Abstract
PURPOSE:
The response to the COVID-19 pandemic has affected the management of patients with cancer. In this pooled retrospective analysis, we describe changes in management patterns for patients with cancer diagnosed with COVID-19 in two academic institutions in the San Francisco Bay Area.
MATERIALS AND METHODS:
Adult and pediatric patients diagnosed with COVID-19 with a current or historical diagnosis of malignancy were identified from the electronic medical record at the University of California, San Francisco, and Stanford University. The proportion of patients undergoing active cancer management whose care was affected was quantified and analyzed for significant differences with regard to management type, treatment intent, and the time of COVID-19 diagnosis. The duration and characteristics of such changes were compared across subgroups.
RESULTS:
A total of 131 patients were included, of whom 55 were undergoing active cancer management. Of these, 35 of 55 (64%) had significant changes in management that consisted primarily of delays. An additional three patients not undergoing active cancer management experienced a delay in management after being diagnosed with COVID-19. The decision to change management was correlated with the time of COVID-19 diagnosis, with more delays identified in patients treated with palliative intent earlier in the course of the pandemic (March/April 2020) compared with later (May/June 2020) (OR, 4.2; 95% CI, 1.03 to 17.3; P = .0497). This difference was not seen among patients treated with curative intent during the same timeframe.
CONCLUSION:
We found significant changes in the management of cancer patients with COVID-19 treated with curative and palliative intent that evolved over time. Future studies are needed to determine the impact of changes in management and treatment on cancer outcomes for patients with cancer and COVID-19.
INTRODUCTION
Oncology practice has been transformed in response to the COVID-19 pandemic. Initial retrospective studies from China, where SARS-CoV-2 originated, suggested that patients with cancer may be more susceptible to contracting the virus partially through healthcare-related spread and have excess mortality from COVID-19 illness compared with patients without cancer.1,2 In response to the perceived vulnerability of patients with cancer in an acute pandemic, oncologists have postponed chemotherapy treatments, delayed curative surgeries, abbreviated radiotherapy, and switched intravenous therapies to oral therapies.3 Although there was little evidence to support these measures, protective policies were broadly used to decrease healthcare exposure among these patients who were often immunocompromised. More recently, publication of large, prospective cohorts of patients with cancer have helped define risk factors for mortality in this patient population.1,4-8
Given the uncertainty about optimal management of patients with cancer during the pandemic, guidelines based on expert consensus have been published to guide clinicians.9-16 However, during the early months of the pandemic, there was limited guidance on how to manage cancer-directed treatment for patients with COVID-19 infection,17,18 and oncologists had to rely on local and regional public health authorities to make recommendations to their patients. Although there have been some reports based on oncologist surveys that have revealed changes in attitudes toward therapy due to COVID-19 concerns,19-24 to the best of our knowledge the implications of COVID-19 infection on the cancer-directed management of patients with cancer are unknown.
Herein, we report a pooled retrospective cohort study of patients with cancer and COVID-19 diagnosis at Stanford University (SU) and the University of California, San Francisco (UCSF), and describe the incidence, nature, and reasons for changes in cancer-directed management. We hypothesize that the incidence and duration of change in cancer management vary according to clinical factors, and that these findings will inform future oncology practice for cancer patients with COVID-19 diagnosis.
MATERIALS AND METHODS
Patient Selection
Demographic and clinical data were obtained from electronic medical records. At UCSF and SU, patients with a current or historical pathologic diagnosis of an invasive cancer were identified using the electronic medical record and the Stanford Cancer Registry, respectively. Patients must have also tested positive for SARS-CoV-2 by either quantitative polymerase chain reaction testing (at SU) or reverse transcriptase polymerase chain reaction (at UCSF), with the first positive test date occurring between March 1, 2020, and June 30, 2020. The date of first positive test date was defined as the index date. Patients with localized skin cancers were excluded; age was not an exclusion criterion. Both sites obtained institutional review board approval, and deidentified patient data were shared in aggregate between institutions in a Health Insurance Portability and Accountability Act–compliant manner.
Definition of Baseline Characteristics
Demographic data included age at index date, race, ethnicity, and sex. Cancer-related data included cancer type, stage (per American Joint Committee on Cancer Staging System 8th Edition), and most recent cancer treatment or diagnostic procedure. Active cancer was defined as cancer detectable on the most recent imaging study or laboratory test within 1 year prior to the index date and included patients receiving adjuvant therapy. Active management was defined as the receipt of cancer-directed therapy or the diagnostic procedure within 2 months prior to the index date.
Changes in Cancer Management
For all patients, including those without active cancer, cancer management changes due to COVID-19 occurring after the index date were determined from documentation in the electronic medical record. Delays were calculated starting from the planned date of the next therapy or procedure to the actual date that therapy or procedure was given. We collected information on the nature of management that was changed (diagnostic procedure, surgery, radiation therapy, and systemic therapy), route of systemic therapy (intravenous [IV], or intramuscular [IM], or orally [PO]), intent of treatment (palliative v curative), reason for change (clinical, ie, due to COVID-19 illness, v nonclinical), and duration of delay (measured from the date of the first anticipated dose or procedure that was foregone to date of resumption). For diagnostic procedure changes, intent of treatment was based on most aggressive therapy reasonable for the stage of disease at index date.
COVID-19–Related Outcomes
For all patients, we determined reason for COVID-19 testing (screening v not) and whether the patient was hospitalized due to COVID-19, need for critical care, length of stay, complications from COVID-19, and death.
Statistical Methods
Clinical and demographic characteristics were summarized in contingency tables. To illustrate the context of potential delays in cancer management, an alluvial diagram was created for all patients. The diagram included whether a change in cancer management occurred, nature of management, time of initial COVID-19 diagnosis, and intent of treatment. Time of initial diagnosis was dichotomized as either March 1 to April 30 or May 1 to June 30. The rationale for this cutoff is that relaxation of suspended clinical procedures at both Stanford and UCSF started in mid-April and continued into early May. For example, at elective procedures, surgeries resumed on April 21 at both Stanford and UCSF; elective radiology resumed on April 21 at Stanford and on May 4 at UCSF.
Odds ratio and their CIs were computed using Baptista-Pike in prism, and a χ2 test was used to compute the significance of the difference in odds. Among patients who experienced a delay in cancer management, we compared the incidence of delay between subgroups (date of COVID-19 diagnosis, nature of management, intent of treatment, and reason for delay) using the χ2 test. We also calculated median and interquartile ranges for the duration of delay and compared medians between the same subgroups using the Wilcoxon rank-sum test. Individual durations of delay were illustrated using a swimmer plot, stratified by the reason for delay. P < .05 was considered significant for statistical testing. No multiple testing adjustments were performed given the small sample size. Analyses were performed using statistical software R or Prism 8.
RESULTS
Patient Characteristics and Outcomes
Baseline characteristics at the time of diagnosis are given in the Data Supplement (online only). Between March 1 and June 30, 2020, a total of 131 patients with a cancer diagnosis tested positive for SARS-CoV-2 at Stanford and UCSF (Data Supplement). The mean age at presentation was 60 (range, 3-97) years. Sex was evenly split: 68 (52%) male and 63 (48%) female. A range of ethnicities were represented, including non-Hispanic White (60 patients, 46%), Hispanic (39 patients, 30%), African American (5 patients, 4%), and Asian (20 patients, 15%).
A diverse mix of cancer types were seen, with the most common being solid malignancies: breast (24 patients, 18%), GI (21 patients, 16%), and genitourinary (10 patients, 15%) cancers. Twenty-nine patients (22%) had hematologic malignancies. Sixty-nine (53%) had active cancer and 50 (38%) received anticancer treatment (intravenous or oral therapy) in the last 2 months. Forty-one patients (31%) had been diagnosed with stage I cancer at the time of cancer diagnosis, and 28 (21%) had metastatic (stage IV) disease. Staging information was not applicable or unknown for 35 patients (27%). Patient outcomes related to COVID-19 illness are described in the Data Supplement. Fifty patients (38%) required hospitalization related to COVID-19. Of the 50 hospitalized patients, 19 (15%) required ICU care and 9 (7%) died of complications of COVID-19. Of the nine patients who died, 4 (44%) were undergoing active cancer treatment at the time of COVID-19 diagnosis.
Implications of COVID Infection on Cancer Management
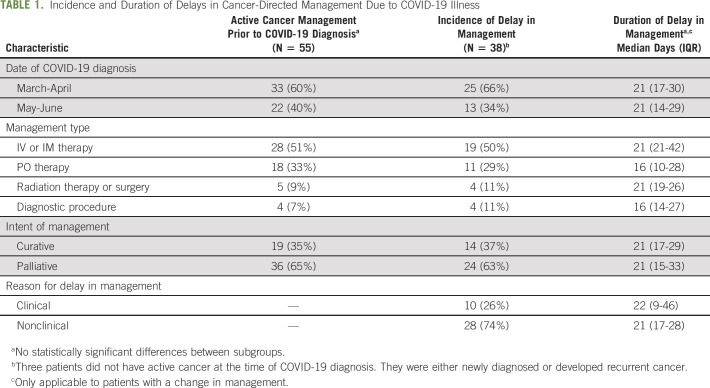
Infection from COVID-19 affected cancer management for the majority of patients with active cancer. Of the 69 patients with active cancer, 55 (78%) patients were undergoing active cancer management in the 2 months preceding the COVID-19 diagnosis. Of the 55 patients undergoing active cancer management, 35 (64%) experienced a change in their cancer management plan. All these changes represented a delay in management, with one patient also experiencing a switch from IV to PO chemotherapy during chemotherapy delay as a result of the progression of disease. An additional three patients who were not undergoing active cancer management, but were either newly diagnosed with cancer or developed recurrent cancer, experienced a delay in cancer management. Among the 38 patients who had a delay in cancer management, most were diagnosed with COVID-19 in March to April (25 of 38, 66%). The most common management was IV or IM systemic therapy (19 of 38, 50%). The intent of treatment was palliative for the majority of patients (24 of 38, 63%) (Table 1). The patients who were receiving palliative intent therapy all had metastatic or incurable relapsed or refractory disease (in the case of a solid tumor primary) or hematologic malignancy. In total, two patients with hematologic malignancies had potentially curable disease with allogeneic hematopoietic stem-cell transplantation but were undergoing palliative intent chemotherapy due to age and/or disease status. The most common reason for delay in management or treatment was not guided by clinical factors (74%, Table 1). Clinical factors included concerns about the individual patient's status: for example, the need to recover from a recent hospitalization or desire for the resolution of illness before administering potentially myelosuppressive therapy, even among minimally symptomatic patients. In all cases, these clinical reasons were due to COVID diagnosis or related complications. Nonclinical reasons were all related to the requirement for a repeat negative COVID-19 test, resolution of symptoms, and/or clearance from an infectious disease specialist prior to starting or resuming treatment or undergoing a procedure. Nonclinical reasons were therefore all due to institutional guidelines, which required documented COVID-19 clearance prior to entry to patient care areas. There was no difference in the incidence of delay among any of these subgroups (Table 1).
TABLE 1.
Incidence and Duration of Delays in Cancer-Directed Management Due to COVID-19 Illness
The alluvial plot illustrates the incidence of delay based on the intent of treatment and timing of COVID-19 diagnosis (Fig 1A) and highlights the fact that a disproportionately greater number of patients who were diagnosed with COVID-19 in March to April experienced a delay in receiving palliative treatment. This prompted a post hoc investigation into factors correlated with management changes. As shown in Figure 1B, there was no significant difference in the incidence of a delay in management based on the timing of COVID diagnosis or treatment intent alone; however, for patients on palliative intent therapy, there were more delays among those diagnosed with COVID-19 earlier (March/April) rather than later (May/June) (OR, 4.2; 95% CI, 1.03 to 17.3; P = .0497) (Fig 1B). This association was not found among patients undergoing curative intent treatment (OR, 0.89; 95% CI, 0.13 to 5.58; P = .91). There were no significant differences in the incidence of delays in management when comparing institution or routes of administration of systemic therapies. In addition, we did not find any significant differences in the occurrence or duration of delays in management based on race or ethnicity, that is, White versus non-White and Hispanic versus non-Hispanic. This analysis was not done for Black/African American versus non-Black/African American given the low frequency of the former.
Fig 1.
Abbreviations: pts, patients. (A) Alluvial plot of management changes stratified by treatment type, intent of treatment, and month of COVID-19 diagnosis. IV or IM: intravenous or intramuscular systemic therapy, oral: oral systemic therapy, other: surgery, radiation, or diagnostic procedure. (B) Bar graphs of management changes, divided by the month of diagnosis and separated by treatment intent. *indicates P < .05.
Duration of Delays in Management due to COVID Infection
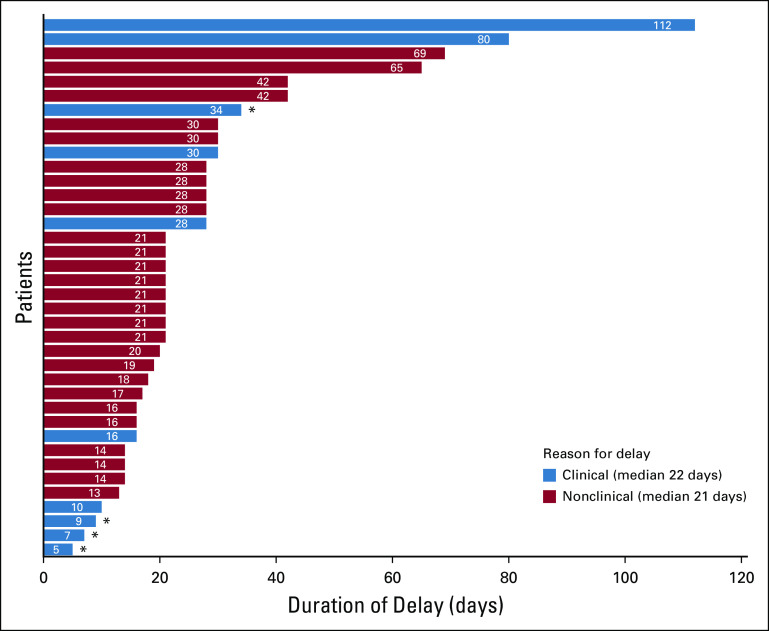
Overall, the median duration of delays in cancer management was 21 days with a range of 5-112 days (Table 1). There was no statistically significant difference in the duration of delay based on predefined subgroups (Table 1). A swimmer plot of individual durations of delay, categorized by reason for delay, can be found in Figure 2 (clinical reason, median duration 22 days; nonclinical reason, median duration 21 days). Of note, three patients were diagnosed with new or recurrent cancer at the index date; the date of first anticipated dose or procedure was estimated based on what could be reasonably expected with normal, pre-COVID-19 practices.
Fig 2.
Swimmer plot of the duration of delay in cancer-directed management due to COVID-19 illness among patients with cancer. Bars are color coded by reason for delay, orange representing a clinical reason and blue representing a nonclinical reason. *indicates patient death.
In this retrospective cohort study of two institutions, we found that patients diagnosed with cancer and COVID-19 were likely to experience a delay in cancer-directed management. No differences in the incidence or duration of delay were found based on the timing of COVID-19 diagnosis, type of management, intent of treatment, or reason for delay. However, a post hoc analysis suggested that among patients undergoing palliative therapies, those who were diagnosed with COVID-19 early in the pandemic had more frequent delays than those diagnosed later. These findings illustrate the effects of COVID-19 diagnosis and related policy changes on the receipt of timely cancer care.
The majority of patients (64%) undergoing active cancer management who were diagnosed with COVID-19 experienced a delay or modification in care at both institutions. There were multiple reasons for delays, including reasons not directly related to the patient's clinical care, such as the need for documentation of COVID-19 clearance prior to being admitted to any clinical site. Such policies and provider decisions led to an overall median duration of a delay of 21 days, with a wide range (13-69 days). Such delays are nontrivial in the care of patients with cancer.25-27 In some cases, the delay was prolonged because of persistent positive tests or difficulty scheduling testing. These barriers to testing may have been exacerbated by variable turnaround times in COVID-19 testing. In California and other regions of the country, delays in testing turnaround for various reasons have led to a recent spike in time from specimen collection to test the result of up to 14 days that vary by testing location.28 These testing issues may differentially affect certain populations such as those living in rural areas,29 possibly exacerbating known disparities. The need for broadly accessible and rapid, yet specific testing is further highlighted by the findings presented here.
Among the possible reasons for delaying treatment was the concern that immunosuppressive systemic therapy could also worsen the course of the infectious illness or lead to complications. We found that therapy had a tendency to be delayed less frequently later in the course of the pandemic, when oncologists had gained some experience and may have had access to more data on the risks of cancer treatments in COVID-19–positive patients, especially for those treated with palliative intent.4,5 This highlights the continued need for large collaborative efforts, such as the COVID-19 and Cancer Consortium,4,8 TERAVOLT,30 National Cancer Institute COVID-19 in Cancer Patients Study,31 and ASCO's Survey on COVID-19 in Oncology,32 that will contribute to making available a more precise identification of factors to assist patients and their oncologists to make informed decisions about plans of care.
Our findings are limited to the San Francisco Bay Area, where local COVID prevalence was relatively controlled. As of August 2020, there has not yet been a surge of COVID-19 cases that overwhelmed the available healthcare delivery systems. This was not necessarily what was expected in March 2020, at the very beginning of the national COVID-19 epidemic. This uncertainty, as well as examples of dire shortages of personnel and resources in the Northeast of the United States,6 likely influenced many of the decisions made by local healthcare professionals and administrators that are highlighted in this study. Although COVID-19 is a global problem, we have learned that care delivery is variable and highly dependent on local and regional factors.33-35 For those with overwhelmed healthcare systems, reduced resources may be an issue.35 Patient fear of exposure through the healthcare system may also play a role.36 Further studies from diverse geographic regions may provide additional insight into how the location affects local policies and informs treatment decisions.
Larger cohort studies are needed to validate and extend our findings. Our study is retrospective and limited by sample size. In addition, medical record review is not entirely sensitive in identifying delays in cancer care, as some may not been documented. The occurrence of delays may therefore be higher than reported. Some of the observed trends, such as differences in management depending on the route of administration of the intended treatment, would benefit from a larger sample size to draw more definitive conclusions. The inclusion of patients from two neighboring academic centers may limit external validity; however, it highlights local trends. Finally, it will take time to fully appreciate the impact COVID-19 has had on cancer outcomes. Although we identified a delay for a significant majority of patients, we are unable to comment on its impact, if any, on clinical outcomes.
COVID-19 has changed many aspects of cancer care delivery, and the full, lasting impact of these changes remains largely unknown. Our study represents an early effort to quantify management changes due to COVID-19 in affected patients and further characterize the factors that lead to management changes in a real-world population. Institutional policies, such as the need for documentation of COVID-19 clearance prior to being allowed to enter a clinical facility, may contribute to management delays and may ultimately affect patient outcomes. Further collaborative studies will help us understand the full impact of local and regional policies on many dimensions of patient care that meaningfully contribute to health outcomes.
EQUAL CONTRIBUTION
J.T.-Y.W., D.H.K., M.J.G., V.S.K., L.S., S.A.S. contributed equally to this work.
SUPPORT
No relevant research funding support. IRB approval #55679.
AUTHOR CONTRIBUTIONS
Conception and design: Julie Tsu-Yu Wu, Daniel H., Kwon, Michael J. Glover, Vadim S. Koshkin, Lidia Schapira, Sumit A. Shah
Provision of study materials or patients: Vadim S. Koshkin
Collection and assembly of data: Julie Tsu-Yu Wu, Daniel H., Kwon, Michael J. Glover, Solomon Henry, Douglas Wood
Data analysis and interpretation: Julie Tsu-Yu Wu, Daniel H., Kwon, Michael J. Glover, Daniel L. Rubin, Vadim S. Koshkin, Lidia Schapira, Sumit A. Shah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Changes in Cancer Management due to COVID-19 Illness in Patients With Cancer in Northern California
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniel L. Rubin
Consulting or Advisory Role: Roche/Genentech
Research Funding: GE Healthcare, Philips Healthcare
Patents, Royalties, Other Intellectual Property: Several pending patents on AI algorithms
Vadim S. Koshkin
Consulting or Advisory Role: Janssen, AstraZeneca, Dendreon, Gerson Lehrman Group, Guidepoint Global, Clovis Oncology, Pfizer/EMD Serono, Seattle Genetics/Astellas
Research Funding: Clovis Oncology, Nektar, Endocyte
Travel, Accommodations, Expenses: AstraZeneca, Janssen
Lidia Schapira
Consulting or Advisory Role: Rubedo, Bluenote Therapeutics
Sumit A. Shah
Stock and Other Ownership Interests: Grand Rounds
Honoraria: Janssen
Consulting or Advisory Role: Natera
Research Funding: Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak Cancer Discov 10783–7912020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China JAMA Oncol 61108–11102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards M, Anderson M, Carter P, et al. The impact of the COVID-19 pandemic on cancer care Nat Cancer 1565–5672020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study [Internet] Lancet 365P1907–P19182020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study Lancet 3951919–19262020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System Cancer Discov 10935–9412020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City Ann Oncol 311088–10892020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera DR, Peters S, Panagiotou OA, et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: A COVID-19 and Cancer Consortium (CCC19) cohort study Cancer Discov 101514–15272020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonoff M, Backhus L, Boffa DJ, et al. COVID-19 guidance for triage of operations for thoracic malignancies: A consensus statement from thoracic surgery outcomes research network J Thorac Cardiovasc Surg 160601–6052020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coles CE, Aristei C, Bliss J, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic Clin Oncol 32279–2812020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curigliano G, Cardoso MJ, Poortmans P, et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic Breast 528–162020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curigliano G, Banerjee S, Cervantes A, et al. Managing cancer patients during the COVID-19 pandemic: An ESMO interdisciplinary expert consensus Ann Oncol 311320–13352020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guckenberger M, Belka C, Bezjak A, et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement Radiother Oncol 146223–2292020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maniakas A, Jozaghi Y, Zafereo ME, et al. Head and neck surgical oncology in the time of a pandemic: Subsite-specific triage guidelines during the COVID-19 pandemic Head Neck 421194–12012020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perini GF, Fischer T, Gaiolla RD, et al. How to manage lymphoid malignancies during novel 2019 coronavirus (COVID-19) outbreak: A Brazilian task force recommendation Hematol Transfus Cell Ther 42103–1102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Society of Clinical Oncology (ASCO) ASCO Special Report: A Guide to Cancer Care Delivery During the COVID-19 PandemicMay 19, 2020https://www.asco.org/sites/new-www.asco.org/files/content-files/2020-ASCO-Guide-Cancer-COVID19.pdf [Google Scholar]
- 17.Desai A, Sachdeva S, Parekh T, et al. COVID-19 and cancer: Lessons from a pooled meta-analysis JCO Global Oncol 557–5592020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Haar J, Hoes LR, Coles CE, et al. Caring for patients with cancer in the COVID-19 era Nat Med 26665–6712020 [DOI] [PubMed] [Google Scholar]
- 19.Gill S, Hao D, Hirte H, et al. Impact of COVID-19 on Canadian medical oncologists and cancer care: Canadian Association of Medical Oncologists survey report Curr Oncol 2771–742020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oba A, Stoop TF, Löhr M, et al. Global survey on pancreatic surgery during the COVID-19 pandemic Ann Surg 272e87–e932020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saab R, Obeid A, Gachi F, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on pediatric oncology care in the Middle East, North Africa, and West Asia Region: A report from the Pediatric Oncology East and Mediterranean (POEM) Group. Cancer. 10.1002/cncr.33075 [epub ahead of print on July 10, 2020] [DOI] [PMC free article] [PubMed]
- 22.Tagliamento M, Spagnolo F, Poggio F, et al. Italian survey on managing immune checkpoint inhibitors in oncology during COVID‐19 outbreak. Eur J Clin Invest. 2020;50:e13315. doi: 10.1111/eci.13315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ürün Y, Hussain SA, Bakouny Z, et al. Survey of the impact of COVID-19 on oncologists’ decision making in cancer JCO Glob Oncol 1248–12572020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterhouse DM, Harvey RD, Hurley P, et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: Findings from an American Society of Clinical Oncology Survey JCO Oncol Pract 16417–4212020 [DOI] [PubMed] [Google Scholar]
- 25.Bleicher RJ.Timing and delays in breast cancer evaluation and treatment Ann Surg Oncol 252829–28382018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coca-Pelaz A, Takes RP, Hutcheson K, et al. Head and neck cancer: A review of the impact of treatment delay on outcome Adv Ther 35153–1602018 [DOI] [PubMed] [Google Scholar]
- 27.Sud A, Jones ME, Broggio J, et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic Ann Oncol 311065–10742020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. https://www.sfchronicle.com/bayarea/article/A-lost-opportunity-Some-Bay-Area-test-15468141.php Why the time to get back COVID-19 test results in bay area ranges from 2 weeks to 2 days [Internet]
- 29.Souch JM, Cossman JS.A commentary on rural-urban disparities in COVID-19 testing rates per 100,000 and risk factors J Rural Health37:188-190, 2021 [DOI] [PMC free article] [PubMed]
- 30.Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): First results of an international, registry-based, cohort study Lancet Oncol 21914–9222020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cancer Institute (NCI) NCI COVID-19 in Cancer Patients Study (NCCAPS): A Longitudinal Natural History Study [Internet] 2020. https://clinicaltrials.gov/ct2/show/NCT04387656 [Google Scholar]
- 32.ASCO Survey on COVID-19 in Oncology (ASCO) Registry [Internet] ASCO; 2020. https://www.asco.org/asco-coronavirus-information/coronavirus-registry [Google Scholar]
- 33.Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands Lancet Oncol 21750–7512020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcum M, Kurtzweil N, Vollmer C, et al. COVID-19 pandemic and impact on cancer clinical trials: An academic medical center perspective Cancer Med 96141–61462020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasquez L, Sampor C, Villanueva G, et al. Early impact of the COVID-19 pandemic on paediatric cancer care in Latin America Lancet Oncol 21753–7552020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karacin C, Bilgetekin I, B Basal F, et al. How does COVID-19 fear and anxiety affect chemotherapy adherence in patients with cancer Future Oncol 162283–22932020 [DOI] [PMC free article] [PubMed] [Google Scholar]