INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is the only curative therapy for many patients with high-risk acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). While the risk of transplant-related mortality has decreased over the past 40 years, disease relapse remains one of the most significant adverse events following HCT, with up to 50% of patients with AML relapsing after HCT.1 Outcomes for patients who relapse after HCT are poor.1-3 The therapeutic benefit of HCT is believed to reside in part through an immune-mediated graft versus malignancy (GVM),4 and over the years, many attempts to improve outcomes have focused on investigating and exploiting this phenomenon. In this review, we will first review the biology of relapse after HCT, with a focus on recent discoveries. In the second part, we will evaluate strategies to prevent and treat relapsed disease post-HCT, highlighting novel molecularly targeted and immunologic treatments.
CONTEXT
Key Objective
Acute myeloid leukemia (AML) relapse after allogeneic stem-cell transplantation represents the most common form of treatment failure. This review summarizes recent findings about the mechanisms of AML relapse after transplantation, as well as therapies to prevent or treat post-transplant relapse.
Knowledge Generated
AML relapse after transplantation is associated with several factors, including active disease before transplantation, conditioning intensity, loss of antigen presentation by relapsing AML cells, and immune cell exhaustion. Studies of post-transplantation maintenance, either with hypomethylating agents or tyrosine kinase inhibitors, have shown some encouraging results. However, standard strategies to treat overt relapse with chemotherapy or donor lymphocyte infusion rarely result in long-term disease control.
Relevance
New therapies to prevent or treat AML relapse after transplantation are urgently needed. Strategies to reverse or circumvent immune escape by AML cells—for example, by using bispecific T cell–engaging molecules—may prove effective in this regard.
THE BIOLOGY OF RELAPSE AFTER ALLOGENEIC HCT
Leukemia Burden Pre-HCT
It has been long appreciated that the presence of active AML at the time of transplantation is associated with a higher risk of relapse. More recently, it has been reported that even low levels of minimal residual disease (MRD) at the time of transplantation lead to worse outcomes.5 Whether additional chemotherapy to eradicate MRD before HCT mitigates this risk has never been directly tested, but several lines of evidence suggest that aggressive chemotherapy before HCT may be of benefit. First, in CTN 0901, patients receiving myeloablative conditioning regimens had a lower relapse rate than patients who received reduced-intensity conditioning.6 Second, patients with high-risk or secondary AML who received induction and consolidation with (CPX-351) not only had better postinduction disease control than patients who received 7 + 3, but this translated to a lower post-HCT relapse rate as well.7 It is likely, therefore, that deeper remissions before HCT will lead to lower relapse risk as has been seen, for example, in multiple myeloma.
Given these findings, accurate MRD monitoring before and after HCT is crucial. Molecular MRD monitoring for mutated genes, fusion genes, and/or overexpressed genes can be performed via multicolor flow cytometry, quantitative reverse transcriptase polymerase chain reaction (PCR), digital droplet PCR, or next-generation sequencing (NGS).8 Given its improved sensitivity, reduced cost and turnaround time, ability to track multiple molecular markers and clonal hierarchy at once and guide molecularly directed therapies (ie, FLT3 inhibitor or isocitrate dehydrogenase [IDH] inhibitors), and utility in predicting clinical outcome in HCT, error-corrected NGS is becoming feasible for routine clinical MRD estimations before and after HCT.9
Disease Characteristics
Several cytogenetic abnormalities and gene mutations are predictive of relapse in patients with AML and MDS undergoing HCT. Poor-risk cytogenetics associated with a significantly increased incidence of AML relapse after HCT includes patients with monosomal or complex karyotypes as well as inv(3)(q21q26)/t(3;3)(q21;q26), del(5q), t(10;11)(p11-14;q13-23), t(6;11)(q27;q23), and abnormalities in chromosome 17p.10,11 In MDS, abnormalities of chromosome 3 [ie, inv(3), t(3q)] or chromosome 7 [-7, del(7q)], either alone or as part of a complex karyotype, result in inferior leukemia-free survival after HCT.12 At the gene level, AML patients with TP53 and FLT3-ITD mutations exhibit inferior outcomes with a higher risk of relapse.10,13-15 Likewise, mutations in TP53 as well as TET2, ASXL1, RUNX1, and RAS are independently associated with an increased risk of relapse after HCT for MDS.16-19
The biological mechanisms mediating the increased risk of relapse after HCT in patients with AML and MDS with the cytogenetic and molecular disease states discussed above remain poorly characterized. In addition to its role as a tumor suppressor, TP53 regulates several innate and adaptive immune responses including antigen processing and presentation, cytokine production, type 1 interferon signaling, and expression of immune inhibitory receptors.20 Recently, the examination of bone marrow samples obtained from patients with MDS or AML with TP53 mutations demonstrated higher CD8+ T cell, ICOShigh/PD-1neg regulatory T cell (Treg), and natural killer (NK) cell infiltration, with increased expression of immune checkpoints (PD-L1, TIGIT, and LAG3) and interferon-gamma (IFNg) signaling compared with other AML subtypes.21-24
Downregulation of Tumor Cell HLA Expression
First reported in 2009, loss of mismatched HLA loci in AML cells is associated with relapse in haploidentical HCT (haplo-HCT) through impairment of donor T cell recognition.25 This loss, observed in roughly one third of relapses after haplo-HCT, is copy number neutral, representing a form of acquired somatic uniparental disomy (ie, loss of a chromosomal region followed by replacement with its homologous copy). Overall, the expression level of HLA molecules is unchanged, including major histocompatibility complex (MHC) class I, which may limit the activation of antitumor NK cells.26 Loss of mismatched HLA is observed in about one third of haploidentical transplants and more frequently occurs in patients with active disease at the time of transplant, increased donor T cell dose, and longer time to relapse, and in patients with chronic graft-versus-host disease (cGVHD), all factors that may lead to increased GVM and therefore selective pressure against mismatched HLA genes.27-29 Genetic loss of mismatched HLA alleles has also been observed (albeit less frequently) in mismatched unrelated donor HCT,30-32 matched unrelated donor HCT,31-33 and mismatched related donor HCT.34
In addition to loss of HLA genes, a wide variety of genetic changes occur in AML cells from patients who relapse after HCT. It has long been observed that chromosomal and other structural abnormalities disappear and emerge at relapse after HCT, reflecting evolution of the malignant clone.32,35-39 More recently, panel sequencing of myeloid malignancy–associated genes has also demonstrated gain and loss of driver mutations in MDS and AML cells after HCT.14,40-44 These changes resemble the spectrum of mutations seen after chemotherapy and, unlike the loss of HLA genes described above, are not specific for post-HCT relapse.40,45-49 Somewhat surprisingly, given the role of alloimmunity in preventing relapse, mutations in genes that govern the immune response are not commonly seen in AML relapse after HCT.31,40
At the level of gene expression, however, two groups have recently reported significant dysregulation of immune genes in AML cells at relapse after HCT.40,50 These studies compared matched diagnosis and post-HCT relapse patient samples and found downregulated surface and RNA expression of MHC class II molecules and associated genes in 30%-50% of samples.40,50 A higher dose of infused donor T cells correlated with a higher likelihood of HLA class II downregulation, and in both studies AML blasts with a low MHC class II expression failed to stimulate HLA-mismatched T cells in vitro, suggesting that downregulation of MHC class II genes contributes to the ability of AML cells to evade immune effectors in relapse after HCT. As observed in previous models of HCT, IFNg restored HLA class II expression, raising the possibility that IFNg treatment could resensitize AML cells to donor immune cell killing.40,50,51 These results are consistent with those of smaller previous reports of MHC loss at relapse.34,52
Inhibitory Immune Checkpoint Molecule Modulation
In addition to alterations in HLA expression on tumor cells, modulation of immune checkpoint molecules on both tumor cells and T cells has been described as another important mechanism of relapse post-HCT. Several groups have reported upregulation of immune-inhibitory genes such as PD-L1 (CD274), B7-H3 (CD276), and PVRL2 (CD155) on AML blasts relapsing after HCT compared with diagnosis.50,53 Expression of immune-inhibitory genes on AML cells is associated with the upregulation of exhaustion markers on T cells after HCT, including PD-1, TIM-3, TIGIT, and KLRG-1.50,53-57 Noviello et al58 found increased evidence of T cell exhaustion in memory stem and central memory T cells from the bone marrow of patients who relapsed compared with controls, and these exhausted bone marrow–infiltrating T cells at relapse expressed a restricted T cell receptor (TCR) repertoire and impaired effector functions. The upregulation of inhibitory immune checkpoint markers has led to several therapeutic trials of immune checkpoint blockade to treat AML relapse after HCT, as described below.
PREVENTION AND TREATMENT OF RELAPSE POST-HCT
Established Strategies for Enhancing Donor Immunity Post-HCT
One of the simplest methods of enhancing donor immunity and treating relapse after HCT is early withdrawal of immunosuppression, which can restore donor hematopoiesis and transient remissions in some patients with frank relapse after HCT.59-61 This strategy may be most effective in the setting of incomplete donor chimerism61 or as a strategy to prevent relapse after HCT for high-risk disease.62,63 Conversely, the use of in vivo T cell depletion with antithymocyte globulin or alemtuzumab for graft-versus-host disease (GVHD) prophylaxis is associated with increased relapse risk in some studies, although not all.64-66 Similarly, administration of fresh immune effector cells as a donor lymphocyte infusion (DLI) can be given to prevent relapse in high-risk patients (prophylactic or preventive DLI) or to treat overt hematologic relapse. In the prophylactic/preventative setting, DLI is associated with overall survival (OS) rates of 40%-70% with GVHD rates of 15%-60%, which compare favorably to expected outcomes in transplant of high-risk AML.67-69 For patients with evidence of MRD after transplant, DLI can reduce the incidence of relapse, with OS rates of approximately 60% and grade II-IV acute GVHD (aGVHD) rates of 21%-28%.70,71 In contrast, when used to treat overt relapse after HCT, DLI (usually administered with chemotherapy) is associated with worse outcomes, with OS rates at 2 years of approximately 20% and grade II-IV aGVHD rates of 22%-43% in large, registry-based retrospective analyses.72-74 In these studies, response to DLI with or without chemotherapy was best in patients with late relapses, suggesting that patients with longer remissions may be more likely to benefit from graft versus leukemia (GVL). Conversely, patients with relapse < 6 months after HCT have particularly poor outcomes, with long-term OS of 5%-10%. Taken together, these observations suggest that DLI may be most effective when tumor burden is low and that in the setting of overt relapse DLI is of limited benefit.
Second HCT represents another option for some patients with MDS/AML relapse after an initial HCT. Registry studies suggest OS rates of 17%-49% and grade II-IV aGVHD rates of 26%-53% with this approach.72,75,76 Use of myeloablative conditioning regimens is associated with high rates of nonrelapse mortality (NRM), ranging from 30% to 50%.76-78 Although the use of a different donor may enhance the GVM effect by providing immune effector cells with more favorable alloreactivity, several retrospective studies have reported similar outcomes between a second HCT from the same and a different donor.75,79,80 A 2018 European Society of Blood and Marrow Transplantation registry study comparing second HCT versus DLI for relapsed AML showed similar OS rates (15% for DLI v 19% for HCT). Although NRM (27% v 10%) and grade II-IV aGVHD rates (37% v 20%) were higher for the group receiving HCT, these differences were not seen when the analysis was restricted to patients receiving therapy in CR.75 In summary, while a subset of patients have some benefit from DLI or second transplant, new treatment approaches are urgently needed for AML relapse after HCT.
Checkpoint Inhibitors
Given the efficacy of checkpoint inhibitors in solid tumor oncology and the evidence of T cell exhaustion in AML relapse after HCT, there has been considerable interest in the use of checkpoint blockade in the post-HCT setting. Early phase trials showed promising results with ipilimumab in patients with both lymphoid and myeloid malignancies relapsing after HCT.81-83 In contrast, early trials of the PD-1 blocker nivolumab suggested a significantly increased risk of toxicities including severe GVHD in HCT patients.84-87 Further study will be required to determine if the choice of checkpoint blockade agent or use of lower doses might reduce the toxicity seen so far with these drugs, but at this time concerns for toxicity have limited their widespread use after HCT.88
Hypomethylating Agents
Based on preclinical studies suggesting their ability to mitigate GVHD and enhance the GVM effect, hypomethylating agents (HMAs) have been studied intensely in the post-HCT setting over the past 10-15 years.89-92 Several small phase I/II studies have shown that HMAs may prevent relapse in patients with high-risk or MRD-positive MDS and AML post-HCT, with relapse rates ranging from 17% to 65%, relapse-free survival (RFS) rates of 46%-63%, and OS rates of 49%-77%.90,93-97 Neutropenia and thrombocytopenia are common significant toxicities of HMA maintenance. While promising, these results await confirmation in larger, randomized trials. A number of trials have also evaluated the use of azacitidine in overtly relapsed disease.99-103 Complete response (CR) rates in these studies range from 13% to 23% with a few long-term responses, although many of the patients received concurrent DLI. Thus, it appears that HMA treatment has a limited ability to enhance antitumor immune cell activity in patients with active disease after HCT and may be considered as maintenance therapy after HCT.
Histone Deacetylase Inhibitors
Histone deacetylase (HDAC) may promote GVM activity while reducing GVHD after HCT in the following ways: (1) increasing the expression of tumor antigens, MHC class I and II, and costimulatory molecules on malignant cells; (2) reducing the secretion of proinflammatory cytokines; (3) promoting the recovery of the intestinal barrier function after conditioning; and (4) expanding CD4+ Tregs.104 HDAC inhibitor monotherapy and combination therapy have shown limited efficacy against R/R AML.105-107 However, maintenance therapy with the pan-HDAC inhibitor panobinostat alone or in combination with a DLI after HCT for AML or MDS yielded an encouraging 2-year OS and RFS of 81% and 75%, respectively.108 A randomized phase III trial to assess the efficacy of panobinostat maintenance therapy after HCT is ongoing (ClinicalTrials.gov identifier: NCT04326764).
Lenalidomide
Trials evaluating maintenance therapy with lenalidomide early (within 3 months) after T cell–replete HCT were unsuccessful due to the high rate of aGVHD.109,110 More recently, combination therapy with azacitidine and lenalidomide in patients with AML or MDS who had relapsed after HCT yielded a CR rate of 40% (6/15; CR, n = 3; complete remission with incomplete hematologic recovery [CRi], n = 3), which was better than that in historical controls receiving only azacitidine.111 Since only three patients developed aGVHD (grade II, n = 2; grade III, n = 1) upon receipt of the combination regimen, it is possible that azacitidine mitigates the lenalidomide-mediated exacerbation of GVHD.
Tyrosine Kinase Inhibitors
A number of studies have suggested that tyrosine kinase inhibitors have activity in preventing or treating relapse after HCT in AML patients with activating FLT3 mutations. Recently, results from randomized trials comparing sorafenib maintenance with placebo after HCT were reported. In the phase II Sormain trial, patients treated with sorafenib had significantly better RFS (85%) compared with patients who received placebo (53.3%).112 Likewise, a significantly increased RFS was observed in the phase III trial (56.6% RFS for nonmaintenance v 78.9% RFS with sorafenib at 24 months post-HCT).113 Currently, the second-generation FLT3 inhibitor gilteritinib, which has shown single-agent activity in nontransplant relapsed refractory (R/R) AML, is also being studied as maintenance in FLT3-mutated AML.114 Finally, for patients with overt relapse after HCT, sorafenib monotherapy has shown modest activity in several smaller studies and case reports, with CR rates of 23%-38%, although these responses are generally short-lived.115,116 Similar responses were reported for AML patients who relapsed after HCT and were treated with gilteritinib or quizartinib.117,118
The first-generation FLT3 inhibitors such as sorafenib are relatively nonspecific for FLT3 and target other molecules and pathways such as c-Kit, platelet-derived growth factor receptor, vascular endothelial growth factor receptor, Janus kinase 2, and RAS/RAF/MEK pathway to induce direct killing of malignant cells.119 FLT3 inhibitors can also promote the GVM effect after HCT by inducing interleukin-15 (IL-15) production from FLT3-ITD+, but not non-ITD, AML cells via inhibition of the ATF4 transcription factor.120 This IL-15 production promoted the GVM activity of donor CD8+ T cells in both mice and humans.120 Furthermore, FLT3 inhibitors can modulate immunological responses by decreasing the number of immunosuppressive CD4+Foxp3+ Tregs and myeloid-derived suppressor cells (MDSCs) and inhibiting dendritic cell (DC) proliferation, maturation, and function.121-123 Therefore, sorafenib may enhance GVM activity without increasing GVHD lethality because of its ability to reduce the numbers of immunosuppressive cell subsets (Tregs and MDSCs) and DCs while concomitantly enhancing the efficacy of leukemia-reactive CD8+ T cells via the release of IL-15.
IDH Inhibitors
Ivosidenib and enasidenib, small-molecule inhibitors of IDH 1 and 2, respectively, have been approved by the FDA for use as single agents in patients with R/R AML. Although limited information is available about the response rate in the 43 transplanted R/R AML patients treated with ivosidenib, 10 of 29 (34%) patients who relapsed with AML after HCT achieved a CR upon treatment with enasidenib.124 Both ivosidenib and enasidenib are currently being tested as maintenance therapy for IDH1/2-mutant myeloid malignancies following HCT (ClinicalTrials.gov identifier: NCT03515512 and NCT03564821).
Venetoclax
Byrne et al125 recently reported the outcomes for 21 patients with myeloid diseases who relapsed after HCT and were treated with venetoclax salvage chemotherapy in combination with a HMA (n = 16) or low-dose cytarabine (LDAC) (n = 5). An overall CR/CRi rate of 42.1% (n = 8/19) was reported for 19 evaluable patients with five responses in the HMA cohort and three in the LDAC group. Two studies testing venetoclax in combination with azacitidine as maintenance therapy after HCT in patients with AML are ongoing (ClinicalTrials.gov identifier: NCT04161885 and NCT04128501).
Tumor Vaccines
The period immediately after HCT may provide the best window for tumor vaccination because of the low tumor burden and the presence of fresh immune effector cells.126 A pilot study evaluating the delivery of a Wilms' Tumor-1 (WT1) peptide-loaded donor-derived dendritic cell vaccine given concurrently with DLI to patients relapsing after HCT was safe and feasible with the evidence of WT-1–specific T cell responses.127 More recently, Lichtenegger et al128 reported antigen-specific responses and prolonged RFS compared with a historical control cohort (1,084 v 396 days) following vaccination of 10 patients with high-risk AML post-HCT with TLR7/8-matured DCs transfected with RNA encoding two AML-associated antigens, WT1 and PRAME, as well as CMVpp65. Although a few cases have been reported of overt AML relapse treated successfully with tumor vaccines,129 it is likely that vaccination will prove more effective at preventing relapse than treating overtly relapsed AML.
Cellular Therapies Post-HCT
Many groups have attempted to improve outcomes after HCT by improving the alloimmune effect of donor cells toward residual recipient AML cells. One early approach was treatment with immune-stimulating cytokines such as interferon alpha, which has been reported to stimulate NK and T cell effector functions in preclinical studies.130,131 While these treatments were well-tolerated, results were mixed and likely hampered by the short in vivo half-life of these agents in their unmodified form, and current efforts have focused on modified long-acting cytokine agonists such as ALT-803, described below.
A second approach to improving the GvL effect to prevent or treat relapse is through graft engineering, the transfer of specific immune cell subsets. Nikiforow et al132 reported a 43% CR rate (9/21; n = 11 with AML) with a 33% incidence of clinically significant GVHD upon infusion of donor lymphocytes depleted of CD25+ T regulatory cells. Since memory T cells cause significantly less GVHD than naive T cells, a phase I clinical trial of 15 patients evaluated the infusion of donor CD8+ memory T cells in patients relapsing after HCT (8 of 15 having AML).133 Seven of the 15 patients achieved a CR, and only one patient developed grade II GVHD after the infusion.
In contrast to allogeneic T cells, NK cells do not cause GVHD and they have been shown to have anti-AML activity in the nontransplant setting where concurrent cytokine treatment is used to improve their duration and activity.134-136 NK cell infusions administered in the peritransplant period have been shown to be safe and well-tolerated in early-phase clinical trials.137-139 Additionally, Choi et al137 noted that donor-derived NK cell infusion after reduced-intensity conditioning haplo-HCT was associated with reduced relapse and no increase in GVHD rates when compared with historical controls. Since a limitation of NK cells is their relatively low frequency in grafts and their short in vivo persistence, Ciurea et al140 used feeder cells expressing membrane-bound IL-21 to expand NK cells ex vivo and reported a low incidence of relapse with limited GVHD upon infusion into patients before and after haplo-HCT. In a similar approach, cytokine-induced memory-like NK cells from haploidentical donors can be generated by ex vivo culture with IL-12, IL-15, and IL-18 and exhibit enhanced antitumor activity.141 Clinical trials using these cells as a prophylactic DLI and for treatment of frank relapse are ongoing (ClinicalTrials.gov identifier: NCT02782546 and NCT03068819). To expand NK cells in vivo, Romee et al142 administered ALT-803, an IL-15 superagonist, in a phase I study of 33 patients relapsing > 60 days post-HCT and reported the activation and proliferation of CD8+ T and NK cells with an overall response rate of 19%.
Direct engineering of T cells to target AML cells by transduction with T cell receptors previously identified to recognize leukemia-associated antigens (TCR transgenic T cells) or with chimeric antigen receptors (CAR-T cells) may provide robust AML cell killing after HCT. With a median follow-up of 44 months, Chapuis et al143 recently reported no relapse in 12 patients with high-risk, heavily pretreated AML treated with WT1-specific TCR transgenic CD8+ T cells after transplantation. Currently, no trials of CAR-T cells for AML in the post-HCT relapse setting have been reported, but the reports of anti-CD19 CAR-T cells used after HCT suggest that this approach may be effective and well-tolerated with a low incidence of GVHD.144,145
Bispecific Antibodies
Bispecific antibodies work by engaging tumor cells with immune effector cells and directly activating immune cells independently of MHC/TCR interactions. In acute lymphoblastic leukemia (ALL), treatment with the bispecific CD19xCD3 agent blinatumomab has been shown to be safe and effective, including after HCT.146 In AML, several early-phase clinical studies are testing bispecific antibodies targeting CD33, CD123, and CLL-1 in patients with R/R disease, including patients who relapsed after HCT.147 A potential advantage of bispecific antibodies in the post-HCT setting is that since they do not rely on MHC/TCR interactions, they would be predicted to work in cases where HLA expression has been lost after relapse post-HCT. Indeed, Rovatti et al148 showed in a preclinical model that an anti-CD33/CD3 bispecific antibody was able to restore T cell activation by AML cells that had lost their mismatched HLA haplotype after haploidentical transplantation. In addition, since T cell engagement leads to IFNg release within the tumor microenvironment, it is possible that the IFNg-induced restoration of MHC class II expression on AML cells could contribute to bystander immune cell killing. We have generated data using both an anti-CD3/CD123 bispecific molecule (flotetuzumab, MGD006) and anti-CD123 CAR-T cells that suggest that targeting AML cells in this fashion leads to the upregulation of MHC class II expression on surrounding AML blasts (unpublished data, see Fig 1). This reinduction of HLA class II expression can potentially restore the GVM effect and effectively treat a subset of relapsed AML post-HCT.
FIG 1.
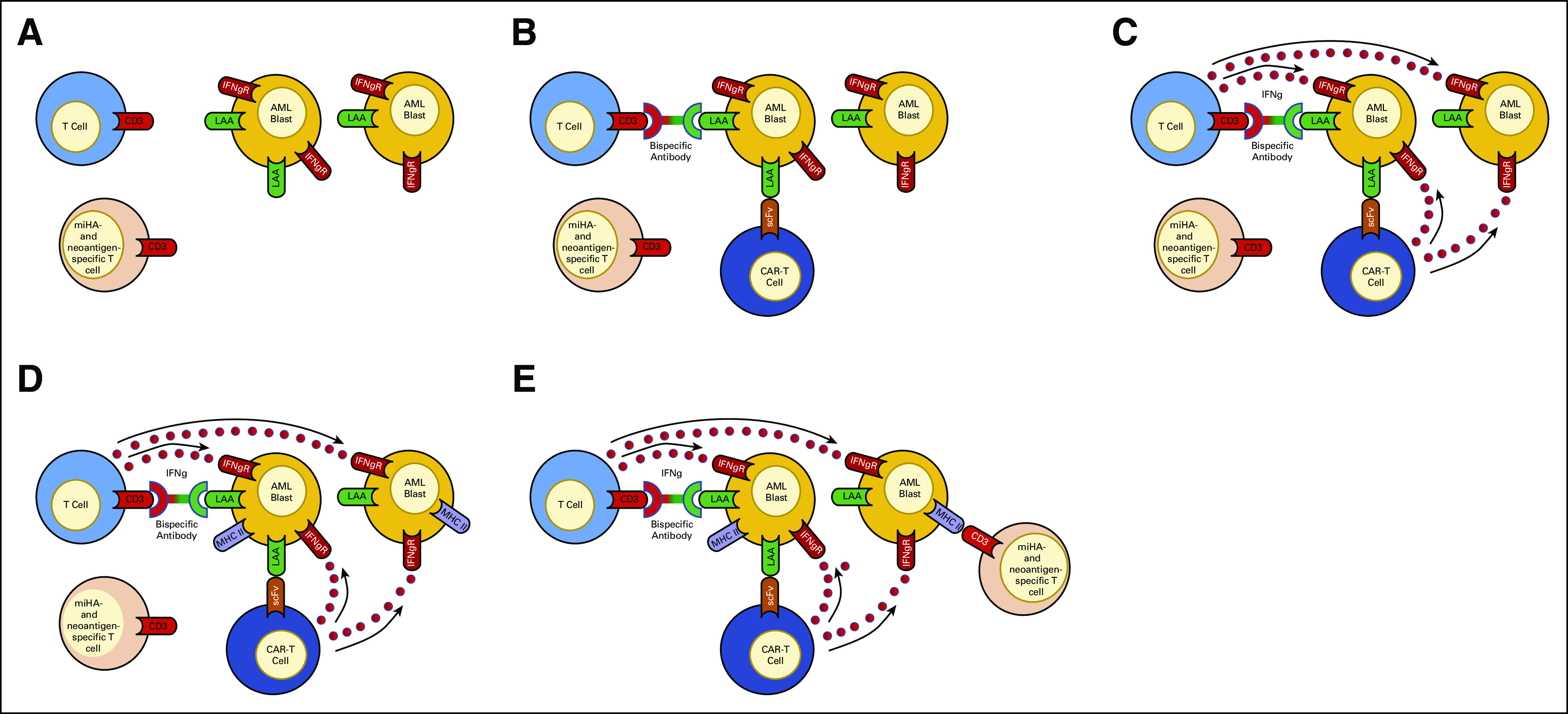
T cell immunotherapy can upregulate MHC class II expression on AML blasts. (A) In 30%-50% of AML patients relapsing after allogeneic hematopoietic cell transplantation, AML blasts have a decrease or loss of surface MHC class II expression, impairing the ability of miHA- and neoantigen-specific T cells to recognize AML blasts and exert a GVM effect.27,38 (B) Introduction of a bispecific antibody or genetically engineered T cell (ie, CAR T cell) capable of recognizing LAAs on AML blasts will lead to non-MHC–restricted T cell recognition of the AML blasts. (C) Non-MHC–restricted T cell recognition of AML blasts will activate the T cells and stimulate the release of IFNg. (D) The release of IFNg will upregulate MHC class II surface expression on surrounding AML blasts, including both blasts that are recognized and unrecognized by the T cell immunotherapy. (E) Upregulation of MHC class II expression on AML blasts will allow for miHA- and neoantigen-specific T cells to recognize and kill the AML blasts, leading to a GVM effect. AML, acute myelogenous leukemia; CAR-T cell, chimeric antigen receptor T cell; GVM, graft-versus-malignancy; IFNg, interferon gamma; IFNgR, interferon gamma receptor; LAA, leukemia-associated antigen; MHC II, major histocompatibility antigen class II molecule; miHA, minor histocompatibility antigen; scFv, single-chain variable fragment.
Gemtuzumab Ozogamicin
Several case reports and small trials testing gemtuzumab ozogamicin (GO) monotherapy and combination therapy in maintenance or salvage settings suggest that the drug exhibits clinical activity after HCT, but some concerns of myelosuppression and hepatic veno-occlusive disease were noted.149 Recently, Genthon et al150 reported an overall response rate of 72% (13/18) with a 1-year OS of 54% in patients with AML relapsing after HCT who were treated with GO in combination with intensive chemotherapy. However, all patients experienced grade III-IV neutropenia and thrombocytopenia. Ongoing clinical trials are testing fractionated dosing of GO alone to treat measurable residual disease (ClinicalTrials.gov identifier: NCT03737955) or GO in combination with CPX-351 chemotherapy to treat relapse (ClinicalTrials.gov identifier: NCT03904251).
DISCUSSION
Relapse after HCT portends a very poor prognosis, and current management approaches such as DLI and second HCT have a modest response rate and significant risk of toxicity. Recent research has identified genetic and epigenetic changes that have resulted in the downregulation of HLA molecules and upregulation of inhibitory checkpoint molecules. These changes suggest a model wherein loss of immune effector cell function contributes to relapse and suggests possible approaches for preventing or treating relapsed malignancies that exploit the GvL effect after HCT.
SUPPORT
Supported by grants from the NIH/NCI: K08 Program grant (CA222630-01, to M.J.C.), NCI Research Specialist Awards (R50 CA211466, to M.P.R.), a Genomics of Acute Myeloid Leukemia Program Project grant (P01 CA101937, to J.F.D.), an NCI Outstanding Investigator Award (R35 CA197561, to J.F.D.), and a Specialized Program of Research Excellence in Acute Myeloid Leukemia grant (P50 CA171963, to J.F.D.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: John F. DiPersio
Administrative support: John F. DiPersio
Provision of study materials or patients: John F. DiPersio
Collection and assembly of data: Joseph C. Rimando, John F. DiPersio, Matthew J. Christopher, Michael P. Rettig
Data analysis and interpretation: Joseph C. Rimando, Michael P. Rettig, John F. DiPersio, Matthew J. Christopher
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Biology of Disease Relapse in Myeloid Disease: Implication for Strategies to Prevent and Treat Disease Relapse After Stem-Cell Transplantation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I =Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Joseph C. Rimando
Stock and Other Ownership Interests: Merck
Michael P. Rettig
Consulting or Advisory Role: Rivervest
Research Funding: MacroGenics, Amphivena Therapeutics, Maxcyte, BiolineRx
Patents, Royalties, Other Intellectual Property: MPR has pending patent applications (PCT/US2017/059777, integrin inhibitors and chemokine receptor agents; PCT/US2017/059733, integrin antagonists) and reports royalties received from the patent applications during the conduct of the study
John F. DiPersio
Stock and Other Ownership Interests: Magenta Therapeutics, WUGEN
Honoraria: Incyte
Consulting or Advisory Role: Cellworks, Rivervest, Magenta Therapeutics, Incyte
Research Funding: Amphivena Therapeutics, Macrogenics, Incyte, WUGEN, BiolineRx, Maxcyte, Bigelow Aerospace
Patents, Royalties, Other Intellectual Property: CD7 and CD2 Knockout for CART to CD7 and CDL, Duvelisib for treatment of cytokine release syndrome (CRS), NT-17 to enhance CART Survival, Novel WU mobilizing compounds, Selection of IMPDH Mutant Stem Cells, IFNg, upregulate MHCII for relapsed AML, Dextran based molecules to detect CAR-T cells, Combining integrin inhibitor with chemokine binders, 016131, JAK and calcineurin inhibition, solid organ transplant, VLA4, gro-b, Triple Combination - CXCR2, VLA-4, gro-b, Targeting IFNR/CSCR3 in GVHD, WU/SLU compounds VLA4 and CXCR2
Travel, Accommodations, Expenses: Incyte, Macrogenics, Magenta Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National Cancer Institute's Second International Workshop on the biology, prevention, and treatment of relapse after hematopoietic stem-cell transplantation: Part III. Prevention and treatment of relapse after allogeneic transplantation Biol Blood Marrow Transplant 204–132014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mielcarek M, Storer BE, Flowers MED, et al. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation Biol Blood Marrow Transplant 131160–11682007 [DOI] [PubMed] [Google Scholar]
- 3.Pollyea DA, Artz AS, Stock W, et al. Outcomes of patients with AML and MDS who relapse or progress after reduced intensity allogeneic hematopoietic cell transplantation Bone Marrow Transplant 401027–10322007 [DOI] [PubMed] [Google Scholar]
- 4.Dickinson AM, Norden J, Li S, et al. Graft-versus-leukemia effect following hematopoietic stem cell transplantation for leukemia. Front Immunol. 2017;8:496. doi: 10.3389/fimmu.2017.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley SA, Wood BL, Othus M, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: A meta-analysis Haematologica 102865–8732017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott BL. Long-term follow up of BMT CTN 0901, a randomized phase III trial comparing myeloablative (MAC) to reduced intensity conditioning (RIC) prior to hematopoietic cell transplantation (HCT) for acute myeloid leukemia (AML) or myelodysplasia (MDS) (MAvRIC trial) Biol Blood Marrow Transplant. 2020;26:S11. [Google Scholar]
- 7.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia J Clin Oncol 362684–26922018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party Blood 1311275–12912018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoest JM, Shirai CL, Duncavage EJ. Sequencing-based measurable residual disease testing in acute myeloid leukemia. Front Cell Dev Biol. 2020;8:249. doi: 10.3389/fcell.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canaani J, Labopin M, Itala-Remes M, et al. Prognostic significance of recurring chromosomal abnormalities in transplanted patients with acute myeloid leukemia Leukemia 331944–19522019 [DOI] [PubMed] [Google Scholar]
- 11.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel Blood 129424–4472017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Witte T, Bowen D, Robin M, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: Recommendations from an international expert panel Blood 1291753–17622017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: A retrospective analysis J Clin Oncol 30735–7412012 [DOI] [PubMed] [Google Scholar]
- 14.Luskin MR, Carroll M, Lieberman D, et al. Clinical utility of next-generation sequencing for oncogenic mutations in patients with acute myeloid leukemia undergoing allogeneic stem cell transplantation Biol Blood Marrow Transplant 221961–19672016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid C, Labopin M, Socie G, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation Blood 1262062–20692015 [DOI] [PubMed] [Google Scholar]
- 16.Bejar R, Stevenson KE, Caughey B, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation J Clin Oncol 322691–26982014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della Porta MG, Galli A, Bacigalupo A, et al. Clinical effects of driver somatic mutations on the outcomes of patients with myelodysplastic syndromes treated with allogeneic hematopoietic stem-cell transplantation J Clin Oncol 343627–36372016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation N Engl J Med 376536–5472017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshizato T, Nannya Y, Atsuta Y, et al. Genetic abnormalities in myelodysplasia and secondary acute myeloid leukemia: Impact on outcome of stem cell transplantation Blood 1292347–23582017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz-Fontela C, Mandinova A, Aaronson SA, et al. Emerging roles of p53 and other tumour-suppressor genes in immune regulation Nat Rev Immunol 16741–7502016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dufva O, Polonen P, Bruck O, et al. Immunogenomic landscape of hematological malignancies Cancer Cell 38380–399.e132020 [DOI] [PubMed] [Google Scholar]
- 22.Vadakekolathu J, Minden MD, Hood T, et al. Immune landscapes predict chemotherapy resistance and immunotherapy response in acute myeloid leukemia. Sci Transl Med. 2020;12:eaaz0463. doi: 10.1126/scitranslmed.aaz0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams P, Basu S, Garcia-Manero G, et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia Cancer 1251470–14812019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vadakekolathu J, Lai C, Reeder S, et al. TP53 abnormalities correlate with immune infiltration and associate with response to flotetuzumab immunotherapy in AML. Blood Adv. 2020 doi: 10.1182/bloodadvances.2020002512. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation N Engl J Med 361478–4882009 [DOI] [PubMed] [Google Scholar]
- 26.Zeiser R, Vago L.Mechanisms of immune escape after allogeneic hematopoietic cell transplantation Blood 1331290–12972019 [DOI] [PubMed] [Google Scholar]
- 27.Crucitti L, Crocchiolo R, Toffalori C, et al. Incidence, risk factors and clinical outcome of leukemia relapses with loss of the mismatched HLA after partially incompatible hematopoietic stem cell transplantation Leukemia 291143–11522015 [DOI] [PubMed] [Google Scholar]
- 28.Grosso D, Johnson E, Colombe B, et al. Acquired uniparental disomy in chromosome 6p as a feature of relapse after T-cell replete haploidentical hematopoietic stem cell transplantation using cyclophosphamide tolerization Bone Marrow Transplant 52615–6192017 [DOI] [PubMed] [Google Scholar]
- 29.Villalobos IB, Takahashi Y, Akatsuka Y, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation Blood 1153158–31612010 [DOI] [PubMed] [Google Scholar]
- 30.Hirabayashi K, Kurata T, Horiuchi K, et al. Loss of mismatched HLA on the leukemic blasts of patients with relapsed lymphoid malignancies following bone marrow transplantation from related donors with HLA class II mismatches in the graft versus host direction Pediatric Blood Cancer 63709–7112016 [DOI] [PubMed] [Google Scholar]
- 31.Jan M, Leventhal MJ, Morgan EA, et al. Recurrent genetic HLA loss in AML relapsed after matched unrelated allogeneic hematopoietic cell transplantation Blood Adv 32199–22042019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waterhouse M, Pfeifer D, Pantic M, et al. Genome-wide profiling in AML patients relapsing after allogeneic hematopoietic cell transplantation Biol Blood Marrow Transplant 171450–1459.e12011 [DOI] [PubMed] [Google Scholar]
- 33.Toffalori C, Cavattoni I, Deola S, et al. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT Blood 1194813–48152012 [DOI] [PubMed] [Google Scholar]
- 34.Stölzel F, Hackmann K, Kuithan F, et al. Clonal evolution including partial loss of human leukocyte antigen genes favoring extramedullary acute myeloid leukemia relapse after matched related allogeneic hematopoietic stem cell transplantation Transplantation 93744–7492012 [DOI] [PubMed] [Google Scholar]
- 35.Bacher U, Haferlach T, Alpermann T, et al. Comparison of cytogenetic clonal evolution patterns following allogeneic hematopoietic transplantation versus conventional treatment in patients at relapse of AML Biol Blood Marrow Transplant 161649–16572010 [DOI] [PubMed] [Google Scholar]
- 36.Ertz-Archambault N, Kosiorek H, Slack JL, et al. Cytogenetic evolution in myeloid neoplasms at relapse after allogeneic hematopoietic cell transplantation: Association with previous chemotherapy and effect on survival Biol Blood Marrow Transplant 23782–7892017 [DOI] [PubMed] [Google Scholar]
- 37.Schmidt-Hieber M, Blau IW, Richter G, et al. Cytogenetic studies in acute leukemia patients relapsing after allogeneic stem cell transplantation Cancer Genet Cytogenet 198135–1432010 [DOI] [PubMed] [Google Scholar]
- 38.Testa JR, Mintz U, Rowley JD, et al. Evolution of karyotypes in acute nonlymphocytic leukemia Cancer Res 393619–36271979 [PubMed] [Google Scholar]
- 39.Yeung CCS, Gerds AT, Fang M, et al. Relapse after allogeneic hematopoietic cell transplantation for myelodysplastic syndromes: Analysis of late relapse using comparative karyotype and chromosome genome array testing Biol Blood Marrow Transplant 211565–15752015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christopher MJ, Petti AA, Rettig MP, et al. Immune escape of relapsed AML cells after allogeneic transplantation N Engl J Med 3792330–23412018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duncavage EJ, Jacoby MA, Chang GS, et al. Mutation clearance after transplantation for myelodysplastic syndrome N Engl J Med 3791028–10412018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacoby MA, Duncavage EJ, Chang GS, et al. Subclones dominate at MDS progression following allogeneic hematopoietic cell transplant. JCI Insight. 2018;3:e98962. doi: 10.1172/jci.insight.98962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quek L, Ferguson P, Metzner M, et al. Mutational analysis of disease relapse in patients allografted for acute myeloid leukemia. Blood Adv. 2016;1:193. doi: 10.1182/bloodadvances.2016000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vosberg S, Hartmann L, Metzeler KH, et al. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation is associated with gain of WT1 alterations and high mutation load Haematologica 103e581–e5842018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing Nature 481506–5102012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farrar JE, Schuback HL, Ries RE, et al. Genomic profiling of pediatric acute myeloid leukemia reveals a changing mutational landscape from disease diagnosis to relapse Cancer Res 762197–22052016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch P, Zhang Y, Tang R, et al. Genetic hierarchy and temporal variegation in the clonal history of acute myeloid leukaemia. Nat Commun. 2016;7:12475. doi: 10.1038/ncomms12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kronke J, Bullinger L, Teleanu V, et al. Clonal evolution in relapsed NPM1-mutated acute myeloid leukemia Blood 122100–1082013 [DOI] [PubMed] [Google Scholar]
- 49.Sood R, Hansen NF, Donovan FX, et al. Somatic mutational landscape of AML with inv(16) or t(8;21) identifies patterns of clonal evolution in relapse leukemia Leukemia 30501–5042015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toffalori C, Zito L, Gambacorta V, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation Nat Med 25603–6112019 [DOI] [PubMed] [Google Scholar]
- 51.Matte-Martone C, Liu J, Zhou M, et al. Differential requirements for myeloid leukemia IFN-γ conditioning determine graft-versus-leukemia resistance and sensitivity J Clin Invest 1272765–27762017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dermime S, Mavroudis D, Jiang YZ, et al. Immune escape from a graft-versus-leukemia effect may play a role in the relapse of myeloid leukemias following allogeneic bone marrow transplantation Bone Marrow Transplant 19989–9991997 [DOI] [PubMed] [Google Scholar]
- 53.Norde WJ, Maas F, Hobo W, et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation Cancer Res 715111–51222011 [DOI] [PubMed] [Google Scholar]
- 54.Dama P, Tang M, Fulton N, et al. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J Immunother Cancer. 2019;7:175. doi: 10.1186/s40425-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hutten TJA, Norde WJ, Woestenenk R, et al. Increased coexpression of PD-1, TIGIT, and KLRG-1 on tumor-reactive CD8+ T cells during relapse after allogeneic stem cell transplantation Biol Blood Marrow Transplant 24666–6772018 [DOI] [PubMed] [Google Scholar]
- 56.Kong Y, Zhang J, Claxton DF, et al. PD-1hiTIM-3+ T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation Blood Cancer J 5e330–e3302015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L, Chang Y-J, Xu L-P, et al. Reversal of T cell exhaustion by the first donor lymphocyte infusion is associated with the persistently effective antileukemic responses in patients with relapsed AML after Allo-HSCT Biol Blood Marrow Transplant 241350–13592018 [DOI] [PubMed] [Google Scholar]
- 58.Noviello M, Manfredi F, Ruggiero E, et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat Commun. 2019;10:1065. doi: 10.1038/s41467-019-08871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elmaagacli AH, Beelen DW, Trenn G, et al. Induction of a graft-versus-leukemia reaction by cyclosporin A withdrawal as immunotherapy for leukemia relapsing after allogeneic bone marrow transplantation Bone Marrow Transplant 23771–7771999 [DOI] [PubMed] [Google Scholar]
- 60.Oran B, Giralt S, Couriel D, et al. Treatment of AML and MDS relapsing after reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation Leukemia 212540–25442007 [DOI] [PubMed] [Google Scholar]
- 61.Rosenow F, Berkemeier A, Krug U, et al. CD34(+) lineage specific donor cell chimerism for the diagnosis and treatment of impending relapse of AML or myelodysplastic syndrome after allo-SCT Bone Marrow Transplant 481070–10762013 [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Cai Y, Jiang J, et al. Early tapering of immunosuppressive agents after HLA-matched donor transplantation can improve the survival of patients with advanced acute myeloid leukemia Ann Hematol 97497–5072018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abraham R, Szer J, Bardy P, et al. Early cyclosporine taper in high-risk sibling allogeneic bone marrow transplants Bone Marrow Transplant 20773–7771997 [DOI] [PubMed] [Google Scholar]
- 64.Kröger N, Solano C, Wolschke C, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease N Engl J Med 37443–532016 [DOI] [PubMed] [Google Scholar]
- 65.Rubio MT, D'Aveni-Piney M, Labopin M, et al. Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine iv-busulfan myeloablative regimen: A report from the EBMT Acute Leukemia Working Party. J Hematol Oncol. 2017;10:31. doi: 10.1186/s13045-016-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soiffer RJ, LeRademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies Blood 1176963–69702011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jedlickova Z, Schmid C, Koenecke C, et al. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation Bone Marrow Transplant 51663–6672016 [DOI] [PubMed] [Google Scholar]
- 68.Schmid C, Labopin M, Schaap N, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia—A matched pair analysis by the Acute Leukaemia Working Party of EBMT Br J Haematol 184782–7872019 [DOI] [PubMed] [Google Scholar]
- 69.Solomon SR, Sizemore CA, Zhang X, et al. Preemptive DLI without withdrawal of immunosuppression to promote complete donor T-cell chimerism results in favorable outcomes for high-risk older recipients of alemtuzumab-containing reduced-intensity unrelated donor allogeneic transplant: A prospective phase II trial Bone Marrow Transplant 49616–6212014 [DOI] [PubMed] [Google Scholar]
- 70.Mo X-D, Zhang X-H, Xu L-P, et al. Comparison of outcomes after donor lymphocyte infusion with or without prior chemotherapy for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation Ann Hematol 96829–8382017 [DOI] [PubMed] [Google Scholar]
- 71.Yan C-H, Liu D-H, Liu K-Y, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation Blood 1193256–32622012 [DOI] [PubMed] [Google Scholar]
- 72.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: A Center for International Blood and Marrow Transplant Research Study Biol Blood Marrow Transplant 21454–4592015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyamoto T, Fukuda T, Nakashima M, et al. Donor lymphocyte infusion for relapsed hematological malignancies after unrelated allogeneic bone marrow transplantation facilitated by the Japan Marrow Donor Program Biol Blood Marrow Transplant 23938–9442017 [DOI] [PubMed] [Google Scholar]
- 74.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: A retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party J Clin Oncol 254938–49452007 [DOI] [PubMed] [Google Scholar]
- 75.Kharfan-Dabaja MA, Labopin M, Polge E, et al. Association of second allogeneic hematopoietic cell transplant vs donor lymphocyte infusion with overall survival in patients with acute myeloid leukemia relapse. JAMA Oncol. 2018;4:1245. doi: 10.1001/jamaoncol.2018.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruutu T, de Wreede LC, van Biezen A, et al. Second allogeneic transplantation for relapse of malignant disease: Retrospective analysis of outcome and predictive factors by the EBMT Bone Marrow Transplant 501542–15502015 [DOI] [PubMed] [Google Scholar]
- 77.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant Bone Marrow Transplant 34721–7272004 [DOI] [PubMed] [Google Scholar]
- 78.Michallet M, Tanguy ML, Socié G, et al. Second allogeneic haematopoietic stem cell transplantation in relapsed acute and chronic leukaemias for patients who underwent a first allogeneic bone marrow transplantation: A survey of the Société Française de Greffe de moelle (SFGM) Br J Haematol 108400–4072000 [DOI] [PubMed] [Google Scholar]
- 79.Duncan CN, Majhail NS, Brazauskas R, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes Biol Blood Marrow Transplant 21151–1582015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orti G, Sanz J, Bermudez A, et al. Outcome of second allogeneic hematopoietic cell transplantation after relapse of myeloid malignancies following allogeneic hematopoietic cell transplantation: A retrospective cohort on behalf of the Grupo Español de Trasplante Hematopoyetico Biol Blood Marrow Transplant 22584–5882016 [DOI] [PubMed] [Google Scholar]
- 81.Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation Blood 1131581–15882009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation N Engl J Med 375143–1532016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou J, Bashey A, Zhong R, et al. CTLA-4 blockade following relapse of malignancy after allogeneic stem cell transplantation is associated with T cell activation but not with increased levels of T regulatory cells Biol Blood Marrow Transplant 17682–6922011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: High response rate but frequent GVHD Blood 130221–2282017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McDuffee E, Aue G, Cook L, et al. Tumor regression concomitant with steroid-refractory GvHD highlights the pitfalls of PD-1 blockade following allogeneic hematopoietic stem cell transplantation Bone Marrow Transplant 52759–7612017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh AK, Porrata LF, Aljitawi O, et al. Fatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin's lymphoma Bone Marrow Transplant 511268–12702016 [DOI] [PubMed] [Google Scholar]
- 87.Wang A, Kline J, Stock W, et al. Unexpected toxicities when nivolumab was given after allogeneic stem cell transplantation. Blood. 2019;134:1956. doi: 10.1016/j.bbmt.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 88.Soiffer RJ.Checkpoint inhibition to prevent or treat relapse in allogeneic hematopoietic cell transplantation Bone Marrow Transplant 54798–8022019 [DOI] [PubMed] [Google Scholar]
- 89.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia Blood 116129–1392010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Craddock C, Jilani N, Siddique S, et al. Tolerability and clinical activity of post-transplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial Biol Blood Marrow Transplant 22385–3902016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML) Blood 1193361–33692012 [DOI] [PubMed] [Google Scholar]
- 92.Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): Potential role in the transplantation setting Blood 115107–1212010 [DOI] [PubMed] [Google Scholar]
- 93.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: A dose and schedule finding study Cancer 1165420–54312010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El-Cheikh J, Massoud R, Fares E, et al. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT Bone Marrow Transplant 52918–9212017 [DOI] [PubMed] [Google Scholar]
- 95.Platzbecker U, Middeke JM, Sockel K, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): An open-label, multicentre, phase 2 trial Lancet Oncol 191668–16792018 [DOI] [PubMed] [Google Scholar]
- 96.Platzbecker U, Wermke M, Radke J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial Leukemia 26381–3892012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pusic I, Choi J, Fiala MA, et al. Maintenance therapy with decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome Biol Blood Marrow Transplant 211761–17692015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reference deleted [Google Scholar]
- 99.Czibere A, Bruns I, Kröger N, et al. 5-Azacytidine for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome who relapse after allo-SCT: A retrospective analysis Bone Marrow Transplant 45872–8762010 [DOI] [PubMed] [Google Scholar]
- 100.Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia Cancer 1151899–19052009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lübbert M, Bertz H, Wäsch R, et al. Efficacy of a 3-day, low-dose treatment with 5-azacytidine followed by donor lymphocyte infusions in older patients with acute myeloid leukemia or chronic myelomonocytic leukemia relapsed after allografting Bone Marrow Transplant 45627–6322010 [DOI] [PubMed] [Google Scholar]
- 102.Schroeder T, Czibere A, Platzbecker U, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation Leukemia 271229–12352013 [DOI] [PubMed] [Google Scholar]
- 103.Tessoulin B, Delaunay J, Chevallier P, et al. Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT Bone Marrow Transplant 49567–5712014 [DOI] [PubMed] [Google Scholar]
- 104.Kim S, Santhanam S, Lim S, et al. Targeting histone deacetylases to modulate graft-versus-host disease and graft-versus-leukemia. Int J Mol Sci. 2020;21:4281. doi: 10.3390/ijms21124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DeAngelo DJ, Walker AR, Schlenk RF, et al. Safety and efficacy of oral panobinostat plus chemotherapy in patients aged 65 years or younger with high-risk acute myeloid leukemia. Leuk Res. 2019;85:106197. doi: 10.1016/j.leukres.2019.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schaefer EW, Loaiza-Bonilla A, Juckett M, et al. A phase 2 study of vorinostat in acute myeloid leukemia Haematologica 941375–13822009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schlenk RF, Krauter J, Raffoux E, et al. Panobinostat monotherapy and combination therapy in patients with acute myeloid leukemia: Results from two clinical trials Haematologica 103e25–e282018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bug G, Burchert A, Wagner EM, et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST Trial) Leukemia 312523–25252017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kneppers E, van der Holt B, Kersten MJ, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: Results of the HOVON 76 Trial Blood 1182413–24192011 [DOI] [PubMed] [Google Scholar]
- 110.Sockel K, Bornhaeuser M, Mischak-Weissinger E, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): Results of the LENAMAINT trial Haematologica 97e34–e352012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Craddock C, Labopin M, Robin M, et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia Haematologica 101879–8832016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burchert A, Bug G, Finke J, et al. Sorafenib as maintenance therapy post allogeneic stem cell transplantation for FLT3-ITD positive AML: Results from the randomized, double-blind, placebo-controlled multicentre Sormain Trial. Blood. 2018;132:661. [Google Scholar]
- 113.Xuan L, Wang Y, Huang F, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: An open-label, multicentre, randomised phase 3 trial Lancet Oncol 211201–12122020 [DOI] [PubMed] [Google Scholar]
- 114.Levis MJ, Hamadani M, Logan BR, et al. BMT CTN protocol 1506: A phase 3 trial of gilteritinib as maintenance therapy after allogeneic hematopoietic stem cell transplantation in patients with FLT3-ITD+ AML. Blood. 2019;134:4602. [Google Scholar]
- 115.De Freitas T, Marktel S, Piemontese S, et al. High rate of hematological responses to sorafenib in FLT3-ITD acute myeloid leukemia relapsed after allogeneic hematopoietic stem cell transplantation Eur J Haematol 96629–6362016 [DOI] [PubMed] [Google Scholar]
- 116.Metzelder SK, Schroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses Leukemia 262353–23592012 [DOI] [PubMed] [Google Scholar]
- 117.Cortes JE, Khaled S, Martinelli G, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A multicentre, randomised, controlled, open-label, phase 3 trial Lancet Oncol 20984–9972019 [DOI] [PubMed] [Google Scholar]
- 118.Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML N Engl J Med 3811728–17402019 [DOI] [PubMed] [Google Scholar]
- 119.Antar AI, Otrock ZK, Jabbour E, et al. FLT3 inhibitors in acute myeloid leukemia: Ten frequently asked questions Leukemia 34682–6962020 [DOI] [PubMed] [Google Scholar]
- 120.Mathew NR, Baumgartner F, Braun L, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells Nat Med 24282–2912018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hipp MM, Hilf N, Walter S, et al. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses Blood 1115610–56202008 [DOI] [PubMed] [Google Scholar]
- 122.Lin JC, Huang WP, Liu CL, et al. Sorafenib induces autophagy in human myeloid dendritic cells and prolongs survival of skin allografts Transplantation 95791–8002013 [DOI] [PubMed] [Google Scholar]
- 123.Whartenby KA, Calabresi PA, McCadden E, et al. Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease Proc Natl Acad Sci U S A 10216741–167462005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stein EM, DiNardo CD, Fathi AT, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib Blood 133676–6872019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Byrne M, Danielson N, Sengsayadeth S, et al. The use of venetoclax-based salvage therapy for post-hematopoietic cell transplantation relapse of acute myeloid leukemia Am J Hematol 951006–10142020 [DOI] [PubMed] [Google Scholar]
- 126.Hosen N, Maeda T, Hashii Y, et al. Wilms tumor 1 peptide vaccination after hematopoietic stem cell transplant in leukemia patients. Stem Cell Investig. 2016;3:90. doi: 10.21037/sci.2016.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shah NN, Loeb DM, Khuu H, et al. Induction of immune response after allogeneic Wilms' tumor 1 dendritic cell vaccination and donor lymphocyte infusion in patients with hematologic malignancies and post-transplantation relapse Biol Blood Marrow Transplant 222149–21542016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lichtenegger FS, Schnorfeil FM, Rothe M, et al. Toll-like receptor 7/8-matured RNA-transduced dendritic cells as post-remission therapy in acute myeloid leukaemia: Results of a phase I trial. Clin Transl Immunology. 2020;9:e1117. doi: 10.1002/cti2.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kitawaki T, Kadowaki N, Kondo T, et al. Potential of dendritic-cell immunotherapy for relapse after allogeneic hematopoietic stem cell transplantation, shown by WT1 peptide- and keyhole-limpet-hemocyanin-pulsed, donor-derived dendritic-cell vaccine for acute myeloid leukemia Am J Hematol 83315–3172008 [DOI] [PubMed] [Google Scholar]
- 130.Anguille S, Lion E, Willemen Y, et al. Interferon-α in acute myeloid leukemia: An old drug revisited Leukemia 25739–7482011 [DOI] [PubMed] [Google Scholar]
- 131.Robb RJ, Kreijveld E, Kuns RD, et al. Type I-IFNs control GVHD and GVL responses after transplantation Blood 1183399–34092011 [DOI] [PubMed] [Google Scholar]
- 132.Nikiforow S, Kim HT, Daley H, et al. A phase I study of CD25/regulatory T-cell-depleted donor lymphocyte infusion for relapse after allogeneic stem cell transplantation Haematologica 1011251–12592016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Muffly L, Sheehan K, Armstrong R, et al. Infusion of donor-derived CD8+ memory T cells for relapse following allogeneic hematopoietic cell transplantation Blood Adv 2681–6902018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer Blood 1053051–30572005 [DOI] [PubMed] [Google Scholar]
- 135.Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8:357ra123. doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia J Clin Oncol 28955–9592010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Choi I, Yoon SR, Park S-Y, et al. Donor-derived natural killer cells infused after human leukocyte antigen–haploidentical hematopoietic cell transplantation: A dose-escalation study Biol Blood Marrow Transplant 20696–7042014 [DOI] [PubMed] [Google Scholar]
- 138.Jaiswal SR, Zaman S, Nedunchezhian M, et al. CD56-enriched donor cell infusion after post-transplantation cyclophosphamide for haploidentical transplantation of advanced myeloid malignancies is associated with prompt reconstitution of mature natural killer cells and regulatory T cells with reduced incidence of acute graft versus host disease: A pilot study Cytotherapy 19531–5422017 [DOI] [PubMed] [Google Scholar]
- 139.Lee DA, Denman CJ, Rondon G, et al. Haploidentical natural killer cells infused before allogeneic stem cell transplantation for myeloid malignancies: A phase I trial Biol Blood Marrow Transplant 221290–12982016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ciurea SO, Schafer JR, Bassett R, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation Blood 1301857–18682017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berrien-Elliott MM, Wagner JA, Fehniger TA.Human cytokine-induced memory-like natural killer cells J Innate Immun 7563–5712015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Romee R, Cooley S, Berrien-Elliott MM, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation Blood 1312515–25272018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chapuis AG, Egan DN, Bar M, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant Nat Med 251064–10722019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brudno JN, Somerville RPT, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease J Clin Oncol 341112–11212016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation Blood 1224129–41392013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia N Engl J Med 376836–8472017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shang Y, Zhou F. Current advances in immunotherapy for acute leukemia: An overview of antibody, chimeric antigen receptor, immune checkpoint, and natural killer. Front Oncol. 2019;9:917. doi: 10.3389/fonc.2019.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rovatti PE, Zito L, Draghi E, et al. Exploiting an anti-CD3/CD33 bispecific antibody to redirect donor T cells against HLA loss leukemia relapses. Blood. 2019;134:513. [Google Scholar]
- 149.Appelbaum FR, Bernstein ID.Gemtuzumab ozogamicin for acute myeloid leukemia Blood 1302373–23762017 [DOI] [PubMed] [Google Scholar]
- 150.Genthon A, Brissot E, Malard F, et al. Gemtuzumab ozogamicin combined with intensive chemotherapy in patients with acute myeloid leukemia relapsing after allogenic stem cell transplantation Clin Lymphoma Myeloma Leuk 20791–7962020 [DOI] [PubMed] [Google Scholar]


