32 it can be calculated that approximately 2% of the general population might have impaired DPD activity, conferring increased risk of FU and capecitabine toxicity.31
Interpretation of the results of DPYD genotyping.
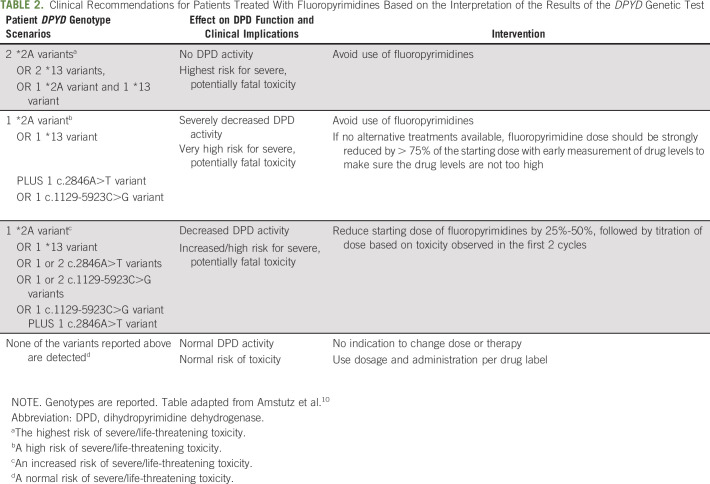
Based on the analysis of the Clinical Pharmacogenetics Implementation Consortium,10 if patients are carriers of 1 or more alleles of these 4 variants before starting FU therapy, several types of interventions are recommended. A user-friendly version of the interpretation of the test results is provided in Table 2. Relative to the recommendation table of Amstutz et al,10 this modified table has removed reference to Activity Scores assigned to combinations of variants and associated rating schemes (in supplementary section of Amstutz et al10). It also does not report the DPYD metabolizer status; the intermediate status could be misleading because it indicates an average metabolizing activity that can be confused as being normal, but for this gene it is associated with increased risk of severe or fatal toxicity. We have divided the genotypes, their effect on DPD function, and the relative intervention into 4 levels based on risk (Table 2). In addition, Table 2 has also removed references to a phenotyping test to establish the starting dose for the high-risk section.10
TABLE 2.
Clinical Recommendations for Patients Treated With Fluoropyrimidines Based on the Interpretation of the Results of the DPYD Genetic Test
Prevalence of FU-related toxic deaths in carriers of DPYD variants.
The US National Institutes of Health has estimated that approximately 1,300 deaths per year are attributable to DPD deficiency, on the basis of a prevalence of 0.5% deaths in patients treated with FU annually.33 This commonly cited document is “Public Teleconference Regarding Licensing and Collaborative Research Opportunities for: Methods and Compositions Relating to Detecting Dihydropyrimidine Dehydrogenase (DPD).”33 It does not report results from any studies. The only available study, based on a limited sample size, found that approximately 10% (5 of 48) of DPYD*2A carriers died of fluoropyrimidine-related toxicity.12
The specificity and sensitivity of DPYD genetic testing.
Testing for p.D949V has a sensitivity of 2%, specificity of 100%, positive predictive value of 86%, and negative predictive value of 51%.17 Testing for *2A, *13, and p.D949V combined has similar rates of predictive values.15 This is because patients who are carriers of these alleles have a high risk of developing severe toxicity (high specificity). Conversely, patients who are not carriers might still develop severe toxicity (low sensitivity), which cannot be predicted.
Effect of DPYD testing on reduction of the risk of severe FU-related toxicity.
Two large prospective studies have evaluated the effect of preemptive DPYD genotyping and dose adjustment. Relative to historical controls, the prevalence of grade 3 or higher toxicity in DPYD*2A carriers was reduced from 73% (35 of 48) to 28% (5 of 18), with a 5-fold reduction in the prevalence of GI toxicity.12 In another study, the relative risk of severe toxicity compared with historical controls was 1.31 versus 2.87 for *2A carriers, no toxicity versus 4.30 for c.1679T>G carriers, 2.00 versus 3.11 for c.2846A>T carriers, and 1.69 versus 1.72 for c.1236G>A carriers.11
Preservation of antitumor efficacy of reduced doses of FU.
A concern of oncologists is the possibility that a dose reduction of FU in DPYD carriers can negatively affect the efficacy of treatment. However, if dose reduction in DPYD carriers is compensated by a normalization in systemic exposure to active drug, the dose reductions might not affect the efficacy of the treatment. In patients with colorectal cancer treated in the adjuvant setting, 71.5% of patients (23 of 32) who were DPYD*2A carriers were disease-free at 36 months versus 73.3% of patients (2,088 of 2,848) who were not DPYD*2A carriers.15
Oncology guidelines and testing for DPYD.
In the National Comprehensive Cancer Network guidelines, the risk of severe toxicity conferred by DPYD variants is acknowledged, but pretreatment genotyping for DPYD variants is not supported because the evidence behind it is deemed to be controversial. The American Society of Clinical Oncology also does not provide any guidelines on DPYD testing. However, on April 30, 2020, the European Society for Clinical Oncology “recommended that patients should be tested for the lack of the enzyme DPD before starting cancer treatment with fluorouracil given by injection or infusion (drip) or with the related medicines, capecitabine and tegafur.”34(p1)
Drug label information in the United States about DPD deficiency.
The label of Adrucil (FU injection) states: “Rarely, unexpected severe toxicity (eg, stomatitis, diarrhea, neutropenia, and neurotoxicity) associated with 5-fluorouracil has been attributed to deficiency of dipyrimidine dehydrogenase activity.”3 The labels of Xeloda (capecitabine, for oral use) and FU (injection solution) states: “Withhold or permanently discontinue XELODA/fluorouracil in patients with evidence of acute early-onset or unusually severe toxicity, which may indicate near complete or total absence of DPD activity. No XELODA/fluorouracil dose has been proven safe in patients with absent DPD activity.”4
Laboratories providing DPYD testing.
For health professionals interested in ordering a DPYD genetic test, we provide a reference to the National Institute of Health (NIH) Genetic Testing Registry.35 This resource lists 54 Clinical Laboratory Improvement Amendments–certified laboratories (43 in the United States and 11 in Europe) that provide DPYD testing. Approaches provided for testing vary and include sequencing of exons of DPYD (as part of a larger list of genes) and genotyping of DPYD variants. Testing for DPYD variants is often included in gene panels for different indications, including pharmacogenetic scans, prenatal analyses, and diagnosis of neurologic and metabolic diseases, and rare disorders. DPYD is also included in tumor gene panels, and careful analysis of the source of material (matching germline DNA v tumor DNA only) should be conducted before ordering the test.
In this concise, albeit comprehensive report, we have checked the facts related to the use of DPYD genotyping in patients treated with fluoropyrimidines. This report does not intend to provide recommendations either for or against DPYD testing. Instead, it provides several elements of knowledge to inform decisions about testing. In this regard, we recommend that practicing oncologists familiarize themselves with the subject of DPD deficiency and its clinical implications by using this document before they search for more specialized information from other sources, for example, Amstutz et al10 and National Institutes of Health.28 We also recommend that for every patient who is considered for treatment with a fluoropyrimidine, in addition to evaluating laboratory/clinical data indicative of a DPD deficiency, a close evaluation of any family history of DPD deficiency be made insofar as patients with cancer presenting with a DPD deficiency are likely to be asymptomatic. Because patients with cancer might undergo personal or diagnostic genomic analyses for other purposes, they should be asked whether they have been genotyped before and for what genes. Doing so will allow health professionals to evaluate whether the information about DPYD genetic variants has been already obtained. Similarly, we also recommend careful review of patient electronic medical records for any genetic analysis being conducted and stored. In the assessment of risks of severe toxicity versus benefits of improved survival, the ethnic origin of the patients also should be considered, because the frequency of DPYD variants conferring risk varies significantly. Regarding the commercial sources for DPYD genetic testing, we recommend that practitioner oncologists refer to the NIH Genetic Testing Registry. Consultation with genetic and pharmacogenetic experts can supplement the decision about the selection of the most appropriate platform for genetic testing. Finally, from a health policy standpoint, we recommend improving the understanding of the clinical implications of DPYD testing by harmonizing clinical guidelines on DPYD genetic testing across oncology organizations.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: Federico Innocenti
Administrative support: Federico Innocenti
Provision of study materials or patients: Federico Innocenti
Collection and assembly of data: Federico Innocenti, Sarah C. Mills
Data analysis and interpretation: Federico Innocenti, Sarah C. Mills, Joseph Ciccolini, Heinz-Josef Lenz, Gerard Milano
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
All You Need to Know About DPYD Genetic Testing for Patients Treated With Fluorouracil and Capecitabine: A Practitioner-Friendly Guide
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Federico Innocenti
Consulting or Advisory Role: Symberix, Emerald Lake Safety
Patents, Royalties, Other Intellectual Property: United States Provisional Application: ”Methods of identifying risk of bevacizumab-induced proteinuria and hypertension,” Innocenti F, Quintanilha J, Lin D, Owzar K, Wang J. Filed on September 20, 2019, serial number 62/903,442. United States Patent: “Flavopiridol drug combinations and methods with reduced side effects,” Ratain MJ, Innocenti F, Iyer L. Filed on April 12, 2001, serial number 09/835,082. United States Patent: “Optimization of cancer treatment with irinotecan,” Ratain MJ, Innocenti F, Karabatsos P, Grimsley C, Di Rienzo A. Filed on February 12, 2003, serial number 60/446,942
Hanna Sanoff
Research Funding: Bayer (Inst)
Joseph Ciccolini
Honoraria: Genentech (Inst)
Travel, Accommodations, Expenses: Roche
Heinz-Josef Lenz
Honoraria: Merck Serono, Roche, Bayer, Boehringer Ingelheim, Isofol, GSK
Consulting or Advisory Role: Merck Serono, Roche, Bayer, BMS, GSK
Travel, Accommodations, Expenses: Merck Serono, Bayer
Gerard Milano
Honoraria: BMS, Merck, Servier, Pierre Fabre Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tuchman M, Stoeckeler JS, Kiang DT, et al. Familial pyrimidinemia and pyrimidinuria associated with severe fluorouracil toxicity N Engl J Med 313245–2491985 [DOI] [PubMed] [Google Scholar]
- 2.Diasio RB, Beavers TL, Carpenter JT.Familial deficiency of dihydropyrimidine dehydrogenase. Biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity J Clin Invest 8147–511988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Food and Drug Administration: Highlights of prescribing information [fluorourcil]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/012209s040lbl.pdf.
- 4. Food and Drug Administration: Highlights of prescribing information [Xeloda]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf.
- 5.Lévy E, Piedbois P, Buyse M, et al. Toxicity of fluorouracil in patients with advanced colorectal cancer: Effect of administration schedule and prognostic factors J Clin Oncol 163537–35411998 [DOI] [PubMed] [Google Scholar]
- 6. Food and Drug Administration: Highlights of prescribing information [Vistogard]. https://www.vistogard.com/Vistogard/files/49/49833e0e-4376-4b07-b379-52b9d74b78f3.pdf.
- 7.Mukherjee KL, Boohar J, Wentland D, et al. XVI. Metabolism of 5-fluorouracil-2-C14 and 5-fluoro-2′-deoxyuridine-2-C14 in cancer patients Cancer Res 2349–661963 [Google Scholar]
- 8.Miura K, Kinouchi M, Ishida K, et al. 5-FU metabolism in cancer and orally-administrable 5-FU drugs Cancers (Basel) 21717–17302010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Sanna’a NA, Van Kuilenburg AB, Atrak TM, et al. Dihydropyrimidine dehydrogenase deficiency presenting at birth J Inherit Metab Dis 28793–7962005 [DOI] [PubMed] [Google Scholar]
- 10.Amstutz U, Henricks LM, Offer SM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 update Clin Pharmacol Ther 103210–2162018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henricks LM, Lunenburg CATC, de Man FM, et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: A prospective safety analysis Lancet Oncol 191459–14672018 [DOI] [PubMed] [Google Scholar]
- 12.Deenen MJ, Meulendijks D, Cats A, et al. Upfront genotyping of DPYD*2A to individualize fluoropyrimidine therapy: A safety and cost analysis J Clin Oncol 34227–2342016 [DOI] [PubMed] [Google Scholar]
- 13.Innocenti F. DPYD variants to predict 5-FU toxicity: The ultimate proof. J Natl Cancer Inst. 2014;106:dju351. doi: 10.1093/jnci/dju351. [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer Ann Oncol 271386–14222016 [DOI] [PubMed] [Google Scholar]
- 15.Lee AM, Shi Q, Pavey E, et al. DPYD variants as predictors of 5-fluorouracil toxicity in adjuvant colon cancer treatment (NCCTG N0147) J Natl Cancer Inst. 2014;106:dju298. doi: 10.1093/jnci/dju298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosmarin D, Palles C, Church D, et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: Investigation in the QUASAR2 study, systematic review, and meta-analysis J Clin Oncol 321031–10392014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boige V, Vincent M, Alexandre P, et al. DPYD genotyping to predict adverse events following treatment with fluorouracil-based adjuvant chemotherapy in patients with stage III colon cancer: A secondary analysis of the PETACC-8 randomized clinical trial JAMA Oncol 2655–6622016 [DOI] [PubMed] [Google Scholar]
- 18.Meulendijks D, Henricks LM, Sonke GS, et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: A systematic review and meta-analysis of individual patient data Lancet Oncol 161639–16502015 [DOI] [PubMed] [Google Scholar]
- 19.Páez D, Salazar R, Tabernero J.DPYD genotype-guided fluoropyrimidines dose: Is it ready for prime time? Ann Oncol 282913–29142017 [DOI] [PubMed] [Google Scholar]
- 20.Lunenburg CATC, Henricks LM, Guchelaar HJ, et al. Prospective DPYD genotyping to reduce the risk of fluoropyrimidine-induced severe toxicity: Ready for prime time Eur J Cancer 5440–482016 [DOI] [PubMed] [Google Scholar]
- 21.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer N Engl J Med 3502343–23512004 [DOI] [PubMed] [Google Scholar]
- 22.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07 J Clin Oncol 252198–22042007 [DOI] [PubMed] [Google Scholar]
- 23.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial JAMA 3172392–24012017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boisdron-Celle M, Capitain O, Faroux R, et al. Prevention of 5-fluorouracil-induced early severe toxicity by pre-therapeutic dihydropyrimidine dehydrogenase deficiency screening: Assessment of a multiparametric approach Semin Oncol 4413–232017 [DOI] [PubMed] [Google Scholar]
- 25.Grem JL.5-Fluorouracil: Forty-plus and still ticking. A review of its preclinical and clinical development Invest New Drugs 18299–3132000 [DOI] [PubMed] [Google Scholar]
- 26.Meulendijks D, van Hasselt JGC, Huitema ADR, et al. Renal function, body surface area, and age are associated with risk of early-onset fluoropyrimidine-associated toxicity in patients treated with capecitabine-based anticancer regimens in daily clinical care Eur J Cancer 54120–1302016 [DOI] [PubMed] [Google Scholar]
- 27.Rothenberg ML, Meropol NJ, Poplin EA, et al. Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: Summary findings of an independent panel J Clin Oncol 193801–38072001 [DOI] [PubMed] [Google Scholar]
- 28. National Institutes of Health: Dihydropyrimidine dehydrogenase deficiency. https://ghr.nlm.nih.gov/condition/dihydropyrimidine-dehydrogenase-deficiency.
- 29.Mattison LK, Fourie J, Desmond RA, et al. Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African-Americans compared with Caucasians Clin Cancer Res 125491–54952006 [DOI] [PubMed] [Google Scholar]
- 30.Innocenti F, Vokes EE, Ratain MJ.Irinogenetics: What is the right star? J Clin Oncol 242221–22242006 [DOI] [PubMed] [Google Scholar]
- 31.Kosinski C, Wen J, Nhan H, et al. Allele frequencies of two dihydropyrimidine dehydrogenase (DPYD) risk variants, c.1905+1G>A (*2A) and c.2846A>T (D949V), in a direct-to-consumer genetic database. J Clin Oncol. 2020;38(suppl; abstr 2) [Google Scholar]
- 32.23andMe Personal Genome Service (PGS) package insert. https://permalinks.23andme.com/pdf/package_insert_v5_not_IVD.pdf.
- 33. Federal Register: Public teleconference regarding licensing and collaborative research opportunities for: Methods and compositions relating to detecting dihydropyrimidine dehydrogenase (DPD). https://www.federalregister.gov/documents/2008/07/03/E8-15182/public-teleconference-regarding-licensing-and-collaborative-research-opportunities-for-methods-and.
- 34. European Medicines Agency: New testing and treatment recommendations for fluorouracil, capecitabine, tegafur and flucytosine. https://www.ema.europa.eu/en/documents/referral/fluorouracil-fluorouracil-related-substances-article-31-referral-new-testing-treatment_en.pdf.
- 35. National Institutes of Health: GRT: Genetic Testing Registry. https://www.ncbi.nlm.nih.gov/gtr/
- 36.Lee AM, Shi Q, Alberts SR, et al. Association between DPYD c.1129-5923 C>G/hapB3 and severe toxicity to 5-fluorouracil-based chemotherapy in stage III colon cancer patients: NCCTG N0147 (Alliance) Pharmacogenet Genomics 26133–1372016 [DOI] [PMC free article] [PubMed] [Google Scholar]