PURPOSE:
There is a concern that influenza vaccination could increase the incidence of immune-related adverse events (irAEs) in patients with cancer receiving immune checkpoint inhibitors. The aim of our study was to determine the safety of influenza vaccination in this patient population.
PATIENTS AND METHODS:
We retrospectively identified patients who received at least 1 dose of pembrolizumab during any influenza season from September 2014 to August 2017 and reviewed medical records for irAEs. The primary endpoint was the incidence of irAEs. We used multivariable logistic regression and cumulative incidence curve with competing risks for comparison.
RESULTS:
Among 162 patients with cancer included in this study, 70 patients (43.2%) received at least 1 influenza vaccination. The vaccinated group was significantly older (P = .002) and received more cycles of pembrolizumab (P = .006). The incidence of any grade irAEs in the vaccinated group trended toward being lower (25.7% v 40.2%; P = .07) compared with the nonvaccinated group. Influenza vaccination was independently associated with fewer irAEs, with an odds ratio of 0.4 (95% CI, 0.2 to 0.9; P = .03) in multivariable analyses. The vaccinated group was less likely to have irAEs compared with the nonvaccinated group (24.7% v 34.4% at 12 months; P = .05), with death as a competing risk. The median irAE-free duration in the vaccinated group was longer than the nonvaccinated group (not reached v 28 months; P = .037).
CONCLUSION:
Influenza vaccination in patients with cancer receiving immune checkpoint inhibitor therapy was not associated with increased irAEs. This supports the safety of influenza vaccination in this patient population.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have become a mainstay of cancer immunotherapy in recent years for a number of solid and hematologic malignancies, such as melanoma, lung cancer, renal cell carcinoma, Hodgkin lymphoma, and many others.1 The most commonly used ICI, pembrolizumab, prevents the tumor-directed downregulation of cancer-specific cytotoxic T cells by blocking the interaction between programmed death receptor-1 (PD-1) and its ligand.2 For some patients, treatment with an ICI can provide prolonged tumor control, even in previously refractory disease.3
However, ICIs can lead to immune-related adverse events (irAEs), presumably due to nonspecific activation of the immune system targeting any healthy tissue and organ.1 In a series of 88 patients with advanced melanoma treated with pembrolizumab, 12.5% experienced grade 3/4 irAEs. The most common irAEs involved the skin, GI system, and endocrine system, which led to the use of systemic immunosuppression in 25% of the patients.4
Annual influenza vaccinations are recommended by the Center for Disease Control and Prevention (CDC), especially for patients receiving cancer-directed therapies and those who can have increased risk of complications from influenza.5 A Cochrane review of influenza vaccines in patients with cancer demonstrated lower influenza rates and lower risk of death with vaccination.6 Specifically, high-dose (compared with standard-dose) influenza vaccine has been shown in meta-analysis to reduce influenza infection risk without serious adverse events in patients over age 65 years.7 It has previously been shown that for patients who receive ipilimumab (monoclonal antibody against cytotoxic T-lymphocyte–associated antigen 4) and influenza vaccine, most demonstrated an increase in humoral response to influenza B.8 Several retrospective studies have reported conflicting results on irAE frequency in patients who receive an ICI; however, these studies either had a short follow-up, lack of a comparator, or lack of a reliable way to confirm influenza vaccination status.9,10 In our study, we retrospectively assessed the prevalence of irAEs in patients with cancer receiving pembrolizumab from our institution who did or did not receive an annual influenza vaccination while receiving ICI therapy.
PATIENTS AND METHODS
This study was approved by the Mayo Clinic Institutional Review Board. Patients with cancer who received at least 1 dose of pembrolizumab at our institution during influenza season (defined as September through March) between September 2014 and August 2017 were identified from the Mayo Clinic medical oncology pharmacy database. This retrospective study included patients age 18 years or older with available influenza vaccination record as well as treatment and follow-up at Mayo Clinic in Rochester, MN. Influenza vaccination status was verified by either the Mayo Clinic electronic medical record (EMR) or the Minnesota Immunization Information Connection, which is an online database of statewide vaccinations from multiple institutions.11 Patients’ demographic and clinical data were retrospectively collected from their medical records. Patients were excluded from the study if records were not available from the Mayo Clinic EMR or the Minnesota Department of Health. Patients who received influenza vaccination within 30 days before initiation of pembrolizumab or during pembrolizumab treatment were assigned to the vaccinated group, as done previously.10 All other patients were assigned to the nonvaccinated group.
The primary endpoint of this study was the incidence of irAEs in the vaccinated group compared with the nonvaccinated group. Grading of irAEs was based on the Common Terminology Criteria for Adverse Events, version 4.0, which was used based on database inclusion until August 2017.12 Time to irAE was defined as the time between pembrolizumab treatment initiation and the occurrence of irAE for the nonvaccinated group and the time between vaccination and the occurrence of an irAE for the vaccinated group. The follow-up duration was defined as the time between the date of pembrolizumab initiation and the date of last follow-up or death. Death from non-irAEs was used as a competing risk when cumulative incidence curves for irAEs were calculated.
Continuous variables were summarized as median and interquartile range and compared with Wilcoxon rank sum test. Categorical variables were summarized as count and percentage and compared with Fisher’s exact test. Logistic regression was used to evaluate variables predicting the occurrence of irAEs. Taking duration of follow-up into consideration, cumulative incidence curves for competing risks were used to compare incidence of irAEs between the vaccinated and nonvaccinated groups. irAE-free duration was estimated using the Kaplan-Meier method and compared with log-rank test. All statistical tests were 2-sided. A P value less than .05 was considered statistically significant. All statistical analyses were performed using R 3.5.3.
RESULTS
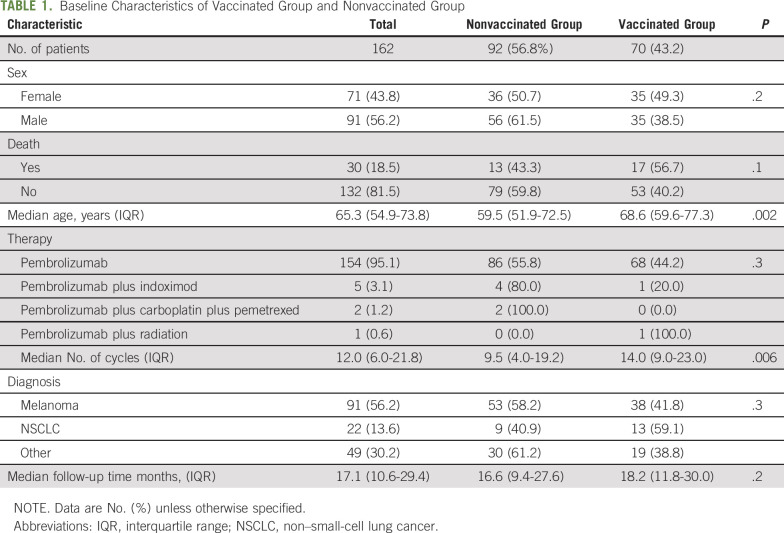
A total of 162 patients were included in this study. Baseline demographic and clinical features are summarized in Table 1. The majority of patients in the study had melanoma (n = 53; 56.2%), followed by non–small-cell lung cancer (NSCLC; n = 22; 13.6%). All patients had stage IV disease. Ninety-two patients (56.8%) did not receive influenza vaccination during any of the influenza seasons while receiving pembrolizumab or within 30 days before pembrolizumab treatment initiation. More women than men were vaccinated (49.3% v 38.5%, respectively). Seventy patients (43.2%) received at least 1 CDC-approved inactivated influenza vaccination without adjuvant. Within the vaccination cohort, 9 patients (12.7%) received influenza vaccines in 2 flu seasons, 7 patients (10%) received influenza vaccines in 3 flu seasons, 56.7% of the vaccinated patients received high-dose (trivalent) vaccines, 35.8% received quadrivalent vaccines, and 7.5% received vaccines with unspecified type. The vaccinated group had a median age of 68.6 years, significantly older than 59.5 years in the nonvaccinated group (P = .002). The vaccinated group received a median of 14 cycles of pembrolizumab, which was significantly more than 9.5 cycles in the nonvaccinated group (P = .006). The median nearest cycle of pembrolizumab treatment was 6 (interquartile range [IQR], 3-9) when the influenza vaccination was given. A total of 3 patients (1.9%) had laboratory-confirmed influenza infection via polymerase chain reaction, and 1 (0.6%) had documented influenza-like symptoms. Only 1 patient with influenza infection (1.4%) was seen in the vaccinated group.
TABLE 1.
Baseline Characteristics of Vaccinated Group and Nonvaccinated Group
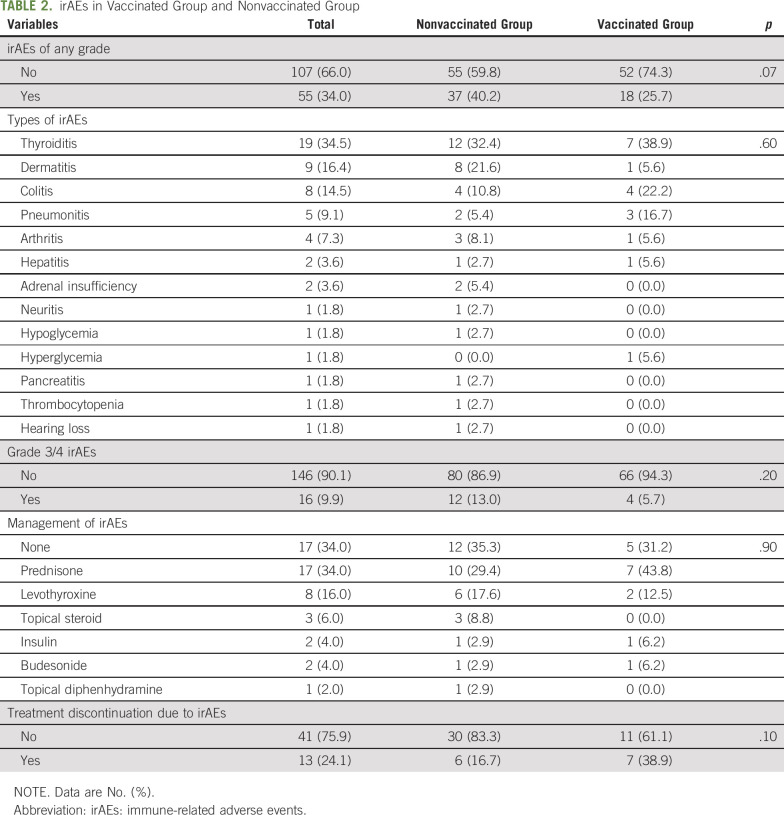
Patients who received an influenza vaccination while receiving pembrolizumab did not have an increase in any grade irAEs and numerically had fewer irAEs than patients who were not vaccinated (P = .07; Table 2). Four patients (5.7%) in the vaccinated group experienced grade 3/4 irAEs, similar to 12 patients (13.0%) in the nonvaccinated group (P = .2). No grade 5 irAEs were observed. The most frequent irAEs while receiving pembrolizumab were thyroiditis (n = 19), dermatitis (n = 9), and colitis (n = 8; Table 2). The median number of cycles of pembrolizumab to the occurrence of an irAE was 6.5 (IQR, 3.0-12.0). The median time from influenza vaccination to an irAE was 3.1 months (IQR, 2.0-5.2 months). There was no difference in irAE management (P = .9) or the rate of treatment discontinuations because of irAEs (P = .1) between the 2 groups. There were 17 patients (24.3%) who died as a result of their disease at the end of follow-up in the vaccinated group, similar to 14.1% in the nonvaccinated group (P = .1).
TABLE 2.
irAEs in Vaccinated Group and Nonvaccinated Group
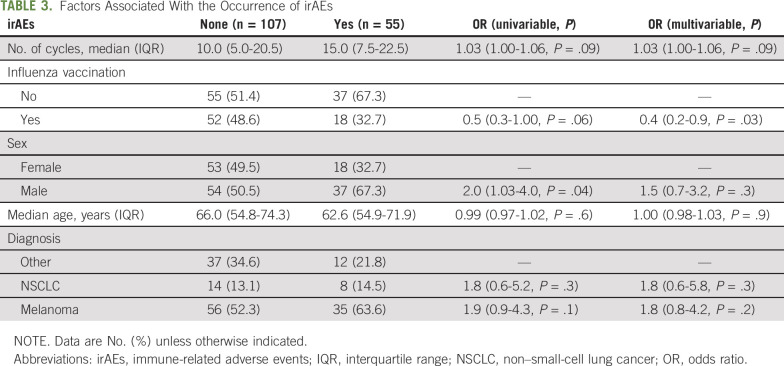
A logistic regression model was used to assess the association between the occurrence of irAEs and other clinical factors. There was no statistically significant association between the type of vaccines and incidence of irAEs (P = .2). As shown in Table 3, influenza vaccination was independently associated with fewer irAEs, with an odds ratio of 0.4 (95% CI, 0.2 to 0.9; P = .03) after adjusting for the number of pembrolizumab cycles, sex, age, and type of cancer diagnosis.
TABLE 3.
Factors Associated With the Occurrence of irAEs
Taking time of follow-up into consideration, irAE-free duration was not reached in the vaccinated group, which was significantly longer than a median of 28 months (95% CI, 13.6 months to not reached; P = .037) in the nonvaccinated group (Appendix Fig A1A, online only). When death was considered as a competing risk, as shown in Appendix Figure A1B, there was a trend toward lower probability in the vaccinated group to have irAEs (24.7% at 12 months) compared with the nonvaccinated group (34.4% at 12 months; P = .05). The probability of death was similar between the 2 groups (P = .8).
DISCUSSION
In this study, we found a trend toward lower incidence of irAEs in patients with cancer who received pembrolizumab and influenza vaccinations compared with that observed in the nonvaccinated group. The absolute overall incidence of any grade irAEs (34%) and grade 3/4 irAEs (9.9%) was comparable to previously published clinical trials and a meta-analysis.13,14 The same applied to the median time from vaccination to irAEs (3.1 months) observed in the vaccinated group.13 In addition, the distribution of the types of irAEs was consistent with clinical experience and previous observations, with thyroid dysfunction being the most common irAE, followed by dermatitis, colitis, and pneumonitis in order of decreasing incidence.13 Thereby, the patient population in our study was representative.
The cohort in this study represented mostly patients with melanoma and NSCLC, but also included several other types of cancers. This was similar to the patient distribution receiving pembrolizumab treatment between 2014 and 2017 when ICIs were first approved for melanoma. The vaccinated group in our cohort was significantly older compared with the nonvaccinated group, consistent with higher influenza vaccination rates in the older general population.15 It is possible that immunosenescence in older patients may play a role in response to influenza vaccination, as well the development of irAEs from ICIs. But when we stratified our patients into older and younger groups using a median age of 65 years as a cutoff, we did not observe a significant difference in irAEs of any grade (P = .4). This was consistent with data obtained from the Food and Drug Administration Adverse Event Reporting System database showing that age was not associated with a higher risk of most irAEs.16 Pembrolizumab, for instance, had a comparable safety profile related to irAEs in patients older and younger than 75 years of age.17 Our vaccinated group received significantly more cycles of pembrolizumab compared with the nonvaccinated group, despite a similar distribution of underlying cancer diagnoses. This observation could have been due to fewer irAEs and irAE-related treatment delay in the vaccinated group or longer clinical benefit. It could also represent a selection bias in this retrospective study, because healthier patients (who could tolerate more cycles of treatment) may have been more likely to be vaccinated. For the latter, we adjusted the number of treatment cycles in our multivariable analyses.
Our study did not support the finding of a significantly increased rate of irAEs in patients with cancer receiving influenza vaccination and nivolumab from a previous study with limited sample size.18 Several groups have attempted to address this concern. In a small study, 52% of 23 patients with lung cancer who received nivolumab and influenza vaccine experienced irAEs; 26% had grade 3/4 toxicities, significantly higher than historical control.18 A retrospective study showed that influenza vaccine did not negatively affect immunotherapy efficacy, although it appeared to be ineffective in reducing influenza syndrome.19 Other studies have concluded that there is no increase in irAEs with ICIs. One study included 127 patients with lung cancer receiving nivolumab, 47 of whom received influenza vaccination. There was no difference in the incidence or severity of irAEs or with clinical outcomes with vaccination.10 The second study included 370 patients receiving ICI therapy who received influenza vaccination, and only 20% experienced an irAE of any grade, and 8% experienced a grade 3/4 irAE.9 We observed similar findings of no increased irAEs from influenza vaccination in this patient population. This finding was confirmed in multivariable logistic regression, adjusting for other clinical factors as potential confounders. We also took the duration of follow-up and potential competing risk from death into consideration in our analyses, which confirmed our findings with similar levels of statistical significance. The median follow-up duration in our study was longer than in previous studies,9,10 which allowed us to capture more delayed irAEs, if present. Other potential confounders not available for review in our study included reasons for receiving or not receiving influenza vaccination (ie, possibility of self-selection) and variable patient reporting of symptoms. Because this was a retrospective study, there may have been variability of recording in the medical record for low-grade irAEs; however, clinically significant and serious irAEs, such as those requiring intervention or hospitalizations, would have been documented because patients were treated at our institution.
Compared with previous studies, our study had rigorous confirmation of both positive and negative influenza vaccination status, and long-term follow-up on a real-world patient population. The observed trend of decreased irAEs from receiving influenza vaccination warrants further confirmatory studies with a larger patient population. Our findings demonstrated no increase in irAEs associated with annual influenza vaccination in patients receiving an ICI and further support planned patient care quality initiatives to educate clinicians on influenza vaccination in the population of patients with cancer.
APPENDIX
Fig A1.
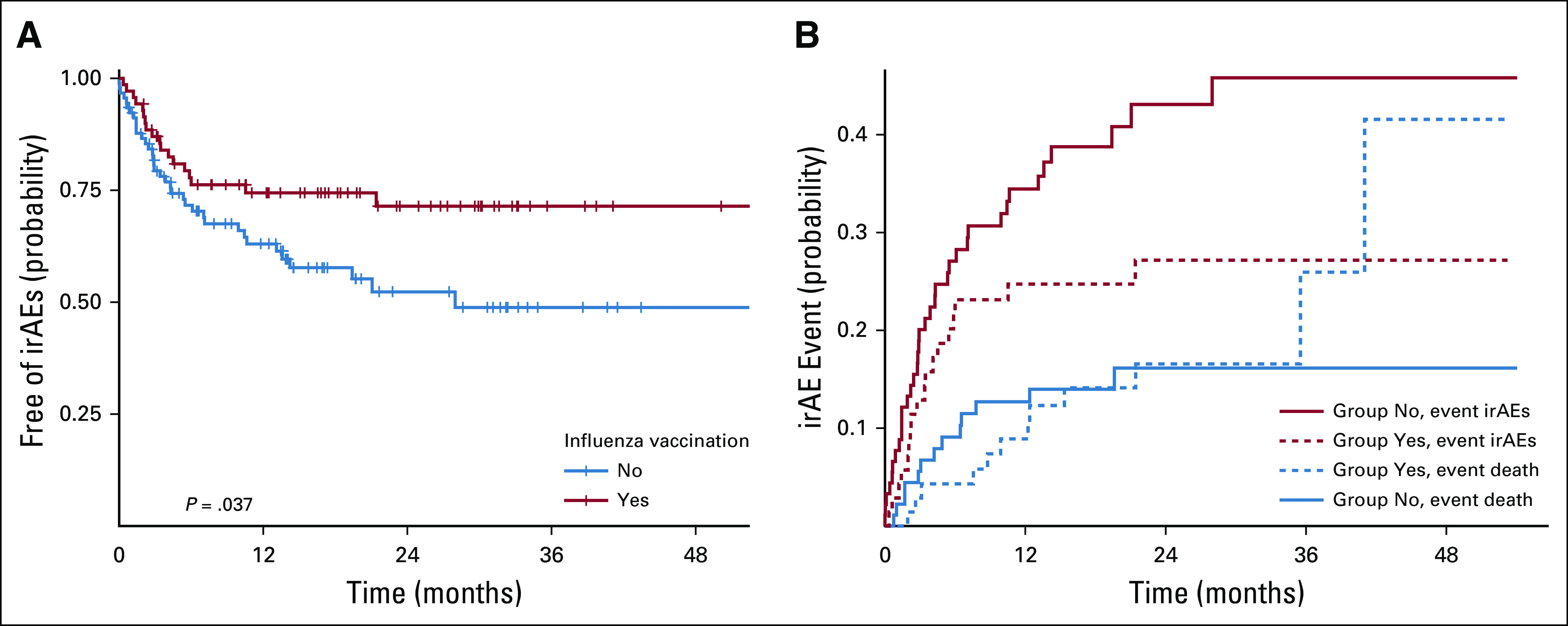
(A) Probability of being free of immune-related adverse events (irAEs), stratified by influenza vaccination status. (B) Cumulative incidence for competing risks from irAEs and death, stratified by vaccinated group and nonvaccinated group.
Footnotes
J.J.F. and T.P.H. are joint first authors. E.L.S. and H.X. are joint senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Jarrett J. Failing, Thanh P. Ho, Siddhartha Yadav, Neil Majithia, Irbaz Bin Riaz, John Y. Shin, Erin L. Schenk, Hao Xie
Collection and assembly of data: Jarrett J. Failing, Thanh P. Ho, Siddhartha Yadav, Neil Majithia, Irbaz Bin Riaz, John Y. Shin, Erin L. Schenk, Hao Xie
Data analysis and interpretation: Jarrett J. Failing, Thanh P. Ho, Hao Xie
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Safety of Influenza Vaccine in Patients With Cancer Receiving Pembrolizumab
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Erin L. Schenk
Honoraria: Takeda, Genentech
Consulting or Advisory Role: AbbVie
Speakers' Bureau: Physicians' Education Resource
Other Relationship: Elsevier
No other potential conflicts of interest were reported.
REFERENCES
- 1. doi: 10.1016/j.ejca.2015.11.016. Michot JM, Bigenwald C, Champiat S, et al: Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 54:139-148, 2016. [DOI] [PubMed] [Google Scholar]
- 2. doi: 10.1056/NEJMe1205943. Ribas A: Tumor immunotherapy directed at PD-1. New Eng J Med 366:2517-2519, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Borcoman E, Nandikolla A, Long G, et al: Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Ed Book 169-178, 2018. [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.2217/mmt-2017-0028. So AC, Board RE: Real-world experience with pembrolizumab toxicities in advanced melanoma patients: A single-center experience in the UK. Melanoma Manage 5:MMT05, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. doi: 10.1200/JOP.18.00567. El Ramahi R, Freifeld A: Epidemiology, diagnosis, treatment, and prevention of influenza infection in oncology patients. J Oncol Pract 15:177-184, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Bitterman R, Eliakim-Raz N, Vinograd I, et al. : Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev 2:CD008983, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. doi: 10.1016/j.vaccine.2017.03.092. Wilkinson K, Wei Y, Szwajcer A, et al: Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine 35:2775-2780, 2017. [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.1097/CJI.0b013e31823aa41c. Weber JS, Hamid O, Chasalow SD, et al: Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother 35:89-97, 2012. [DOI] [PubMed] [Google Scholar]
- 9. doi: 10.1093/cid/ciz202. Chong CR, Park VJ, Cohen B, et al: Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis 70:193-199, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. doi: 10.1016/j.ejca.2018.09.012. Wijn DH, Groeneveld GH, Vollaard AM, et al: Influenza vaccination in patients with lung cancer receiving anti-programmed death receptor 1 immunotherapy does not induce immune-related adverse events. Eur J Cancer 104:182-187, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Minnesota Department of Health: Minnesota Immunization Information Connection (MIIC). https://www.health.state.mn.us/people/immunize/miic/index.html.
- 12.Zhang S, Liang F, Tannock I: Use and misuse of common terminology criteria for adverse events in cancer clinical trials. BMC Cancer 16:392, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1200/JCO.2015.66.1389. Weber JS, Hodi FS, Wolchok JD, et al: Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol 35:785-792, 2017. [DOI] [PubMed] [Google Scholar]
- 14.El Osta B, Hu F, Sadek R, et al. : Not all immune-checkpoint inhibitors are created equal: Meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 119:1-12, 2017 [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention: General population vaccination coverage. https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm.
- 16. Myint Z, Qasrawi A, O'Neal R, et al: Immune-related adverse events for different age groups using the FDA Adverse Event Reporting System. J Clin Oncol 37:e14256-e14256, 2019 (15; suppl) [Google Scholar]
- 17. doi: 10.1016/j.lungcan.2019.07.004. Nosaki K, Hosomi Y, Saka H, et al: 103O_PRSafety and efficacy of pembrolizumab (pembro) monotherapy in elderly patients (pts) with PD-L1–positive advanced NSCLC: Pooled analysis from KEYNOTE-010, -024, and -042. Ann Oncol 30, 2019 (suppl 2) [DOI] [PubMed] [Google Scholar]
- 18. doi: 10.1186/s40425-018-0353-7. Läubli H, Balmelli C, Kaufmann L, et al: Influenza vaccination of cancer patients during PD-1 blockade induces serological protection but may raise the risk for immune-related adverse events. J Immunother Cancer 6:40, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. doi: 10.2217/imt-2018-0080. Bersanelli M, Giannarelli D, Castrignanò P, et al: INfluenza Vaccine Indication During therapy with Immune checkpoint inhibitors: A transversal challenge. The INVIDIa study. Immunotherapy 10:1229-1239, 2018. [DOI] [PubMed] [Google Scholar]