Abstract
PURPOSE
Germline testing (GT) for prostate cancer (PCA) is now central to treatment and hereditary cancer assessment. With rising demand for and shortage of genetic counseling (GC), tools to deliver pretest informed consent across practice settings are needed to improve access to GT and precision care. Here, we report on Evaluation and Management for Prostate Oncology, Wellness, and Risk (EMPOWER), a patient-choice study for pretest video-based genetic education (VBGE) versus GC to inform urgent practice needs.
PATIENTS AND METHODS
Men with PCA or at risk for PCA (family history of PCA) were eligible and could choose pretest VBGE or GC. Outcomes included decisional conflict for GT, change in genetics knowledge, satisfaction, and intention to share results with family and/or providers. Descriptive statistics summarized results with counts and percentages for categorical variables and mean ± standard deviation for continuous variables. Data were compared with Fisher's exact, chi-squared, or Wilcoxon two-sample tests. Mean change in genetics knowledge was compared with t tests. The significance level was set a priori at .05.
RESULTS
Data on the first 127 participants were analyzed. Characteristics were White (85.8%), bachelor's degree (66.9%), and PCA diagnosis (90.6%). The majority chose VBGE (71%) versus GC (29%; P < .001). No differences were observed in decisional conflict for GT or satisfaction. Cancer genetics knowledge improved in both groups without significant difference (+0.9 VBGE, +1.8 GC, P = .056). Men who chose VBGE had higher intention to share GT results (96.4% VBGE v 86.4% GC, P = .02). Both groups had high rates of GT uptake (VBGE 94.4%, GC 92%).
CONCLUSION
A substantial proportion of men opted for pretest VBGE, with comparable patient-reported outcomes and uptake of GT. The results support the use of pretest video to address the critical GC shortage in the precision era.
INTRODUCTION
Germline testing (GT) for prostate cancer (PCA) is rapidly increasing, with a central role in determining eligibility for precision therapy among men with advanced PCA and increasing role in informing PCA screening strategies.1-6 Furthermore, GT provides key information on hereditary cancer risk for men and their families.1,4 Pathogenic variants in several DNA repair genes, particularly BRCA1 and BRCA2, may determine which men with metastatic, castration-resistant PCA could respond to poly (ADP-ribose) polymerase (PARP) inhibitors. In 2020, the US Food and Drug Administration (FDA) approved olaparib and rucaparib for men with metastatic, castration-resistant PCA who carry BRCA pathogenic variants after progression on standard therapies on the basis of demonstrated clinical activity.7,8 Olaparib also received FDA approval for men with pathogenic variants in several additional DNA repair genes, such as ATM, CHEK2, PALB2, BARD1, BRIP1, CDK12, CHEK1, FANCL, PP2R2A, RAD51B/C/D, and RAD54L.7 In the PCA screening setting, men with pathogenic variants in BRCA2 are recommended to start screening at age 40 years, with a similar consideration for men with pathogenic variants in BRCA1.6 Furthermore, GT provides information on hereditary cancer syndromes such as hereditary breast and ovarian cancer and Lynch syndrome, which can lead to multiple cancer risks for men and their male and female blood relatives.6,9 Current guidelines recommend that all men with metastatic PCA, high-risk localized disease, and intraductal or cribriform histology, or who are of Ashkenazi Jewish ancestry (with any stage of PCA) have GT.5,6 Additional testing criteria are based on family cancer history, including the breast, ovary, prostate, uterus, colon or rectum, pancreas, upper bowel, kidney, and specific skin cancers due to the potential links to hereditary cancer syndromes.5,6 Therefore, many thousands of men are now eligible for GT with implications for precision therapy and screening.
CONTEXT
Key Objective
Thousands of men are eligible for prostate cancer germline testing (GT) to inform precision therapy, screening, and hereditary cancer risk, necessitating alternate delivery models of pretest genetic education given the shortage of genetic counseling (GC). This study evaluated a pretest genetic education video to determine patient choice for video versus GC and assessed key patient-reported outcomes to support clinical use for enhanced access to GT.
Knowledge Generated
Among 127 males, the majority chose pretest video versus GC. No differences were observed in decisional conflict for genetic testing and satisfaction. Men who chose video reported higher intention to share results with family. Both groups had high uptake of GT.
Relevance
These novel results in the setting of prostate cancer GT reveal high uptake of pretest video, support use of pretest video as part of informed consent for GT, and address the critical GC shortage.
With this rise in volume of men needing genetic testing, there is increasing demand for genetic counseling (GC). Best practice dictates that individuals undergo appropriate pretest informed consent to make an informed decision for genetic testing.1,5,6,10 Pretest discussions need to include understanding of cancer inheritance, purpose of testing, risks and benefits of testing, multigene panel options, types of potential results, implications of results for treatment, screening, and cancer management, implications of hereditary cancer risk for blood relatives, genetic discrimination laws, and possible reproductive implications.1,10 Classically, these pretest discussions have been conducted solely by genetic counselors, who are trained professionals in the principles and practice of genetic testing with attention to ethical delivery of care.11 However, with the current increase in men in need of GT, there is an increasing need for alternate delivery of pretest informed consent in nongenetic practices as referral of all men to GC is currently not sustainable. In 2019, there were 5,250 genetic counselors reported in the United States,12 which is insufficient to meet the rising demand of patients in need of GC. Indeed, there is a rise of urologists and oncologists ordering GT in their practice to have timely results to inform therapy and management of men with PCA. One study reported that approximately 23% of urologists surveyed ordered genetic testing in their practice or had a combination approach of practice ordering testing or referral to GC.13 Another study surveying academic oncologists reported that 15% performed their own pretest counseling, ordering of testing, and post-test disclosure, while an additional 45% used a combination approach of practice ordering testing and referral to GC.14 As such, approaches to delivery of pretest information are needed to facilitate nongenetic practices and providers in the genetic evaluation process.
Various alternate delivery approaches to GC have been reported.15 Telephone-based GC has been shown to be noninferior to standard GC with regard to patient-reported outcomes, particularly in the setting of breast and ovarian cancer genetic testing.16,17 Telegenetics with video-based GC has also been reported to have high patient satisfaction compared with in-person GC.18 However, there has been a scarcity of literature regarding tools to facilitate delivery of pretest information by urologists and oncologists to accelerate the genetic evaluation process and meet the rising need of patients in need of PCA GT.
The Evaluation and Management for Prostate Oncology, Wellness, and Risk (EMPOWER) study was therefore developed as a patient-choice study to evaluate video-based genetic education (VBGE) versus GC among men with PCA or at risk for PCA referred for GT. The goal was to determine key patient-reported outcomes of men who chose video or GC for pretest informed consent to help streamline genetics care delivery across practice disciplines with attention to best practice. Here, we report interim results on the first cohort of participants to inform practice given the rapidly developing need for GT in PCA precision medicine.
PATIENTS AND METHODS
Study Process and IRB Approval
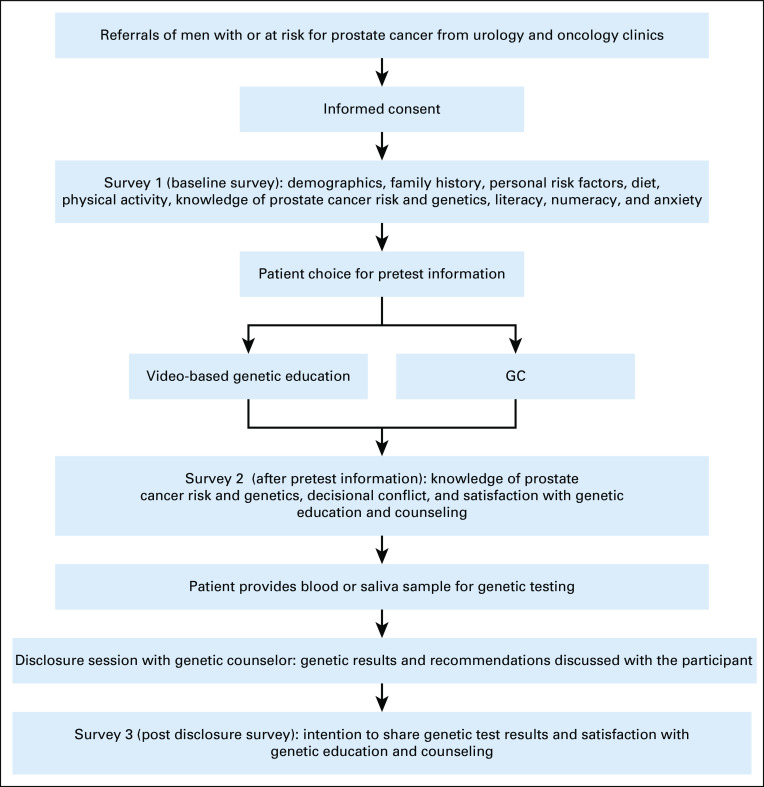
The EMPOWER study was approved by the Institutional Review Board at Thomas Jefferson University. Men with a personal history of PCA and unaffected males who were at higher risk for PCA (family history of PCA) were eligible for the study. Upon referral, the study coordinator contacted men for interest in participating in the study. Men could choose VBGE or a GC session with a genetic counselor. Upon informed consent, men were asked to complete three surveys during the course of their participation: baseline survey (survey 1; No. = 127 completed), post-VBGE and post-GC counseling survey (survey 2; No. = 100 completed), and postresults disclosure survey (survey 3; No. = 78 completed). Participants who chose to proceed with genetic testing underwent multigene testing of 51 genes through Invitae. Genes tested included ABRAXAS1, APC, ATM, AXIN2, BAP1, BARD1, BLM, BMPR1A, BRCA1, BRCA2, BRIP1, CDK4, CDKN2A, CHEK2, EPCAM, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, GREM1, HOXB13, MLH1, MLH3, MRE11, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, POLD1, POLE, PTEN, RAD50, RAD51C, RAD51D, SMAD4, SMARCA4, SMARCB1, SMARCE1, STK11, TP53, WRN, WT1, and XRCC2. Figure 1 displays the study flow. All participants received post-test GC for disclosure of genetic results.
FIG 1.
Evaluation and Management for Prostate Oncology, Wellness, and Risk study flow. GC, genetic counseling.
Genetic Education Video
A short video (11 minutes, 19 seconds) was created by the Jefferson Cancer Genetics team that addressed cancer inheritance, purpose of testing, risks and benefits of testing, multigene panel options, types of potential results, implications of results for treatment, screening, and cancer management, implications of hereditary cancer risk for blood relatives, genetic discrimination laws, and possible reproductive implications.1,10 A link to the video was sent to men who chose VBGE to view, with the opportunity to ask questions to the study coordinator before proceeding with genetic testing.
Survey Measures
Baseline survey.
Survey 1 included demographic information (age, race, education, marital status, and ethnicity), reason for choosing VBGE or GC, and family history. Knowledge of cancer genetics was assessed using a 14-item knowledge scale adapted from prior studies of cancer genetics (Cronbach's alpha .92).19-21 Preliminary results from this survey have been published in a PCA genetics context.21 Respondents answered each statement by marking True or False. Each correct answer was scored with a point, with higher scores reflecting greater knowledge. Health literacy was measured using three items from the Short Test of Functional Health Literacy in Adults.22 These items are as follows: (1) How often do you have someone help you read hospital materials? (2) How confident are you filling out medical forms by yourself? (3) How often do you have problems learning about your medical condition because of difficulty understanding written information? Responses were on a five-point Likert scale. Numeracy was assessed using a validated measure of three probability questions and was scored as the total number of correct responses.23 Anxiety was assessed using the Generalized Anxiety Disorder-7 scale, a seven-item scale to assess a patient's overall anxiety status.24 Responses were on a four-point scale (not at all sure, several days, over half the days, and nearly every day) with higher responses indicating greater anxiety (Cronbach's alpha .92).24
Survey after pretest information.
Survey 2 was administered after participants viewed the pretest genetic education video or had pretest GC. The survey readministered questions regarding knowledge of cancer genetics to assess change in knowledge.19-21 Decisional conflict for genetic testing was assessed using the validated Decisional Conflict Scale.25 This survey includes 16 questions with responses on a five-point scale (yes, probably yes, unsure, probably no, and no) with higher scores indicating greater decisional conflict (Cronbach's alpha .78-.86).25 Satisfaction was assessed using the validated Genetic Counseling Satisfaction Scale.26 This survey includes six items with responses on a five-point scale (strongly disagree, somewhat disagree, uncertain, somewhat agree, and strongly agree) with higher scores indicative of greater satisfaction (Cronbach's alpha .83).26
Postdisclosure survey.
Survey 3 was administered after participants received their genetic results and recommendations by a genetic counselor. Satisfaction with the process was readministered as on survey 2.26 Intention to share results with primary care provider (Will you share your genetic test results with your primary care provider? Yes, no, don't know) and family members (Will you discuss your genetic test results with your family? Yes, no, don't know) was assessed using a single question for each.
Statistical Analysis
The cohort characteristics were summarized with counts and percentages for categorical variables and means and standard deviations for continuous variables. The comparison of these characteristics by genetic education type (VBGE v GC) was conducted with the Fisher's exact test, chi-squared test, or two-sample t test. The proportions of participants who receive VBGE and GC were calculated, and the proportion of participants who received VBGE was reported with its 95% CI. Also, this proportion was compared against the threshold of 50% with the exact binomial test. The total score for knowledge of cancer genetics, decisional conflict score, and satisfaction at postgenetic education were summarized by means and standard deviations and compared by genetic education type with or two-sample t test. The significance level of all tests was a priori set at .05. All the analyses were performed with SAS 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
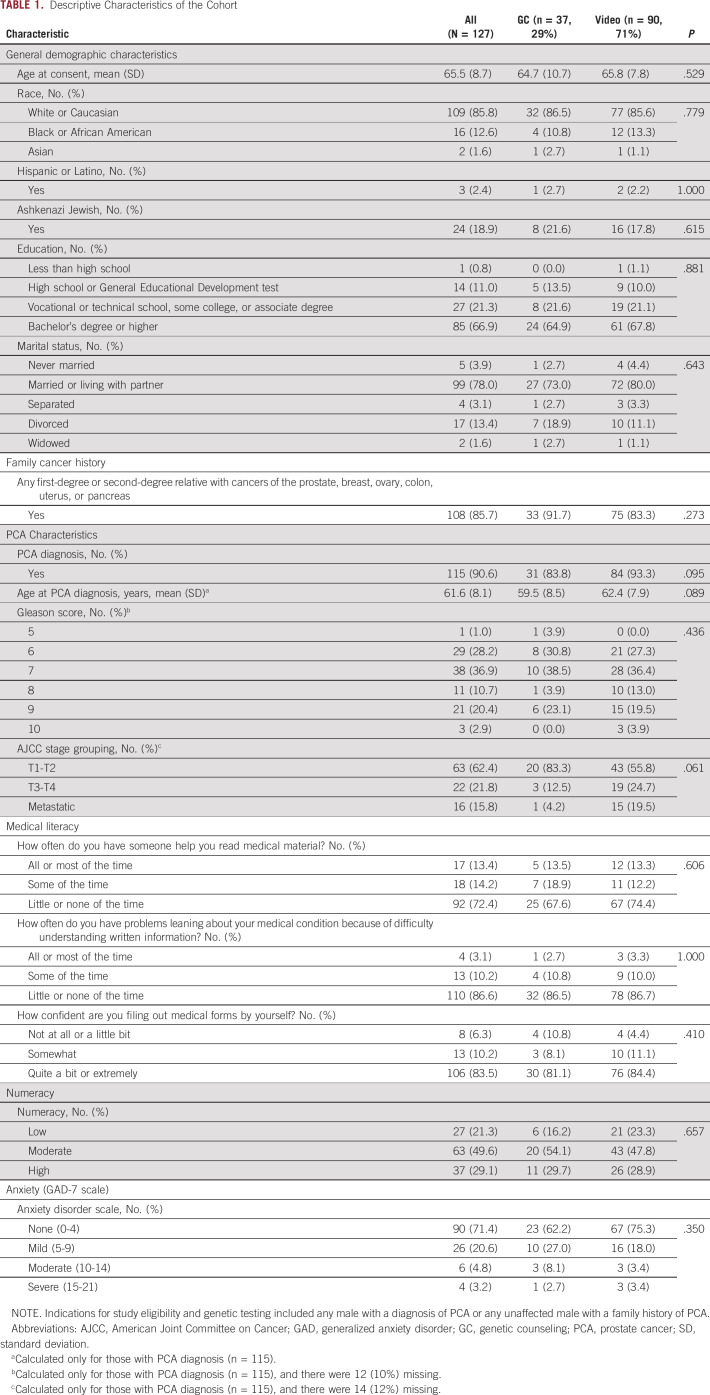
The EMPOWER study is ongoing, and this report includes data on the first 127 participants with complete data available for analysis. Table 1 summarizes the characteristics of the cohort. The majority were White (85.8%), had a bachelor's degree (66.9%), were married or living with a partner (78.0%), had a current or prior diagnosis of PCA (90.6%), and had a mean age of PCA diagnosis of 61.6 ± 8.1 years. The majority (85.7%) had a family cancer history. No significant differences were observed in characteristics between men who chose VBGE or GC. Importantly, no differences were noted in baseline medical literacy, numeracy, or anxiety between participants choosing VBGE or GC.
TABLE 1.
Descriptive Characteristics of the Cohort
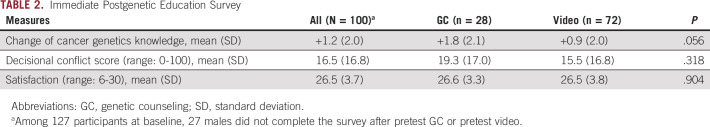
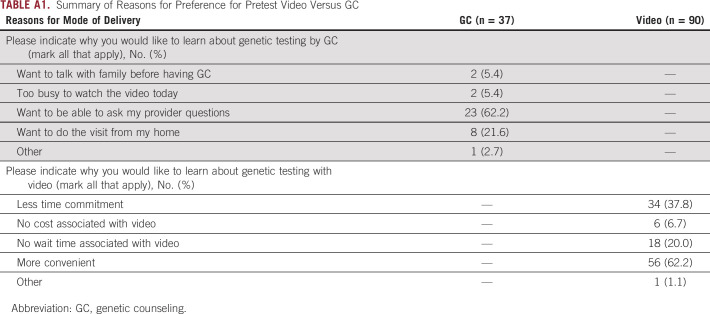
The majority of men preferred VBGE (n = 90, 71%) versus GC (n = 37, 29%; P < .001). Major reasons for choosing VBGE among the 90 males included more convenience (62.2%), less time commitment (37.8%), and no wait time associated with video (20.2%). The GC arm was conducted in-person, by telehealth, or by phone (n = 37). Major reasons for choosing GC included ability to ask questions to a genetics provider (62.2%) and preference or ability to do the visit from home (for telehealth or phone options [21.6%]; Appendix Table A1). No significant differences were observed in decisional conflict for genetic testing (P = .318) or satisfaction with the process (P = .904) between men who chose VBGE or GC (Table 2). Cancer genetics knowledge improved in both groups though slightly greater in the GC group of borderline significance (+0.9 VBGE, +1.8 GC, P = .056). Both groups had high rates of GT uptake (VBGE 94.4%, GC 92%).
TABLE 2.
Immediate Postgenetic Education Survey
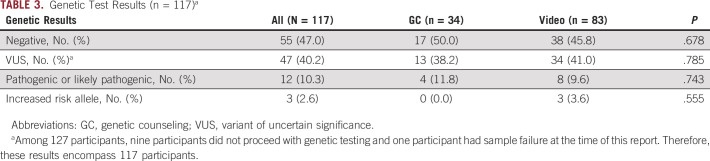
Genetic results are shown in Table 3. Of 127 participants, nine participants did not proceed with genetic testing and one participant had sample failure at the time of this report. Therefore, these results encompass 117 participants. Overall, 10.3% of the cohort had a pathogenic or likely pathogenic variant identified (n = 12) along with three participants who had an increased risk allele identified (2.6%). Variants of uncertain significance were identified in 40.2% (n = 47), and 47% (n = 55) had negative genetic test results (Table 3). No differences in genetic results were observed by modality of pretest genetic education and counseling.
TABLE 3.
Genetic Test Results (n = 117)a
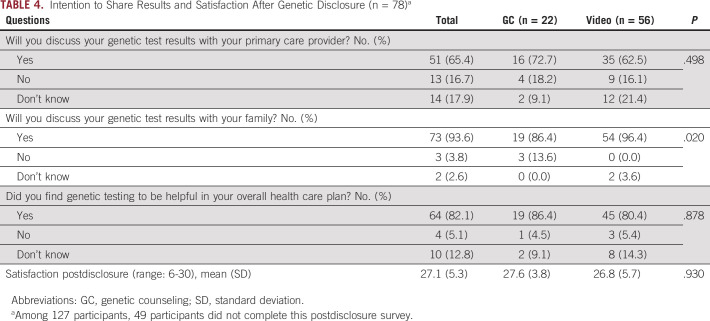
A third survey was administered after receipt of genetic results by a genetic counselor for both arms of the study, and 78 men completed this postdisclosure survey (Table 4). Men in both groups had high intention to share results with their family members, with a significantly higher percentage among men who underwent pretest VBGE compared with GC (96.4% VBGE v 86.4% GC, P = .02). There was also high intention to share GT results with primary care providers in both groups (62.5% VBGE v 72.7% GC), although not significantly different. Satisfaction scored highly among men in both groups with no differences observed. Furthermore, over 80% of men in both groups reported genetic testing to be helpful in their overall health care plan (Table 4).
TABLE 4.
Intention to Share Results and Satisfaction After Genetic Disclosure (n = 78)a
DISCUSSION
With the rise in precision medicine indications for PCA treatment and recognition of multiple genes involved in PCA hereditary risk,1-4,7,8 there has been a significant need to address the relative shortage of GC.12 Expansion of GT guidelines and current FDA approvals for PARP inhibitors has led to many thousands of men being eligible for GT and in need of information to make informed decisions for testing.1,5-8 Furthermore, millions of men who are PCA survivors are also now eligible for GT to assess hereditary cancer risk for themselves and their families.27 This rising need for GT has placed a strain on the health care system, with long wait times for GC which hinders rapid return of test results. As a result, urologists and oncologists have increasingly started ordering GT in their practices and have a need for tools to provide men with appropriate pretest information to make an informed decision for genetic testing to adhere to best practice recommendations.1,5,6,10 Therefore, this report details interim results from the EMPOWER patient-choice study of VBGE versus GC to address the critical need for pretest genetic education tools in nongenetic practices given the growth of testing indications.
Our results show that the majority of men chose pretest video over GC when making a decision for genetic testing. Convenience, less time commitment, and no wait times for a GC appointment were noted as reasons for choosing pretest video, which are important real-world aspects to consider. Importantly, no differences were noted in key patient-reported outcomes for making a decision for genetic testing, including decisional conflict and satisfaction, which are previously published validated outcomes supporting genetic delivery models.16-18 Improvement in cancer genetics knowledge was observed in both groups and was notably higher among men who chose pretest GC of borderline significance; however, this may be a function of lower baseline cancer genetics knowledge among this group. Pretest GC may be more beneficial for men who have lower cancer genetics knowledge to ask questions to a GC and ensure understanding before proceeding with genetic testing. Our results also showed that a significantly higher percentage of men who chose VBGE intended to share result with their families, although this was also high in the GC group. This is of key importance given the hereditary nature of genetic testing, with implications for cascade testing of blood relatives, cancer risk information, and cancer screening recommendations that need to be delivered to male and female relatives.1,10 Overall, there was high uptake of over 90% among men who chose pretest video or pretest GC, indicating that a video-based strategy did not lead to lower genetic testing.
Aspects to implementing pretest video in urology and oncology practices need to be considered. Time to view the video, space to view the video privately, and practice expertise in genetics to answer patient questions about genetic testing are needed to ensure that men understand considerations of genetic testing and have their questions answered.1,10 If the video is viewed outside of the clinical appointment, then the follow-up to address questions and to coordinate test ordering are important implementation aspects to consider. Overall, nongenetic practices who opt for using a pretest video and order genetic testing need to gain working knowledge of genetic testing and build collaborations with GC to ensure appropriate referral of patients in the pretest and post-test setting for comprehensive recommendations.1,10
There are some considerations and limitations to note. The results are from a patient-choice strategy rather than a randomized trial, although this is reflective of real-world practice. Our results provide key information regarding men's actual choice for pretest video which was substantially higher than for pretest GC, supporting resource development of pretest video in nongenetic practice settings. Furthermore, our results of patient-reported outcomes are an important next step in care delivery assessment precedented by prior published randomized trials where outcomes such as decisional conflict and satisfaction supported genetic delivery models. The majority of study participants were White and college-educated; therefore, it is imperative to study digital solutions to pretest genetic delivery across diverse populations to ensure generalizability. Furthermore, there is increasing recognition of digital barriers in health care particularly coming to light in the COVID-19 pandemic and postpandemic era relevant to underserved populations which need to be addressed.28 Our results are also an interim update but were necessary to report at this time given the emerging critical practice need for tools and strategies to streamline pretest informed consent, with recent FDA approvals for PARP inhibitors among men with metastatic disease.
In conclusion, our results support the use of pretest genetic education videos in nongenetic practices to address the shortage of GC and advance GT to capitalize on the progress in precision medicine. Further research in diverse populations and across practice settings is warranted.
ACKNOWLEDGMENT
We would like to thank all participants of the EMPOWER study. We would also like to thank Invitae for providing genetic testing for study participants, which was covered by study funding.
Appendix
TABLE A1.
Summary of Reasons for Preference for Pretest Video Versus GC
Jessica Russo
Stock and Other Ownership Interests: Agenus
Colette Hyatt
Stock and Other Ownership Interests: GenomeSmart
Consulting or Advisory Role: GenomeSmart
William K. Kelly
Honoraria: Janssen Oncology, Bayer
Consulting or Advisory Role: Merck Sharp & Dohme
Research Funding: Novartis, Janssen Oncology, Bayer, Exelixis, Seattle Genetics, Endocyte, Amgen, BioClin Therapeutics, Sarah Cannon Research Institute, Roche
Travel, Accommodations, Expenses: Janssen Oncology, Merck Sharp & Dohme
Anne Calvaresi
Consulting or Advisory Role: Tolmar
Speakers' Bureau: Janssen Oncology, Deciphera
Nathan R. Handley
Research Funding: Nektar
Irvin H. Hirsch
Stock and Other Ownership Interests: Keystone Mobile Partners
Edouard J. Trabulsi
Consulting or Advisory Role: GenomeDx
Speakers' Bureau: Johnson & Johnson, Janssen, Astellas Medivation, Pfizer
Leonard G. Gomella
Consulting or Advisory Role: Astra Zeneca/Merck
Patents, Royalties, Other Intellectual Property: Patents Held by Thomas Jefferson University
Veda N. Giri
Stock and Other Ownership Interests: Novopyxis
Honoraria: Invitae, Ambry Genetics
Speakers' Bureau: Janssen
No other potential conflicts of interest were reported.
SUPPORT
Supported by SKCC TIPS Pilot Funding (Grant No. 27000-908088) and NCI Cancer Center Support Grant No. (5P30CA056036-17).
J.R. and C.M. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Colette Hyatt, Anne Calvaresi, Mark Mann, Veda N. Giri
Administrative support: Jessica Russo, Donald Preate
Provision of study materials or patients: Colette Hyatt, William K. Kelly, Irvin H. Hirsch, Costas D. Lallas, Donald Preate, Thenappan Chandrasekar, Leonard G. Gomella
Collection and assembly of data: Jessica Russo, Carey McDougall, Nicholas Bowler, Laura Gross, Anne Calvaresi, Costas D. Lallas, Mark Mann, Patrick J. Mille, Donald Preate, Miranda Tsang, Thenappan Chandrasekar, Perry R. Weiner, Veda N. Giri
Data analysis and interpretation: Nicholas Bowler, Ayako Shimada, William K. Kelly, Nathan R. Handley, Irvin H. Hirsch, Joseph K. Izes, Costas D. Lallas, James Ryan Mark, Edouard J. Trabulsi, Thenappan Chandrasekar, Leonard G. Gomella, Veda N. Giri
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jessica Russo
Stock and Other Ownership Interests: Agenus
Colette Hyatt
Stock and Other Ownership Interests: GenomeSmart
Consulting or Advisory Role: GenomeSmart
William K. Kelly
Honoraria: Janssen Oncology, Bayer
Consulting or Advisory Role: Merck Sharp & Dohme
Research Funding: Novartis, Janssen Oncology, Bayer, Exelixis, Seattle Genetics, Endocyte, Amgen, BioClin Therapeutics, Sarah Cannon Research Institute, Roche
Travel, Accommodations, Expenses: Janssen Oncology, Merck Sharp & Dohme
Anne Calvaresi
Consulting or Advisory Role: Tolmar
Speakers' Bureau: Janssen Oncology, Deciphera
Nathan R. Handley
Research Funding: Nektar
Irvin H. Hirsch
Stock and Other Ownership Interests: Keystone Mobile Partners
Edouard J. Trabulsi
Consulting or Advisory Role: GenomeDx
Speakers' Bureau: Johnson & Johnson, Janssen, Astellas Medivation, Pfizer
Leonard G. Gomella
Consulting or Advisory Role: Astra Zeneca/Merck
Patents, Royalties, Other Intellectual Property: Patents Held by Thomas Jefferson University
Veda N. Giri
Stock and Other Ownership Interests: Novopyxis
Honoraria: Invitae, Ambry Genetics
Speakers' Bureau: Janssen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Giri VN, Knudsen KE, Kelly WK, et al. Implementation of germline testing for prostate cancer: Philadelphia Prostate Cancer Consensus Conference J Clin Oncol 382798–28112020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019 Eur Urol 77508–5472020 [DOI] [PubMed] [Google Scholar]
- 3.Lowrance W, Breau R, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline. https://www.auanet.org/guidelines/advanced-prostate-cancer
- 4.Genetics of Prostate Cancer (PDQ®)–Health Professional Version. National Cancer Institute; www.cancer.gov [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network . National Comprehensive Cancer Network Clinical Guidelines in Oncology (NCCN Guidelines®): Prostate Cancer (Version 2.2021) NCCN.org [Google Scholar]
- 6.National Comprehensive Cancer Network . National Comprehensive Cancer Network Clinical Guidelines in Oncology (NCCN Guidelines®): Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 2.2021) NCCN.org [DOI] [PubMed] [Google Scholar]
- 7.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer N Engl J Med 3822091–21022020 [DOI] [PubMed] [Google Scholar]
- 8.Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration J Clin Oncol 383763–37722020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . National Comprehensive Cancer Network Clinical Guidelines in Oncology (NCCN Guidelines®): Genetic/Familial High-Risk Assessment: Colorectal (Version 1.2020) NCCN.org [Google Scholar]
- 10.Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: Updated recommendations of the National Society of Genetic Counselors J Genet Couns 21151–1612012 [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control Genomics and precision health: Genetic counseling. https://www.cdc.gov/genomics/gtesting/genetic_counseling.htm
- 12.Abacan M, Alsubaie L, Barlow-Stewart K, et al. The global state of the genetic counseling profession Eur J Hum Genet 27183–1972019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loeb S, Byrne N, Walter D, et al. Knowledge and practice regarding prostate cancer germline testing among urologists: Gaps to address for optimal implementation. Cancer Treat Res Commun. 2020;25:100212. doi: 10.1016/j.ctarc.2020.100212. [DOI] [PubMed] [Google Scholar]
- 14.Paller CJ, Antonarakis ES, Beer TM, et al. Germline genetic testing in advanced prostate cancer; practices and barriers: Survey results from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium Clin Genitourin Cancer 17275–282.e12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoll K, Kubendran S, Cohen SA.The past, present and future of service delivery in genetic counseling: Keeping up in the era of precision medicine Am J Med Genet C Semin Med Genet 17824–372018 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer J Clin Oncol 32618–6262014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinney AY, Steffen LE, Brumbach BH, et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-Year follow-up J Clin Oncol 342914–29242016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchanan AH, Datta SK, Skinner CS, et al. Randomized trial of telegenetics vs. in-person cancer genetic counseling: Cost, patient satisfaction and attendance J Genet Couns 24961–9702015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erblich J, Brown K, Kim Y, et al. Development and validation of a Breast Cancer Genetic Counseling Knowledge Questionnaire Patient Educ Couns 56182–1912005 [DOI] [PubMed] [Google Scholar]
- 20.Miesfeldt S, Jones SM, Cohn W, et al. Men's attitudes regarding genetic testing for hereditary prostate cancer risk Urology 5546–502000 [DOI] [PubMed] [Google Scholar]
- 21.Giri VN, Obeid E, Hegarty SE, et al. Understanding of multigene test results among males undergoing germline testing for inherited prostate cancer: Implications for genetic counseling Prostate 78879–8882018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chew LD, Bradley KA, Boyko EJ.Brief questions to identify patients with inadequate health literacy Fam Med 36588–5942004 [PubMed] [Google Scholar]
- 23.Schwartz LM, Woloshin S, Black WC, et al. The role of numeracy in understanding the benefit of screening mammography Ann Intern Med 127966–9721997 [DOI] [PubMed] [Google Scholar]
- 24.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder Arch Intern Med 1661092–10972006 [DOI] [PubMed] [Google Scholar]
- 25.O'Connor AM. User Manual–Decisional Conflict Scale (10-item Question Format) Ottawa, ON, Canada: Ottawa Hospital Research Institute; 1993. http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf.20 [Google Scholar]
- 26.DeMarco TA, Peshkin BN, Mars BD, et al. Patient satisfaction with cancer genetic counseling: A psychometric analysis of the Genetic Counseling Satisfaction scale J Genet Couns 13293–3042004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prostate Cancer Statistics. Cancer.Net of conquer cancer ® The ASCO Foundation. www.cancer.net
- 28.Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: Digital health access inequities during the COVID-19 pandemic in New York City J Urban Health 98183–1862021 [DOI] [PMC free article] [PubMed] [Google Scholar]