Abstract
Background
Uncertainty underlies the effectiveness of somatostatin analogues for slowing the progression of polycystic kidney or liver disease.
Methods
Eligible studies included randomized controlled trials (RCTs) evaluating somatostatin analog as therapy for patients with polycystic kidney disease (PKD) or polycystic liver disease (PLD) compared to placebo or standard therapy. Two reviewers independently screened studies identified from databases (MEDLINE, EMBASE, Cochrane Database), clinical trial registries, and references from pertinent articles and clinical practice guidelines. Outcome measurements were changes in total liver volume (TLV), total kidney volume (TKV), and estimated glomerular filtration rate (eGFR).
Results
Of 264 nonduplicate studies screened, 10 RCTs met the inclusion criteria. The body of evidence provided estimates warranting moderate confidence. Meta-analysis of 7 RCTs including a total of 652 patients showed that somatostatin analogs are associated with a lower %TLV growth rate compared to control (mean difference, -6.37%; 95% CI -7.90 to -4.84, p<0.00001), and with a lower %TKV growth rate compared to control (mean difference, -3.66%; 95% CI -5.35 to -1.97, p<0.0001). However, it was not associated with a difference in eGFR decline (mean difference, -0.96 mL/min./1.73m2; 95% CI -2.38 to 0.46, p = 0.19).
Conclusions
Current body of evidence suggests that somatostatin analogs therapy slows the increase rate of TLV and TKV in patients with PKD or PLD compared to control within a 3-year follow-up period. It does not seem to have an effect on the change in eGFR. Somatostatin analogs therapy can be a promising treatment for ADPKD or ADPLD, and we need to continue to research its effectiveness for ADPKD or ADPLD.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most frequent inherited kidney disease and is worldwide the fourth leading cause of end-stage renal disease (ESRD) in adults [1, 2]. ADPKD is a multisystemic disorder and patients often present with extrarenal manifestations such as polycystic liver disease (PLD) [3–5]. Nonetheless, PLD can also arise in the absence of polycystic kidneys or in the presence of few renal cysts, which is denominated isolated polycystic liver disease (PCLD) or autosomal dominant polycystic liver disease (ADPLD) [6–12].
Evidence supports that somatostatin may blunt cyst development by acting at multiple levels: inhibition of secretin release by the pancreas [13], inhibition of secretin-induced cAMP generation and fluid secretion in cholangiocytes [14–16], vasopressin-induced cAMP generation and water permeability in collecting ducts by its effects on G protein-coupled receptors (Gi subtype), and suppression of the expression of IGF-1, vascular endothelial growth factor, and other cystogenic growth factors causing downstream signaling of their receptors [17–21]. Therefore, theoretically, somatostatin analogs could provide benefit for both these diseases.
Some randomized clinical trials (RCTs) have evaluated the therapeutic effectiveness of somatostatin analogues in these clinical contexts. Moreover, three previous meta-analysis were done to estimate the effectiveness of this therapeutic option [22–24]. Two of them reported that somatostatin analogs attenuated the total kidney volume (TKV) increase rate and did not altered estimated glomerular filtration rate (eGFR) [23, 24]. However, the body of evidence has continued to grow and this meta-analysis did not analyzed the effect of these drugs on the total liver volume (TLV). Another previous meta-analysis reported that somatostatin analogs attenuated TLV, but it did not show demonstrated an improvement in TKV and eGFR [22]. This last study used absolute volumes of kidney and liver which may lead to an under- or over-estimated effect size due to highly heterogeneous baseline volumes between studies. To try to overcome these limitations, we conducted a systematic review and meta-analysis of RCTs using percentage change instead of absolute volumes to assess effectiveness of somatostatin analogs therapy regarding the progression of polycystic kidney or liver disease.
Methods
This study was conducted following guidance provided by the Cochrane Handbook for systematic reviews [25]; and it is reported in accordance to the recommendations set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) work group (S1 Table, S1 Fig). The protocol of this study has been registered the PROSPERO international registry (CRD42018105336). This study protocol is accessed by the Web address (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=105336).
Eligibility criteria
We included RCTs published in peer review journals that compared a somatostatin analog against control in adult patients with PKD or PLD within a follow-up timeframe of at least 6 months. The outcomes of interest TLV, TKV or eGFR of patients with PKD or PLD.
Data sources and search strategy
A comprehensive literature search strategy, with input from study investigators, was designed and carried out by an expert librarian (P.J.E.) using MEDLINE, EMBASE, The Cochrane Database of Systematic Reviews, and The Cochrane Central Register of Controlled Trials databases. The timeframe was from each database inception to May 15, 2018 with no language restriction. The literature search strategy was updated on November 4, 2020 using the same database and the same method by an experienced librarian (E.G.). The complete search strategy can be found on the supplementary material (S2 Table).
Study selection
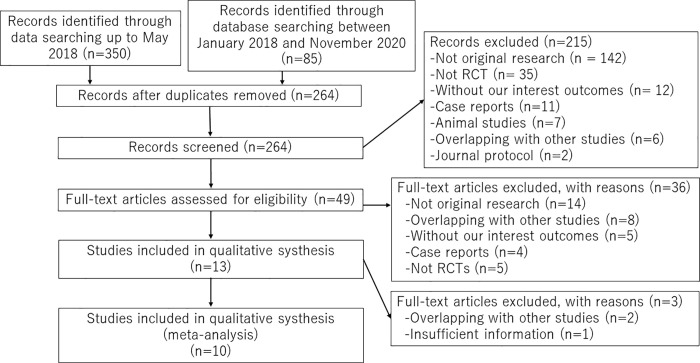
The selection process consisted of a title and abstract screening phase and a full- text screening phase (Fig 1). In both phases, each reference was screened independently by two reviewers using standardized pilot-tested instructions. As part of calibration, eligibility criteria were iterated for clarity and consistency. In the title and abstract screening level, both reviewers must have agreed to exclude an article; conflicts were included. Disagreements at the full-text screening phase were resolved by a consensus between both reviewers. When reviewers couldn’t reach consensus, a third reviewer was consulted (YU). List of excluded studies are presented in S3 Table. A total of 10 studies were included in this study (Table 1).
Fig 1. Process of study selection.
Table 1. Characteristics of the included studies.
| Author, year Study | Country | Study design | N total (% males) | N Somatostatin therapy (% males) | N Control therapy (% males) | Age (mean ± SD) years old | Intervention | Control | Outcome(s) | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|---|
| Ruggenenti et al., 2005 | Italy | Single-center crossover RCT | 12 (75) | 12 (75.0) | 12 (75.0) | 44.5 (35–58) † | Octreotide 40mg every 28 days | Placebo | TKV, eGFR | 6 months |
| van Keimpema et al., 2009 (LOCK CYST) | Netherlands and Belgium | Multicenter parallel RCT | 54 (13) | 27 (11.1) | 27 (14.8) | Intervention: 49.6 (34.4–64.8) ‡ | Lanreotide 120mg every 28days | Placebo | TLV, TKV | 6 months |
| Control: 50.3 (32.6–68.1) ‡ | ||||||||||
| Caroli et al., 2010 | Italy | Multicenter crossover RCT | 12 (75) | 12 (75) | 12 (75) | 44.5 (35–58) † | Octreotide 40mg every 28 days | Placebo | TLV, TKV | 6 months |
| Hogan et al., 2010 | USA | Single-center parallel RCT | 42 (14.3) | 28 (17.9) | 14 (7.1) | 49.9 ± 8.38 | Octreotide 40mg every 28 days | Placebo | TLV, TKV, eGFR, QoL | 1 year |
| Caroli et al., 2013 (ALADIN) | Italy | Multicenter parallel RCT | 79 (46.8) | 40 (42.5) | 39 (51.3) | 36.98 ± 8.0 | Octreotide 40mg every 28 days | Placebo | TKV, eGFR | 3 years |
| Pisani et al., 2016 | Italy | Multicenter parallel RCT | 27 (37) | 14 (36) | 13 (38) | 33.37 ± 8.61 | Octreotide 40mg every 28 days | Placebo | TLV | 3 years |
| Meijer et al., 2018 (DIPAK 1) | Netherlands | Multicenter parallel RCT | 305 (46.6) | 153 (46.4) | 152 (46.7) | 48.34 ± 7.29 | Lanreotide 120mg every 28days | Standard care only | eGFR | 2.3 years |
| TKV | ||||||||||
| QoL | ||||||||||
| Perico et al., 2019 (ALADIN 2) | Italy | Multicenter parallel RCT | 100 (57.0) | 51 (60.8) | 49 (53.1) | 49.33 ± 9.07 | Octreotide 40mg every 28 days | Placebo | TKV | 3 years |
| eGFR | ||||||||||
| Van Aerts et al., 2019 | Netherlands | Multicenter parallel RCT | 175 (45.7) | 93 (43.0) | 82 (48.8) | 48.15 ± 6.56 | Lanreotide 120mg every 28 days | Standard care only | htTLV, htTLKV, QoL | 2.3 years |
| Hogan et al., 2020 | USA | Single center randomized clinical trail | 48 (10.4) | 33 (6.1) | 15 (20.0) | 50.55 ± 8.37 | Pasireotide 60mg every 28 days | Placebo | % Change in TLV | 1 year |
* Median (IQR).
† Median (range).
‡ 95% CI. RCT = Randomized Controlled Trial. TLV = Total Liver Volume. TKV = Total Kidney Volume. eGFR = estimated Golmerular Filtration Rate. QoL = Quality of Life.
Outcomes
Our main outcome was to investigate the effect of somatostatin analogs on the TLV, TKV and eGFR in ADPKD or ADPLD patients. TLV and TKV are considered important clinical outcomes in patients with PKD because they are closely related to their quality of life [26, 27]. As the kidney or liver volume may be associated with complications of PKD, such as cyst infection, these outcomes could also predict morbidity and mortality [28]. As the baseline characteristics of the patients are variable (e.g., absolute TLV, TKV and age), we decided to conduct a meta-analysis using change in percentage of TLV and TKV between baseline and follow up (ΔTLV% or ΔTKV%) instead of the absolute value of TLV or TKV, as the change in percentage could be less influenced by the variability in baseline characteristics across studies. Regarding eGFR, we aimed to meta-analyze eGFR as the absolute value since this variable is more standardized across populations.
Data collection and management
The data from RCTs was extracted using a standardized form. Data extraction was done in a duplicated and independent manner by two reviewers (TS and FJB) after a pilot phase in which reviewers were calibrated. Data on inclusion criteria for each trial, patient demographics, baseline characteristics, sample size, intervention characteristics (type of somatostatin analog and doses), follow-up time, outcome measurements (TLV, TKV, eGFR), and loss to follow-up rate was extracted. If available, the number of events in each trial was extracted and attributed to the arm to which patients were randomized.
Risk of bias and confidence in the body of evidence
We used the Cochrane risk of bias assessment tool to assess the risk of bias of the primary studies (S2 Fig). This tool takes into consideration seven domains, (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective outcome reporting, and (7) other sources of bias. Two reviewers (TS and FJB) independently assessed each study´s quality by examining these domains. Disagreements between the reviewers were resolved by consensus. When reviewers couldn’t reach consensus, a third reviewer was consulted (YU or RRG). The overall confidence or overall quality of evidence for each outcome was appraised by discussion between the two extractors using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (S4 Table). This approach takes into account the risk of bias of the individual studies, inconsistency in the results, indirectness, imprecision and other considerations to provide a global assessment of the confidence merited by the body of evidence [29].
Statistical analysis
Assumptions and calculations for meta-analysis
The summary of TLV, TKV and eGFR is shown in Table 2. Six of the 10 articles reported the rate of change in TLV (ΔTLV) and/or TKV (ΔTKV). Perico et al. and Temmerman et al. reported median and quartile ranges of the rate of change in TLV and TKV (%), we estimated the mean and SD of the rate of change in TLV and TKV under the assumption that the distribution was normal. Meijer et al. reported the rate of change in height adjusted TLV and TKV (%), but we considered it was equal to the rate of change in TLV and TKV (%). Caroli et al. reported actual TKV of each patient, but they reported only mean and standard deviation (SD) of TKV in their article in 2010. Caroli et al. reported mean and standard error (SE) of TKV in their article in 2013. We estimated the rate of change in TKV from these values. We presented the calculation process in S5–S7 Tables.
Table 2. Total kidney, liver volumes, and estimated glomerular filtration rate reported in (or estimated from) the included studies.
| Study, year | Follow-up | Somatostatin | Control | ||||
|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Estimated or Reported Change % (mean ± SD) | Pre-treatment | Post-treatment | Estimated or Reported Change % (mean ± SD) | ||
| Total Liver Volumes (mL) | |||||||
| LOCKCYST, 2009 | 6 months | 4606 (547–8665)‡ | 4471 (542–8401)‡ | -2.9 ± 15.5 | 4689 (613–8765)‡ | 4896 (739–9053)‡ | 1.6 ± 12.8 |
| Caroli, 2010 | 6 months | 1595 ± 478 | 1524 ± 453 | -4.03 ± 3.33 | 1580 ± 487 | 1594 ± 480 | 1.23 ± 6.46 |
| Hogan, 2010 | 1 year | 5907.7 ± 2915.0 | 5557.1 ± 2659.4 | -5.0 ± 6.77 | 5373.9 ± 3565.4 | 5360.6 ± 3330.9 | 0.9 ± 8.33 |
| Pisani, 2016 | 3 years | 1609.7 ± 501.2 | 1479.5 ± 470.9 | -7.8 ± 7.4 | 1693.0 ± 470.7 | 1837.2 ± 748.5 | 6.1 ± 14.1 |
| Van Aerts, 2019 | 6 months | 2781 (2272–4230)* | -1.99 ± 3.30 ¶ | 2389 (2168–3029)* | 3.92 ± 3.50 ¶ | ||
| Hogan, 2020 | 1 year | 2582 ± 1381 | 2479 ± 1317 | -3.4 ± 7.3 | 2387 ± 759 | 2533 ± 770 | 6.3 ± 7.0 |
| Total Kidney Volumes (mL) | |||||||
| Ruggenenti, 2005 | 6 months | 2551 ± 1053 | 2622 ± 1111 | 2.2 ± 3.7 | 2461 ± 959 | 2623 ± 1021 | 5.9 ± 5.4 |
| LOCKCYST, 2009 | 6 months | 1000 (-39-2039)‡ | 983 (-62~2028) ‡ | -1.5 ± 31.1 | 1115 (-519~2748) ‡ | 1165 (-541~2871) ‡ | 3.4 ± 28.0 |
| Hogan, 2010 | 1 year | 1142.9 ± 826.9 | 1128.5 ± 796 | 0.25 ± 7.53 | 803 ± 269.1 | 873.5 ± 306.2 | 8.61 ± 10.07 |
| ALADIN, 2013 | 1 year | 1556.9 ± 1035.1† | 1603.1 ± 176.1† | 2.97 ± 7.41 | 2161.2 ± 1274.9† | 2304.9 ± 224.6† | 6.65 ± 5.31 |
| 3 years | 1556.9 ± 1035.1† | 1672.7 ± 202.0† | 14.14 ± 19.95 | 2161.2 ± 1274.9† | 2621.0 ± 271.0† | 21.02 ± 32.41 | |
| DIPAK 1, 2018 | 1 year | 2046 (1383–2964)* | N/A | 4.15 ± 5.20 | 1874 (1245–2868)* | N/A | 5.56 ± 5.06 |
| ALADIN 2, 2019 | 1 year | 2338.9 (1967.6–3807.4)* | 2513.3 (2023.6–3923.5)* | 5.2 ± 6.37 | 2591.0 (1959.3–3855.7)* | 2935.1 (2197.1–4094.4)* | 8.8 ± 6.15 |
| 3 years | |||||||
| 2338.9 (1967.6–3807.4)* | 3043.9 (2337.3–5470.6)* | 29.9 ± 21.33 | 2591.0 (1959.3–3855.7)* | 3613.8 (2584.1–4866.8)* | 37.1 ± 23.26 | ||
| Hogan, 2020 | 1 year | 534 ± 343 | 523 ± 325 | -1.4 ± 3.5 | 397 ± 159 | 417 ± 177 | 3.9 ± 4.5 |
| Estimated Glomerular Filtration Rate (mL/min./1.73m 2 ) | |||||||
| Ruggenenti, 2005 | 6 months | 59.5 ± 25.2 | 54.0 ± 23.6 | -5.5 ±10.75 | 57.9 ± 22.49 | 57.7 ± 25.7 | -0.2 ± 7.33 |
| Hogan, 2010 | 1 year | 68.1 ± 26.53 | 64.6 ± 25.66 | -5.1 ± 15.46 | 70.8 ± 28.08 | 65.7 ± 26.40 | -7.2 ± 13.21 |
| ALADIN, 2013 | 1 year | 88.68 ± 3.93† | 77.86 ± 4.23† | -10.82 ± 7.03 | 77.77 ± 5.30† | 72.16 ± 5.45† | -5.61 ± 4.09 |
| 3 years | 88.68 ± 3.93† | 76.33 ± 4.66† | -3.85 ± 3.17 | 77.77 ± 5.30† | 64.64 ± 6.51† | -4.95 ± 4.13 | |
| DIPAK 1, 2018 | 1 year | 51.0 ± 11.5 | -3.53 ± 2.93 | 51.4 ± 11.2 | -3.46 ± 1.39 | ||
| ALADIN 2, 2019 | 1 year | 27.9 (23.5–32.1)* | 22.5 (17.3~27.7)* | -6.2 ± 2.5 | 25.8 (19.5~33.2)* | 20.2 (14.7~28.1)* | -6.5 ± 0.8 |
| 3 years | 27.9 (23.5–32.1)* | 14.9 (11.3~20.2)* | -4.26 ± 2.00 | 25.8 (19.5~33.2)* | 15.0 (7.5~24.4)* | -4.19 ± 2.52 | |
| Hogan, 2020 | 1 year | 74 ± 24 | 73 ± 22 | -0.3 ± 14 | 74 ± 18 | 73 ± 22 | -2.2 ± 17.7 |
Mean±SD.
*Median (IQR).
† mean ± SE.
‡ 95%CI.
¶ hight adjusted TLV
Summary measures and data synthesis
We estimated the mean differences (MD) and SD in rate of ΔTLV, ΔTKV (%), and eGFR (mL/min./1.73m2), and pooled all the studies’ effect size using a random-effects model as described by DerSimonian and Kacker [30]. We chose random-effects model as our main method of analysis because of its conservative summary of estimates and incorporation of between- and within-study variability. To assess heterogeneity of treatment effect among trials, we used the I2 statistic; this represents the proportion of heterogeneity of treatment effect across trials that are not attributable to chance or random error. A value of 50% reflects significant heterogeneity and could be due to real differences in study populations, protocols, interventions, or outcomes [31]. Finally, we performed a sensitivity analysis excluding studies with a cross-over design from the meta-analysis to see if the estimate changed because these studies could introduce bias by the presence of a carry-over effect of the intervention. The p value threshold for statistical significance was set at .05 for effect sizes. Analyses were conducted using features on RevMan version 5.3 (The Nordic Cochrane Center, Copenhagen, Denmark). Finally, we analyzed the inter-observer agreement in the full text screening phase by calculating Cohen’s kappa coefficient.
Results
Characteristics of included studies
A total of 10 RCTs that included a total of 854 patients were included in the qualitative and quantitative analysis [32–41]. We excluded the pooled study regarding LOCKCYST I TRIAL by Temmerman et al. in 2013 that overlapped patients with the study by Van Keimpema et al. in 2009. The full characteristics of the included studies are summarized in Table 1. Somatostatin analog was administered by intramuscular or subcutaneous injections in all studies; therefore, placebo was not administered in some studies. In these studies, blinding of participants and personnel was not done. Cohen’s kappa coefficient resulted in substantial inter-observer agreement (κ = .71).
Total Liver Volume (TLV)
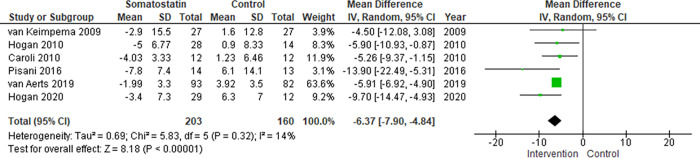
On the meta-analysis of 6 studies assessing the effect on TLV (363 patients), Somatostatin analog was associated with lower %TLV growth rate compared to control that was not statistically significant: MD -6.37% (95% CI -7.90 to -4.84, p<0.00001; I2 = 14%) (Fig 2).
Fig 2. Meta-analysis of TLV.
Total Kidney Volume (TKV)
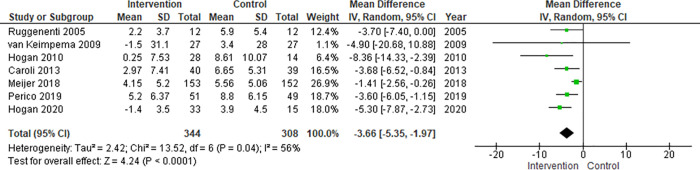
On the meta-analysis of 7 studies assessing the effect on TKV (652 patients), Somatostatin analog was significantly associated with lower %TKV growth rate compared to control: MD, -3.66% (95% CI -5.35 to -1.97, p<0.0001; I2 = 56%) (Fig 3).
Fig 3. Meta-analysis of TKV.
Estimated Glomerular Filtration Rate (eGFR)
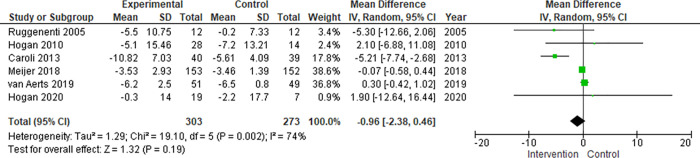
On the meta-analysis of 6 studies assessing the effect on eGFR (576 patients), somatostatin analog showed a slight decrease in eGFR compared to control that was not statistically significant: MD -0.96 mL/min./1.73m2 (95% CI -2.38 to 0.46, p = 0.19; I2 = 74%) (Fig 4).
Fig 4. eGFR.
Adverse events
We present the percentage change (frequency in somatostatin group–frequency in control group) of each adverse event. Symptoms such as cholelithiasis/cholecystitis, gastrointestinal symptoms, and hepatic or renal cyst infection were more frequent in somatostatin group than control group. We summarized serious adverse events associated with somatostatin analog in Table 3. All adverse events are presented in S8 Table.
Table 3. Severe adverse events; % in somatostatin group vs. % in control group.
| Study, year | Biliary complications | Gastrointestinal complications | Infection | Others* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cholelithiasis | Cholecystitis | Abdominal pain, Epigastric pain, Gastroenteritis | Constipation | Diarrhea | Gastroenteritis, epigastric pain | Hepatic /Renal cyst infection | Hepatic / Renal cyst hemorrhage | Urinary tract infection, pyelonephritis | ||
| Ruggenenti, 2005 | 8.3 vs. 0 | N/A | N/A | N/A | 25.0 vs. 0 | N/A | N/A | N/A | N/A | N/A |
| LOCKCYST, 2009 | N/A | N/A | 59.0 vs. 0.0 | 4 vs. 0 | 70.0 vs. 21.0 | N/A | N/A | N/A | N/A | 7.0 vs. 0 |
| Hogan, 2010 | N/A | N/A | 50.0 vs. 21.0 | N/A | 61.0 vs. 28.0 | N/A | N/A | N/A | N/A | N/A |
| ALADIN, 2013 | 5.0 vs. 0 | 5.0 vs. 0 | N/A | N/A | N/A | N/A | 2.5 vs. 0 | 2.4 vs. 0 | 2.5 vs. 0 | |
| Pisani, 2016 | 7.1 vs. 0 | 7.1 vs. 0 | N/A | N/A | N/A | N/A | 7.1 vs. 0 | 14.2 vs. 0 | N/A | 7.1 vs. 0 |
| DIPAK 1, 2018 | 0.7 vs. 0 | N/A | N/A | N/A | N/A | 1.3 vs. 0 | N/A | N/A | 2.0 vs. 0 | |
| Van Aerts, 2019 | 1.1 vs. 0 | N/A | N/A | N/A | N/A | 1.1 vs. 0 | 8.7 vs. 2.2 | N/A | 1.1 vs. 0 | 1.1 vs. 0 |
| ALADIN 2, 2019 | N/A | N/A | N/A | N/A | N/A | N/A | 2.0 vs. 0 | N/A | 29.4 vs. 16.3 | |
| Hogan, 2020 | N/A | N/A | 48.5 vs 40 | 3 vs 6.7 | 51.5 vs 53.3 | N/A | N/A | 3 vs 0 | 9.1 vs 0 | |
* Other all adverse events except for biliary, gastrointestinal, and infectious complications.
Sensitivity analyses
In the outcome of TLV, we excluded the study by Caroli et al., 2010. Results suggested that somatostatin analog was still significantly associated with a lower %TKV growth rate compared with control: MD -6.89% (95% CI -9.11 to -4.68, p<0.00001; I2 = 29%) (S3-1 Fig).
We conducted a sensitivity analysis by study design excluding the studies that had a cross-over design because these studies may introduce some bias due to presence of carry-over effect. In the outcome of TKV, we excluded the studies of Ruggenenti et al., 2005. This demonstrated that somatostatin analog was still significantly associated with a lower %TKV growth rate compared with control: MD -3.73% (95% CI -5.66 to -1.79, p = 0.0002; I2 = 62%) (S3-2 Fig).
Finally, in the outcome of eGFR, we excluded the study by Ruggenenti et al., 2009. This showed that somatostatin analog was still not associated with a lower eGFR decline: MD -0.79 mL/min./1.73m2 (95% CI -2.20 to 0.63, p = 0.28; I2 = 77%) (S3-3 Fig).
Risk of bias and confidence in the overall body of evidence
All of the studies included a low rate of lost to follow-up, representing low risk of attrition bias in some RCTs (S9 Table, S2 Fig). Random sequence generation and allocation concealment were graded as low risk of bias for the majority of the studies. Blinding of participants and personnel represented a concern in 5 studies. However, outcome assessment was blinded in almost all of the studies. Finally, studies using a crossover design might have introduced bias through the “carryover effect” but this was analyzed through a sensitivity analysis, and we concluded these studies were unlikely to introduce bias through this phenomenon because the estimates did not change significantly.
The overall quality of the evidence was considered high for the efficacy in TLV (S4 Table); and moderate for TKV due to inconsistency of results. Regarding the eGFR outcome, quality of the evidence was graded as low due to inconsistency of results and indirectness. The result of risk of bias assessment is presented in S2 Fig.
Discussion
Main findings
We found a significant reduction in change in TLV and TKV. This difference was relatively large (6.37% for ΔTLV and 3.66% for ΔTKV). We consider that this should be considered as a significant clinical benefit to the patients. We observed no difference in the eGFR decline; our analysis suggested a high heterogeneity for this outcome which could be partially explained by a different direction of the effect estimates in the included studies.
Comparison with previous studies
Compared with a previous meta-analysis, we estimated the effect of somatostatin analogues on TLV or TKV using the change in percentage instead of absolute values [22]. We considered this methodology more appropriate because baseline TLV or TKV is highly heterogeneous among studies and therefore the analysis could be over- or under-estimated, and this could lead to inaccurate efficacy estimates. Other previous meta-analyses have concluded controversial results regarding the efficacy of this intervention. Our results resonate with those of two previous meta-analysis that found no difference in the eGFR decline rate after the intervention. Conversely, our results differ from those in a previous analysis that suggested that this intervention shows no benefit in TKV. We consider that our meta-analysis was more sensitive to detect the effectiveness of somatostatin analogues on TLV and TKV in comparison to the previous analysis that used the absolute values of TLV and TKV because we used the change in percentage of TLV and TKV for the analysis. This suggests that somatostatin analogs are more likely to be effective for early-stage patients or patients with a smaller TLV or TKV.
Implications for clinical practice and research
TLV and TKV are considered important clinical outcomes in patients with PKD because they are closely related to their quality of life, morbidity and mortality [26–28]. Our results suggest that somatostatin analogs are effective for slowing growth of PKD or PLD, however the treatment effect of somatostatin analogs could vary among individual patients. The average age of the enrolled patients in the study by Pisani 2020 was the youngest in all studies and the effect of somatostatin analogs on TLV was the strongest among all studies. Meijer et al. also reported that somatostatin analogs were more effective for patients whose age (≤45) than those whose age (>45) though it was not statistically significant [38]. Among the enrolled RCTs of this meta-analysis, van Aerts et al., presented a subgroup analysis that suggested that patients ≤ 45 years old seemed to have more benefits from somatostatin analogs compared to those > 45, however this effect was not statistically significant [40]. These suggests that somatostatin analogs are more effective on younger patients, which was consistent with the report by Gevers et al., They reported that young female patients (48 years old and younger) seemed to have the most substantial effect of somatostatin analogs in a pooled analysis [42]. Some studies reported multiple pregnancies and exogenous estrogens as risk factors for growth of hepatic cysts [43, 44]. Gevers et al. mentioned that premenopausal status may be an independent risk factor for polycystic liver growth due to hormonal influence [42]. Cholangiocyte proliferation is considered one of the major contributors to hepatic cystogenesis and is significantly increased by estrogens in vitro [45–47]. Thus, liver cysts grow rapidly in young women and somatostatin analogs may be the more effective for such patients with extensive cyst proliferation. It has also been suggested that estrogens may enhance the ability of somatostatin analogs to inhibit cyclic adenosine monophosphate production in cholangiocytes, and can increase susceptibility to somatostatin analogs therapy in fertile women [42]. Taken together, we hypothesize that young women may receive the most benefit from somatostatin analogs; however, the primary studies lack subgroup analyses. Moreover, our results suggest that TKV seems to be less effected and eGFR does not seem to be affected by somatostatin analog therapy. Nonetheless, longer follow-up periods could be useful to further clarify any effectiveness on TKV and eGFR.
The frequency of reported adverse events with somatostatin analog therapy was high in all RCTs. However, the rate of adverse events seems to be almost same as the rates reported in other clinical trials using somatostatin analogs for other diseases, such as acromegaly and neuroendocrine tumors [48].
End stage renal disease (ESRD) and death may be the most important outcomes. The study by Perico et al., reported that 3 patients in the intervention group (5.9%) progressed to end stage renal disease compared to 8 (16.9%) in the placebo group. In the study of Meijer et al., 1 patient in the intervention group died but they report that this patient was diagnosed with lung cancer during the study. However, only 4 studies (Meijer et al., Perico et al., and Hogan et al.) considered ESRD as an outcome. Additionally, death was reported by 2 studies (Meijer et al. and Van Aerts et al.), none of the studies considered it as an outcome S10 Table. As such, there is scarcity of information regarding these patient-important outcomes and therefore, we were unable to assess the effectiveness of somatostatin analogs on ESRD or death. PKD or PLD are slowly progressive diseases and longer study periods may be necessary to evaluate these outcomes.
Basic optimized treatments for ADPKD include rigorous blood pressure control and various dietary changes [49]. Disease modifying treatment for ADPKD is currently very limited, but tolvaptan (a vasopressin V2 receptor antagonist) has been approved in several countries. According to the TEMPO 3:4 study, the rate of any adverse events was 97.9% among patients who received tolvaptan and a total of 15.4% of the patients who received tolvaptan permanently discontinued the trial drug due to adverse events associated with the drug [50]. The common adverse events of tolvaptan are thirsty and polyuria. On the other hand, the common adverse events of somatostatin analogs are digestive problems and the rate of discontinuation in patients who received somatostatin analogs as a trial drug was up to 15%. Taken together, the tolerability of somatostatin analogs may not be worse than that of tolvaptan.
Cost for somatostatin analogs is as high as that for tolvaptan ($8,011 for tolvaptan, $7,960 for 40mg octreotide LAR, and $10,144 for 120mg lanreotide per month in the U.S.). However, as there are no studies head-to-head trials comparing somatostatin analog and tolvaptan, we were not able to compare tolvaptan versus somatostatin analogs directly. Tolvaptan is generally ineffective for slowing progression of PLD because vasopressin V2-receptor is unique for kidney, therefore somatostatin analog may be the only current available drug to slow progression of PLD. In that sense, somatostatin analog use could be more justified in patients with PLD. Moreover, somatostatin analogs and tolvaptan could provide synergistic effect if used together. If true, the required dose of somatostatin analogs could be reduced if used along with tolvaptan. Nonetheless, further studies are necessary to clarify the relationship between somatostatin analogs and tolvaptan.
Ultimately, we consider that somatostatin analogs could be effective even though the disadvantages -relatively high adverse event frequency and high therapy cost- it may carry. However, more studies are needed to further define which particular patients could experience the greatest benefit by this therapy.
Strengths and limitations
The systematic approach of this review and the thorough search strategy strengthens our study. Moreover, the moderate confidence of our estimates and the fact that the average age of patients in 7 of 10 studies was similar, also provide strength to our results.
There are also some limitations in this study. First, the follow-up periods were variable (ranged from 6 months to 3 years). This difference in follow up time might have affected the results of our meta-analysis. The baseline characteristics of enrolled patients were also variable among each study. For example, baseline TLV and TKV were variable even though they may be important predictive factors. The average TKV ranged from 1000 to 2600 mL. The range of mean TLV of included studies was more variable. As such, the mean TLV was about 1590 mL in the study by Caroli et al. in 2010, whereas that in the study by Hogan et al. in 2010 was about 5900 mL. However, it seems that there was not any association between baseline TLV/TKV and the effectiveness of the intervention. In addition, other important baseline characteristics on enrolled patients such as genetics characteristics, blood pressure and concomitant medications are unknown in many studies. Moreover, some studies included patients with PLD instead of PKD. Also, two of the 10 RCTs had crossover design. Additionally, since all of the studies included in this meta-analysis were conducted in Europe or the U.S. and the included patients were mostly Caucasians, we cannot truly generalize these results to the other races or other regions of the world. Besides, the number of included studies is not large, and this made it difficult to evaluate the risk of publication bias. At last, as the number of studies included in this meta-analysis is limited, accumulation of studies would be necessary to make stronger conclusions.
Conclusion
The body of evidence shows that somatostatin analog therapy slows increase rate of TLV and TKV in patients with PLD or PKD compared to control group within a 3-year follow-up period. However, somatostatin analogs are associated with severe adverse events and high costs. More evidence is needed to further define to which patients could this therapy be justified, and which patients would receive the greatest benefit from it.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
(DOCX)
(DOCX)
Acknowledgments
TS and FJB were scholars of the class of Systematic Reviews and Meta-Analysis in Mayo Clinic’s Graduate School, which was directed by: Victor Montori, MD, MSc; Colin West, MD, PhD; and M. Hassan Murad, MD in 2018, and this study is followed by the policy of this class. Analysis and interpretation of data in this study was supported by Satista (Kyoto, Japan). We thank Ms. Lisa E. Vaughan for providing us the data about the study by Dr. Hogan in 2020.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by JSPS KAKENHI in the form of a grant awarded to TS (JP19K17758) and in part by the Ministry of Health, Labour and Welfare of Japan in the form of a Grant-in-Aid for Progressive Renal Disease Research and Okinaka Memorial Institute for Medical Research, Toranomon Hospital in the form of funds awarded to TS.
References
- 1.Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–85. Epub 2008/10/04. doi: 10.1056/NEJMcp0804458 . [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–301. doi: 10.1016/S0140-6736(07)60601-1 . [DOI] [PubMed] [Google Scholar]
- 3.Pirson Y. Extrarenal Manifestations of Autosomal Dominant Polycystic Kidney Disease. Advances in Chronic Kidney Disease. 2010;17(2):173–80. doi: 10.1053/j.ackd.2010.01.003 . [DOI] [PubMed] [Google Scholar]
- 4.Chauveau D, Fakhouri F, Grunfeld JP. Liver involvement in autosomal-dominant polycystic kidney disease: therapeutic dilemma. J Am Soc Nephrol. 2000;11(9):1767–75. Epub 2000/08/31. doi: 10.1681/ASN.V1191767 . [DOI] [PubMed] [Google Scholar]
- 5.Milutinovic J, Fialkow PJ, Rudd TG, Agodoa LY, Phillips LA, Bryant JI. Liver cysts in patients with autosomal dominant polycystic kidney disease. Am J Med. 1980;68(5):741–4. Epub 1980/05/01. doi: 10.1016/0002-9343(80)90266-1 . [DOI] [PubMed] [Google Scholar]
- 6.Suwabe T, Chamberlain AM, Killian JM, King BF, Gregory AV, Madsen CD, et al. Epidemiology of autosomal-dominant polycystic liver disease in Olmsted county. JHEP Rep. 2020;2(6):100166. Epub 2020/11/05. doi: 10.1016/j.jhepr.2020.100166; PubMed Central PMCID: PMC7593615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iglesias DM, Palmitano JA, Arrizurieta E, Kornblihtt AR, Herrera M, Bernath V, et al. Isolated polycystic liver disease not linked to polycystic kidney disease 1 and 2. Dig Dis Sci. 1999;44(2):385–8. Epub 1999/03/04. doi: 10.1023/a:1026623005401 . [DOI] [PubMed] [Google Scholar]
- 8.Pirson Y, Lannoy N, Peters D, Geubel A, Gigot JF, Breuning M, et al. Isolated polycystic liver disease as a distinct genetic disease, unlinked to polycystic kidney disease 1 and polycystic kidney disease 2. Hepatology. 1996;23(2):249–52. Epub 1996/02/01. doi: 10.1002/hep.510230208 . [DOI] [PubMed] [Google Scholar]
- 9.Karhunen PJ, Tenhu M. Adult polycystic liver and kidney diseases are separate entities. Clin Genet. 1986;30(1):29–37. Epub 1986/07/01. doi: 10.1111/j.1399-0004.1986.tb00565.x . [DOI] [PubMed] [Google Scholar]
- 10.Berrebi G, Erickson RP, Marks BW. Autosomal dominant polycystic liver disease: a second family. Clin Genet. 1982;21(5):342–7. Epub 1982/05/01. doi: 10.1111/j.1399-0004.1982.tb01381.x . [DOI] [PubMed] [Google Scholar]
- 11.Sotaniemi EA, Luoma PV, Jarvensivu PM, Sotaniemi KA. Impairment of drug metabolism in polycystic non-parasitic liver disease. Br J Clin Pharmacol. 1979;8(4):331–5. Epub 1979/10/01. doi: 10.1111/j.1365-2125.1979.tb04714.x ; PubMed Central PMCID: PMC1429841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comfort MW, Gray HK, Dahlin DC, Whitesell FB Jr. Polycystic disease of the liver: a study of 24 cases. Gastroenterology. 1952;20(1):60–78. Epub 1952/01/01. . [PubMed] [Google Scholar]
- 13.Li JP, Lee KY, Chang TM, Chey WY. MEK inhibits secretin release and pancreatic secretion: roles of secretin-releasing peptide and somatostatin. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G890–6. Epub 2001/04/09. doi: 10.1152/ajpgi.2001.280.5.G890 . [DOI] [PubMed] [Google Scholar]
- 14.Gong AY, Tietz PS, Muff MA, Splinter PL, Huebert RC, Strowski MZ, et al. Somatostatin stimulates ductal bile absorption and inhibits ductal bile secretion in mice via SSTR2 on cholangiocytes. Am J Physiol Cell Physiol. 2003;284(5):C1205–14. Epub 2003/04/05. doi: 10.1152/ajpcell.00313.2002 . [DOI] [PubMed] [Google Scholar]
- 15.Ferjoux G, Bousquet C, Cordelier P, Benali N, Lopez F, Rochaix P, et al. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J Physiol Paris. 2000;94(3–4):205–10. Epub 2000/11/23. doi: 10.1016/s0928-4257(00)00206-0 . [DOI] [PubMed] [Google Scholar]
- 16.Tietz PS, Holman RT, Miller LJ, LaRusso NF. Isolation and characterization of rat cholangiocyte vesicles enriched in apical or basolateral plasma membrane domains. Biochemistry. 1995;34(47):15436–43. Epub 1995/11/28. doi: 10.1021/bi00047a007 . [DOI] [PubMed] [Google Scholar]
- 17.Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008;286(1–2):230–7. Epub 2008/03/25. doi: 10.1016/j.mce.2008.02.002 . [DOI] [PubMed] [Google Scholar]
- 18.Friedlander G, Amiel C. Somatostatin and alpha 2-adrenergic agonists selectively inhibit vasopressin-induced cyclic AMP accumulation in MDCK cells. FEBS Lett. 1986;198(1):38–42. Epub 1986/03/17. doi: 10.1016/0014-5793(86)81180-2 . [DOI] [PubMed] [Google Scholar]
- 19.Winkler SN, Torikai S, Levine BS, Kurokawa K. Effect of somatostatin on vasopressin-induced antidiuresis and renal cyclic AMP of rats. Miner Electrolyte Metab. 1982;7(1):8–14. Epub 1982/01/01. . [PubMed] [Google Scholar]
- 20.Mountokalakis T, Levy M. Effect of somatostatin on renal water handling in the dog. Can J Physiol Pharmacol. 1982;60(5):655–64. Epub 1982/05/01. doi: 10.1139/y82-090 . [DOI] [PubMed] [Google Scholar]
- 21.Forrest JN Jr., Reichlin S, Goodman DB. Somatostatin: an endogenous peptide in the toad urinary bladder inhibits vasopressin-stimulated water flow. Proc Natl Acad Sci U S A. 1980;77(8):4984–7. Epub 1980/08/01. doi: 10.1073/pnas.77.8.4984 ; PubMed Central PMCID: PMC349974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths J, Mills MT, Ong AC. Long-acting somatostatin analogue treatments in autosomal dominant polycystic kidney disease and polycystic liver disease: a systematic review and meta-analysis. BMJ Open. 2020;10(1):e032620. Epub 2020/01/12. doi: 10.1136/bmjopen-2019-032620; PubMed Central PMCID: PMC6955551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolignano D, Palmer SC, Ruospo M, Zoccali C, Craig JC, Strippoli GF. Interventions for preventing the progression of autosomal dominant polycystic kidney disease. Cochrane Database Syst Rev. 2015;(7):CD010294. Epub 2015/07/15. doi: 10.1002/14651858.CD010294.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myint TM, Rangan GK, Webster AC. Treatments to slow progression of autosomal dominant polycystic kidney disease: systematic review and meta-analysis of randomized trials. Nephrology (Carlton). 2014;19(4):217–26. Epub 2014/01/28. doi: 10.1111/nep.12211 . [DOI] [PubMed] [Google Scholar]
- 25.Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Training. 2021. [Google Scholar]
- 26.Miskulin DC, Abebe KZ, Chapman AB, Perrone RD, Steinman TI, Torres VE, et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1–4: a cross-sectional study. Am J Kidney Dis. 2014;63(2):214–26. Epub 2013/11/05. doi: 10.1053/j.ajkd.2013.08.017 ; PubMed Central PMCID: PMC4075014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suwabe T, Ubara Y, Mise K, Kawada M, Hamanoue S, Sumida K, et al. Quality of life of patients with ADPKD-Toranomon PKD QOL study: cross-sectional study. BMC Nephrol. 2013;14:179. Epub 2013/08/28. doi: 10.1186/1471-2369-14-179; PubMed Central PMCID: PMC3765978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suwabe T, Ubara Y, Hayami N, Yamanouchi M, Hiramatsu R, Sumida K, et al. Factors Influencing Cyst Infection in Autosomal Dominant Polycystic Kidney Disease. Nephron. 2019;141(2):75–86. Epub 2018/12/05. doi: 10.1159/000493806 . [DOI] [PubMed] [Google Scholar]
- 29.Ryan R HS. How to GRADE the quality of the eidence. In Version 3.0 ed. 2016.
- 30.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. Epub 2006/06/30. doi: 10.1016/j.cct.2006.04.004 . [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Epub 2003/09/06. doi: 10.1136/bmj.327.7414.557 ; PubMed Central PMCID: PMC192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney International. 2005;68(1):206–16. doi: 10.1111/j.1523-1755.2005.00395.x . [DOI] [PubMed] [Google Scholar]
- 33.van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137(5):1661–8.e1-2. doi: 10.1053/j.gastro.2009.07.052 . [DOI] [PubMed] [Google Scholar]
- 34.Caroli A, Antiga L, Cafaro M, Fasolini G, Remuzzi A, Remuzzi G, et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clinical Journal of The American Society of Nephrology: CJASN. 2010;5(5):783–9. doi: 10.2215/CJN.05380709 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. Journal of the American Society of Nephrology. 2010;21(6):1052–61. doi: 10.1681/ASN.2009121291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caroli A, Perico N, Perna A, Antiga L, Brambilla P, Pisani A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet. 2013;382(9903):1485–95. doi: 10.1016/S0140-6736(13)61407-5 . [DOI] [PubMed] [Google Scholar]
- 37.Pisani A, Sabbatini M, Imbriaco M, Riccio E, Rubis N, Prinster A, et al. Long-term Effects of Octreotide on Liver Volume in Patients With Polycystic Kidney and Liver Disease. Clinical Gastroenterology & Hepatology. 2016;14(7):1022–30.e4. doi: 10.1016/j.cgh.2015.12.049 . [DOI] [PubMed] [Google Scholar]
- 38.Meijer E, Visser FW, van Aerts RMM, Blijdorp CJ, Casteleijn NF, D’Agnolo HMA, et al. Effect of Lanreotide on Kidney Function in Patients With Autosomal Dominant Polycystic Kidney Disease: The DIPAK 1 Randomized Clinical Trial. JAMA. 2018;320(19):2010–9. Epub 2018/11/14. doi: 10.1001/jama.2018.15870 ; PubMed Central PMCID: PMC6248170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perico N, Ruggenenti P, Perna A, Caroli A, Trillini M, Sironi S, et al. Octreotide-LAR in later-stage autosomal dominant polycystic kidney disease (ALADIN 2): A randomized, double-blind, placebo-controlled, multicenter trial. PLoS medicine. 2019;16(4):e1002777. doi: 10.1371/journal.pmed.1002777; PubMed Central PMCID: PMC6450618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Aerts RMM, Kievit W, D’Agnolo HMA, Blijdorp CJ, Casteleijn NF, Dekker SEI, et al. Lanreotide Reduces Liver Growth In Patients With Autosomal Dominant Polycystic Liver and Kidney Disease. Gastroenterology. 2019;157(2):481–91 e7. Epub 2019/04/26. doi: 10.1053/j.gastro.2019.04.018 . [DOI] [PubMed] [Google Scholar]
- 41.Hogan MC, Chamberlin JA, Vaughan LE, Waits AL, Banks C, Leistikow K, et al. Pansomatostatin Agonist Pasireotide Long-Acting Release for Patients with Autosomal Dominant Polycystic Kidney or Liver Disease with Severe Liver Involvement: A Randomized Clinical Trial. Clin J Am Soc Nephrol. 2020;15(9):1267–78. Epub 2020/08/28. doi: 10.2215/CJN.13661119 ; PubMed Central PMCID: PMC7480539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gevers TJ, Inthout J, Caroli A, Ruggenenti P, Hogan MC, Torres VE, et al. Young women with polycystic liver disease respond best to somatostatin analogues: a pooled analysis of individual patient data. Gastroenterology. 2013;145(2):357–65.e1-2. doi: 10.1053/j.gastro.2013.04.055 . [DOI] [PubMed] [Google Scholar]
- 43.Chapman AB. Cystic disease in women: clinical characteristics and medical management. Adv Ren Replace Ther. 2003;10(1):24–30. Epub 2003/03/05. doi: 10.1053/jarr.2003.50005 . [DOI] [PubMed] [Google Scholar]
- 44.Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology. 1990;11(6):1033–7. Epub 1990/06/01. doi: 10.1002/hep.1840110619 . [DOI] [PubMed] [Google Scholar]
- 45.Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, et al. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119(6):1681–91. Epub 2000/12/13. doi: 10.1053/gast.2000.20184 . [DOI] [PubMed] [Google Scholar]
- 46.Alvaro D, Onori P, Alpini G, Franchitto A, Jefferson DM, Torrice A, et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol. 2008;172(2):321–32. Epub 2008/01/19. doi: 10.2353/ajpath.2008.070293 ; PubMed Central PMCID: PMC2312356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strazzabosco M, Somlo S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology. 2011;140(7):1855–9, 9 e1. Epub 2011/04/26. doi: 10.1053/j.gastro.2011.04.030 ; PubMed Central PMCID: PMC3109236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ludlam WH, Anthony L. Safety review: dose optimization of somatostatin analogs in patients with acromegaly and neuroendocrine tumors. Adv Ther. 2011;28(10):825–41. Epub 2011/10/04. doi: 10.1007/s12325-011-0062-9 . [DOI] [PubMed] [Google Scholar]
- 49.Chebib FT, Torres VE. Recent Advances in the Management of Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol. 2018;13(11):1765–76. Epub 2018/07/28. doi: 10.2215/CJN.03960318 ; PubMed Central PMCID: PMC6237066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–18. Epub 2012/11/06. doi: 10.1056/NEJMoa1205511 ; PubMed Central PMCID: PMC3760207. [DOI] [PMC free article] [PubMed] [Google Scholar]