Supplemental Digital Content is available in the text
Keywords: cabozantinib, hepatocellular carcinoma, meta-analysis, ramucirumab, regorafenib
Abstract
Background:
The present network meta-analysis was conducted to perform an indirect comparison among ramucirumab, regorafenib, and cabozantinib in patients with advanced hepatocellular carcinoma (HCC) progressed on sorafenib treatment.
Methods:
A systematic review through Medline, Embase, and Cochrane library was developed, with eligible randomized clinical trials been included. Hazard ratios (HRs) including progression-free survival (PFS), overall survival (OS), odds ratios of disease control rate (DCR), objective response rate (ORR), and adverse events were compared indirectly with network meta-analysis using random model in software STATA version 13.0.
Results:
A total of 4 randomized clinical trials including 2137 patients met the eligibility criteria and enrolled. Indirect comparisons showed that there was no statistical difference observed in the indirect comparison of PFS, OS, ORR, or DCR among agents of regorafenib, cabozantinib, and ramucirumab in advanced HCC patients with elevated α-fetoprotein (AFP) (400 ng/mL or higher). However, in patients with low-level AFP (lower than 400 ng/mL), regorafenib was the only agent associated with significant superiority in OS, compared with placebo (hazard ratio 0.67, 95% CI, 0.50–0.90).
Conclusions:
The present network meta-analysis revealed that there might be no statistical difference observed in the indirect comparison of PFS, OS, ORR, or DCR among regorafenib, cabozantinib, or ramucirumab in advanced HCC patients with elevated AFP (400 ng/mL or higher). However, in patients with low-level AFP (lower than 400 ng/mL), regorafenib might be associated with significant superiority in OS, compared to placebo, which need further investigation in clinical practice.
1. Introduction
Liver cancer has emerged as the fourth leading cause of cancer related death after lung cancer, colorectal cancer, and gastric cancer in 2015 all over the world. Globally, hepatocellular carcinoma (HCC) was the most common type in primary site of liver cancer.[1] Risk factors including viral infections, such as hepatitis B and hepatitis C virus, alcohol, and certain external sources might be responsible for the cause of HCC.[2,3] Treatment strategies for patients diagnosed with HCC include surgical resection, ablation, and transplantation, which have already been well established for the early stage disease according to guidelines.[4,5] However, the systematic therapy measure for patients diagnosed with advanced disease is limited.
Sorafenib, the only drug which has been shown to significantly improve survival time, was recommended as preferable choice in patients with advanced/unresectable HCC.[6,7] However, after that, several attempts of other targeted agents or regimens were failed to obtain a positive outcome in the treatment of advanced HCC.[8,9] In most recent years, lenvatinib, a small molecule inhibitor of multiple receptor tyrosine kinases, was approved as the first-line option in patients diagnosed with unresectable HCC in the USA, China, and Japan.[10] The results of the trial REFLECT revealed that median time to progression for patients in the lenvatinib group (8.9 months, 95% CI 7.4–9.2) was statistically longer than in the sorafenib group (3.7 months, 95% CI 3.6–5.4; hazard ratio [HR] 0.60, 95% CI 0.51–0.71). However, median overall survival (OS) for lenvatinib (13.6 months, 95% CI 12.1–14.9) was shown similar to sorafenib (12.3 months, 10.4–13.9; HR 0.92, 95% CI 0.79–1.06).[11] The extension of disease control duration in first-line treatment did not transform into long-term benefit, which suggested that further-line treatment might also be significant during the whole management of advanced HCC.
In terms to further-line systematic therapy, several agents including cabozantinib, regorafenib, and ramucirumab (for patients with increased α-fetoprotein concentrations) have been recently recommended for patients diagnosed with advanced HCC progressed on sorafenib treatment. As one of the oral small molecule inhibitors of multiple receptor tyrosine kinases, regorafenib has been proved effective among several carcinomas including metastatic colorectal carcinoma,[12] advanced gastrointestinal stromal tumors,[13] as well as advanced HCC.[14] Cabozantinib was primarily designed for patients with metastatic medullary thyroid carcinoma.[15] Based on the encouraging results of OS benefit (HR 0.76, 95% CI 0.63–0.92), cabozantinib was also recommended for patients diagnosed with advanced and progressing HCC.[16] Ramucirumab, a human IgG1 monoclonal antibody, which inhibits the ligand activation of vascular endothelial growth factor receptor 2, was also approved for the further-line treatment in advanced HCC patients with increased α-fetoprotein (AFP) concentrations (400 ng/mL or higher) according to the positive results of REACH-2 trial.
Recently, there was a network meta-analysis conducted to evaluate the efficacy of agents as second-line treatments in patients with HCC.[17] However, a majority of drugs such as Tivantinib,[18] Codrituzumab,[19] Axitinib,[20] Everolimus,[21] Brivanib,[22] ADI-PEG 20,[23] and S-1[24] included in the meta-analysis were not approved because of their negative results of clinical trials. It seems meaningless to compare the efficacy or safety of such agents in an indirect comparison. In addition, the shortage of AFP-based subgroup analysis in former publication might lead to potential controversy for physician. According to guidelines of the National Comprehensive Cancer Network, other than immunotherapy drugs, only 3 agents including ramucirumab, regorafenib, and cabozantinib were recommended as further-line treatment in patients diagnosed with advanced HCC who had progressed on sorafenib treatment. However, because of the deficiency of detailed efficacy and safety profile with a phase III study momentarily, immunotherapy agents were not included in the present indirect analysis. Hence, given that lack of options in this scenario or head-to-head studies comparing the 3 agents, we performed the present network meta-analysis to assess the efficacy and safety of ramucirumab, regorafenib, and cabozantinib for the treatment in patients with advanced HCC progressed on sorafenib treatment.
2. Patients and methods
2.1. Literature search strategy
A systematic literature review among databases including MEDLINE, EMBASE, and Cochrane Library was conducted up to April 2019 with the main key word “Ramucirumab,” “Regorafenib,” “Cabozantinib,” “hepatocellular,” and their similarities. The searching procedure was confined to published, original, randomized, and placebo controlled clinical researches, and fully published in English. The present network meta-analysis was conducted in the light of the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, within all the results reported on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[25]
2.2. Inclusion and exclusion criteria
Inclusion criteria:
-
1.
Fully published, randomized, and placebo controlled clinical trials with ramucirumab, regorafenib, or cabozantinib in patients with advanced HCC;
-
2.
Participants who had been evaluated as disease progression after receiving sorafenib or intolerant to sorafenib, were assigned to receive one of the agents (ramucirumab, regorafenib, or cabozantinib) as experiment or placebo treatment in the group control randomly in the included studies;
-
3.
All of the following results should be reported: progression-free survival (PFS), OS, disease control rate (DCR), objective response rate (ORR), and adverse events.
Accordingly, the following exclusion criteria were adopted:
-
1.
Non-controlled or single-arm studies.
-
2.
Ongoing researches without full data published.
-
3.
Any systematic review, correspondence, or case reports.
-
4.
The language was not in English.
2.3. Data extraction and outcomes
The available data from the available literatures was extracted by 2 investigators independently (Chen and Wang), with any controversy resolved by consensus of them. The necessary information extracted from the available literatures including: names of the trials, number of patients, gender, median age, ECOG performance status, race of the included patients, potential cause of the cancer, and Barcelona clinic liver cancer stage of the patients. The primary outcomes evaluated in the present study were PFS (randomization to progression of any causes or death regardless of any causes) and OS (randomization to death regardless of any causes). Secondary endpoints covered ORR (evaluated as complete response or partial response), DCR (including ORR status and stable disease), severe adverse events (SAEs), fatal adverse events (FAEs), treatment events (TEs, patients suffered dose reduction, death during the therapy or within 30 days of treatment discontinuation), and specified adverse events (increased ALT, fatigue, hypertension, and vomiting).
2.4. Quality assessment of the available literatures
The criteria of Cochrane Collaboration's tool for assessing risk of bias of randomized clinical trials was adopted for the quality assessment in the present study, within evaluative items as allocation concealment, random sequence generation, binding of outcome assessments, binding of participants and personnel, selective reporting, incomplete outcome data, and others.
2.5. Statistical analysis
All the data in the present research was analyzed with software STATA version 13.0 (StataCorp, College Station, Texas). HRs were extracted for the evaluation of OS and PFS, with the variance estimates calculated from the reported CIs. Efficacy of OS and PFS was evaluated for indirect comparison with random effect model. Besides, ORR, DCR, SAEs, FAEs, TEs, and specified adverse events were calculated with odds ratios (ORs). In addition, we further conducted a subgroup analysis by the level of AFP to reduce the potential bias on patient selection. In terms to comparison, network meta-analysis methods (STATA network) were adopted for indirect comparisons among ramucirumab, regorafenib, cabozantinib, and placebo. In addition, the software RevMan version 5.3 (CochraneCorp, UK) was adopted for the description of Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram, risk of bias graph, and risk of bias summary. Other figures in the present paper were presented with software STATA version 13.0.
2.6. Patient and public involvement
No patient involved.
3. Results
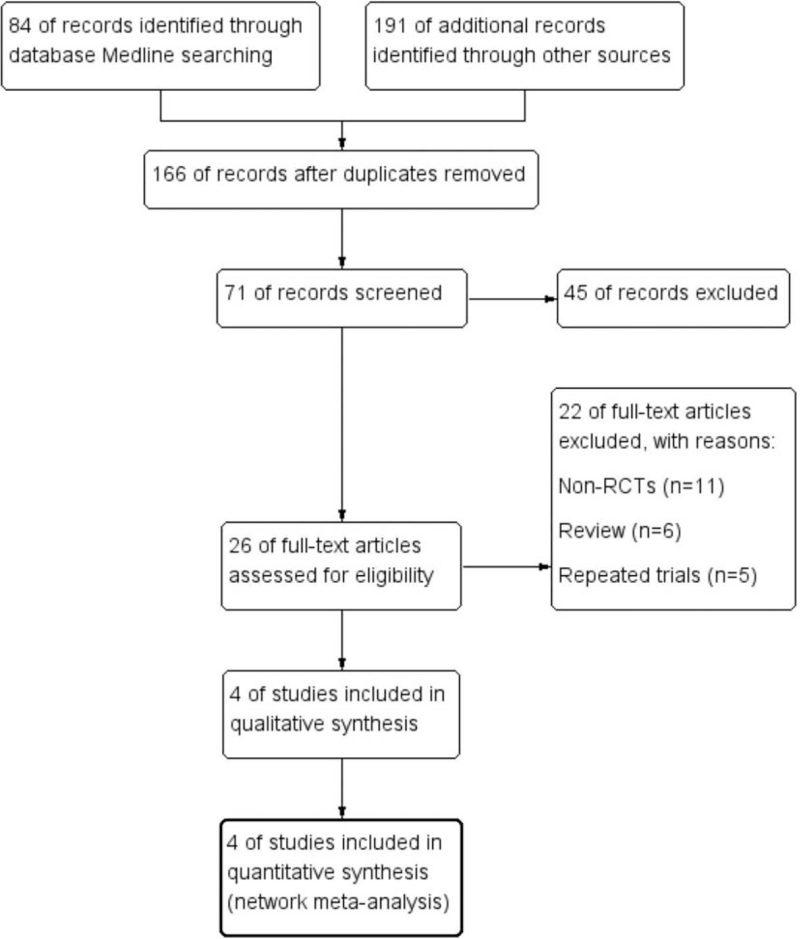
Through databases searching including EMBASE, MEDLINE, and Cochrane Library, a total of 275 potential literatures were initially investigated. One hundred nine publications were excluded because of the duplications. One hundred forty researches were further removed with the property of prospective, randomized, placebo controlled characteristic of the trials. After full text carefully reviewed, a total of 4 clinical trials were considered eligible for the final analysis. The flow diagram which detailed the enrollment of the included literatures was shown in Figure 1.
Figure 1.
Study selection procedure with PRISMA flow diagram. PRISMA = Preferred Reporting Items For Systematic Reviews and Meta-analyses.
3.1. Quality assessment
The results of quality evaluation revealed that all of the included researches satisfied the items including allocation concealment, random sequence generation, binding of outcome assessments, binding of participants and personnel, selective reporting, incomplete outcome data and others, with results presented in Appendix, http://links.lww.com/MD2/A404.
3.2. Study design and the population characteristics
A total of 4 studies (marked REACH, REACH-2, RESORCE, and CELESTIAL) including 2137 patients were identified available in the present network meta-analysis. All of the patients included were refractory patients who had been resistant to sorafenib treatment or intolerant to sorafenib. To be specific, in the REACH and REACH-2 trials, patients randomly received ramucirumab or placebo at 8 mg/kg intravenously over 1 hour every 2 weeks until progressive disease. While in RESORCE, patients received oral regorafenib 160 mg or placebo once daily. In the study CELESTIAL, patients received either a 60 mg of cabozantinib or placebo once daily. All the patients included in the present study received best supportive care (BSC) until progressive disease, death, unacceptable toxicity, or withdrawal of consent by the patients. The baseline clinic-pathological characteristics for the included researches were presented in Table 1.
Table 1.
Baseline characteristics of the studies included in the present study.
| REACH | REACH 2 | RESORCE | CELESTIAL | |||||
| Ram | PLA | Ram | PLA | Reg | PLA | Cab | PLA | |
| Number | N = 283 (%) | N = 282 (%) | N = 197 (%) | N = 95 (%) | N = 379 (%) | N = 194 (%) | N = 470 (%) | N = 237 (%) |
| Median age | 64 | 62 | 64 | 64 | 64 | 62 | 64 | 64 |
| Gender | ||||||||
| Male | 236 (83) | 242 (86) | 154 (78) | 79 (83) | 333 (88) | 171 (88) | 379 (81) | 202 (85) |
| Female | 47 (17) | 40 (14) | 43 (22) | 16 (17) | 46 (12) | 23 (12) | 91 (19) | 35 (15) |
| ECOG PS | ||||||||
| 0 | 159 (56) | 153 (54) | 113 (57) | 55 (58) | 247 (65) | 130 (67) | 245 (52) | 131 (55) |
| 1 | 124 (44) | 129 (46) | 84 (43) | 40 (42) | 132 (35) | 64 (33) | 224 (48) | 106 (45) |
| Race | ||||||||
| Asian | 131 (46) | 135 (48) | 102 (52) | 45 (47) | 156 (41) | 78 (40) | 116 (25) | 59 (25) |
| White | 139 (49) | 137 (49) | 60 (30) | 31 (33) | 138 (36) | 68 (35) | 231 (49) | 108 (46) |
| Other | 13 (5) | 10 (4) | 35 (18) | 19 (20) | 85 (23) | 48 (25) | 123 (26) | 70 (30) |
| Cause of cancer | ||||||||
| Hepatitis B | 100 (35) | 101 (36) | 71 (36) | 36 (38) | 143 (38) | 73 (38) | 178 (38) | 89 (38) |
| Hepatitis C | 77 (27) | 77 (27) | 48 (24) | 28 (29) | 78 (21) | 41 (21) | 113 (24) | 55 (23) |
| Other | 106 (37) | 104 (37) | 78 (40) | 31 (33) | 158 (41) | 80 (41) | 179 (38) | 93 (39) |
| BCLC stage | ||||||||
| B | 33 (12) | 34 (12) | 34 (17) | 20 (21) | 53 (14) | 22 (11) | NR | NR |
| C | 250 (88) | 248 (88) | 163 (83) | 75 (79) | 325 (86) | 172 (89) | NR | NR |
BCLC = Barcelona clinic liver cancer stage; Cab = cabozantinib; ECOG PS = eastern cooperative oncology group performance status; NR = not reported; PLA = placebo; Ram = ramucirumab; Reg = regorafenib.
The model of the comparison developed with network was presented in Figure 2. All the drugs including ramucirumab, regorafenib, and cabozantinib were compared with placebo, separately.
Figure 2.
Network of the comparisons.
3.3. Efficacy
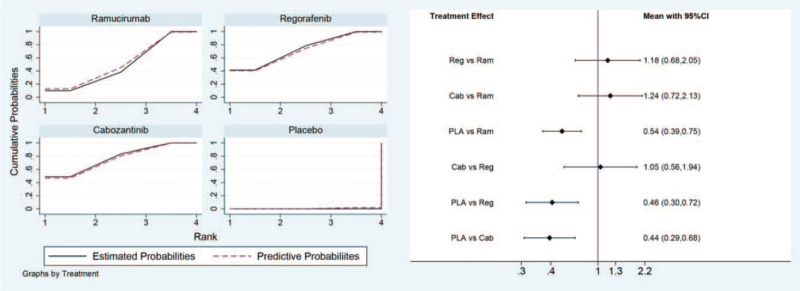
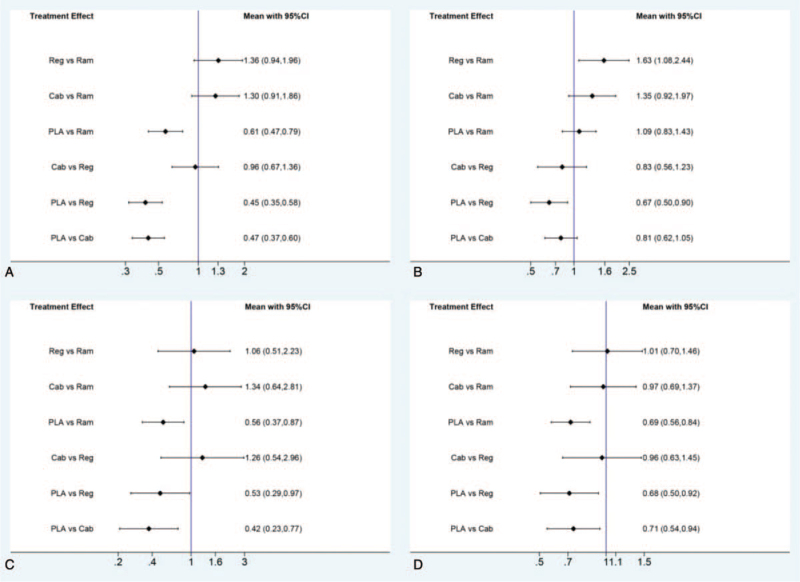
For PFS in the direct and indirect comparison, all the 3 drugs were showed beneficial when compared with placebo (HR for ramucirumab 0.54, 95% CI, 0.39–0.75; HR for regorafenib 0.46, 95% CI, 0.30–0.72; HR for cabozantinib 0.44, 95% CI, 0.29–0.68). No statistical difference was observed in the indirect comparison between ramucirumab and regorafenib (HR 1.18, 95% CI, 0.68–2.05), nor in the comparison between regorafenib and cabozantinib (HR 1.05, 95% CI, 0.56–1.94), or in the comparison between ramucirumab and cabozantinib (HR 1.24, 95% CI, 0.72–2.13). All the results of comparison for PFS were presented in Figure 3.
Figure 3.
Direct and indirect comparisons for PFS among Ram (ramucirumab), Reg (regorafenib), Cab (cabozantinib), and PLA (placebo). PFS = progression-free survival.
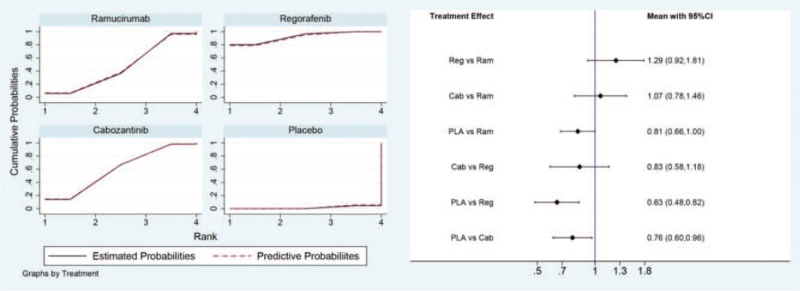
For OS in the direct and indirect comparison, all the 3 drugs showed beneficial when compared to placebo (HR for ramucirumab 0.81, 95% CI, 0.66–1.00; HR for regorafenib 0.63, 95% CI, 0.48–0.82; HR for cabozantinib 0.76, 95% CI, 0.60–0.96). However, no statistical difference was observed in the between ramucirumab and regorafenib (HR 1.29, 95% CI, 0.92–1.81), nor in the comparison between regorafenib and cabozantinib (HR 0.83, 95% CI, 0.58–1.18), or in the comparison between ramucirumab and cabozantinib (HR 1.07, 95% CI, 0.78–1.46). All the results of comparison for OS were presented in Figure 4.
Figure 4.
Direct and indirect comparisons for OS among Ram (ramucirumab), Reg (regorafenib), Cab (cabozantinib), and PLA (placebo). OS = overall survival.
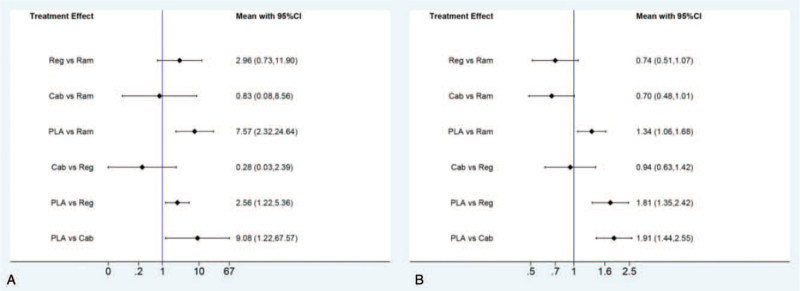
In the indirect analysis of efficacy including ORR and DCR, there is no statistical difference observed for ORR or DCR among ramucirumab, regorafenib, and cabozantinib. The results were presented in Figure 5.
Figure 5.
Direct and indirect comparisons for ORR (A) and DCR (B) among Ram (ramucirumab), Reg (regorafenib), Cab (cabozantinib), and PLA (placebo). DCR = disease control rate; ORR = objective response rate.
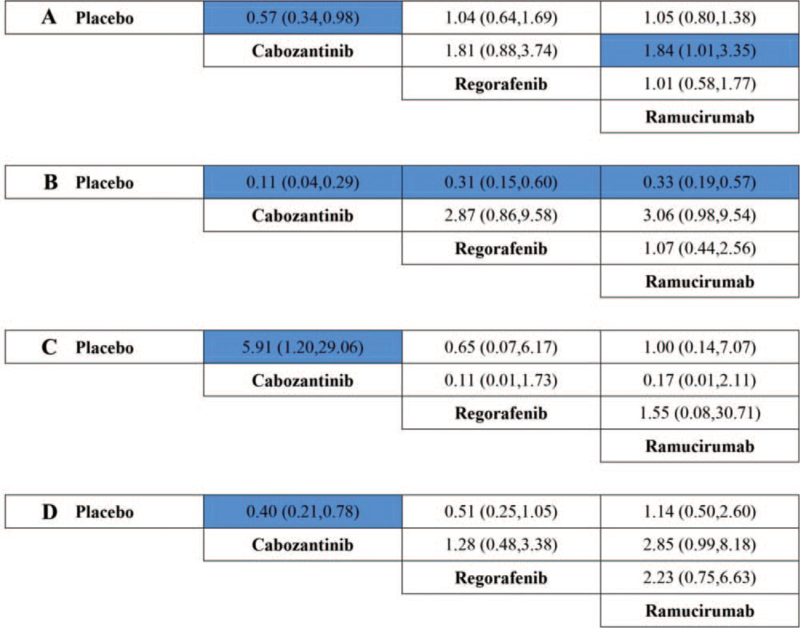
3.4. Subgroup analysis by AFP level
Because of the diversity of AFP level among the included literatures, we further conducted a subgroup analysis by AFP level. As a result, in patients with low-level AFP (lower than 400 ng/mL), regorafenib was the only agent associated with significant superiority in OS, compared to placebo (HR 0.67, 95% CI, 0.50–0.90). However, in patients with high level AFP (400 ng/mL or higher), no significant difference was observed for PFS or OS in the indirect comparison between ramucirumab and regorafenib (HR for PFS, 1.06, 95% CI, 0.51–2.23; HR for OS, 1.01, 95% CI, 0.70–1.46), nor in the comparison between regorafenib and cabozantinib (HR for PFS, 1.26, 95% CI, 0.54–2.96; HR for OS, 0.96, 95% CI, 0.63–1.45), or in the comparison between ramucirumab and cabozantinib (HR for PFS, 1.34, 95% CI, 0.64–2.81; HR for OS, 0.97, 95% CI, 0.69–1.37). The outcomes were presented in Figure 6.
Figure 6.
Direct and indirect comparisons for PFS (A and C) and OS (B and D) by AFP level (A and B for low-level AFP; C and D for high level AFP), among Ram (ramucirumab), Reg (regorafenib), Cab (cabozantinib), and PLA (placebo). AFP = α-fetoprotein; OS = overall survival; PFS = progression-free survival.
3.5. Safety
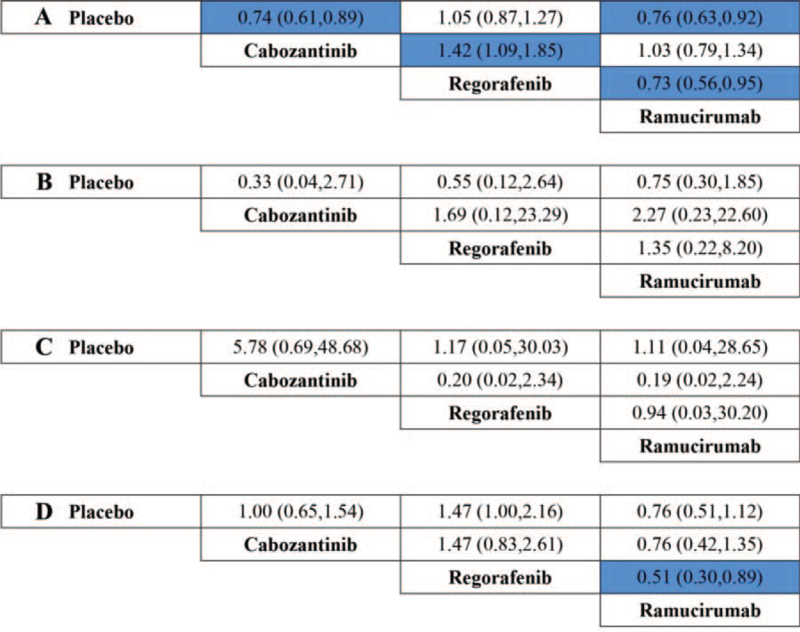
For safety analysis, direct and indirect comparisons of SAEs, FAEs, and TEs (patients suffered dose reduction, death during the therapy or within 30 days of treatment discontinuation) were conducted among drugs including ramucirumab, regorafenib, cabozantinib, and placebo. Regorafenib was shown a statistically lower risk of SAEs when compared to cabozantinib (OR 0.71 95% CI, 0.54–0.92) or ramucirumab (OR 0.73, 95% CI, 0.56–0.95). Moreover, regorafenib was also shown lower risk of death during the therapy or within 30 days of treatment discontinuation comparing to ramucirumab (OR 0.51, 95% CI, 0.30–0.89). All the results of direct and indirect comparison among ramucirumab, regorafenib, cabozantinib, and placebo were presented in Figure 7.
Figure 7.
Treatment comparison for SAEs (A), FAEs (B), and TEs (patients suffered dose reduction, (C); death during the therapy or within 30 days of treatment discontinuation, (D) (ORs, 95% CI). FAEs = fatal adverse events; OR = odds ratio; SAEs = severe adverse events; TEs = treatment events.
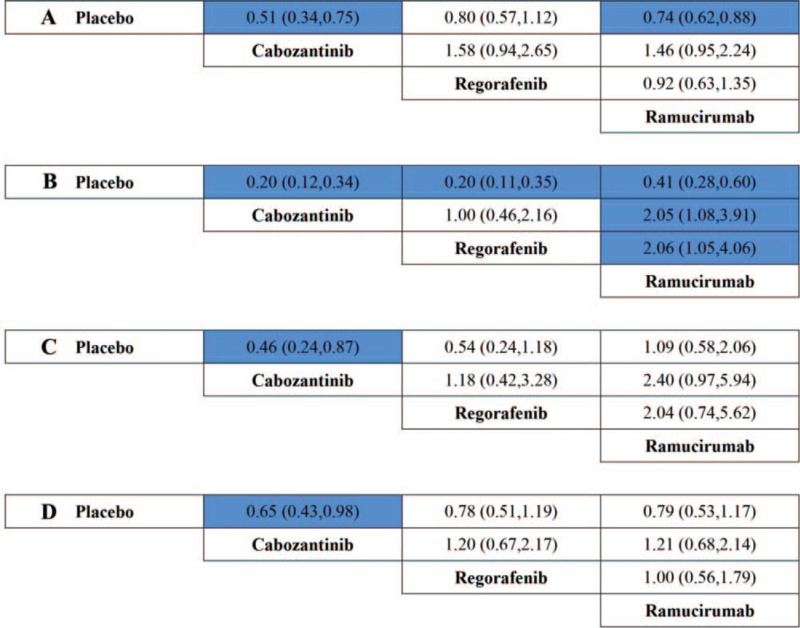
In addition, common adverse events including elevated ALT, hypertension, vomiting, and fatigue at all grade and high grade were also compared among ramucirumab, regorafenib, cabozantinib, and placebo. Ramucirumab was shown a statistically lower risk of hypertension at all grades when compared to regorafenib (OR 0.49 95% CI, 0.25–0.96) or cabozantinib (OR 0.49, 95% CI, 0.26–0.93) (Fig. 8). In addition, ramucirumab was also showed a statistically lower risk of elevated ALT at high grade when compared to cabozantinib (OR 0.54 95% CI, 0.30–0.99) (Fig. 9).
Figure 8.
Treatment comparison for elevated ALT (A), hypertension (B), vomiting (C), and fatigue (D) at all grades (ORs, 95% CI). ORs = odds ratios.
Figure 9.
Treatment comparison for elevated ALT (A), hypertension (B), vomiting (C), and fatigue (D) at high grade (ORs, 95% CI). OR = odds ratio.
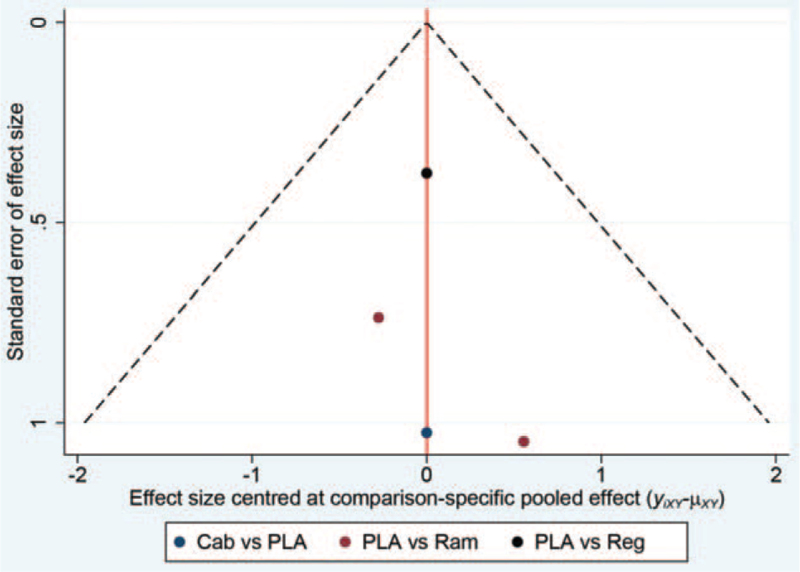
3.6. Publication bias
Funnel plot of network meta-analysis did not suggest statistical publication bias in the present study (Fig. 10).
Figure 10.
Funnel plot for the detection of publication bias (Ram, ramucirumab; Reg, regorafenib; Cab, cabozantinib; PLA, placebo).
4. Discussion
The present network meta-analysis revealed that there was no statistical difference observed in the indirect comparison of PFS, OS, ORR, or DCR among agents of regorafenib, cabozantinib, and ramucirumab in advanced HCC patients with elevated AFP (400 ng/mL or higher). However, in those patients with low-level AFP (lower than 400 ng/mL), regorafenib might be the only agent associated with significant superiority in OS when compared to placebo.
The majority of HCC patients were diagnosed with advanced or metastatic disease at the time of first visit, which have already lost an opportunity to receive curative operation or radical local treatment. Systematic therapy has been emerged as the only appropriate strategy to prolong the survival time in such patients. However, clinical efficacy with cytotoxic drugs did not show a promising result in patients with advanced or metastatic HCC, whether in short term efficacy (ORR, DCR), or long term ones (PFS, OS).[26,27] At the moment, systematic therapy with multi-targeted agents has often been the primary choices for patients with advanced HCC. Sorafenib has been approved for the first-line administration because of the positive results of the 2 randomized, placebo-controlled, phase III trials.[6,7] In recent years, lenvatinib was also recommended as the first-line choice for advanced or metastatic HCC according to a phase III randomized non-inferiority study.[11] Though lenvatinib showed a longer PFS than sorafenib (HR, 0.66; 95% CI, 0.57–0.77), median survival time for lenvatinib was 13.6 months, compared to 12.3 months for sorafenib (HR, 0.92; 95% CI, 0.79–1.06), indicated that lenvatinib was not superior to sorafenib in OS.[11] The benefit of PFS did not transform into the profit on OS, which revealed that further-line treatment might be also significant in clinical practice.
AFP was deemed as a significant tumor marker to predict prognosis in patients with HCC.[28] There were several researches reported that low level of AFP was often showed a better prognosis than those with elevated levels of AFP.[28,29] In the present study, because the relative trial on ramucirumab (REACH-2) only recruited HCC patients with increased AFP concentrations (400 ng/mL or higher),[30] we hence further conducted a subgroup analysis by the level of AFP to reduce the potential bias. We developed indirect comparisons on PFS and OS among the 3 drugs in patients with high level AFP (400 ng/mL or higher), and low-level AFP (lower than 400 ng/mL), respectively (Fig. 6). As a result, in patients with high level AFP, there was no statistical difference observed on PFS or OS among regorafenib, cabozantinib, and ramucirumab. However, in patients with low-level AFP, regorafenib was revealed as the only agent which significantly prolonged the OS, compared to placebo (HR, 0.67; 95% CI, 0.50–0.90). Though the outcomes were dated from the subgroup-based indirect comparison, which need further identification, we still suggested that regorafenib might be more appropriate in advanced HCC patients with low-level AFP (lower than 400 ng/mL).
The liver is the main detoxifying organ, which metabolize almost all of the oral drugs including anti-cancer agents. In patients with advanced HCC, the selection of drugs fit for liver function might be more significant. In the present study, we also conducted indirect comparisons on safety among regorafenib, cabozantinib, and ramucirumab. As a result, it was revealed that regorafenib was shown a statistically lower risk of SAEs and death during the therapy or within 30 days of treatment discontinuation when compared to cabozantinib or ramucirumab. However, the advanced HCC cases with sorafenib intolerance were excluded from the RESORCE trial. As the similarity on molecular structure between regorafenib and sorafenib, the superior results of regorafenib on SAE should be prudently interpreted. In addition, ramucirumab was shown a statistically lower risk of hypertension at all grades, and elevated ALT at high grade when compared to cabozantinib and regorafenib. Although advanced HCC patients with uncontrolled arterial hypertension were excluded from eligibility criteria in the REACH trial and REACH-2 trial we still suggested that ramucirumab might be more appropriate for patients with hypertension or abnormal liver function.
In addition, there were also immunotherapy drugs approved as second-line treatment for patients with advanced HCC. Nivolumab, an anti-PD-1 antibody, was approved by FDA because of the positive results of the trial CheckMate 040.[31] In that study, median OS and 6 month OS rates for the included patients were 13.2 months and 75%, respectively. Subsequent analyses from the trial CheckMate 040 presented a median DoR (duration of response) as 17 months inpatients who had not received sorafenib treatment (n = 80), and 19 months in patients who had been treated with sorafenib previously (n = 182). Because of the single-armed design of the trial, and the negative outcomes of the phase III, randomized researches for nivolumab and pembrolizumab,[32] we did not include the immunotherapy agents in the present study. In addition, there are also some other trials ongoing to evaluate the efficacy of emerging regimens or drugs in patients with advanced HCC, especially in patients who had progressed on sorafenib. Bevacizumab has shown efficacy in several phase II, single-armed trials. The reported PFS for single bevacizumab or bevacizumab-based regimens ranged from 2.7 to 6.9 months.[33–37] However, without controlled, randomized studies further confirmed, the outcomes should be prudently approached. In addition, chemotherapy regimen of FOLFOX4 (infusional fluorouracil, leucovorin, and oxaliplatin) was also revealed effective on PFS (HR, 0.62; 95% CI, 0.49 to 0.79), compared to doxorubicin in Asian patients with advanced HCC.[38] However, because of the negative results of OS, the regimen was not preferentially recommended. In the most recent, the trial IMbrave 150, which was conducted to evaluate the efficacy of atezolizumab combined with bevacizumab (TA) as first-line treatment in patients with HCC, published its initial results.[39] The PFS in patients received TA treatment was 6.8 months to 4.3 months in sorafenib group (HR 0.59, P < .0001). The OS in TA group was not reached to 13.2 months in sorafenib group (HR 0.58, P = .0006). Even so, we did not include the promising study because of its first-line schedule, which against the design of the present study.
4.1. Study limitations
There are several limitations existed in the present study. The most obvious one was the heterogeneity, which might be caused by the diversity of AFP level, and the inclusion criteria. Though we have conducted a subgroup analysis by AFP level to reduce the influence, the heterogeneity on inclusion criteria might be impossible to be eliminated. To be specific, the RESORCE trial of regorafenib allowed recruitment of only patient who tolerated sorafenib, while the remaining trials allowed also intolerant patients. A significant proportion of intolerant patients might have taken sorafenib only for a few weeks making the “second line” drug actually a “frontline” treatment, which comes to be the inclusion bias. In addition, the RESORCE trial used mRECIST, rather than RECIST 1.1 to establish PFS. Patients with multiple liver lesions increasing over time but with a hypovascular pattern are classified as PD according to RECIST 1.1 and SD (or even PR) according to mRECIST so that PFS can be longer in the later case, which may lead to the misleading for the comparison of PFS. Hence, we choose OS, rather than PFS for the main outcome in the conclusion. Finally, interested data was not obtained from individual patients of each trial, which would have resulted in a comprehensive analysis.
4.2. Conclusion and recommendations
In summary, the present network meta-analysis revealed that there might be no statistical difference observed in the indirect comparison of PFS, OS, ORR, or DCR among agents of regorafenib, cabozantinib, and ramucirumab in advanced HCC patients with elevated AFP (400 ng/mL or higher). However, in patients with low-level AFP (lower than 400 ng/mL), regorafenib might be the only agent associated with significant superiority in OS, compared to placebo. Hence, regorafenib might be more appropriate for HCC patients with low-level AFP (lower than 400 ng/mL). However, ramucirumab might be more suitable for patients with hypertension, or liver disease, as ramucirumab was shown a statistically lower risk of hypertension, as well as elevated ALT. We hope the results of the present study could provide some implication in clinical practice. However, owing to the indirect comparisons, the outcomes in the present study should be rationally explained and treated, which need further identification in direct comparisons.
Acknowledgments
The authors thank all the patients enrolled in the clinical trials included in the present study.
Author contributions
Conceptualization: Jianxin Chen.
Data curation: Jianxin Chen, Junhui Wang.
Formal analysis: Jianxin Chen.
Investigation: Fangwei Xie.
Methodology: Junhui Wang.
Project administration: Jianxin Chen.
Software: Junhui Wang, Fangwei Xie.
Supervision: Junhui Wang, Fangwei Xie.
Writing – original draft: Jianxin Chen.
Writing – review & editing: Jianxin Chen, Fangwei Xie.
Footnotes
Abbreviations: AFP = α-fetoprotein, DCR = disease control rate, FAE = fatal adverse event, HCC = hepatocellular carcinoma, HR = hazard ratio, OR = odds ratio, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, SAE = severe adverse event, TE = treatment event.
How to cite this article: Chen J, Wang J, Xie F. Comparative efficacy and safety for second-line treatment with ramucirumab, regorafenib, and cabozantinib in patients with advanced hepatocellular carcinoma progressed on sorafenib treatment: A network meta-analysis. Medicine. 2021;100:38(e27013).
JW and FX contributed equally to the present study.
The authors have no funding and conflicts of interest to disclose.
The ethical approval was waived as the present study being a systematic review. This article does not contain any studies with animals performed by any of the authors.
Informed consent was not available in the present study as it was a systematic review.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].Akinyemiju T, Abera S, Ahmed M, et al. C. Global Burden of Disease Liver Cancer. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol 2017;3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127: (Suppl 1): S35–50. [DOI] [PubMed] [Google Scholar]
- [3].de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology 2015;62:1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bruix J, Sherman M. American Association for the Study of Liver. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].European Association For The Study Of The Liver, European Organisation For Research And Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- [6].Llovet JM, Ricci S, Mazzaferro V, et al. S.I.S. Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [7].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [8].Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res: an official journal of the American Association for Cancer Research 2014;20:2072–9. [DOI] [PubMed] [Google Scholar]
- [9].Kelley RK. Brivanib and FOLFOX in hepatocellular carcinoma: finding the common themes among negative trials. J Clin Oncol: official journal of the American Society of Clinical Oncology 2013;31:3483–6. [DOI] [PubMed] [Google Scholar]
- [10].Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs 2019;79:665–74. [DOI] [PubMed] [Google Scholar]
- [11].Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–73. [DOI] [PubMed] [Google Scholar]
- [12].Grothey A, Van Cutsem E, Sobrero A, et al. C.S. Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. [DOI] [PubMed] [Google Scholar]
- [13].Demetri GD, Reichardt P, Kang YK, et al. G.S. Investigators. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bruix J, Qin S, Merle P, et al. R. Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- [15].Houvras Y, Wirth LJ. Cabozantinib in medullary thyroid carcinoma: time to focus the spotlight on this rare disease. J Clin Oncol: official journal of the American Society of Clinical Oncology 2011;29:2616–8. [DOI] [PubMed] [Google Scholar]
- [16].Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bakouny Z, Assi T, El Rassy E, Nasr F. Second-line treatments of advanced hepatocellular carcinoma: systematic review and network meta-analysis of randomized controlled trials. J Clin Gastroenterol 2019;53:251–61. [DOI] [PubMed] [Google Scholar]
- [18].Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018;19:682–93. [DOI] [PubMed] [Google Scholar]
- [19].Abou-Alfa GK, Puig O, Daniele B, et al. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol 2016;65:289–95. [DOI] [PubMed] [Google Scholar]
- [20].Kang YK, Yau T, Park JW, et al. Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann Oncol 2015;26:2457–63. [DOI] [PubMed] [Google Scholar]
- [21].Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57–67. [DOI] [PubMed] [Google Scholar]
- [22].Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509–16. [DOI] [PubMed] [Google Scholar]
- [23].Abou-Alfa GK, Qin S, Ryoo BY, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 2018;29:1402–8. [DOI] [PubMed] [Google Scholar]
- [24].Kudo M, Moriguchi M, Numata K, et al. S-1 versus placebo in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE): a randomised, double-blind, multicentre, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:407–17. [DOI] [PubMed] [Google Scholar]
- [25].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [26].Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 2005;97:1532–8. [DOI] [PubMed] [Google Scholar]
- [27].Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol 2008;15:1008–14. [DOI] [PubMed] [Google Scholar]
- [28].Kang SH, Kim DY, Jeon SM, et al. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. Eur J Gastroenterol Hepatol 2012;24:849–56. [DOI] [PubMed] [Google Scholar]
- [29].Park H, Park JY. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. BioMed Res Int 2013;2013:310427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhu AX, Kang YK, Yen CJ, et al. R.-S. Investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282–96. [DOI] [PubMed] [Google Scholar]
- [31].El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhu AX, Finn RS, Edeline J, et al. K.-. Investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. [DOI] [PubMed] [Google Scholar]
- [33].Hsu CH, Yang TS, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer 2010;102:981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol: official journal of the American Society of Clinical Oncology 2008;26:2992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sun W, Sohal D, Haller DG, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011;117:3187–92. [DOI] [PubMed] [Google Scholar]
- [36].Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol: official journal of the American Society of Clinical Oncology 2009;27:843–50. [DOI] [PubMed] [Google Scholar]
- [37].Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol: official journal of the American Society of Clinical Oncology 2006;24:1898–903. [DOI] [PubMed] [Google Scholar]
- [38].Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol: official journal of the American Society of Clinical Oncology 2013;31:3501–8. [DOI] [PubMed] [Google Scholar]
- [39].Finn RS, Qin S, Ikeda M, et al. I. M. Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–905. [DOI] [PubMed] [Google Scholar]