Abstract
PURPOSE
To prospectively evaluate the effectiveness of risk-adapted preemptive tocilizumab (PT) administration in preventing severe cytokine release syndrome (CRS) after CTL019, a CD19 chimeric antigen receptor T-cell therapy.
METHODS
Children and young adults with CD19-positive relapsed or refractory B-cell acute lymphoblastic leukemia were assigned to high- (≥ 40%) or low- (< 40%) tumor burden cohorts (HTBC or LTBC) based on a bone marrow aspirate or biopsy before infusion. HTBC patients received a single dose of tocilizumab (8-12 mg/kg) after development of high, persistent fevers. LTBC patients received standard CRS management. The primary end point was the frequency of grade 4 CRS (Penn scale), with an observed rate of ≤ 5 of 15 patients in the HTBC pre-defined as clinically meaningful. In post hoc analyses, the HTBC was compared with a historical cohort of high-tumor burden patients from the initial phase I CTL019 trial.
RESULTS
The primary end point was met. Seventy patients were infused with CTL019, 15 in the HTBC and 55 in the LTBC. All HTBC patients received the PT intervention. The incidence of grade 4 CRS was 27% (95% CI, 8 to 55) in the HTBC and 3.6% (95% CI, 0.4 to 13) in the LTBC. The best overall response rate was 87% in the HTBC and 100% in the LTBC. Initial CTL019 expansion was greater in the HTBC than the LTBC (P < .001), but persistence was not different (P = .73). Event-free and overall survival were worse in the HTBC (P = .004, P < .001, respectively). In the post hoc analysis, grade 4 CRS was observed in 27% versus 50% of patients in the PT and prior phase I cohorts, respectively (P = .18).
CONCLUSION
Risk-adapted PT administration resulted in a decrease in the expected incidence of grade 4 CRS, meeting the study end point, without adversely impacting the antitumor efficacy or safety of CTL019.
INTRODUCTION
CD19-targeted chimeric antigen receptor (CAR) T-cells have demonstrated impressive clinical efficacy in treating relapsed or refractory B-cell acute lymphoblastic leukemia (r/r B-ALL).1-6 In August 2017, tisagenlecleucel (initially developed as CTL019) became the first CAR T-cell product to receive approval by the US Food and Drug Administration (FDA).7 Since then, the use of tisagenlecleucel has been broadened to increasing numbers of patients at additional centers worldwide. Although this therapy has been transformative, it is also associated with potentially life-threatening toxicities, the most common of which is cytokine release syndrome (CRS).8-12
CONTEXT
Key Objective
Cytokine release syndrome (CRS) is the most significant toxicity of CD19-directed chimeric antigen receptor (CAR) T-cell therapy including the Food and Drug Administration-approved product tisagenlecleucel. Cytokine (interleukin-6) blockade with tocilizumab is the only approved therapy for CRS. It is unclear whether earlier administration of tocilizumab may mitigate grade 4 CRS and/or compromise antitumor efficacy or safety, including neurotoxicity. To address this gap, we conducted a prospective trial of preemptive tocilizumab administration at the time of first fever in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (ALL) who were at high risk for grade 4 CRS based on high tumor burden.
Knowledge Generated
We observed a greater than one-third decrease in expected grade 4 CRS rates, meeting the predetermined primary end point. We did not detect an impact on neurotoxicity, efficacy, or CAR-T persistence.
Relevance
Preemptive tocilizumab may decrease the risk of severe CRS in patients with ALL and high tumor burden. This approach may improve safety and broaden applicability of CAR T-cells.
CRS has been observed in the majority of patients treated with CTL019 and tisagenlecleucel within the first 14 days after infusion. In the phase II global study of tisagenlecleucel (ELIANA), 77% of patients developed CRS, with 21% developing grade 4 CRS (Penn scale).2 Overall, 47% required intensive care unit (ICU) admission for CRS management. The principal risk factor identified for severe CRS is the baseline bone marrow tumor burden. In the initial phase I trial of CTL019, 50% of patients with ≥ 40% bone marrow blasts developed grade 4 CRS.1,13
CRS results from the activation of infused CAR T-cells, which leads to the release of inflammatory cytokines, immune system activation, and systemic inflammation.14 A primary CRS driver, interleukin (IL)-6, can be targeted with tocilizumab, an anti-IL-6 receptor antibody, and life-threatening CRS rapidly reversed in many cases.1,9,10,12 Tocilizumab is, thus, standard management for severe CRS and is FDA-approved for this indication.7,15 However, it is unknown whether tocilizumab can be used preemptively to mitigate the risk of severe CRS or whether early use prior to significant expansion of CAR-T would impact efficacy of CTL019. Several groups, including Seattle Children's and Kite Pharma, have started to investigate earlier tocilizumab administration after other CD19 CARs.16,17 Their results suggest that early intervention may decrease severe CRS but underscore the need to prospectively evaluate optimal tocilizumab timing in a widely used CAR T-cell product.
We conducted a prospective clinical trial to determine the effectiveness of risk-adapted preemptive tocilizumab (PT) administration in decreasing the expected incidence of grade 4 CRS in children and young adults receiving CTL019. We also conducted a secondary comparison with a historic cohort of patients with high tumor burden who received standard CRS management. We hypothesized that earlier administration of tocilizumab in patients with high tumor burden may decrease the incidence of grade 4 CRS without compromising antitumor efficacy.
METHODS
Patients and Study Design
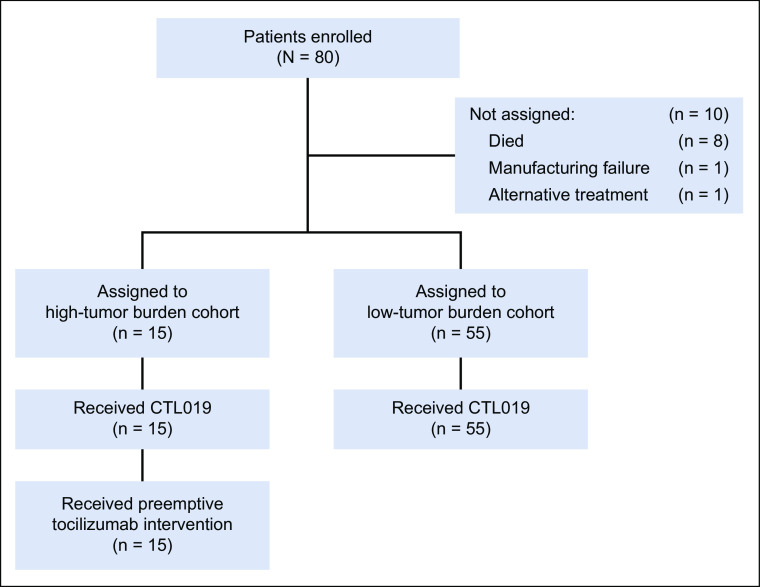
We conducted a two-cohort, open-label pilot study of risk-adapted PT after CTL019 in children and young adults with CD19-positive r/r B-ALL (Fig 1). Eligibility criteria and study procedure details are available in the Data Supplement (online only).
FIG 1.
Screening, enrollment, and treatment.
Patients received lymphodepleting chemotherapy with fludarabine and cyclophosphamide followed by a pre-infusion bone marrow aspirate or biopsy to assess tumor burden. Tumor burden was defined as the highest blast percentage measured by aspirate, biopsy morphology, or multiparameter flow cytometry for minimal residual disease (MRD).18 Patients with ≥ 40% bone marrow blasts were assigned to the high-tumor burden cohort (HTBC). Patients with < 40% blasts were assigned to the low-tumor burden cohort (LTBC).
Patients in both cohorts were infused with CTL019 over 1-2 days. HTBC patients received a single dose of tocilizumab (8-12 mg/kg) at the time of developing high, persistent fever, defined as two temperatures ≥ 38.5°C in a 24-hour period, measured at least 4 hours apart. For patients with progression of clinical CRS, further care was given per standard CRS management guidelines. LTBC patients who developed clinical CRS received standard CRS management (see the Data Supplement for a table with details).
The study Protocol (online only) was approved by the institutional review boards of the Children's Hospital of Philadelphia and the University of Pennsylvania. Patients or their guardians provided written informed consent. The study was registered with ClinicalTrials.gov (NCT02906371). A Reproducibility Supplement with the full annotated computer code for the primary analysis is available online.19
Assessments and End Points
The primary end point was the incidence of grade 4 CRS (Penn scale).20 Based on CRS rates in historical cohorts, we prospectively defined ≤ 5 of 15 HTBC patients with grade 4 CRS as a clinically meaningful result. Secondary end points included overall response rate (ORR), duration of remission (DOR), event-free survival (EFS), CAR T-cell expansion and persistence, and safety events. See the Data Supplement for details about secondary end points and the statistical analysis.
Comparative Analyses
A comparison of the PT HTBC with patients with high tumor burden from the initial phase I CTL019 trial1 (phase I HTBC; NCT01626495) was also conducted (Data Supplement).
RESULTS
Patients
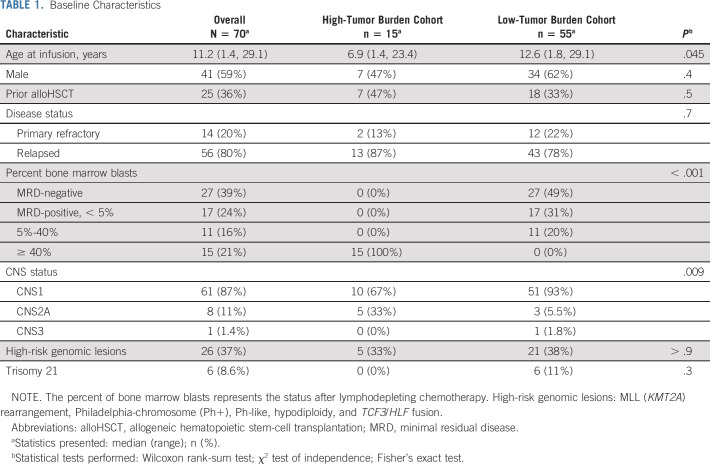
Eighty patients were screened and enrolled between August 26, 2016, and January 30, 2019 (Fig 1). Of those, 70 were assigned to HTBC (n = 15) or LTBC (n = 55) based on the pre-infusion bone marrow aspirate or biopsy and received CTL019 (HTBC: median, 5.0 × 106, range, 1.1-15 × 106 CAR-T/kg; LTBC: median, 5.0 × 106, range, 0.56-19 × 106 CAR-T/kg; P = .5). The median duration of follow-up was 24 months (range, 5-36) after infusion. Baseline patient characteristics are shown in Table 1.
TABLE 1.
Baseline Characteristics
Cytokine Release Syndrome
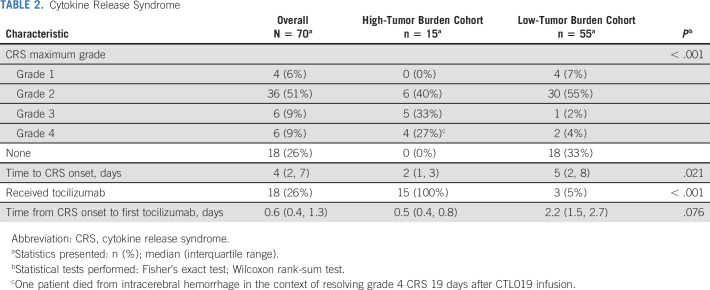
Table 2 shows CRS rates, graded on the Penn scale. A retrospective comparison with the American Society for Transplantation and Cellular Therapy CRS Consensus Grading Scale is available in the Data Supplement.11 All 15 HTBC patients developed ≥ grade 2 CRS and received the PT intervention. Median CRS onset was 2 days (interquartile range [IQR], 1 to 3) after infusion, and PT was administered at a median of 0.5 days (IQR, 0.4 to 0.8) later. Four HTBC patients evolved to grade 4 CRS (27%, 95% CI, 8 to 55), meeting the predefined primary study end point of ≤ 5 of 15 patients. One patient died 19 days after infusion from cerebral hemorrhage in the context of coagulopathy and resolving grade 4 CRS that had included congestive heart failure requiring extracorporeal membrane oxygenation support. Further description of interventions required for CRS management is included in the comparison with the historical cohort below.
TABLE 2.
Cytokine Release Syndrome
Among the 55 LTBC patients, 37 (67%) developed any-grade CRS and two (3.6%, 95% CI, 0.4 to 13) evolved to grade 4 CRS. MRD status (positive or negative) at infusion was not associated with CRS (P = .2, Data Supplement). Three patients (5.5%) received tocilizumab at a median of 2.2 days (IQR, 1.5 to 2.7) after CRS onset.
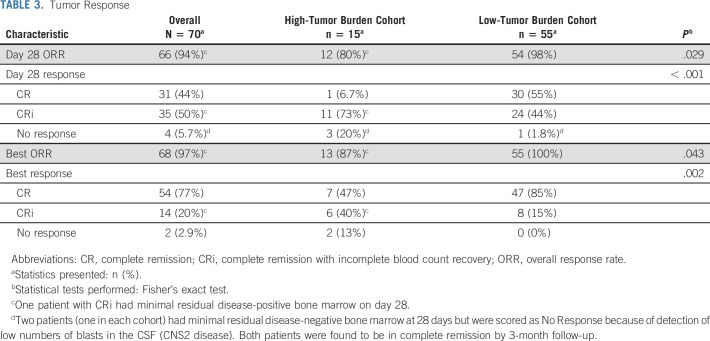
Tumor Response
In the full study cohort, the per-protocol ORR was 94% at day 28; 93% of patients were also negative for MRD, defined as < 0.01% blasts by flow cytometry (Table 3). Two patients were classified as nonresponders at day 28 because of the presence of blasts in the CSF (CNS2), but in both cases, blasts cleared by 3 months without further therapy, leading to a best ORR of 97%. In the HTBC, the best ORR was 87% and the MRD-negative complete response (CR) rate was 80%. In the LTBC, the best ORR was 100% and all responders were MRD-negative.
TABLE 3.
Tumor Response
Among the 68 patients with CR or complete response with incomplete blood count recovery (CRi), the median DOR was not reached (Fig 2A). Among HTBC patients, the probability of continued remission was 49% (95% CI, 27 to 88) at 12 months and 39% (95% CI, 19 to 82) at 24 months. Of the 13 HTBC patients with CR or CRi, nine experienced morphologic relapse. Two additional patients received new cancer therapy for the emergence of MRD and were censored at that time.
FIG 2.
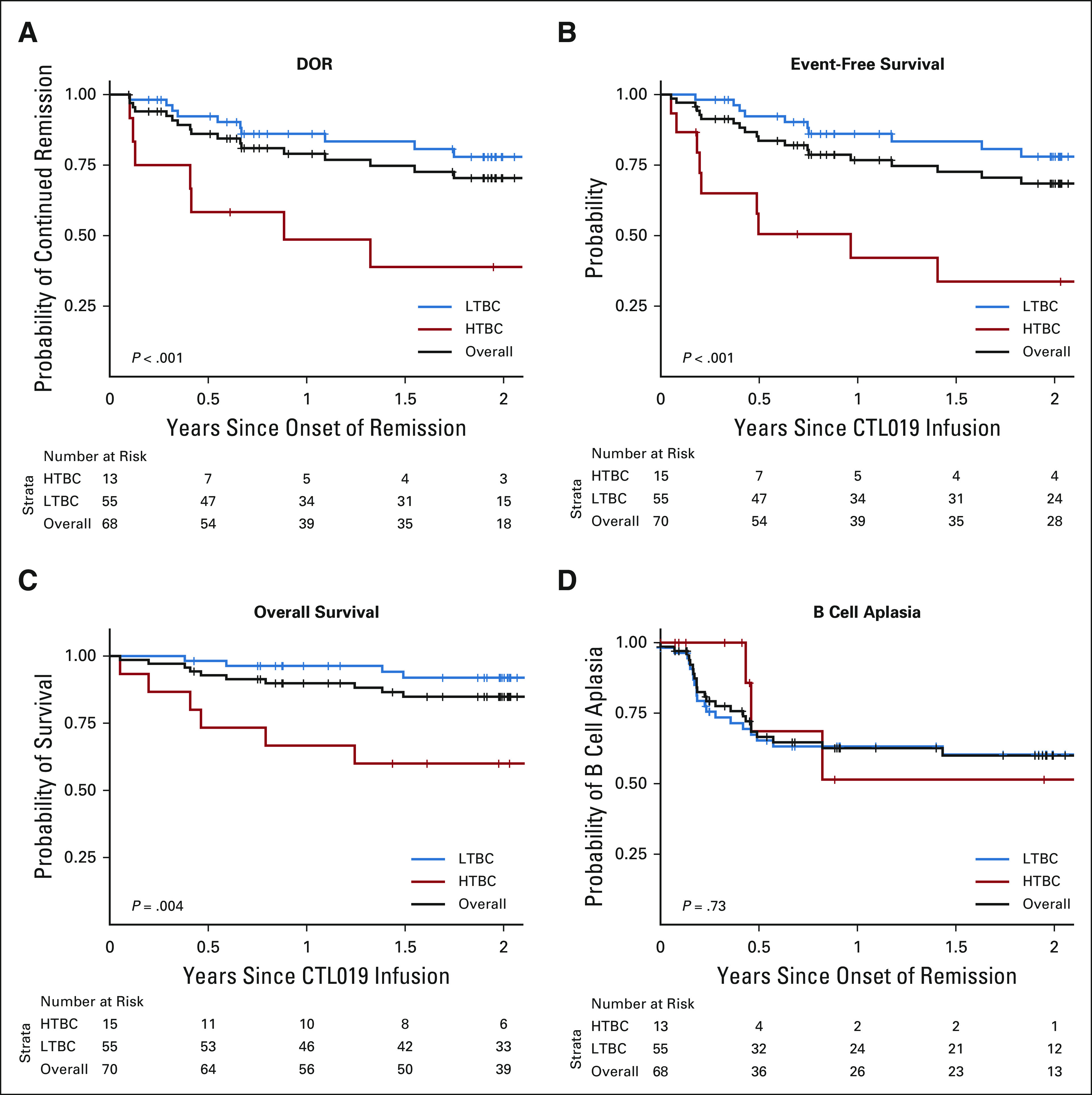
DOR, event-free survival, overall survival, and B-cell aplasia for the overall study population and broken down by cohort. (A) DOR in the 68 patients who had a response (either complete remission or complete remission with incomplete hematologic recovery), defined as the time from the first response to morphologic relapse (9 patients in the HTBC, 10 in the low-tumor burden cohort [LTBC]) or death (none). Data for 12 patients were censored for DOR, either for allogeneic stem-cell transplant (3 LTBC), or for alternative treatments (2 HTBC, 7 LTBC). (B) Event-free survival, defined as the time from CTL019 infusion to one of the following events: no response (1 HTBC), morphologic relapse (9 HTBC, 10 LTBC), or death (1 HTBC). The same data were censored as for the DOR. (C) Overall survival. By the end of follow-up, 12 patients had died (6 HTBC, 6 LTBC). (D) B-cell aplasia, defined as the time to the emergence of ≥ 1% CD19-positive B cells in a bone marrow aspirate or ≥ 3% B cells by peripheral blood flow cytometry (3 HTBC, 20 LTBC). Data were censored for patients who had relapsed disease (3 HTBC, 2 LTBC). DOR, duration of remission; HTBC, high-tumor burden cohort; LTBC, low-tumor burden cohort.
Among LTBC patients, the probability of continued remission was 86% (95% CI, 77 to 96) at 12 months and 78% (95% CI, 67 to 91) at 24 months. Of the 55 CR or CRi patients, 10 experienced morphologic relapse. Seven additional patients received additional cancer therapy for emergence of MRD (n = 3) or loss of CTL019 persistence (n = 4). Six patients underwent allogeneic hematopoietic stem-cell transplantation (alloHSCT) while in CR, including two with early B-cell recovery and one who received an unconditioned CD34-selected stem-cell infusion for persistent bone marrow aplasia. There was a trend toward a greater proportion of CD19-negative relapses in the HTBC (P = .057, Data Supplement).
In the HTBC, the EFS rate was 42% (95% CI, 23 to 79) at 12 months and 34% (95% CI, 16 to 73) at 24 months (Fig 2B). Overall survival (OS) was 67% (95% CI, 47 to 95) at 12 months and 60% (95% CI, 40 to 91) at 24 months (Fig 2C). In the LTBC, the EFS rate was 86% (91% CI, 77 to 96) at 12 months and 78% (95% CI, 67 to 91) at 24 months (Fig 2B). OS was 96% (95% CI, 92 to 100) at 12 months and 92% (95% CI, 85 to 100) at 24 months (Fig 2C).
B-Cell Aplasia
All patients who achieved CR or CRi developed B-cell aplasia. The median time to B-cell recovery was not reached (Fig 2D). The probability of continued B-cell aplasia at 6 months was 69% (95% CI, 40 to 100) in the HTBC versus 65% in the LTBC (95% CI, 54 to 80; P = .73).
CTL019 Expansion
Peripheral blood CAR T-cell abundance, measured by flow cytometry and quantitative polymerase chain reaction, is shown in Figure 3. To compare CAR T-cell expansion in HTBC versus LTBC, the area under the curve and maximum concentration of CAR-T transgenes over the first 28 days were calculated. Expansion was greater in the HTBC by both measures (P < .001, Figs 3E and 3F).
FIG 3.
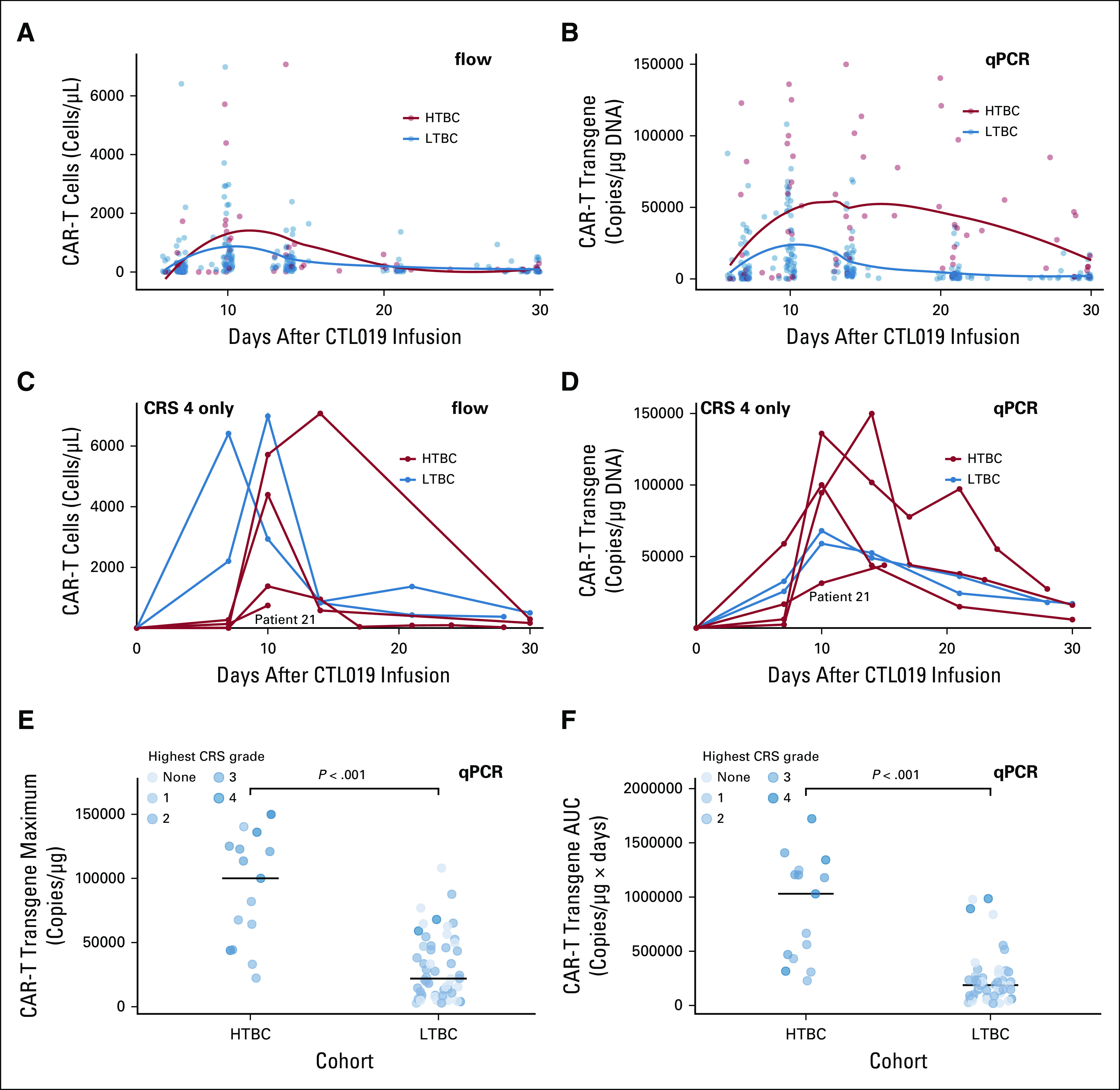
CTL019 expansion. (A) and (B) Scatterplots of circulating CAR T-cells measured by flow cytometry (flow) or qPCR over time during the first month after CTL019 infusion. Trends for each cohort were generated by locally estimated scatterplot smoothing (loess) and are shown as solid lines. In the HTBC, CAR-T expansion peaked at 10 days (interquartile range, 10 to 17 by flow and 10 to 18 by qPCR). In the LTBC, CAR-T expansion also peaked at 10 days; however, the interquartile range was narrower (10-12) by both flow and qPCR. Thus, the distribution of time-to-peak CAR-T expansion was wider in the high-tumor burden cohort (P = .027 for qPCR, P = .2 for flow). (C) and (D) Individual CAR T-cell expansion profiles for the six patients who experienced grade 4 cytokine release syndrome (CRS) measured by flow or qPCR. Of note, patient 21, who died on study day 19, had the lowest peak CAR-T expansion in this group. (E) Maximum concentration and (F) Area under the curve of CAR-T transgenes measured by qPCR for the first month after CTL019 infusion, broken down by cohort. P values were derived from Wilcoxon rank-sum tests. CRS, cytokine release syndrome; HTBC, high-tumor burden cohort, LTBC, low-tumor burden cohort; qPCR, quantitative polymerase chain reaction.
Early Cytokine Response to CTL019 Infusion
To evaluate whether cytokine profiling in LTBC patients might identify additional patients who could benefit from PT, we compared maximum cytokine levels from days 0 to 3 after CTL019 infusion in patients who would subsequently develop grade 4 CRS versus those who would not (Data Supplement). The cytokines most strongly associated (P < .2) with grade 4 CRS in the LTBC were IL-6, vascular endothelial growth factor, IP-10, IL-8, G-CSF, monocyte chemoattractant protein-1 (MCP-1), IL-1RA, and IL-2R. In addition, the performance of two previously developed prediction models for development of grade 4 CRS14 was evaluated for LTBC patients (Data Supplement). One patient who developed grade 4 CRS on day 2 after infusion was excluded from this analysis. Model E was 98% accurate (sensitivity 100%, specificity 98%). Model C was 73% accurate. Both models correctly predicted the development of CRS in the one remaining LTBC patient who went on to develop grade 4 CRS.
Safety
Treatment-related neurological events occurred in 19 patients (27%), 9 (60%) in the HTBC and 10 (18%) in the LTBC (CTCAE grading, Data Supplement). Grade 3 and 4 events occurred in four patients (HTBC, n = 2; LTBC, n = 2) and one patient (HTBC), respectively. Grade 3-4 neurotoxicity was also retrospectively graded on the immune effector cell-associated neurotoxicity syndrome scale (Data Supplement).11 Other adverse events are detailed in the Data Supplement.
Comparison with a Historical High-Tumor Burden Cohort
Patients
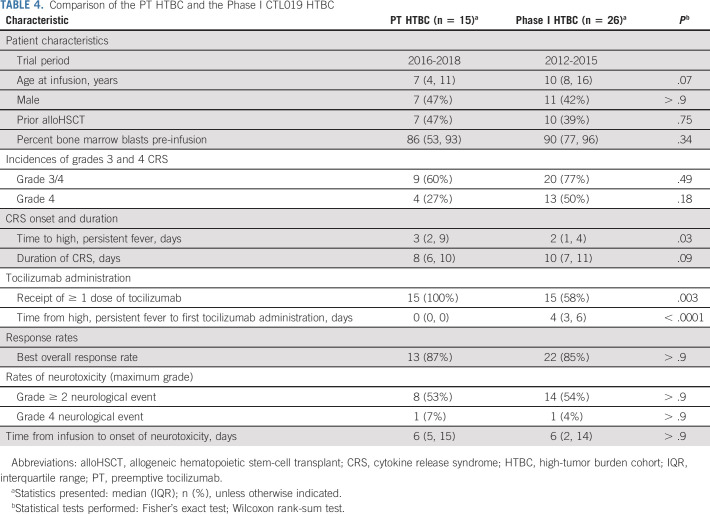
For the comparative analyses, the PT HTBC consisted of the 15 patients in the HTBC. The phase I HTBC included 26 (of 62 infused) patients from the phase I CTL019 trial who met the high-tumor burden criteria. The PT and phase I HTBCs were similar in terms of age, sex, prior alloHSCT, and bone marrow blasts pre-infusion (Table 4).
TABLE 4.
Comparison of the PT HTBC and the Phase I CTL019 HTBC
Incidence and clinical description of CRS
All patients in both cohorts developed ≥ grade 2 CRS and the duration of CRS was similar (PT, 8 days [IQR, 6 to 10]; phase I, 10 days [IQR, 7 to 11], P = .092). Grade 4 CRS was observed in 4 of 15 (27%) PT HTBC patients versus 13 of 26 (50%) phase I HTBC patients (relative risk [RR], 0.53 [95% CI, 0.21 to 1.34], P = .18) (Table 4). Multivariable log binomial regression models were developed to adjust for baseline characteristics, but none of the models significantly altered the unadjusted relative risk.
In the phase I HTBC, grade 4 CRS was associated with earlier onset of fever (P = .04). An additional analysis compared severe CRS among patients with early onset of CRS, defined as development of high, persistent fever by day 4 after infusion. In this restricted sample, grade 4 CRS was observed in 4 of 9 (44%) versus 13 of 21 (62%) patients in the PT and phase I HTBC, respectively (RR, 0.72 [95% CI, 0.32 to 1.60], P = .42).
ICU admission was required for eight (53%) PT HTBC patients and 18 (69%) phase I HTBC patients within 30 days after infusion (P = .34) (Data Supplement). Among patients admitted to the ICU, median cumulative length of stay was similar between PT (9 days, IQR, 5 to 11) and phase I HTBC (11 days, IQR, 7 to 19, P = .18). The use of specific ICU resources was also similar, with a trend toward fewer days of vasoactive medications in the PT HTBC (PT, 4 days [IQR, 3 to 5]; phase I, 6 days [IQR, 5 to 9], P = .05).
Tocilizumab and other cytokine blockade
All 15 PT HTBC patients received the PT dose, at a median of 0 days (IQR, 0 to 0) after developing high, persistent fever. Six (23%) patients received an additional dose of tocilizumab because of clinical CRS progression and one (7%) received multiple additional doses. Of the 26 phase I HTBC patients, 15 (58%) received ≥ 1 dose of tocilizumab at a median of 4 days (IQR, 3 to 6) after developing high, persistent fever. Five (33%) patients received multiple doses.
Five (33%) PT HTBC patients received corticosteroids, including four of four patients with grade 4 CRS. One patient also received siltuximab, basiliximab, and anakinra. Eight (31%) phase I HTBC patients received corticosteroids, including 8 of 13 patients with grade 4 CRS. One patient also received etanercept, and one patient received siltuximab.
Efficacy
CAR-T expansion was similar in both cohorts (P = .9, Data Supplement). In addition, the cohorts had similar ORR (PT, 87%; phase I, 85%, P > .9) and 24-month probabilities of EFS (P = .75) and OS (P = .20) were similar between the cohorts (Data Supplement).
Neurotoxicity
Neurotoxicity rates were similar between cohorts (Table 4). Eight (53%) PT HTBC patients experienced a ≥ grade 2 neurological event versus 14 (54%) phase I HTBC patients (P > .9). One patient from each cohort had grade 4 neurotoxicity.
DISCUSSION
CRS is the most common severe toxicity associated with CAR T-cells. As tisagenlecleucel (originally developed as CTL019) is now commercially available at many centers worldwide, it is crucial to develop strategies to mitigate the risk of severe CRS.
In this prospective study of children and young adults treated with CTL019 for relapsed or refractory B-ALL, we found that risk-adapted PT administration decreased the expected incidence of grade 4 CRS, meeting the predefined study end point. In a secondary comparison with a historical cohort of high-tumor burden patients who received standard CRS management, we found a clinically meaningful decrease in the incidence of grade 4 CRS from 50% to 27%. Although we were likely able to prevent some cases of severe CRS, patients who developed grade 4 CRS despite PT administration were as critically ill as patients who did not receive PT. Importantly, PT did not impact the excellent ORR of CTL019, nor did it affect CTL019 expansion, persistence, or long-term efficacy. This intervention was safe with no increase in frequency or severity of neurotoxicity.
Two groups have reported their experiences with earlier tocilizumab administration. Seattle Children's published results of an intervention with tocilizumab and/or dexamethasone during periods of mild CRS in a subset of pediatric patients with B-ALL enrolled on their PLAT-02 trial of SCRI-CAR19v1, an anti-CD19 CAR.16 The 20 patients who received the intervention had a 15% incidence of severe CRS compared with a 30% incidence among an earlier cohort who did not receive the intervention (P = .29). Similar to our study results, the intervention did not negatively impact antitumor efficacy or safety. However, in contrast to our study, their definition of severe CRS was not based on a specific CRS grading system and the CAR T-cell dose, a known predictor of CRS in SCRI-CAR19v1, was lower in the intervention group.5 The ZUMA-1 axicabtagene ciloleucel trial also reported preliminary data from a prophylactic tocilizumab intervention given to 31 patients in a safety expansion cohort.17 They found that the frequency of grade ≥ 3 CRS may be lower with prophylactic tocilizumab, but grade ≥ 3 neurotoxicity rates may be greater. Based on these findings, the intervention was discontinued. Our study, however, had key differences including the intervention timing (preemptive v prophylactic tocilizumab), patient population (pediatric v adult), disease (B-ALL v lymphoma), and CAR T-cell product, with axicabtagene ciloleucel having higher neurotoxicity risks.
EFS and OS in the PT HTBC were similar to the phase I HTBC, suggesting that PT did not negatively impact survival outcomes. However, we did see lower long-term DOR, EFS, and OS in the HTBC compared with the LTBC. These inferior outcomes in the HTBC occurred despite an excellent ORR (87%), greater CTL019 expansion, and similar persistence to the LTBC. It is also important to note that LTBC patients, including those who were MRD-negative at infusion, had excellent DOR and CAR-T persistence using CTL019. This is the first report from our group of the association of high tumor burden with worse long-term survival; however, several trials of other CD19 CARs have also found similar results.21-23 The etiology of this association is unclear, but we did find a trend toward a greater proportion of CD19-negative relapses in the HTBC. It is possible that higher tumor burden is associated with larger clonal heterogeneity, which then increases the risk of antigen escape.24 In addition, the HTBC consisted of patients with extremely refractory disease. Thus, we do not conclude that further efforts to decrease disease burden in highly refractory patients prior to infusion is warranted, either to decrease CRS risk or to potentially improve DOR. Rather, our results highlight the need to better understand mechanisms of relapse in this population, mitigate toxicity through pre-emption, and develop strategies to prevent CD19-negative relapses.
We confirmed that high tumor burden remains a strong risk factor for the development of severe CRS and that severe CRS is uncommon in patients with low tumor burden. Only 2 of 55 (4%) of LTBC patients developed grade 4 CRS, reinforcing that a risk-adapted approach to PT administration is appropriate. We also used previously developed cytokine prediction models to test whether cytokine levels could identify the few LTBC patients at risk for severe CRS, but the analysis was limited by the small number of events.14 In addition, the clinical utility of measuring cytokines depends on the availability of clinically validated cytokine testing with rapid turn-around times.
The current study has some limitations that should be considered in interpreting the results. Although we met the predefined primary study end point for grade 4 CRS, it was infeasible to add a contemporaneous control group. Given changes in referral patterns, the number of patients with ≥ 40% tumor burden has decreased over time.25 Instead, we performed a comparison with a well-matched historical cohort, but these analyses had limitations inherent to using historical controls and were not powered to demonstrate statistically significant differences.26 In addition, although our results are informative to the many patients being treated with CTL019 and tisagenlecleucel, they should not be extrapolated to other CAR T-cell products without further study.
In conclusion, we found that preemptive administration of tocilizumab to patients with high tumor burden decreased the expected incidence of severe CRS, meeting the primary end point, without compromising the antitumor efficacy or safety of CTL019. In the time since this clinical trial was initiated, CTL019 received FDA approval and is now available at a large number of centers. Thus, these results are applicable to broad groups of patients at risk for severe CRS, the critical tisagenlecleucel toxicity. Further research is needed to confirm our findings across centers and in other patient populations. In addition, as new CAR constructs are developed, it will be important to determine optimal tocilizumab timing for each product.
SUPPORT
Supported by NCI grant 5P01CA214278, the Frontier Program at Children's Hospital of Philadelphia, the St. Baldrick's-Stand Up To Cancer Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT-27-17), The V Foundation, the Gerdin Fund, and investigator-initiated funding from Novartis Pharmaceuticals Corporation.
CLINICAL TRIAL INFORMATION
S.K. and R.M.M. contributed equally to the manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Yimei Li, David M. Barrett, Simon F. Lacey, Shannon L. Maude, Regina McGuire, Amanda M. DiNofia, Stephan A. Grupp
Financial support: David T. Teachey, Stephan A. Grupp
Administrative support: Stephan A. Grupp
Collection and assembly of data: Stephan Kadauke, Regina M. Myers, Richard Aplenc, Diane Baniewicz, Colleen Callahan, Joseph G. Dolan, Regina McGuire, Simon F. Lacey, Shannon L. Maude, Laura S. Motley, Gerald B. Wertheim, Lisa Wray, Amanda M. DiNofia, Stephan A. Grupp
Data analysis and interpretation: Stephan Kadauke, Regina M. Myers, Yimei Li, Richard Aplenc, David M. Barrett, Allison Barz Leahy, Joseph G. Dolan, Julie C. Fitzgerald, Whitney Gladney, Simon F. Lacey, Hongyan Liu, Shannon L. Maude, David T. Teachey, Amanda M. DiNofia, Stephan A. Grupp
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Risk-Adapted Preemptive Tocilizumab to Prevent Severe Cytokine Release Syndrome After CTL019 for Pediatric B-Cell Acute Lymphoblastic Leukemia: A Prospective Clinical Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stephan Kadauke
Employment: Gilead Sciences, Immunomedics, (I)
Honoraria: MEI Pharma, Deciphera, Novo Nordisk, Exelixis, (I)
Research Funding: Miltenyi Biotec
Travel, Accommodations, Expenses: Novartis
(Optional) Open Payments Link: https://openpaymentsdata.cms.gov/physician/397333
Richard Aplenc
Honoraria: Sigma-Tau
Travel, Accommodations, Expenses: Sigma-Tau
David M. Barrett
Travel, Accommodations, Expenses: Therakos
Colleen Callahan
Consulting or Advisory Role: Novartis
Speakers' Bureau: PeerView Institute
Simon F. Lacey
Consulting or Advisory Role: Gilead Sciences
Research Funding: Tmunity Therapeutics, Inc, Cabaletta, Novartis Institutes for BioMedical Research
Patents, Royalties, Other Intellectual Property: Patents and IP related to CTL019 (Kymriah) assigned by the University of Pennsylvania to Novartis
Shannon L. Maude
Consulting or Advisory Role: Novartis, Kite Pharma, Wugen
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis, Kite Pharma, Wugen
Laura S. Motley
Stock and Other Ownership Interests: Novartis, Thermo Fisher Scientific, Bristol-Myers Squibb
David T. Teachey
Consulting or Advisory Role: Janssen, La Roche, Sobi
Research Funding: Novartis
Gerald B. Wertheim
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Stephan A. Grupp
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Janssen, CBMG, TCR2 Therapeutics, Humanigen, Roche, Vertex, Adaptimmune, Allogene
Research Funding: Novartis, Kite/Gilead, SERVIER, Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: UPenn Toxicity management patent
No other potential conflicts of interest were reported.
REFERENCES
- 1.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia N. Engl. J. Med 3711507–15172014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia N Engl J Med 378439–4482018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor t-cell therapy for adults with acute lymphoblastic leukemia J Clin Oncol 38415–4222020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients J Clin Invest 1262123–21382016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults Blood 1293322–33312017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial Lancet 385517–5282015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome.
- 8.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma N Engl J Med 38045–562019 [DOI] [PubMed] [Google Scholar]
- 9.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia N Engl J Med 3681509–15182013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome Blood 124188–1952014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells Biol Blood Marrow Transplant 25625–6382019 [DOI] [PubMed] [Google Scholar]
- 12.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol. 2016;34(15_suppl):3011-3011. [Google Scholar]
- 14.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia Cancer Discov 6664–6792016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell‐induced severe or life‐threatening cytokine release syndrome Oncologist 23943–9472018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy Blood 1342149–21582019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-hodgkin lymphoma (NHL) Blood. 2017;130(suppl 1):1547. [Google Scholar]
- 18.Wood BL.Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry Cytometry B Clin Cytom 9047–532016 [DOI] [PubMed] [Google Scholar]
- 19. Reproducibility supplement: preemptive tocilizumab to mitigate CRS after CTL019 ( NCT02906371). https://skadauke.github.io/early-toci.
- 20.Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11:35. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL Blood 1342361–23682019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Liu J, Xu M, et al. Treatment response, survival, safety, and predictive factors to chimeric antigen receptor T cell therapy in Chinese relapsed or refractory B cell acute lymphoblast leukemia patients Cell Death Dis 111–132020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia N Engl J Med 378449–4592018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruella M, Maus MV.Catch me if you can: Leukemia escape after CD19-directed T cell immunotherapies Comput Struct Biotechnol J 14357–3622016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers RM, Fitzgerald JC, DiNofia AM, et al. Trends in inpatient and intensive care resource utilization after chimeric antigen receptor T cell therapy for pediatric acute lymphoblastic leukemia from 2012-2019. Blood. 2019;134(suppl_1):61. [Google Scholar]
- 26.Diehl LF, Perry DJ.A comparison of randomized concurrent control groups with matched historical control groups: Are historical controls valid? J Clin Oncol 41114–11201986 [DOI] [PubMed] [Google Scholar]