Continued smoking after cancer diagnosis increases the risk of cancer recurrence, treatment-related toxicities, treatment failure, and death.1-9 Apart from disease site and stage, continued smoking is the strongest predictor of mortality in patients with cancer.9,10 Quitting smoking can increase long-term survival, reduce total symptom burden, decrease treatment toxicity, optimize postoperative outcomes in patients requiring surgery, and improve quality of life.3,5,11-15
Despite these considerable benefits, multiple challenges remain to helping patients with cancer quit smoking. In particular, most do not receive evidence-based tobacco treatment services. Clinical practice guidelines recommend that oncologists assess patient readiness to quit and assist in making a quit attempt, yet < 40% of oncologists report treating or referring their patients to tobacco treatment.16 If patients do not express interest in quitting, typically, referrals to treatment are not made. Thus, receipt of treatment is entirely dependent on patient readiness to opt in to treatment. However, patients can benefit from treatment even if they do not have an initial desire to quit.17 Tobacco treatment doubles the likelihood of abstinence even among patients unwilling to quit at initial assessment, an effect size similar to that among patients who wanted to quit.18 Thus, opt-out approaches to tobacco treatment in cancer care have been proposed.19 In this approach, which is based on the concept of presumed consent, all patients with cancer who smoke are automatically referred to tobacco treatment, irrespective of their intent to quit. Presumed consent assumes that individuals want to participate in services that may improve their health unless they expressly decline (ie, opt out); failure to oppose treatment is considered as consent. The opt-out approach also has the practical advantage of not depending upon clinicians initiating discretionary referrals.
To improve the reach of tobacco treatment services, the National Cancer Institute launched the Cancer Center Cessation Initiative to integrate tobacco treatment as a routine element of cancer care.20 Several participating cancer centers have begun using an opt-out approach to refer all patients to tobacco treatment, with encouraging results in terms of increasing the reach and effectiveness of tobacco treatment for patients with cancer.21-25 Although the potential benefits of referral are clear, there are also important questions about the ethical justifiability of such practices, such as whether doing so violates patient autonomy or results in unintended consequences. Although the seminal paper proposing the opt-out approach discussed some of these issues, and generated lively correspondence, no study has yet explored in detail the ethical considerations in implementing this approach.19,26-28
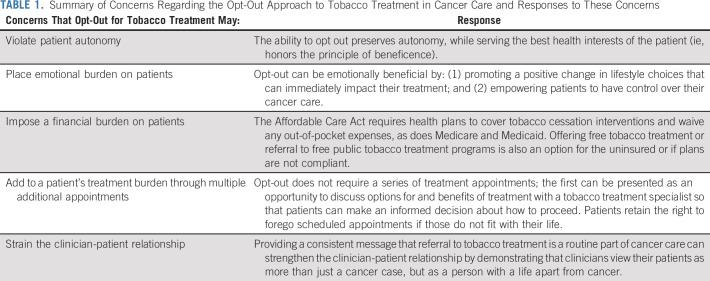
In this work, we examine several ethical considerations in using a presumed consent with opt-out approach to tobacco treatment, referred to hereafter as opt-out. Ethics involves weighing consequences, goods, and the rights of moral agents. The opt-out approach presupposes that certain goods and consequences are worth pursuing in general, while preserving a way for patients to refuse services if they so choose. We argue that although the opt-out approach shapes how patients exercise their liberty and choice, it is rooted in patient welfare in terms of improved outcomes. This intention aligns with the duty of health professionals (beneficence) and implies that systems may be set up toward that end so long as a right to refuse is preserved (autonomy). We also argue that practice protections can ensure patient choices are honored and the dignity of patients with cancer who choose not to pursue treatment is preserved. As a point of reference, the arguments in this work are summarized in Table 1.
TABLE 1.
Summary of Concerns Regarding the Opt-Out Approach to Tobacco Treatment in Cancer Care and Responses to These Concerns
DOES THE OPT-OUT APPROACH TO TOBACCO TREATMENT REFERRAL VIOLATE PATIENT AUTONOMY?
Opt-out approaches can influence health behavior and positively impact patient care.29,30 In medical settings, examples of opt-out approaches include mandatory influenza vaccination for healthcare workers, routine administration of pneumococcal vaccine for eligible hospitalized patients, default registration for organ donation, and obtaining patient samples for population biobanks as part of routine care.31-34 The utilization of the opt-out approach among cancer centers has steadily increased. It shows promising results in terms of increasing patient attendance to tobacco treatment and subsequent quit rates compared with the traditional opt-in approach, although further work is necessary to evaluate long-term cessation efficacy.21-25,35-37 Although increasing quit rates among patients with cancer is clearly beneficial, the possibility of interfering with patient autonomy is real.
Autonomy is defined as the ability of an individual to act freely in accordance with a self-chosen plan such that they are free of controlling influences that determine their action or inhibit self-directedness.38 The act of referring a patient who uses tobacco without taking into consideration intent to quit, or obtaining explicit consent prior to referral, is inherently controlling. Opt-out may thus appear to limit patient choice. Instead of actively choosing to consent or immediately decline a tobacco treatment appointment, patients are required to take additional steps to refuse treatment. The opt-out approach leverages medical authority and the position of power to divert patients toward an action they did not actively choose. Such limitations of patient autonomy could lead to what has been called a dignitary harm—someone being wronged by virtue of the disrespect implied in delimiting their choices. Additionally, patients may believe that the provision of care could be jeopardized if they do not comply.
However, we argue that patients still retain freedom of choice and that the opt-out approach differs from a mandate. We also argue that it is not coercive—denying smokers cancer treatment until they quit smoking would be coercive. It is reasonable that clinicians presume that patients want to improve their health. Although clinicians may sometimes misestimate which services will achieve this goal, there is no doubt that quitting is beneficial. The opt-out approach intentionally structures choices, the so called choice architecture, on the basis of a presumption of beneficence—a central ethical principle in medicine to maximize patient welfare.34,39 It is based on soft paternalism—shaping choices while maintaining freedom.34,40 Clinicians often assume that patients want proven interventions, such as mammograms, colonoscopies, and vaccines, among others. Systems for preventive care are designed on this assumption, as the act of pursuing medical care implies the desire to maximize health outcomes. The opt-out approach provides patients access to evidence-based treatment that will maximize the possibility of successful cancer treatment—while still allowing patients to exercise autonomy by not scheduling or canceling a tobacco treatment appointment.
Potential threats to autonomy with the opt-out approach can be managed. Respecting autonomy requires clinicians to honor patient agency by avoiding undue influence or coercion and respecting individual rights.38 Moreover, protections need to be in place to honor and respect the dignity of patients who decide to continue to smoke during cancer treatment. To limit dignitary harms, patients should be made aware of their right to decline (opt out) without real or perceived negative consequences to their care and be provided practical opportunities to do so.
UNINTENDED CONSEQUENCES OF THE OPT-OUT APPROACH
Practical consequences must be considered prior to implementing an opt-out approach. One concern is the emotional burden opt-out may place on patients, such as emotional guilt, shame, or embarrassment for their actions.41-43 Patients may blame themselves or perceive that their clinician blames them for getting cancer, increasing anxiety and fear.43 However, we argue that the opt-out approach is unlikely to cause an additional emotional burden as many patients who use tobacco already feel shame or guilt resulting from an internal belief that their lifestyle may have contributed to their cancer.41 Rather, referral to tobacco treatment promotes a positive change in lifestyle choices that can immediately impact their treatment and may empower patients to have some measure of control over their cancer care, which may in turn be emotionally beneficial.
The opt-out approach may impose a financial burden on some patients. Patients of low socioeconomic status or uninsured (who tend to have a higher prevalence of tobacco use) may be at greater risk of financial strain if tobacco treatment generates out-of-pocket costs, potentially worsening healthcare inequalities arising from tobacco use.44 Conversely, opt-out could reduce disparities by reducing clinician referral bias and other barriers to treatment. The Affordable Care Act requires health plans to cover tobacco cessation interventions and waive any out-of-pocket expenses.45 In practice, tobacco treatment is not always totally covered. One approach would be to waive these co-payments for patients as elimination of Medicare co-payments increases enrollment into tobacco treatment among lower-income patients.27,46 Offering free tobacco treatment is an option for the uninsured or if plans are not compliant. If offering free tobacco treatment is not feasible, patients can be referred to free public tobacco treatment programs, such as online support from sites like that in ref. 47 and telephone support from state quitlines. Finally, costs associated with tobacco treatment may be recovered if cessation is achieved, even if only temporarily, by reducing the purchase of tobacco products and risk of future tobacco-related healthcare expenses.
Other consequences include potential forms of psychological and dignitary harms, such as adding to a patient's treatment burden through additional appointments; the potential to strain the clinician-patient relationship; and shame, embarrassment, or loss of self-esteem if they do not succeed in quitting. As a result, patients may become discouraged or annoyed and lose confidence in their clinician, healthcare team, or the medical institution. This may further impact their desire to comply with not only tobacco treatment but other treatments as well. However, our own experience has found that consistent messages from clinicians and other members of the healthcare team that a referral is a routine part of cancer care can in fact strengthen the clinician-patient relationship by demonstrating that clinicians view their patients as more than just a cancer case.25 Indeed, referral can be presented as an opportunity to discuss options for and benefits of treatment with a tobacco treatment specialist so that patients can make an informed decision about how to proceed. However, more research is necessary to explore these potential deleterious effects and devise consistent mitigating strategies.
There are also potential beneficial consequences. Making autonomous decisions requires patients with capacity to weigh options and choose one that is aligned with their values and desires. Patients with cancer who use tobacco are confronted with an additional challenge on the basis of logistics of when clinicians assess readiness to quit. Receiving news of a cancer diagnosis is an emotionally difficult situation. In this situation, patients are challenged with concentrating on crucial information such as cancer stage and treatment options.48 At such a juncture, the patient may well be unable to adequately assess the information being conveyed about the benefits of quitting, limiting their capacity to make decisions when assessed for readiness to quit, and consequently may refuse tobacco treatment. The opt-out approach does not require an immediate decision to purse tobacco treatment. It permits a patient's cancer treatment to move forward but offers them time to consider relevant facts, process the information, and discuss tobacco treatment further with tobacco treatment specialists after the initial emotional shock subsides, without a commitment to quit.
In conclusion, we consider the primary ethical concern with using the opt-out approach for tobacco treatment as balancing the preservation of autonomy with the principle of beneficence. The medical community has a duty to promote the well-being of patients while allowing them to accept or refuse care. The benefits of improved cancer treatment outcomes outweigh any inconvenience to a patient if they choose to opt out of their tobacco treatment appointment. The traditional approach to tobacco treatment requires patients to actively opt in to care—placing a greater importance on autonomy at the potential expense of beneficence. The opt-out approach honors both principles, serving the best health interests of the patient while protecting autonomy. As with any treatment approach, there are potential unintended consequences, including psychological, financial, or dignitary harms. However, experience to date suggests that these consequences are manageable and are outweighed by the benefits of this approach. Each healthcare system implementing this approach needs to assess their practice, and their patient population, to understand how best to technically implement automatic referrals and minimize unintended consequences. We conclude that implementing a presumed consent with opt-out approach for tobacco treatment is ethically justifiable and should be seriously considered by healthcare systems that serve patients with cancer.
SUPPORT
Supported by an administrative supplement to the Mayo Clinic Comprehensive Cancer Center from the NCI (P30CA015083-44S2). This publication was also supported by CTSA Grant Number TL1 TR002380 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
AUTHOR CONTRIBUTIONS
Financial support: David O. Warner
Administrative support: David O. Warner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Presumed Consent With Opt-Out: An Ethical Consent Approach to Automatically Refer Patients With Cancer to Tobacco Treatment Services
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Joshua W. Ohde
Stock and Other Ownership Interests: Pfizer, Sanofi, Abbvie, Johnson & Johnson, Schrodinger
No other potential conflicts of interest were reported.
REFERENCES
- 1.Garces YI, Hays JT.Tobacco dependence: Why should an oncologist care? J Clin Oncol 211884–18862003 [DOI] [PubMed] [Google Scholar]
- 2.Cox LS, Africano NL, Tercyak KP, et al. Nicotine dependence treatment for patients with cancer Cancer 98632–6442003 [DOI] [PubMed] [Google Scholar]
- 3.Gritz ER, Toll BA, Warren GW.Tobacco use in the oncology setting: Advancing clinical practice and research Cancer Epidemiol Biomarkers Prev 233–92014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Underwood JM, Townsend JS, Tai E, et al. Persistent cigarette smoking and other tobacco use after a tobacco-related cancer diagnosis J Cancer Surviv 6333–3442012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppone LJ, Mustian KM, Morrow GR, et al. The effect of cigarette smoking on cancer treatment-related side effects Oncologist 161784–17922011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter V, Jansen L, Hoffmeister M, et al. Smoking and survival of colorectal cancer patients: Systematic review and meta-analysis Ann Oncol 251517–15252014 [DOI] [PubMed] [Google Scholar]
- 7.Warren GW, Kasza KA, Reid ME, et al. Smoking at diagnosis and survival in cancer patients Int J Cancer 132401–4102013 [DOI] [PubMed] [Google Scholar]
- 8.O'Malley M, King AN, Conte M, et al. Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer J Thorac Oncol 9917–9262014 [DOI] [PubMed] [Google Scholar]
- 9.Jassem J.Tobacco smoking after diagnosis of cancer: Clinical aspects Transl Lung Cancer Res 8S50–S582019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karam-Hage M, Cinciripini PM, Gritz ER.Tobacco use and cessation for cancer survivors: An overview for clinicians CA Cancer J Clin 64272–2902014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsadius D, Hedelin M, Johansson KA, et al. Tobacco smoking and long-lasting symptoms from the bowel and the anal-sphincter region after radiotherapy for prostate cancer Radiother Oncol 101495–5012011 [DOI] [PubMed] [Google Scholar]
- 12.Richardson GE, Tucker MA, Venzon DJ, et al. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers Ann Intern Med 119383–3901993 [DOI] [PubMed] [Google Scholar]
- 13.Passarelli MN, Newcomb PA, Hampton JM, et al. Cigarette smoking before and after breast cancer diagnosis: Mortality from breast cancer and smoking-related diseases J Clin Oncol 341315–13222016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Neill AC, Haykal S, Bagher S, et al. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction J Plast Reconstr Aesthet Surg 691349–13552016 [DOI] [PubMed] [Google Scholar]
- 15.Bjarnason GA, Mackenzie RG, Nabid A, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: A prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3) Int J Radiat Oncol Biol Phys 73166–1722009 [DOI] [PubMed] [Google Scholar]
- 16.Price SN, Studts JL, Hamann HA.Tobacco use assessment and treatment in cancer patients: A scoping review of oncology care clinician adherence to clinical practice guidelines in the US Oncologist 24229–2382019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahill K, Lancaster T, Green N. Stage-based interventions for smoking cessation. Cochrane Database Syst Rev. 2010;11:Cd004492. doi: 10.1002/14651858.CD004492.pub4. [DOI] [PubMed] [Google Scholar]
- 18.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 19.Richter KP, Ellerbeck EF.It's time to change the default for tobacco treatment Addiction 110381–3862015 [DOI] [PubMed] [Google Scholar]
- 20.Croyle RT, Morgan GD, Fiore MC.Addressing a core gap in cancer care: The NCI Moonshot Program to help oncology patients stop smoking N Engl J Med 380512–5152019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gali K, Pike B, Kendra MS, et al. Integration of tobacco treatment services into cancer care at Stanford. Int J Environ Res Public Health. 2020;17:2101. doi: 10.3390/ijerph17062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahhas GJ, Wilson D, Talbot V, et al. Feasibility of implementing a hospital-based “opt-out” tobacco-cessation service Nicotine Tob Res 19937–9432017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolan M, Ridgeway JL, Ghosh K, et al. Design, implementation, and evaluation of an intervention to improve referral to smoking cessation services in breast cancer patients Support Care Cancer 272153–21582019 [DOI] [PubMed] [Google Scholar]
- 24.Jenssen BP, Leone F, Evers-Casey S, et al. Building systems to address tobacco use in oncology: Early benefits and opportunities from the cancer center cessation initiative J Natl Compr Canc Netw 17638–6432019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jose T, Ohde JW, Hays JT, et al. Design and pilot implementation of an electronic health record-based system to automatically refer cancer patients to tobacco use treatment. Int J Environ Res Public Health. 2020;17:4054. doi: 10.3390/ijerph17114054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashcroft RE.The ethics of an opt-out default in tobacco treatment Addiction 110389–3902015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotz D.Implementation of a new 'opt-out' default for tobacco treatment is urgently needed, but requires free access to evidence-based treatments Addiction 110387–3882015 [DOI] [PubMed] [Google Scholar]
- 28.Baker TB, Fiore MC.Treating more smokers, more of the time, more successfully Addiction 110388–3892015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel MS, Volpp KG, Asch DA.Nudge units to improve the delivery of health care N Engl J Med 378214–2162018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpern SD, Ubel PA, Asch DA.Harnessing the power of default options to improve health care N Engl J Med 3571340–13442007 [DOI] [PubMed] [Google Scholar]
- 31.Greene MT, Fowler KE, Ratz D, et al. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Netw Open. 2018;1:e180143. doi: 10.1001/jamanetworkopen.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevention of pneumococcal disease: Recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep 461–241997 [PubMed] [Google Scholar]
- 33.Giesbertz NAA, Bredenoord AL, van Delden JJM. Inclusion of residual tissue in biobanks: Opt-in or opt-out? PLoS Biol. 2012;10:e1001373. doi: 10.1371/journal.pbio.1001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunstein CR, Thaler RH. Nudge: Improving Decisions about Health, Wealth, and Happiness. New Haven, CT: Yale University Press; 2008. [Google Scholar]
- 35.Faseru B, Ellerbeck EF, Catley D, et al. Changing the default for tobacco-cessation treatment in an inpatient setting: Study protocol of a randomized controlled trial. Trials. 2017;18:379. doi: 10.1186/s13063-017-2119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warren GW, Marshall JR, Cummings KM, et al. Automated tobacco assessment and cessation support for cancer patients Cancer 120562–5692014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notier AE, Hager P, Brown KS, et al. Using a quitline to deliver opt-out smoking cessation for cancer patients JCO Oncol Pract 16e549–e5562020 [DOI] [PubMed] [Google Scholar]
- 38.Childress TLBaJF . Principles of Biomedical Ethics. ed 4. New York, NY: Oxford University Press; 2019. [Google Scholar]
- 39.Cohen S.Nudging and informed consent Am J Bioeth 133–112013 [DOI] [PubMed] [Google Scholar]
- 40.Thaler RH, Sunstein CR.Libertarian paternalism Am Econ Rev 93175–1792003 [Google Scholar]
- 41.LoConte NK, Else-Quest NM, Eickhoff J, et al. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer Clin Lung Cancer 9171–1782008 [DOI] [PubMed] [Google Scholar]
- 42.Weiss J, Yang H, Weiss S, et al. Stigma, self-blame, and satisfaction with care among patients with lung cancer J Psychosoc Oncol 35166–1792017 [DOI] [PubMed] [Google Scholar]
- 43.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: Qualitative study. BMJ. 2004;328:1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiscock R, Bauld L, Amos A, et al. Socioeconomic status and smoking: A review Ann N Y Acad Sci 1248107–1232012 [DOI] [PubMed] [Google Scholar]
- 45.Tobacco Control Legal Consortium . How the Affortable Care Act Affects Tobacco Use and Control. 2015. https://www.publichealthlawcenter.org/sites/default/files/resources/tclc-fs-aca-&-tobacco-control-2014_0.pdf [Google Scholar]
- 46.Young-Wolff KC, Adams SR, Klebaner D, et al. Evaluating the impact of eliminating copayments for tobacco cessation pharmacotherapy Med Care 56912–9182018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smokefree. smokefree.gov [Google Scholar]
- 48.Ogawa A, Kondo K, Takei H, et al. Decision-making capacity for chemotherapy and associated factors in newly diagnosed patients with lung cancer Oncologist 23489–4952018 [DOI] [PMC free article] [PubMed] [Google Scholar]