Supplemental Digital Content is available in the text
Keywords: cascade stomach, gastric morphology, gastroesophageal reflux, radiographs of the upper gastrointestinal tract
Abstract
The study's aim was to determine if there was an association between gastric morphology and gastroesophageal reflux (GER). Few published studies have investigated the relationship between gastric morphology and the risk of GER.
A total of 777 patients were randomly selected from 3000 to 3300 patients who presented at a medical center in Taipei for annual health checkups from early 2008 through to late 2010 and underwent a series of radiographs of the upper gastrointestinal tract (UGI). GER was recorded during the real-time fluoroscopic study. Thirty-nine participants had a follow-up endoscopy, and another 164 participants were followed up by a second UGI series 12 +/ −1.5 months later, from late 2008 through to early 2022. All participants completed a lifestyle and symptom questionnaire. The variables included current smoking and alcohol consumption. Participants who had heartburn and dysphagia were included in the study. Additionally, all participants underwent a limited physical examination which recorded age, sex, body mass index, and total cholesterol and triglyceride levels.
All participants were classified into types 1 to 6 based on the gastric morphology determined from the first UGI. Cascade stomach is recognized by characteristic findings on UGI. Gastric types 2 and 3 tend to appear as cascade stomachs and were significantly associated with GER (P < .05) compared with the other groups. Morphologic type 5 appeared as an elongated sac extending downward into the pelvic cavity and was less likely to develop GER (P < .001). The results of follow-up studies by UGI and endoscopy were similar to those of the first UGI. Gastric morphologic type 2 was significantly associated, and type 5 was usually not associated, with GER and erosive esophagitis (P < .05) compared with the other groups, by both UGI and endoscopy.
Gastric morphologic types 2 and 3, with cascade stomach, might provide a relatively easy method for the development of the GER phenomenon. Gastric morphologic type 5 appeared as an elongated sac that might reduce the incidence of the GER phenomenon. The study suggested that gastric morphologic type could influence the occurrence of GER.
1. Introduction
Gastroesophageal reflux (GER) is generally defined as the retrograde passage of gastric contents into the esophagus.[1] Chronic cough, likely due to GER, even without associated gastrointestinal symptoms, has been reported.[2] The reflux may also result in extra-gastrointestinal complications, including dental erosions, laryngitis, cough, asthma, sinusitis, and idiopathic pulmonary fibrosis.[3] GER is the involuntary movement of gastric contents into the esophagus.[4] It is a common disease that occurs in one-third of the population in the United States.[4] However, reflux is only considered a disease when it causes common or severe symptoms or when it produces damage.[4] Physiological reflux (reflux in normal individuals) is brief, relatively infrequent, tends to occur after meals, and is caused by a sudden relaxation of the lower esophageal sphincter (LES).[4] This type of relaxation, called transient spontaneous LES relaxation, is also the major mechanism of reflux in patients with GER disease (GERD).[4,5] Most other reflux events in patients with GERD occur when resting LES pressure is inadequate to resist pressure within the stomach.[4] Hence, these findings may indicate the important role of gastric morphology in patients with reflux events.
The diagnosis of GERD is complex. On endoscopy, only about one-third to one-half of patients with GERD have positive findings, such as erosions and ulcers. Most patients with GERD symptoms have no obvious mucosal breaks.[6] Clinical history and questionnaire data are inadequate for a definitive diagnosis of GERD.[6] Conclusive evidence of reflux in esophageal testing includes advanced grade erosive esophagitis (The Los Angeles classification system of GERD; grades C and D), long-segment Barrett mucosa, peptic strictures on endoscopy, or distal esophageal acid exposure time > 6% on ambulatory pH or pH-impedance monitoring.[6] Thus, making a definitive diagnosis in lesser grades of reflux esophagitis might still pose a challenge with current tools.
Radiographs of the upper gastrointestinal (UGI) barium swallow examination allows significant evaluation of esophageal peristalsis and the presence and extent of GER.[7] Even minor episodes of reflux should be considered an indication for further study.[4] However, there has been a decline in the number of barium procedures in recent years due to wider availability of cross-sectional imaging modalities.[8] Authors have advocated that barium esophagrams are no longer necessary to make a diagnosis of GERD.[9] Only a few researchers have recommended double-contrast swallow in conditions requiring better mucosal detail, such as GERD and its complications.[8] Still, some authors recommend an upper GI series to diagnose structural or functional peristaltic abnormalities (including GER) of the esophagus, stomach, and duodenum.[10–13]
In persons with cascade stomach (CS), barium initially pools in the retroflexed gastric fundus and fills it, after which the barium “cascades” into the body of the stomach.[13] Kusano et al[14] hypothesized that the disappearance or persistence of the ridge after inflation of the stomach was related to the extent of CS and that it might not only be related to reflux symptoms, but also to esophageal mucosal injury. CS has been identified as an independent risk factor for endoscopic reflux esophagitis.[14] Gastroptosis is diagnosed by the downward displacement of the stomach in a UGI study in a standing position, with the greater curve of the stomach partly projecting below the level of the iliac crests.[15] Previous reports suggested that gastroptosis may protect against dyspeptic symptoms, rather than causing functional dyspepsia.[13,16]
The abovementioned studies raise a question. Is there an association between gastric morphology and the risk of GER? The pathophysiology of GERD is complex. Over the past few decades, most studies on the pathogenesis of reflux esophagitis have concentrated primarily on how abnormalities in anti-reflux mechanisms allow acidic gastric juice to reflux into the esophagus.[17] Very few articles have focused on the differences in gastric morphology (anatomy) that may provoke or lessen GER episodes although one article reported that gastric morphology is related to upper GI symptoms.[13] A UGI series is not currently recognized as a standard tool to confirm GERD. The aim of this study was to determine the role of gastric morphology in GER. In view of the results of recent studies, this study was designed to evaluate the association between gastric morphology and GER.
2. Methods
2.1. Study population
A total of 777 patients were randomly selected from 3000 to 3300 patients who present annually for general medicine routine health checkups. They were first assessed from 2008 through to 2010 and the follow-ups occurred from late 2008 through to early 2022.
The inclusion criterion for patient selection was age between 19 and 75 years. These participants received health surveys freely, and were not referred from clinicians for specific reasons, such as GERD. Thirty-nine participants returned in a self-motivated follow-up for endoscopy and another 164 participants returned for a follow-up radiographic UGI series 12+/−1.5 months later, from late 2008 through to early 2011 (Fig. 1).
Figure 1.
The study flow diagram. UGI = radiographs of the upper gastrointestinal tract.
The exclusion criteria in patient selection were any precipitating factors that might be associated with esophageal strictures such as a history of scleroderma, nasogastric intubation, Zollinger–Ellison Syndrome, body irradiation, and ingestion of caustic substances; use of drugs such as quinidine, potassium chloride, alendronate, and aspirin or other nonsteroidal anti-inflammatory agents; and any related history of strictures[7,18] except reflux stricture. Patients with gastrointestinal obstructive lesions which could increase the incidence of GER, such as a history of gastrointestinal surgery, were also excluded because any GER findings in these patients could be due to a pre-existing condition. Some poor-quality radiographic images were also excluded from the study. These were often due to some patients inadequately following orders resulting in improper or unstable positions on the examination table and leading to motion artifacts on images. In addition, a few patients could not take in sufficient amounts of barium contrast meal and/or effervescent granules orally. None of the patients were pregnant during the study period. Informed consent was obtained from all the study participants. Gastric morphology and GER were diagnosed by a single author (YJW) who has more than 20 years of experience in reading barium X-ray films. The study protocol was approved by the ethics committee and research board of Cathay General Hospital (CGHIRB No.: CGH-P10400 August 30, 2013) to be conducted in a medical center in Taipei, Taiwan. The study was conducted in accordance with the Declaration of Helsinki.
2.2. Study design
Kusano et al[13] performed barium studies to classify gastric morphology with the focus on the association between CS and upper GI symptoms. We designed this retrospective, observational study to extend and classify all the different gastric morphologic types in our subjects. All subjects fasted for at least 8 hours before double-contrast UGI. Each patient ingested 4 g of effervescent granules with a small amount of water. Buscopan (scopolamine butylbromide) was not used in our study because it exerts a parasympatholytic action if used and the peristalsis of the observed gastrointestinal tract would be unnatural.[19] The esophagus, stomach, and duodenum were made visible on X-ray film by a liquid suspension (approximately 180 mL of barium meal). We also paid more attention to visualizing whether GER or lesions occurred over the esophageal-gastric junction (EGJ). We assessed the UGI findings based on real-time fluoroscopic and workstation imaging analyses (Impax DS3000, Agfa HealthCare, Mortsel, Belgium). The subjects were in the standing position in the final stage of the UGI study, either on anterior-posterior projection or occasionally on the right anterior oblique view, especially for CS. The angle (if present) between the medial fundus axis and the body axis was measured by drawing lines along these structures on the radiographs. The subject's body position was sometimes slowly adjusted under fluoroscopy to obtain an ideal view (particularly for anatomical types 2 and 3).[19]
All participants completed a lifestyle and symptom questionnaire. An assistant conducted interviews using an extensive written questionnaire completed by all participants. The variables included current smoking (an adult who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes),[20] alcohol consumption (current alcohol consumption; participants were asked the following question: at present, on how many days per week do you drink alcohol? Possible answers: 1–2 days/3–4 days/5–6 days/7 days),[21] and exercise for more than 30 minutes at least twice weekly. Participants who had heartburn (excluding those with coronary arterial disease) were included, and dysphagia was defined as occurrence at least once per month in the past year. Additionally, all participants underwent a limited physical examination which recorded age, sex, body mass index (BMI), and serum plasma total cholesterol and triglyceride levels. (Tables 1 and 2). Normal weight was defined as a BMI between 20 and 25 kg/m2, overweight as a BMI between 25 and 30 kg/m2, and obesity as a BMI above 30 kg/m2.[22]
Table 1.
Demographics, baseline characteristics, plasma makers, and symptoms among no GER, GER-s, and GER-d groups. (N = 777).
| Variables | No GER (n = 436) | GER-s (n = 200) | GER-d (n = 141) | P value |
| Age (yr) | 40.89 ± 9.38 | 41.59 ± 10.34 | 46.18 ± 9.83†,‡ | <.001∗ |
| Sex | <.001∗ | |||
| Male | 185 (42.43%) | 143 (71.50%)† | 119 (84.40%)†,‡ | |
| Female | 251 (57.57%) | 57 (28.50%) | 22 (15.60%) | |
| Body mass index | <.001∗ | |||
| Normal (<25 kg/m2) | 357 (81.88%) | 140 (70%)† | 60 (42.55%)†,‡ | |
| Overweight (25–30 kg/m2) | 69 (15.83%) | 52 (26%) | 67 (47.52%) | |
| Obese (>30 kg/m2) | 10 (2.29%) | 8 (4%) | 14 (9.93%) | |
| Waistline, cm | 76.12 ± 9.67 | 80.73 ± 8.97† | 87.18 ± 9.20†,‡ | <.001∗ |
| Current smoking | <.001∗ | |||
| Yes | 77 (17.66%) | 64 (32%)† | 54 (38.3%)† | |
| No | 359 (82.34%) | 136 (68%) | 87 (61.0%) | |
| Current drinking | <.001∗ | |||
| Yes | 149 (34.17%) | 91 (45.50%)† | 71 (50.35%)† | |
| No | 287 (65.83%) | 109 (54.50%) | 70 (49.65%) | |
| r-GT > 65 | .003∗ | |||
| Yes | 19 (4.4%) | 20 (10%)† | 16 (11.3%)† | |
| No | 417 (95.6%) | 180 (90%) | 125 (88.7%) | |
| Current drinking + r-GT > 65 | <.001∗ | |||
| Yes | 9 (2.06%) | 12 (6%)† | 13 (9.22%)† | |
| No | 427 (97.94%) | 188 (94%) | 128 (90.78%) | |
| Morphologic type | ||||
| 1 | 189 (43.34%) | 100 (50%) | 53 (37.59%) | .069 |
| 2 | 23 (5.28%) | 59 (29.50%)† | 61 (43.26%)† | <.001∗ |
| 3 | 20 (4.60%) | 18 (9%) | 16 (11.35%)† | .010∗ |
| 4 | 6 (1.38%) | 0 (0%) | 1 (0.71%) | NA |
| 5 | 197 (45.18%) | 21 (10.50%)† | 10 (7.09%)† | <.001∗ |
| 6 | 1 (0.23%) | 2 (1.00%) | 0 (0%) | NA |
| Chronic disease | ||||
| Hypertension | 25 (5.73%) | 23 (11.50%)† | 31 (21.99%)†,‡ | <.001∗ |
| Diabetes mellitus | 8 (1.83%) | 10 (5.00%) | 9 (6.38%)† | .015† |
| Exercise, yes (%) | 278 (63.76%) | 130 (65.00%) | 90 (63.83%) | |
| Plasma markers | ||||
| Hs-CRP | 0.07 (0.04–0.14) | 0.08 (0.05–0.17) | 0.12 (0.06–0.21)†,‡ | <.001∗ |
| Total cholesterol | 184.5 (165–207) | 189 (166.5–208) | 200 (173–225)†,‡ | .013∗ |
| Triglycerides | 93.0 (69.0–130.5) | 102 (80–159)† | 133 (90–190)†,‡ | <.001∗ |
| Symptoms | ||||
| Heartburn | 44 (10.09%) | 22 (11%) | 36 (25.53%)†,‡ | <.001∗ |
| Dysphagia | 24 (5.50%) | 9 (4.50%) | 26 (18.44%)†,‡ | <.001∗ |
Data are presented as mean± SD for age and waistline, median (interquartile range: Q1 to Q3) for plasma markers, and n (%) for other categorical variables
Differences among groups were compared using one-way ANOVA with Bonferroni post-hoc test for age and waistline; Kruskal-Wallis test with Mann-Whitney U post-hoc test for plasma markers because the data were not normally distributed; Pearson Chi-square or Fisher’ s exact test if any cell numbers were less than five in categorical ones.
GER = gastroesophageal reflux, GER-d = GER episodes noted, complicated with either mucosal erosions or strictures or both, over the distal esophagus, GER-s = GER episode observed, with smooth distal esophageal contour without mucosal erosions, strictures or scarring of the lower esophagus, Hs-CRP = high-sensitivity C- reactive protein, NA = not assessed, No GER = no reflux was observed, and the esophageal contour was smooth, r-GT = r-glutamyl transferase.
P < .05, indicates significant differences among groups.
P < 0.0167, indicates a significant difference compared with the †no GER.
P < 0.0167, indicates a significant difference compared with the ‡ GER-s group.
Table 2.
Results of logistic regression analysis for the influence factors of GER-s and GER-d.
| Univariable logistic regression | Multivariable logistic regression | |||
| Variables | OR (95% CI) | P value | OR (95% CI) | P value |
| Age (yr) | 1.027 (1.012–1.042) | <.001∗ | – | |
| Sex | ||||
| Female | 1.0 (reference) | 1.0 (reference) | ||
| Male | 4.500 (3.283–6.167) | <.001∗ | 2.070 (1.415, 3.026) | <.001∗ |
| BMI | ||||
| Normal (<25 kg/m2) | 1.0 (reference) | – | ||
| Overweight (25–30 kg/m2) | 3.078 (2.184–4.340) | <.001∗ | – | |
| Obese (>30 kg/m2) | 3.927 (1.823–8.458) | <.001∗ | – | |
| Smoking | 2.467 (1.770–3.439) | <.001∗ | – | |
| Drinking | 1.743 (1.304–2.331) | <.001∗ | – | |
| r-GT> 65 | 2.591 (1.458–4.604) | .001∗ | – | |
| Current Drinking + rGT> 65 | 3.754 (1.728–8.153) | .001∗ | – | |
| Morphologic type | ||||
| 1 | 1.064 (0.800–1.415) | .672 | – | |
| 2 | 9.750 (6.063–15.680) | <.001∗ | 6.261 (3.798–10.322) | <.001∗ |
| 3 | 2.304 (1.301–4.080) | <.05∗ | 1.867 (1.022–3.411) | .042∗ |
| 4 | 0.211 (0.025–1.759) | .150 | – | |
| 5 | 0.121 (0.080–0.184) | <.001∗ | 0.302 (0.188–0.485) | .008∗ |
| 6 | 2.566 (0.232–28.421) | .442 | – | |
| Exercise | ||||
| No | 1.0 (reference) | – | ||
| Yes | 1.033 (0.769–1.389) | .828 | ||
| Chronic disease | ||||
| Hypertension | 3.093 (1.881–5.087) | <.001∗ | 2.114 (1.220–3.663) | .008∗ |
| Diabetes mellitus | 3.157 (1.365–7.302) | <.001∗ | – | |
| Plasma markers | ||||
| Hs-CRP | 1.438 (0.928–2.227) | .104 | – | |
| Total cholesterol | 1.006 (1.002–1.010) | <.05∗ | – | |
| Triglyceride | 1.006 (1.004–1.008) | <.001∗ | – | |
Results were represented as estimated OR with the respective 95% CI. Variables with significant association in the univariate logistic regression analysis were included in multivariable logistic regression analysis with the forward conditional method.
BMI = body mass index, CI = confidence interval, GER-d = GER episodes noted, complicated with either mucosal erosions or strictures or both, over the distal esophagus, GER-s = GER episode observed, with smooth distal esophageal contour without mucosal erosions, strictures or scarring of the lower esophagus, Hs-CRP = high-sensitivity C- reactive protein, OR = odds ratio, r-GT = r-glutamyl transferase.
Indicates a significant association between the variable and GER-s and GER-d. (P < .05).
2.3. Definition of UGI findings over the EGJ region
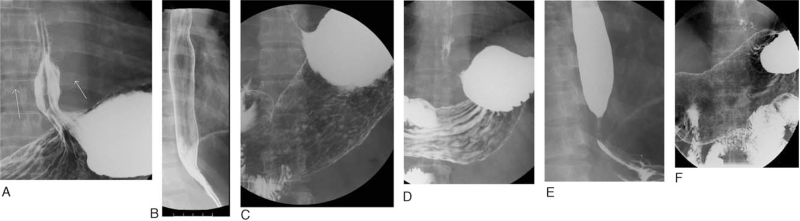
The GER phenomenon was recorded immediately when found (Fig. 2 A) during the real-time fluoroscopic study. Here, we classified UGI findings over the EGJ into 3 groups: no reflux was observed, and the esophageal contour was smooth (no GER), GER episode observed, with smooth distal esophageal contour without mucosal erosions, strictures or scarring of the lower esophagus (GER-s), and GER episodes noted, complicated with either mucosal erosions or strictures or both, over the distal esophagus (GER-d). No GER meant no reflux was observed, and the esophageal contour was smooth (Fig. 2 B). GER-s was defined as a GER episode without mucosal erosions, strictures, or scarring of the lower esophagus (Fig. 2 C). GER-d had a GER episode complicated with either mucosal erosions (Fig. 2 D) or strictures (Fig. 2 E) or both erosions and strictures (Fig. 2 F) over the distal esophagus, [7,18] suggesting local wall injury and/or postinjury changes. We defined these based on our experience of more than ten thousand UGI series studies and obeyed the barium swallow examination critical evaluation and radiographic findings of the presence and extent of GER, and complications including esophagitis and stricture.[7,18]
Figure 2.
Definition of UGI findings over the EGJ region. The GER phenomenon was recorded immediately when found (A) during the real-time fluoroscopic study. Here, we classified UGI findings over the EGJ into 3 groups: no GER, GER-s, and GER-d. No GER meant no reflux was observed, and the esophageal contour was smooth (B). GER-s was defined as a GER episode without mucosal erosions, strictures, or scarring of the lower esophagus (2C). GER-d had a GER episode, complicated with either mucosal erosions (D) or strictures (E) or both erosions and strictures (F) over the distal esophagus[7,18]; suggesting local wall injury and/or post-injury changes. EGJ = esophageal-gastric junction, GER = gastroesophageal reflux, GER-d = GER episodes noted, complicated with either mucosal erosions or strictures or both, over the distal esophagus, GER-s = GER episode observed, with smooth distal esophageal contour without mucosal erosions, strictures or scarring of the lower esophagus, no GER = no reflux was observed, and the esophageal contour was smooth.
2.4. Statistical methods and analysis
The subjects’ demographics, baseline characteristics, plasma markers, and symptoms were presented as means ± standard deviations (SDs) for age and waistline, medians and (interquartile ranges: Q1–Q3) for plasma markers; n (%) for other categorical variables by no GER, GER-s, and GER-d groups. Differences among groups were compared using the one-way ANOVA with the Bonferroni posthoc test for age and waistline, the Kruskal–Wallis test with Mann–Whitney U post-hoc test for plasma markers because the data were not normally distributed, Pearson Chi-square or Fishers exact test if any cell numbers were less than five in categorical variables. Furthermore, the association of morphological types with sex, or with GER status considering sex, was conducted using Pearson Chi-square or Fisher exact test. The follow-up status from UGI and endoscopy examinations were also summarized in a frequency table by group (baseline GER status). The difference in follow-up status, considering baseline status (groups), was assessed using the McNemar test. All statistical assessments were two-sided and considered significant at P < .05. An adjusted significance level of 0.0167 (0.05/3) was also considered for posthoc pair-wise comparisons. Statistical analyses were performed using SPSS software (version 15.0; SPSS Inc., Chicago, IL).
3. Results
3.1. The characteristics of each subtype of gastric morphology
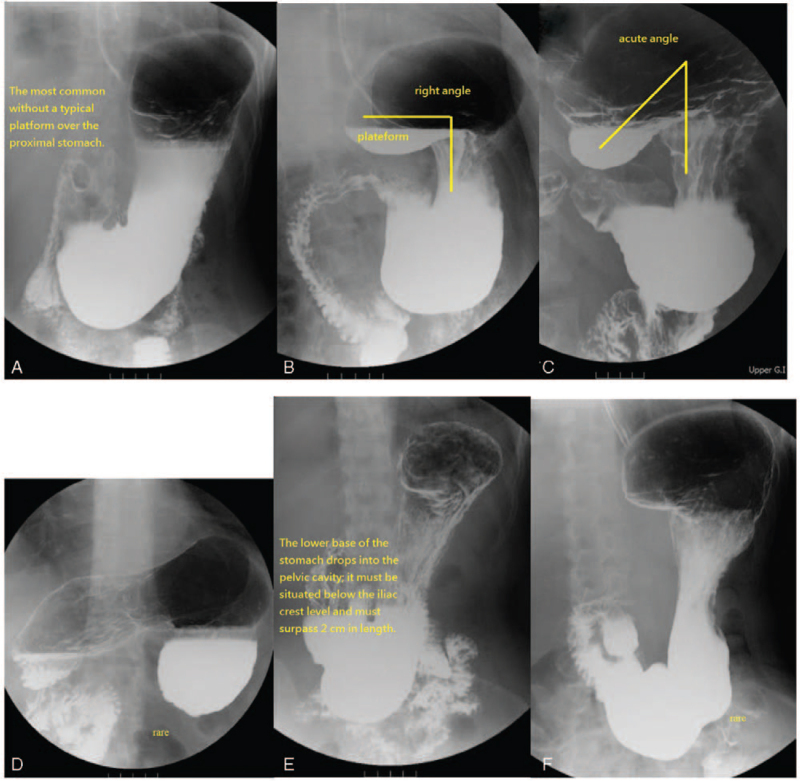
The characteristics and definition of each subtype of gastric morphology are shown in Figure 3.[19]
Figure 3.
The characteristics of each subtype of gastric morphology. Type 1 was the most common and had no typical platform over the proximal stomach, either the platform over the medial fundus was less than 3.8 cm in length (or was not discernible) or the angle of the medial fundus axis and the body axis of the stomach were more than 100° (A). Type 2 had a typical horizontal platform formation (more than 3.8 cm) over the medial fundus, and the angle of the medial fundus and body axis of the stomach appeared as a right angle (or nearly). The platform of the medial fundus (horizontal base) was more than 3.8 cm in length, and the angle of the medial fundus and body axis of the stomach were between 80° and 100°; this type may allow barium meal stasis on the platform (B). Type 3 revealed an acute angle between the fundus and the body axes of the stomach with platform formation (more than 3.8 cm) over the medial fundus. The platform of the medial fundus had an upward-facing concave shape; the horizontal line of the concave up platform was more than 3.8 cm in length, and the angle of the medial fundus axis and body axis of the stomach were less than 80°. There was easy retention of barium in the fundus (C). Type 4; the fundus is upside down (D). ∗For Types 1–4, the gastric base must be either above the level of the iliac crest or must not exceed 2 cm in length below the iliac crest. Type 5 had no remarkable platform. The lower base of the lower gastric portion that dropped into the pelvic cavity must be situated below the iliac crest level and must surpass 2 cm in length. The platform over the medial fundus was less than 3.8 cm in length or not discernible, and the angle of the medial fundus portion axis and body axis of the stomach must be more than 100°. The lower base of the lower gastric portion (antrum) that drops into the pelvic cavity must be situated below the level of the iliac crest and must always exceed 2 cm in length. Type 6: The lower base of the lower gastric portion drops into the pelvic cavity, was situated below the level of the iliac crest, and always exceeded 2 cm in length; otherwise, the criteria for the fundus and body are the same as that of type 2 (F). ∗Types 4 and 6 are rare.
3.2. Demographics, baseline characteristics, plasma markers, and symptoms among no GER, GER-s, and GER-d groups.
Table 1 summarizes the subjects’ demographics and baseline characteristics by group. The incidence of GER-d, diagnosed on the basis of the UGI radiography series, was 19% (141/777), while 26% (200/777) were diagnosed with GER-s and the remaining 55% (436/777) had no GER (Table 1). The age was an average of 40.89 years (SD = 9.38) in the no GER group; 41.59 years (SD = 10.34) in the GER-s group; and 46.18 years (SD = 9.83) in the GER-d group. The dispersion in BMI, waistline, current smoking, hypertension, and serum triglyceride levels were all significantly different among the no GER, GER-s, and GER-d groups. (P < .05).
For the morphologic (anatomical) type, both GER-s and GER-d groups had a higher proportion of type 2 morphology than the no GER group. (Type 2: GER-s 29.5%; GER-d 43.26%; no GER 5.28%, P < .001). There was a lower proportion of type 5 in both the GER-s and GER-d groups than in the no GER group. (Type 5: no GER 45.06%; GER-s 10.50%, GER-d 7.09%, P < .001). The no GER group seemed to have a lower proportion of subjects with chronic diseases like hypertension and diabetes mellitus (both P < .05). For the plasma markers, the GER-d group seemed to have higher levels of high-sensitivity C- reactive protein, total cholesterol, and triglyceride than the no-GER and GER-s groups. Furthermore, it was also found that triglycerides were higher in the GER-s group than in the no GER group (P values < .05). Symptoms like heartburn and dysphagia were present in higher proportions in the GER-d group than in the no GER and GER-s groups (P values < .0167) (Table 1). Reflux was more likely to occur in the supine position.
3.3. Results of logistic regression analysis for the influencing factors of GER-s and GER-d
Table 2 presents the logistic regression analysis that was conducted to identify the influencing factors associated with GER-s or GER-d. The univariate logistic regression analysis showed that age, male sex, high BMI (overweight, obesity), smoking, drinking, r-glutamyl transferase > 65, morphologic types 2, 3, and 5, chronic diseases (hypertension and diabetes mellitus), and plasma markers (total cholesterol and triglyceride) were significantly associated (all P < .05). Multivariate logistic regression analysis showed that GER-s and GER-d may be positively associated with male sex (odds ratio [OR] = 2.07, 95% confidence interval [CI] = 1.4 to 3.0, P < .05), morphologic type 2 (OR = 6.3, 95% CI = 3.8 to 10.3, <.001), morphologic type 3 (OR = 1.9, 95% CI = 1.0 to 3.4, P = .042), and hypertension (OR = 2.1, 95% CI = 1.2 to 3.7, P = .008) and negatively associated with morphologic type 5 (OR = 0.3, 95% CI = 0.2 to 0.5, P = .008) (Table 2).
3.4. Endoscopy and UGI series follow-up results
Table 3 displays the characteristics of 39 participants who followed up the first UGI series with a self-motivated endoscopy 12 ± 1.5 months later. Esophagitis was diagnosed more frequently in patients with morphologic type 2 (P < .05; P = .002). None of the type 5 cases (10 out of 39) were diagnosed with erosive esophagitis (Table 3).
Table 3.
Characteristics of 39 subjects with endoscopic follow-up.
| Variables | No esophagitis (n = 28) | Erosive esophagitis (n = 11) | P value |
| Morphologic type | |||
| 1 (n = 16) | 12 (42.9%) | 4 (36.4%) | 1.000 |
| 2 (n = 10) | 3 (10.7%) | 7 (63.6%) | <.05∗ |
| 3 (n = 3) | 3 (10.7%) | 0 | NA |
| 5 (n = 10) | 10 (35.7%) | 0 | NA |
Esophagitis presentation among gastric morphologic types 1,2,3, and 5; 12 + -1.5 months after the first UGI series. Esophagitis can be categorized into grades A to D according to the Los Angeles classification: Grade A = 6, B = 3, C = 1, and D = 1.
NA = not assessed, UGI = radiographs of the upper gastrointestinal tract.
Type 2 (P < .05; P = .002) shows significant differences among groups.
Table 4 shows the results of 164 subjects followed up by UGI series 12 ± 1.5 months after the first UGI series. Morphologic type 2 was present in higher proportions in the GER-s and GER-d groups than in the no GER group (P < .001) (Table 4).
Table 4.
Summary of 164 subjects’ follow-up by UGI series study 12 + -1.5 months after the first UGI series.
| Variables | No GER (n = 102) | GER-s (n = 20) | GER-d (n = 42) | P value |
| Morphologic type | ||||
| 1 (n = 75) | 49 (48.0%) | 11 (55.0%) | 15 (35.7%) | .271 |
| 2 (n = 39) | 8 (7.8%) | 9 (45.0%) | 22 (52.4%) | <.001∗ |
| 3 (n = 13) | 8 (7.8%) | 0 | 5 (11.9%) | .327 |
| 5 (n = 37) | 37 (36.3%) | 0 | 0 | NA |
GER-d = GER episodes noted, complicated with either mucosal erosions or strictures or both, over the distal esophagus, GER-s = GER episode observed, with smooth distal esophageal contour without mucosal erosions, strictures or scarring of the lower esophagus, no GER = no reflux was observed, and the esophageal contour was smooth, NA = not assessed, UGI = radiographs of the upper gastrointestinal tract.
Indicates a significant association between GER-s, GER-d and Type 2 morphology (P < .001).
The results of the follow-up studies by UGI and endoscopy were similar to those of the first UGI.
4. Discussion
The GER phenomenon observed during the X-ray fluoroscopic study could be physiological reflux or GERD.[4] The physiological reflux type of relaxation, called transient spontaneous LES relaxation, is also the predominant mechanism of reflux in patients with GERD.[4] While transient spontaneous relaxation is responsible for 98% of reflux events in normal individuals, it accounts for about 60% of reflux events in patients with GERD.[4] However, even minor episodes of GER during radiological study is an indication for further study.[4] Psychosocial factors contribute to symptoms in functional dyspepsia. The intensity of functional dyspepsia symptoms is related to the degree of impairment of quality of life.[23] Of all adults, 30 to 40% experience symptoms of upper abdominal pain or discomfort but an organic cause is found in only a minority who seek medical care.[24,25]
Over the past four decades, most studies on the pathogenesis of reflux esophagitis have emphasized primarily how abnormalities in antireflux mechanisms allow acidic gastric juice to reflux into the esophagus.[17] Our objective was to provide new evidence to describe the relationship between the GER phenomenon and gastric morphology. The aim of our study was predominantly to determine whether there was a difference in the development of the GER phenomenon among these different morphologic types in the same UGI series study condition. Double-contrast radiography of the upper GI tract is noninvasive and allows physicians to directly observe the entire morphology, including the esophagus, stomach, duodenum, as well as the barium flow of the GER in real-time[7,18] thus enhancing the realization of the anatomic effect. We found that morphologic type 2 and 3 stomachs, particularly type 2, in many cases provided a relatively easy method for the development of the GER phenomenon. This might be due to the proximity of the medial platform, close to the EGJ, and prolonged barium meal retention. Participants with types 2 and 3 were more likely to be overweight/obese (P < .001) and more were male.[19] Gastric morphologic type 5 appeared as an elongated sac that propelled food material directly downward into the pelvic cavity, far away from the EGJ; thus, this mechanism may reduce the incidence of the GER phenomenon.[19] Additionally, the proportion of women with type 5 was significantly higher than that of men; participants with this type were less likely to be overweight/obese.[19] The GER phenomenon was more commonly observed during X-ray fluoroscopic study in the supine position (Fig. 4) than in the standing and prone positions. Based on our analysis, using univariate and multivariate logistic regression, we determined that morphologic type 2 was the most significant predictor of GER and erosive esophagitis at endoscopy (Tables 1–4). Type 5 was the most significantly low risk for development of GER. Type 3 also had a significantly higher rate of detection of the GER phenomenon in the UGI series (Tables 1 and 2). Indeed, type 5, by definition, had the lower base of the lower gastric portion dropped into the pelvic cavity by more than 2 cm below the iliac crest level, which denoted a trend to develop gastroptosis. Therefore, our type 5 had the lowest rate of identification of the GER phenomenon in the UGI series (Tables 1 and 2), and none (10 out of 39) had erosive esophagitis on endoscopy (Table 3).
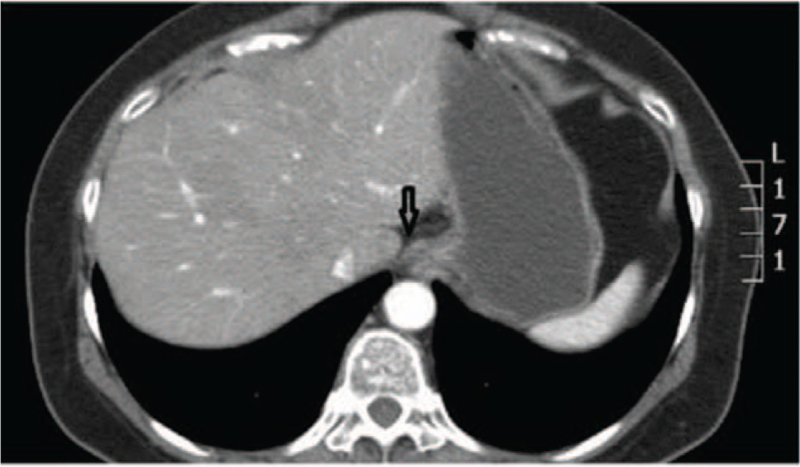
Figure 4.
Lower esophageal sphincter pressure is necessary to resist the pressure within the stomach, particularly the proximal stomach, and it is also close to the EGJ (arrow) in the supine position. EGJ = esophageal-gastric junction.
What is the basis by which we developed these six types of gastric morphology? The stomach consists of five main topographic regions: the cardia, fundus, body, antrum, and pylorus. There are differences in the properties and physiological functions between the proximal and distal stomach.[26] The proximal stomach is an area of special interest for the transient relaxation of the LES, and its likely modulation, which forms a reservoir for the meal and allows the gastric volume to increase.[5,27] The distal stomach is less compliant to low-level distension than the proximal stomach.[26] Disruption of proximal gastric vagal afferent function can occur in high-fat diet induced obesity,[27] which suggests that if a person has a disturbance in the proximal gastric vagal afferent function, he or she would need to eat more food before feeling the same degree of fullness than a healthy individual.[27] The results may change gastric morphology with increases in gastric fundus volume (reservoir).[19] Abdominal or intra-abdominal adiposity, which denotes visceral fat within the abdominal cavity, may be more frequently observed in men than in women.[28] A greater proportion of men have android (ie, upper body) fat distribution than premenopausal women.[19] Evaluation of intra-abdominal fat could be important for assessing gastric anatomy,[13] and hormonal factors should be investigated in the future to determine whether or not they are responsible for the significant difference in gastric morphologic types between females and males.[19] However, managing ingested nutrients in the gastrointestinal tract is a complex process that is closely regulated by both humoral and neural mechanisms.[29] One likely explanation for the changes in gastric morphology related to increased BMI may be due to the increase in the maximum radius of the gastric fundus (such as due to disruption of gastric vagal afferent function). It also proved another potential effect that is, differences in the properties and functions between the proximal and distal stomach.[19] We found that if an individual's BMI increases above the cutoff values indicating overweight or obesity, the proximal stomach may extend to the lesser sac (such as morphologic type 3) because other parts of the stomach are surrounded by solid organs that is, the spleen, liver, pancreas, and diaphragm, and have difficulty expanding at these sites.[19]
Epidemiological estimates of the prevalence of GERD are based primarily on the typical symptoms of heartburn and regurgitation.[30] A systematic review found the prevalence of GERD to be 10% to 20% in the Western world, with a lower prevalence in Asia.[30] Clinically troublesome heartburn is seen in about 6% of the population. The prevalence of regurgitation was reported to be 16%.[30] The rates of heartburn and dysphagia were higher in the GER-d group than in the no GER and GER-s group (all P values < .0167) in our study (Table 1). However, clinical history and questionnaire data are inadequate to make a definitive diagnosis of GERD in the current opinion.[6] These correlations of our radiological findings and symptoms are just useful to approach GERD; they cannot make the diagnosis. A wide variety of questionnaires are accessible for the study of GERD, and their selection depends on the aspect to be evaluated: diagnosis, therapeutic response, or quality of life. While they are useful as an initial diagnostic approach, none of them can be used as a single diagnostic test for GERD[6,31] Our radiological findings also demonstrated that subjects with obesity, smoking, and alcohol consumption were more likely to develop GER phenomena. Prior studies have demonstrated that heartburn and dysphagia are the main symptoms of GERD.[32] Obesity, smoking, and alcohol consumption are risk factors for the development of GERD.[33–36] The purpose of our study was predominantly to determine how these different morphologic groups could provoke or reduce the development of the GER phenomenon in the same UGI series study condition. The significant correlation in the statistics in our study still needs further elucidation, such as through continuous pH monitoring. In addition, certain factors (Tables 1 and 2) such as the high waistline measurement, plasma markers (total cholesterol, triglyceride), hypertension, and diabetes mellitus were more likely to demonstrate GER under fluoroscopic study. We think these associations are understandable because these factors were strongly correlated with obesity.[37–39] Regarding demographic factors in GERD; prevalence could be similar between the sexes, but men can have more severe reflux disease and thus might have more reflux esophagitis than women.[30] However, our UGI study showed that men, and people of advancing age, were more likely to have the GER phenomenon.
Our study has some limitations. Firstly, our sample contained a limited number of racial minorities. Secondly, patient radiation exposure is a concern. The UGI series, in the past, has been part of a health checkup in Japan and at our hospitals.[13,19] Additional research aimed at confirming these associations could be performed using prior data from recent decades. UGI radiography is currently not an advisable measure for screening because of radiation exposure. The use of radiographic examinations has rapidly declined. Thirdly, only 39 subjects followed up with endoscopy 12 ± 1.5 months after the first UGI, which might be related to the limited number of participants (Supplemental Digital Content (xls), http://links.lww.com/MD2/A417, Supplemental Digital Content, http://links.lww.com/MD2/A418).
5. Conclusion
Kusano et al[14] studies suggested that CS may not only be related to upper GI symptoms (in combination with UGI series studies) but also to esophageal reflux esophagitis (findings by endoscopic health screening). Although, gastrointestinal fluoroscopy (UGI series studies) is no longer the main imaging technique that it was 20 to 30 years ago,[12] a recent opinion suggested that CS is one of the main causes of incomplete resection of the gastric fundus in laparoscopic sleeve gastrectomy, which in turn is accountable for primary dilation and is one of the major determining factors of weight regain and GERD after laparoscopic sleeve gastrectomy.[40] The use of UGI studies could be valuable in certain patients, such as the evaluation of cascade stomach before laparoscopic sleeve gastrectomy, but this still needs further validation. One previous study suggested that obesity and sex could alter the presentation of gastric anatomic type.[19] Subsequently, the gastric morphologic type could affect the risk of GER. Our morphologic types 2 and 3 were significantly associated with the GER phenomenon compared with other types, particularly type 2, which might be due to the medial platform structure of the gastric fundus with barium retention, and close location to the EGJ. Morphologic type 5 was less likely to develop the GER phenomenon than the other types, which may be due to its elongated gastric structure with the stomach base dropping into the pelvic cavity. This study suggests that gastric morphology may play an important role in modulating GER.
Author contributions
Conceptualization: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng, Hsin-Fan Chiang.
Data curation: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng, Hsin-Fan Chiang.
Formal analysis: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng, Hsin-Fan Chiang.
Investigation: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng, Hsin-Fan Chiang.
Methodology: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng, Hsin-Fan Chiang.
Project administration: She-Meng Cheng, Yu-Jen Wang, Suk-Ping Ng, Hsin-Fan Chiang.
Resources: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng, Hsin-Fan Chiang.
Software: Suk-Ping Ng.
Supervision: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng.
Validation: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng.
Visualization: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng.
Writing – original draft: She-Meng Cheng, Yu-Jen Wang, Suk-Ping Ng.
Writing – review & editing: She-Meng Cheng, Kun-Long Hung, Yu-Jen Wang, Suk-Ping Ng.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CS = cascade stomach, EGJ = esophageal-gastric junction, GER = gastroesophageal reflux, GERD = gastroesophageal reflux disease, GER-d = GER episodes noted, complicated with either mucosal erosions or strictures or both, over the distal esophagus, GER-s = GER episode observed, with smooth distal esophageal contour without mucosal erosions, strictures or scarring of the lower esophagus, LES= lower esophageal sphincter, no GER = no reflux was observed, and the esophageal contour was smooth, OR = odds ratio, SD = standard deviation, UGI = radiographs of the upper gastrointestinal tract.
How to cite this article: Cheng SM, Hung KL, Wang YJ, Ng SP, Chiang HF. Influence of gastric morphology on gastroesophageal reflux in adults: an observational study. Medicine. 2021;100:38(e27241).
The authors have no funding and conflicts of interest to disclose.
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Data used in our study are included in this published article and its supplementary files.
Abbreviations in the spreadsheets (Excel): Gender; Age; BMI = body mass index; waistline TriG= Serum Triglyceride Levels (mg/dL); T Cholesterol = Serum Total Cholesterol Levels (mg/dL); H.B= Heartburn; Dys= Dysphagia; H/T= Hypertension; Exe= Exercise; Smoke = Smoking; Wine= Alcohol drinking; DM= Diabetes Mellitus; M1 M2 M3 M4 M5 M6: M= Morphological type; GER = gastroesophageal reflux; GER-s = GER episode observed with smooth distal esophageal contour, without mucosal erosions, strictures, or scarring of the lower esophagus; GER-d = GER episodes noted, complicated with either mucosal erosions or strictures or both over the distal esophagus.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Rybak A, Pesce M, Thapar N, Borrelli O. Gastro-esophageal reflux in children. Int J Mol Sci 2017;18:1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kahrilas PJ, Altman KW, Chang AB, et al. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest 2016;150:1341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Azer SA, Reddivari AK. Reflux Esophagitis. Treasure Island, Florida: StatPearls Publishing; 2020. [Google Scholar]
- [4]. Gastroesophageal Reflux Disease. https://www.hopkinsmedicine.org/gastroenterology_hepatology/_pdfs/esophagus_stomach/gastroesophageal_reflux_disease.pdf Accessed June 28, 2021. Johns Hopkins Medicine. [Google Scholar]
- [5].Penagini R, Carmagnola S, Cantù P, Alloca M, Bianchi PA. Mechanoreceptors of the proximal stomach: Role in triggering transient lower esophageal sphincter relaxation. Gastroenterology 2004;126:49–56. [DOI] [PubMed] [Google Scholar]
- [6].Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Canon CL, Morgan DE, Einstein DM, Herts BR, Hawn MT, Johnson LF. Surgical approach to gastroesophageal reflux disease: what the radiologist needs to know. RadioGraphics 2005;25:1485–99. [DOI] [PubMed] [Google Scholar]
- [8].Debi U, Sharma M, Singh L, Sinha A. Barium esophagogram in various esophageal diseases: a pictorial essay. Indian J Radiol Imaging 2019;29:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Badillo R, Francis D. Diagnosis and treatment of gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther 2014;5:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Upper Gastrointestinal Series. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/upper-gastrointestinal-series Accessed June 28, 2021. Johns Hopkins Medicine. [Google Scholar]
- [11].Giles B. Gastrointestinal Imaging: The Requisites. Elsevier/Saunders, 4th EditionPhiladelphia, United States:2013. [Google Scholar]
- [12].Levine MS, Trenkner SW. Training the next generation in luminal gastrointestinal radiology: a call to arms. AJR Am J Roentgenol 2011;196:362–6. [DOI] [PubMed] [Google Scholar]
- [13].Kusano M, Hosaka H, Moki H, et al. Cascade stomach is associated with upper gastrointestinal symptoms: a population-based study. Neurogastroenterol Motil 2012;24:451–5. [DOI] [PubMed] [Google Scholar]
- [14].Kusano M, Hosaka H, Yasuoka H, et al. New endoscopic classification of cascade stomach, a risk factor for reflux esophagitis. J Gastroenterol 2017;52:211–7. [DOI] [PubMed] [Google Scholar]
- [15].van Welie AJM, Klein WM, Draaisma JMT. The clinical or radiographic diagnosis of gastroptosis: still relevant? Gastro Open 2017;2:14–9. [Google Scholar]
- [16].Kusano M, Moki F, Hosaka H, et al. Gastroptosis is associated with less dyspepsia, rather than a cause of dyspepsia, in Japanese persons. Intern Med 2011;50:667–71. [DOI] [PubMed] [Google Scholar]
- [17].Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 2009;137:1776–84. [DOI] [PubMed] [Google Scholar]
- [18].Luedtke P, Levine MS, Rubesin SE, Weinstein DS, Laufer I. Radiologic diagnosis of benign esophageal strictures: a pattern approach. Radiographics 2003;23:897–909. [DOI] [PubMed] [Google Scholar]
- [19].Wang YJ, Hung KL, Yang JN, Wang TC, Chin CH. Gastric anatomic type is associated with obesity and gender. Obes Facts 2016;9:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].National Health Interview Survey. https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm Accessed June 28, 2021. National Center for Health Statistics. [Google Scholar]
- [21].Weyerer S, Schäufele M, Wiese B, et al. Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing 2011;40:456–63. [DOI] [PubMed] [Google Scholar]
- [22].Sasaki A, Wakabayashi G, Yonei Y. Current status of bariatric surgery in Japan and effectiveness in obesity and diabetes. J Gastroenterol 2014;49:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miwa H, Kusano M, Arisawa T, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol 2015;50:125–39. [DOI] [PubMed] [Google Scholar]
- [24].El-Serag HB, Talley NJ. Systemic review: the prevalence and clinical course of functional dyspepsia. Aliment Pharmacol Ther 2004;19:643–54. [DOI] [PubMed] [Google Scholar]
- [25].Talley NJ, Silverstein MD, Agreus L, Nyren O, Sonnenberg A, Holtmann G. AGA technical review: evaluation of dyspepsia. Gastroenterology 1998;114:582–95. [DOI] [PubMed] [Google Scholar]
- [26].Lee KJ, Vos R, Janssens J, Tack J. Differences in the sensorimotor response to distension between the proximal and distal stomach in humans. Gut 2004;53:938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kentish SJ, O’Donnell TA, Frisby CL, Li H, Wittert GA, Page AJ. Altered gastric vagal mechanosensitivity in diet-induced obesity persists on return to normal chow and is accompanied by increased food intake. Int J Obes (Lond) 2014;38:636–42. [DOI] [PubMed] [Google Scholar]
- [28].Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol 2011;8:340–7. [DOI] [PubMed] [Google Scholar]
- [29].Farré R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol 2013;108:698–706. [DOI] [PubMed] [Google Scholar]
- [30].Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28. [DOI] [PubMed] [Google Scholar]
- [31].Ciriza de Los RC. Questionnaires for the diagnosis of gastroesophageal reflux disease: are they really useful? Rev Esp Enferm Dig 2016;108:171–3. [DOI] [PubMed] [Google Scholar]
- [32].Cho YK, Lee JS, Lee TH, et al. The relationship of the post-reflux swallow-induced peristaltic wave index and esophageal baseline impedance with gastroesophageal reflux disease symptoms. J Neurogastroenterol Motil 2017;23:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet 2006;367:2086–100. [DOI] [PubMed] [Google Scholar]
- [34].Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med 2005;143:199–211. [DOI] [PubMed] [Google Scholar]
- [35].Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the etiology of gastro-oesophageal reflux. Gut 2004;53:1730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pehl C, Frommherz M, Wendl B, Pfeiffer A. Gastroesophageal reflux induced by white wine: the role of acid clearance and “rereflux”. Am J Gastroenterol 2002;97:561–7. [DOI] [PubMed] [Google Scholar]
- [37].Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013;5:1218–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jiang SZ, Lu W, Zong XF, Ruan HY, Liu Y. Obesity and hypertension. Exp Ther Med 2016;12:2395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 2014;7:587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bernante P, Balsamo F, Rottoli M, et al. Cascade stomach as a risk factor for incomplete resection of the gastric fundus in laparoscopic sleeve gastrectomy: a point of technique. Obes Surg 2020;30:5139–41. [DOI] [PubMed] [Google Scholar]