Abstract
Background
Intraamniotic inflammation is associated with up to 40% of preterm births, most notably in deliveries occurring prior to 32 weeks’ gestation. Despite this, there are few treatment options allowing the prevention of preterm birth and associated fetal injury. Recent studies have shown that the small, non-competitive allosteric interleukin (IL)-1 receptor inhibitor, rytvela, may be of use in resolving inflammation associated with preterm birth (PTB) and fetal injury. We aimed to use an extremely preterm sheep model of chorioamnionitis to investigate the anti-inflammatory efficacy of rytvela in response to established intra-amniotic (IA) lipopolysaccharide (LPS) exposure. We hypothesized that rytvela would reduce LPS-induced IA inflammation in amniotic fluid (AF) and fetal tissues.
Methods
Sheep with a single fetus at 95 days gestation (estimated fetal weight 1.0 kg) had surgery to place fetal jugular and IA catheters. Animals were recovered for 48 hours before being randomized to either: i) IA administration of 2 ml saline 24 hours before 2 ml IA and 2 ml fetal intravenous (IV) administration of saline (Saline Group, n = 7); ii) IA administration of 10 mg LPS in 2 ml saline 24 hours before 2 ml IA and 2 ml fetal IV saline (LPS Group, n = 10); 3) IA administration of 10 mg LPS in 2 ml saline 24 hours before 0.3 mg/fetal kg IA and 1 mg/fetal kg fetal IV rytvela in 2 ml saline, respectively (LPS + rytvela Group, n = 7). Serial AF samples were collected for 120 h. Inflammatory responses were characterized by quantitative polymerase chain reaction (qPCR), histology, fluorescent immunohistochemistry, enzyme-linked inmmunosorbent assay (ELISA), fluorescent western blotting and blood chemistry analysis.
Results
LPS-treated animals had endotoxin and AF monocyte chemoattractant protein (MCP)-1 concentrations that were significantly higher at 24 hours (immediately prior to rytvela administration) relative to values from Saline Group animals. Following rytvela administration, the average MCP-1 concentrations in the AF were significantly lower in the LPS + rytvela Group relative to in the LPS Group. In delivery samples, the expression of IL-1β in fetal skin was significantly lower in the LPS + rytvela Group compared to the LPS Group.
Conclusion
A single dose of rytvela was associated with partial, modest inhibition in the expression of a panel of cytokines/chemokines in fetal tissues undergoing an active inflammatory response.
Background
Preterm birth (PTB; delivery before 37 weeks of completed gestation) is a major cause of neonatal mortality and morbidity, with the greatest rates of death and significant disease seen in premature deliveries occurring prior to 32 weeks’ gestation [1, 2]. PTB is a multifactorial syndrome [1, 2]. Many studies have investigated the role of intraamniotic inflammation in PTB; comparatively few studies have evaluated the efficacy of antenatal treatments for infection and inflammation-associated prematurity [1, 3, 4].
Interleukin (IL)-1 is a potent pro-inflammatory cytokine, [4, 5]. IL-1β concentration and bioactivity in amniotic fluid of women is related to preterm labor (PTL), infection [6], and PTB [7]. Intra-amniotic (IA) injection of 10 mg lipopolysaccharide (LPS) caused elevated concentrations of IL-1 in the amniotic fluid, chorioamnionitis, lung inflammation, and systemic inflammation in studies using preterm ovine model. Several studies have previously reported investigations into the efficacy of IL-1 targeting agents to prevent PTB and fetal injury [4, 5], using agents that competitively antagonize the IL-1 receptor and therefore likely block all downstream IL-1 signal transduction, including NF-κB activation [4]. This approach may risk interruption to important physiological processes such as cytoprotection and immune-surveillance [8].
Rytvela is a selective allosteric inhibitor of IL-1 receptor signalling [9, 10]. Allosteric antagonists interact with the receptor at a site remote from the orthosteric binding site, allowing the ligand to bind its receptor. They are biased signal inhibitors; i.e. they selectively inhibit some but not all intracellular signals of the receptor [11, 12]. A previous study showed rytvela selectively inhibited IL-1Racp downstream stress-associated protein kinase JNK, mitogen-activated protein kinases p38 and Rho/Rho GTPase/Rho-associated coiled-coil-protein kinase pathway, without affecting NF-κB activation [9]. Therefore, unlike other IL-1-targeting agents, rytvela can exert functional selectivity [11]. Accordingly, rytvela likely does not inhibit NF-κB [10] or suppress immune-surveillance. Due to its small size, rytvela is expected to have good bioavailability [13]. Recent studies demonstrated that rytvela (1 mg/kg) reduced PTB and perinatal death, and reduced inflammation in the fetal brain, lung and colon of mice exposed to LPS [4, 9, 10]. No evidence of reproductive toxicity was detected and rytvela was shown to have a promising pharmacological profile [4, 5, 10].
It remains to be determined if rytvela allows for control of established intrauterine inflammation in extremely preterm fetal lambs–an established model for human fetal inflammation/infection. Making this determination is important because: i) patients in preterm labour are commonly diagnosed with intrauterine infection / inflammation after already presenting with symptoms; and ii) infection and inflammation are most commonly associated with early preterm delivery and fetal injury. In this experiment, rytvela was administrated to extremely preterm ovine fetuses 24 hours after LPS exposure to simulate a potential clinical intervention scenario. While clinically rytvela would be given maternally, in this study direct fetal and intramniotic administration of rytvela was employed to ensure accurate intraamniotic dosing. We hypothesized that antenatal administration of rytvela treatment would reduce intra-amniotic and fetal inflammation.
Methods
Animals
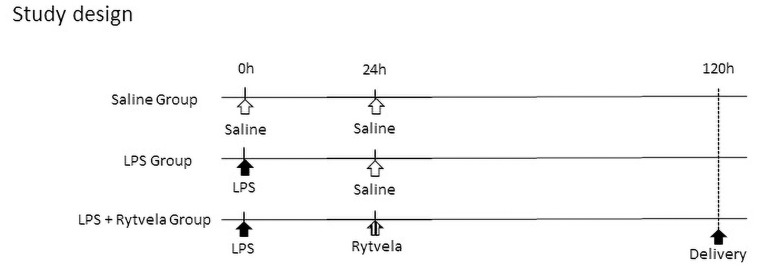
Animal studies were approved by The University of Western Australia’s Animal Ethics Committee (approval RA.3.100.1638). Ewes with a singleton fetus at 95 days of gestation (term is ~150 days) were fasted overnight before undergoing recovery surgery to place fetal jugular and intra-amniotic (IA) catheters, as previously reported [14]. After a 48-hour recovery, animals were randomized to one of the following groups (Fig 1): i) a single IA bolus of 2 ml saline 24 hours before both 2 ml IA and 2 ml fetal intravenous (IV) injection of saline (Saline Group, n = 7); ii) IA injection of 10 mg LPS from Escherichia coli (055:B5; Sigma Aldrich, St Louis, Missouri) in 2 ml saline 24 hours before 2 ml IA and 2 ml fetal IV injection of saline (LPS Group, n = 10); 3) IA injection of LPS in 2 ml saline 24 hours before 0.3 mg/kg IA and 1 mg/kg fetal IV injection of rytvela, both in 2 ml saline vehicle (LPS + rytvela Group, n = 7). Rytvela was administered into the fetal circulation and amniotic cavity to target systemic and localized (amniotic fluid-exposed) inflammation, with dosing based on previous efficacious use in a small animal model [5, 9, 10]. Animal wellbeing was monitored daily, with free access to food and water.
Fig 1. Study design.
The LPS Group and the LPS + Rytvela Group animals received an IA injection of 10 mg LPS at 0 hour. The Saline Group animals received an equivalent volume of saline solution. The LPS + rytvela Group received both IA and fetal intravenous injection of rytvela at 24 hours after LPS exposure. The Saline Group and the LPS Group animals received an equivalent volume of saline solution. IA, intra-amniotic; LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein.
Amniotic fluid (AF) was serially sampled from amniotic catheters at 0, 12, 24, 48, 72, and 120 hours after the first administration of either saline or LPS. Animals were euthanized with an intravenous bolus (160 mg/kg) of pentobarbital at 120 hours. Fetal cord blood was collected at delivery for blood chemistry and ELISA analysis. Fetal tissues were snap frozen in liquid nitrogen for protein or messenger ribonuclease acid (mRNA) expression analyses. For histological analyses, fetal tissue was placed in cassettes and fixed in 10% neutral buffered formalin before being processed by paraffin embedding.
Endotoxin quantification
Endotoxin concentrations in amniotic fluid were measured by using a Pierce™ Chromogenic Endotoxin Quant Kit (Thermo Fisher, A39552), according to manufacturer’s instructions. Each plate was read at 405 nm on a Spectramax plate reader (Molecular Devices LLC, Sunnyvale, CA).
Enzyme-linked inmmunosorbent assay
Quantification of the cytokine IL-1 in amniotic fluid, cord blood at delivery, and fetal lung and IL-8 in amniotic fluid and cord plasma were measured by using a Sheep ELISA Kits (CUSABIO, CSB-E10115Sh), according to manufacturer’s protocols. Each plate was read at 450 nm on a Spectramax plate reader (Molecular Devices LLC, Sunnyvale, CA). The software package Curve Expert, version 1.4 (CUSABIO), was used to make a standard curve. Similarly, quantification of the chemokine monocyte chemoattractant protein (MCP)-1 and cytokine tumor necrosis factor (TNF)-α in amniotic fluid and cord plasma were measured by using a Kingfisher Biotech (Saint Paul, MN) ELISA Development Kits, according to the manufacture’s protocol. Each plate was read at 450 nm on a Spectramax plate reader (Molecular Devices LLC, Sunnyvale, CA).
Tissue RNA isolation
DNA-free total RNA was isolated from frozen fetal tissues (lung right lower lobe, internal groin skin, colon, chorioamnion and brain cortex) using RNeasy® Plus Mini Kit (QIAGEN, Hilden, Germany), according to the manufacture’s protocol. The concentration and quality of extracted RNA was quantified with a Qubit 2.0 fluorometer (Life Technologies, Carlsbad, CA).
Quantitative polymerase chain reaction
Ovine-specific PCR primers and hydrolysis probes for IL-1β, IL-6, TNF-α and MCP-1 (Thermofisher) were used to perform quantitative PCR reactions on RNA from fetal lung, skin, colon, chorioamnion and brain cortex tissue. Fetal lung, skin, chorioamnion and colon were selected for analysis as they are exposed to the amniotic fluid directly or via swallowing and have been shown to initiative a pro-inflammatory response following IA LPS administration. Inflammation of the fetal brain in the setting of preterm birth is of significant interest given its association with neuro-developmental damage.
Reactions were conducted using a Step One Real-Time PCR system and probe/primer sets from Life Technologies with RNA normalised to 25 ng/μl. Reaction cycling conditions were as follows: 1 × 15 min reverse transcription [50°C], 1 × 20 seconds initial denaturation [95°C], followed by 40 cycles of 3 seconds denaturation [95°C] and 30 seconds annealing [60°C]. All reactions were performed in 96 well fast plates on a ViiA7 real-time PCR themocycler (Life Technologies) and in triplicate. Averaged quantitation cycle (Cq) values were normalized against averaged Cq values of ribosomal protein 18s. Data were processed to generate fold changes using a 2-ddCq method and were tested for significance.
Histology of fetal lung and chorion-amnion
The right upper lobe of each lung was inflation fixed with 4% paraformaldehyde at 30 cmH2O pressure. The inflammation of both fetal lung and chorion-amnion was evaluated using hematoxylin and eosin (H&E)-stained sections and was graded in a blinded fashion by a scoring methods follows: Fetal lung was assessed using three 5-μm sections from each animal as 0 (no inflammatory cells), 1 (a few inflammatory cells), 2 (moderate influx of cells) or 3 (extensive influx of inflammatory cells), as previously published [15, 16].
Chorioamnion rolls were fixed in 4% paraformaldehyde. Chorioamnionitis was classified using a modified grading system based on that reported by Redline et. al. [17]. Histological staining was used to determine the localization of inflammatory cells and (where present) tissue damage. The grade evaluation also identifies the intensity of the inflammatory cell infiltration [17, 18]. The chorioamnion of each animal was graded based on this classification as previously reported [19]: 0 (no chorioamnionitis), 1 (localization: within choriodecidua; intensity: individual or small clusters of inflammation cell), 2 (localization: within choriodecidua; intensity: the three or more chorionic microabscesses which are defined as inflammatory cell ≥ 10 × 20 cells), 3 (localization: choriodecidua and/or amnion, intensity: same as score = 2), 4 (localization: necrosis of the amniotic epithelium; intensity: same as score = 2). Six 5-μm sections from each animal were evaluated at random and an average score was calculated for each animal.
Fluorescent immunohistochemical analysis of fetal brain
The left hemisphere was fixed in 4% paraformaldehyde and sectioned in 4-mm serial section (5 serial sections in total) along the coronal plane from the anterior to posterior sections according to the method of Banker and Larroche [20]. The second serial section was selected to be embedded in paraffin and stained using following primary antibodies: glial fibrillary acidic protein (GFAP) (1:1000, ab7260, Abcam), ionized calcium-binding adapter molecule 1 (Iba-1) (1:500, ab178846, Abcam), oligodendrocyte transcription factor 2 (Olig-2) (1:2000, NBP1-28667, Novusbio) and neuronal nuclei (NeuN) (1:750, ab128886, Abcam). Alexa Fluor® 488 (1:10000, ab150077, Abcam) was used as secondary antibody and nuclei were stained with DAPI (F6057, Sigma-Aldrich). Microphotographs were captured using a confocal microscope (Nikon A1Si). Five random, non-overlapping fields per section were assessed for each antigen target. Numbers of GFAP-, Iba-1-, Olig-2- and NeuN-positive / DAPI-positive cells in subcortical white matter were counted manually by single investigator blinded to treatment.
Western blot analysis of fetal brain tissue
Proteins from the right hemisphere cortex were extracted using RIPA lysis buffer (Thermo Fisher, 89901) and a cocktail of protease inhibitors (Merck, 04693116001) following cold maceration on a Precellys 24 tissue homogenizer. Protein concentration was quantified with a Pierce™ Rapid Gold BCA Protein Assay Kit (Thermo Fisher, A53226). 15 μg of total protein per well was resolved using a NuPAGE™ 10% Bris-Tris Gel and electrotransferred to PVDF membrane according to manufacturer’s instructions. Membranes were blocked with blocking buffer (Thermo Fisher, 37565) for 30 minutes at room temperature and incubated with No-Stain™ Protein labeling Reagent (A44717, Thermo Fisher) according to the manufacture’s instructions. Membranes were then incubated overnight at 4°C on a rocker platform with following primary antibodies: GFAP (1:5000, ab7260, Abcam), Iba-1 (1:1000, ab178846, Abcam), Olig-2 (1:3000, NBP1-28667, Novusbio) and NeuN (1:1000, ab128886, Abcam). Western blot enhancer (SuperSignal™ Western Blot Enhancer, 46640, Thermo Fisher) was used for Olig-2 according to the manufacture’s protocol. Membranes were washed for 30 minutes in PBS-Tween before being incubated with Alexa Fluor® 488 (1:10000, ab150077, Abcam) for 1 hour at room temperature. Membranes was analyzed with iBright™ FL1000 imaging system (Thermo Fisher). Each band volume was normalized against the total protein amounts and each membrane was compensated by the total protein amounts of quality control animal.
Hematology
Complete blood counts (CBC) were performed by VetPath Laboratory Services using an automated Coulter counter customized for sheep (Ascot, Perth, Western Australia).
Statistical analysis
Data were analysed for distribution, variance and skew. All values are expressed as either mean ± one standard deviation or median ± IQR. Analyses were performed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp. Armonk, NY.). Paired comparisons were performed with t-tests or Mann-Whitney tests. The comparison of more than two groups was tested by one-way ANOVA analysis of variance with multiple post-hoc comparisons used by Tukey’s t or Kruskal-Wallis post-hoc analyses. Significance was accepted at p < 0.05.
Results
There were no significant differences in birthweight, cord blood pH, pCO2, or lactate at delivery between the three treatment groups (Table 1). No increase of fetal edema or ascites as a result of lipopolysaccharide exposure or rytvela administration was detected. Variability in the group sizes used to analyse time-course samples obtained by catheter sampling reflect changes in fetal positioning which impact catheter patency.
Table 1. Delivery data.
| Arterial fetal arterial cord blood | |||||
|---|---|---|---|---|---|
| Group | Sex (M/F) | Delivery Wt. (kg) | pH | pCO2 (mmHg) | Lactate (mmol/L) |
| Saline Group (n = 7) | 3/4 | 1.1 ± 0.1 | 7.1 ± 0.1 | 78.0 ± 8.5 | 4.4 ± 0.9 |
| LPS Group (n = 10) | 6/4 | 1.2 ± 0.2 | 7.1 ± 0.1 | 75.8 ± 9.1 | 4.1 ± 1.3 |
| LPS + Rytvela Group (n = 7) | 7/0 | 1.2 ± 0.1 | 7.1 ± 0.1 | 81.3 ± 13.9 | 4.4 ± 1.1 |
Endotoxin quantification
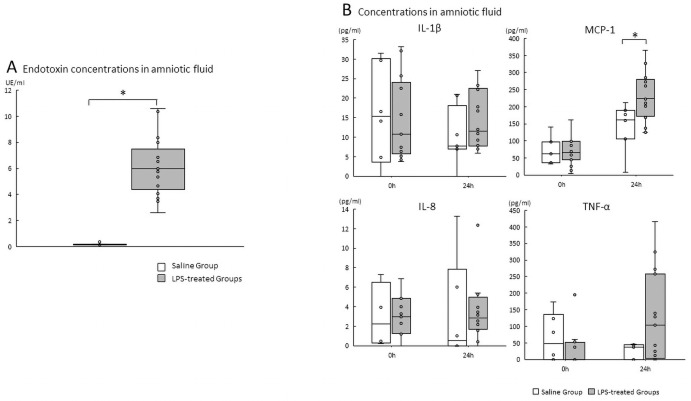
Endotoxin concentrations from the LPS-treated Groups (the LPS Group and the LPS + rytvela Group) at 10 hours after LPS administration in AF were significantly increased compared to AF samples from the Saline Group. (Saline Group vs. LPS-treated Groups p<0.000) (Fig 2A).
Fig 2.
A. Endotoxin concentrations in amniotic fluid. Plots show median, 25th, and 75th percentile ranges of AF endotoxin concentrations at 24 hours post-administration as boxes and 5th to 95th percentile as error bars. *p < .05 vs. the Saline Group. n = 6–10 animals/group. AF, amniotic fluid; LPS, lipopolysaccharide. B. Cytokines/chemokine concentrations in amniotic fluid from 0 to 24 hours after either saline or LPS. A, amniotic fluid IL-1β, IL-8, MCP-1, and TNF-α concentrations. *p < .05 between the Saline Group and the LPS-treated Groups (LPS Group and LPS + rytvela Group). Plots show median, 25th, and 75th percentile ranges as boxes and 5th to 95th percentile as error bars. n = 4–8 animals/group. AF, amniotic fluid; LPS, lipopolysaccharide; ELISA, Enzyme-linked inmmunosorbent assay.
ELISA
Pre-intervention analysis
We evaluated the effect of IA administration of LPS or saline from 0 to 24 hours (before randomization to treatment by either saline or rytvela). No difference was detected in AF concentrations in IL-1β, IL-8, MCP-1 or TNF-α at 0 hours between the Saline-treated and LPS-treated animals. AF MCP-1 concentrations in the LPS-treated animals increased significantly at 24 hours compared with Saline-treated animals (p = 0.016) (Fig 2B). No significant differences were detected in IL-1β, IL-8 and TNF-α concentrations in AF between Saline-treated animals and LPS-treated animals at 24 hours.
Post-intervention analysis
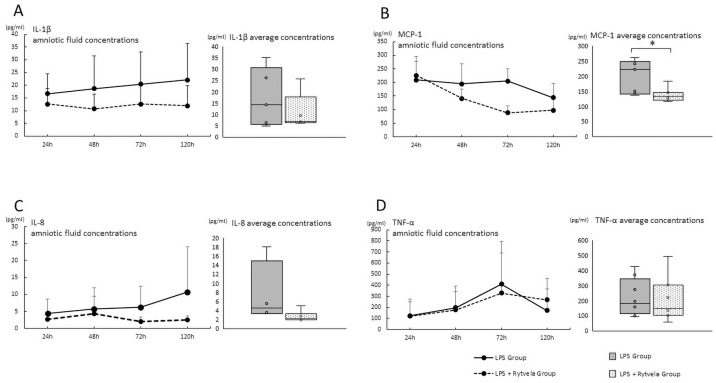
We then compared the average concentration of cytokines/chemokines at time points between the LPS Group and the LPS + rytvela Group (Fig 3). The average MCP-1 concentration in AF from the LPS + rytvela Group decreased significantly compared to the LPS Group (p = 0.002) (Fig 3B). There were no significant differences in IL-1β, IL-8 and TNF-α AF concentrations (Fig 3A, 3C and 3D).
Fig 3. Cytokines/chemokine concentrations in amniotic fluid after the administration of either saline or rytvela).
Cytokines/chemokine concentration in AF at each time point plotted as mean ± SD. Each group’s average concentration is shown in bar charts. A, IL-1β; B, MCP-1; C, IL-8; D, TNF-α. The average concentration was compared between the LPS Group and the LPS + rytvela Group. *p < .05 between the LPS Group and the LPS + rytvela Group. n = 3–7 animals/group. AF, amniotic fluid; SD, standard deviation; IL, Interleukin; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor.
At delivery
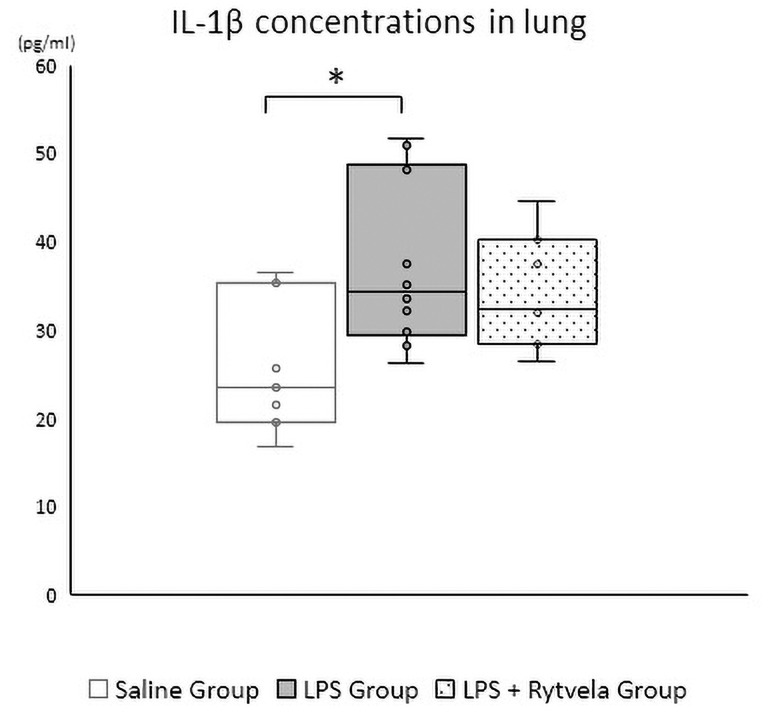
There was a significant difference in IL-1β protein concentration in fetal lung tissue at delivery between the Saline Group and the LPS Group (p = 0.023). No difference was detected between the Saline Group and the LPS + rytvela Group (p = 0.129) (Fig 4).
Fig 4. IL-1β protein concentrations in fetal lung at delivery.
*p < .05 vs. the Saline Group. Plots show median, 25th, and 75th percentile ranges as boxes and 5th to 95th percentile as error bars. n = 7–10 animals/group IL, Interleukin.
qPCR
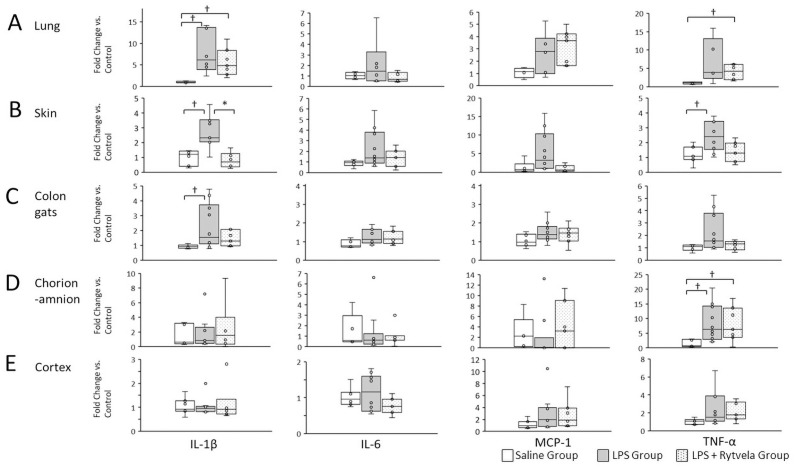
Results are shown in Fig 5. Statistically significant increases were detected in fetal lung IL-1β mRNA in both the LPS and LPS + rytvela Groups relative to the Saline Group animals (Saline Group vs. LPS Group p = 0.043; Saline Group vs. LPS + rytvela Group p = 0.02). TNF-α mRNA in the fetal lung was significantly increased in the LPS + rytvela Group animals compared to the Saline Group animals (Saline Group vs. LPS + rytvela Group p = 0.029) (Fig 6A). IL-1β mRNA in the fetal skin increased significantly in the LPS Group in comparison to the Saline Group (Saline Group vs. LPS Group p = 0.004). Moreover, IL-1β mRNA concentration in fetal skin was significantly lower in the LPS + rytvela Group compared to the LPS Group (LPS Group vs. LPS + Rytvela Group p = 0.002) (Fig 6B).
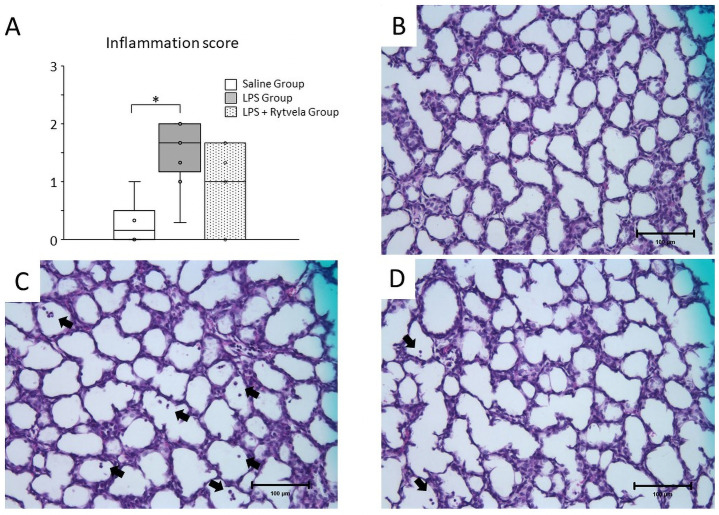
Fig 5. Histological assessment of fetal lung.
A, Plots show median, 25th, and 75th percentile ranges as boxes and 5th to 95th percentile as error bars. LPS Group increased inflammation score in fetal lung tissue relative to Saline Group. *p < .05 between the Saline Group and the LPS Group. Representative sections of tissue from B, Saline Group (score = 0); C, LPS Group (score = 2); D, LPS + rytvela Group (score = 1). Inflammatory cells are indicated by arrows. Scale bar: 100 μm. n = 7–10 animals/group. LPS, lipopolysaccharide.
Fig 6. Messenger RNA expression in at delivery.
Relative expression of mRNA transcripts for IL-1β, IL-6, MCP-1, and TNF-α in A, fetal lung; B, skin; C, colon; D, chorioamnion; E, cortex. †p < .05 vs the Saline Group. *p < .05 between the LPS Group and the LPS + rytvela Group. Plots show median, 25th, and 75th percentile ranges as boxes and 5th to 95th percentile as error bars. n = 7–10 animals/group. IL, Interleukin; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor.
The LPS Group had a significant increase in fetal skin TNF-α mRNA concentration compared with the Saline Group animals (Saline Group vs. LPS Group p = 0.016) (Fig 6B). Similar to fetal lung and skin, there were significant increases in IL-1β mRNA in the colon of LPS Group animals relative to the Saline Group animals (Saline Group vs. LPS Group p = 0.045) (Fig 6C). No differences were identified in TNF-α mRNA concentration in colon tissue between the three groups.
There were no significant differences in fetal chorioamnion in IL-1β mRNA concentration among the three groups; however, significant increases were detected in TNF-α mRNA concentration in the chorion-amnion of both the LPS Group and the LPS + rytvela Group animals relative to the Saline Group animals (Saline Group vs. LPS Group p = 0.01; Saline Group vs. LPS + Rytvela Group p = 0.03) (Fig 6D). There were no differences in IL-1β, TNF-α, IL-6 or MCP-1 mRNA detected in fetal brain (cortex), among the three groups (Fig 6E).
Histology and fluorescent immunohistochemistry
Inflammation in the fetal lung and chorioamnion was evaluated by blind scoring of inflammatory cells by a single investigator. Inflammatory cell infiltration was detected in fetal lung in both the LPS Group and the LPS + rytvela Group; in contrast, few or no inflammatory cells were detected in the Saline Group animals (Fig 5B to 5D for representative images). The inflammation score of the LPS Group was significantly higher than that of the Saline Group, while there was no significant difference between the Saline Group and the LPS + rytvela Group animals (Saline Group vs. LPS Group p = 0.005; Saline Group vs. LPS + rytvela Group p = 0.142) (Fig 5A).
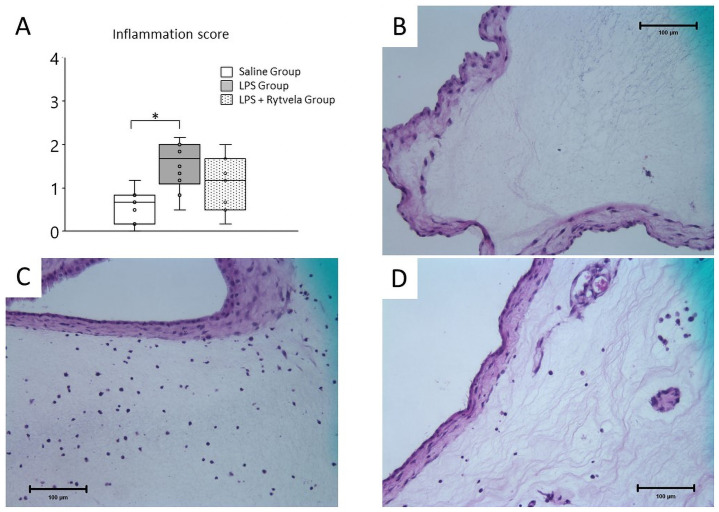
Similarly, inflammatory cells were detected in the chorion-amnion of both the LPS Group and the LPS + rytvela Group (Fig 7B to 7D for representative images). The inflammation score was significantly increased in the LPS Group relative to the Saline Group (Saline Group vs. LPS Group p = 0.006). The inflammation score in the LPS + rytvela Group was comparable to that of the Saline Group (Saline Group vs. LPS + Rytvela Group p = 0.23) (Fig 7A).
Fig 7. Histological assessment of chorioamnion.
A, Plots show median, 25th, and 75th percentile ranges as boxes and 5th to 95th percentiles as error bars. The LPS Group increased inflammation score in chorion-amnion tissue relative to the Saline Group. *p < .05 between the Saline Group and the LPS Group. Representative sections of tissue from B, Saline Group (score = 0); C, LPS Group (score = 2); D, LPS + rytvela Group (score = 1). Scale bar: 100 μm. n = 7–10 animals/group. LPS, lipopolysaccharide.
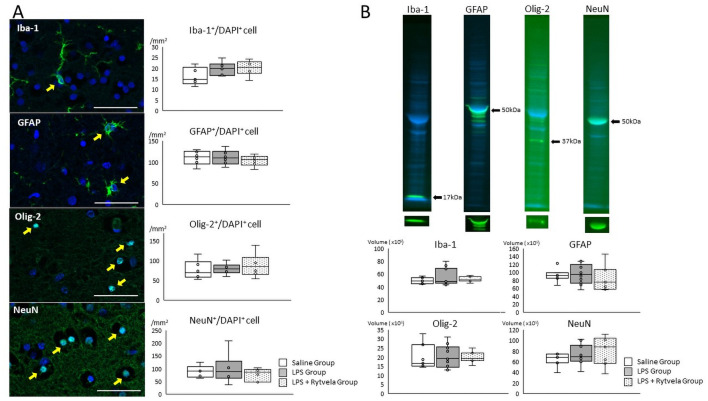
Fluorescent immunohistochemistry and western blotting was performed to assess fetal brain injury. Previous studies showed the reaction of fetal brain exposed to LPS are activated microglia and astrocyte, maturation arrest or death of oligodendrocyte, and axonal loss or death of neurons [21–23]. Therefore, as for analysis of the cortex, inflammation/injury was assessed by counting Iba-1-, GFAP-, Olig-2- and NeuN-positive / DAPI positive cells in subcortical white matter. There were no significant differences in the numbers of Iba-1-, GFAP-, Olig-2-, and NeuN-positive / DAPI positive cells in subcortical white matter among the three groups. (Fig 8A).
Fig 8. Fluorescent immunohistochemistry and western blotting assessment of fetal brain.
A, Representative photomicrographs show Iba-1-, GFAP-, Olig-2- and NeuN- positive (green) / DAPI positive (blue) cells in subcortical white matter of the Saline Group animals. Positive cells are indicated by arrows. Scale bar: 50 μm. Box plots show the numbers of counting Iba-1-, GFAP-, Olig-2- and NeuN- positive / DAPI positive cells of three groups. B, Representative images show Iba-1, GFAP, Olig-2 and NeuN specific band (green) and total protein stained band (blue) of the quality control animal (Saline Group animal). Box plot show the band volume of the each antibody against the total protein amounts. Plots show median, 25th, and 75th percentile ranges as boxes and 5th to 95th percentile as error bars. n = 6–8 animals/group. Iba-1, ionized calcium-binding adapter molecule 1; GFAP, glial fibrillary acidic protein; Olig-2, oligodendrocyte transcription factor 2; NeuN, neuronal nuclei.
Fluorescent western blotting
Results are shown in Fig 8B. Iba-1, GFAP, Olig-2 and NeuN specific bands (green bands) were observed and each band volume was normalized to total protein amounts (blue bands). There was no significant differences of the band volume of Iba-1, GFAP, Olig-2 and NeuN in fetal cortex (Fig 8B) between groups.
Haematology
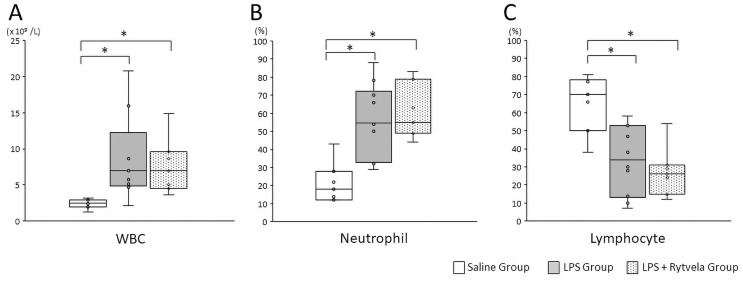
There was a significant increase in the total white blood cell count at delivery in both the LPS Group and the LPS + rytvela Group fetuses relative to the Saline Group animals (Saline Group vs. LPS Group p = 0.036; Saline Group vs. LPS + rytvela Group p = 0.029) (Fig 9A). Similarly, the ratio of fetal neutrophils to total white blood cells at delivery was significantly increased in both the LPS Group and the LPS + rytvela Group animals compared with the Saline Group animals (Saline Group vs. LPS Group p = 0.001; Saline Group vs. LPS + Rytvela Group p = 0.001). The ratio of lymphocytes to total white blood cells was significantly decreased in both the LPS Group and the LPS + rytvela Group animals when compared with the Saline Group animals (Saline Group vs. LPS Group p = 0.003; Saline Group vs. LPS + rytvela Group p = 0.001) (Fig 9B and 9C).
Fig 9. Haematological assessments.
Plots show median, 25th, and 75th percentile ranges as boxes and 5th to 95th percentile as error bars. Intra-amniotic injection of LPS affected A, total white blood cell (WBC) counts; B, ratio of neutrophil; C, ratio of lymphocyte from cord blood. LPS, lipopolysaccharide. n = 7–9 animals/group.
Discussion
Primary findings
These data show that, in extremely preterm sheep fetuses, the introduction of LPS to the amniotic cavity induced chorioamnionitis and increased tissue inflammation. The combined intraamniotic and fetal intravenous administration of rytvela was associated with a modest partial reduction in fetal and intraamniotic inflammation even 24 hours following LPS exposure, relative to treatment with saline only. As such, with further optimization (dose, timing, route of administration) of rytvela may show promise as treatment for intra-amniotic inflammation.
Results
Amniotic fluid inflammation
LPS-associated increases in TNF-α expression were noted in fetal tissues, although not in the AF as previously reported [24]. Similarly, differences in IL-1β expression were only detected in fetal tissue. Previous studies found that IL-1β induced MCP-1 expression in human cells via both NF-κB-mediated and alternative signaling pathways [25], while significantly increased MCP-1 concentration in the AF of women were associated with evidence of intra-amniotic infection [26]. Moreover, studies have also shown that the concentration of MCP-1 in AF from women who delivered preterm without apparent intra-amniotic infection was significantly higher than those who delivered at term delivery [26, 27]. Therefore, MCP-1 is a useful biomarker to the existence of intraamniotic infection regardless of whether the antigen is identified or not. The amount of MCP-1 protein detected was significantly elevated in the LPS-treated animals compared with the Saline Group. Moreover, in the LPS + rytvela Group, MCP-1 average AF concentrations were significantly decreased, relative to values in the LPS Group, suggesting a partial inhibition of inflammation in response to rytvela treatment. We and others have previously demonstrated that extended exposure to intrauterine inflammation negatively affects fetal outcome [15, 21, 28]. As such, reduction of intraamniotic inflammation may reduce the risk and/or severity of fetal injury.
No statistically significant increase in either IL-1β or IL-8 was detected in the AF between LPS and non-LPS groups prior to treatment. Although this reason for this observation is not known exactly, it is possible that the timing of sampling was too early or too late to optimally capture increase expression. It is also possible that an increase in baseline inflammation deriving from fetal and maternal surgery increased the noise in the analysis, partly masking a difference in AF inflammation between groups.
Fetal tissue inflammation
Animals in the LPS Group had significant increases in IL-1β mRNA expression in all three organs assessed (skin, lung and colon) that were exposed to the AF (and thus directly to LPS), compared to the Saline Group, whereas there were no differences detected between the LPS + rytvela Group and the Saline Group animals, suggesting some degree of resolution of inflammation signaling by rytvela. Similarly, ELISA results showed that IL-1β concentrations in fetal lung from the LPS Group were significantly increased compared to the Saline Group, while no difference was found between the LPS + rytvela Group and the Saline Group.
The LPS Group and the LPS + rytvela Group animals both had significantly increased WBC counts and altered neutrophil and lymphocyte ratios. Histopathological evaluation of the fetal lung and the chorioamnion also showed the inflammation score increased significantly in the LPS Group compared to the Saline Group, while there was no significant differences between the LPS + rytvela Group and the Saline Group–although the range of scores therein was increased. Moreover, IL-1β mRNA expression in the skin of fetuses in the LPS + rytvela Group was significantly lower than that of animals from the LPS Group. Previous studies have identified the fetal skin is as a pro-inflammatory organ following intrauterine endotoxin exposure in the preterm sheep [29, 30]. We previously demonstrated that selective exposure of fetal skin + amnion to LPS induce acute systemic inflammation [31] and significant elevation of mRNA expression for IL-1β in fetal skin persisted till 15 days after LPS exposure [32], while that in fetal lung persisted till only 2days after LPS exposure [15]. As such, significant decrease of IL-1β mRNA expression in rytvela-treated fetal skin can be taken as suggestive of a beneficial effect of rytvela.
Taken together, these data suggest a modest reduction in fetal inflammation associated with the administration of rytvela. Given the exploratory nature of the dosing employed in this study and the persistent half-life of endotoxin in the intraamniotic environment, more frequent administration of rytvela may offer improved dampening of inflammatory responses.
Clinical implications
It is widely accepted that intra-amniotic infection can cause acute neonatal mortality as well as long-term adverse outcomes. Currently, if the intra-amniotic infection and inflammation are suspected on the basis of a patient presenting with maternal fever, vaginal discharge, fetal distress or PTL, responses are broadly limited to the use of antibiotics and/or the management of delivery. A means of rapidly resolving intrauterine inflammation would be of potential use and likely to improve neonatal outcomes.
There have been several studies investigating the efficacy of IL-1 targeting agents to prevent PTB and fetal injury [4, 5, 9, 10]. The agents used in these previous studies all antagonized IL-1 actions competitively and blocked all downstream signal transduction [4], including that of NF-κB; this is thought to affect immune-surveillance and may result in an increased risk of fetal and maternal infection [8, 33]. Therefore, the functional selectivity of rytvela may allow for a reduction in the risk of adverse effects.
Agents such as rytvela regulate inflammatory responses but do not resolve the underlying infection or sequester pro-inflammatory antigen (in contrast to antibiotics such as polymyxin-B). These agents also have limited half-lives in vivo. Thus, any efficacious treatment regimen will likely be multi-faceted to allow for resolution of infection, inflammation signaling, and inhibition of further inflammatory stimulation. Additionally, in clinical practice there is a lack of effective and established diagnostic tests for women who are at risk of intra-amniotic infection leading to PTB, especially before 32 weeks gestation. As such, the successful application of agents such as rytvela (in combination with antimicrobial agents) will likely require a tandem improvement in the reliability of diagnostic tests for women at risk of intrauterine infection and PTL.
Research implications
This study suggests potential efficacy of rytvela for treatment of intraamniotic infection during pregnancy. It is unclear whether IA or IV administration alone was sufficient for transient resolution of inflammation, or whether treatment of both the fetus and the amniotic fluid is necessary. As noted above, a significant amount of work is still required to optimize dose, timing, and duration of rytvela treatment. Ongoing development of this approach should focus on enhancing efficacy without additional adverse side-effects, in both sterile inflammation (i.e. LPS) and in model systems wherein an active infection is established.
Limitations
Data presented herein should be interpreted in light of several considerations. Firstly, as a surgical model, the impact of LPS and peptide exposures may be somewhat masked by background inflammation–with the surgery likely causing a higher background level of inflammation. Secondly, the limited number of animals used in this study (by virtue of the intensive experimental design) combined with the wide group standard deviations of the ELISA results mean that these data should be interpreted with appropriate caution. Thirdly, as the dosing regimen adopted was exploratory and designed to inform subsequent experiments it should not be considered optimal. Lastly, a lack of brain inflammation in the present study restricts our ability to assess the potential neuro-protective benefits of rytvela. The reasons for this are unclear, but may relate to the early gestational age of the fetuses used in this study, the comparatively short period between LPS exposure and sample collection, and the region of brain assessed.
A number of factors including the inability of LPS to cross cell-cell barriers, a lack of multi-antigen stimulation, and an absence of viable infection mean that host responses to LPS likely differ from viable microorganisms. The half-life of LPS in the amniotic fluid is 1.7 days [34] and inflammation is gradually reduced without any intervention, which may not reflect clinical intra-amniotic infection. Despite this limitation, results showing the rytvela administration even 24 h following LPS exposure was associated with decreased inflammation in the AF and fetal tissues is still significant.
Given the intensive nature of studies employing the chronically catheterized sheep model, we were able to select only one dose of rytvela, based on the mouse literature, and were restricted in terms of our group sizes. The timing of rytvela administration (24h after LPS) was designed to allow capture a broad cross-section of protein, molecular and histological data from multiple tissues over a 5-day time course. Given the ontology of intrauterine inflammation in the sheep model, studies targeting individual tissues (especially the brain) and signals at specified time points will be required for more granular assessments. Irrespective, the data presented herein will inform future studies testing other rytvela dosing levels and timing relative to LPS administration to optimize both. These will also compare the efficacy of fetal IV-only vs. amniotic fluid-only administration of rytvela and need to assess the adverse effect using rytvela-only model.
Conclusions
We report that administration of the peptide rytvela partially reduced fetal and amniotic fluid inflammation following IA LPS challenge in an ovine model of chorioamnionitis. Given that dosing was exploratory and inflammation already established, these findings may be cautiously viewed as promising. Additional work focusing on optimizing dosing regimen may allow for a more comprehensive resolution of inflammation associated with preterm birth and fetal injury.
Supporting information
(PDF)
Acknowledgments
We would like to thank Sara and Andrew Ritchie (Icon Agriculture, Darkan, Western Australia) for their expertise and providing date-mated sheep for this study, and Dr Christiane Quiniou for providing and validating the rytvela used in this study and for valuable advice on its storage and handling.
Data Availability
All relevant data are within the manuscript. Raw western images are in attached file.
Funding Statement
DO, JK, MK. This work was supported by funding from the National Health and Medical Research Council (NHMRC; GNT1145295). MK. This work was supported by funding from the Women and Infants Research Foundation.
References
- 1.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol. 2004;190(1):147–51. Epub 2004/01/30. doi: 10.1016/j.ajog.2003.07.012 . [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35. Epub 2016/11/15. doi: 10.1016/S0140-6736(16)31593-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taguchi A, Yamashita A, Kawana K, Nagamatsu T, Furuya H, Inoue E, et al. Recent Progress in Therapeutics for Inflammation-Associated Preterm Birth: A Review. Reprod Sci. 2017;24(1):7–18. Epub 2015/12/03. doi: 10.1177/1933719115618282 . [DOI] [PubMed] [Google Scholar]
- 4.Nadeau-Vallee M, Obari D, Quiniou C, Lubell WD, Olson DM, Girard S, et al. A critical role of interleukin-1 in preterm labor. Cytokine Growth Factor Rev. 2016;28:37–51. Epub 2015/12/20. doi: 10.1016/j.cytogfr.2015.11.001 . [DOI] [PubMed] [Google Scholar]
- 5.Nadeau-Vallee M, Chin PY, Belarbi L, Brien ME, Pundir S, Berryer MH, et al. Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice. J Immunol. 2017;198(5):2047–62. Epub 2017/02/06. doi: 10.4049/jimmunol.1601600 . [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160(5 Pt 1):1117–23. Epub 1989/05/01. doi: 10.1016/0002-9378(89)90172-5 . [DOI] [PubMed] [Google Scholar]
- 7.Puchner K, Iavazzo C, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, et al. Mid-trimester amniotic fluid interleukins (IL-1beta, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo. 2011;25(1):141–8. Epub 2011/02/02. . [PubMed] [Google Scholar]
- 8.Ostensen M, Khamashta M, Lockshin M, Parke A, Brucato A, Carp H, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8(3):209. Epub 2006/05/23. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quiniou C, Sapieha P, Lahaie I, Hou X, Brault S, Beauchamp M, et al. Development of a novel noncompetitive antagonist of IL-1 receptor. J Immunol. 2008;180(10):6977–87. Epub 2008/05/06. doi: 10.4049/jimmunol.180.10.6977 . [DOI] [PubMed] [Google Scholar]
- 10.Nadeau-Vallee M, Quiniou C, Palacios J, Hou X, Erfani A, Madaan A, et al. Novel Noncompetitive IL-1 Receptor-Biased Ligand Prevents Infection- and Inflammation-Induced Preterm Birth. J Immunol. 2015;195(7):3402–15. Epub 2015/08/26. doi: 10.4049/jimmunol.1500758 . [DOI] [PubMed] [Google Scholar]
- 11.Christopoulos A, May LT, Avlani VA, Sexton PM. G-protein-coupled receptor allosterism: the promise and the problem(s). Biochem Soc Trans. 2004;32(Pt 5):873–7. Epub 2004/10/21. doi: 10.1042/BST0320873 . [DOI] [PubMed] [Google Scholar]
- 12.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. Epub 2006/06/29. doi: 10.1124/jpet.106.104463 . [DOI] [PubMed] [Google Scholar]
- 13.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–7. Epub 2008/06/14. doi: 10.1016/j.jri.2008.04.002 . [DOI] [PubMed] [Google Scholar]
- 14.Kemp MW, Musk GC, Saito M. Chapter 35—Animal Models for the Study of Infection-Associated Preterm Birth. In: Conn PM, editor. Animal Models for the Study of Human Disease. Boston: Academic Press; 2013. p. 863–88. [Google Scholar]
- 15.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280(3):L527–36. Epub 2001/02/13. doi: 10.1152/ajplung.2001.280.3.L527 . [DOI] [PubMed] [Google Scholar]
- 16.Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C, et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol. 2000;182(2):401–8. Epub 2000/02/29. doi: 10.1016/s0002-9378(00)70231-6 . [DOI] [PubMed] [Google Scholar]
- 17.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–48. Epub 2004/01/08. doi: 10.1007/s10024-003-7070-y . [DOI] [PubMed] [Google Scholar]
- 18.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. Epub 2015/10/03. doi: 10.1016/j.ajog.2015.08.040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder CC, Wolfe KB, Gisslen T, Knox CL, Kemp MW, Kramer BW, et al. Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep. Am J Obstet Gynecol. 2013;208(5):399.e1–8. Epub 2013/02/16. doi: 10.1016/j.ajog.2013.02.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banker BQ, Larroche JC. Periventricular leukomalacia of infancy. A form of neonatal anoxic encephalopathy. Arch Neurol. 1962;7:386–410. Epub 1962/11/01. doi: 10.1001/archneur.1962.04210050022004 . [DOI] [PubMed] [Google Scholar]
- 21.Gussenhoven R, Westerlaken RJJ, Ophelders D, Jobe AH, Kemp MW, Kallapur SG, et al. Chorioamnionitis, neuroinflammation, and injury: timing is key in the preterm ovine fetus. J Neuroinflammation. 2018;15(1):113. Epub 2018/04/21. doi: 10.1186/s12974-018-1149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67(4):287–94. Epub 2012/03/03. doi: 10.1111/j.1600-0897.2012.01110.x . [DOI] [PubMed] [Google Scholar]
- 23.Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol. 2015;11(4):192–208. Epub 2015/02/18. doi: 10.1038/nrneurol.2015.13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp MW, Molloy TJ, Usuda H, Woodward E, Miura Y, Payne MS, et al. Outside-in? Acute fetal systemic inflammation in very preterm chronically catheterized sheep fetuses is not driven by cells in the fetal blood. Am J Obstet Gynecol. 2016;214(2):281 e1–e10. Epub 2015/09/27. doi: 10.1016/j.ajog.2015.09.076 . [DOI] [PubMed] [Google Scholar]
- 25.Lim JH, Um HJ, Park JW, Lee IK, Kwon TK. Interleukin-1beta promotes the expression of monocyte chemoattractant protein-1 in human aorta smooth muscle cells via multiple signaling pathways. Exp Mol Med. 2009;41(10):757–64. Epub 2009/06/30. doi: 10.3858/emm.2009.41.10.082 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17(6):365–73. Epub 2005/07/13. doi: 10.1080/14767050500141329 . [DOI] [PubMed] [Google Scholar]
- 27.Tarca AL, Fitzgerald W, Chaemsaithong P, Xu Z, Hassan SS, Grivel JC, et al. The cytokine network in women with an asymptomatic short cervix and the risk of preterm delivery. Am J Reprod Immunol. 2017;78(3). Epub 2017/06/07. doi: 10.1111/aji.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res. 2000;48(6):782–8. Epub 2000/12/05. . [DOI] [PubMed] [Google Scholar]
- 29.Boonkasidecha S, Kannan PS, Kallapur SG, Jobe AH, Kemp MW. Fetal skin as a pro-inflammatory organ: Evidence from a primate model of chorioamnionitis. PLoS One. 2017;12(9):e0184938. Epub 2017/09/29. doi: 10.1371/journal.pone.0184938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–91. Epub 2009/09/19. doi: 10.1038/nri2622 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp MW, Kannan PS, Saito M, Newnham JP, Cox T, Jobe AH, et al. Selective exposure of the fetal lung and skin/amnion (but not gastro-intestinal tract) to LPS elicits acute systemic inflammation in fetal sheep. PLoS One. 2013;8(5):e63355. Epub 2013/05/22. doi: 10.1371/journal.pone.0063355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Saito M, Jobe A, Kallapur SG, Newnham JP, Cox T, et al. Intra-amniotic administration of E coli lipopolysaccharides causes sustained inflammation of the fetal skin in sheep. Reprod Sci. 2012;19(11):1181–9. Epub 2012/05/19. doi: 10.1177/1933719112446079 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keelan JA, Khan S, Yosaatmadja F, Mitchell MD. Prevention of inflammatory activation of human gestational membranes in an ex vivo model using a pharmacological NF-kappaB inhibitor. J Immunol. 2009;183(8):5270–8. Epub 2009/09/29. doi: 10.4049/jimmunol.0802660 . [DOI] [PubMed] [Google Scholar]
- 34.Newnham JP, Kallapur SG, Kramer BW, Moss TJ, Nitsos I, Ikegami M, et al. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol. 2003;189(5):1458–66. Epub 2003/11/25. doi: 10.1067/s0002-9378(03)00758-0 . [DOI] [PubMed] [Google Scholar]