Abstract
Rationale:
Phosphaturic mesenchymal tumor (PMT) is a rare neoplasm causing oncogenic osteomalacia. Surgery remains the definitive treatment for PMT, and radiotherapy is seldom employed. However, surgery for PMT involving the head and neck is often difficult due to the local invasion and complicated anatomy. We report the first case of PMT, which was successfully treated with the combination of radiotherapy and supplementation of activated vitamin D.
Patient concerns:
A 55-year-old woman suffered from pain in the hip and bilateral femur. Serum phosphate and calcium decreased to abnormal levels. Serum alkaline phosphatase and fibroblast growth factor 23 increased to abnormal levels. The hearing loss of the right ear had continued and a middle ear tumor was revealed.
Diagnoses:
Subsequent biopsy provided the diagnosis of PMT that caused oncogenic osteomalacia. These clinical and pathological characteristics were consistent with and provided the final diagnosis of benign PMT.
Interventions:
Surgery of the PMT was difficult and the patient underwent radiotherapy. The prescribed dose was 36 Gy in 10 fractions. Simultaneously, the patient started supplementation of 1,25-dihydroxyvitamin D3 (1–2 μg/day) and continued for 2 years.
Outcomes:
Near-complete resolution of the symptoms was achieved and abnormal laboratory values recovered. At 5 years of follow-up, the irradiated tumor showed no regrowth. Severe hearing loss of the right ear was not observed.
Lessons:
Radiotherapy was effective for the PMT and could be an important treatment option for inoperable cases.
Keywords: fibroblast growth factor 23, oncogenic osteomalacia, phosphaturic mesenchymal tumor, radiotherapy, somatostatin receptor 2
1. Introduction
Phosphaturic mesenchymal tumor (PMT) is a rare neoplasm causing oncogenic osteomalacia characterized by increased renal phosphate excretion and hypophosphatemia.[1] PMTs arising in the head and neck are extremely rare, mostly in the extremities and appendicular skeleton.[2,3] Although surgery remains the definitive treatment, radiotherapy is an alternative treatment for inoperable patients. So far, there have been no reports of successful treatment of PMT with radiotherapy. We herein report a patient presenting with oncogenic osteomalacia due to a PMT in the middle ear, who was successfully treated with the combination of radiotherapy and supplementation of activated vitamin D.
2. Case report
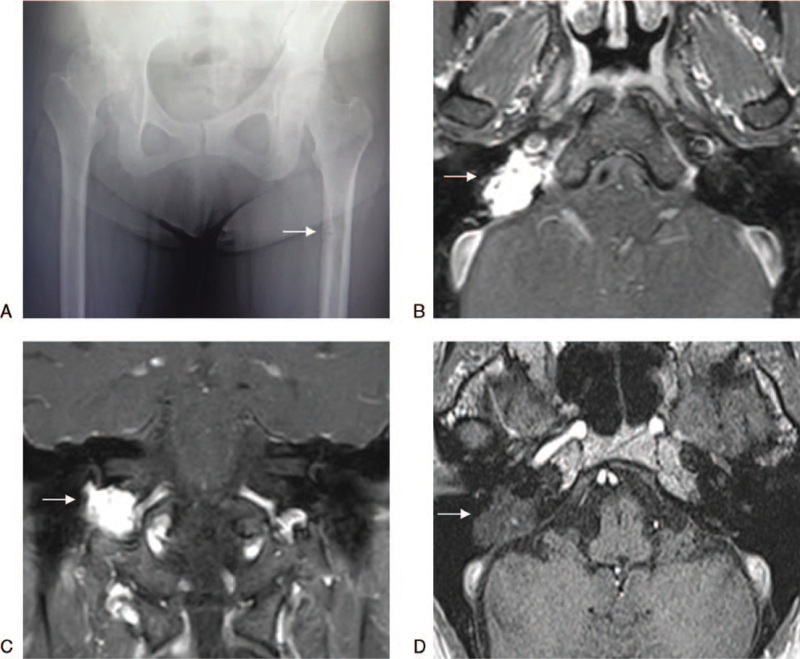
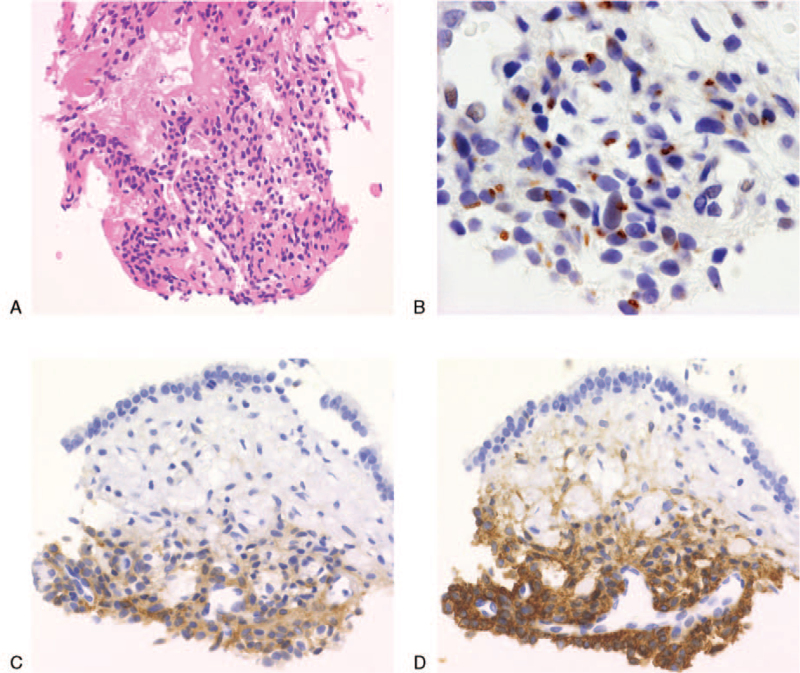
A 55-year-old woman visited an orthopedic surgery department with a complaint of pain in the hip and bilateral femurs. There was no history of trauma, and abnormal findings on plain radiography were not identified except for a mild radiolucent shadow in the left femur (Fig. 1A). In additional magnetic resonance imaging (MRI), a linear hypointensity consistent with the mild radiolucent shadow in the left femur was detected on T1-weighed images and provided the diagnosis of occult fracture. The blood test revealed decreases in serum phosphate and calcium levels to 2.2 mg/dL and 8.4 mg/dL, respectively. The additional blood test revealed significant increases in serum alkaline phosphatase and fibroblast growth factor 23 (FGF23) levels to 535 U/L and 1140 pg/mL (normal range < 30 pg/mL), respectively. The hearing loss of the right ear had continued for many months before the occurrence of pain in the hip and bilateral femurs, so the patient had an otolaryngological consultation at the same time. Otoscopic examination and MRI of the cerebellopontine angle revealed a mass in the right middle ear, measuring 1.5 × 1.5 × 1.0 cm in the largest dimensions (Fig. 1B, C). Subsequently, a biopsy of the mass was performed for histological diagnosis. Necrosis and mitotic figures indicating malignancy were absent. Short spindle-shaped cells proliferating around the branched microvessels presented a hemangiopericytoma-like growth pattern (Fig. 2A). Tumor cells stained positive for FGF23 (Fig. 2B), somatostatin receptor 2 (Fig. 2C), and cluster of differentiation 56 (Fig. 2D) but negative for h-caldesmon on immunohistochemistry. The pathological diagnosis was PMT. These clinical and pathological characteristics were consistent with and provided the final diagnosis of benign PMT.
Figure 1.
A: Plain radiography of the hip and femur. The arrow represented the mild radiolucent shadow. B: Transverse magnetic resonance imaging (MRI) before radiotherapy, gadolinium-enhanced fat saturation T1-weighted. The arrow represented the tumor and the size was 1.5 × 1.5 × 1.0 cm. C: Coronal MRI before radiotherapy, gadolinium-enhanced fat saturation T1-weighted. The arrow represented the tumor and local invasion. D: Transverse MRI at 5 years after radiotherapy, non-enhanced T1-weighted. The arrow represented the irradiated tumor.
Figure 2.
A: Short spindle-shaped cells proliferating around the branched microvessels presented a hemangiopericytoma-like growth pattern. Hematoxylin and eosin staining (×40). B: Tumor cells showed positive expressions of fibroblast growth factor 23, a dot-like pattern (×100). C: Tumor cells showed positive expressions of somatostatin receptor 2 (×40). D: Tumor cells showed positive expressions of cluster of differentiation 56 (×40).
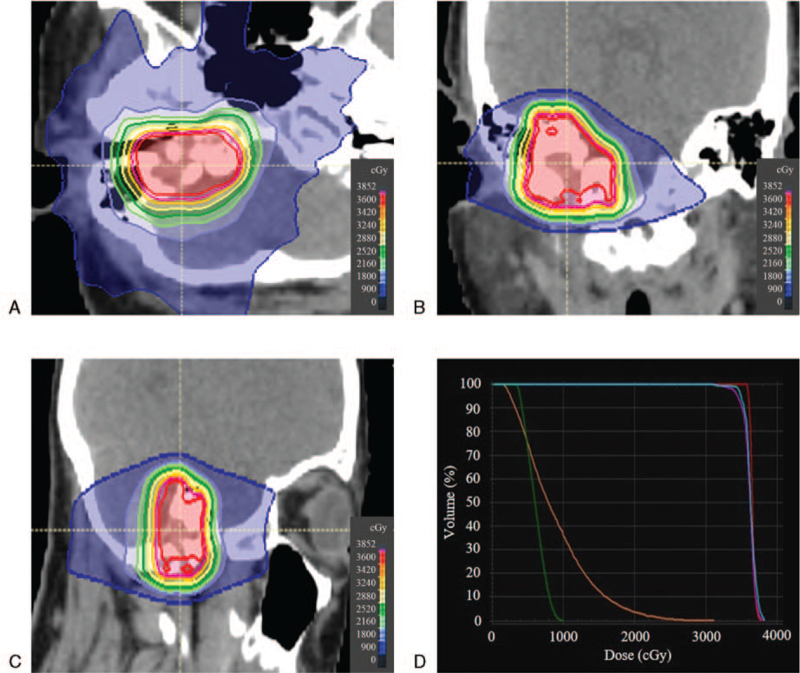
The patient underwent radiotherapy because surgery was difficult due to the local invasion. The gross tumor volume (3.8 cm3) was the visible lesion on MRI. Taking the direction of tumor invasion into consideration, the clinical target volume (10.8 cm3) was adjusted based on anatomical structures. The planning target volume (20.9 cm3) was defined with a 3-mm margin around the clinical target volume. Subsequent planning and treatments were carried out with the Tomotherapy version 5.0.1 treatment planning station and TomoTherapy HDA system (Accuray, Inc, Sunnyvale, CA). The TomoHelical mode was employed. The prescribed dose was 36 Gy in 10 fractions over 2 weeks once a day to cover 50% of the planning target volume. The field width, pitch, normal modulation factor, and irradiation time was 1.0 cm, 0.43, 2.0, and 5.6 minutes, respectively. Our method of helical tomotherapy was previously described in detail.[4] The constraints for normal organs were equal to the tolerance dose of normal tissue in radiotherapy.[5]Figure 3 showed the dose distributions and dose volume histogram of radiotherapy. Radiotherapy was performed with no acute toxicity. Simultaneously, the patient started supplementation of 1,25-dihydroxyvitamin D3 (1–2 μg/day) and continued for 2 years.
Figure 3.
Radiotherapy planning using helical tomotherapy. A: Isodose distribution in the transverse plane. B: Isodose distribution in the coronal plane. C: Isodose distribution in the sagittal plane. D: Dose volume histogram. Red, pink, blue, green, and orange lines represented gross tumor volume, planning target volume, right inner ear, left inner ear, and brain stem, respectively.
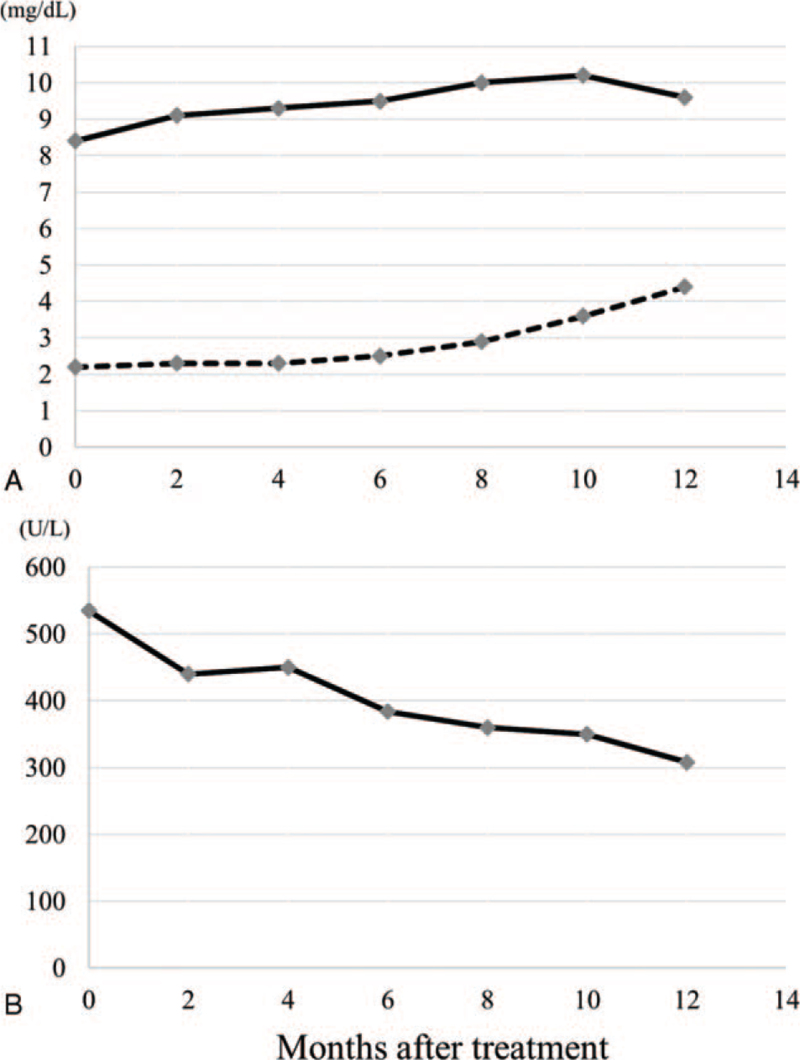
After radiotherapy, the patient was followed every 2 months during the first year, and at intervals of 6 or 12 months thereafter. Regular follow-up included pure-tone audiometry, blood tests, and MRI. At 2 months of follow-up, serum calcium recovered dramatically owing to the supplementation of 1,25-dihydroxyvitamin D3. At 8 months, normalization of serum phosphate and alkaline phosphatase levels was achieved and maintained thereafter. Figure 4 showed changes in serum calcium, phosphate, and alkaline phosphatase after treatment. At 2 years, the patient achieved near-complete resolution of bone pain. At 5 years, repeat MRI showed no regrowth of the irradiated tumor (Fig. 1D). Concerning the hearing acuity of the right ear, the Gardner-Robertson scale was used to evaluate the hearing level. Pre-treatment hearing level was scale II (38 dB). Posttreatment hearing level was scale II (40 dB) at 1 year of follow-up, scale II (46 dB) at 2 years, scale II (48 dB) at 3 years, scale III (56 dB) at 4 years, and scale III (52 dB) at 5 years, respectively. No other chronic toxicities were observed.
Figure 4.
Changes in serum calcium, phosphate, and alkaline phosphatase after treatment. A: The solid line represented the changes in serum calcium (normal range, 8.7–10.3 mg/dL). The broken line represented the changes in serum phosphate (normal range, 2.5–4.7 mg/dL). B: The solid line represented the changes in serum alkaline phosphatase (normal range, 115–350 U/L).
3. Discussion
Oncogenic osteomalacia is known as a rare and curable cause of osteomalacia characterized by increased renal phosphate excretion and hypophosphatemia.[1] PMT is a rare neoplasm arising in bone and soft tissue that inappropriately produces FGF23, which has phosphaturic activity inhibiting renal tubular reabsorption of phosphate and renal conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (active form of vitamin D). This results in hypophosphatemia and ultimately bone loss.[6] PMT is considered the most common cause of oncogenic osteomalacia.[1] PMTs arising in the head and neck are extremely rare, mostly in the extremities and appendicular skeleton.[2,3] PMT is usually benign but the local invasiveness is a characteristic feature.[3]
The definitive treatment for oncogenic osteomalacia is surgical resection of the causative PMT,[7,8] and radiotherapy is seldom employed. However, surgery for PMT involving the head and neck is often difficult due to the local invasion and complicated anatomy,[9] as the present case exhibited. Radiotherapy should be considered as an alternative treatment for inoperable cases. Cases of PMTs treated with radiotherapy were summarized in Table 1.[10–12] It was reported postoperative radiotherapy for benign PMT was effective to improve oncogenic osteomalacia. Although the detailed mechanism of the effectiveness of radiotherapy for benign PMT was still unclear, the obstruction and fibrosis of the tumor vessels could occur thus inhibiting the growth, similar to those observed in other hormone- or cytokine-producing tumors.[13,14] Considering the mechanism, radiotherapy could take some time to resolve the symptoms compared with surgery. Although PMTs and acoustic tumors were different types of tumors, the tumor location and surrounding normal tissues of the present case were similar to acoustic tumors. Considering the PMT was benign tumor, the employed dose was decided based on the excellent local control rates of acoustic tumors.[15,16] We considered the dose was probably enough to control benign PMT. It was considered that the employed dose was appropriate to prevent severe toxicities of normal organs around the target. Various studies on fractionated stereotactic radiotherapy for acoustic tumors have been reported in literature.[17,18] Especially in hearing outcomes, it was reported that the mean cochlear dose <40 Gy was a significant factor associated with hearing preservation.[17] Indeed, severe hearing loss was not observed in the present case. Malignant transformation from benign PMT was reported in the long-time follow-up.[19] Therefore, we will carefully continue the follow-up of the patient.
Table 1.
Cases of phosphaturic mesenchymal tumor treated with radiotherapy.
| Case | Author | Age, gender | Location | Pathology | Surgery | Radiotherapy | OO |
| 1 | Shah et al[10] | 60, male | Ethmoid sinus | Benign PMTMCT | Endoscopic resection | Postoperative, 54 Gy/30fxs | Cured |
| 2 | Lee et al[11] | 52, male | Ethmoid sinus | Benign PMTMCT | Endoscopic resection | Postoperative, unknown dose | Cured |
| 3 | Uramoto et al[12] | 48, male | Tongue | Malignant PMTMCT | Marginal resection | Salvage, 66 Gy/33fxs | Cured |
fxs = fractions, OO = oncogenic osteomalacia, PMTMCT = phosphaturic mesenchymal tumor, mixed connective tissue variant.
4. Conclusion
This was the first case report showing successful treatment of benign PMT with the combination of radiotherapy and supplementation of activated vitamin D. This report would serve to increase the awareness of this uncommon disease and could be a reference for employing radiotherapy in future inoperable cases.
Acknowledgment
We would like to thank all staffs at Narita Memorial Hospital and our hospitals.
Author contributions
TT contributed to collect data and draft the manuscript. NT contributed to collect data and revise the manuscript. YS contributed to the treatment of oncogenic osteomalacia. SB contributed to the pathological diagnosis of phosphaturic mesenchymal tumor. MF contributed to the staining of histopathology specimens. CS contributed to generate the radiotherapy planning. YS contributed to translate and revise the manuscript. All authors read and approved the final manuscript.
Data curation: Taiki Takaoka, Natsuo Tomita, Yoji Shido, Satoshi Baba, Mayu Fukushima, Chikao Sugie.
Investigation: Yoji Shido, Satoshi Baba, Mayu Fukushima, Chikao Sugie.
Writing – original draft: Taiki Takaoka.
Writing – review & editing: Natsuo Tomita, Yuta Shibamoto.
Footnotes
Abbreviations: FGF23 = fibroblast growth factor 23, MRI = magnetic resonance imaging, PMT = phosphaturic mesenchymal tumor.
How to cite this article: Takaoka T, Tomita N, Shido Y, Baba S, Fukushima M, Sugie C, Shibamoto Y. Radiotherapy for a rare phosphaturic mesenchymal tumor in the middle ear presenting with oncogenic osteomalacia: a case report. Medicine. 2021;100:38(e27284).
Written informed consent was obtained from the patient for this case report and accompanying images.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Folpe AL, Fanburg-Smith JC, Billings SD, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol 2004;28:01–30. [DOI] [PubMed] [Google Scholar]
- [2].Deep NL, Cain RB, McCullough AE, Hoxworth JM, Lal D. Sinonasal phosphaturic mesenchymal tumor: case report and systematic review. Allergy Rhinol 2014;5:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Komínek P, Stárek I, Geierová M, Matoušek P, Zeleník K. Phosphaturic mesenchymal tumour of the sinonasal area: case report and review of the literature. Head Neck Oncol 2011;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sugie C, Shibamoto Y, Ayakawa S, et al. Craniospinal irradiation using helical tomotherapy: evaluation of acute toxicity and dose distribution. Technol Cancer Res Treat 2011;10:187–95. [DOI] [PubMed] [Google Scholar]
- [5].Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22. [DOI] [PubMed] [Google Scholar]
- [6].Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 2001;98:6500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu S, Zhou X, Song A, et al. Successful treatment of tumor-induced osteomalacia causing by phosphaturic mesenchymal tumor of the foot. Medicine (Baltimore) 2019;98:e16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu S, Zhou X, Song A, Huo Z, Wang Y, Liu Y. Surgical treatment of recurrent spinal phosphaturic mesenchymal tumor-induced osteomalacia: a case report. Medicine (Baltimore) 2020;99:e18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fong PY, Tan TY, Kiong KL. Concurrent chemoradiation in locally advanced primary middle ear lymphoepithelial carcinoma: an effective treatment modality case report. J Otolaryngol Head Neck Surg 2021;50:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shah R, Lila AR, Jadhav RS, et al. Tumor induced osteomalacia in head and neck region: single center experience and systematic review. Endocr Connect 2019;8:1330–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee JY, Park HS, Han S, et al. Localization of oncogenic osteomalacia by systemic venous sampling of fibroblast growth factor 23. Yonsei Med J 2017;58:981–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Uramoto N, Furukawa M, Yoshizaki T. Malignant phosphaturic mesenchymal tumor, mixed connective tissue variant of the tongue. Auris Nasus Larynx 2009;36:104–5. [DOI] [PubMed] [Google Scholar]
- [13].Rieken S, Habermehl D, Welzel T, et al. Long term toxicity and prognostic factors of radiation therapy for secreting and non-secreting pituitary adenomas. Radiat Oncol 2013;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tomita N, Kodaira T, Tomoda T, Nakajima K, Murao T, Kitamura K. A case of cervical multicentric Castleman disease treated with intensity-modulated radiation therapy using helical tomotherapy. Jpn J Radiol 2012;30:349–53. [DOI] [PubMed] [Google Scholar]
- [15].Sakanaka K, Mizowaki T, Arakawa Y, et al. Hypofractionated stereotactic radiotherapy for acoustic neuromas: safety and effectiveness over 8 years of experience. Int J Clin Oncol 2011;16:27–32. [DOI] [PubMed] [Google Scholar]
- [16].Williams JA. Fractionated stereotactic radiotherapy for acoustic neuromas. Int J Radiat Oncol Biol Phys 2002;54:500–4. [DOI] [PubMed] [Google Scholar]
- [17].Bennion NR, Nowak RK, Lyden ER, Thompson RB, Li S, Lin C. Fractionated stereotactic radiation therapy for vestibular schwannomas: dosimetric factors predictive of hearing outcomes. Pract Radiat Oncol 2016;6:e155–62. [DOI] [PubMed] [Google Scholar]
- [18].Murai T, Kamata SE, Sato K, et al. Hypofractionated stereotactic radiotherapy for auditory canal or middle ear cancer. Cancer Control 2016;23:311–6. [DOI] [PubMed] [Google Scholar]
- [19].Morimoto T, Takenaka S, Hashimoto N, Araki N, Myoui A, Yoshikawa H. Malignant phosphaturic mesenchymal tumor of the pelvis: a report of two cases. Oncol Lett 2014;8:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]