Abstract
Approximately 5–7% of non–small cell lung cancer (NSCLC) cases harbor an anaplastic lymphoma kinase (ALK) fusion gene and may benefit from ALK inhibitor therapy. To detect ALK fusion genes, we developed a novel test using reverse transcription polymerase chain reaction (RT-PCR) for the ALK kinase domain (KD). Since ALK expression is mostly silenced in the adult with the exception of neuronal tissue, the normal lung tissue, mesothelial lining, and inflammatory cells are devoid of ALK transcript, making ALK KD RT-PCR an ideal surrogate test for ALK fusion transcripts in lung or pleural effusion. The test was designed with a short PCR product (197 bp) to work for both malignant pleural effusion (MPE) and formalin-fixed, paraffin-embedded (FFPE) NSCLC samples. Using ALK IHC as a reference, the sensitivity of the test was 100% for both MPE and FFPE. The specificity was 97.6% for MPE and 97.4% for FFPE. Two false positive cases were found. One was a metastatic brain lesion which should be avoided in the future due to intrinsic ALK expression in the neuronal tissue. The other one resulted from ALK gene amplification. Due to potential false positivity, subsequent confirmation tests such as fluorescence in situ hybridization or multiplex PCR would be preferable. Nevertheless, the test is simple and inexpensive with no false negativity, making it a desirable screening test. It also offers an advantage over multiplex RT-PCR with the capability to detect novel ALK fusions. Indeed through the screening test, we found a novel ALK fusion partner (sperm antigen with calponin homology and coiled-coil domains 1 like gene, SPECC1L) with increased sensitivity to crizotinib in vitro. In summary, a novel RNA-based ALK KD analysis was developed for ALK rearrangement screening in MPE and FFPE specimens of NSCLC. This simple inexpensive test can be implemented as routine diagnostics.
Introduction
Lung cancer is the leading cause of cancer-related death worldwide, despite improvements in relevant detection methods and treatment regimens. Personalized therapy through the selection of patients who are likely to respond to a particular therapeutic agent may improve patient survival [1]. The most successful example is the identification of activating mutations of the EGFR gene in patients with non–small cell lung cancer (NSCLC) for the administration of EGFR-kinase–targeting drugs [2]. Thus, the application of targeted therapies for NSCLC patients based on biomarker analysis is expected to increase.
Soda et al. discovered the fusion of the anaplastic lymphoma kinase (ALK) gene with echinoderm microtubule–associated protein like 4 (EML4) in NSCLC as a novel molecular target for cancer therapy [3]. The reported incidence of ALK rearrangement ranges from 5% to 7% in unselected NSCLC patients, with 29% in the subset of young patients with adenocarcinoma who are never or light smokers. In addition, ALK rearrangement is mutually exclusive with EGFR and KRAS mutations [4]. However, clinicopathologic characteristics are insufficient for identifying relevant patients, and molecular testing is becoming the mainstream laboratory test for analyzing ALK status [5]. The recent introduction of an ALK inhibitor in therapy for patients with ALK rearrangement further necessitates the development of molecular testing to identify patients who may benefit from the ALK targeted therapy [6]. Moreover, fusions of different ALK partners or even different fusion points with the same partner may result in differential sensitivity to structurally diverse ALK kinase inhibitors [7]. Thus, the detection of ALK rearrangement is crucial for providing quality care for patients with NSCLC in routine clinical service.
Currently, three traditional techniques are available for ALK rearrangement detection: immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and reverse transcription PCR (RT-PCR) [5, 8, 9]. In addition, ALK fusion detection can be incorporated in large panels of next generation sequencing, such as the FDA approved FoundationOne CDx (Foundation Medicine, Cambridge, MA). Each traditional method has its advantages and limitations, especially for formalin-fixed, paraffin-embedded (FFPE) tissue specimens. Interpretation of IHC results can be challenging. Positivity for ALK IHC staining was denoted by the presence of strong granular cytoplasmic staining in any percentage of positive tumor cells using VENTANA ALK (D5F3) CDx Assay. Some background staining also may be observed within normal mucosa in NSCLC (including mucin) and in necrotic tumor areas, which should be excluded from the clinical evaluation. RT-PCR is highly sensitive; however, the technique cannot identify rearrangements with unknown partners and requires high-quality RNA, which is often degraded in FFPE material. FISH was originally approved for ALK testing for NSCLC, but it is impractical because it is high cost, time consuming, and expertise dependent and requires specialized equipment [10]. Robust ALK FISH results may be difficult to achieve for small biopsy specimens containing less than 100 interpretable tumor cells. ALK FISH, such as the Vysis Break Apart ALK FISH assay, normally have a cut-off value of ≥ 15 per 100 cells (≥ 15%) for a positive result [11].
This study aims to overcome the obstacles of ALK fusion gene detection by RT-PCR in the context of suboptimal RNA quality and unknown fusion partners of ALK. Various ALK fusion genes have been reported for anaplastic large cell lymphomas, inflammatory myofibroblastic tumors, and lung adenocarcinomas [12, 13]. Given that all ALK fusion genes are within the kinase domain (KD), the sequence might be indispensable for its oncogenic activity. On the basis of this hypothesis, a novel RT-PCR analysis targeting ALK KD was designed for screening ALK fusion genes in malignant pleural effusion (MPE) or lung tissues. The positive cases can then be confirmed through sequencing or IHC, as appropriate. With this strategy, a high specificity (97.6% for MPE and 97.4% for FFPE) and 100% sensitivity (for both MPE and FFPE) were achieved in ALK gene rearrangement screening without the observation of breakpoint sequences. The results demonstrated 92.3% concordance rate (24/26) with those obtained using an ALK RGQ RT-PCR kit and Rotor-Gene Q series built-in software version 2.3.1 (Build 2). Even though the principle was the same, we do not know the primer design of the Therascreen ALK RGQ RT-PCR kit because it is confidential. The in-house primer set used in this study was independently developed in our laboratory. The efficacy supports application of the molecular test for ALK rearrangement detection in patients with NSCLC.
Materials and methods
Patients
Effusion with abnormal cytology (malignant, suspicious, and atypical) was performed through centrifugation, and the cell pellet was stored for molecular testing. From January 2011 to December 2015, 144 patients with pleural effusion were admitted to our hospital. Their cytology diagnoses included 142 for “malignancy” and 2 for “suspicion of malignancy.” Among the 144 MPE cases, 50% were male and 50% were female. Cytological types included 77 adenocarcinoma (53.5%), 2 suspicion of malignancy (1.4%), 62 malignancy (43.1%), and 3 NSCLC not otherwise specified (NSCLC-NOS) (2.1%). Of the 84 cases that had biopsy tissue proof (n = 34) or were TTF-1 positive (n = 50), all were adenocarcinoma cases; the other 60 cases were transported from referral hospital.
A total of 190 paraffin-embedded NSCLC tissue samples were examined; 55% were from male patients and 45% were from female patients. A total of 164 samples (86.3%) were adenocarcinomas and 26 (13.7%) were NSCLC-NOS. A total of 101 samples (53.2%) harbored an EGFR mutant, whereas 89 samples (46.8%) were EGFR wild-type (EGFR-wt) samples; only 47 samples had sufficient RNA for molecular testing. FFPE samples were collected from December 2013 to May 2014. All patients provided written informed consent. This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (IRB #: A-BR-101-129 and B-BR-104-089).
Extracting RNA from cell pellets and FFPE
RNA was extracted from stored cell pellets by using a QIAamp RNeasy kit (Qiagen, Hilden, Germany), QIAamp RNA FFPE tissue kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions.
RNA quality control
For quality control, two primer pairs were used to amplify the GAPDH (165 bp) and β2-microglobulin (256 bp) genes from 30 ng of each sample. Primers for GAPDH were GAA GGT GAA GGT CGG AGT C and TGG AAT TTG CCA TGG GTG GA. Primers for β2-microglobulin were TGG AGG CTA TCC AGC GTA CT and CGG CAG GCA TAC TCA TCT TT. PCR was conducted in a mixture of PCR primers (10 μM each) in a 25-μL reaction by using a thermal cycler (G-Storm; GMI, Inc., Ramsey, MN, USA). PCR conditions were as follows: 95°C for 5 min, followed by 35 cycles each of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s, with a final 10-min extension at 72°C. Gel electrophoresis was conducted using 2% agarose gel. Details were as previously described [14].
Constructing plasmid clones of ALK KD and mixed cell lines
The pGEMT easy vector plasmids containing ALK exons 20 to 28 (4080–5020; NM_004304.3) were derived from OVCAR-3 cell lines through PCR cloning. The sequence of each plasmid was confirmed using Sanger sequencing. To determine the detection limit of ALK KD screening at the RNA level, a 10-fold dilution series was prepared, ranging from 105 copies to 100 copy of the standard preparation.
ALK KD screening
ALK KD screening was amplified using primers, as follows.
ALK-forward: TCAAGTCCTTCCTCCGAGAG
ALK-reverse: CAATCTTGGCCACTCTTCCA
PCR was conducted in a mixture of PCR primers (10 μM each), 1 μL of cDNA (100 ng of RNA converted to cDNA), and a DNA polymerase (Super Therm Gold Master Mix; Bionovas Biotechnology, Toronto, Ontario, Canada). Cycling was performed as follows: 95°C for 5 min, followed by 30 cycles each of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s for MPE samples, and 35 cycles for FFPE samples. The PCR amplicons of 197 bp were examined used 2% agarose gel.
Immunohistochemistry
In brief, 4-μm–thick sections were cut from the tissue samples and placed on poly-L-lysine–coated glass slides. All slides were stained with an ALK antibody (clone D5F3 Cell Signaling Technology) diluted to 1:50 by using a Ventana Ultraview DAB detection kit in a Ventana BenchMark XT processor (Ventana Medical Systems, Inc., Tucson, AZ, USA). Antigen retrieval was conducted at 37°C for 16 min through the standard automated process in Ventana BenchMark XT. Two pathologists analyzed and classified all slides. Samples were deemed IHC positive if tumor-specific staining of any intensity was present in ≥10% of the tumor cells [15]. Two pathologists conducted IHC scoring (C-L Ho and N-H Chow).
Detecting the EML4-ALK fusion gene through multiplex PCR
The procedure for EML4-ALK multiplex PCR, adapted from Sanders et al. [16] and Soda et al. [3], was performed using Super Therm Gold Master Mix (Bionovas Biotechnology) in one tube. The primers were the same as those described previously [14].
Fluorescence in situ hybridization
For ALK break-apart FISH, ALK rearrangements were detected in FFPE specimens through FISH by using a commercially available break-apart probe for the ALK gene (Vysis LSI ALK Dual Color, Abbott Molecular, Abbott Park, IL, USA) in accordance with the manufacturer’s instructions. The probe hybridized to band 2p23 on either side of the ALK gene breakpoint. Spectrum green was used to label 5’ ALK signals, and spectrum orange was used to label 3’ ALK signals. Criteria for the probe signal interpretation of at least 50 interphase nuclei were as follows: 1. separated green and orange signals or a single red signal in at least 15% of the analyzed tumor cells indicated rearranged ALK; 2. overlapping red and green signals (yellow) indicated cells in which ALK was not rearranged.
For sperm antigen with calponin homology and coiled-coil domains 1 like gene (SPECC1L)-ALK FISH, dual-color FISH assays were performed on unstained tumor sections (4 μm) by using two Blue FISH probe sets (Illumina, San Diego, CA, USA). The SPECC1L (RP 11-80O7 bacterial artificial chromosome clone) was labeled with Chromatide Alexa Fluor 488 to produce a green probe, and the ALK (RP 11-328L16 bacterial artificial chromosome clone) was labeled with Cy3 to produce an orange probe. The FISH assays were performed using Paraffin Pretreatment Reagent Kit II (Abbott Molecular). In brief, the slide was deparaffinized, dehydrated, and then incubated in a pretreatment solution (NaSCN) at 90°C for 15 min, followed by digestion with proteases (250 mg) at 37°C for 20 min. The slide was air dried, and the SPECC1L-ALK DNA probes were codenatured at 75°C for 10 min, followed by hybridization at 37°C for 48 h. Posthybridization washing was performed with a 0.4× sodium chloride–sodium citrate (SSC) buffer (pH 7.0) and 2× SSC/0.05% Tween 20 buffer (pH 7.0); the slide was counterstained with 4’,6-diamidino-2-phenylindole. Fluorescence signals were detected using a fluorescence microscope (AxioImager Z2, Zeiss, Germany). Image analysis was performed using the Isis FISH imaging system (Meta-Systems, Germany).
5’-rapid amplification cDNA end
Fusion genes that yielded positive ALK KD screening results for ALK FISH but negative results for EML4-ALK were subjected to 5’-rapid amplification cDNA end (5’-RACE) analysis, which was performed using the SMARTer RACE cDNA Amplification Kit (Clontech, CA, USA) in accordance with the manufacturer’s protocol. The procedure for 5’-RACE primers was adapted from Wong et al. [17]. The first cDNA strand was obtained from 500 ng of the total RNA and cDNA amplified by PCR with a universal primer and an ALK-SP1 primer (5’-CATGAGGAAATCCAGTTCGT-3’) in ALK exon 22. The tailed cDNA was amplified through PCR with an oligo-dT anchor primer and ALK-SP2 primer (5’-TCAGAGCACACTTCAGGCAG-3’) in ALK exon 22. Nested PCR amplification was conducted using the anchor primer and the ALK-SP3 primer (5’-GTTGGGCATTCCGGACACCT-3’), which spanned the ALK exon 21 region. The amplified PCR fragments were subjected to DNA sequencing to identify the fusion partner.
ALK Analysis by RGQ RT-PCR
Human lung tumor RNA was extracted from FFPE tissue of NSCLC. The ALK RGQ RT-PCR kit (Qiagen, Valencia, California) was uses Scorpions technology, enabling the detection of RNA transcripts encoding the ALK tyrosine KD and control region of the ABL1 RNA transcript. The kit is designed to detect the aberrant expression of mRNA encoding the ALK tyrosine KD. Analysis was conducted using the Rotor-Gene Q series built-in software version 2.3.1 (Build 2) for the ALK RUO Kit. Real-time curves were generated using FAM-labeled probes for the control tube (ABL1, as a control) and each mutation in separate tubes. If a sample had a CT value higher than the cutoff (35.9), it was classified as negative and beyond the assay’s detection limit.
Plasmid constructs
To express the vectors of SPECC1LL, SPECC1LL-ALK, and EML4-ALK, cDNA were cloned in pGEM®-T vectors (Promega).
Cell lines
Human embryonic kidney cell lines HEK293 were maintained in Dulbecco’s modified Eagle’s medium (Hyclone) supplemented with 1% AA (Caisson) and 10% FBS (GeneTex) and cultured at 37°C with 5% CO2.
Cell viability
Cell viability and proliferation were measured using the WST-1 solution (Roche). HEK293 cells infected with retroviruses were seeded into 24-well plates (1 × 103) in 500 μL of medium containing 10% FBS and incubated at 37°C in 5% CO2. After 24 h, the medium was removed and 500 μL of fresh medium containing a different drug concentration was added. After 48 h of treatment, 500 μL of the medium was removed and 250 μL of fresh medium was added. Subsequently, 25 μL of the WST-1 solution was added to each well. Cells were then incubated for 4 h at 37°C in 5% CO2; subsequently, absorbance at 492 nm was measured using a microplate ELISA reader.
Colony formation assays for drug treatment
Cells were seeded at a final density of 1 × 103 cells per well in 6-cm cell culture plates containing fresh medium with puromycin (GeneDirex, Taiwan) and allowed to attach overnight. After 24 h, 2 mL of the medium was removed and 2 mL of fresh medium containing a different drug concentration was added (10 nM and 20 nM). After 48 h of treatment, 2 mL of the medium was removed and 2 mL of fresh medium was added. After 14 days, viable colonies were washed twice in phosphate buffered saline, fixed with 4% paraformaldehyde for 15 min, and stained with a crystal violet solution for 60 min. The colonies were then counted.
Statistical analysis
Patient characteristics (age, gender, and histology) were tabulated in relation to the mutation status. Fisher’s exact test was used to analyze associations between patient characteristics and the presence of EGFR mutations and ALK rearrangement. Significance was set at p < 0.001 (two-sided). SPSS 17.0 for Windows (SPSS Inc., Chicago, IL) was used for all analyses.
Results
Patient characteristics
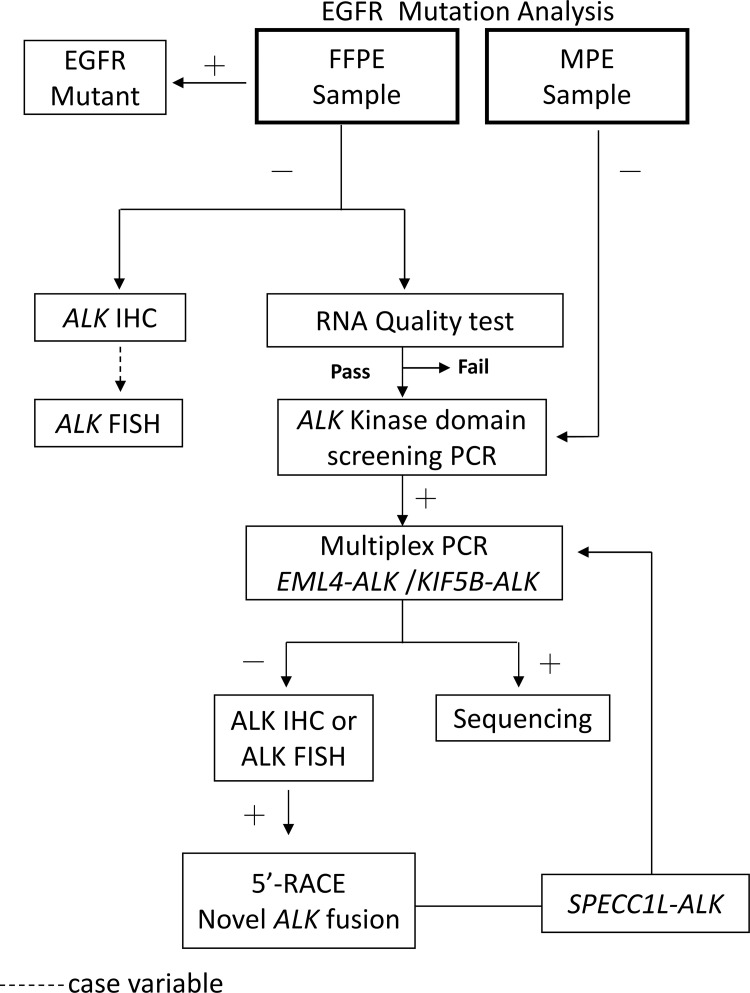
The mean ages of patients were 69.8 years (31–100 years) and 63.9 years (40–90 years) in the MPE and FFPE groups, respectively Table 1. The patient characteristics of patient cohorts are summarized in Table 1. The ALK rearrangement status was not associated with sex or age. The incidence of EGFR mutations was 67.4% and 53.2% for MPE (n = 144) and FFPE (n = 190) groups, respectively. The workflow of the determination of the ALK status and the populations identified are depicted in Fig 1. All cases of EGFR-wt were submitted for ALK KD screening and EML4-ALK multiplex PCR tests Table 2.
Table 1. Patient characteristics features of patient cohorts in relation to EGFR and ALK status.
| EGFR mutation | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malignancy pleural effusion (n = 144) | Formalin-fixed paraffin-embedded (n = 190) | Malignancy pleural effusion (n = 47) | Formalin-fixed paraffin-embedded (n = 89) | ||||||||||||||||||||
| Positive (n = 97) | Negative (n = 47) | p | Positive (n = 101) | Negative (n = 89) | p | Positive (n = 5) | Negative (n = 42) | p | Positive (n = 9) | Negative (n = 80) | p | ||||||||||||
| Age | |||||||||||||||||||||||
| Mean | 69.3 | 70.1 | NS | 67.1 | 62.3 | NS | 56.4 | 69 | <0.001 | 55.8 | 68.5 | <0.001 | |||||||||||
| Range | 35–100 | 31–92 | 41–92 | 40–90 | 38–68 | 31–92 | 38–76 | 42–90 | |||||||||||||||
| Gender | |||||||||||||||||||||||
| Male | 43 (29.9%) | 29 (20.1%) | NS | 48 (25.3%) | 57 (30.0%) | NS | 2 (4.3%) | 27 (57.4%) | NS | 4 (4.5%) | 44 (49.4%) | NS | |||||||||||
| Female | 54 (37.5%) | 18 (12.5%) | 53 (27.9%) | 32 (16.8%) | 3 (6.4%) | 15 (31.9%) | 5 (5.6%) | 36 (40.4%) | |||||||||||||||
NS: Not Significant.
Bold fonts indicate significant p values.
Fig 1. Flow chart for detecting ALK rearrangement in MPE and FFPE samples.
Table 2. Summary of ALK rearrangement detection in malignant pleural effusion (MPE) and formalin fixed paraffin embedded (FFPE).
| Malignant Pleural Effusion (MPE) (n = 144) | ||||
| EGFR Mutant | EGFR Wild type | |||
| (n = 97; 67.4%) | (n = 47; 32.6%) | |||
| ALK Kinase | EML4-ALK (mtPCR) | ALK IHC | ||
| domain screen | ||||
| 12.8% (6a/47) | 10.6% (5/47) | 13.6% (3/22) | ||
| Formalin Fixed Paraffin Embedded (FFPE) (n = 190) | ||||
| EGFR Mutant | EGFR Wild type | |||
| (n = 101; 53.2%) | (n = 89; 46.8%) | |||
| ALK Kinase domain screen | EML4-ALK (mtPCR) | ALK IHC | ||
| 21.3% (10bc/47) | 17.0% (8/47) | 10.1% (9c/89) | ||
| aCase E2211: one of six was ALK low copy number gain | ||||
| bCase E1154: one of ten was Brain metastasis | ||||
| cCase E1757: one of ten was SPECC1L-ALK | ||||
| Special Case | ALK Kinase | EML4-ALK (mtPCR) | ALK IHC | ALK FISH |
| domain screen | ||||
| aCase E2211 | Positive | Negative | Negative | Negative |
| ALK low copy number gain | (Faintly ALK stain) | (ALK copy number>3) | ||
| bCase E1154 | Positive | Negative | Negative | Negative |
| Brain metastasis | ||||
| cCase E1757 | Positive | Negative | Positive | Positive |
| SPECC1L-ALK | (Novel fusion detected by 5’-RACE) | |||
NA: not variable; 5’-RACE: 5’-rapid amplification cDNA end
Determination of assay sensitivity by using cloned DNA fragments
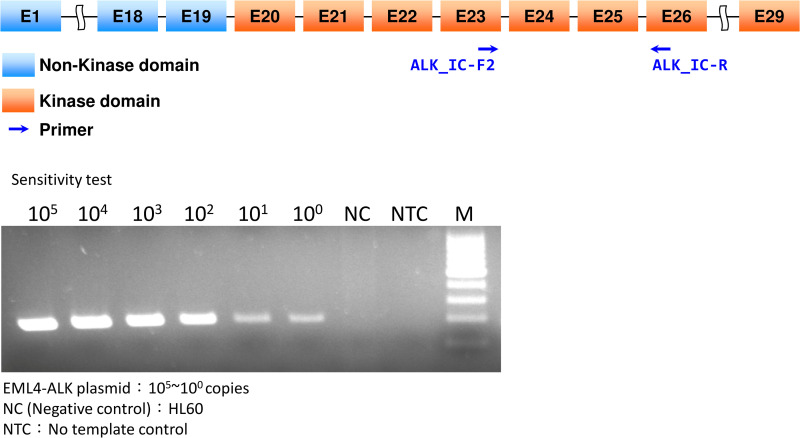
To examine the sensitivity of the ALK KD screening test, cloned ALK DNA fragments were serially diluted in the genomic DNA of HL-60 cells with different gene copies. The analytical sensitivity of the screening test was estimated at one copy of mutation in a normal background Fig 2.
Fig 2. ALK KD screening and sensitivity.
Cloned DNA fragments containing the ALK KD region (ALK exons 20 to 28 (4080–5020; NM_004304.3), serially diluted in genomic DNA from HL-60 cells). The arrows indicate the ALK primer of the ALK KD region. PCR was conducted to determine analytical sensitivity; plasmid from ALK KD and HL-60 was mixed in ratios ranging from 105 to 100 copies. The analytical sensitivity of ALK KD screening was estimated using one copy of mutations in a normal background.
ALK KD screening and EML4-ALK multiplex PCR in MPE and FFPE samples
ALK KD screening found 6 positive cases from 47 cases (12.8%) of EGFR-wt MPE, whereas the EML4-ALK multiplex PCR test yielded a positive rate of 10.6% (5/47) Table 2. For the EGFR-wt FFPE samples (n = 47), the ALK KD screening test identified 10 cases (21.3%) and the EML4-ALK multiplex PCR test identified 8 cases (17.0%).
Analysis of discordant cases in MPE and FFPE samples
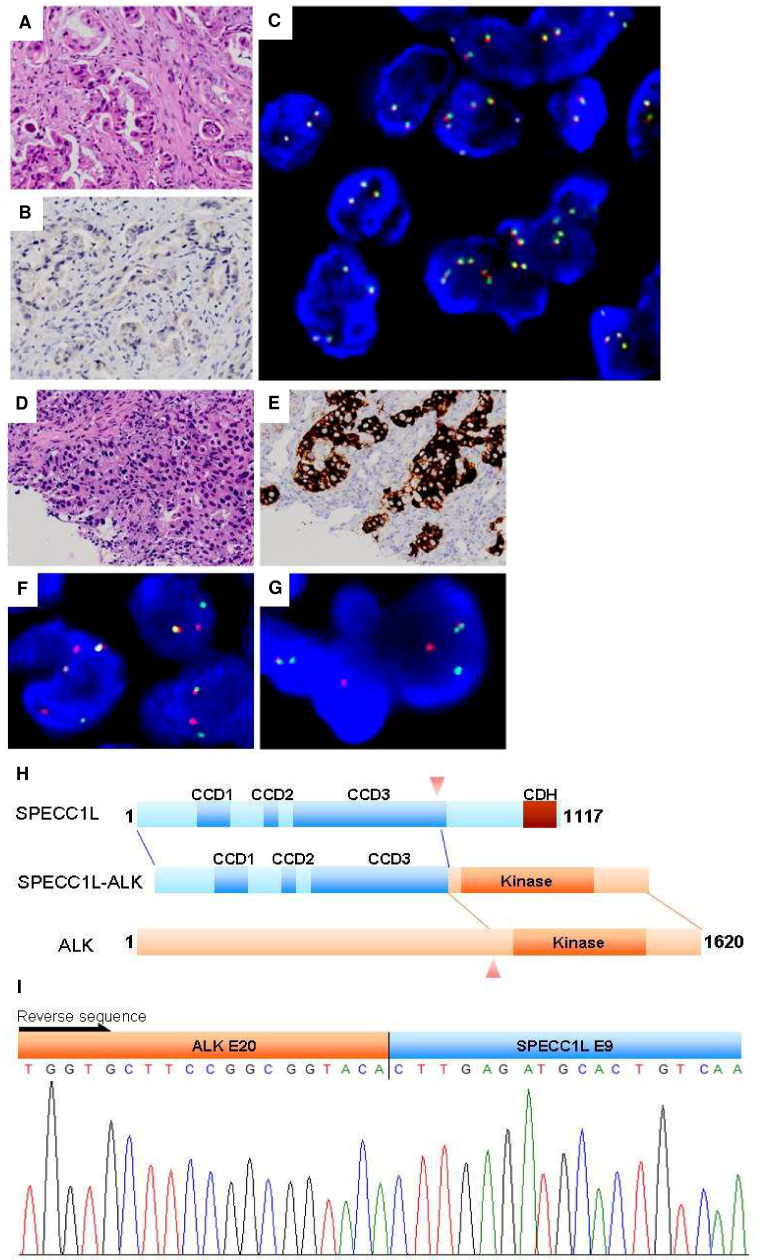
In the MPE group, case E2211 yielded a positive result from KD screening, whereas EML4-ALK multiplex PCR produced a negative result Table 2. Further investigation revealed faint IHC staining Fig 3. However, FISH revealed no break apart; many have more than 2 fused signals.
Fig 3. Analysis of ALK status by ALK KD screening, IHC, and FISH.
FFPE discrepancy cases. Case E1154 brain metastasis: (A) H&E stain, (B) ALK IHC, (C) ALK break-apart probes. No break-apart event was identified. Many cells showed more than 2 fused signals. Case E1757 SPECC1L-ALK: (D) H&E stain, (E) ALK IHC, (F) ALK break-apart probes, (G) SPECC1L-ALK probe in tumor cell containing the SPECC1L-ALK fusion (one orange/green (yellow) fusion signal was observed), (H) schematic representation of the SPECC1L-ALK protein, (I) 5’-RACE and sequencing confirmed.
Two cases of FFPE showed a discrepancy Table 2. Case E1154 was found to be positive through KD screening, but EML4-ALK multiplex PCR analysis produced a negative result. Because the tumor was retrieved from brain metastasis, false positivity could be explained by contaminated ALK mRNA transcripts from adult brain tissue [18]. Case E1757 was found to contain a novel partner of SPECC1L Fig 3. Both 5’-RACE and a sequencing experiment confirmed that exon 9 of SPECC1L was fused to exon 20 of the ALK gene. FISH analysis of tumor cells by using SPECC1L-ALK DNA probes showed a fusion of orange (ALK) and green (SPECC1L) into yellow signals.
Discrepancy between the ALK RGQ RT-PCR kit and ALK KD screening results
A subset of 26 FFPE samples had sufficient residual cDNA after screening for the presence of ALK KD. Cases subjected to parallel ALK RGQ RT-PCR testing included 11 positive cases and 15 negative cases. All ALK KD-positive samples confirmed to have mutations (n = 11) were supported by the ALK RGQ RT-PCR test (100% sensitivity). By contrast, 13 of 15 ALK KD-negative samples were confirmed by the ALK RGQ RT-PCR test (86.7% sensitivity). The concordance between the two methods was 92.3% (p < 0.0001) Table 3.
Table 3. Comparison of ALK KD sequencing and ALK RGQ RT-PCR kit results for FFPE samples.
| Method | ALK KD sequencing | ||
|---|---|---|---|
| ALK RGQ RT-PCR kit | WT | MT | |
| WT | 13 | 0 | |
| MT | 2a | 11 | |
WT: wild type; MT: mutant type.
aTwo cases with ALK CT of 35.88 and 34.58 were near the ALK cutoff CT of 35.9.
Significance set at p < 0.001.
Of note, two cases showing ALK signals at the late phase (CT 35.88 and 34.58) of the ALK RGQ RT-PCR test (cutoff CT ≤ 35.9) were confirmed to be negative by ALK IHC and thus were concluded to be false positive results. Our limited data suggest no false negativity for the ALK KD screening test.
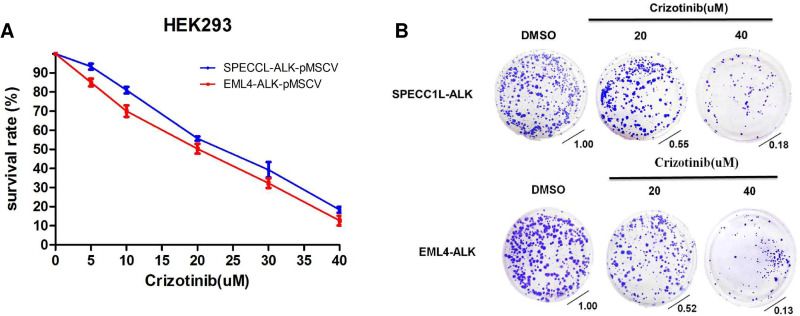
Effect of crizotinib on growth in HEK293-SPECC1LL-ALK and HEK293-EML4-ALK
To investigate the potential impact of a novel ALK fusion variant, WST-1 cell viability assay was performed on HEK293-SPECC1LL-ALK and HEK293-EML4-ALK stable cells after treatment with crizotinib, an ALK and c-ros oncogene 1 inhibitor. Crizotinib dose-dependently inhibited the growth and survival of both stable cell lines (in vitro, Fig 4).
Fig 4. Effect of crizotinib on growth in HEK293-SPECC1L-ALK and HEK293-EML4-ALK.
HEK293-SPECC1L-ALK and HEK293-EML4-ALK were incubated in complete medium for the drug concentration after 48 h of treatment with (A) WST-1 and (B) colony formation analysis. Data are presented as means ± SD from three independent experiments.
Discussion
Activating mutations as well as genomic amplification have become critical for identifying patients with NSCLC who are suitable for molecular targeted therapy. However, NSCLC with ALK gene rearrangement constitutes approximately 5%–7% of all NSCLC patients [19]. Therefore, an efficient and accurate screening test for ALK rearrangements is crucial for identifying appropriate candidates for ALK inhibitor therapy.
The rationale of this ALK KD screening test is based on the premise that wild-type ALK is constitutively silent in most adult tissues and inflammatory cells [20–22]. As a result, detection of ALK KD in adult lung tissue or pleural effusion indicates aberrant ALK expression. The technology is simple, rapid, and cost effective for detecting aberrant mRNA expression of ALK KD. We demonstrated that the ALK KD screening strategy provides comparable sensitivity to that of ALK RGQ RT-PCR testing for MPE (12.8% vs. 10.6%, respectively) and FFPE (21.3% vs. 17.0%, respectively) in patients with EGFR-wt. Current CAP/AMP guidelines recommend prioritizing testing of EGFR mutations followed by ALK assays. The detection rates of the ALK KD screening test are similar to those reported by Shaw AT., et al. [4] (approximately 13%), who focused on a subset of patients without EGFR and KRAS gene mutations, but substantially higher than those reported by Soda et al. [3] (approximately 5%), who examined patients with unselected lung adenocarcinoma.
The ALK KD screening test has several advantages over current products. First, our strategy can detect the presence of ALK fusion genes without knowledge of fusion partners. Second, EGFR mutation and ALK gene fusion are mutually exclusive events in lung adenocarcinoma [12, 13]. Our finding that cases with positive ALK gene fusion were all negative for EGFR mutations concurs with this notion. Thus, this laboratory test may be especially suitable for screening ALK gene rearrangements in EGFR-wt MPE or FFPE by using the same collection of extracted RNA.
In FFPE samples, ALK KD screening and EML4-ALK multiplex PCR tests yielded discrepant results for two cases. One false positive example could be explained by included brain tissue [18, 23]. The other one was revealed to be a new ALK fusion variant, a benefit of using this novel technique on FFPE samples [24]. Our discovery adds SPECC1L to the list of ALK fusion partners [3, 25–29]. Because of its sensitivity to crizotinib in vitro, the ALK inhibitor should be considered for patients with SPECC1L-ALK NSCLC. Given that one-fourth of ALK-positive cases might be underdiagnosed by FISH or IHC examination alone [30], the RNA-based ALK KD screening test may be a simple alternative for routine practice. This investigation provides further support for our hypothesis that RNA is a more favorable material for comprehensive molecular diagnoses in MPE [14].
Of note, current guidelines do not recommend the use of RT-PCR technology for detecting ALK rearrangement in FFPE material because of the higher failure rate in RNA-based assay due to RNA easy degrade [24]. In contrast to combined analysis of ALK KD and the control ABL1 gene in the ALK RGQ RT-PCR kit, RNA quality assessment with GAPDH (165 bp) and β2-microglobulin (256 bp) genes was chosen as our standard to select qualified samples for ALK testing. With this approach, most of the MPE samples (143/144, 99.3%) and FFPE samples (185/190, 97.4%; 5 μm, 3 sections) were favorable for testing. Our study provides a cost-effective alternative to next-generation sequencing for evaluating clinical molecular pathology in laboratories.
In this study, ALK rearrangement was associated with patients’ age but not associated with gender. The results agree with a prospective ALK screening study reporting a substantial association of younger ages with ALK rearrangements. In case of gender, conflicting findings were reported [31–33]. Further investigation is required to explain the discrepancy; however, the small sample size, selection bias, or ethnic difference of our study might account for the difference.
Even though this study put emphasis on testing economy, our ALK KD test still holds its value in a scenario where cost is not a major concern. In fact, a primer set for the ALK kinase domain can be incorporate into a multiplex PCR. If properly designed, it can give rise to a distinct band different from other specific fusions; or in a more sophisticate system, a different color or tag can be assigned to the kinase domain product. In this way, the kinase domain primers can help to detect potential novel fusions, thus eliminating the main concern of a multiplex PCR which normally can only detect known fusion events.
In summary, a novel RNA-based ALK KD analysis method has been successfully developed for ALK rearrangement screening in MPE and FFPE specimens of NSCLC. The laboratory test is simple and practical with potential to identify the rare occurrence of ALK amplification and new rearrangement partners, if substantiated by 5’RACE. The technique also has the advantage of joint analysis of EGFR and ALK gene rearrangements in NSCLC through the use of the same collection of RNA.
Supporting information
(XLSX)
(ZIP)
Acknowledgments
This manuscript was edited by Wallace Academic Editing. We thank Molecular Medicine Core Lab, Research Center of Clinical Medicine, National Cheng Kung University Hospital, for providing services that include technical support and assistance with experimental design and data analysis using the ABI 3500 Dx Genetic Analyzer.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This manuscript was supported by research grants MOST 110-2320-B-006-026 from the Ministry of Science and Technology, TAIWAN; NCKUH-11008004, NCKUH-11008014 and NCKUH-11008018 from the National Cheng Kung University Hospital, Tainan, TAIWAN.
References
- 1.Mitsudomi T, Suda K, Yatabe Y. Surgery for NSCLC in the era of personalized medicine. Nature reviews Clinical oncology. 2013;10(4):235–44. Epub 2013/02/27. doi: 10.1038/nrclinonc.2013.22 . [DOI] [PubMed] [Google Scholar]
- 2.Siegelin MD, Borczuk AC. Epidermal growth factor receptor mutations in lung adenocarcinoma. Laboratory investigation; a journal of technical methods and pathology. 2014;94(2):129–37. Epub 2014/01/01. doi: 10.1038/labinvest.2013.147 . [DOI] [PubMed] [Google Scholar]
- 3.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. Epub 2007/07/13. doi: 10.1038/nature05945 . [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53. Epub 2009/08/12. doi: 10.1200/JCO.2009.22.6993 ; PubMed Central PMCID: PMC2744268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang NN, Liu YT, Ma L, Wang L, Hao XZ, Yuan Z, et al. The molecular detection and clinical significance of ALK rearrangement in selected advanced non-small cell lung cancer: ALK expression provides insights into ALK targeted therapy. Plos One. 2014;9(1):e84501. Epub 2014/01/10. doi: 10.1371/journal.pone.0084501; PubMed Central PMCID: PMC3880316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awad MM, Shaw AT. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol. 2014;12(7):429–39. Epub 2014/10/17. ; PubMed Central PMCID: PMC4215402. [PMC free article] [PubMed] [Google Scholar]
- 7.Heuckmann JM, Balke-Want H, Malchers F, Peifer M, Sos ML, Koker M, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18(17):4682–90. Epub 2012/08/23. doi: 10.1158/1078-0432.CCR-11-3260 . [DOI] [PubMed] [Google Scholar]
- 8.Wynes MW, Sholl LM, Dietel M, Schuuring E, Tsao MS, Yatabe Y, et al. An International Interpretation Study Using the ALK IHC Antibody D5F3 and a Sensitive Detection Kit Demonstrates High Concordance between ALK IHC and ALK FISH and between Evaluators. Journal of Thoracic Oncology. 2014;9(5):631–8. doi: 10.1097/JTO.0000000000000115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu S, Yu Y, Fu S, Ren H. Cost-effectiveness of ALK testing and first-line crizotinib therapy for non-small-cell lung cancer in China. PLoS One. 2018;13(10):e0205827. Epub 2018/10/24. doi: 10.1371/journal.pone.0205827; PubMed Central PMCID: PMC6198972 support in the form of salaries for authors [Shijun Fu and Hongye Ren], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The study sponsor does not alter our adherence to PLOS ONE policies on sharing data and materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shackelford RE, Vora M, Mayhall K, Cotelingam J. ALK-rearrangements and testing methods in non-small cell lung cancer: a review. Genes & cancer. 2014;5(1–2):1–14. Epub 2014/06/24. doi: 10.18632/genesandcancer.3 ; PubMed Central PMCID: PMC4063252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Z, Wang L, Tang G, Medeiros LJ. Fluorescence in Situ Hybridization (FISH) for Detecting Anaplastic Lymphoma Kinase (ALK) Rearrangement in Lung Cancer: Clinically Relevant Technical Aspects. Int J Mol Sci. 2019;20(16). Epub 2019/08/16. doi: 10.3390/ijms20163939; PubMed Central PMCID: PMC6720438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723–33. Epub 2009/01/27. doi: 10.1002/cncr.24181 . [DOI] [PubMed] [Google Scholar]
- 13.Wu SG, Kuo YW, Chang YL, Shih JY, Chen YH, Tsai MF, et al. EML4-ALK translocation predicts better outcome in lung adenocarcinoma patients with wild-type EGFR. J Thorac Oncol. 2012;7(1):98–104. Epub 2011/11/30. doi: 10.1097/JTO.0b013e3182370e30 . [DOI] [PubMed] [Google Scholar]
- 14.Chen YL, Lee CT, Lu CC, Yang SC, Chen WL, Lee YC, et al. Epidermal Growth Factor Receptor Mutation and Anaplastic Lymphoma Kinase Gene Fusion: Detection in Malignant Pleural Effusion by RNA or PNA Analysis. PLoS One. 2016;11(6):e0158125. Epub 2016/06/29. doi: 10.1371/journal.pone.0158125; PubMed Central PMCID: PMC4924845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mino-Kenudson M, Chirieac LR, Law K, Hornick JL, Lindeman N, Mark EJ, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res. 2010;16(5):1561–71. Epub 2010/02/25. doi: 10.1158/1078-0432.CCR-09-2845 ; PubMed Central PMCID: PMC2831135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders HR, Li HR, Bruey JM, Scheerle JA, Meloni-Ehrig AM, Kelly JC, et al. Exon scanning by reverse transcriptase-polymerase chain reaction for detection of known and novel EML4-ALK fusion variants in non-small cell lung cancer. Cancer Genet. 2011;204(1):45–52. Epub 2011/03/02. doi: 10.1016/j.cancergencyto.2010.08.024 . [DOI] [PubMed] [Google Scholar]
- 17.Wong DW, Leung EL, Wong SK, Tin VP, Sihoe AD, Cheng LC, et al. A novel KIF5B-ALK variant in nonsmall cell lung cancer. Cancer. 2011;117(12):2709–18. Epub 2011/06/10. doi: 10.1002/cncr.25843 . [DOI] [PubMed] [Google Scholar]
- 18.Palmer RH, Vernersson E, Grabbe C, Hallberg B. Anaplastic lymphoma kinase: signalling in development and disease. Biochem J. 2009;420(3):345–61. Epub 2009/05/23. doi: 10.1042/BJ20090387 ; PubMed Central PMCID: PMC2708929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clinical epidemiology. 2014;6:423–32. Epub 2014/11/28. doi: 10.2147/CLEP.S69718 ; PubMed Central PMCID: PMC4242069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbour KC, Riely GJ. Diagnosis and Treatment of Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer. Hematol Oncol Clin North Am. 2017;31(1):101–11. Epub 2016/12/04. doi: 10.1016/j.hoc.2016.08.012 ; PubMed Central PMCID: PMC5154547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuyama N, Sakamoto K, Sakata S, Dobashi A, Takeuchi K. Anaplastic large cell lymphoma: pathology, genetics, and clinical aspects. J Clin Exp Hematop. 2017;57(3):120–42. Epub 2017/12/28. doi: 10.3960/jslrt.17023 ; PubMed Central PMCID: PMC6144189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata M, Hatachi Y, Ogata T, Satake H, Imai Y, Yasui H. Effectiveness of Crizotinib for Inflammatory Myofibroblastic Tumor with ALK mutation. Intern Med. 2019;58(7):1029–32. Epub 2018/11/20. doi: 10.2169/internalmedicine.1640-18 ; PubMed Central PMCID: PMC6478978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb TR, Slavish J, George RE, Look AT, Xue L, Jiang Q, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther. 2009;9(3):331–56. Epub 2009/03/12. doi: 10.1586/14737140.9.3.331 ; PubMed Central PMCID: PMC2780428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Archives of pathology & laboratory medicine. 2013;137(6):828–60. Epub 2013/04/05. doi: 10.5858/arpa.2012-0720-OA ; PubMed Central PMCID: PMC4162344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ou SH, Bartlett CH, Mino-Kenudson M, Cui J, Iafrate AJ. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology. The oncologist. 2012;17(11):1351–75. Epub 2012/09/20. doi: 10.1634/theoncologist.2012-0311 ; PubMed Central PMCID: PMC3500356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi K, Choi YL, Togashi Y, Soda M, Hatano S, Inamura K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15(9):3143–9. Epub 2009/04/23. doi: 10.1158/1078-0432.CCR-08-3248 . [DOI] [PubMed] [Google Scholar]
- 27.Jung Y, Kim P, Keum J, Kim SN, Choi YS, Do IG, et al. Discovery of ALK-PTPN3 gene fusion from human non-small cell lung carcinoma cell line using next generation RNA sequencing. Genes Chromosomes Cancer. 2012;51(6):590–7. Epub 2012/02/16. doi: 10.1002/gcc.21945 . [DOI] [PubMed] [Google Scholar]
- 28.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23. Epub 2007/12/22. doi: 10.1038/nrc2291 . [DOI] [PubMed] [Google Scholar]
- 29.Togashi Y, Soda M, Sakata S, Sugawara E, Hatano S, Asaka R, et al. KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only. PloS one. 2012;7(2):e31323. Epub 2012/02/22. doi: 10.1371/journal.pone.0031323; PubMed Central PMCID: PMC3275577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabillic F, Gros A, Dugay F, Begueret H, Mesturoux L, Chiforeanu DC, et al. Parallel FISH and immunohistochemical studies of ALK status in 3244 non-small-cell lung cancers reveal major discordances. J Thorac Oncol. 2014;9(3):295–306. Epub 2014/02/13. doi: 10.1097/JTO.0000000000000072 . [DOI] [PubMed] [Google Scholar]
- 31.Lee JO, Kim TM, Lee SH, Kim DW, Kim S, Jeon YK, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011;6(9):1474–80. Epub 2011/06/07. doi: 10.1097/JTO.0b013e3182208fc2 . [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Jang SJ, Chung DH, Yoo SB, Sun P, Jin Y, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PloS one. 2013;8(10):e76999. Epub 2013/11/07. doi: 10.1371/journal.pone.0076999; PubMed Central PMCID: PMC3806726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Yang T, Wei S, Wang J, Wang M, Wang Y, et al. Clinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancer. PloS one. 2013;8(1):e52093. Epub 2013/01/24. doi: 10.1371/journal.pone.0052093; PubMed Central PMCID: PMC3544857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.