Abstract
INTRODUCTION:
Racial/ethnic disparities in breast cancer survival are well documented, but the influence of health care institutions is unclear. We therefore examined the effect of hospital characteristics on survival.
METHODS:
Harmonized data pooled from 5 case-control and prospective cohort studies within the California Breast Cancer Survivorship Consortium were linked to the California Cancer Registry and the California Neighborhoods Data System. The study included 9,701 patients with breast cancer who were diagnosed between 1993 and 2007. First reporting hospitals were classified by hospital type—National Cancer Institute (NCI) –designated cancer center, American College of Surgeons (ACS) Cancer Program, other—and hospital composition of the neighborhood socioeconomic status and race/ethnicity of patients with cancer. Multivariable Cox proportional hazards models adjusted for clinical and patient-level prognostic factors were used to examine the influence of hospital characteristics on survival.
RESULTS:
Fewer than one half of women received their initial care at an NCI-designated cancer center (5%) or ACS program (38%) hospital. Receipt of initial care in ACS program hospitals varied by race/ethnicity—highest among non-Latina White patients (45%), and lowest among African Americans (21%). African-American women had superior breast cancer survival when receiving initial care in ACS hospitals versus other hospitals (non-ACS program and non–NCI-designated cancer center; hazard ratio, 0.67; 95% CI, 0.55 to 0.83). Other hospital characteristics were not associated with survival.
CONCLUSION:
African American women may benefit significantly from breast cancer care in ACS program hospitals; however, most did not receive initial care at such facilities. Future research should identify the aspects of ACS program hospitals that are associated with higher survival and evaluate strategies by which to enhance access to and use of high-quality hospitals, particularly among African American women.
INTRODUCTION
Racial/ethnic disparities in survival of breast cancer in the United States are large and persistent.1 A growing body of research has attempted to identify the major factors that contribute to the differences in breast cancer survival between racial/ethnic groups in the United States and propose targets for interventions to reduce disparities in outcomes. A number of factors have been proposed, ranging from genetic and biologic to demographic and socioeconomic factors.2,3 In addition to individual patient characteristics, the influence of institutional characteristics, such as the health care delivery system and indicators of hospital performance, on racial/ethnic disparities in cancer outcomes has received some attention.3,4
Racial/ethnic disparities in access to high-quality health care have been reported for a number of conditions, including cancer, acute myocardial infarction, congestive heart failure, and pneumonia. These studies are consistent in their conclusion that non-Latina (NL) African American patients are more likely to receive care in lower-performing hospitals.5-10 African American patients are also more likely to be treated by physicians who are not board certified and to experience difficulties in accessing clinical specialists and high-quality diagnostic imaging.11 Proximity to a hospital has been shown to be significantly associated with hospital choice,12 but African American patients are more likely than NL White patients to undergo high-risk surgical procedures at low-quality hospitals, despite living close to high-quality hospitals.13
Studies specifically focused on access to care for patients with breast cancer are limited. Keating et al12,14 reported that minority patients with breast cancer are more likely than NL White patients to undergo surgery in hospitals with a high proportion of minority patients, a high proportion of Medicaid-insured patients, low rates of adjuvant radiation therapy, and poor survey measures of patient experience. Compared with NL White patients with breast cancer, minority patients are also less actively involved in physician and hospital selection, and rely more on physician referral and health plans than reputation.15 Minority patients are also less likely to use both high-volume hospitals and surgeons for procedures, including breast cancer surgery, that have an established volume-outcome relationship.7,16 As survival disparities persist in studies that have adjusted for socioeconomic status (SES) and/or health insurance status,14-16 hospital-level factors may play an independent role in racial/ethnic differences in breast cancer outcomes beyond individual-level access to care.
To our knowledge, the extent to which institutional characteristics, such as hospital type—National Cancer Institute (NCI) cancer center designation and American College of Surgeons (ACS) Cancer Program—and patient racial/ethnic and socioeconomic composition, might explain racial/ethnic disparities in breast cancer survival has yet to be examined. We hypothesized that patients with reporting hospitals designated as NCI and ACS cancer centers and programs will have better survival as a result of more rigorous standards, and that patients seen at hospitals with higher proportions of minority patients and those with lower SES will have worse survival because of the poor quality of care. We used data from the California Breast Cancer Survivorship Consortium (CBCSC) that consisted of patient interview, cancer registry, and institutional and neighborhood data from 5 studies of breast cancer to explore this question. CBCSC includes a diverse population of more than 10,000 patients with breast cancer who were diagnosed in California, with substantial representation of African American, Latina, and Asian American women, and is an ideal resource to address the role of the institutional environment in disparities in breast cancer outcomes.
METHODS
Study Sample
Analyses use a cohort of female patients who were diagnosed with breast cancer from 1993 through 2007 who participated in one of 5 studies included in the CBCSC—described in full previously.17-19 Briefly, harmonized data were used from 3 case-control studies (Asian American Breast Cancer Study, Women’s Contraceptive and Reproductive Experiences Study, and San Francisco Bay Area Breast Cancer Study) and two prospective cohort studies (California Teachers’ Study and the Multiethnic Cohort). Questionnaire, California Cancer Registry (CCR), and neighborhood data were harmonized to provide detailed information on patients’ sociodemographic, behavioral, reproductive, tumor, and treatment characteristics. Protocols for the CBCSC study were approved by the institutional review boards at all participating institutions and by the California State Institutional Review Board (Committee for the Protection of Human Subjects).
CCR obtains vital status and underlying cause of death through hospital follow up and linkage to state and national mortality databases. Follow-up time was defined as the time from the date of diagnosis to study end date (December 31, 2010), last known contact, or death, whichever came first. Mean follow-up time was 9.1 years. A total of 10,521 patients with breast cancer were potentially eligible for analysis. Patients with in situ breast cancer (n = 22), a previous cancer before their breast cancer diagnosis (n = 779), or fewer than 30 days of follow up (n = 19) were excluded. The final study sample included 9,701 patients with breast cancer.
Hospital Characteristics
CCR provides the first hospital to report each cancer case, which is also the initial treating facility for 98% of the study sample. Reporting hospitals were classified on the basis of three characteristics: type of hospital, composition of the neighborhood SES (nSES) of patients with cancer, and racial/ethnic composition of patients with cancer.
Hospital type was categorized consistent with the NCI cancer center designation (as of 201020) and ACS Cancer Program categories21 as NCI Cancer Center, ACS Cancer Program (academic comprehensive cancer program, comprehensive community cancer program, community cancer program), or other. The one hospital with both NCI and ACS designations was classified as an NCI-designated cancer center hospital. ACS accreditation requires stringent adherence to best practices standards for the timely delivery of specific treatments—for example, surgical approaches and medical and radiation therapies.22,23 NCI-designated cancer centers also meet rigorous standards in the delivery of cancer treatments, with particular focus on transdisciplinary, advanced research across the cancer control spectrum.
Hospital-level sociodemographic composition measures consisting of race/ethnicity and nSES were derived from patient-level CCR data for all cancer cases diagnosed from 1993 through 2007.3 Patient race/ethnicity from the CCR—self-reported or assigned by provider/health care system staff—was defined as NL White, NL African American, Latino, or Asian American/Pacific Islander.17 With these data, for each hospital we calculated the percentage of patients with cancer within each racial/ethnic group. Categories of hospital patients’ with cancer racial/ethnic composition were based on the median distribution of cases diagnosed from 1993 to 2007 and were defined as follows: high minority (≥ 2.3% African American, ≥ 3.7% Asian American, or ≥ 10.2% Latino, and < 75% NL White), predominantly NL White (< 2.3% African American, < 3.7% Asian American, or < 10.2% Latino, and ≥ 75% NL White), or mixed (all other combinations). For the regression models, we combined predominantly NL White and mixed categories as a result of sample size.
CCR assigns each patient’s nSES for their address at diagnosis with a composite measure developed using principal components analysis of 1990 and 2000 census block group data on education, occupation, employment, household income, poverty, and rent and house values.24 Components are summed and the composite SES score was categorized according to quintiles (Q) of the state-wide distribution, from high (Q5) to low (Q1). Similarly, with these data, for each hospital we calculated the percentage of patients with cancer—all cancer sites, diagnosed 1993 to 2007—within each quintile of nSES. Hospital patients’ with cancer nSES composition was categorized as follows: low (≥ 50% of patients with cancer residing in low SES neighborhoods [Q1 or Q2] and < 50% residing in high SES neighborhoods [Q4 or Q5]), high (≥ 50% of patients residing in high SES neighborhoods and < 50% residing in low SES neighborhoods), or mixed (all other combinations).
Statistical Analysis
Covariates were obtained from questionnaire and CCR data, and included tumor factors (American Joint Committee on Cancer stage, histology, grade, tumor size, and estrogen receptor and progesterone receptor status), subsequent tumors, first course of treatments (surgery, radiation, and/or chemotherapy), comorbidities (history of hypertension or diabetes), sociodemographic factors (age at diagnosis, race/ethnicity, education, marital status, and nSES), and prediagnosis behavioral and reproductive factors (body mass index, smoking, alcohol consumption, or number of full-term pregnancies).
Multivariable Cox proportional hazards regression models were used to examine the association between hospital characteristics and overall and breast cancer–specific survival, adjusted for clustering by hospital. The sandwich estimator of the covariance structure was applied to regression models.25 The assumption of proportional hazards for covariates was assessed by including interaction terms with time and assessing their significance using likelihood ratio tests. Models were stratified by stage at diagnosis and study—Asian American Breast Cancer Study, Women’s Contraceptive and Reproductive Experiences Study, San Francisco Bay Area Breast Cancer Study, California Teachers’ Study, and the Multiethnic Cohort—to allow the baseline hazards within each model to vary by these factors that violated the proportional hazards assumption. Association between each hospital characteristic and overall and breast cancer–specific survival was first assessed in baseline models, minimally adjusted for age and year of diagnosis and clustering by hospital. Models were then sequentially adjusted for covariates in the following order: tumor characteristics, first course of treatment, clinical factors, sociodemographic characteristics, and behavioral/reproductive factors. Racial/ethnic heterogeneity was tested by checking the significance of interactions between race/ethnicity, and institutional factors were assessed using Wald Type 3 tests. Significant interactions were observed between race/ethnicity and type of hospital (P = .040) and nSES composition (P = .049) for breast cancer-specific mortality; therefore, we present results stratified by race/ethnicity. Analyses were conducted using SAS (version 9.3; SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
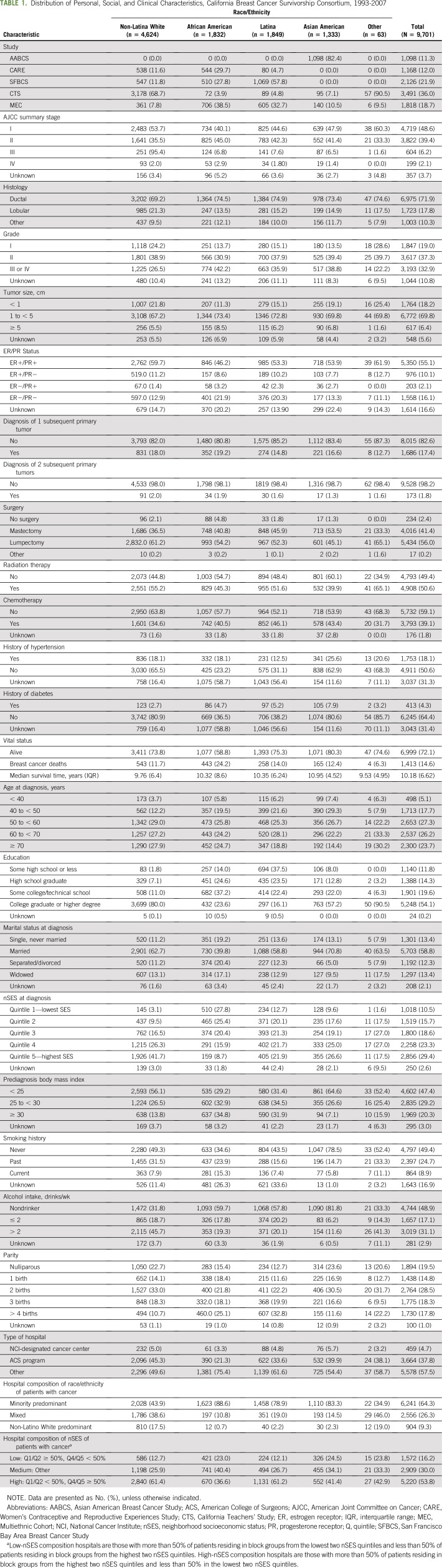
A total of 9,701 women were included in the study: 4,624 NL White (48%), 1,832 African American (18%), 1,849 Latina (19%), 1,333 Asian American (14%), and 63 other (1%) women. The majority of women were age 50 years or older at diagnosis (77%), diagnosed with early-stage disease (American Joint Committee on Cancer stage I and II; 88%), had a college degree or higher (54%), were married at the time of diagnosis (58%), and lived in a high-SES neighborhood at diagnosis (53%). Before diagnosis, approximately one half were never smokers (49%), nondrinkers (49%), and normal weight or underweight (47%; Table 1). Among all women, 72% were alive at the end of the study period, with the highest proportion among Asian Americans (80%) and the lowest among African American women (59%). Almost two thirds of women (64%) were diagnosed in hospitals with a high minority racial/ethnic composition, and this status varied by race/ethnicity as follows: 44% among NL Whites, 89% among African Americans, 79% among Latinas, and 83% among Asian Americans. Overall, 16% were diagnosed in low-SES hospitals, but the proportion varied by racial/ethnic group: 23% of African Americans and 25% of Asian Americans were diagnosed in low-SES hospitals compared with 13% of NL Whites and 12% of Latinas. Only 5% were diagnosed in an NCI-designated cancer center hospital and 38% in an ACS program hospital (NL Whites, 45%; African Americans, 21%; Latinas, 34%; Asian Americans, 40%).
TABLE 1.
Distribution of Personal, Social, and Clinical Characteristics, California Breast Cancer Survivorship Consortium, 1993-2007
Hospital Characteristics and Survival
Hospitals of NCI-designated cancer centers (70%) and ACS programs (50%) had a higher proportion of patients from high-SES neighborhoods than did other hospitals (34%; Data Supplement). The majority of NCI-designated cancer center hospitals (80%) and other hospitals (52%) served a higher proportion of minority patients than did ACS program–designated hospitals (42%). Hospital composition of patient race/ethnicity and nSES were modestly correlated (Pearson’s r = 0.20).
In baseline models (adjusted for age at diagnosis, year of diagnosis, and clustering by hospital), hospital nSES composition was associated with overall and breast cancer–specific survival: Women whose reporting hospitals served primarily high-nSES patients had superior overall survival (hazard ratio [HR], 0.83; 95% CI, 0.73 to 0.94) and breast cancer–specific survival (HR, 0.84; 95% CI, 0.72 to 0.99) than women whose reporting hospitals served primarily low-nSES patients (Fig 1 and Data Supplement). The association between hospital nSES composition and overall survival remained statistically significant after adjusting for tumor characteristics, but became nonsignificant after additional adjustment for sociodemographic, treatment, and behavioral factors. No association between hospital nSES composition and breast cancer–specific survival remained after adjustment for tumor characteristics. When stratified by race/ethnicity, association between high hospital nSES composition and overall survival was present for NL White women only (HR, 0.84; 95% CI, 0.71 to 0.99), but not after additional adjustment for sociodemographic treatment and behavioral factors. Hospital racial/ethnic composition was not associated with either overall or breast cancer–specific survival.
Fig 1.
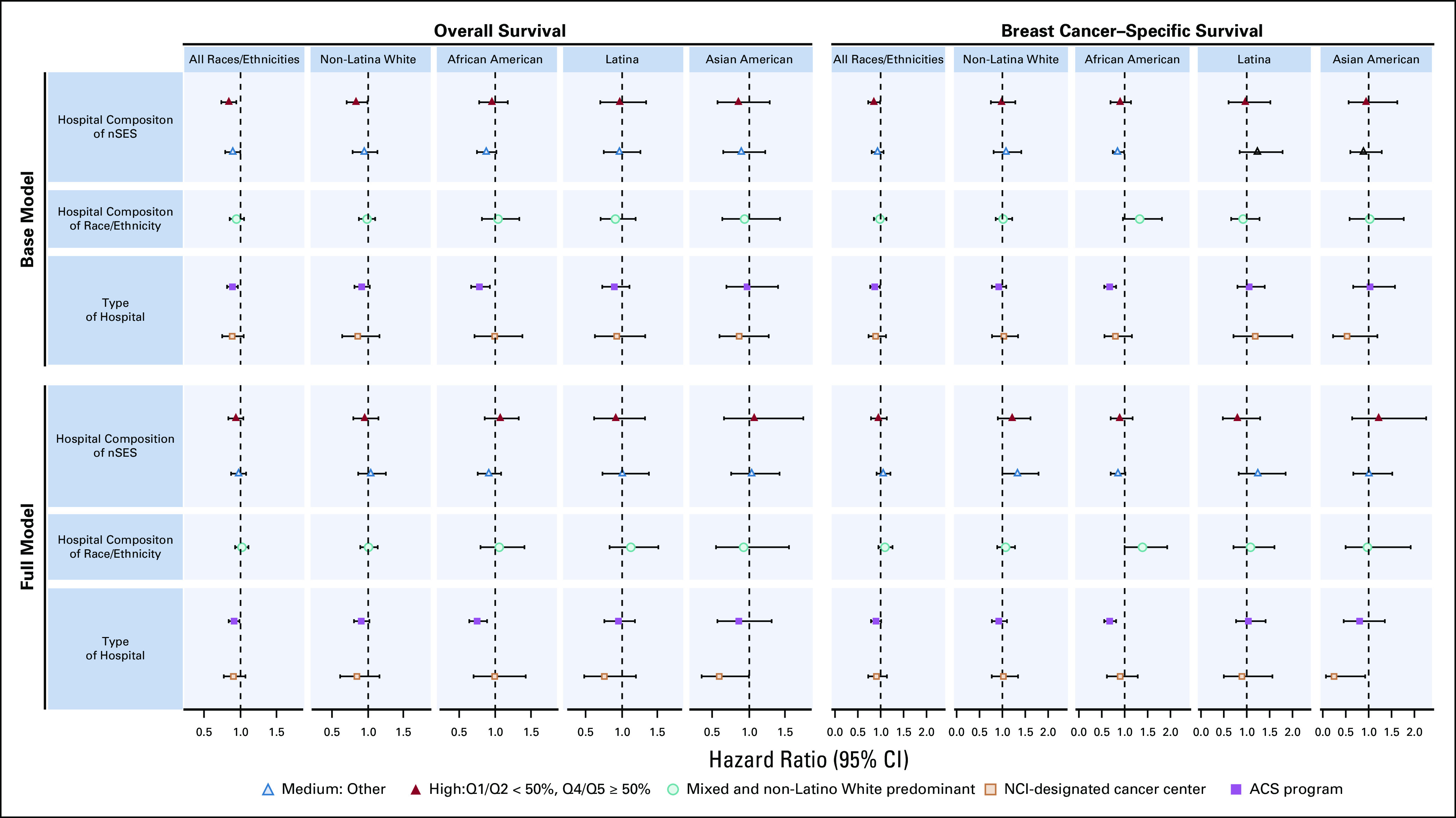
Associations between hospital factors and survival after breast cancer, California Breast Cancer Survivorship Consortium (CBCSC), 1993-2007. ACS, American College of Surgeons; NCI, National Cancer Institute; nSES, neighborhood socioeconomic status; Q, quintile.
Women who received care in an ACS program hospital experienced superior overall survival (HR, 0.88; 95% CI, 0.80 to 0.96) and breast cancer–specific survival (HR, 0.87; 95% CI, 0.77 to 0.98) than women who were diagnosed in a non–NCI cancer center/non-ACS hospital. This association persisted in overall survival models adjusted for tumor, sociodemographic, treatment, and behavioral factors (fully adjusted HR, 0.91; 95% CI, 0.84 to 0.99). Association between ACS program hospital and breast cancer–specific survival remained after adjustment for tumor characteristics, but not after additional adjustment for other covariates. There were no significant differences in overall or breast cancer–specific survival between women who were diagnosed in an NCI-designated cancer center hospital and those diagnosed in a non–NCI cancer center/non-ACS hospital.
When stratified by race/ethnicity, the superior survival association with being diagnosed and initially treated in an ACS program hospital was present only for African American patients. This association remained statistically significant after adjustment for all covariates, for both overall (HR, 0.76; 95% CI, 0.64 to 0.90) and breast cancer–specific (HR, 0.67; 95% CI, 0.55 to 0.83) survival.
Racial/Ethnic Disparities in Breast Cancer Survival
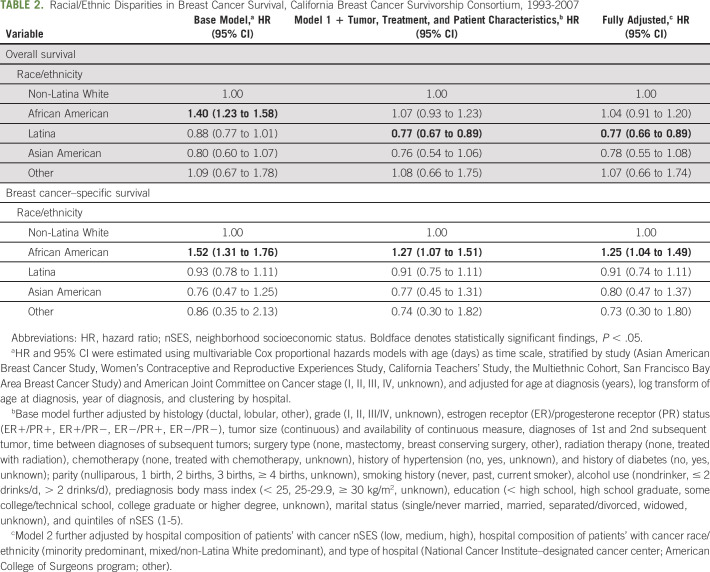
In minimally adjusted models for overall survival, African American women had worse (HR, 1.40; 95% CI, 1.23 to 1.58) and Latina women marginally superior (HR, 0.88; 95% CI, 0.77 to 1.01) survival compared with NL White women (Table 2). After adjusting for personal, tumor, and clinical factors, the association for African American women was fully attenuated and no longer statistically significant (HR, 1.07; 95% CI, 0.93 to 1.23). For Latina women, the association was both statistically significant and stronger (HR, 0.77; 95% CI, 0.67 to 0.89). In models additionally adjusted for institutional factors, the superior association for Latina women remained (HR, 0.77; 95% CI, 0.66 to 0.89). For all models, Asian American women did not have statistically significantly different survival compared with NL White women.
TABLE 2.
Racial/Ethnic Disparities in Breast Cancer Survival, California Breast Cancer Survivorship Consortium, 1993-2007
For breast cancer–specific survival, only African American women experienced worse survival compared with NL White women (minimally adjusted HR, 1.52; 95% CI, 1.31 to 1.76). Adjusting for personal, tumor, and clinical factors somewhat attenuated the association (HR, 1.27; 95% CI, 1.07 to 1.51). In a fully adjusted model including institutional factors, the association persisted (HR, 1.25; 95% CI, 1.04 to 1.49).
DISCUSSION
In this large study of 9,701 racially/ethnically diverse patients with breast cancer diagnosed in California, we found that the hospital type was significantly associated with overall and breast cancer–specific survival in African American women, independent of a comprehensive range of individual-level factors. African American women who were diagnosed with breast cancer in institutions accredited by ACS had a survival advantage over African American women diagnosed in hospitals that were not accredited by either ACS or NCI. However, we also observed that the racial/ethnic or socioeconomic patient compositions of the reporting hospitals were not associated with overall or breast cancer–specific survival. Despite considering a range of tumor, treatment, sociodemographic, behavioral, and institutional factors, we found that African American women experienced worse breast cancer survival than NL White patients.
Our finding that African American women whose reporting hospitals were ACS accredited have better survival is striking and potentially actionable. In this study, as in many others,1-4 African American women had worse overall and breast cancer–specific survival after adjusting for known prognostic factors. Prior studies, including our own, have demonstrated that triple-negative breast cancer is substantially more prevalent in African Americans, and triple-negative breast cancer and human epidermal growth factor receptor 2–overexpressing tumors are slightly more prevalent among Hispanics compared with NL White patients.26-28 However, although differential diagnoses of these more aggressive tumor subtypes do account for some of the survival disparities, research shows that they do not account for all, and such social factors as SES are just as influential, if not more.3,27,28 For example, African Americans may be exposed to more social stressors and have less social support and/or community resources. Among Latinas with breast cancer in California and Texas, we observed better survival among women residing in ethnic enclaves and high-SES neighborhoods, suggesting that social support and community resources may contribute to improved outcomes.29
This racial/ethnic survival disparity has increased over recent decades, despite the development of increasingly effective breast cancer treatments, which suggests that disparities in access to high-quality care may be an important cause.30 In addition to ACS accreditation requiring stringent adherence to treatment best practices, adherence is closely monitored by a rapid quality reporting system.22 A recent study demonstrated higher adherence to quality measures among ACS-accredited versus nonaccredited hospitals.23 Why should a survival benefit with ACS-accredited care be most apparent for African American women? It may be that a structured approach to best practices adherence is particularly effective in mitigating both conscious and unconscious racial bias among clinicians; African American women may be most vulnerable to such bias and gain most from its correction.31 Future research should seek to identify specific aspects of ACS-accredited hospitals that are most beneficial in terms of patient survival and determine how they can be scaled across facilities to reach more African American women.
Although ACS status was associated with superior survival, neither the hospital racial/ethnic nor nSES composition of their patients with cancer was associated with survival in multivariable models. These factors may represent local concentrations of social and behavioral factors that are associated with breast cancer–specific and overall survival and have been included in models as patient-level prognostic factors. We may need to focus on other aspects of the health care setting—and in greater detail on specific therapies—to understand further the role of the health care environment on survival outcomes. Lastly, the sequential models demonstrated that tumor characteristics and sociodemographic factors are most important in explaining racial/ethnic survival disparities.2
To our knowledge, this is the first evaluation of the potential role of selected hospital characteristics on survival for breast cancer separately for four major racial/ethnic groups in the United States—NL Whites, African Americans, Latinas, and Asian Americans. It is a large study with almost 10,000 patients with breast cancer, with data on tumor and treatment characteristics, sociodemographic and other risk factors, and neighborhood and hospital factors from study questionnaires and cancer registry. This pooled data set has long-term follow up, and it is increasingly recognized that late mortality is important in breast cancer.32 Whereas there are important strengths to this study, there are a few limitations to note. Although the diverse study sample may not represent the current US population, thus limiting the generalizability of study findings, this study provides important insights for understanding survival disparities across racial/ethnic groups, especially for Latinas and Asian Americans, the two fastest growing segments of the US population.33 In addition, the representation of these breast cancer cases was previously evaluated; the study sample had a lower proportion of advanced and estrogen receptor–negative tumors, as well as some differences in nSES, compared with incident breast cancer cases in California.17 While cancer registry data provide an opportunity to examine hospital characteristics, the data are limited to the reporting hospital, which may not be the best measure of the breast cancer care setting, as a large majority of breast cancer care is conducted in the outpatient setting, especially care beyond first course treatment—that is, treatment beyond the first 6 months. Additional hospital characteristics were not available through CCR. Cancer registry data on human epidermal growth factor receptor 2/neu status were not available for patients—diagnosed 1993 to 2007—included in this study, and information on specific treatment regimens—for example, chemotherapy drugs, duration, dose, and adherence—was not available; therefore, we were not able to examine whether there were tumor subtype and treatment differences across health care settings. Data were also not available to assess comorbidities beyond hypertension and diabetes across the pooled studies. The case-control and cohort studies contributed patients that were diagnosed 13 to 26 years ago. Given improvements in treatment efficacy and increases in cost—for example, trastuzumab, atezolizumab, and other targeted therapies, and the increasing use of oral regimens with large copays, such as aromatase inhibitors, palbociclib, and capecitabine—disparities in survival may be greater for patients diagnosed today. However, other more contemporary data sources do not have the same detailed information on important patient characteristics that we report here, notably sociodemographic factors. In addition, racial/ethnic disparities in breast cancer survival have not lessened over time.1
Our finding that African American women have superior breast cancer–specific survival when cared for in ACS-accredited hospitals has clinical implications. Patient navigation programs that direct African American women to ACS-accredited hospitals may be beneficial and clinical trials can be designed to test this hypothesis. In the long term, studies should identify the key aspects of care in ACS-accredited hospitals and how best to extend them to all health care delivery systems. In the short term, clinicians and patients should know that hospitals meeting high quality metrics may be particularly helpful for African American women, who have greater risk of death after breast cancer diagnosis than other women in the United States.
ACKNOWLEDGMENT
The authors thank all the study participants for their contributions in the five California-based studies. Clinical and tumor characteristics and mortality data were obtained from the California Cancer Registry.
PRIOR PRESENTATION
Presented at the Seventh AACR Conference on The Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, San Antonio, TX, November 9-12, 2014.
SUPPORT
The Asian American Breast Cancer Study was supported by California Breast Cancer Research Program (CBCRP) Grants No. 1RB-0287, 3PB-0120, and 5PB-0018. The San Francisco Bay Area Breast Cancer Study was supported by National Cancer Institute Grants No. R01-CA63446 and R01-CA77305, by US Department of Defense Grant No. DAMD17-96-1-6071, and by CBCRP Grants No. 4JB-1106 and 7PB-0068. The Women’s Contraceptive and Reproductive Experiences Study was funded by the National Institute of Child Health and Human Development through a contract with University of Southern California (N01-HD-3-3175). The California Teachers Study was funded by the California Breast Cancer Act of 1993, National Cancer Institute Grants No. R01-CA77398 and K05-CA136967 (L.B.), and the California Breast Cancer Research Fund (contract 97-10500). The Multiethnic Cohort Study was supported by National Cancer Institute Grants No. R01-CA54281, R37-CA54281, and UM1-CA164973. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s SEER Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HSN26120100035C awarded to the University of Southern California, and contract HHSN26120100034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries under agreement #1U58 DP000807-01 awarded to the Public Health Institute. This work was supported by grants from the California Breast Cancer Research Program: 16ZB-8001 (A.H.W.), 16ZB-8002 (S.L.G.), 16ZB-8003 (L.B.), 16ZB-8004 (M.L.K.), and 16ZB-8005 (K.R.M.).
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, the California Department of Health Services, the National Cancer Institute, or the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
AUTHOR CONTRIBUTIONS
Conception and design: Salma Shariff-Marco, Cheryl Vigen, Scarlett Lin Gomez, Allison W. Kurian
Financial support: Scarlett Lin Gomez
Provision of study materials or patients: Esther M. John, Marilyn L. Kwan, Leslie Bernstein, Anna H. Wu
Collection and assembly of data: Salma Shariff-Marco, Esther M. John, Iona Cheng, Cheryl Vigen, Marilyn L. Kwan, Leslie Bernstein, Anna H. Wu
Data analysis and interpretation: Salma Shariff-Marco, Libby Ellis, Juan Yang, Jocelyn Koo, Theresa H.M. Keegan, Iona Cheng, Yani Lu, Anna H. Wu, Allison W. Kurian
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hospital Characteristics and Breast Cancer Survival in the California Breast Cancer Survivorship Consortium
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Theresa H.M. Keegan
Research Funding: Shire (Inst)
Cheryl Vigen
Stock and Other Ownership Interests: Aetna, UnitedHealthcare
Anna H. Wu
Leadership: ImaginAb
Stock and Other Ownership Interests: ImaginAb
Consulting or Advisory Role: ImaginAb
Patents, Royalties, Other Intellectual Property: Royalties from Mustang Bio via University of California, Los Angeles
Scarlett Lin Gomez
Employment: Bioinspire (I)
Stock and Other Ownership Interests: Amgen (I), Bioinspire (I)
Allison W. Kurian
Research Funding: Myriad Genetics (Inst)
Other Relationship: Ambry Genetics, Color Genomics, GeneDx/BioReference, InVitae, Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state CA Cancer J Clin 67439–4482017 [DOI] [PubMed] [Google Scholar]
- 2.Sposto R, Keegan TH, Vigen C, et al. The effect of patient and contextual characteristics on racial/ethnic disparity in breast cancer mortality Cancer Epidemiol Biomarkers Prev 251064–10722016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis L, Canchola AJ, Spiegel D, et al. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics J Clin Oncol 3625–332018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JD, Huang Y, Ananth CV, et al. Influence of treatment center and hospital volume on survival for locally advanced cervical cancer Gynecol Oncol 139506–5122015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnato AE, Lucas FL, Staiger D, et al. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes Med Care 43308–3192005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner J, Chandra A, Staiger D, et al. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients Circulation 1122634–26412005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JH, Zingmond DS, McGory ML, et al. Disparities in the utilization of high-volume hospitals for complex surgery JAMA 2961973–19802006 [DOI] [PubMed] [Google Scholar]
- 8.Lucas FL, Stukel TA, Morris AM, et al. Race and surgical mortality in the United States Ann Surg 243281–2862006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: Examination of the hospital quality alliance measures Arch Intern Med 1671233–12392007 [DOI] [PubMed] [Google Scholar]
- 10.Jha AK, Orav EJ, Li Z, et al. Concentration and quality of hospitals that care for elderly black patients Arch Intern Med 1671177–11822007 [DOI] [PubMed] [Google Scholar]
- 11.Bach PB, Pham HH, Schrag D, et al. Primary care physicians who treat blacks and whites N Engl J Med 351575–5842004 [DOI] [PubMed] [Google Scholar]
- 12.Keating NL, Kouri EM, He Y, et al. Location isn’t everything: Proximity, hospital characteristics, choice of hospital, and disparities for breast cancer surgery patients Health Serv Res 511561–15832016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimick J, Ruhter J, Sarrazin MV, et al. Black patients more likely than whites to undergo surgery at low-quality hospitals in segregated regions Health Aff (Millwood) 321046–10532013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating NL, Kouri E, He Y, et al. Racial differences in definitive breast cancer therapy in older women: Are they explained by the hospitals where patients undergo surgery? Med Care 47765–7732009 [DOI] [PubMed] [Google Scholar]
- 15.Freedman RA, Kouri EM, West DW, et al. Racial/ethnic differences in patients’ selection of surgeons and hospitals for breast cancer surgery JAMA Oncol 1222–2302015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein AJ, Gray BH, Schlesinger M.Racial and ethnic differences in the use of high-volume hospitals and surgeons Arch Surg 145179–1862010 [DOI] [PubMed] [Google Scholar]
- 17.Wu AH, Gomez SL, Vigen C, et al. The California Breast Cancer Survivorship Consortium (CBCSC): Prognostic factors associated with racial/ethnic differences in breast cancer survival Cancer Causes Control 241821–18362013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shariff-Marco S, Yang J, John EM, et al. Intersection of race/ethnicity and socioeconomic status in mortality after breast cancer J Community Health 401287–12992015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng I, Shariff-Marco S, Koo J, et al. Contribution of the neighborhood environment and obesity to breast cancer survival: The California Breast Cancer Survivorship Consortium Cancer Epidemiol Biomarkers Prev 241282–12902015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute Find a cancer center. http://www.cancer.gov/researchandfunding/extramural/cancercenters/find-a-cancer-center
- 21.American College of Surgeons American College of Surgeons cancer program. https://www.facs.org/quality-programs/cancer
- 22.Knutson AC, McNamara EJ, McKellar DP, et al. The role of the American College of Surgeons’ cancer program accreditation in influencing oncologic outcomes J Surg Oncol 110611–6152014 [DOI] [PubMed] [Google Scholar]
- 23.Miller ME, Bleicher RJ, Kaufman CS, et al. Impact of breast center accreditation on compliance with breast quality performance measures at Commission on Cancer–accredited centers Ann Surg Oncol 261202–12112019 [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Schupp CW, Harrati A, et al. Developing an Area-Based Socioeconomic Measure From American Community Survey Data. Fremont, CA: Cancer Prevention Institute of California; 2014. [Google Scholar]
- 25.Lin DY, Wei L-J.The robust inference for the Cox proportional hazards model J Am Stat Assoc 841074–10781989 [Google Scholar]
- 26.Kurian AW, Fish K, Shema SJ, et al. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12:R99. doi: 10.1186/bcr2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez ME, Gomez SL, Tao L, et al. Erratum to: Contribution of clinical and socioeconomic factors to differences in breast cancer subtype and mortality between Hispanic and non-Hispanic white women. Breast Cancer Res Treat. 2017;166:195. doi: 10.1007/s10549-017-4455-6. [DOI] [PubMed] [Google Scholar]
- 28.Martínez ME, Gomez SL, Tao L, et al. Contribution of clinical and socioeconomic factors to differences in breast cancer subtype and mortality between Hispanic and non-Hispanic white women Breast Cancer Res Treat 166185–1932017[Erratum: Breast Cancer Res Treat 166:195, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shariff-Marco S, Gomez SL, Canchola AJ, et al. Nativity, ethnic enclave residence, and breast cancer survival among Latinas: Variations between California and Texas. Cancer. doi: 10.1002/cncr.32845. [epub ahead of print on March 17, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeSantis CE, Miller KD, Goding Sauer A, et al. Cancer statistics for African Americans, 2019 CA Cancer J Clin 69211–2332019 [DOI] [PubMed] [Google Scholar]
- 31.Blair IV, Steiner JF, Fairclough DL, et al. Clinicians’ implicit ethnic/racial bias and perceptions of care among Black and Latino patients Ann Fam Med 1143–522013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years N Engl J Med 3771836–18462017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown A. U.S. Hispanic and Asian populations growing, but for different reasons. Pew Research Center; https://www.pewresearch.org/fact-tank/2014/06/26/u-s-hispanic-and-asian-populations-growing-but-for-different-reasons/ [Google Scholar]