Abstract
Sweet liking (heightened preference for highly-sweet solutions) is linked to Alcohol Use Disorder (AUD) and relapse, as well as attitudes towards sweet foods – use of sugar to cope with negative affect (sweet-cope), and impaired control over sweets consumption (sweet-control). This prospective analysis of individuals with AUD (N=26) participating in an Alcohol and Drug partial hospitalization program observed increases in self-reported sugar consumption and sweet craving from Time 1 (T1) to Time 2 (T2; 4 weeks later). Sweet-cope (T1) predicted T2 sweet craving. In an exploratory cross-lagged panel model, sweet-cope predicted sugar consumption and sweet craving at T1 and T2, and alcohol craving at T2. This pattern of results suggests the hypothesis that use of sugar to regulate negative affect may prove a novel, modifiable risk mechanism of the association between sweet liking and relapse. Sweet-cope may also prove an intervention target for improving nutrition and weight-related factors in early recovery. Future research in larger sample sizes is needed.
Keywords: Alcohol Craving, Alcohol Use Disorder, Sugar Consumption, Early Recovery
Introduction
Alcohol Use Disorder (AUD) remains a pressing public health concern (Mokdad et al., 2018), and high relapse rates underscore the need for continued research to elucidate novel treatment targets and improve recovery outcomes (Anton et al., 2006; Brandon, Vidrine, & Litvin, 2007). A heightened preference for very sweet sucrose solutions – termed “sweet liking” – is a candidate endophenotype strongly associated with AUD and family history of alcoholism that may increase relapse risk (Kampov-Polevoy et al., 2014; Kampov-Polevoy, Garbutt, & Janowsky, 1999; Salvatore, Gottesman, & Dick, 2015). “Sweet likers” endorse a preference for the highest molar sucrose concentration (0.83 or 0.88 M), measured in a range from 0.05 M to 0.83 or 0.88 M, equivalent to 2.5 times the sweetness of soft drinks (Eiler et al., 2018). Garbutt et al. (2009) found sweet likers to take 10 times longer to attain two consecutive days of abstinence compared to those not preferring highly sweet solutions, a clinical association supported in a recent trial (Garbutt, Kampov-Polevoy, Kalka-Juhl, & Gallop, 2016). Such findings suggest that being a sweet liker may play a key role in elevated relapse rates.
The phenotypic trait of sweet liking is unlearned, and has been detected within several hours of birth (Mennella, Bobowski, & Reed, 2016). Research suggests that sweet liking influences attitudes toward sweet foods. For example, Kampov-Polevoy and colleagues (2006) developed the Sweet Taste Questionnaire, which assesses attitudes about the consumption of sweets to cope with negative affect and impaired control over eating sweets, and found that sweet liking (determined by a laboratory assessment of preference for highly sweet sucrose tastants) was strongly associated with both of these factors. However, these attitudes toward sweet foods have not been examined among patients with AUD, despite sweet liking being a potential risk for poorer recovery outcomes.
Indeed, each of these attitudes toward sweets share substantial functional parallels with mechanisms implicated in alcohol use and relapse; consuming sweets to cope could be related to self-medication (use of alcohol to soothe negative affect), and impaired control over eating sweets may be related to affect dysregulation and (Khantzian, 2003) loss of control (Kampov-Polevoy, Garbutt, & Janowsky, 1999). Further, eating pathology (e.g., the pathological use of food to self-medicate negative affect) is comorbid with up to 60% of substance use disorders (von Ranson & Cassin, 2007), where loss of control over food intake (including, but not exclusive to sweets) has been identified as present well before excessive alcohol intake develops (Goodman, 2008). Such findings suggest that individuals high on these attitudes toward sweets may use sweet foods as a functional substitute during periods of increased negative affect or alcohol craving in the early recovery period. However, the associations between attitudes toward sweets, sugar consumption, sweet craving, and craving for alcohol has received little empirical attention in treatment-seeking samples.
Preliminary studies have observed increases in sugar consumption, sweet cravings, and alcohol cravings in early recovery (Alarcon et al., 2020; Gottfredson & Sokol, 2020). For instance, among male inpatients in alcohol detoxification, a 37% increase in sugar consumption was reported from treatment entry to three weeks later alongside significant correlations between alcohol cravings and sweet cravings observed in the second week (Junghanns et al., 2005). Two recent studies extend these findings. Alarcon and colleagues (2020) found that among patients in alcohol detoxification and subsequent rehabilitation across a 45-day timeframe, only those reporting substantive increases in sweet craving evidenced an objective increase in sweet products stored in their rooms, as well as weight gain. Complementing these findings, a recent study of adults in early recovery (most with AUD) using ecological momentary assessment (EMA) methodology found only those who scored high on a factor termed ‘addiction propensity’ consumed more added sugar and gained more weight over time. The addiction propensity factor used in this study comprised items consistently linked with a range of behavioral addictions, including family history of SUD, trait impulsivity, self-control, and food addiction. Sweet taste phenotype has been associated with novelty seeking and impulsive behaviors in humans, and may underlie or moderate such findings (Lange, Kampov-Polevoy, & Garbutt, 2010; Weafer, Burkhardt, & de Wit, 2014). Moreover, attitudes towards sweets may represent a proximal, more readily modifiable mechanism of these risk associations than sweet taste phenotype, given the latter’s heritability and stability.
Patients in early recovery with substance disorders (including AUD) have reported substitution of sweets for substances to improve mood and soothe cravings (Cowan & Devine, 2008). Two studies that investigated the link between sugar consumption and relapse in early recovery conceptualized sweets as an aid to abstinence, rather than a potential hindrance, and found preliminary support for this hypothesis (Stickel et al., 2016; Yung, Gordis, & Holt, 1983). This conceptualization reflects clinical lore and Alcoholics Anonymous’ (AA) longstanding recommendation that sweets be used to help manage alcohol cravings in early recovery (i.e., approximately first 6 months of sobriety; Living Sober, 1975). However, one study suggests consuming sweets in early recovery poses a risk for treatment outcomes — patients with AUD entering treatment and randomized to three different recommendations for sugar consumption found that those told to avoid sugar reported the greatest abstinence rates (83%) relative to the groups instructed to consume a balanced diet (58%) or to use sweets to cope with alcohol craving (53%; Krahn et al., 2006). Yet, this study did not assess actual consumption of sugar nor did it take into account the effects of specific attitudes towards sweets (i.e., using sweets to cope and experiencing impaired control of sweets).
These overall mixed findings may reflect the variable presence of unmeasured attitudes toward sweets in different samples. For instance, those with a greater tendency to consume sweets to regulate negative affect or who have impaired control of sweets may experience increased sugar consumption and sweets and/or alcohol craving, increasing risk for poorer subsequent alcohol treatment outcomes. Equally possible is that such risk sequelae occur independent of attitudes towards sweets (and/or sweet taste phenotype). Yet, no research to date has examined associations between “self-medicating” negative affect with sweets, or impaired control of eating sweets, with sugar consumption, sweet craving, or alcohol craving across the early recovery period – or assessed the temporality of these associations. Importantly, both consuming sweets to cope and impaired control over eating sweets are, if found to be a risk in early recovery, potentially modifiable with targeted interventions.
The current study provides a preliminary, hypotheses-generating examination of the concurrent and temporal associations between attitudes toward sweets, sugar consumption, and cravings for sweets and alcohol among a sample of patients with AUD starting alcohol treatment and over the course of the first month of recovery. Our aims are 3-fold: 1) to examine changes from baseline (starting alcohol treatment; T1) to one month later (T2) in sugar consumption, sweets craving, and alcohol craving; 2) to determine whether attitudes toward sweets (T1) – using sweets to cope (sugar-cope), and impaired control over sweets (sugar-control) – predict these changes; and capitalizing on the advantages of cross-lagged panel statistical methods, to explore 3a) whether attitudes towards sweets predicts T2 sugar consumption, sweet craving, and alcohol craving, holding all other modeled factors constant, and 3b) the temporality of associations between the latter constructs over time, controlling for attitudes towards sweets. Specifically, we hypothesize that increases in sugar consumption, sweet craving, and alcohol craving will be observed over time (H1), and that attitudes towards sweets will predict these changes at T2 in regression analyses (H2) as well as in a cross-lagged panel model (CLPM) that examines the temporality of these constructs controlling for attitudes towards sweets (H3). For the CLPM, we posited no a priori hypotheses regarding the patterns between sugar consumption, sweet craving, and alcohol craving after controlling for attitudes towards sweets.
Materials and Methods
Participants and Procedures
Recruitment.
Participants with AUD (N=26, 77.2% women, 95.5% White, age = M. 40.3, S.D. 10.2) were recruited from the Alcohol and Drug Partial Hospitalization program (ADP) at a large psychiatric hospital in the Northeast. Recruitment occurred between January and February of 2019. The ADP runs Monday through Friday from 9:00am to 3:30pm. Patients in ADP receive individual counseling, group therapy covering 3–4 topics per day, medication management with an attending psychiatrist, and case management over the course of 5–10 days (approximately 7 days, on average). Patients did not receive any nutritional/diet information or counseling during their time in the program. During the patient’s stay, aftercare treatment is coordinated, which often includes outpatient therapy and pharmacotherapy. Patients with a primary diagnosis of AUD, between the ages of 18–65, who did not have current psychotic symptoms or suicidal ideation were eligible to be enrolled.
Research staff reviewed medical records of ADP patients for potential eligibility. Individuals who appeared to be eligible were then approached during their time in ADP, and were given a brief description of the study. The baseline assessment (Time 1; T1) was conducted during the participant’s time in ADP. Participants completed questionnaires assessing eating habits, food and alcohol cravings, and substance use. Approximately 4 weeks after ADP discharge (Time 2; T2), participants completed the same set of measures. The study was approved by the study site’s Institutional Review Board. Participants were paid $50 for each of the T1 and T2 assessments.
Measures
Alcohol Consumption.
The Timeline Follow Back (TLFB; Sobell & Sobel, 1992) was administered to assess frequency of alcohol use over the previous 90 days at T1 and last month at T2. The TLFB uses anchor dates to prompt participant recall, and was used in the present study to assess relapse incidence.
Attitudes toward Sweet Foods.
The Sweet Taste Questionnaire (STQ; Alexey B. Kampov-Polevoy et al., 2006) is a 12-item measure assessing attitudes and behaviors surrounding sugar consumption on a scale of 1 (strongly disagree) to 7 (strongly agree). The STQ forms 5 constructs. For the purpose of this study, we utilize the items that assess sugar consumption’s mood-altering effects (omega = 0.97) and the perception of control of sweet consumption (omega = 0.86).
Sugar Consumption.
Sugar consumption was measured by the 8 sugar-related items of the Dietary Screening Questionnaire module of the National Health and Nutrition Examination Survey (Centers for Disease Control and Prevention, 2020). The DSQ asks participants the frequency of consuming common sweet foods (e.g., candy, pastries, ice cream) over the previous 30 days using a 9-point scale of “never” to “6+ times/day”. These responses are then converted into estimated intake of added sugars (in teaspoon equivalents) using an algorithm developed by the National Cancer Institute (NCI; NCI, 2020).
Sweet Craving.
Sweet craving was assessed by the 10-item Craving Experience Questionnaire (May et al., 2014). Participants rated their craving experience over the previous 4 weeks on a Likert scale to 0 (not at all) to 10 (extremely), with omega coefficients of 0.98 at baseline and follow-up.
Alcohol Craving.
The Penn Alcohol Craving Scale (PACS; (Flannery, Volpicelli, & Pettinati, 1999) is a 5-item measure of past week alcohol craving assessed on a scale of 0 (low craving) to 6 (high craving), with omega coefficients of 0.94 at baseline and 0.96 at follow-up.
Data Analysis
All analyses were conducted in R version 4.0 using the mice and lavaan packages (R Core Team, 2020; Rosseel, 2012; van Buuren & Groothuis-Oudshoorn, 2010). First, variable distributions were evaluated for normality and skewness, and missing data were imputed. Zero-order correlations were then used to assess associations between relapse (a theoretically-relevant covariate), whether attitudes towards sweets (using sweets to cope, i.e., sweet-cope, and impaired control over sweets, i.e., sweet-control, both assessed at T1), and pre-treatment (T1) and post-treatment (T2) sweet craving, sugar consumption, and alcohol craving.
To test Hypothesis 1, whether increases in sweet craving, sugar consumption, and alcohol craving were observed from pre-treatment (T1) to one month later (T2), we conducted paired-samples t-tests. To test Hypothesis 2, whether sweet-cope or sweet-control predicted changes in sugar consumption, sweet craving, and alcohol craving over time, we conducted a series of multiple regression analyses. In two sets of models, sweet-cope and sweet-control were examined as predictors of T2 sweet craving, sugar consumption, and alcohol craving, controlling for T1 levels of the dependent variable. We also controlled for relapse incidence, defined as participant report of alcohol consumption over the 4-week study timeframe using the Timeline Follow-Back, given associations in the empirical literature between relapse and sweet taste that suggest this factor may confound results (e.g., Garbutt et al., 2016). Each model was conducted separately because the use of sweets to cope with negative affect and impaired control over eating sweets reflect distinct correlates of sweet liking (Kampov-Polevoy et al., 2006).
To test Hypothesis 3, whether sweet-cope and sweet-control predict T2 study constructs, holding all other modeled factors constant, we aimed to fit two exploratory conditional cross-lagged panel models (CLPM) to the data, controlling for sweet-cope (model 1) and sweet-control (model 2). The CLPM tested regression paths from sweet-cope and sweet-control to T1 and T2 sugar consumption, sweet craving, and alcohol craving (aim 3a, Hypothesis 3). The CLPM also tested the temporality of autoregressive and cross-lagged paths between scaled scores of T1 and T2 sugar consumption, sweet craving, and alcohol craving over time, after accounting for sweet-cope and sweet-control in respective models (exploratory inquiry, aim 3a). Autoregressive coefficients characterize the stability of constructs across time (e.g., T1 sugar consumption predicts T2 sugar consumption), while cross-lagged regression coefficients indicate reciprocal associations of constructs across time (e.g., T1 sweet craving predicts T2 sugar consumption; Newsom, 2015). Conditioning on T1 sweet-cope generates a less biased estimate of the autoregressive and cross-lagged effects in the model, as this approach controls for the variability explained in T1 and T2 variables by sweet-cope. Moreover, this approach generates regression path estimates for the effect of sweet-cope on both sets of variables at each timepoint, a central question in this study. By controlling for all other constructs in the model, a CLPM allows for a better understanding of the possible mechanistic pathways among variables over time (Newsom, 2015). Missing data were imputed using multiple imputation (m = 10 datasets) for the main analyses and full-information maximum likelihood for the exploratory analysis.
Results
Participants (N=26) were 77.2% women, 95.5% White, with an average age of 40.3 (SD = 10.2). Seven participants relapsed between T1 and T2. Sugar consumption variables at both time points had issues of non-normality due to one participant who consumed high amounts of sugar. Missing data diagnostics showed 9.48% of the data were missing. Descriptive statistics can be viewed in Table 1.
Table 1.
Descriptive statistics and t-test results
| Variable | T1 (M/SD) | T2 (M/SD) | T1 (N) | T2 (N) | T1 (Skew/Kurt) | T2 (Skew/Kurt) | Mean Diff. | t | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|---|---|
| Sweet-Cope | 24.08 (11.20) | - | 26 | - | −0.24 / 1.52 | - | - | - | - | - |
| Sweet-Control | 17.54 (6.97) | - | 26 | - | −0.15 / 0.81 | - | - | - | - | - |
| Sugar Consumption | 19.97 (15.88) | 25.04 (23.15) | 25 | 22 | 3.30 / 11.48 | 3.38 / 11.62 | 3.74 | 2.25 | 0.04 | 0.20 |
| Sweet Craving | 2.90 (2.74) | 3.75 (2.65) | 26 | 22 | 0.54 / −1.21 | 0.18 / −1.33 | 0.82 | 1.43 | 0.17 | 0.30 |
| Alcohol Craving | 16.38 (7.21) | 16.23 (7.55) | 26 | 22 | 0.05 / −1.08 | −0.02 / −0.71 | −0.05 | −0.03 | 0.86 | −0.01 |
Note: Degrees of freedom equal to 25 for all tests, imputed data used for all t-tests.
Sugar consumption significantly increased (p = .04) post-treatment (T2) relative to pre-treatment (T1), with a small effect size. Sweet craving (p = .17; see Table 1) and alcohol craving (p= .86) did not change from T1 to T2, both showing small effect sizes. Alcohol craving did not change from T1 to T2. Sweet-cope was significantly correlated with T1 and T2 sweet craving (r = .64, p < .001; r=.58, p < .001) and sugar consumption (r = .41, p = .03; r= .43, p = .03). Sweet-control was significantly correlated only with T1 sweet craving (r = .43, p = .03). T1 sweet craving was correlated with T1 sugar consumption (r = .44, p = .02) and marginally, T2 sweet craving (r = .38, p = .08) and sugar consumption (r = .35, p = .08). Alcohol craving and relapse were unrelated to any construct.
To test our hypothesis that sweet-cope and sweet-control predict changes in sweet craving, sugar consumption, and alcohol craving over time, we ran six regression models, controlling for relapse rates and baseline levels of each dependent variable. Sweet-cope predicted T2 sweet craving (β = .13, SE = .05, t = 2.52, p = .04). T1 sugar consumption was also a predictor of T2 sugar consumption in both models (sugar-cope, β = 1.28, SE = .10, t = 12.40, p<.001; sugar-control, β = 1.31, SE = .10, t = 13.70, p <.001). No other significant results emerged, and relapse did not predict outcomes in any model.
Last, we used an exploratory Cross-Lagged Panel Model (CLPM) to examine sweet-cope as a predictor of T2 sugar consumption, sweet craving, and alcohol craving, an approach that holds the relationships among all other modeled factors constant (Newsom, 2015). This model also allowed us to examine the temporality of changes in sugar consumption and sweet cravings from T1 to T2 and associations with alcohol cravings, while controlling for sweet-cope. Because sweet-control was a non-significant predictor of study outcomes of interest, we excluded this construct from the final CLPM analysis. While alcohol craving was unrelated to other study constructs, and did not change over time, we elected to retain this construct in our analysis given strong theoretical rationale.
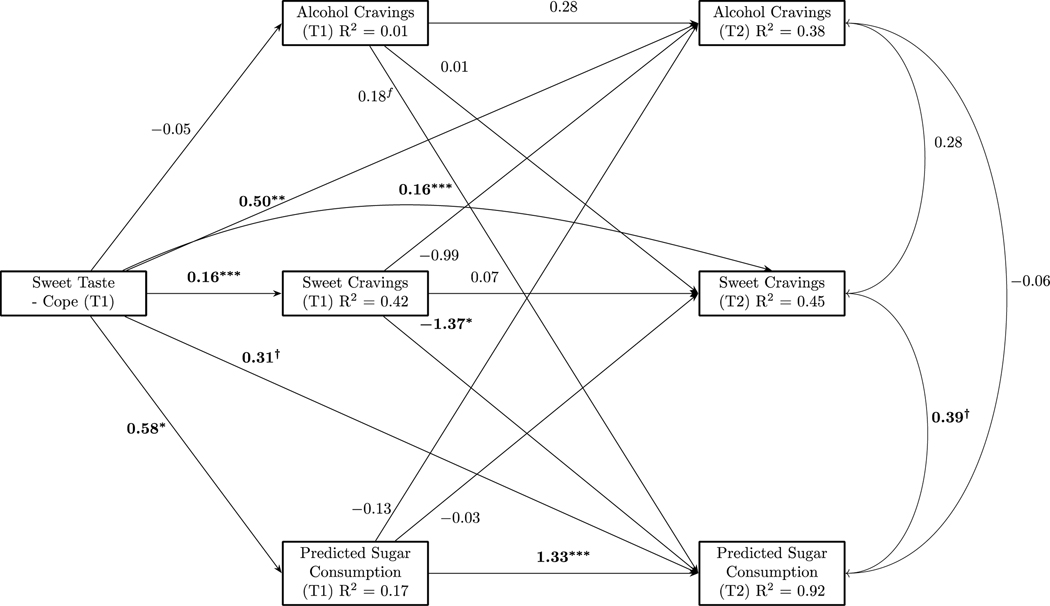
This CLPM showed an excellent fit based on the χ2 test, CFI, and RMSEA fit indices, χ2(1) = 0.00, p = 0.99, CFI = 1.00, RMSEA = 0.00 90% CI [0.00, 0.00] (Hu & Bentler, 1999). A fully parameterized model was estimated and a non-significant pathway was fixed to the estimate found in the fully parameterized model to allow one degree of freedom for model estimation (Harlow, 2014). Missing data (9.48%) were imputed using full-information maximum likelihood. Figure 1 shows the sweet-cope model. Sweet-cope was associated with T1 sweet craving (p < .001) and sugar consumption (p < .05). Sweet-cope predicted T2 sweet craving (p < .001), alcohol craving (p < .01), and sugar consumption (marginally; p < .10). Only one auto-regressive path was significant; T1 sugar consumption predicted sugar consumption at T2 (p < .001). In a cross-lagged path, T1 sweet craving negatively predicted T2 sugar consumption (p < .05). No other significant or marginal pathways emerged.
Figure 1.
Exploratory cross-lagged panel model, conditioning on pre-treatment mood altering effects of sweet foods (sweet-cope). Standardized estimates for regression coefficients and correlations shown. † = p ≤ .10, * = p < .05, ** = p < .01, *** = p < .001. Correlations at T1 not shown for simplicity of figure; there was one marginal association between T1 sugar consumption and sweet craving (r = .26, p = .06).
Discussion
Emerging research connects sweet liking phenotype (i.e., sweet liking, referring to a preference for the highest molar sucrose concentration) with subjective sugar liking and consumption in people without AUD, and with alcohol craving and relapse risk in AUD samples. Our study represents the first effort to integrate these literatures by examining changes in sugar consumption, sugar craving, and alcohol craving during a four-week period in early recovery, alongside concurrent and prospective associations between these factors and pre-treatment attitudes towards sweets that are strongly linked to sweet liking – using sweets to cope (sweet-cope) and impaired control over sweets (sweet-control).
Our first hypothesis was partially supported. Sugar consumption and sweet craving increased with small effect sizes, replicating other recent studies that have observed increased sugar cravings and consumption in the early recovery period (Alarcon et al., 2020; Gottfredson & Sokol, 2020). Ours is among the first studies to demonstrate increased sugar consumption using a validated dietary assessment; earlier studies utilized qualitative and less rigorously assessed reports (Junghanns et al., 2005). However, alcohol craving did not change across the four-week study period, in contrast to reductions seen from admission to 3-month discharge in a residential addiction program (Stohs, Schneekloth, Geske, Biernacka, & Karpyak, 2019), perhaps because four weeks remains early in the recovery process.
Our second hypothesis was partially supported; use of sweets to cope predicted change in sweet craving at T2, but not sugar consumption or alcohol craving. This marks the first such empirical observation in early recovery, and adds to Alarcon et al.’s (2020) findings that patients in alcohol withdrawal programs who reported increased sugar craving purchased more sweet products over time than did those reporting less craving. Pending further research, these findings suggest that targeting sweet-cope may help reduce sugar consumption in early recovery and improve corresponding health concerns common in this population (e.g., nutrient deficiencies, weight gain; Cowan & Devine, 2008; Jeynes & Gibson, 2017).
Our third hypothesis was largely supported. Using an exploratory cross-lagged panel model, the reported use of sweets to cope with negative affect at treatment entry (T1) predicted simultaneous increases in T2 sugar consumption (marginally), sweet craving, and alcohol craving, accounting for all other modeled constructs. The tested model does not permit inference of mediation or causality, however, sweet-cope’s relationship with sugar consumption, sweet craving, and alcohol craving strongly supports continued research to elucidate these pathways. For instance, it is possible that a predisposition to use sweets to alleviate negative affect in early recovery may reflect a latent dispositional tendency to cope with negative affect via a range of substances, whether alcohol or sugar (e.g., Gottfredson & Sokol, 2020). Our results also support the hypothesis that the general tendency to use sweets for emotional coping may lead to heightened alcohol craving and relapse in early recovery independent of actual sugar consumption.
In the context of extant empirical literature, our results also suggest the possibility of other, more complex pathways. For our exploratory aim, few autoregressive or cross-lagged associations were observed between T1 and T2 sugar consumption, sweet craving, and alcohol craving after accounting for sweet-cope. This pattern of results suggests that sweet-cope may better be conceptualized as a distal predictor, moderator, or other mechanism of the tested relationships in future research. One hypothesis is that the connection between sweet-cope and alcohol craving or relapse could be mediated or moderated by sugar consumption and/or sweets craving, a research inquiry well-suited to assessment using ambulatory and EMA methods. Additionally, sweet-cope may moderate the relationships between sugar consumption, sweet craving, and/or alcohol craving or relapse.
As with AUD, scientists have conceptualized findings on the reward value of sugar within the “reward deficiency syndrome” framework, a phenomena of impaired brain reward circuitry leading to hypodopaminergic functioning in those with addictive behaviors (Blum, Thanos, & Gold, 2014; Leggio et al., 2011). Sweet liking may be a novel indicator or moderator of reward deficiency syndrome and contribute to relapse in early recovery through neurophysiological mechanisms as well as the modifiable risk factor studied here – use of sweets to regulate negative affect. Sweet-cope may in turn increase likelihood of sugar craving, consumption, and both alcohol craving and use (Hansson et al., 2019). Prospective evidence linking sweet liking to impulsive behavior, novelty seeking, and alcohol-related problems supports this hypothesis (Kampov-Polevoy et al., 2014; Lange, Kampov-Polevoy, & Garbutt, 2010; Weafer, Burkhardt, & de Wit, 2014), as does the recent evidence linking predisposition to behavioral addiction (including heritable and phenotypic factors) and sugar cravings to increased sweet consumption, acquisition, and weight gain in early recovery from drug and alcohol use (Alarcon et al., 2020; Gottfredson & Sokol, 2020). These risk sequelae may be particularly salient under conditions of stress or negative affect, an interesting avenue for future investigation.
Clinical Implications
Sweet likers who use sugar to cope in early recovery may experience increased risk of alcohol craving either directly or through sugar consumption and/or craving. These results raise the possibility that reducing the use of sweets to cope and providing strategies to manage sweet cravings, including decreasing sugar consumption, could be novel targets for mitigating relapse risk, an important topic of future investigation. Moreover, even in sweet likers who do maintain abstinence shorter-term, it is possible that increased consumption of sugar to regulate negative affect may interact with loss of control over sugar intake and reward deficiency to maintain the maladaptive reward pathways implicated in AUD. These sequelae could lead to uptake of other maladaptive and rewarding behaviors that engender distress and poor health, thereby increasing long-term risk of relapse in a cyclical fashion. Indeed, compelling evidence has recently been presented for how sugar may act as a gateway substance to other psychoactive drugs (Liester & Moore Liester, 2015), and animal research suggests the combination of sweet liking preference and intermittent access to sugar can activate a state with strong parallels to addiction, including cross-sensitization effects to alcohol and amphetamine (Avena & Hoebel, 2003; Avena, Carrillo, Needham, Leibowitz, & Hoebel, 2004). Such findings underscore a critical need for further research to examine whether and/or how sugar consumption may impact the continuum of addictive behaviors among sweet likers with AUD in recovery. Larger studies, with longer follow-up that capture lapse and relapse episodes would be an important next step.
A strength of the present study is its examination of a factor linked to sweet liking in prior research – use of sugar to self-medicate negative affect (Kampov-Polevoy et al., 2006). While sweet liking phenotype is partially heritable (Salvatore et al., 2015) and a known target for pharmacological intervention (Naltrexone; Garbutt et al., 2016), sweet-cope may offer avenues for non-pharmacological intervention to reduce sugar consumption and potentially prevent relapse in early recovery for those susceptible. Pending further research, use of sugar to regulate negative affect could, like other forms of behavior that serve a self-medication function (e.g., Binge Eating Disorder, AUD), be treated with Cognitive-Behavioral Therapy or other affect regulation therapies (Hilbert, Petroff, Herpertz, & Pietrowsky, 2019).
Last, among women with AUD, stress and drinking to cope are stronger predictors of drinking and relapse than among men (Nolen-Hoeksema, 2004; Peltier et al., 2019). Eating disorders are also a common AUD comorbidity among women (Gadalla & Piran, 2007), and use of sweets and alcohol to cope – as well sweet liking – may prove shared risk mechanisms underlying these sequelae. Indeed, use of sweets to cope was reported greater among women in the validation sample for the STQ than among men (Kampov-Polevoy et al., 2006). Our sample, while small, comprised 77.2% women. To optimally inform treatment, future research is needed to elucidate gender differences in sweet-cope and differential associations with sweet liking and sugar consumption, alcohol craving, and relapse, as well as comorbid eating pathology.
Limitations
Limitations of this study include the small sample size, the upper confidence interval of the RMSEA value being above the desired threshold of 0.10, and the use of self-report measures. Further, while our measure of factors related to sweet liking (sweet-cope and sweet-control) was objectively associated with sweet liking phenotype in the validation article (Kampov-Polevoy et al., 2006), we can only infer each factor as a proxy of sweet liking as we did not objectively assess sweet liking via sucrose tastants. Moreover, given that several of the results were at the p < .10 level, these findings should be considered hypothesis-generating, despite results being consistent with extant literature and theory. Future research in this area and replication of our findings in adequately powered samples will be needed to directly test these hypotheses. Additionally, while our use of a validated self-report dietary screener to assess sugar consumption is a relative strength, a comprehensive assessment of dietary intake (e.g., 24-hour dietary recall, comprehensive food frequency questionnaire) would more accurately capture sugar intake related to carbohydrate and other macronutrient consumption. Last, the cross-lagged panel model was considered exploratory because it is generally inadvisable to conduct path analyses on small sample sizes (Newsom, 2015). However, the unique aspect of these data is assessment over the critical period post-discharge for AUD treatment, to inform hypothesis generation. Thus, we proceeded with fitting the CLPM, and acknowledge that fitting the complex model to this sample has limited application for interpretation of findings and needs to be followed up with fully-powered research.
Conclusions
Taken together, our findings suggest that use of sweets to regulate negative affect may play a key role in sugar consumption in early recovery, and may predict increases in sweet and alcohol cravings. A predisposition to use sweets to regulate negative affect may be directly implicated in subsequently increased alcohol craving, sweet craving, and sugar consumption. However, the overall pattern of results suggests more complex and potentially mechanistic relationships between the use of sweets to cope and sugar consumption, sweet craving, and alcohol craving over time. Our results strongly support continued investigation to better elucidate the contributions of this sweet-taste related component to relapse and quality of life in early recovery, as well as intervention development efforts targeting these factors.
Acknowledgments
Funding: This work was supported through a National Institutes of Health Cardiovascular Behavioral and Preventive Medicine Training Grant awarded to the Miriam Hospital, Providence, RI (T32 HL076134). Dr. Miranda’s effort was supported by grant K24 AA026326.
Footnotes
Disclosure statement: The authors report no conflict of interest.
References
- Alarcon R, Tiberghien M, Trouillet R, Pelletier S, Luquiens A, Ahmed SH, … Perney P. (2020). Sugar intake and craving during alcohol withdrawal in alcohol use disorder inpatients. Addiction Biology, (March), 2–7. 10.1111/adb.12907 [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, … COMBINE Study Research Group. (2006). Combined pharmacotherapies and behavioral interventions for alcohol dependence. JAMA, 295(17), 2003–2017. 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- Avena NM, & Hoebel BG (2003). A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience, 122(1), 17–20. 10.1016/S0306-4522(03)00502-5 [DOI] [PubMed] [Google Scholar]
- Avena Nicole M., Carrillo CA, Needham L, Leibowitz SF, & Hoebel BG (2004). Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol, 34(2–3), 203–209. 10.1016/j.alcohol.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Blum K, Thanos PK, & Gold MS (2014). Dopamine and glucose, obesity, and reward deficiency syndrome. Frontiers in Psychology, 5. 10.3389/fpsyg.2014.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, & Litvin EB (2007). Relapse and relapse prevention. Annual Review of Clinical Psychology, 3, 257–284. 10.1146/annurev.clinpsy.3.022806.091455 [DOI] [PubMed] [Google Scholar]
- Cowan J, & Devine C. (2008). Food, eating, and weight concerns of men in recovery from substance addiction. Appetite, 50(1), 33–42. 10.1016/j.appet.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Eiler WJA, Dzemidzic M, Soeurt CM, Carron CR, Oberlin BG, Considine RV, … Kareken DA (2018). Family history of alcoholism and the human brain response to oral sucrose. NeuroImage: Clinical, 17, 1036–1046. 10.1016/j.nicl.2017.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, & Pettinati HM (1999). Psychometric properties of the Penn Alcohol Craving Scale. Alcoholism: Clinical and Experimental Research, 23(8), 1289–1295. 10.1111/j.1530-0277.1999.tb04349.x [DOI] [PubMed] [Google Scholar]
- Gadalla T, & Piran N. (2007). Co-occurrence of eating disorders and alcohol use disorders in women: A meta analysis. Archives of Women’s Mental Health, 10(4), 133–140. 10.1007/s00737-007-0184-x [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoy AB, Kalka-Juhl LS, & Gallop RJ (2016). Association of the sweet-liking phenotype and craving for alcohol with the response to naltrexone treatment in alcohol dependence a randomized clinical trial. JAMA Psychiatry, 73(10), 1056–1063. 10.1001/jamapsychiatry.2016.2157 [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Osborne M, Gallop R, Barkenbus J, Grace K, Cody M, … Kampov-Polevoy AB (2009). Sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol and Alcoholism, 44(3), 293–300. 10.1093/alcalc/agn122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. (2008). Neurobiology of addiction: An integrative review. Biochemical Pharmacology, 75(1), 266–322. 10.1016/j.bcp.2007.07.030 [DOI] [PubMed] [Google Scholar]
- Gottfredson NC, & Sokol R. (2020). Explaining excessive weight gain during early recovery from addiction. Substance Use and Misuse, 54(5), 769–778. 10.1080/10826084.2018.1536722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Gründer G, Hirth N, Noori HR, Spanagel R, & Sommer WH (2019). Dopamine and opioid systems adaptation in alcoholism revisited: Convergent evidence from positron emission tomography and postmortem studies. Neuroscience and Biobehavioral Reviews, 106, 141–164. 10.1016/j.neubiorev.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Harlow LL (2014). The essence of multivariate thinking: Basic themes and methods, (2nd ed.). Routledge/Taylor & Francis Group. 10.4324/9781315832746 [DOI] [Google Scholar]
- Hilbert A, Petroff D, Herpertz S, & Pietrowsky R. (2019). Meta-analysis of the efficacy of psychological and medical treatments for Binge-Eating Disorder. Journal of Consulting and Clinical Psychology, 87(1), 91–105. 10.1037/ccp0000358 [DOI] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Jeynes KD, & Gibson EL (2017). The importance of nutrition in aiding recovery from substance use disorders: A review. Drug and Alcohol Dependence, 179, 229–239. 10.1016/j.drugalcdep.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Rink L, Wetterling T, & Driessen M. (2005). The consumption of cigarettes, coffee and sweets in detoxified alcoholics and its association with relapse and a family history of alcoholism. European Psychiatry, 20(5–6), 451–455. 10.1016/j.eurpsy.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy A, Lange L, Bobashev G, Eggleston B, Root T, & Garbutt JC (2014). Sweet-liking is associated with transformation of heavy drinking into alcohol-related problems in young adults with high novelty seeking. Alcoholism: Clinical and Experimental Research, 38(7), 2119–2126. 10.1111/acer.12458 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, & Janowsky DS (1999). Association between preference for sweets and excessive alcohol intake: A review of animal and human studies. Alcohol and Alcoholism, 34(3), 386–395. 10.1093/alcalc/34.3.386 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy Alexey B., Alterman A, Khalitov E, & Garbutt JC (2006). Sweet preference predicts mood altering effect of and impaired control over eating sweet foods. Eating Behaviors, 7(3), 181–187. 10.1016/j.eatbeh.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Khantzian EJ (2003). The self-medication hypothesis revisited: The dually diagnosed patient. Primary Psychiatry, 10(9), 47–48, 53–54. [Google Scholar]
- Krahn D, Grossman J, Henk H, Mussey M, Crosby R, & Gosnell B. (2006). Sweet intake, sweet-liking, urges to eat, and weight change: Relationship to alcohol dependence and abstinence. Addictive Behaviors, 31(4), 622–631. 10.1016/j.addbeh.2005.05.056 [DOI] [PubMed] [Google Scholar]
- Lange LA, Kampov-Polevoy AB, & Garbutt JC (2010). Sweet liking and high novelty seeking: Independent phenotypes associated with alcohol-related problems. Alcohol and Alcoholism, 45(5), 431–436. 10.1093/alcalc/agq040 [DOI] [PubMed] [Google Scholar]
- Leggio L, Addolorato G, Cippitelli A, Jerlhag E, Kampov-Polevoy AB, & Swift RM (2011). Role of feeding-related pathways in alcohol dependence: A focus on sweet preference, NPY, and ghrelin. Alcoholism: Clinical and Experimental Research, 35(2), 194–202. 10.1111/j.1530-0277.2010.01334.x [DOI] [PubMed] [Google Scholar]
- Liester MB, & Moore Liester JD (2015). Is sugar a gateway drug? Journal of Drug Abuse, 01(01), 1–8. 10.21767/2471-853x.10008 [DOI] [Google Scholar]
- May J, Andrade J, Kavanagh DJ, Feeney GFX, Gullo MJ, Statham DJ, … Connor JP (2014). The Craving Experience Questionnaire: A brief, theory-based measure of consummatory desire and craving. Addiction, 109(5), 728–735. 10.1111/add.12472 [DOI] [PubMed] [Google Scholar]
- Mennella JA, Bobowski NK, & Reed DR (2016). The development of sweet taste: From biology to hedonics. Reviews in Endocrine and Metabolic Disorders, 17(2), 171–178. 10.1007/s11154-016-9360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, … Murray CJL (2018). The State of US Health, 1990–2016. JAMA, 319(14), 1444. 10.1001/jama.2018.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health and Nutrition Examination Survey Questionnaire. (2020). Hyattsville, MD. [Google Scholar]
- NCI. (2020). Data processing & scoring procedures using current methods. Retrieved from https://epi.grants.cancer.gov/nhanes/dietscreen/scoring/current/
- Newsom JT (2015). Longitudinal Structural Equation Modeling. New York, NY: Routledge/Taylor & Francis Group. [Google Scholar]
- Nolen-Hoeksema S. (2004). Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review, 24(8), 981–1010. 10.1016/j.cpr.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, & McKee SA (2019). Sex differences in stress-related alcohol use. Neurobiology of Stress, 10(January). 10.1016/j.ynstr.2019.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rosseel Y. (2012). lavaan: An R package for Structural Equation Modeling. Journal of Statistical Software, 48(2), 1–36. 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Salvatore JE, Gottesman II, & Dick DM (2015). Endophenotypes for Alcohol Use Disorder: An update on the field. Current Addiction Reports, 2(1), 76–90. 10.1007/s40429-015-0046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobel MB (1992). Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In Litten RZ (Ed.), Measuring Alcohol Consumption (pp. 41–72). The Humana Press. [Google Scholar]
- Stickel A, Rohdemann M, Landes T, Engel K, Banas R, Heinz A, & Muller CA (2016). Changes in nutrition-related behaviors in alcohol-dependent patients after outpatient detoxification: The role of chocolate. Substance Use and Misuse, 51(5), 545–552. 10.3109/10826084.2015.1117107 [DOI] [PubMed] [Google Scholar]
- Stohs ME, Schneekloth TD, Geske JR, Biernacka JM, & Karpyak VM (2019). Alcohol craving predicts relapse after residential addiction treatment. Alcohol and Alcoholism, 54(2), 167–172. 10.1093/alcalc/agy093 [DOI] [PubMed] [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K. (2010). mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 1–68. [Google Scholar]
- von Ranson KM, & Cassin SE (2007). Eating disorders and addiction: Theory and evidence. In Rubin JS (Ed.), Eating disorders and weight loss research (pp. 1–37). Nova Science Publishers, Inc. [Google Scholar]
- Weafer J, Burkhardt A, & de Wit H. (2014). Sweet taste liking is associated with impulsive behaviors in humans. Frontiers in Behavioral Neuroscience, 8(JUNE), 1–6. 10.3389/fnbeh.2014.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung L, Gordis E, & Holt J. (1983). Dietary choices and likelihood of abstinence among alcoholic patients in an outpatient clinic. Drug and Alcohol Dependence, 12(4), 355–362. 10.1016/0376-8716(83)90007-8 [DOI] [PubMed] [Google Scholar]