Supplemental Digital Content is available in the text
Keywords: herbal medicine, meta-analysis., mild cognitive impairment, the mini mental state examination, systematic evaluation
Abstract
Introduction:
The prevalence of mild cognitive impairment (MCI) in the elderly population aged 60 to 84 years ranges from 6.7% to 25.2%, and the effective prevention and reversal of MCI progression to Alzheimer disease (AD) is crucial. The mini mental state examination (MMSE) is the most commonly used screening tool in Chinese outpatient clinics, with sufficient sensitivity and specificity to allow useful stratification from average to abnormal with adequate consideration of age and education.
Objective:
To investigate the clinical significance of Chinese herbs on MMSE scores in MCI patients and discuss the effectiveness of Chinese herbs through pharmacology.
Methods:
Three English databases and 4 Chinese databases we have searched, and the risk of bias was assessed according to the Cochrane tool. Statistics will be used for heterogeneity assessment, sensitivity analysis, data synthesis, funnel plot generation and subgroup analysis. If sufficiently homogeneous studies are found, a Meta-analysis will be performed, with subgroups describing any differences.
Results:
A total of 21 studies were included, 4 studies were placebo-controlled, 14 Chinese Herbal Medicines (CHMs) were compared with other cognitive improvements, 3 CHMs were combined with other medications, and the results of 17 studies favored the herbal group.
Conclusion:
The results indicate that herbal medicine can improve MMSE scores, and herbal medicine combined with other drugs that can improve cognition can significantly improve MMSE scores, but there are methodological flaws in the study. Experimental studies have found a basis for the ability of herbs to improve cognition and memory impairment, and herbal medicine has great potential to improve MCI cognition. Keywords mild cognitive impairment, herbal medicine, MMSE, systematic evaluation, meta-analysis. PROSPERO international prospective register of systematic reviews protocol registration number: CRD42020202368
1. Introduction
Mild cognitive impairment (MCI) is a complex clinical entity that manifests significant deficits in attention, learning, memory, processing speed, and semantic language,[1] and it is a transitional state between normal aging and Alzheimer's disease (AD).[2] This state can progress to dementia, primarily in Alzheimer's disease.[3] The prevalence of MCI ranges from approximately 6.7% to 25.2% in the 60 to 84 -year old population.[4] In Asia, on average, 10.5 out of 100 people aged 60 years or older will progress to MCI each year.[5] Alzheimer's disease (AD) is the most common form of dementia, accounting for almost 60% to 70% of dementia cases,[6] and the prevalence of dementia worldwide is expected to double every 20 years, from 46.8 million in 2015 to 131.5 million in 2050, with most of the increase occurring in low- and middle-income countries.[7] China currently has more than 10 million people with dementia and already has the highest number of patients in the world.[8] Each year, 16% of MCI patients develop dementia,[9] and the progression of MCI to dementia is associated with poor treatment outcomes and a heavy financial burden on families and society. Therefore, it is crucial to prevent and reverse the progression of MCI to AD effectively. Slowing or even reversing the progression of MCI may facilitate early intervention and ultimately prevent AD. The US Federal Drug Administration approves cholinesterase inhibitors (e.g., donepezil, rivastigmine, galantamine) for the treatment of mild to moderate Alzheimer's disease. These drugs have also been used clinically to treat MCI; unfortunately, they are ineffective in MCI, and they do not delay the onset of dementia.[10] Studies have shown improved semantic memory in aMCI patients treated with ionotropic glutamate receptor antagonists (meantime),[11] but more evidence is still needed to support this, and the pros and cons of pharmacological treatment of MCI are open to debate.[12,13] Based on the favorable efficacy and safety profile of EGb761, an extract of Ginkgo biloba, the Asian Clinical Expert Group on Neurocognitive Disorders agreed to include it as part of the treatment of MCI.[14] EGB761VR has been approved in several EU countries and is the only pharmacological treatment recommended in the existing guidelines for the symptomatic treatment of MCI.[15] In recent years, Chinese herbs have shown great potential in the prevention and treatment of cognitive decline.[16–19] Studies have shown that diet and dietary supplements have a positive additive role in preventing cognitive decline,[20–22] and exercise may slow down the rate of cognitive decline in MCI.[23–25] In addition to common drugs such as cholinesterase inhibitors and ionotropic glutamate receptor antagonists, herbal medicines have been commonly used in clinical practice in China. In contrast, the calcium channel blockers nimodipine, piracetam, aniracetam, and olanzapine are also used synergistically with the above drugs to improve cognitive function.[26,27] A practical method for clinicians to grade cognitive status, called the mini mental state examination (MMSE), was first proposed by Professor Marshal Folstein in 1975[28] and includes temporal orienting force, place Orientation, immediate memory, attention and computation, delayed memory, language, visual-spatial. Another question is a short test that takes 7 to 10 minutes to complete and is still the most well-researched instrument today.[29] The most commonly used screening tool on an outpatient basis, although Montreal Cognitive Assessment (MOCA) has been shown to be more sensitive in screening and diagnosing MCI compared to MMSE,[30,31] both tests are accurate in the detection of AD.[32] By observing how the MMSE changes over time, rather than a single measurement, the transition from the MCI stage to dementia can be better predicted,[33] although most of the time, it is used in combination with other outcome measures to improve the accuracy of the diagnosis of MCI.[34]
2. Methods
Data retrieval MCI clinical trials were searched using Chinese and English databases from inception to July 31, 2020: Cochrane Library, EMbase, PubMed, China Biomedical Literature Database, China National Knowledge Infrastructure Project, China Wanfang Medical Database (WANFANG, DATA), and China Vip Database. Search terms were grouped into 4 groups:
-
1.
intervention (including herbal medicine, herbal medicine, a combination of Chinese, and Western medicine, etc.),
-
2.
clinical status (including cognitive impairment, memory impairment, memory loss, etc.),
-
3.
study design (including a clinical trial, placebo, randomized, double-blind, controlled, etc.), and
-
4.
outcome measure: MMSE. Searches were performed in accordance with the Cochrane Collaboration Network requirements. (See Supplemental Digital Content, http://links.lww.com/MD2/A455, search strategy, which illustrates the database search formula.)
2.1. Incorporation criteria
2.1.1. Types of participants
Participants diagnosed with MCI or mild neurocognitive impairment based on valid criteria: patient or informed report, or a validated clinician finding of cognitive impairment, presence of objective evidence of impairment in 1 or more cognitive domains (from cognitive tests), complex instrumental daily abilities can be mildly impaired, but maintain independent daily living skills, and have not yet reached the diagnosis of dementia. Diagnostic criteria for inclusion of subjects in this study with no restrictions on age or sex included the Mayo diagnostic criteria,[35] Petersen diagnostic criteria[36] and 2018 guideline update[4] diagnostic criteria for MCI due to Alzheimer's disease developed by the National Institute on Aging (NIA) and Alzheimer's Association (ADA) group in 2011,[37] 2013 the annual Diagnostic and Statistical Manual of Mental Disorders in the United States (DSM-VI), among others.[38]
2.1.2. Interventions
The intervention group consists of: any form of single herbal medicine, herbal preparation, or combination of herbal and non-herbal medicine, excluding ginkgo biloba extracts and purified compounds of plant origin as the primary test intervention in the study, such as stilbene A and purified extract (EGb 761) present in the test group must be used in combination with herbal medicine, with no restrictions on the dose, dosage form, frequency of use, or duration of treatment. The control group could be placebo or drug treatment, with no restrictions on the mode of administration or dose.
2.1.3. Outcome indicators
There are many outcome indicators for MCI, including neuropsychological assessment, biomarkers, and neuroimaging. Although biomarker and neuroimaging methods are well consolidated in the medical community, they are expensive, invasive, and potentially dangerous for the diagnosis and observation of this disease[39]; the most commonly used is the neuropsychological assessment, which has the advantage of being convenient, quick and cost-effective, and is the preferred observational indicator in large samples of MCI clinical trials, with the disadvantage that the results are subjective. The more commonly used MMSE neuropsychological assessment scale was chosen for this study to observe the change in scores before and after treatment.
2.1.4. Type of study design
All randomized controlled trials studying herbal medicine or a combination of traditional Chinese and Western medicine in patients with mild cognitive impairment, with or without blinding, had no restrictions on study duration, background, or publication language.
2.2. Exclusion criteria
Studies with mean baseline MMSE scores below 20 in either the experimental or control groups were excluded, as participants with scores below 20 are typically classified as having mild AD. There are also studies of repeated publication, non-clinical randomized controlled trials with other complementary therapies such as yoga, massage, tai chi, qi gong, acupuncture, etc. studies with AD, VaD, healthy young participants, or other Studies of people with dementia and missing data.
2.3. Screening and data extraction for inclusion
In the study the literature screening was carried out independently by 2 researchers, according to the literature screening flowchart stated by PRISMA.[40] Apparent discrepancies were eliminated, and the screened out literature was further searched, read in full, and screened again according to the inclusion and ranking criteria, and evaluated in full for studies that met the main criteria. Data from trials that met the inclusion criteria were extracted into a preconstructed spreadsheet containing the necessary information for the study, including, among other things, first author, title, year of publication, trial site, diagnostic criteria, sample size, the mean age of participants, study duration, intervention (composition, dose), control (drug, dose), data information (baseline, end of treatment, follow-up)—outcome indicators, number of shedding, adverse effects, etc. When the data in the original document are deviated or incomplete, the original author should be contacted to obtain further relevant information and exclude the original document for which specific data cannot be obtained. The data were extracted in the original language. Chinese names were translated using Hanyu Pinyin and scientific names of herbs based on authoritative pharmacopeia.[41]
2.4. Risk of bias assessment
According to the literature evaluation criteria provided in the Cochrane Handbook 5. 1. 0,[42,43] the methodological quality of the included studies was evaluated based on random sequence generation, allocation concealment, blinding of researchers and subjects, blinding of outcome evaluators, completeness of outcome data, selective reporting, and other factors that may affect the validity of the results. The final results were divided into 3 levels: “low risk of bias,” “unclear,” “high risk of bias” and the quality assessment was still cross-checked independently by 2 researchers. Any disagreements will be discussed and adjudicated with the third reviewer to reach a final agreement, and the assessment will be reported in a grade summary of the final findings table.
2.5. Data analysis
Statistical analysis was performed using RevMan 5.3 software provided by the Cochrane Collaboration. The relative risk (RR) and 95% CI were used for the bicategorical data; the weighted mean difference (WMD) was used for the continuous data. Suppose there was no significant heterogeneity (P > .1, I2 < 50%), the combined effect volume was calculated by fixed-effects model; if there was heterogeneity (P < .1, I2 > 50%), but the groups were judged to be clinically consistent and needed to be combined, the combined effect volume was calculated by the random-effects model, and subgroup analysis was performed to find the heterogeneity between the studies. Heterogeneous sources; if heterogeneity was excessive, only descriptive analysis was performed. The large number of papers included in this study (more than 10) requires a funnel plot to detect publication bias. To determine the stability of the outcome measures, a sensitivity analysis was performed by comparing the differences between the combined effect measures using a literature-by-life exclusion method.
3. Results
3.1. Literature search process
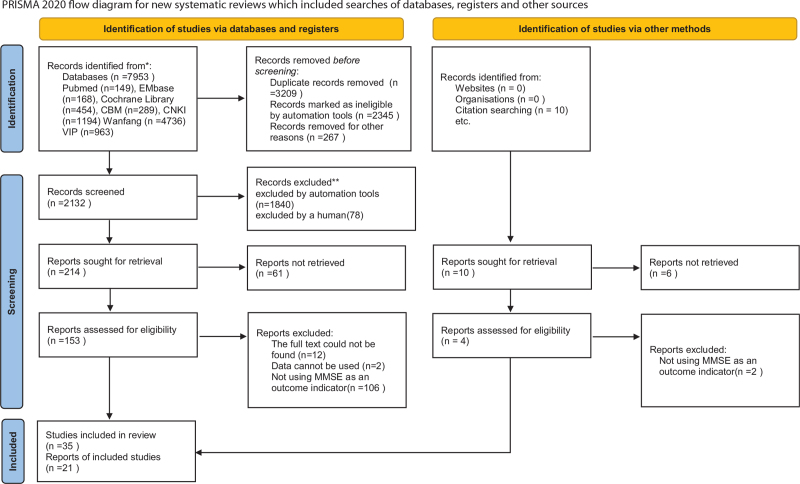
According to the search strategy, an initial search of 7953 documents was conducted, all of which were obtained through electronic search, leaving 2142 documents after weight picking and 153 documents after reading the title, abstract, and keywords; after reading the full text of the literature, 118 studies were excluded, 11 did not find the full text, and 106 did not use MMSE as an outcome measure. Thirty five pieces of literature were included in the quality evaluation, of which 7 high-quality papers described changes in MMSE using graphs and language, did not report specific changes in scores, did not receive a response after contacting the authors, 1 study used CHM as a control group, 6 were non-RCT studies, and finally 21 pieces of literature were included for data analysis. (See Fig. 1, which illustrates the research retrieval process).
Figure 1.
The research retrieval process.
3.2. General characteristics of the included studies
Of the 21 studies included, all conducted in China, 17 were published in Chinese journals, and 4 in English journals, including a total of 1560 patients (treatment group: 830 patients, control group: 730 patients). Four studies used CHM versus placebo in the treatment group[44–47]; 14 studies[48–61] used CHM versus other drugs in the treatment group; 3 studies used CHM versus Chinese medicine plus other drugs versus other drugs.[62–64] Only 1 study followed the subjects for 24 months, and 16 study pairs reported the number of years of education, 9 of which reported the mean number of years of education of the patients. (See Table 1, which illustrates the basic information of included studies, and see Supplemental Table S1, http://links.lww.com/MD2/A454, which illustrates the data of included studies.)
Table 1.
Basic information of included studies.
| AEs | CHM composition | |||||
| Author; Published Date; location; | Sample size; Duration (in weeks) | Intervention(Chinese herbal formulae/dosage) | Control/dosage | Experimental Group | Control group | |
| Zhang 2016 | 30/30; 96 | Bushen capsule; 4 capsules 3/d | placebo capsule; 4 capsules 3/d | Decreased appetite constipation | Mild nausea constipation | Rou cong rong (Cistanche deserticola), Ze xie (Rhizoma Alismatis), and He shou wu (Polygonum multiflorum thumb) |
| Zhang 2014 | 16/12/13; 12 | CCRC capsule; 4 grains for each time, 3/d | placebo capsule 4 grains for each time, 3/d | Decreased appetite | Mild nausea | He shou wu (Polygonum multiflorum), He ye (lotus leaf), Rou cong rong (Cistanche deserticola), Di long (Earthworm), Lou lu (Stemmacantha uniflora) |
| Zhou 2007 | 42/38/37; 48 | Shenyin Oral Liquid (10 mL/piece); 2/d | Placebo Oral Liquid (10 mL/piece); 2/d | None | Facial pimples with itching | Dang shen (Codonopsis pilosula), Yin xing ye (Gnkgo biloba leaves), Long yan (euphoria longan), Da zao (Fructus Ziziphi Jujubae) |
| Wu 2016 | 62/16 12 | Bushen Jianpi Huatan Pills, 9 g/time, 2 /d | Placebo Pills, 9 g/time, 2 /d | Swollen gums | constipation | Bai zhu (Atractylodes macrocephala), Ji nei jin (Gallus gallus domesticus) |
| Zhang 2018 | 30/30; 12 | Qingnao Yizhi Granules;10 g/pack/time;3/d | Nimodipine Tablets 30 mg/time, 3/d | None | None | Shi chang pu (Acorus tatarinowii), Yu jin (Radix Rurcumae), Lou lu (Rhizoma leucorrhizae), Ji xue teng (Caulis Spatholobus), Fu ling (Poria cocos), Dang gui (Angelica sinensis), Huang jing (Polygonatum), Di long (Earthworm), Gan cao (Licorice), |
| Zhao 2009 | 30/30 12 | Jinkui Shenqi Pills Modified Pills 9 g, 2/d | Nimodipine Tablets 30 mg/time, 3/d | Not reported | Not reported | Di huang (Rehmannia glutinosa), Shan yao (yam), Shan zhu yu (Cornus officinalis), Ze xie (Rhizoma Alismatis), Fu ling (Poria cocos), Mu dan pi (peony bark), Fu zi (aconite), Gui zhi (Cinnamon Twig), Yi zhi ren (Alpinia Oxyphylla Miq), Rou cong rong (Cistanche deserticola) |
| Cui 2015 | 16/16 12 | Naofucong: 150 mL/pack, 1pack /time, 2/d | Nimodipine Tablets 30 mg/time, 3/d | None | None | Renshen (Radix Ginseng)He shou wu (Polygonum multiflorum), Shui zhi (leech), Dan shen (Salvia miltiorrhiza), Huang lian (Coptis chinensis) |
| Guo2010 | 32/32/15; 12 | Huanglian Wendan Decoction 200 mL; 2/d Aniracetam analog capsules 2 grains for each time 3/d | Aniracetam capsules (0.1g/ capsule) 2 grains f 3/d, Mock decoction 200 mL; 2/d | None | None | Ban xia (Pinellia ternata)Fu ling (Poria cocos) Chen pi (dried tangerine or orange peel) Zhu ru (siliceous accretions formed under the skin of bamboo) Shi chang pu (Acorus tatarinowii) Chuan xiong (Ligusticum wallichii) Yuan zhi (polygala root) |
| Zhong 2007 | 83/83; 12 | Shenwu Capsule (5 capsules each time); 3/d | Aniracetam capsules (2 capsules /time) and Aniracetam Capsule Simulator (3capsules/ time); 3/day | Not reported | Not reported | He Shou wu (Polygonum multiflorum), Ren shen (Panax ginseng), Shi chang pu (Acorus tatarinowii) |
| Gu 2015 | 50/50 12 | Dihuang Yizhi decoction: 1pack/time, 2/d | Aniracetam capsules 200mg/time, 3/d | Not reported | Not reported | Di huang (Radix Rehmanniae), Zi he che (dried human placenta), Dan shen (Salvia miltiorrhiza), Shi chang pu (Acorus tatarinowii), Gui jia jiao (Tortoise shell glue), Fu shen (poria with hostwood), Yi zhi ren (Alpinia Oxyphylla Miq) |
| Chen 2016 | 43/43 12 | Kaixin Yizhi decoction 120 m/ time,2/d | Aniracetam capsules 200 mg/time, 3/d | Not reported | Not reported | Tai zi shen (Panax japonicus), Bai zi ren (Platycadus orientali), Tu si zi (The seed of Chinese dodder), Yuan zhi (polygala root), Fu ling (Poria cocos), Shi chang pu (Acorus tatarinowii), Ba ji tian (Morinda officinalis L), Gan cao (Radix liquiritiae) |
| Wang 2011 | 45/35 12 | Shouwu Yanshou pill, 6 g /time, 3 /d, | Aniracetam capsules 0.4 g/time,3/d | None | None | He shou wu (Polygonum multiflorum), Nv zhen zi (Ligustrum lucidum), Han mo lian (Eclipta), Sang ye (Mulberry leaves), Sang shen zi (Fructus Mori Albae), Du zhong (Eucommia ulmoides), Tu si zi (The seed of Chinese dodder), Huai niu xi (Achyranthes bidentata), Xi xian cao (Siegesbeckiae), Ren dong teng (Lonicera japonica), Jin ying zi (Fructus Rosae Laevigatae), Shu di (Radix Rehmanniae), Hei zhi ma (Semen Sesami nigrum) |
| Zhu 2010 | 50/50 12 | Xuan yun ning tablets, 2 tablets/time, 3/d | Piracetam Tablets 0.8 g/time, 3/d | Insomnia, | decreased appetite, palpitation, Alanine aminotransferase was slightly increased [(46–49) μ g/L] | Zei xie (Alisma orientalis)Bai zhu (Atractylodes macrocephala), Fu ling (Poria cocos), Chen pi (dried tangerine or orange peel), Ban xia (Pinellia ternata), Nv zhen zi (Ligustrum lucidum), Han mo lian (Eclipta), Ju hua (Chrysanthemum), Niu xi (Achyranthes bidentata), Gan cao (Radix liquiritiae) |
| Li 2016 | 30/30 12 | Shenwu Granules, 1 pack/time, 3/d; Naofukang tablets placebo 0.8 g/time, 3/d | Piracetam Tablets 0.8 g/time, 3/d; Shenwu Granules Placebo,1 pack /time, 3/d | Nausea, dry mouth | None | Ren shen (Panax Ginseng), He shou wu (Polygonum multiflorum), Dan shen (Salvia miltiorrhiza), Chuan xiong (Ligusticum wallichi), Chi shao (Radix Paeoniae Rubra), Hong hua (safflower)Shi chang pu (Acorus tatarinowii), Yuan zhi (polygala root), Shu di (Radix Rehmanniae), Shan zhu yu (Cornus officinalis)Shan yao (Chinese yam), Zei xie (Alisma orientalis), Yi zhi ren (Alpinia Oxyphylla Miq), Chen pi (dried tangerine or orange peel) |
| Lin 2020 | 47/41 12 | Tiaobu Xinshen Recipe, 150 mL/time; 2/d | Donepezil hydrochloride 5 mg/time 1/d | Not reported | Not reported | He shou wu (Polygonum multiflorum), Huang jing (Rhizoma Polygonatum), Huang qi (Astragalus membranaceus), Dang gui (Angelica sinensis), Gou qi zi (Fruit of Chinese wolfberry), Wu wei zi (Schisandra chinensis), Yuan zhi (polygala root), Shan zhu yu (Cornus officinalis), Dan shen (Salvia miltiorrhiza), Shi chang pu (Acorus tatarinowii), Chi shao (Radix Paeoniae Rubra) |
| Miao 2012 | 48/24 12 | Bushen Huatan Quyu granules, 1 pack / time, 2/d | Donepezil Hydrochloride, 5 mg/time, 1/d | Insomnia Nausea Diarrhea | Insomnia Dreaminess Nausea Diarrhea | Renshen(Radix Ginseng), Yin yang huo(Herba Epimedii Brevicornus), Yuanzhi(Radix polygalae) |
| Chen 2017 | 35/34 24 | Shenzhi oral liquid, 1/time, 2/d | Huperzine A tablets: 50 μ g/tablet, 2 tablets/time, 2/d | Not reported | Not reported | Dang shen(Codonopsis pilosula), Yuan zhi (polygala root), Gui zhi (Ramulus Cinnamomi), Bai shao (Radix Paeoniae Alba), Gan cao (Radix liquiritiae) |
| Sheng 2016 | 32/32 24 | Xintiaoxingfang granules, 1 pack/time, 2/d | Huperzine A tablets: 50 μ g/tablet,2 tablets/time, 2/d | Not reported | Not reported | Huang qi (Astragalus membranaceus), Hong jing tian (Rhodiola rosea), Yuan zhi (polygala root), Gui zhi (Ramulus Cinnamomi), Bai shao (Radix Paeoniae Alba), Gan cao (Radix liquiritiae) |
| Wang 2012 | 26/26 12 | Donepezil Hydrochloride, 5 mg/time,1/d; nimodipine, 40 mg /time,3/day, Guipi Decoction 200 mL daily, twice a d | Donepezil Hydrochloride, 5 mg/time,1/d; nimodipine, 40 mg /time, 3/d, | Not reported | Not reported | Ren shen (Panax Ginseng), Fu ling (Poria cocos), Di huang (Radix Rehmanniae), Bai zhu (Atractylodes macrocephala), Long yan rou (euphoria longan)Huang qi (Astragalus membranaceus), Suan zao ren (Semen Ziziphi Spinosae), Yuan zhi (polygala root), Shan zhu yu (Cornus officinalis), He shou wu (Polygonum multiflorum), Shi chang pu (Acorus tatarinowii), Huang qi (Astragalus membranaceus), Mu xiang (Radix Aucklandiae), Wu wei zi (Schisandra chinensis) |
| Xu 2013 | 30/30 12 | Nimodipine tablets 30 mg, 3/d, Qingli zengzhiyin: 150 mL/time, 2/d | Nimodipine Tablets 30 mg/time, 3/d | Abdominal discomfort | Head swelling | He shou wu (Polygonum multiflorum), Gou qi zi (The fruit of Chinese wolfberry), Yi zhi ren (Alpinia Oxyphylla Miq), Dang gui (angelica), Dan shen (Salvia miltiorrhiza), Hong hua (Safflowe), Shi chang pu (Acorus tatarinowii), Yu jin (radix curcumae), Chuan xiong (Ligusticum wallichi), Gan cao (Radix liquiritiae) |
| Wu 2017 | 53/49; 8 | Citicoline Sodium Tablets 0.2 g, 3/d and Dream sweet oral liquid 20 mL, 2/d | Citicoline Sodium Tablets 0.2 g, 3/d | Mild constipation | None | Ci wu jia (Acanthopanax), Huang jing (Polygonatum), Can e (Silkworm Moth), Sang shen (Mulberry), Dang shen (Codonopsis pilosula), Huang qi (Astragalus membranaceus), Sha ren (Amomum), Gou qi zi (Lycium Barbarum), Shan zha (Hawthorn), Shu di huang (Rehmannia glutinosa), Yin yang huo (Herba Epimedii Brevicornus), Chen pi (dried tangerine or orange peel), Fuling (Poria), Ma qian zi (Cymbidium), Ban xia (Pinellia), Ze xie (Rhizoma Alismatis), Shan yao (Yam) |
From left to right are the author's name, publication year, sample size, drug dosage, adverse events, and herbal composition, AEs = adverse events, CHM = Chinese herbal medicine.
3.3. Evaluation of the quality of the included studies
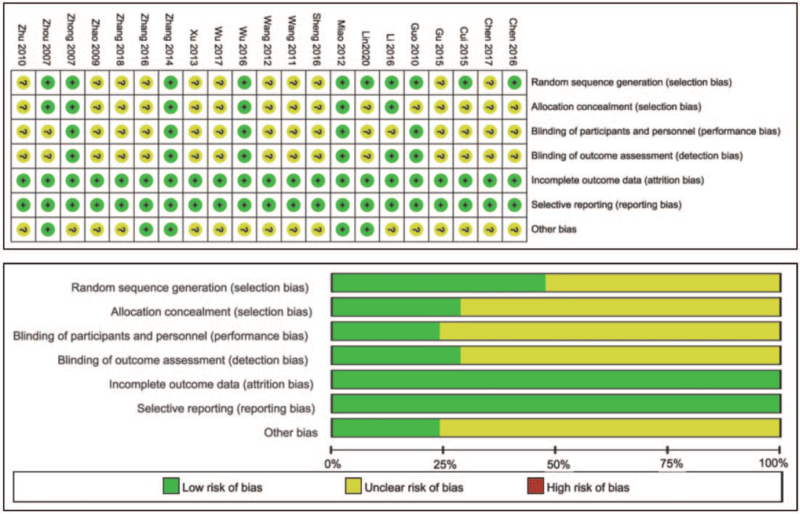
All 21 included studies that were described as RCTs, of which 9 specified an appropriate method of sequence generation and 6 referred to assignment concealment. All studies mentioned blinding, but only 6 specifically described blinding; all studies had complete outcome data, counted and described dropouts and lost patients, and did not selectively report results; 4 studies declared no conflicts of interest, and 17 did not declare conflicts of interest. (See Fig. 2, which illustrates the risk bias of the included studies).
Figure 2.
The risk bias of the included studies.
4. Comparative results
The collation revealed that the control groups included in the study were different, according to which they could be divided into 3 main groups:
- 1.
- 2.
-
3.
herbal combined with other drugs and other drugs groups[62–64]; the changes in MMSE scores in the experimental and control groups after the end of treatment were compared between the groups collated and analyzed. (See Table 2 which lists the Comparison result at the end of treatment: CHM VS Control group, Within 2 groups); because the Chinese herbal formulae in the experimental group were all compound drugs, each with many individual herbal medicines (single Chinese herbal medicines (CHMs)) in the mix, these studies emerged as Many duplicate herbs, and therefore the individual herbs that appeared were categorized and summarized, and
-
4.
the 10 drugs with the highest frequency of occurrence in the 21 studies were organized and summarized for a systematic evaluation concerning current herbal pharmacology studies.
Table 2.
Comparison result at the end of treatment: CHM VS Control group, within 2 groups.
| Comparison | Participants; Duration (in weeks) | Change between CHM and Control Group MD (95% CI) | Change within CHM group; MD (95% CI) | Change within Control group; MD (95% CI) |
| 1. CHM vs Placebo | ||||
| Zhang 2016 | 96;60 | 1.33 [−0.34,3.00] | 0.68 [−0.80,2.16] | −1.32[−2.52, −0.12] |
| Zhang 2014 | 12;28 | 0.68 [−0.67,2.03] | 1.50 [0.52, 2.48] | 0.50 [−0.95, 1.95] |
| Zhou 2007 | 48;79 | 0.64 [−0.09,1.37] | 2.22 [1.68, 2.76] | 1.81 [1.11. 2.51] |
| Wu 2016 | 12;78 | 2.53 [0.92, 4.14] | 1.35 [0.68, 2.02] | −0.60 [−2.77,1.57] |
| 2. CHM vs Other drugs | ||||
| CHM vs Nimodipine | ||||
| Zhang 2018 | 12;60 | 3.15 [1.30, 5.00] | 4.68 [2.17,7.19] | 2.12 [0.79, 3.45] |
| Zhao 2009 | 12;60 | 0.32 [−0.09, 0.73] | 1.35 [0.68,2.02] | −0.60 [−2.77,1.57] |
| Cui 2015 | 12;32 | 0.13 [−0.92,1.18] | 2.00 [1.16, 2.84] | 2.06 [1.16, 2.96] |
| CHM vs Aniracetam | ||||
| Guo 2010 | 12;64 | 1.09 [0.18, 2.00] | 2.91 [2.21, 3.61] | 1.94 [1.24, 2.64] |
| Zhong 2007 | 12;166 | −0.06 [−0.61, 0.49] | 0.77 [0.16, 1.38] | 0.57 [0.01, 1.13] |
| Gu2015 | 12;100 | −0.39 [−1.04, 0.26] | 1.00 [0.13, 1.87] | 0.63 [0.02, 1.24] |
| Chen 2016 | 12;86 | 0.24 [−0.41, 0.89] | 0.42 [−0.04, 0.88] | 0.97 [0.41, 1.53] |
| Wang 2011 | 12;80 | 1.41 [0.44, 2.38] | 3.40 [2.47, 4.33] | 1.68 [0.73, 2.63] |
| CHM vs Piracetam | ||||
| Zhu 2010 | 12;100 | 0.90 [0.37, 1.43] | 2.48 [1.94, 3.02] | 1.58 [1.04, 2.12] |
| Li 2016 | 12;60 | 0.29 [−0.23, 0.81] | 1.00 [0.49, 1.51] | −0.97 [−1.44, −0.50] |
| CHM vs Donepezil | ||||
| Lin 2020 | 12;88 | −0.21 [−0.95,0.53] | 1.57 [0.79, 2.35] | 1.57 [0.84, 2.03] |
| Miao 2012 | 12;72 | −0.17 [−0.80, 0.46] | 0.11 [−0.34, 0.56] | 0.36 [−0.48, 1.20] |
| CHM vs Huperzine | ||||
| Chen 2017 | 24;69 | 0.54 [−049, 1.57] | 1.72 [1.47,1.97] | 2.22 [2.03, 241] |
| Sheng 2016 | 24;64 | 0.03 [−0.22, 0.28] | 2.63 [1.35,3.91] | 2.18 [1.16, 3.20] |
| 3. CHM + Other drugs vs Other drugs | ||||
| Wang 2012 | 12;52 | 2.72 [0.32, 5.12] | 4.68 [2.17,7.19] | 2.12 [0.79, 3.45] |
| Xu 2013 | 12;60 | 1.00 [−0.14, 2.14] | 1.40 [0.50,2.30] | 0.42 [0.52, 1.36] |
| Wu 2017 | 8;102 | 3.94 [3.52, 4.36] | 8.15 [7.62,8.68] | 4.04 [3,48, 4.60] |
4.1. Chinese herbal medicine versus placebo
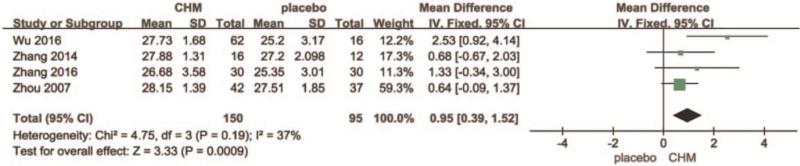
There were 4 studies in this group,[44–47] with a treatment duration of 12 to 96 weeks, 245 participants at baseline, and 3 studies using randomized numbers and table-blinded double simulations, which were assessed as high-quality studies.[45–47] The other study only mentioned randomization and blinding without specifying the method, and the risk was unclear.[44] After a heterogeneity test, I2 = 37% <50% and P = .19>0.1 for the Q-test, suggesting no heterogeneity between groups. A fixed-effects model could be selected for meta-analysis, which showed that the MD = 0.95, 95% confidence interval [0.39, 1.52], Z = 3.33, and P = .0009<.05 for the 4 literature summaries were statistically significant, and the results were significantly biased towards the CHM group. In one of the 2 12-week studies, Bushen Jianpi Huatan Pills had a significantly higher MMSE score (MD = 2.53 [0.92, 4.14]),[47] whereas the change in Compound congrongyizhi capsule was not very significant (MD = [−0.67, 2.03]). In the remaining 2 studies, Bushen capsule treatment for 96 weeks (MD = 1.33 [−0.34, 3.00]) and Senin Oral Liquid treatment for 48 weeks (MD = −0.64 [−0.09, 1.52]),[44–46] there was no significant pattern in the length of treatment cycle or change in MMSE score can be followed. All changes in scores in the placebo group were not significant. (See Fig. 3, which illustrates the forest plot for Chinese Herbal Medicines versus Placebo at end of treatment).
Figure 3.
Forest plot for Chinese Herbal Medicines versus Placebo at end of treatment.
4.2. Chinese herbal medicine versus other treatments
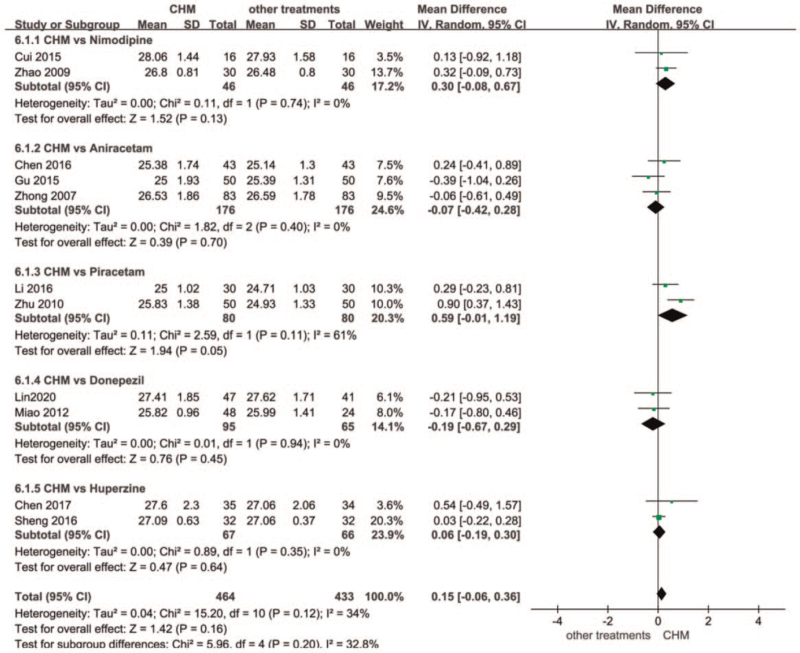
There were 14 studies in this group, treatment duration was 12 to 24 weeks, there were 1101 participants at baseline, and 3 studies were assessed as high-quality studies using randomized numerical and tabular double-blind, double simulations.[52,57,59] After heterogeneity test, I2 = 34%, Q test P = .12, indicating that there is no heterogeneity between the groups, and the overall result is biased towards the CHM group, indicating that the CHM group is better than other drug intervention groups. The research found that the intervention can be based on Divided into 5 subgroups. (See Fig. 4, which illustrates the forest plot for Chinese Herbal Medicines versus other treatments at end of treatment).
Figure 4.
Forest plot for Chinese Herbal Medicines versus other treatments at end of treatment.
The first group of traditional Chinese medicine was compared with nimodipine (CHM vs Nimodipine), a total of 2 studies, the treatment time was 12 weeks. The results show that I2 = 0% in the summarization of the 2 literatures, and the P = .74 of the Q test indicates no difference. Jinkui Shenqi Pills MD [50] = 0.32 [−0.09, 0.73], Naofucong MD[51] = 0.13[-0.92, 1.18], the overall MD of the 2 studies was 0.30, 95% confidence interval [−0.08, 0.67], the results were obviously biased towards the CHM group, Z = 152, but P = .13.
The second group of traditional Chinese medicine compared with Aniracetam (CHM vs Aniracetam) has a total of 5 studies. Excluding the studies with higher heterogeneity, 3 studies remain. The treatment time was also 12 weeks, I2 = 0%, P = .40, suggesting that the heterogeneity between the groups is very low. One study Kaixin Yizhi decoction MD 0.24 [−0.41, 0.89] [55] the results were significantly more focused on the CHM group. The results of the remaining 2 studies Shenwu Capsule MD = −0.06[−0.61, 0.49][53] and Dihuang Yizhi decoction MD = −0.39[−1.04,0.26] [54] are slightly more important than the aniracetam group, 3 items The overall MD of the study was −0.07, 95% confidence interval [−0.42, 0.28], Z = 0.39, P = .70.
The third group of traditional Chinese medicine vs. Piracetam (CHM vs. Piracetam) There were 2 studies, the treatment time was 12 weeks, I2 = 61%>50%, P = .11>0.1 of the Q-test suggested that the difference between the groups was not significant, which may be related to the sample size and results. The MMSE scores of the 2 studies were higher than piracetam, and the more participants Xuan Yun Ning tablets MD = 0.90 [0.37, 1.43][56] scored higher than Shenwu Granules MD = 0.29 [−0.23,0.81],[57] which had fewer participants. The MD = 0.59, 95% confidence interval [−0.01, 1.19], Z = 1.94, P = .05 for both studies was not statistically significant and needed to be supported by more evidence.
The fourth group of CHM versus Donepezil (CHM vs. Donepezil) had 2 studies with a treatment duration of 12 weeks, I2 = 0%<50%, P = .94>0.1 in Q-test suggesting no difference between the groups, 2 studies Tiaobu Xinshen Recipe MD = −0.21[−0.95,0.53][58] and The results of Bushen Huatan Quyu granule −0.17 [−0.80,0.46][59] were biased towards the donepezil group MD = −0.19, 95% confidence interval [−0.67,0.29], Z = 0.76, P = .45, which was not statistically significant.
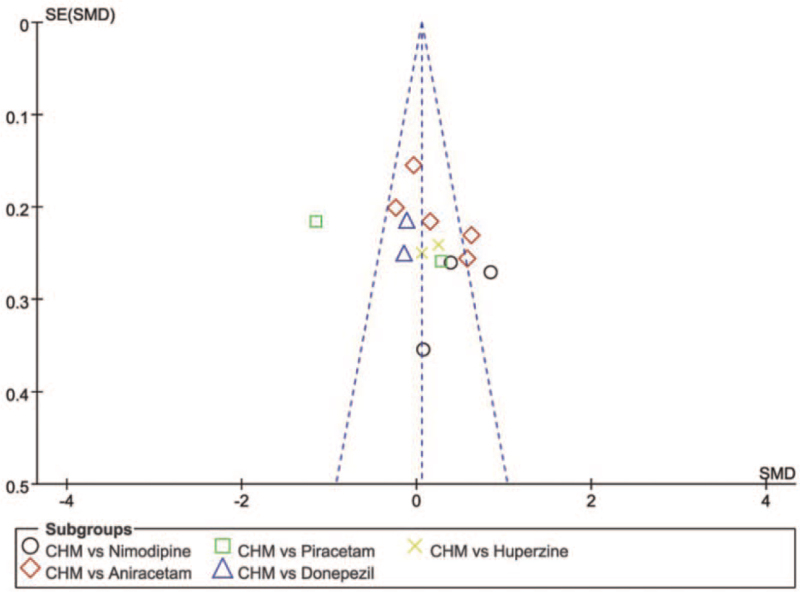
The fifth group of traditional Chinese medicine vs. Huperzine A (CHM vs. Huperzine) had a total treatment duration of 24 weeks, I2 = 0%<50%, P = .35>0.1 of Q-test suggested no difference between the groups; Shenzhi oral liquid MD = 0.54[−049, 1.57][60] had significantly higher MMSE scores. The 2 groups’ scores were not significantly different MD = 0.06, 95% confidence interval [−0.19, 0.30], Z = 0.47, P = .64, and were not statistically significant. There were 14 studies in this group, and publication bias was assessed using funnel plots, with most of the studies having good symmetry in the distribution on both sides of the funnel plot, and 2 studies being significantly asymmetric, suggesting possible bias. (See Fig. 5, which illustrates the funnel chart of the Chinese Herbal Medicines group versus the other treatments group).
Figure 5.
The funnel chart of the Chinese Herbal Medicines versus the other treatments.
4.3. Chinese herbal medicine plus versus other treatments
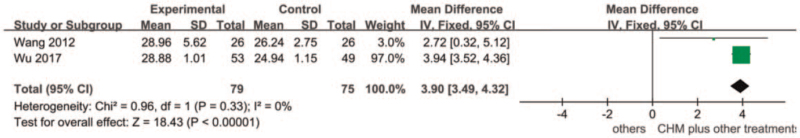
This group consisted of 2 studies,[62,64] with a treatment duration of 8 to 12 weeks and 154 participants at baseline, 2 studies only mentioned randomization and blinding without specifying methods, and the risk is unclear. After heterogeneity testing, I2 = 0% and P = .33 for the Q-test, There is no heterogeneity between groups. The fixed-effect model was selected for meta-analysis, and the results showed that MD = 3.90, 95% confidence interval [3.49, 4.32] Z = 2.28, and P = <.00001, which were statistically significant. The results were significantly more focused on traditional Chinese medicine combined with other Drug group. (See Fig. 6, which illustrates the meta-analysis of Chinese Herbal Medicines plus other treatment group versus other treatments group).
Figure 6.
Forest plot for Chinese Herbal Medicines plus other treatment versus other treatments at end of treatment.
4.4. Characteristics of the Chinese herbal medicine included in the study
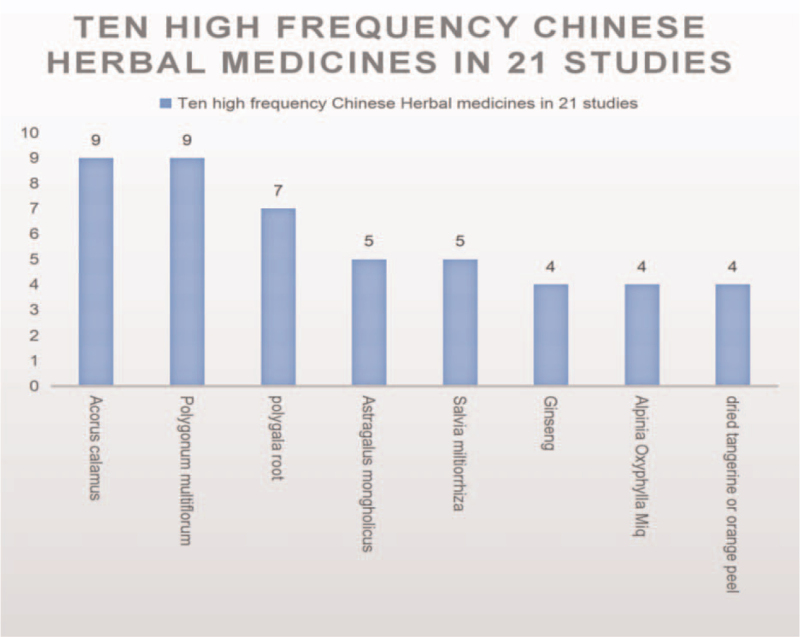
The herbal medicines that appeared in the study were organized and summarized into a total of 69 drugs, of which 63 were botanical, and 6 were animal. The top 8 drugs that appeared most frequently were: Acorus calamus, Polygonum multiflorum, polygala root, Astragalus mongholicus, Salvia miltiorrhiza, Ginseng, Alpinia Oxyphylla Miq, and dried tangerine or orange peel. (See Fig. 7, which illustrates ten high frequency Chinese Herbal Medicines in 21 studies).
Figure 7.
Ten high frequency Chinese Herbal Medicines in 21 studies.
A search of these 8 drugs revealed that many experiments had elucidated these drugs’ mechanism of action. Some studies have shown that the calamus can effectively prevent stress-induced cognitive dysfunction and anxiety behavior,[65] the volatile oil of the calamus may induce hippocampal neuron regeneration in mice and promote hippocampal growth[66] its extract has anticholinesterase activity[67] can also be able to alleviate AB transgenesis. Cognitive deterioration in Drosophila.[68] The tetrahydro diphenyl-2-O-β-D-glucoside in Polygala tenuifolia Willd has a significant neuroprotective effect against glutamate-induced hippocampal injury by reducing reactive oxygen species production and stabilizing glutamate-induced mitochondrial membrane potential.[69] Aerial parts of Polygala tenuifolia Willd can, by modulating cholinergic activity, Promotes brain-derived neurotrophic factors and inhibits neuroinflammation and oxidative stress to improve learning and memory deficits.[70] Salvianolic acid B isolated from Salvia miltiorrhiza improved cognitive performance and restored cognitive deficits,[71,72] and total salvianolic acid improved learning ability and memory deficits in mice.[73] Astragaloside not only reduced the over-activation of hippocampal microglia but also inhibited the overexpression of inflammatory cytokines.[74] Ginseng extract improves memory impairment in rats and affects brain tissue histological characteristics,[75] and ginsenoside restores memory impairment and has an anti-brain aging effect.[76] The main compound of the extract of Alpinia oxyphylla Miq has neuroprotective effects.[77] Thenaringin in tangerine peel has antioxidant and anti-inflammatory properties that can protect ischemic brain nerves[78] and inhibit Tau protein hyperphosphorylation in mice.[79]
5. Adverse event
In 21 studies no serious adverse events were reported, 10 reported mild adverse events, and 6 studies in the CHM group reported adverse events, mainly insomnia, swollen gums, nausea, decreased appetite, constipation, diarrhea, and several other mild adverse events, none of which were suspended after the adverse events and no pharmacological interventions were performed, and the participants improved spontaneously. In one study, 11 participants in the piracetam group had mildly elevated glutamyltransferase [(46–49) μg/L], and 2 participants had panic attacks.[56] Three studies had no adverse events, and 8 studies did not report adverse events, with insufficient safety data for statistical analysis.
6. Discussions
This study conducted a comprehensive search of English and Chinese databases and retrieved a total of 35 RCTs on herbal medicine for MCI using MMSE as an outcome measure. Only 21 studies containing MMSE outcome data were meta-analyzed. MMSE scores increased at the end of treatment in all herbal groups, with 18 studies in the experimental group (n = 721) using herbs alone increasing their scores by an average of 1.76 points; 17 studies in the control group (n = 635) using other cognitive-improving drugs, increasing their scores by an average of 1.34 points; and 3 studies in the experimental group (n = 109) using herbs plus other cognitive-improving drugs scoring the mean increase was 4.74 points; in the herbal and placebo groups the mean MMSE score for 1 96-week study increased by only 0.68 points,[44] the mean scores for 2 12-week studies increased by 1.50 and 1.35 points, respectively.[45,47] The score for 1 48-week study increased by 2.22 points, considering that herbal medicine's efficacy may peak at 1 year of treatment, exceeding the efficacy of the treatment tapers off after 1 year. In the herbal and other cognitive improvement drugs group, the results of 3 12-week studies comparing with nimodipine were skewed in favor of the herbal group, with the herbal group scoring significantly ahead of nimodipine by 3.15 points in 1 study[48] and not significantly in the other 2.[49,50] In a 12-week comparison with anisiracetam, MMSE scores in 2 of the studies significantly favored the herbal group. However, the change in scores was not significant in the other 3 studies. The results in the 12-weeks comparison of the piracetam group were heavily weighted towards the CHM, with significant changes in both the CHM and aniracetam groups in 1 of the more heavily attended studies (n = 100) and a decrease in the aniracetam score of -0.9 in the other study (n = 60), rather than an increase. The 2 12-week studies’ scores comparing the donepezil group were both heavily weighted towards donepezil, but the change in scores was not significant. The 2 studies’ scores comparing the stigmata A's were heavily weighted toward the CHM group, and the change in scores was not significant. One 8-week (n = 100) study in the CHM combined with other drugs group improved the mean score by 8.15 points, the largest change in this study. The changes in the other 2 12-week studies were also significant. These studies showed that herbal medicine was significantly better than placebo in improving MMSE scores; although most of the results were in favor of the CHM group, the evidence was insufficient to show that the herbal medicine group possessed a better ability to improve cognition compared to other drugs. The combination of herbal medicine with other cognition-improving medications was able to improve MMSE scores substantially. There was little correlation between the number of participants and final MMSE scores in this study, with greater variation in shorter studies scores. It is noteworthy that some studies have shown that the level of education, age, and gender can make a difference in MMSE score results.[80–82] A mean MMSE score change of 3.7 is an important threshold for AD,[83] but how much the MMSE of MCI changes to be clinically meaningful needs further validation. The MMSE sensitivity to MCI has been much debated in recent years, and although it is less sensitive in screening, it is still a good tool for detection.[29,30] Future studies would be more meaningful if the participants were observed in different stages according to their age and if a protocol for use that is consistent with Chinese conditions was used.[84,85] Although clinical studies have many shortcomings, the results of animal, cellular, and pharmacological studies are encouraging, and in addition to the above-mentioned herbs, many other herbs have been shown to improve cognitive function or protect the nerves, such as resveratrol, saffron, and turmeric, and the extracted compounds are more than sufficient to improve cognition,[86] Poria (Poria cum Radix (Pini) can reduce amyloid-associated motor dysfunction and premature death of AD transgenes in transgenic Drosophila and exert neuroprotective effects,[87] The seeds of Zizyphus jujuba var (Zizyphus jujuba var) alleviate AD-like symptoms in 5Xfad mice and modulate fibrinolytic activity, and similar studies have been conducted.[88] A lot, but that is just the beginning, the ability for these compounds to be absorbed by the body and achieve the desired effect is what really counts as success. The method of identifying active compounds from clinical observations is now often referred to as reverse pharmacology,[89] and the 8 drugs with a high frequency of repetition compiled from the 21 studies in this paper have found some basis through experiments. Some of the drugs with a low frequency of repetition have been discovered through experiments, such as dang ginseng, aconite, and deer antler, while most of the remaining herbs have yet to be discovered. The historical literature on herbal medicine in China dates back to at least 2000 years, with tens of thousands of herbal plants, many of which have pharmacological activity and drug-like properties.[90] 2000 years of documented herbal medicines have been aggregated in authoritative pharmacopeias, counting more than 10,000 herbal medicines,[91] and the herbal medicines mentioned in this study are among them. Discovered treasures.
7. Limitations of this study
These studies varied widely in the number of participants and duration of treatment, with only 4 studies with more than 100 participants, many with low numbers of participants, 1 study in the comparative placebo group lasted 96 weeks, and another lasted 48 weeks. Two studies in the comparative stigmata A group lasted 24 weeks, and the remaining studies were observed for shorter periods. Only 6 studies specifically elaborated on blinding, there was significant heterogeneity between groups when performing the meta-analysis, and many studies were methodologically flawed. Many subjective factors can confound MMSE scores in addition to the patient's education, age, and gender, and these weaknesses limit the strength of the evidence. More high-quality, large-sample clinical studies with clear objective outcome measures (e.g., biomarkers, imaging tests, etc.) are needed to further delve into whether herbs can improve cognition of MCI.
8. Conclusion
The results show that herbal medicine can improve MMSE scores, and herbal medicine combined with other drugs that can improve cognition can significantly improve MMSE scores, but there are methodological flaws in the study. Experimental studies have found a basis for herbs’ ability to improve cognition and memory impairment, and herbal medicine has great potential to improve MCI cognition.
Acknowledgment
Thanks to Professor Yongchang Diwu for putting forward the research direction and quality control of the article.
Author contributions
Conceptualization: Wei Wang.
Data curation: Yuan Zhou.
Investigation: Wei Wang.
Methodology: Wei Wang.
Project administration: Yuan Zhou.
Resources: Qi Liu, Dengkun Wang, Yurui Gou.
Supervision: Yongchang Diwu, Qi Liu.
Writing – original draft: Wei Wang, Tayed Ilsam Sayed.
Writing – review & editing: Wei Wang, Yongchang Diwu.
Footnotes
Abbreviations: AD = Alzheimer disease, CHM = Chinese herbal medicine, CHMs = single Chinese herbal medicines, MCI = mild cognitive impairment, MMSE = the mini mental state examination.
How to cite this article: Wang W, Diwu Y, Liu Q, Zhou Y, Sayed TI, Wang D, Gou Y. Chinese herbal medicine for mild cognitive impairment using mini-mental state examination: a systematic review and meta-analysis. Medicine. 2021;100:38(e27034).
The Disciplinary Innovation Team of Shaanxi University of Traditional Chinese Medicine (No.2019-QN05); Shaanxi Province “Special Support Program” Technology Innovation Leadership Project; National Natural Science Foundation of China (Project Number: 82074503); Shaanxi Province Natural Science Basic Research Program-Major Foundation Funding of the research project (project number: 2017ZDJC-15).
Ethical approval was not required for this study.
The authors have no conflicts of interests to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Chehrehnegar N, Nejati V, Shati M, et al. Early detection of cognitive disturbances in mild cognitive impairment: a systematic review of observational studies. Psychogeriatrics 2020;20:212–28. [DOI] [PubMed] [Google Scholar]
- [2].Petersen RC. Clinical practice. Mild cognitive impairment. N Engl Med 2011;364:2227–34. [DOI] [PubMed] [Google Scholar]
- [3].Prestia A, Caroli A, van der Flier WM, et al. Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology 2013;80:1048–56. [DOI] [PubMed] [Google Scholar]
- [4].Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2018;90:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hussin NM, Shahar S, Yahya HM, Din NC, Singh DKA, Omar MA. Incidence and predictors of mild cognitive impairment (MCI) within a multi-ethnic Asian populace: a community-based longitudinal study. BMC Public Health 2019;19:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].World Health Organization. Alzheimer's disease accounts for 60%-70% of the total number of patients with dementia.[EB/OL].[2020.9.21] https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed January 10, 2021. [Google Scholar]
- [7].Alzheimer Disease International. World Alzheimer Report 2015: The Global Impact of Dementia. London, England. Retrieved from www.alz.co.uk. Accessed January 10, 2021. [Google Scholar]
- [8].Li D, Jinchao W, Li Y, et al. Choline transporters and Alzheimer's disease in Chinese. Progress Biochemistry Biophysics 2014;41:1207–13. [Google Scholar]
- [9].Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 2005;62:1160–7. [DOI] [PubMed] [Google Scholar]
- [10].Cooper C, Li R, Lyketsos C, et al. A systematic review of treatments for mild cognitive impairment. Br J Psychiatry 2013;203:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ilhan Algin D, Dagli Atalay S, Ozkan S, Ozbabalik Adapinar D, Ak Sivrioz I. Memantine improves semantic memory in patients with amnestic mild cognitive impairment: A single-photon emission computed tomography study. J Int Med Res 2017;45:2053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tricco AC, Soobiah C, Berliner S, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ 2013;185:1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moyer VA. U. S. preventive services task force. Screening for cognitive impairment in older adults: U. S. Preventive services task force recommendation statement. Ann Intern Med 2014;160:791–7. [DOI] [PubMed] [Google Scholar]
- [14].Kandiah N, Ong PA, Yuda T, et al. Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: expert consensus on the use of Ginkgo biloba extract, EGb 761®. CNS Neurosci Ther 2019;25:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kasper S, Bancher C, Eckert A, et al. Management of mild cognitive impairment (MCI): the need for national and international guidelines published online ahead of print. World J Biol Psychiatry 2020. 01–16. [DOI] [PubMed] [Google Scholar]
- [16].Wu TY, Chen CP, Jinn TR. Traditional Chinese medicines and Alzheimer's disease published correction appears in Taiwan J Obstet Gynecol. 2011 Sep; 50(3):408. Chen, Chip-Ping corrected to Chen, Chih-Ping. Taiwan J Obstet Gynecol 2011;50:131–5. [DOI] [PubMed] [Google Scholar]
- [17].May BH, Yang AW, Zhang AL, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology 2009;10:109–23. [DOI] [PubMed] [Google Scholar]
- [18].Dong L, May BH, Feng M, et al. Chinese herbal medicine for mild cognitive impairment: a systematic review and meta-analysis of cognitive outcomes. Phytother Res 2016;30:1592–604. [DOI] [PubMed] [Google Scholar]
- [19].Dong L, Hyde AJ, Zhang AL, Xue CC, May BH. Chinese herbal medicine for mild cognitive impairment using montreal cognitive assessment: a systematic review. J Altern Complement Med 2019;25:578–92. [DOI] [PubMed] [Google Scholar]
- [20].McGrattan AM, McEvoy CT, McGuinness B, McKinley MC, Woodside JV. Effect of dietary interventions in mild cognitive impairment: a systematic review. Br J Nutr 2018;120:1388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Román GC, Jackson RE, Gadhia R, Román AN, Reis J. Mediterranean diet: The role of long-chain (-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris) 2019;175:724–41. [DOI] [PubMed] [Google Scholar]
- [22].Olivera-Pueyo J, Pelegrín-Valero C. Dietary supplements for cognitive impairment. Actas Esp Psiquiatr 2017;45: (Supplement): 37–47. [PubMed] [Google Scholar]
- [23].Devenney KE, Sanders ML, Lawlor B, Olde Rikkert MGM, Schneider S. NeuroExercise Study Group. Erratum to: The effects of an extensive exercise programme on the progression of mild cognitive impairment (MCI): study protocol for a randomised controlled trial. BMC Geriatr 2017;17:112.doi: 10.1186/s12877-017-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sungkarat S, Boripuntakul S, Chattipakorn N, Watcharasaksilp K, Lord SR. Effects of tai chi on cognition and fall risk in older adults with mild cognitive impairment: a randomized controlled trial. J Am Geriatr Soc 2017;65:721–7. [DOI] [PubMed] [Google Scholar]
- [25].Biazus-Sehn LF, Schuch FB, Firth J, Stigger FS. Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: a systematic review and meta-analysis. Arch Gerontol Geriatr 2020;89:104048.doi: 10.1016/j.archger.2020.104048. [DOI] [PubMed] [Google Scholar]
- [26]. Tian JZ, ed. Guidelines for the diagnosis and treatment of dementia in China. Beijing: People's Medical Publishing House, 2017:210-212. [Google Scholar]
- [27]. Jia JP, ed. Guidelines for the diagnosis and treatment of dementia and mild cognitive impairment in China. Beijing: People's Health Press, 2015:47-48. [Google Scholar]
- [28].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [29].Patnode CD, Perdue LA, Rossom RC, et al. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020. [PubMed] [Google Scholar]
- [30].Tangalos EG, Petersen RC. Mild cognitive impairment in geriatrics. Clin Geriatr Med 2018;34:563–89. [DOI] [PubMed] [Google Scholar]
- [31].Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993;269:2386–91. [PubMed] [Google Scholar]
- [32].Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's Disease (AD) in the elderly? Int Psychogeriatr 2019;31:491–504. [DOI] [PubMed] [Google Scholar]
- [33].Arevalo-Rodriguez I, Smailagic N, Roqué I, Figuls M, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 2015;2015:CD010783.doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaneko H, Kimura N, Nojima S, Abe K, Aso Y, Matsubara E. Diagnosis of mild cognitive impairment using multiple neuroimaging modalities in addition to the Mini-Mental State Examination. Geriatr Gerontol Int 2019;19:1193–7. [DOI] [PubMed] [Google Scholar]
- [35].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8. [DOI] [PubMed] [Google Scholar]
- [36].Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- [37].Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. fifth editionWashington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- [39].Batistela MS, Josviak ND, Sulzbach CD, de Souza RL. An overview of circulating cell-free microRNAs as putative biomarkers in Alzheimer's and Parkinson's Diseases. Int J Neurosci 2017;127:547–58. [DOI] [PubMed] [Google Scholar]
- [40].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01.doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China (Zhong Hua Ren Min Gong He Guo Yao Dian). Beijing: China Medical Science Press; 2011. [Google Scholar]
- [42].Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. [EB/OL] (2019-07) http://www.training.cochrance.org/handbook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang J, Liu Z, Zhang H, et al. A two-year treatment of amnestic mild cognitive impairment using a compound Chinese medicine: a Placebo Controlled Randomized Trial published correction appears in Sci Rep. 2016 Aug 19;6:30511. Sci Rep 2016;6:28982.doi: 10.1038/srep30511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang J, Wang Z, Xu S, et al. The effects of CCRC on cognition and brain activity in aMCI patients: a pilot placebo controlled BOLD fMRI study. Curr Alzheimer Res 2014;11:484–93. [DOI] [PubMed] [Google Scholar]
- [46].Zhou Ruqian, Lin Shuimiao, Yuan Quan. Clinical study of Shenyin oral liquid in the treatment of mild cognitive impairment in Chinese. Chin J Integrated Traditi Chin Western Med 2007. 793–5. [PubMed] [Google Scholar]
- [47].Wu Dongyue, Tian Jinzhou, Wei Mingqing, et al. A randomized double-blind controlled study on the treatment of mild cognitive impairment with the method of invigorating the kidney and spleen and resolving phlegm in Chinese. Modern J Integrated Traditi Chin Western Med 2016;25:229–31. [Google Scholar]
- [48].Zhang Tingting, Wang Lei, Wang Xingchen. Clinical observation of Qingnaoyizhi granule in the treatment of 30 cases of mild cognitive impairment and memory impairment in Chinese. J Integrated Traditi Chin Western Med Cardio-Cerebrovasc Dis 2018;16:1769–70. [Google Scholar]
- [49].Zhen Z, Liwei Z. Clinical observation on the treatment of cognitive dysfunction in old and young with Jinkuishenqi pills in Chinese. Jilin Traditi Chin Med 2009;29:579–80. [Google Scholar]
- [50].Yazhong C, Furong L, Mengren Z. Clinical observation on the treatment of 16 patients with mild cognitive dysfunction of kidney deficiency and blood stasis type with Naofucong decoction in Chinese. J Traditi Chin Med 2015;56:1577–80. [Google Scholar]
- [51].Renzhen G, Wenquan Z, Zenggang L, et al. Clinical study of Huanglian Wendan Decoction in the treatment of cognitive impairment in the elderly and young with phlegm and turbidity blocking orifice syndrome in Chinese. Chin J Integrated Traditi Chin Western Med 2010;30:33–6. [PubMed] [Google Scholar]
- [52].Jian Z, Aihua Z, Chengzhi Y, Jinzhou T. A clinical controlled study on Shenwu Capsule in the treatment of cognitive impairment in the elderly and young in Chinese. Chin J Chin Materia Med 2007. 1800–3. [PubMed] [Google Scholar]
- [53].Chao G, Ting S, Guojiang M, et al. Clinical observation on treatment of amnestic mild cognitive impairment (kidney essence deficiency and phlegm stasis syndrome in Chinese. Sichuan Tradition Chin Med 2015;33:67–73. [Google Scholar]
- [54].Chen Yongcan, Bai Yu, Wang Yaqun, et al. Clinical observation of kaixinyizhiyin treating mild cognitive dysfunction in Chinese. Chin J Traditi Chin Med 2016;31:5370–2. [Google Scholar]
- [55].Xi W, Huilin S. Clinical observation of Shouwu Yanshoudan in the treatment of cognitive impairment in young and old In Chinese. Hubei J Traditi Chin Med 2011;33:12–3. [Google Scholar]
- [56].Liming L Z. Clinical observation on the treatment of phlegm turbidity obstructing orifice syndrome of cognitive impairment in young and old with Xuanyunning [In Chinese]. China Med Guide 2010;12:2107–9. [Google Scholar]
- [57].Lejun L, Yumei L, Chonghe Y. Randomized double-blind controlled clinical study on the treatment of amnestic mild cognitive impairment with Shenwu granules [In Chinese]. Chin J Basic Med Traditi Chin Med 2016;22:655–7. [Google Scholar]
- [58].Lin ZY, Huang TW, Huang JS, Zheng GY. Tiaobu Xinshen recipe improved mild cognitive impairment of Alzheimer's disease patients with Xin (Heart) and Shen (Kidney) deficiency. Chin J Integr Med 2020;26:54–8. [DOI] [PubMed] [Google Scholar]
- [59].Miao YC, Tian JZ, Shi J, Mao M. Effects of Chinese medicine for tonifying the kidney and resolving phlegm and blood stasis in treating patients with amnestic mild cognitive impairment: a randomized, double-blind and parallel-controlled trial. Zhong Xi Yi Jie He Xue Bao 2012;10:390–7. [DOI] [PubMed] [Google Scholar]
- [60].Jiulin C, Shenwei S, Zhihua Y, Fei S, Liang Z. Observation on the clinical efficacy of Shenzhiling oral liquid in the treatment of mild cognitive impairment [In Chinese]. J Integrated Traditi Chin Western Med Cardio-Cerebrovasc Dis 2017;15:365–8. [Google Scholar]
- [61].Sheng F, Yu Z, Zhou L, Chen J, Wang J. Clinical observation on 64 cases of mild cognitive impairment treated by Xin Tiaoxin decoction in Chinese. Shanghai Med 2016;37:26–8. [Google Scholar]
- [62].Zhuoer L W. Observation on 26 cases of mild cognitive impairment treated with integrated traditional Chinese and western medicine in Chinese. Zhejiang J Traditi Chin Med 2012;47:288. [Google Scholar]
- [63].Xu Suzhi, Wang Jing, Li Yuzhi, Zhu Jianwen, Wang Shaoying. Clinical observation on 30 cases of mild cognitive impairment treated by Qiangli Zengzhi Decoction in Chinese. Hebei Traditi Chin Med 2013;35:1475–6. [Google Scholar]
- [64].Wu Di, Guo Ruijing, Wang Yue. Clinical observation of Tianmeng oral liquid in treating mild cognitive impairment with spleen and kidney deficiency in Chinese. Chin Herbal Med 2017;48:4958–62. [Google Scholar]
- [65].Reddy S, Rao G, Shetty B, Hn G. Effects of acorus calamus rhizome extract on the neuromodulatory system in restraint stress male rats. Turk Neurosurg 2015;25:425–31. [DOI] [PubMed] [Google Scholar]
- [66].Gao N, Liu H, Li S, et al. Volatile oil from acorus gramineus ameliorates the injury neurons in the hippocampus of amyloid beta 1-42 injected mice. Anat Rec (Hoboken) 2019;302:2261–70. [DOI] [PubMed] [Google Scholar]
- [67].Oh MH, Houghton PJ, Whang WK, Cho JH. Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine 2004;11:544–8. [DOI] [PubMed] [Google Scholar]
- [68].Zhang WY, Feng XL, Lu D, Gao H, Yu Y, Yao XS. New lignans attenuating cognitive deterioration of Aβ transgenic flies discovered in Acorus tatarinowii. Bioorg Med Chem Lett 2018;28:814–9. [DOI] [PubMed] [Google Scholar]
- [69].Lee SY, Ahn SM, Wang Z, Choi YW, Shin HK, Choi BT. Neuroprotective effects of 2,3,5,4’-tetrahydoxystilbene-2-O-β-D-glucoside from Polygonum multiflorum against glutamate-induced oxidative toxicity in HT22 cells. J Ethnopharmacol 2017;195:64–70. [DOI] [PubMed] [Google Scholar]
- [70].Wang X, Zhang D, Song W, et al. Neuroprotective effects of the aerial parts of Polygala tenuifolia Willd extract on scopolamine-induced learning and memory impairments in mice. Biomed Rep 2020;13:37.doi: 10.3892/br.2020.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kim DH, Park SJ, Kim JM, et al. Cognitive dysfunctions induced by a cholinergic blockade and Aβ 25-35 peptide are attenuated by salvianolic acid B. Neuropharmacology 2011;61:1432–40. [DOI] [PubMed] [Google Scholar]
- [72].Wang W, Hu W. Salvianolic acid B recovers cognitive deficits and angiogenesis in a cerebral small vessel disease rat model via the STAT3/VEGF signaling pathway. Mol Med Rep 2018;17:3146–51. [DOI] [PubMed] [Google Scholar]
- [73].Shen L, Han B, Geng Y, Wang J, Wang Z, Wang M. Amelioration of cognitive impairments in APPswe/PS1dE9 mice is associated with metabolites alteration induced by total salvianolic acid. PLoS One 2017;12:e0174763.doi: 10.1371/journal.pone.0174763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li M, Li H, Fang F, Deng X, Ma S. Astragaloside IV attenuates cognitive impairments induced by transient cerebral ischemia and reperfusion in mice via anti-inflammatory mechanisms. Neurosci Lett 2017;639:114–9. [DOI] [PubMed] [Google Scholar]
- [75].Al-Hazmi MA, Rawi SM, Arafa NM, Wagas A, Montasser AO. The potent effects of ginseng root extract and memantine on cognitive dysfunction in male albino rats. Toxicol Ind Health 2015;31:494–509. [DOI] [PubMed] [Google Scholar]
- [76].Nam SM, Hwang H, Seo M, et al. Gintonin attenuates D-galactose-induced hippocampal senescence by improving long-term hippocampal potentiation, neurogenesis, and cognitive functions. Gerontology 2018;64:562–75. [DOI] [PubMed] [Google Scholar]
- [77].Xu J, Wang F, Guo J, et al. Pharmacological mechanisms underlying the neuroprotective effects of alpinia oxyphylla miq. on Alzheimer's disease. Int J Mol Sci 2020;21:2071.doi: 10.3390/ijms21062071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Okuyama S, Yamamoto K, Mori H, et al. Neuroprotective effect of Citrus kawachiensis (Kawachi Bankan) peels, a rich source of naringin, against global cerebral ischemia/reperfusion injury in mice. Biosci Biotechnol Biochem 2018;82:1216–24. [DOI] [PubMed] [Google Scholar]
- [79].Okuyama S, Kotani Y, Yamamoto K, et al. The peel of Citrus kawachiensis (kawachi bankan) ameliorates microglial activation, tau hyper-phosphorylation, and suppression of neurogenesis in the hippocampus of senescence-accelerated mice. Biosci Biotechnol Biochem 2018;82:869–78. [DOI] [PubMed] [Google Scholar]
- [80].Bruandet A, Richard F, Bombois S, et al. Cognitive decline and survival in Alzheimer's disease according to education level. Dement Geriatr Cogn Disord 2008;25:74–80. [DOI] [PubMed] [Google Scholar]
- [81].Solias A, Skapinakis P, Degleris N, Pantoleon M, Katirtzoglou E, Politis A. [Mini Mental State Examination (MMSE): determination of cutoff scores according to age and educational level]. Psychiatriki 2014;25:245–56. [PubMed] [Google Scholar]
- [82].Buckwalter JG, Sobel E, Dunn ME, Diz MM, Henderson VW. Gender differences on a brief measure of cognitive functioning in Alzheimer's disease. Arch Neurol 1993;50:757–60. [DOI] [PubMed] [Google Scholar]
- [83].Burback D, Molnar FJ, St John P, Man-Son-Hing M. Key methodological features of randomized controlled trials of Alzheimer's disease therapy. Minimal clinically important difference, sample size and trial duration. Dement Geriatr Cogn Disord 1999;10:534–40. [DOI] [PubMed] [Google Scholar]
- [84].Li H, Jia J, Yang Z. Mini-mental state examination in elderly chinese: a population-based normative study. J Alzheimers Dis 2016;53:487–96. [DOI] [PubMed] [Google Scholar]
- [85].Xie H, Zhang C, Wang Y, et al. distinct patterns of cognitive aging modified by education level and gender among adults with limited or no formal education: a normative study of the mini-mental state examination. J Alzheimers Dis 2016;49:961–9. [DOI] [PubMed] [Google Scholar]
- [86].Cicero AFG, Fogacci F, Banach M. Botanicals and phytochemicals active on cognitive decline: the clinical evidence. Pharmacol Res 2018;130:204–12. [DOI] [PubMed] [Google Scholar]
- [87].Hou Y, Wang Y, Zhao J, et al. Smart Soup, a traditional Chinese medicine formula, ameliorates amyloid pathology and related cognitive deficits published correction appears in PLoS One. 2020 Aug 3;15(8):e0237035. PLoS One 2014;9:e111215.doi: 10.1371/journal.pone.0237035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Park HJ, Jung IH, Kwon H, et al. The ethanol extract of Zizyphus jujuba var. spinosa seeds ameliorates the memory deficits in Alzheimer's disease model mice. J Ethnopharmacol 2019;233:73–9. [DOI] [PubMed] [Google Scholar]
- [89].Patwardhan B, Vaidya ADB, Chorghade M, et al. Reverse pharmacology and systems approaches for drug discovery and development. Curr Bioactive Compounds 2008;4:201–12. [Google Scholar]
- [90].Sucher NJ. The application of Chinese medicine to novel drug discovery. Expert Opin Drug Discov 2013;8:21–34. [DOI] [PubMed] [Google Scholar]
- [91].State Administration of Traditional Chinese Materia Medica. Chinese Materia Medica. Shanghai, China: Shanghai Science and Technology Press; 2005. [Google Scholar]