Supplemental Digital Content is available in the text
Keywords: balanced crystalloids, critical ill patients, meta-analysis, randomized controlled trials, saline
Abstract
Objective:
To compare the safety of balanced crystalloids and saline among critically ill patients in intensive care unit (ICU).
Methods:
The Medline, EMBASE, Web of Science, Cochrane Library databases were systematically searched from the inception dates to May 17, 2020 in order to identify randomized controlled trials which evaluated the safety of balanced crystalloids and saline in critically ill patients. The primary outcome was major adverse kidney events within 30 days (MAKE30). The second outcomes included 30-day mortality, ICU mortality, In-hospital mortality, ICU length of stay, hospital length of stay, creatinine highest before discharge (mg/dl) and needs for renal replacement therapy (RRT).
Results:
A total of nine randomized controlled trials involving 19,578 critical ill patients fulfilled the inclusion criteria. The outcomes of this meta-analysis showed that balanced crystalloids treatment shared the same risk of MAKE30 with saline treatment among critical ill patients [RR = 0.95; 95%CI, 0.88 to 1.01; Z = 1.64 (P = .102)]. The clinical mortality which included 30-day mortality [RR = 0.92; 95%CI, 0.85 to 1.01; Z = 1.78 (P = .075)], ICU mortality [RR = 0.92; 95%CI, 0.83 to 1.02; Z = 1.67 (P = .094)] and In-hospital mortality [RR = 0.93; 95%CI, 0.71 to 1.21; Z = 0.55 (P = .585)] were similar between balanced crystalloids treatment and saline treatment among critical ill patients. Patients who received balanced crystalloids treatment or saline treatment needed the same length of ICU stay [WMD = 0.00; 95%CI, −0.09 to 0.10; Z = 0.09 (P = .932)] and hospital stay [WMD = 0.59; 95%CI, −0.33 to 1.51; Z = 1.26 (P = .209)]. Critical ill patients who received balanced crystalloids treatment or saline treatment had the same level of creatinine highest before discharge [WMD = 0.01; 95%CI, −0.02 to 0.04; Z = 0.76 (P = .446)] and needs for RRT [RR = 1.04; 95%CI, 0.75 to 1.43; Z = 0.21 (P = .830)]. Similar results were obtained in subgroups of trials stratified according to the age of patients (children or adults).
Conclusions:
When compared with saline, balanced crystalloids could not reduce the risk of MAKE30, 30-day mortality, ICU mortality and in-hospital mortality, could not reduce the length of ICU stay, length of hospital stay, the level of creatinine highest before discharge and the needs for RRT among critical ill children and adults. Therefore, it was still too early for balanced crystalloids to replace normal saline among critical ill patients.
1. Introduction
Saline (0.9% sodium chloride) has been commonly used among critical ill patients. In recent years, several studies have reported that the concentration of chloride ions in saline may lead to a series of adverse reactions. Chowdhury et al [1] reported that intravenous infusion of 0.9% saline reduced renal blood flow velocity and renal cortical tissue perfusion which has implications for intravenous fluid therapy among critically ill patients. Waters et al[2] reported that 0.9% saline significantly cause more acidosis on completion of surgery.
Therefore, some studies suggested that balanced crystalloids maybe replaced normal saline in critical ill patients.[3,4] At present, the commonly used balanced crystalloids in clinical practice mainly include lactate Ringer's solution and plasma-Lyte A. Carlo et al[5] reported that balanced solution was responsible of less alteration of plasmatic electrolytes, acid-base equilibrium, kidney function and it might be associated with an early anti-inflammatory mechanisms triggering. Matthew et al[6] recruited 974 patients and reported that saline made up a larger proportion of the isotonic crystalloid given in the saline group than in the balanced crystalloid group, but MAKE30 did not differ between balanced crystalloids and saline.
However, the comparison of balanced crystalloids and saline in critically ill patients was still controversial.[7] Therefore, we conduct this meta-analysis to investigate the safety of balanced crystalloids and saline among critical ill patients in ICU. Different from previous studies, our study excluded those retrospective studies and only included randomized clinical trials (RCTs) about balanced solution versus saline for critically ill patients, the sample size was up to 19,578 participants. Besides, previous meta-analysis had no relative data about children. Our study presented the data about children by sub-group analysis according to the age of patients (children or adults). Furthermore, we presented more indicators, some of which were not even mentioned in previous meta-analysis, such as major adverse kidney events within 30 days (MAKE30), 30-day mortality, ICU mortality, In-hospital mortality, ICU length of stay, hospital length of stay, creatinine highest before discharge(mg/dl) and needs for renal replacement therapy (RRT).
2. Methods
This meta-analysis was performed to compare the safety of balanced crystalloids and saline in critically ill patients according to the Cochrane Handbook for Systematic Reviews of Interventions and presented based on Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines.[8]
2.1. Ethical statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital (Beijing Hospital of Traditional Chinese medicine, Capital Medical University). Since this study was a meta-analysis and did not require any patient-related intervention or experiment, the ethical committee of our hospital had reviewed it without informed consent.
2.2. Search trials
We systematically searched Medline, Cochrane Library, EMBASE and Web of Science from the inception dates to May 17, 2020 by the keywords “balanced crystalloids”, “saline”, “critical ill patients”, “randomized clinical trials” to identify published systematic reviews or meta-analysis which compare the safety of balanced crystalloids and saline in critically ill patients (the complete search strategy was listed in the file of “Supplemental search strategy”, http://links.lww.com/MD2/A416). No language restrictions. We identified original RCTs included in the systematic reviews or meta-analysis. An additional search was performed to identify recently published RCTs (from the inception dates to May 17, 2020) which met the inclusion criteria by using the databases and keywords described above.
2.3. Inclusion and exclusion criteria
Trials were selected based on the following inclusion criteria:
-
1.
Randomized Controlled Trials (RCTs) which compare the safety of balanced crystalloids and saline in critically ill patients.
-
2.
Reported at least one of the following outcomes: MAKE30, 30-day mortality, ICU mortality, In-hospital mortality, ICU length of stay, hospital length of stay, creatinine highest before discharge or needs for RRT.
Exclusion criteria:
-
1.
Trials whose participants were not treat in intensive care unit (ICU).
-
2.
Clinical retrospective studies.
-
3.
Studies included pregnant patients or immunosuppressed patients.
2.4. Risk of bias assessments
The methodological quality of the included trials was assessed independently by two researchers according to Cochrane handbook for systematic reviews of interventions and summarized. The evaluation items included the following components: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias.[9]
2.5. Data extraction
Two researchers independently reviewed the full text of the retrieved articles. Disparities between researchers regarding the inclusion of each trial were resolved by a third researcher. The information for each study included the lead author, publication year, country of origin, study design, sample size, participant characteristics, intervention and outcomes.
2.6. The main endpoints
The primary outcome was MAKE30. The second outcomes included 30-day mortality, ICU mortality, In-hospital mortality, ICU length of stay, hospital length of stay, creatinine highest before discharge(mg/dl) and needs for RRT. MAKE30 was defined as the composite of death, new receipt of renal-replacement therapy, or persistent renal dysfunction (final inpatient creatinine value ≥200% of the baseline value) within 30 days after enrollment.
In this study, we conducted subgroup analysis according to the age in order to investigate the comparison between children and adults. Those children were defined as patients who received treatment in pediatric intensive care units (PICU), adults were defined as patients who received treatment in adult ICU.
2.7. Statistical analysis
This meta-analyses were performed by the software program Stata 12.0. Dichotomous outcomes were analyzed by relative risk (RR) and 95% confidence intervals (CIs). Continuous outcomes were analyzed by weighted mean differences (WMD) and 95% confidence intervals (CIs). Based on the practice recommendation of the Cochrane Handbook,[9] trials with zero events in both the intervention and the control groups were not included in the meta-analysis when RR and WMD were calculated.
Random-effects model or fixed-effects model was used to pool the data according to the statistical heterogeneity by the chi-squared test (I2), if I2≤50%, a fixed-effect model was used; if I2 > 50%, a random-effects model was used.[9] The sensitivity analysis was performed by excluding any single hazard ratio from the analysis. The potential for publication bias was assessed by Begg's test and a visual assessment of a funnel plot. In order to evaluate whether the safe of balanced crystalloids for critically ill patients was modified by the age of patients, we specified subgroups based on the age of patients (children or adults). A P < .05 was considered statistically significant.
3. Results
3.1. Studies retrieved and characteristics
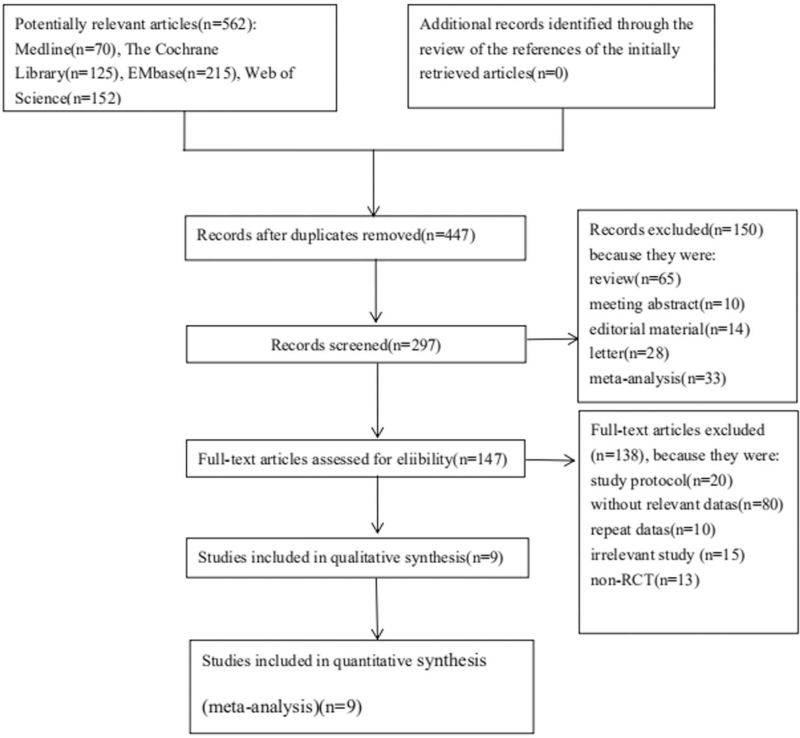
A total of 562 potentially eligible records were identified from the literature searches. After screened the titles and abstracts of these records, 147 trials were reviewed. Eventually, a total of nine trials involving 19,578 participants were ultimately included in the quantitative analysis (Fig. 1). All studies were published in English from 2001 to 2020. The sample size varied from 50 to 15,802 among the included studies. Table 1 reported the details of the study characteristics. Among these 9 RCTs, 5 trials were placebo-controlled, double-blind RCTs, other 4 trials were RCT without blinding. Table 2 showed the assessment of the risk of bias.
Figure 1.
Flow diagram of the literature search and selection process of the studies.
Table 1.
Main characteristics of the studies included in the meta-analysis.
| Study | Locations | Study Design | Follow-up Duration | Numbers of patients | Population | Intervention | Control | Outcomes |
| Williams 2020[10] | 1 PICU in India | P, PG, DB, SC | 28 days | 66 | critically ill children | Balanced crystalloids | Saline | MAKE30, ICU mortality, In-hospital mortality, ICU length of stay, Hospital length of stay, Needs for RRT |
| Balamuth 2019[11] | 1 PICU in USA | P, PG, OL, SC | 90 days | 50 | critically ill children | Balanced crystalloids | Saline | MAKE30, hospital mortality, hospital length of stay |
| Semler 2018[12] | 5 ICUs in United States | P, PG, OL, MC | 60 days | 15802 | critically ill adults | Balanced crystalloids | Saline | MAKE30, 30-day mortality, ICU mortality, ICU length of stay, Creatinine highest before discharge |
| Semler 2017[6] | ICUs in United States | P, PG, OL, MC | 30 days | 974 | critically ill adults | Balanced crystalloids | Saline | MAKE30, ICU mortality, 30-day mortality, ICU length of stay, Needs for RRT, Creatinine highest before discharge |
| Ratanarat 2017[13] | 1 ICU in Thailand | P, PG, OL, SC | 28 days | 181 | critically ill adults | Balanced crystalloids | Saline | Needs for RRT |
| Verma 2016[14] | 3 ICUs in Australia | P, PG, DB, MC | 30 days | 67 | critically ill adults | Balanced crystalloids | Saline | MAKE30, ICU mortality, In-hospital mortality, ICU length of stay, Hospital length of stay, Needs for RRT, Creatinine highest before discharge |
| Young 2015[15] | 4 ICUs in New Zealand | P, PG, DB, MC | 90 days | 2262 | critically ill adults | Balanced crystalloids | Saline | MAKE30, ICU mortality, hospital mortality, ICU length of stay, hospital length of stay, Needs for RRT, Creatinine highest before discharge |
| Young 2014[16] | 1 ICU in United States | P, PG, DB, SC | 30 days | 65 | critically ill adults | Balanced crystalloids | Saline | 30-day mortality, ICU length of stay, hospital length of stay |
| Ngo 2001[17] | 1 PICU in Vietnam | P, PG, DB, SC | 7 days | 111 | critically ill children | Balanced crystalloids | Saline | ICU mortality, hospital mortality |
DB = double-blind, MC = multi-center, OL = open label, P = prospective, PG = parallel group, SC = single-center.
Table 2.
Assessment of the methodological aspects of the included studies.
| Study | Bias Component Sequence generation and allocation concealment | Blinding | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Quality score |
| Williams 2020[10] | Low risk | Double-blind | Low risk | Low risk | Low risk | High |
| Balamuth 2019[11] | Low risk | Open-label | Low risk | Low risk | Low risk | High |
| Semler 2018[12] | Low risk | Open-label | Low risk | Low risk | Low risk | High |
| Semler 2017[6] | Low risk | Open-label | Low risk | Low risk | Low risk | High |
| Ratanarat 2017[13] | Low risk | Open-label | Low risk | Low risk | Low risk | Modern |
| Verma 2016[14] | Low risk | Double-blind | Low risk | Low risk | Low risk | High |
| Young 2015[15] | Low risk | Double-blind | Low risk | Low risk | Low risk | High |
| Young 2014[16] | Low risk | Double-blind | Low risk | Low risk | Low risk | High |
| Ngo 2001[17] | Low risk | Double-blind | Low risk | Low risk | Low risk | High |
3.2. Primary outcomes
3.2.1. Major adverse kidney events within 30 days (MAKE30)
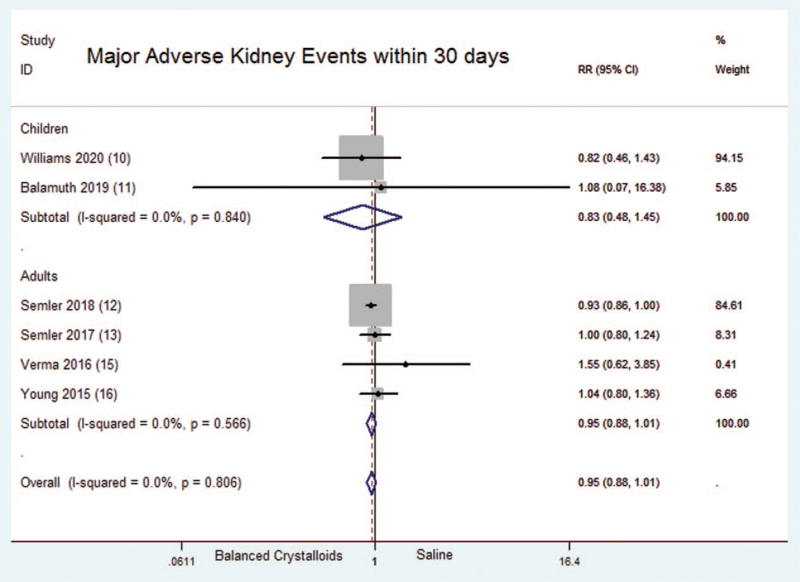
A total of six randomized controlled trials which included 19,049 critical ill patients reported the rate of major adverse kidney events within 30 days. No significant difference was found between balanced crystalloids group and saline group [Fixed-effects model; RR = 0.95; 95%CI, 0.88 to 1.01; Z = 1.64 (P = .102)] (Fig. 2). There was no significant heterogeneity in the results (P = .806; I2 = 0.0%). There was evidence of publication bias (Begg's test, P = .000) (Supplemental Digital Content Figure S1, http://links.lww.com/MD2/A405). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result.
Figure 2.
Major adverse kidney events within 30 days (MAKE30). Forest plot showing the comparison of the balanced crystalloids group vs. saline group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the RR for all studies.
The results of subgroup analysis showed that the rate of MAKE30 had no significantly difference between balanced crystalloids group and saline group among both children and adults [RR = 0.83; 95%CI, 0.48 to 1.45; Z = 0.65 (P = .516); RR = 0.95; 95%CI, 0.88 to 1.01; Z = 1.58 (P = .113)].
3.3. Secondary outcomes
3.3.1. 30-day mortality
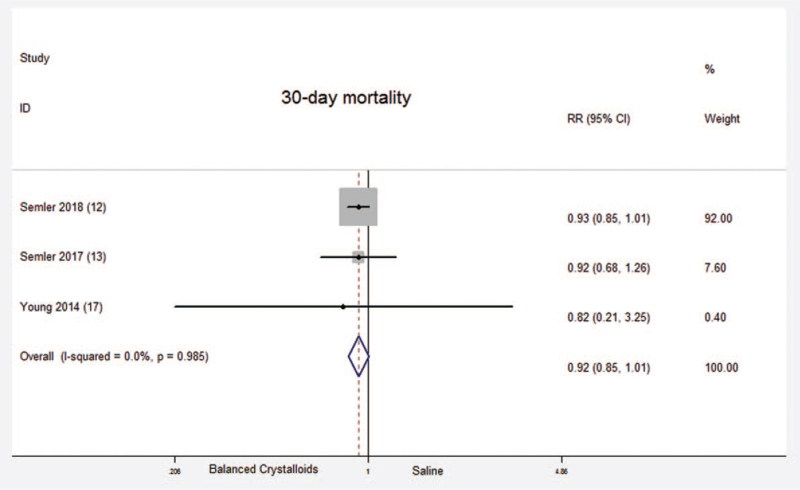
A total of three randomized controlled trials which included 16,822 critical ill patients reported 30-day mortality. No significant difference was found between balanced crystalloids group and saline group [Fixed-effects model; RR = 0.92; 95%CI, 0.85 to 1.01; Z = 1.78 (P = .075)] (Fig. 3). There was no significant heterogeneity in the results (P = .985; I2 = 0.0%). There was evidence of publication bias (Begg's test, P = .004) (Supplemental Digital Content Figure S2, http://links.lww.com/MD2/A406). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result. All of these three trials included adult critical ill patients. Therefore, there was no subgroup analysis between children and adults about 30-day mortality.
Figure 3.
30-day mortality. Forest plot showing the comparison of the balanced crystalloids group vs. saline group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the RR for all studies.
3.4. ICU mortality
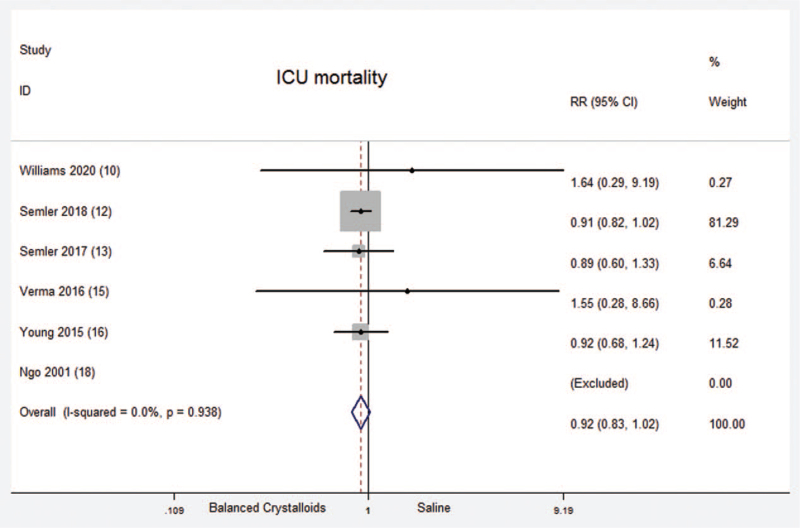
A total of six randomized controlled trials which included 19285 critical ill patients reported ICU mortality. No significant difference was found between balanced crystalloids group and saline group [Fixed-effects model; RR = 0.92; 95%CI, 0.83 to 1.02; Z = 1.67 (P = .094)] (Fig. 4). There was no significant heterogeneity in the results (P = .938; I2 = 0.0%). There was evidence of publication bias (Begg's test, P = .000) (Supplemental Digital Content Figure S3, http://links.lww.com/MD2/A407). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result. Among these six trials, there was only one trial reported the data about children. Therefore, there was no subgroup analysis between children and adults about ICU mortality.
Figure 4.
ICU mortality. Forest plot showing the comparison of the balanced crystalloids group vs. saline group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the RR for all studies.
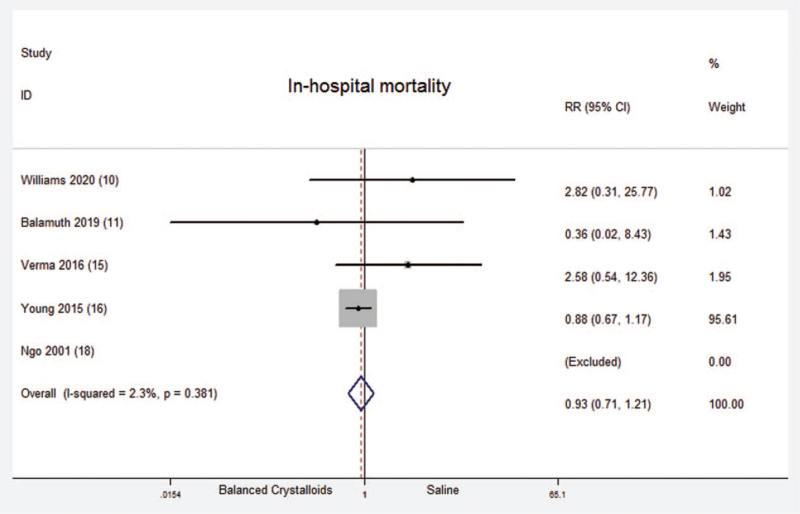
3.5. In-hospital mortality
A total of five randomized controlled trials which included 2556 critical ill patients reported in-hospital mortality. No significant difference was found between balanced crystalloids group and saline group [Fixed-effects model; RR = 0.93; 95%CI, 0.71 to 1.21; Z = 0.55 (P = .585)] (Fig. 5). There was no significant heterogeneity in the results (P = .381; I2 = 2.3%). There was no evidence of publication bias (Begg's test, P = .107) (Supplemental Digital Content Figure S4, http://links.lww.com/MD2/A408). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result. Among these five trials, there was only one trial reported the data about children. Therefore, there was no subgroup analysis between children and adults about in-hospital mortality.
Figure 5.
In-hospital mortality. Forest plot showing the comparison of the balanced crystalloids group vs. saline group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the RR for all studies.
3.6. ICU Length of stay
A total of six randomized controlled trials which included 19,217 critical ill patients reported ICU length of stay. No significant difference was found between balanced crystalloids group and saline group [Random- effects model; WMD = 0.00; 95%CI, −0.09 to 0.10; Z = 0.09 (P = .932)] (Fig. 6). There was significant heterogeneity in the results (P = .000; I2 = 96.4%). There was evidence of publication bias (Begg's test, P = .017) (Supplemental Digital Content Figure S5, http://links.lww.com/MD2/A409). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result. Among these six trials, there was only one trial reported the data about children. Therefore, there was no subgroup analysis between children and adults about ICU length of stay.
Figure 6.
ICU length of stay. Forest plot showing the comparison of the balanced crystalloids group vs. saline group. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the WMD for all studies.
3.7. Hospital length of stay
A total of five randomized controlled trials which included 2491 critical ill patients reported hospital length of stay. No significant difference was found between balanced crystalloids group and saline group [Random-effects model; WMD = 0.59; 95%CI, −0.33 to 1.51; Z = 1.26 (P = .209)] (Supplemental Digital Content Figure S6, http://links.lww.com/MD2/A410). There was significant heterogeneity in the results (P = .000; I2 = 92.4%). There was evidence of publication bias (Begg's test, P = .024) (Supplemental Digital Content Figure S7, http://links.lww.com/MD2/A411). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result.
The results of subgroup analysis showed that the hospital length of stay had no significantly difference between balanced crystalloids group and saline group among both children and adults [WMD = 0.48; 95%CI, −2.46 to 3.42; Z = 0.32 (P = .751); WMD = 0.70; 95% CI, −0.43 to 1.82; Z = 1.22 (P = .223)].
3.8. Creatinine highest before discharge(mg/dl)
A total of four randomized controlled trials which included 19,105 critical ill patients reported creatinine highest before discharge. No significant difference was found between balanced crystalloids group and saline group [Random-effects model; WMD = 0.01; 95%CI, −0.02 to 0.04; Z = 0.76 (P = .446)] (Supplemental Digital Content Figure S8, http://links.lww.com/MD2/A412). There was significant heterogeneity in the results (P = .000; I2 = 98.5%). There was no evidence of publication bias (Begg's test, P = .082) (Supplemental Digital Content Figure S9, http://links.lww.com/MD2/A413). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result. Among these four trials, there was no trial reported the data about children. Therefore, there was no subgroup analysis between children and adults about creatinine highest before discharge.
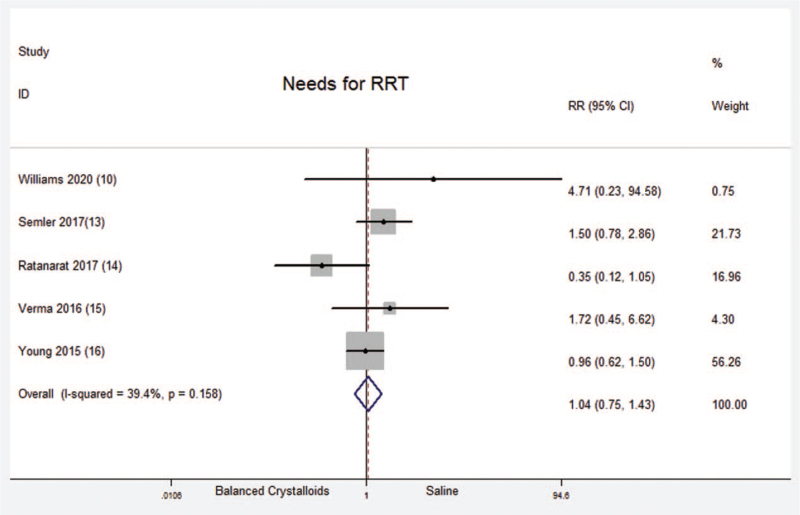
3.9. Needs for renal replacement therapy (RRT)
A total of five randomized controlled trials which included 3550 critical ill patients reported the needs for RRT. No significant difference was found between balanced crystalloids group and saline group [Fixed-effects model; RR = 1.04; 95%CI, 0.75 to 1.43; Z = 0.21 (P = .830)] (Supplemental Digital Content Figure S10, http://links.lww.com/MD2/A414). There was no significant heterogeneity in the results (P = .158; I2 = 39.4%). There was no evidence of publication bias (Begg's test, P = .312) (Supplemental Digital Content Figure S11, http://links.lww.com/MD2/A415). The sensitivity analysis (exclusion of any single hazard ratio from the analysis) didn’t substantively alter the overall result. Among these five trials, there was only one trial reported the data about children. Therefore, there was no subgroup analysis between children and adults about needs for RRT.
4. Discussion
Although a previous study had reported that balanced crystalloids could improve the kidney function of critical ill patients. The efficacy and safety of balanced crystalloids for critical ill patients were still controversial recently. Therefore, we conducted this meta analysis to evaluate the influence of balanced crystalloids on kidney function, the main indexes included MAKE30, creatinine highest before discharge(mg/dl) and needs for renal replacement therapy (RRT). Besides, our study also included other indexes which were common in ICU, such as mortality, ICU length of stay and hospital length of stay in order to investigate the effectiveness of balanced crystalloids for critical ill patients’ clinical prognosis
A total of nine randomized controlled trials involving 19,578 critical ill patients were included in this study. Among these 9 studies, there was one study came from India, four study came from United States, one study came from Thailand, one study came from Australia, one study came from New Zealand and one study came from Vietnam. Most participants were adults, only three trials reported data about children. The outcomes presented that balanced crystalloids treatment shared the same risk of MAKE30 with saline treatment among critical ill patients. The clinical mortality which included 30-days mortality, ICU mortality and in-hospital mortality were similar between balanced crystalloids treatment and saline treatment among critical ill patients. Besides, patients who received balanced crystalloids treatment or saline treatment needed the same length of ICU stay, length of hospital stay, level of creatinine highest before discharge and needs for RRT.
In this study, MAKE30 was defined as the composite of death, new receipt of renal-replacement therapy, or persistent renal dysfunction within 30 days after enrollment. Matthew et al[10] recruited 15,802 critically ill patients and reported that patients who received balanced crystalloids had lower risk of MAKE30 than patients who had received saline (14.3% vs. 15.4%). However, another trial[11] recruited 974 critical ill patients and found that MAKE30 did not differ between balanced crystalloids and saline (24.7% vs 24.6%). Therefore, the risk of MAKE30 between balanced crystalloids and saline remains unknown. This meta analysis included 19,578 critical ill patients and we found that balanced crystalloids treatment shared the same risk of MAKE30 with saline treatment. Therefore, balanced crystalloids did not have obvious advantage in reducing risk of MAKE30 among critical ill patients
Previous studies had reported the in-hospital mortality had no significant difference between balanced crystalloids and saline.[10–11] Alba et al[18] conducted a systematic review and reported that the balanced crystalloids could not reduced the in-hospital mortality when compared with saline. Although previous studies reported the in-hospital mortality, other mortality still remains unclear, such as ICU mortality, 30-day mortality.[19,20,21] In order to further research the mortality between balanced crystalloids and saline, our study collected the data about mortality among these 9 randomized controlled trials and we found that the 30-day mortality, ICU mortality and In-hospital mortality were all similar between balanced crystalloids treatment and saline treatment among critical ill patients. Therefore, our study furtherly confirmed that balanced crystalloids could not reduced the mortality of critical ill patients. Besides, we found that balanced crystalloids did not show significantly advantage in reducing the length of ICU stay and length of hospital stay.
Previous meta analysis had reported the MAKE30 and mortality about balanced crystalloids.[3,4,7,22] However, the level of creatinine highest before discharge and needs for RRT among critical ill patients who had received balanced crystalloids instead of saline remains unknown.[23,24] Our study showed that balanced crystalloids did not significantly reduce the level of creatinine highest before discharge and needs for RRT when compared to saline among critical ill patients. Therefore, the efficacy of balanced crystalloids on the level of creatinine highest before discharge and needs for RRT among critical ill patients still needed further research.
Our study was different from previous studies. Firstly, our study only included RCTs about balanced solution versus saline for critically ill patients and the sample size was up to 19,578 participants which was larger than any previous RCTs. Secondly, our study presented the relative data about children and conducted subgroup analysis according to the age of participants. Thirdly, the outcomes of our study were also different from previous studies, such as our study found that balanced crystalloids treatment had no reduction in the risk of MAKE30 as previously reported. Fourthly, previous studies reported limited outcome indicators, our study included as many outcome measures as possible.
5. Limitations
Our study still had several limitations. Firstly, only 9 RCTs were included in this study according to the inclusion and exclusion critieria, the limited sample size may result with false negative results. Secondly, among these 9 RCTs, 5 trials were placebo-controlled, double-blind RCTs, other 4 trials were RCT without blinding. Those studies that were not blinded may influenced the final results to some extend by subjective factors, such as participants’ psychological cues, anxiety or suspicion. Thirdly, most of those included studies report the data about adult critical ill patients, few studies reported the data about children. Therefore, the efficacy and safety of balanced crystalloids among children maybe in need for large sample studies for further confirmation. Fourthly, in our study, several index had large heterogeneity. In order to analyze the reason of heterogeneity, we conducted sensitivity analysis by excluding any single hazard ratio from the analysis, publication bias analysis by Begg's test and subgroup analysis based on the age of patients (children or adults). However, these methods could not reduce the heterogeneity and we have to use random effect model instead of fixed effects model. Furthermore, we considered that the reason of heterogeneity maybe related to the limited sample size, different medical level of ICU, regional differences which should be further research.
6. Conclusions
When compared with saline, balanced crystalloids could not reduce the risk of MAKE30, 30-day mortality, ICU mortality and in-hospital mortality, could not reduce the length of ICU stay, length of hospital stay, the level of creatinine highest before discharge and the needs for RRT among critical ill children and adults. Therefore, it was still too early for balanced crystalloids to replace normal saline among critical ill patients.
Author contributions
Conceptualization: Yuhan Zhu, Nan Guo, Yu Zhang.
Data curation: Yuhan Zhu, Tengfei Chen.
Formal analysis: Yuhan Zhu, Xusheng Wang, Xin Zhang.
Investigation: Yuhan Zhu, Nan Guo, Xiaoxu Shen.
Methodology: Yuhan Zhu.
Project administration: Yuhan Zhu, Fei Xia.
Resources: Yuhan Zhu, Maifen Song, Siwen Yang, Qingquan Shi.
Software: Yuhan Zhu, Yanqing Wu.
Supervision: Yuhan Zhu.
Validation: Yuhan Zhu.
Visualization: Yuhan Zhu, Zhihai Yang.
Writing – original draft: Yuhan Zhu.
Writing – review & editing: Yuhan Zhu, Zhihai Yang.
Footnotes
Abbreviations: CI = confidence intervals, ICU = intensive care unit, MAKE30 = major adverse kidney events within 30 days, RCT = randomized clinical trials, RR = relative risk, RRT = renal replacement therapy, WMD = weighted mean differences.
How to cite this article: Zhu Y, Guo N, Song M, Xia F, Wu Y, Wang X, Chen T, Yang Z, Yang S, Zhang Y, Zhang X, Shi Q, Shen X. Balanced crystalloids versus saline in critically ill patients: The PRISMA study of a meta-analysis. Medicine. 2021;100:38(e27203).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and Plasma-Lyte (R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surgery 2013;258:1118. [DOI] [PubMed] [Google Scholar]
- [2].Waters JH, Gottlieb A, Schoenwald P, et al. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg 2001;93:817–22. [DOI] [PubMed] [Google Scholar]
- [3].Zwager CL, Tuinman PR, de Growth HJ, et al. Why physiology will continue to guide the choice between balanced crystalloids and normal saline: a systematic review and meta-analysis. Critical Care 2019;23:01–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thiago Domingos C, Cavalcanti AB, Cesar de MS. Balanced crystalloids for septic shock resuscitation. Revista Brasileira De Terapia Intensiva 2016;28:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Volta CA, Trentini A, Farabegoli L, et al. Effects of two different strategies of fluid administration on inflammatory mediators, plasma electrolytes and acid/base disorders in patients undergoing major abdominal surgery: a randomized double blind study. J Inflamm (Lond) 2013;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Semler MW, Wanderer JP, Ehrenfeld JM, et al. Balanced crystalloids versus saline in the intensive care unit: the SALT randomized trial. Am J Respir Crit Care Med 2017;195:1362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zayed YZM, Aburahma AMY, Barbarawi MO, et al. Balanced crystalloids versus isotonic saline in critically ill patients: systematic review and meta-analysis. JIntensive Care 2018;6:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;6:e1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Higgins JPT. Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (updated March 2011). Cochrane Collaboration website. http://training.cochrane.org /handbook.2011. Accessed November 22, 2017. [Google Scholar]
- [10].Williams V, Jayashree M, Nallasamy K, et al. 0.9% saline versus Plasma-Lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): a double-blind randomized controlled trial. Crit Care 2020;24:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Balamuth F, Kittick M, McBride P, et al. Pragmatic pediatric trial of balanced versus normal saline fluid in sepsis: the PRoMPT BOLUS randomized controlled trial pilot feasibility study. Acad Emerg Med 2019;26:1346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Matthew W, Semler, Wesley H, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med 2018;378:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ratanarat R, Sanguanwit PCA, et al. The effects of normal saline versus balanced crystalloid solution as a resuscitation fluid on acute kidney injury in shock patients: a randomized opened label-controlled trial. Intensive Care Med Exp 2017;5:0400. [Google Scholar]
- [14].Verma B, Luethi N, Cioccari L, et al. A multicentre randomised controlled pilot study of fluid resuscitation with saline or Plasma-Lyte 148 in critically ill patients. Crit Care Resusc 2016;18:205–12. [PubMed] [Google Scholar]
- [15].Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA 2015;314:01–10. [DOI] [PubMed] [Google Scholar]
- [16].Young JB, Utter GH, Schermer CR, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg 2014;259:255–62. [DOI] [PubMed] [Google Scholar]
- [17].Nhan Ngo Thi, Phuong Cao Xuan Thanh, Kneen Rachel, et al. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis 2001;2:02. [DOI] [PubMed] [Google Scholar]
- [18].Alba M Antequera Martín, Jesus A Barea Mendoza, Alfonso Muriel, et al. Buffered solutions versus 0.9% saline for resuscitation in critically ill adults and children. Cochrane Database Syst Rev 2019;7:CD012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Semler MW, Wanderer JP, Ehrenfeld JM, et al. Balanced crystalloids versus saline in the intensive care unit: the SALT randomized trial. Am J Respir Crit Care Med 2016;201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Coulthard MG, Long DA, Ullman AJ, et al. A randomised controlled trial of Hartmann solution versus half normal saline in postoperative paediatric spinal instrumentation and craniotomy patients. Arch Dis Child 2012;97:491–6. [DOI] [PubMed] [Google Scholar]
- [21].Burkhard Simma, René Burger, Markus Falk, et al. The release of antidiuretic hormone is appropriate in response to hypovolemia and/or sodium administration in children with severe head injury: a trial of lactated Ringers solution versus hypertonic saline. Anesth Analg 2001;45:295–6. [DOI] [PubMed] [Google Scholar]
- [22].Semler MW, Kellum JA. Balanced crystalloid solutions. Am J Respir Crit Care Med 2018;199:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Andrew F, Natalie M, Swanson MB, et al. Normal saline solution and lactated Ringer's solution have a similar effect on quality of recovery: a randomized controlled trial. Ann Emerg Med 2018;73:S0196064418306279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med 2014;42:1585–91. [DOI] [PubMed] [Google Scholar]