Abstract
To overcome nitrogen deficiency, legume roots establish symbiotic interactions with nitrogen-fixing rhizobia that are fostered in specialized organs (nodules). Similar to other organs, nodule formation is determined by a local maximum of the phytohormone auxin at the primordium site. However, how auxin regulates nodule development remains poorly understood. Here, we found that in soybean, (Glycine max), dynamic auxin transport driven by PIN-FORMED (PIN) transporter GmPIN1 is involved in nodule primordium formation. GmPIN1 was specifically expressed in nodule primordium cells and GmPIN1 was polarly localized in these cells. Two nodulation regulators, (iso)flavonoids trigger expanded distribution of GmPIN1b to root cortical cells, and cytokinin rearranges GmPIN1b polarity. Gmpin1abc triple mutants generated with CRISPR-Cas9 showed the impaired establishment of auxin maxima in nodule meristems and aberrant divisions in the nodule primordium cells. Moreover, overexpression of GmPIN1 suppressed nodule primordium initiation. GmPIN9d, an ortholog of Arabidopsis thaliana PIN2, acts together with GmPIN1 later in nodule development to acropetally transport auxin in vascular bundles, fine-tuning the auxin supply for nodule enlargement. Our findings reveal how PIN-dependent auxin transport modulates different aspects of soybean nodule development and suggest that the establishment of auxin gradient is a prerequisite for the proper interaction between legumes and rhizobia.
In soybean, nodule primordium formation involves GmPIN1-mediated polar auxin transport within primordium cells, and nodule enlargement involves the collaboration of GmPIN9d and GmPIN1-dependent auxin transport within nodule vasculature.
Introduction
The developmental plasticity of plant cells largely depends on the de novo post-embryonic generation of new organs initiated from specialized tissues (Benkova et al., 2003; Yamaguchi et al., 2013; Qi et al., 2014). For example, a lateral root is generated from pericycle cells, via a series of anticlinal divisions, giving rise to lateral root primordia (Benkova and Bielach, 2010). Leaf bulges formed at the flanks of the shoot apical meristem generate leaf primordia (Xiong and Jiao, 2019). Some primordia differentiate into determinate organs with constant cell numbers, whereas others develop into indeterminate organs with a secondary meristem at the tip.
Initiation and positioning of an organ primordium are often instructed by local accumulation of the phytohormone auxin (Benkova et al., 2003; Qi et al., 2014; Wu et al., 2015). Local auxin accumulation within founder cells depends mainly on the coordinated activities of AUXIN1/LIKE-AUXIN1 influx carriers and PIN-FORMED (PIN) efflux carriers in conjunction with other auxin transporters (Mravec et al., 2008). The roles of different PINs are well characterized in the context of auxin transport for organ growth and development in Arabidopsis thaliana, such as PIN1 in flower development (Okada et al., 1991), PIN2 in root gravitropism (Muller et al., 1998), PIN3 in shoot tropism (Friml et al., 2002), PIN4 and PIN7 in embryo development (Benkova et al., 2003; Weijers et al., 2005).
The cellular polarity of PIN proteins is tightly related to the direction of auxin flow. For example, primary root growth requires the acropetal/rootward direction of auxin transport by PIN1 which targets the basal side of the plasma membrane (PM) in stele cells, and the basipetal/shootward direction of auxin transport by PIN2 that targets the apical side of PM in epidermal cells, as well as the coordination of PIN3, PIN4, and PIN7 for auxin lateralization within root stem cells (Feraru and Friml, 2008). Current models in diverse organ patterning explicitly show that a PIN-dependent local auxin gradient participates in nearly all types of organ development processes in plants (Benkova et al., 2003; Wisniewska et al., 2006).
Many legumes (Fabaceae) are capable of symbiotic interaction with soil bacteria (rhizobia) in specialized organs, called nitrogen-fixing root nodules. The initiation of nodules is triggered by specific signaling molecules, lipochitooligosaccharides/Nod factor released by rhizobia, in response to flavonoids secreted from the root hairs of host plants (Suzaki and Kawaguchi, 2014). Subsequently, the rhizobia penetrate the root via an infection thread, reaching the cortex cells through the curled root hairs. In the cortical cells, the rhizobia stimulate cell proliferation and expansion, resulting in a functional nodule on the host root (Popp and Ott, 2011). Later, the entire nodule is surrounding by a continuum of vascular bundles, providing a robust route to exchange water and organic materials between the root and nodule (Livingston et al., 2019). Within the nodule, rhizobia reside as intracellular symbionts, where they convert atmospheric nitrogen into ammonia to overcome nitrogen shortage in the host plant.
Different legume species show substantial variation in nodule organogenesis and nodules are broadly defined as indeterminate versus determinate types. Medicago truncatula develops indeterminate nodules, which harbor active apical meristems that continuously produce new meristem tissue. Lotus japonicus and Glycine max (soybean) generate determinate nodules, whose meristematic activity is lost soon after their initiation (Popp and Ott, 2011). Irrespective of nodule type, these nodules all initiate from a primordium and subsequently develop into diverse organ morphologies. Auxin accumulates at the position of future primordia, to prepare/prime the initiation of nodule primordia (van Noorden et al., 2007; Suzaki et al., 2012; Turner et al., 2013). In soybean, accumulating evidence further illustrates the importance of auxin for nodule development. For example, overexpression of a nodule-expressed auxin biosynthesis enzyme YUCCA2a (GmYUC2a) delays nodule organogenesis (Wang et al., 2019). Moreover, overexpression of the auxin receptor transport inhibitor response1 (GmTIR1) and suppression of auxin response factor8 (GmARF8) increases rhizobia infection events and nodule number (WaNg et al., 2015b; Cai et al., 2017). Besides many similarities between determinate nodule formation in soybean and L. japonicus, species-specific variation exists, such as the pseudoinfections in soybean (Calvert et al., 1984).
It has long been proposed that the auxin maximum in the founder cells of a nodule primordium is controlled by polar auxin transport (PAT) (Hirsch et al., 1989; Mathesius et al., 1998; Kohlen et al., 2018); however, the underlying molecular mechanisms remain elusive. Ng and Mathesius (2018) utilized L. japonicus and M. truncatula as model legumes to study the differential regulation of auxin transport for initiating determinate and indeterminate nodules. They proposed that acropetal auxin transport is not necessary for determinate nodule formation, based on the observation that acropetal auxin transport in the equivalent root segments below the rhizobia-inoculation spot decreases significantly in M. truncatula, while it increases in L. japonicus (Pacios-Bras et al., 2003; Ng et al., 2015; Ng and Mathesius, 2018). This further supported the generation of a pseudonodule in M. truncatula, but not in L. japonicus upon application of auxin transport inhibitors (Ng and Mathesius, 2018). Therefore, the mechanism of determinate nodule formation is somehow different from that of the indeterminate type. So far, much of our knowledge of the regulation of auxin for nodulation derives from the studies of indeterminate nodule development and the knowledge gleaned from lateral root organogenesis. In contrast, the functionality of auxin transport during determinate nodule development remains speculative (Kohlen et al., 2018). Therefore, a better understanding of the PIN-dependent auxin transport module for soybean nodule organogenesis is crucial for discerning the basic nodulation mechanisms and uncovering the different regulatory mechanisms between determinate and indeterminate nodule development.
In this study, we demonstrate that soybean nodule primordium formation involves GmPIN1-mediated PAT. Among all canonical PINs, the GmPIN1 orthologs, GmPIN1b, c, d, were specially expressed in nodule primordium cells during nodule primordium initiation, and their dynamic polarity indicates involvement in the directional auxin flux for nodule primordium formation. We found that the upstream nodulation elicitors, flavonoids, trigger the expanding distribution of stele-restricted GmPIN1b to cortical cells and cytokinin enable the rapid redirection of the auxin stream by rearranging the cellular GmPIN1b polarity. CRISPR/Cas9-based mutagenesis of GmPIN1a, GmPIN1b, and GmPIN1c failed to establish a focused auxin maximum in nodule meristem, resulting in aberrant cortical cell division. Additionally, we found that the soybean-evolved GmPIN9d accumulates strongly in the conjunctive vascular bundles between the root and nodule. In these tissues, GmPIN9d acts synergistically with GmPIN1 to coordinate the auxin supply within the vasculature for nodule enlargement. Our findings reveal the fundamental role of GmPIN-dependent PAT for determinate nodule development.
Results
Auxin accumulates in the nodule primordium and vascular bundles
To visualize the developmental process of soybean nodulation, 7-day-old soybean seedlings were inoculated with the rhizobium strain Bradyrhizobium sp. (BXYD3). The root segments below shoot–root junction area where large amounts of nodules accumulated (Supplemental Figure S1A) were collected in series for further sectioning, at 4 days post-rhizobia inoculation (dpi) until 14 dpi.
Based on cell morphology, we divided nodule development into four stages. At Stage I, rhizobial infection via the curled root hairs determined the position of future nodule primordia and subsequently resulted in cell proliferation in the outer cortex cells, corresponding to nodule primordium initiation. At Stage II, cortical cells underwent a series of anticlinal and periclinal divisions to establish a multi-layered meristem. In particular, the soybean nodule primordium started from the outer cortex layer and proceeded both outward and inward suggesting the presence of a dynamic regulator involved in modulating this dual-directional cell division. At Stage III, the developing nodule continued to produce new cells, and the protruding meristem contained a small amount of bacteroids and visible vascular tissues that connected the host root and nodule. At maturity (Stage IV), the oval nodule was rapidly expanding and became filled with a large amount of bacteroids, with complex vascular bundles enveloping the entire nodule (Figure 1A).
Figure 1.
NPA disrupts auxin distribution and nodule formation. A and B, The 7-day-old WT (A) or DR5V2:GUS transgenic soybean plants (B) were infected by BXYD3, and then incubated in a low-nitrogen hydroponic solution containing 10−7 M NPA or DMSO (as mock control). Root–shoot junctions were collected in series at 4–14 dpi for resin embedding and sectioning. The morphology of stage I to IV soybean nodules was examined by sectioning in the longitudinal direction (A). Red boxes label the primordium area, dotted lines label the vascular bundles, and black arrows highlight the nodule or nodule primordium (A). DR5V2 activity in different nodule stages was stained and captured by sectioning in longitudinal (L) or transverse (T) directions (B). St: root stele. Red dot lines label the sectioning areas in the transverse direction. Red arrows highlight the dividing cortical cells. Scale bars, 100 μm (A and B).
To track auxin distribution at different growth stages of soybean nodules, we generated a stable transgenic soybean line with the synthetic DR5V2:β-glucuronidase (GUS) auxin response reporter (Liao et al., 2015) that shows higher sensitivity to endogenous auxin responses in Arabidopsis than the original DR5:GUS reporter (Ulmasov et al., 1997). A robust auxin gradient with a peak concentration at the tip of the nodule primordium, suggesting an early auxin response, coincides with initial cell divisions of the nodule (Figure 1B, Stage I). When the outer cortex cells were rapidly dividing and expanding, we found an accumulation of DR5V2 activity with its maximum at the apex of the nodule primordium (Figure 1B, Stage II). Afterward, the tip-focused auxin maximum disappeared and a new auxin maximum gradually appeared within the surrounding cells near the root–nodule vascular connection (Figure 1B, Stage III). Within the rapidly expanding Stage IV nodule, auxin was concentrated throughout the nodule vascular bundles (Figure 1B).
The auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) directly elicits the generation of nodule-like structures (pseudonodules) in legume species with indeterminate nodules (Hirsch et al., 1989; Wu et al., 1996; Rightmyer and Long, 2011), but not in those forming determinate nodules (Ng and Mathesius, 2018). We investigated whether NPA influences soybean nodule formation. Therefore, we incubated the rhizobium-colonized DR5V2:GUS seedlings with NPA. NPA blocked auxin efflux and disrupted the auxin gradient, resulting in a strong ectopic DR5V2 activity around the cortical cells (Stage I), pericycle cells (Stage II), and vascular bundles (Stage III) (Figure 1B). A reduced auxin response in the central zone of Stage III primordium was observed (Figure 1B), suggesting a redirection of auxin transport occurs at this stage of nodule development. Interestingly, this was not seen in the NPA-treated plant nodules at the same stage. This ectopic NPA-driven auxin response activation was associated with the onset of rapid cell divisions, resulted in a three-fold increase in small nodules compared with the mock treatment (Figure 1A; Supplemental Figure S1, B–D). Additionally, NPA disrupted vascular development, leading to aberrant nodules and two infection zones within bacteroids (Figure 1A, Stage IV). Collectively, these results suggest that nodule primordium formation requires the establishment of an appropriate auxin gradient and the further nodule development of the nodule vascular bundles involves a dynamic redirection of the auxin transport.
Identification of nodule-expressed GmPINs
The best-characterized regulators of auxin transport are PIN efflux transporters (Petrasek et al., 2006). The Arabidopsis PIN family consists of eight members, divided into canonical and noncanonical types based on the length of their hydrophilic loop and subcellular localization (Viaene et al., 2013). The canonical, PM-localized PINs play a predominant role in determining the directionality of intercellular auxin flow (Adamowski and Friml, 2015). To better understand the regulatory mechanism of auxin transport for nodule development, we were motivated to study the functionality of the nodule-expressed canonical GmPIN ortholog. In soybean, the PIN family has extensively expanded because of the two whole-genome duplication events. A total of 23 GmPINs have been identified in the soybean genome, of which 7 pairs are duplicated genes, namely the GmPIN1, GmPIN2, GmPIN3, GmPIN5, GmPIN6, GmPIN8, and GmPIN9 subfamilies (WaNg et al., 2015a). According to the constructed phylogenetic tree, the canonical GmPIN members comprise GmPIN1 (GmPIN1a, b, c, d, e), GmPIN2 (GmPIN2a, b), and GmPIN3 (GmPIN3a, b, c, d), which showed the highest sequence similarity with Arabidopsis AtPIN1, AtPIN2, and AtPIN3, 4, 7, respectively (Figure 2A; Supplemental File S1). Additionally, the soybean genome contains a specific GmPIN9 group that has apparently co-evolved with GmPIN2 (Figure 2A; Supplemental Files S1 and S2).
Figure 2.
Expression pattern of GmPINs during nodule development. A, The phylogenetic tree of PIN proteins from Arabidopsis (At), Glycine max (Gm) and Klebsormidium flaccidum (Kf) was constructed using MEGA X. These PIN genes were grouped as canonical, noncanonical, and soybean-specific PIN types. B, The transcript level of 23 GmPIN genes was detected by RT-qPCR in nodules at 14 dpi (n≥3), Gmactin11 was the normalization gene. C, pGmPIN1a, b, c, d, e:GUS constructs were introduced in WT soybean plants by hairy root transformation, the positive roots were infected by BXYD3. Root segments were collected at 4–14 dpi. Root sectioning was performed for further histochemical staining. D, Cartoon model represents the spatial and temporal expression pattern (different colors) of GmPIN1s during nodule development. Scale bars, 100 μm (C). Error bar=sd.
To search for nodule-expressed GmPINs, we extracted RNA from nodules at 14 dpi to perform reverse transcription quantitative PCR (RT-qPCR). Among the 23 GmPINs, the canonical PIN orthologs, GmPIN1, GmPIN2, and GmPIN3 genes were detectable in the mature nodule. Additionally, GmPIN5a and GmPIN9d transcripts were strikingly abundant in the mature nodule, suggesting their possible involvement as well (Figure 2B). The hydrophilic loop of GmPIN9 is shorter than the canonical PINs but longer than that of the noncanonical type (Supplemental Figure S2A); hence, the functionality of GmPIN9 during nodule development deserved our further investigation.
GmPIN1, orthologs of auxin efflux carrier AtPIN1, control directional auxin flow for nodule primordium formation
We first examined the expression profiles of canonical GmPINs during nodule development, including GmPIN1, GmPIN2, and GmPIN3. We established pGmPIN1a-e:GUS, pGmPIN2a-b:GUS, and pGmPIN3a-d:GUS constructs, using a GUS reporter individually driven by an approximately 2-kb region upstream of the GmPIN coding sequence. We then introduced them into soybean seedlings via hairy root transformation. Histochemical staining of the root tip revealed that every GmPIN1 was expressed in the root stele, consistent with the expression pattern of AtPIN1 in Arabidopsis root (Supplemental Figure S3A).
Upon rhizobial infection, expression of the GmPIN1b, c, d reporters was detected in the nascent nodule primordia that formed in outer cortical cells (Figure 2C). After a series of cortical cell divisions, the primordium tip-distributed GmPIN1b, c, d gradually accumulated in the surrounding vascular bundles (Figure 2C). In Stage IV, the expression of GmPIN1b, c, d was confined to vascular bundles (Figure 2C). In contrast, GmPIN1a appeared after primordium emergence and its expression was exclusively associated with vascular bundle development, whereas GmPIN1e had an extremely weak signal in nodule primordia (Figure 2C). GmPIN1 expression was associated with the nodule primordium and the nodule vascular bundle (Figure 2D), consistent with the spatial pattern of auxin activity during nodule development. This suggested the possible involvement of GmPIN1 in nodulation.
Other GmPIN members, GmPIN2a and 2b were mainly expressed in the root epidermis and outer cortex cells, and GmPIN3a-d genes were expressed in root vascular cells (Supplemental Figure S3B). In contrast, both GmPIN2 and GmPIN3 mainly accumulated in nodule vascular bundles after the establishment of the nodule primordium; only GmPIN2a and GmPIN3a were detectable in the nodule primordium tip, albeit at extremely low levels (Supplemental Figure S3B). Our analysis of the spatiotemporal expression pattern of canonical GmPINs lent further support for a predominant role of GmPIN1 in nodule development.
The variation in the expression of multiple GmPIN1 orthologs allowed us to evaluate the functional conservation between GmPIN1 and AtPIN1. We established a GmPIN1–GFP fusion protein under the control of the AtPIN1 promoter. We cloned GFP in-frame in the hydrophilic loop of GmPIN1 (Figure 3A) at the comparable position, as in AtPIN1-GFP (Benkova et al., 2003; Wisniewska et al., 2006) and introduced them into the Arabidopsis pin1-En mutant. All the transgenic Arabidopsis pAtPIN1:GmPIN1a-e-GFP plants showed partial rescue of the pin-shaped inflorescence meristem phenotype of the homozygous pin1 mutant (Galweiler et al., 1998), in that they had a high percentage of normal growing stems with intact flowers and siliques (Figure 3C). In the root stele, protein localization of GmPIN1a-e was confined to the basal side of PM (Figure 3B), consistent with the subcellular polarity of AtPIN1. To further confirm the cellular polarity of GmPIN1a-e, we also generated transgenic Arabidopsis plants having GmPIN1a-e-GFP driven by their native GmPIN1a-e promoters. Similar to pAtPIN1:GmPIN1-GFP or in pGmPIN1:GmPIN1-GFP transgenic seedlings, GmPIN1a–e were exclusively confined to the basal PM in root stele cells (Supplemental Figure S3C), thus indicating the conservation of localization between GmPIN1 and AtPIN1 proteins.
Figure 3.
GmPIN1 localization is conserved with AtPIN1 and direct auxin flow during nodule primordium formation. A, Predicted topology of GmPIN1 proteins. The following GmPIN1–GFP constructs were established based on the insertion position of GFP in the hydrophilic loop. B and C, GmPIN1a-e distribution and polarity were visualized in roots of the transgenic Arabidopsis plants (pAtPIN1: GmPIN1-GFP in pin1-En Arabidopsis (At.) mutant background (B and C). The phenotypes of inflorescence and silique (inset boxes) were shown in C. Polar localization of GmPIN1 in the basal side of PM in root stele (inset boxes) is highlighted by white arrows (B). D, GmPIN1b localization was visualized in roots and different stages of nodules in the transgenic soybean plant pGmPIN1b:GmPIN1b-GFP. The polarity of GmPIN1b (with predominant polarity toward nodule apex) was highlighted by white arrows. Dot lines label the area of C1-derived cell layers or vascular bundles. Pictures with a bright field view were shown in the inset boxes of upper panels. The images in lower panels showed the enlarged views of boxed areas in the representative cartoon (1: undifferentiated vascular bundles; 2 and 3: differentiated vascular bundles). Yellow and blue dotted lines represent GmPIN1b signal on periclinal and anticlinal directions at Stage I. E, GmPIN1b localization was visualized in nodule primordium (Stage II) of NPA (10−7 M, 4 days)-treated pGmPIN1b:GmPIN1b-GFP seedlings White arrows label GmPIN1b polarity toward nodule apex. F and G, GmPIN1b signal toward the nodule apex (apical) or perpendicular to the nodule apex (lateral) was individually measured in F (n=136, 189, 89, 129, 136, 189, 89, 129). The comparison ratio of GmPIN1b signal in different nodule primordium stage relative with those in Stage I was shown as a percentage in (F). Polar index of GmPIN1b was quantified by calculation of GmPIN1b signal ratio of apical-targeted GmPIN1b signals relative to them in the lateral sides (G: n=90, 136, 189, 89, 129, 40, 102). Scale bars: 5 μm (B), 50 μm (B, inset box), 5 mm (C), 100 μm (D upper panel, E), 20 μm (D lower panel). Error bar=sd. In D-E: Ep, epidermis; C1, first cortical cell layer; C2, second cortical cell layer; Ed, endodermis; Pc, pericycle; St, stele; VB, vascular bundle. Root tip growth directions (g) were labeled by red arrows (D and E). Nodule primordium apex (apical-targeted GmPIN1b) was labeled by yellow arrows, and the lateral localization (perpendicular to the apical direction) of GmPIN1b was labeled by blue arrows (D and E). P-values were determined by a two-tailed Student’s t test assuming equal variances (***P < 0.001; ****P <0.0001; NS, not significant).
Based on this functional conservation and consistent polarization of the five GmPIN1 proteins, we next focused on GmPIN1b because it was detectable throughout all stages of nodule development, using it as a representative to study the dynamics of GmPIN1 polarity during nodule development. For this, we generated a stable transgenic soybean line transformed with pGmPIN1b:GmPIN1b-GFP. By comparison with wild-type (WT) plants, we were able to distinguish the GFP signal from the strong auto-fluorescence of soybean tissues under the same fluorescence microscopy settings (Supplemental Figure S4). We determined the degree of polar localization of GmPIN1b as the signal ratio of apical relative to the lateral side of individual cells (called the polarity index, p.i.) (Figure 3G). In the soybean root stele, GmPIN1b displayed basal polarity (p.i. = 2.6), consistent with its distribution in Arabidopsis (Figure 3, D and G). During the nodule primordium initiation (Stage I) with visible anticlinal cell divisions, GmPIN1b showed predominant polarization toward the future nodule meristem (p.i. = 3.2); while some of the cells exhibited dual polarization of GmPIN1b, with PM accumulation toward the imminently initiated nodule vascular bundle and toward the primary root tip (Figure 3, D and G). The predominant apical polarity of GmPIN1b toward the primordium apex suggests that auxin is being transported to nodule primordia, while the bi-polar localization of GmPIN1b implies that auxin is partly being directed away from the outer cortical cells.
In Stage II, GmPIN1b polarity was mainly directed to the protruding nodule primordium (p.i. = 2.2); while a proportion of primordia cells displayed the lateral polarity of GmPIN1b, which might result in reduced auxin response at the nodule primordium tip (Figure 3, D and G; Supplemental Figure S5, A). When the nodule primordium was fully established (Stage III), GmPIN1b polarized toward the primordium tip as well as in the lateral direction (p.i. = 1.7) (Figure 3, D and G; Supplemental Figure S5, A), suggesting that GmPIN1 established a re-allocation route for draining auxin away from the tip region. In the mature nodule (Stage IV), the GmPIN1b-GFP signal was restricted within vascular bundles (Figure 3, D and G), corresponding to the depletion of auxin at the primordium tip and its reallocation to the vascular bundles (Figure 1B).
We further examined whether GmPIN1b directs auxin flow toward the central cells of the nodule primordium or away to the peripheral cells by evaluation of apical or lateral GmPIN1b signal at Stages I, II, and III. Compared with the GmPIN1b signal in Stage I, the apical direction of the GmPIN1b signal was significantly reduced to 53% and 62% in Stages II and III + IV, while the lateral GmPIN1b signal was not promoted in the later stages (Figure 3F). The comparison of GmPIN1b polarization at different stages of nodule development further indicated that the re-allocation of auxin away from nodule primordium tip is caused by the reduction in apical polarization of GmPIN1.
In vascular bundles of mature nodules, GmPIN1b was highly polarized within the differentiated vasculature (p.i. = 21.0) but was nonpolar within the undifferentiated vasculature (p.i. = 1.0; Figure 3, D and G). The polarization of GmPIN1b within the mature vasculature of root–nodule junction suggested that auxin might be directionally transported via the nodule vasculature. Interestingly, GmPIN1b polarity was disrupted by NPA. NPA relocated the GmPIN1b polarity to the lateral side that was perpendicular to nodule primordium cells (p.i. = 1.4 in NPA-treated nodule primordia, compared with p.i. = 2.3 in the untreated ones) (Figure 3, E–G; Supplemental Figure S5, B). Consequently, NPA-rearranged GmPIN1 polarity resulted in ectopic formation of nodule primordia (Figures 1, A and 3, E). Taken together, the dynamic polarization of GmPIN1 in the nodule primordium is consistent with the presence of multiple cell proliferation sites during soybean determinate nodule development, which begins in the outer cortex and proceeds outwards as well as inwards.
GmPIN1s regulate nodule primordium formation
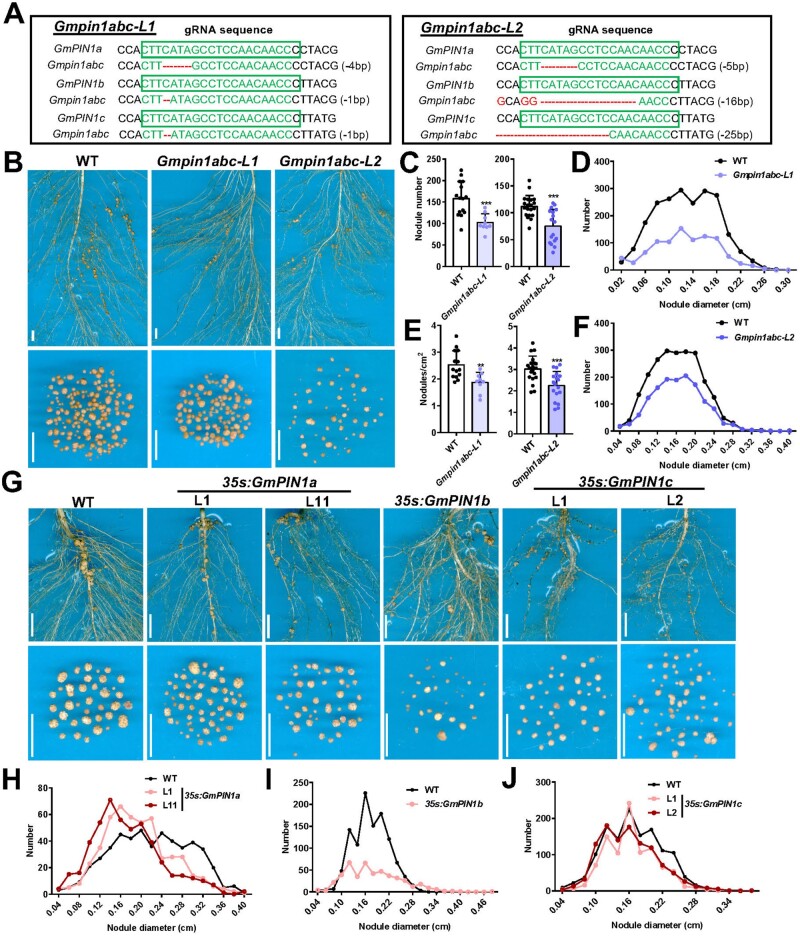
Soybean has evolved five PIN1 orthologues compared with the sole PIN1 identified in Arabidopsis, suggesting functional redundancy of GmPIN1 genes. Among them, GmPIN1a, b, and c showed the closest sequence similarity to AtPIN1 (Figure 2A), while GmPIN1d and e might be an ancestral PIN1 form (Kohlen et al., 2018). Using the CRISPR/Cas9 approach, we designed a sgRNA that simultaneously edits the first exon of GmPIN1a, b, and c, from which, we subsequently obtained two independent, triple mutants of Gmpin1abc (named Gmpin1abc-L1 and -L2) via Agrobacterium-mediated stable transformation in soybean (Figure 4A). These Gmpin1abc mutants displayed slightly shorter primary roots, fewer lateral roots, and had less biomass in their shoot tissues, compared with WT (Supplemental Figure S6, A-D).
Figure 4.
Nodulation phenotypes of Gmpin1abc triple mutant and GmPIN1 overexpression lines. A, Gmpin1abc triple mutants were generated via CRISPR-Cas9 gene-editing approach. The sequence of GmPIN1a, b, and c with the gRNA targeted sites was designed in the first exon of GmPIN1 genes. gRNA targeted sequences were framed by green, and the mutation or deletion sequences were labeled by red. Two independent triple mutant lines with edited genes are shown. B–J, The 7-day-old WT, Gmpin1abc mutants and 35s:GmPIN1a, b, and c lines were infected by BXYD3, and the nodulation phenotypes were analyzed at 14 dpi. Nodule number (per plant) and density (number per cm2) were quantified in (C) (n=15, 9, 20, 17) and (E) (n=15, 9, 20, 17). Profile of nodule diameter was tracked in (D (n=2,138, 945), (F) (n=2,236; 1,404), (H) (n=848, 930, 480), (I) (n=1,111, 471), (J) (n=1,318; 961; 1,096) (all from upper to lower groups). Scale bars: 1 cm (B and G). Error bar=sd. P-values were determined by a two-tailed Student’s t test assuming equal variances (**P < 0.01; ***P < 0.001).
To understand the contribution of GmPIN1 to auxin transport, we examined the auxin transport capacity in Gmpin1abc mutants. Without rhizobium inoculation, Gmpin1abc mutants’ acropetal auxin transport was reduced by 89%, compared with WT (Figure 5H). The decreased acropetal auxin transport in Gmpin1abc mutants resulted in strongly reduced auxin content (46%–53%) compared with WT, and reduced DR5V2:GUS activity (Supplemental Figure S6, E and F). Quantification of the total nodule number (per plant) and nodule density (nodule number per square centimeter of root area) both demonstrated that Gmpin1abc had fewer nodules than the WT (Figure 4, B, C, and E). Despite the reduction in nodulation events in Gmpin1abc mutants, their average nodule size was not significantly changed, as indicated by the diameter distribution profile of more than 1,000 nodules (Figure 4, D and F). The nodulation phenotype of the Gmpin1abc mutant indicates that GmPIN1 is involved in the regulation of nodule formation.
Figure 5.
Loss-of-function and gain-of-function of GmPIN1 disrupted auxin distribution and nodule primordium initiation. A–C and I, The 7-day-old WT, Gmpin1abc-L1 and 35s:GmPIN1a-L11 plants were infected by BXYD3 for additional 5 days of growth. Consistent 1-cm root segments were collected from lateral roots at the root–shoot junction area. Root segments were sectioned in a longitudinal direction and co-stained by Chameleon and Schiff’s fuchsin-sulfite reagents (A). Nodule primordium density and developing nodule density were individually quantified as profiles in B (n=106) and C (n=106). The overall nodule primordium density was analyzed in I (n=106). The middle panels of WT in A show 3× enlarged views of boxed areas in the images of left panels. D, E–G, and H, 0.5-cm root segments below root–shoot junction of 5-day-old WT, Gmpin1abc-L1 and Gmpin1abc-L2 were performed by 48 h continuous BXYD3 spot inoculation (equipped as the cartoon in D). Auxin content in the U and L root segments close to the inoculated position was measured in (G) (uninfected roots were used as controls) (n=5, 3, 3, 4, 4, 3, 5, 5, 4, 4, 4, 4). PAT efficiency was measured in the acropetal direction in H (n=12, 14, 10, 13, 12, 11; red arrow in (D) labeled PAT direction). The comparison ratio of auxin content in L relative with those in U is shown as a percentage in (G), and the comparison ratio of auxin transport capacity in infected groups with uninfected groups was shown as a percentage in (H). Nodule primordium morphology in WT and Gmpin1abc mutants was observed at 5 dpi after spot inoculation (E). Cartoons simulate the cell morphology of each genotype, and green color indicates the dividing cortical cells (E). The percentage in E indicates the proportion of roots with aberrant dividing cortical cells in the total counting roots. F, DR5-V2-GUS construct was introduced in WT, Gmpin1abc-L1 and 35s:GmPIN1a-L11 plants by hairy root transformation, and different stages of nodule were collected at 8 dpi for sectioning. Auxin distribution was visualized by histochemical GUS staining. Scale bars: 200 μm (A), 100 μm (E and F). Error bar=sd. P-values were determined by a two-tailed Student’s t test assuming equal variances (*P <0.05; **P < 0.01; ****P < 0.0001; NS, not significant).
We then generated stable transgenic plants that constitutively overexpressed GmPIN1a-c (35s:GmPIN1a-c). The 35s:GmPIN1a-c lines all exhibited curling roots and an unusual thickening of hypocotyls (Supplemental Figure S7, A and B), features similar but not entirely identical to agravitropic root growth seen in Arabidopsis 35s:AtPIN1 plants (Zhang et al., 2010). Further analysis of nodulation phenotypes showed that the induction of GmPIN1a, b, and c all significantly reduced nodule size (Figure 4, G–J). The retarded growth of 35s:GmPIN1 nodules could be due to the disrupted auxin maximum at nodule primordia, leading to delayed organogenesis, or due to an impaired auxin concentration within the enlarged nodule that impeded nodule expansion.
To understand the causal relationship of the defective nodulation phenotype in Gmpin1abc mutants and gain-of-function GmPIN1 lines, we examined the density of nodule primordia at 5 dpi, when most of the nodules had just emerged as primordia. We divided the nodules into two categories: primordia (without the resident bacteroids) and developing nodules (with bacteroids). Compared with WT, Gmpin1abc mutants had a significantly decreased density of nodule primordia but this did not influence their developing nodules (Figure 5, A–C), further supporting the involvement of GmPIN1 in nodule primordium formation. Strikingly, 35s:GmPIN1a has almost no detectable nodule primordia at 5 dpi (Figure 5, A–C and I).
To further understand the functionality of GmPIN1-mediated auxin transport during nodule primordium formation, we established a spot inoculation system in soybean (Figure 5D) to closely examine auxin transport efficiency and nodule primordium morphology within the spot inoculation area. To this end, 0.5-cm root segments of WT and Gmpin1abc mutants below the root–shoot junction were subjected to a continuous BXYD3 spot inoculation for 48 h (Figure 5D; Supplemental Figure S7C). Auxin concentration was individually measured in the above (upper, U) and below (lower, L) root segments close to the inoculated site. In WT, the rhizobial infection did not influence auxin levels in U root segments, but decreased the auxin content to 72% in the L segments, compared with the uninfected roots (Figure 5G). Auxin concentration was generally decreased in Gmpin1abc roots, compared with WT. In Gmpin1abc mutants, rhizobial inoculation did not change auxin level in the U root segments but caused auxin elevation in the L segments (247% and 196%) (Figure 5G).
The opposite responsiveness of WT and Gmpin1abc intrigued us to further examine auxin transport rates of inoculated root segments. Radiolabeled [3H]-IAA was applied to the U segments and the transported [3H]-IAA was measured in the L segments at 48-h post-inoculation (hpi). In WT or Gmpin1abc-L2 mutants, rhizobial inoculation did not significantly change the efficiency of acropetal auxin transport across the inoculated roots (Figure 5H). It is worth noting that rhizobial inoculation tended to decrease auxin transport in WT (92%), while it tended to increase auxin transport in Gmpin1abc-L1 and L2 mutants (121% and 111%), compared with the uninfected samples (Figure 5H). Reduction in auxin levels below the inoculation site supported the notion that auxin transport regulation happens before the formation of visible nodule primordial, whereas the minor change in acropetal auxin transport upon rhizobium infection suggested that nodule primordium initiation in the outer cortex is not sufficient to influence acropetal auxin transport within the primary root vasculature. The unexpected elevation of auxin level in L segments of Gmpin1abc suggests that a possible upregulation of auxin biosynthesis occurs within inoculated-Gmpin1abc roots.
We further examined nodule primordium morphology at 5 days after rhizobia spot-inoculation. The visible nodule primordium with a series of anticlinal and periclinal divisions were homogenously distributed in inoculated WT root segments, while clusters of aberrantly dividing cortical cells and nodule primordia were discretely distributed in Gmpin1abc mutants (Figure 5E), compared with the normal cortical cells in the uninfected roots (Supplemental Figure S7D). We then introduced DR5V2:GUS in WT, Gmpin1abc mutants, and 35s:GmPIN1a to visualize the auxin distribution. A robust auxin gradient with a peak concentration was present at the tip of WT nodule primordium (Stage I) (Figure 5F). Despite the DR5V2 signal remaining clear in the nodule primordium of Gmpin1abc mutant, the mutant failed to establish a focused auxin gradient in the primordium apex (Stage I) (Figure 5F). The defective auxin maximum in Gmpin1abc resulted in the ectopic formation of nodule primordia, leading to the clustered spacing of nodule primordia. In contrast, overexpression of GmPIN1a in 35s:GmPIN1a roots resulted in ectopic auxin deposition in the cells beneath the primordium tip, which interfered with the directional auxin transport that would guide nodule primordia to grow outward. Consequently, 35s:GmPIN1a exhibited a delay in nodule organogenesis (Figure 5, A and F). These results collectively demonstrate that a GmPIN1-mediated auxin gradient regulates nodule primordium formation.
Flavonoids and cytokinin influence PIN1 distribution and polar localization during nodulation
An auxin maximum and GmPIN1b, c, d expression emerged in nodule primordium founder cells, suggesting the involvement of auxin transport in the initial step of nodule formation. However, GmPIN1a, b, c expression was exclusively restricted within the root vasculature (Supplemental Figure S3A), which pointed to an unknown nodulation signal eliciting the lateralization of GmPIN1 to prime the auxin signal at these founder cells.
Flavonoids are crucial signaling molecules that can trigger the synthesis of Nod factors from rhizobia, ensuring successful symbiotic infection of host plant tissues (Liu and Murray, 2016). A variety of flavonoid compounds have been characterized, and most of them act as inhibitors of auxin transport (Brown et al., 2001; Santelia et al., 2008; Ng et al., 2015) by interfering with PIN expression and its polar localization (Peer et al., 2004; Santelia et al., 2008; Kuhn et al., 2017). According to the multiple steps of signal exchange during nodule formation, different flavonoids may play distinct roles across individual steps of nodulation (Zhang et al., 2009). The isoflavone compound genistein is a well-characterized Nod-factor inducer in soybean (Ip et al., 2001; Subramanian et al., 2006; Lang et al., 2008). We thus checked the effect of genistein on the expression pattern of GmPIN1b, serving as a representative GmPIN1. Soybean plants with pGmPIN1b:GUS showed an expanded distribution of GmPIN1b into their cortical cells by genistein treatment (Supplemental Figure S8, A and C). The flavonoid 7,4′-dihydroxyflavone (DHF) is a Nod gene-inducing flavone compound in M. truncatula, known to function very early during nodulation (Zhang et al., 2009). Application of exogenous DHF to soybean pGmPIN1b:GUS trans-genetic plants also showed an expanded distribution of GmPIN1b into the cortical cells (Supplemental Figure S8, A and C), consistent with the genistein treatment. These results indicated that the isoflavone genistein and flavonoid DHF affect GmPIN1 distribution. Corresponding to the expanded distribution of GmPIN1b, genistein and DHF treatment resulted in a significantly increased DR5V2:GUS signal along the outer cortex and epidermal cells, but a decrease in the quiescent center of the root tip (Supplemental Figure S8, B and D). These data collectively demonstrate that the isoflavone/flavonoid influences GmPIN1 distribution and auxin accumulation, and probably leading to the alteration of auxin transport during nodulation.
Appropriate integration of various phytohormones is essential for determining when and where organogenesis occurs, such as the coordination between auxin and cytokinin (Schaller et al., 2015; Pierre-Jerome et al., 2018; Kurepa et al., 2019). Cytokinin plays a major role in the control of cortical cell divisions during nodule primordium formation (Murray et al., 2007; Reid et al., 2017), which consequently promotes nodule numbers (Supplemental Figure S9, A and B). To understand whether cytokinin functions upstream of auxin, we applied a synthetic cytokinin, 6-benzylaminopurine (6-BA), onto pGmPIN1b:GmPIN1b-GFP. Visualization of GmPIN1b localization revealed that 6-BA rearranged the cellular GmPIN1b polarity that predominantly targeted to primordium tip, resulting in a switch of GmPIN1b polarity to the lateral side that was perpendicular to the primordium cells (Figure 6, A and B). Cytokinin-mediated directional alteration of PIN polarity during nodule meristem formation was consistent with its effect on lateral root initiation (Marhavy et al., 2014). Interestingly, a directional alteration of GmPIN1 polarity by 6-BA treatment resulted in a series of proliferation in the outer cortical cells of WT plants, comparable with the cell morphology in spot inoculated-Gmpin1abc mutants (Figures 5, G and 6, C). Hence, these findings suggested that the cytokinin-regulated re-polarization action of GmPIN1 might facilitate a rapid auxin stream redirection, which provides an efficient approach to initiate nodule primordium. Taken together, the influence of flavonoid and cytokinin on GmPIN1 cellular localization might coordinate an appropriate auxin gradient for nodule organogenesis.
Figure 6.
Cytokinin rearranges GmPIN1b polarity. A and B, Nodule primordium of BXYD3-infected pGmPIN1b:GmPIN1b-GFP soybean plants were analyzed at 8 dpi (with or without 1 μM 6-BA treatment for 16 h). GmPIN1b-GFP signal in the apical (yellow arrows) and lateral (blue arrows) directions of nodule primordium was calculated and shown as lateral/apical ratio in (B) (n=126 from 22 sections, 152 from 36 sections), and the distribution frequency of GmPIN1b signal ratio was shown in the right chart (B, gray dot line marked the cutoff value). The cells with GmPIN1b signal ratio above the cutoff value was highlighted by yellow stars (A). Root tip growth directions (g) were labeled by red arrows (A). C, The 7-day-old WT plants were infected by BXYD3 and then they were irrigated with a low-nitrogen hydroponic solution containing 0.1 μM 6-BA or DMSO (as mock control) for additional 7 days. Cortical root cell morphology was observed by root sectioning. Numbers in the right panels showed 4× enlarged views framed in the left image. The percentage indicates the proportion of roots with a cluster of dividing cortical cells in the total counting roots. Scale bars: 100 μm (A), 500 μm (C). Error bar=sd. P-values were determined by a two-tailed Student’s t test assuming equal variances (****P < 0.0001).
GmPIN9d is a nodule vascular bundle-abundant auxin transporter
The above results confirmed the important role of GmPIN1 during nodule primordium formation. Apart from the typical canonical and noncanonical GmPINs, there was an additional GmPIN9 subfamily conspicuously present in soybean that showed a very close evolutionary relationship with the PIN2 clade (Figure 2A). Our RT-qPCR analysis of GmPIN9a-d expression levels showed that GmPIN9d was extremely enriched in the mature nodule (Figure 7C). In contrast, the transcripts of GmPIN9a, GmPIN9b, GmPIN9c were rather low or not detectable (Figure 7C), making GmPIN9d the most likely candidate involved in nodulation.
Figure 7.
GmPIN9d is specifically expressed in the protoxylem cells of vascular bundles and root–nodule junctions. A, Different tissues of the 10-day-old pGmPIN9d:GUS transgenic soybean plants were in series collected for sectioning and histochemical staining. Xylem cells were indicated by phloroglucin co-staining. Numbers in the right and lower panels indicate the areas labeled in the left image. Pictures in the lower panels showed 3× enlarged views of the images in the upper panels. B, pGmPIN9d:GUS transgenic soybean plants were infected by BXYD3, roots and different stages of nodules were collected for sectioning and histochemical staining. C, Transcript levels of GmPIN9a, b, c, and d genes were detected in different soybean tissues by RT-qPCR, and Gmactin11 was used as the normalization gene. The relative transcript levels are shown by heatmap. Scale bars: 100 μm (A) and 200 μm (B).
We next generated a stable transgenic soybean plant with a GUS reporter gene driven by the 2-kb promoter region of GmPIN9d. A series of cross-sections spanning leaf to root tissues, integrated with histochemical staining, from 10-day-old pGmPIN9d:GUS seedlings demonstrated that GmPIN9d was weakly expressed in aerial tissues but enriched in both root and nodule. Either in the hypocotyl or root organs, GmPIN9d was confined within the vascular bundles (Figure 7, A and B). Histochemical co-staining of xylem cells using phloroglucinol confirmed that GmPIN9d was located in protoxylem cells (Figure 7A). Intriguingly, GmPIN9d was particularly abundant in the conjunctive vascular bundles lying between the root and nodule (Figure 7B), which suggested a potential role of GmPIN9d in the later stage of nodule growth when primordia were already established.
Because GmPIN9d is a soybean-evolved PIN, we tested if GmPIN9d is indeed a functional auxin transporter. To do this, we cloned GmPIN9d-GFP under control of the Arabidopsis PIN2 promoter (pAtPIN2:GmPIN9d-GFP) and introduced it in the Arabidopsis PIN2 knockout mutant, eir1-4 that showed agravitropic roots (Muller et al., 1998). Compared with pAtPIN2:GmPIN2b-GFP which entirely rescued the agravitropic root phenotype of eir1-4 mutant, pAtPIN2:GmPIN9d-GFP displayed a partial restoration (Supplemental Figure S11, A–C), suggesting that GmPIN9d might carry out a comparable function as PIN2 protein for PAT. Importantly, much of the GmPIN9d-GFP localized to the internal structures reminiscent of the endoplasmic reticulum (ER) (Supplemental Figure S11B), suggesting that it either acts at the ER, or that its trafficking to the membrane is impaired in Arabidopsis.
We further generated Gmpin9d mutants using CRISPR/Cas9. Two homozygote mutant alleles were identified, designated Gmpin9d-#1 and 9d-#2, with an early stop codon that arose from a 2-bp and 40-bp deletion, respectively (Figure 8A). The 7-day-old Gmpin9d mutants had slightly decreased the length and density of lateral roots but did not display other obvious developmental defects in adult plants (Figure 8, B; Supplemental Figure S10, A–C). Next, the auxin response reporter DR5V2:GUS was introduced to hairy roots of the WT and the Gmpin9d mutant. The DR5V2:GUS activity was much reduced in both the roots and nodules of Gmpin9d mutant (Figure 8C), supporting the involvement of GmPIN9d in auxin homeostasis.
Figure 8.
GmPIN9d transports auxin in an acropetal direction. A, Gmpin9d mutants were generated via CRISPR-Cas9 gene editing. gRNA targeted sites of GmPIN9d are framed in green, and the mutation or deletion sequences are labeled in red. Two independent mutant lines with editing manner were shown. B, Global morphology of 21 days old Gmpin9d mutants. C, DR5-V2-GUS construct was introduced in WT, Gmpin9d#2 mutant background by hairy root transformation, and the positive hairy roots were infected by BXYD3. Roots and nodules were collected at 14 dpi for sectioning and histochemical staining. D–F, Auxin transport capacity of WT and Gmpin9d mutants was assayed in 2 cm root segments as described in (D), and acropetal and basipetal auxin transportations were tracked by [3H]-IAA (n=9, 12, 6 in (E); n=11, 10, 13 in (F)). G and H, Free IAA level was individually measured in the roots and nodules of WT and Gmpin9d mutants ((G): n=12, 15, 11; (H): n=15, 15, 15). I–K, The 7-day-old WT and Gmpin9d mutants were infected by BXYD3. The nodule primordium density was analyzed at 6 dpi ((I): n=121, 137, 129), the nodule density was measured at 14 dpi ((J): n=8, 8, 7), and the profile of nodule diameter was tracked ((K): n=560, 536, 473). Scale bars: 10 cm (B), 100 μm (C). Error bar=sem in (E–H), sd in (I and J). P-values were determined by a two-tailed Student’s t test assuming equal variances (*P <0.05; **P <0.01; NS, not significant).
The GmPIN9 clade is located between canonical and noncanonical PIN types, according to our phylogenetic analysis (Figure 2A). This raised a problem: might GmPIN9d polarly transport auxin as a canonical PIN, or does it regulate auxin homeostasis as a noncanonical PIN? To address these questions, we measured free and conjugated auxin levels in both the WT and Gmpin9d mutants, using High-Performance Liquid Chromatography (HPLC). In comparison with WT, the free auxin level decreased by ∼50% in roots and 20% in nodules of Gmpin9d mutants (Figure 8, G and H), results consistent with the previous observation of DR5V2 activity (Figure 8C). However, the conjugated form of auxin, indole-3-acetyl-aspartate (IAA-asp), in Gmpin9d mutants occurred at a level comparable with WT (Supplemental Figure S10, D and E), supporting a transporter function of GmPIN9d. Finally, using radiolabeled [3H]-IAA, we measured the auxin transport capacity of GmPIN9d in 2-cm equivalent root segments of the WT and Gmpin9d mutants. Both Gmpin9d-#1 and #2 mutants underwent a significant decrease in auxin transport in the rootward (acropetal) but not shootward (basipetal) direction, in comparison with WT (Figure 8, D–F). Altogether, these results show that GmPIN9d can transport auxin to the root tip.
GmPIN9d-dependent auxin transport is required for nodule enlargement
Although the free auxin level was diminished by ∼50% in roots of Gmpin9d compared with WT, the density of nodules and nodule primordia were not significantly changed (Figure 8, I and J). However, the profile of nodule size in Gmpin9d was slightly shifted compared with WT (Figure 8K), suggesting the involvement of GmPIN9d for nodule expansion. Still, the phenotypic defects of Gmpin9d single mutants were mild might be caused by complicated gene redundancy of the PIN family. The acropetal auxin transport capacity of GmPIN9d was reminiscent of GmPIN1, prompting us to speculate that GmPIN9d might work synergistically with GmPIN1 to achieve PAT within nodule vascular bundles.
We then examined the subcellular localization of GmPIN9d, via visualization of the Arabidopsis transgenic pAtPIN2:GmPIN9d-GFP seedlings. GmPIN9d showed dual localization in both ER and PM by colocalization of GmPIN9d-GFP with FM4-64-stained PM and HDEL-labeled ER (Supplemental Figure S11, D and E). PM-associated PIN proteins are located on the PM, meanwhile, by trafficking among the endomembrane system via endocytosis and exocytosis pathways (Chen et al., 2011; Adamowski and Friml, 2015).
To further investigate whether GmPIN9d is associated with the PM, we applied Brefeldin A (BFA), which is widely used as a vesicle trafficking inhibitor (Geldner et al., 2003). BFA specifically blocks exocytosis but allows endocytosis, aggregating both secretory and endocytic endomembrane cargoes in BFA bodies. BFA treatment specifically aggregated the PM-localized GmPIN2b, but not the ER-resident AtPIN8 in BFA bodies (Supplemental Figure S11F). We found that BFA also resulted in the aggregation of GmPIN9d proteins within BFA compartments (Supplemental Figure S11F). These data in Arabidopsis clearly evinced a dual localization of GmPIN9d in both PM and ER, similar to AtPIN6.
We further detected the in vivo distribution of GmPIN9d in soybean roots and nodules, by placing the GmPIN9d–GFP fusion protein under the control of the native GmPIN9d promoter (Figure 9A). The transformed positive hairy roots were selected based on a dTomato fluorescence-based screening system (Supplemental Figure S12A). In pGmPIN9d:GmPIN9d-GFP positive hairy roots, a strong GFP signal was present in the root protoxylem, with rootward PM localization (Figure 9B). In particular, this GmPIN9d–GFP signal was associated with vascular bundles within the nodule and root–nodule conjunction. The strong polarization of GmPIN9d within root–nodule conjunctive vasculature suggested its possible involvement in directing auxin stream toward nodule (Figure 9B; Supplemental Figure S11G). Such a GmPIN9d-polarized pattern resembles GmPIN1 at a later stage of nodule development, pointing to their synergistic activity. Hence, we introduced the Gmpin9d-RNAi construct in Gmpin1abc triple mutants via the dTomato-based hairy root screening system. The resulting Gmpin1abc Gmpin9d-RNAi showed a comparable nodule density but a much smaller nodule size than Gmpin1abc (Figure 9, C–E; Supplemental Figure S12, B–D). Taken together, the above results demonstrated that GmPIN9d-dependent PAT functions at the later stage of nodule development, collaborating with GmPIN1 to fine-tune nodule enlargement.
Figure 9.
GmPIN9d works synergistically with GmPIN1 to coordinate nodule enlargement. A, GmPIN9d-GFP construct was established based on the insertion position of GFP in the hydrophilic loop. B, pGmPIN9d:GmPIN9d-GFP construct was introduced in WT soybean plant by hairy root transformation, and the positive roots were infected by BXYD3. Roots and nodules were collected at 14 dpi for sectioning, and GmPIN9d-GFP signal was visualized. Cells with polar GmPIN9d are highlighted by white arrows. The numbered images show 5× enlarged views (red boxes) in Supplemental Figure S11, G images. Root tip growth directions are labeled by red arrows, and directions of nodule apex were indicated by yellow arrows. VB, vascular bundle. C–E, WT and Gmpin1abc mutants were transformed by Gmpin9d-RNAi construct (with dTomato-tag). The positive hairy roots were selected based on fluorescence and further infected by BXYD3, and the profile of nodule diameter was tracked in D (n=613, 618, 624) at 14 dpi. The expression level of GmPIN9d was detected by RT-qPCR (E, n=4), Gmactin11 was used as the normalization gene (E). F, Model for PAT in nodule primordium and developing nodule. Stage I, GmPIN1 mainly directs auxin flow to the outer cortical cells, and partial auxin flows away from outer cortical cells. Stages II and III: GmPIN1-dependent auxin stream flows toward the nodule primordium, and some auxin is retrieved to the neighboring cells. Stage IV: auxin stream is transported within vascular bundles toward nodule via GmPIN1 and GmPIN9d-dependent PAT. Auxin accumulation is depicted in green, auxin gradient is indicated in light green, and the presumptive routes of auxin flow are depicted by arrows (red arrows: GmPIN1-mediation; purple arrows: GmPIN9d-mediation). Flavonoid and cytokinin act as the elicitors to mediate rapid auxin stream redirection. Scale bars: 100 μm (B), 1 cm (C). Error bar=sd.
Discussion
Legume plants interact with rhizobia to generate nodule organs, representing a way by which legume species coordinate the mutually beneficial exchange of signals and nutrients with their symbiotic partners. Previous studies have characterized well the crucial roles of Nod factor recognition, nodule inception (NIN) transcription factor, and phytohormone cytokinin in nodule formation (Ng et al., 2015; Schiessl et al., 2019; Bozsoki et al., 2020; Laffont et al., 2020). Here, we showed that dynamic GmPIN1 polarization establishes auxin canalization during nodule primordium development; flavonoids influence GmPIN1 distribution and cytokinin rearranges the cellular GmPIN1 polarity, participating in auxin gradient formation. The later stage of nodule enlargement involves the collaboration of GmPIN9d and GmPIN1-dependent auxin transport within nodule vasculature (Figure 9F). Our data thus provide insights into auxin-regulated nodule development in legume plants.
Establishment of an auxin maximum is required for determinate nodule primordium formation
Auxin accumulation appears in the early stage during nodule primordium formation, when the rhizobia-elicited infection threads start to grow in the proliferating cortical cells of L. japonicus (Suzaki et al., 2012), suggesting that nodule primordium formation is tightly accompanied by an auxin response. In soybean, overexpression of a nodule-expressed auxin biosynthesis enzyme GmYUC2a increases auxin content by approximately two-fold, but severely delays nodule formation (Wang et al., 2019). These data suggest that maintenance of appropriate auxin concentration and distribution is a prerequisite for determinate nodule primordium formation. If auxin transport is not required for determinate nodule primordium formation, there should be a burst of local auxin biosynthesis at the nodule primordium site. YUCCA flavin monooxygenases are the major enzymes catalyzing IAA synthesis (Mashiguchi et al., 2011; Stepanova et al., 2011; Zhao, 2018). In soybean, GmYUC2a is the most abundant nodule-expressed YUC among all GmYUCs (Wang et al., 2019); however, GmYUC2a displays uniformly high activity in all participating cell layers during nodulation, including the root hairs, proliferating cortex cells, and nitrogen fixation zone (Wang et al., 2019). It is unlikely that a localized auxin maximum, restricted to nodule primordium founder cells, can be established solely relying on GmYUC2a. PIN-mediated PAT has been well characterized and deemed necessary for almost all organogenesis processes in plants. This raises the hypothesis that PIN-mediated auxin transport could also contribute to the formation of determinate nodules.
Subramanian and colleagues mentioned that no measurable inhibition of auxin transport occurred at the site of rhizobial inoculation where flavonoids/isoflavonoids accumulate in soybean root, and they concluded that the effects of flavonoids on auxin redistribution is not required for soybean nodulation (Subramanian et al., 2007). Our study confirmed that acropetal auxin transport is not significantly affected in the rhizobia-inoculated root segments (Figure 5H). Different kinds of flavonoids are synthesized at distinct stages of nodule formation (Mathesius et al., 1998), and the fluorescent flavonoids were visualized in different cell layers of distinct legumes. For instance, flavonoids were found in the inner cortical cells of pea (Pisum sativum) and alfalfa (Medicago sativa), but they were found in the outer cortical cells of siratro (Macroptilium atropurpureum) (Mathesius et al., 1998). These data suggest that different flavonoids play diverse roles during different stages of nodule formation and in different species of legume plants.
Flavonoids have a general inhibitory effect on auxin transport (Buer et al., 2013; Ng et al., 2015; Zhang et al., 2021), and acropetal auxin transport was disrupted during indeterminate nodule formation where high amounts of flavonoids were synthesized. However, soybean nodules initiate in the cells of the outer cortex, and flavonoid compounds are probably also synthesized at the outer cortex of soybean roots, lying far from the phloem. This could explain why acropetal auxin transport is not affected during nodule primordium formation in soybean. To understand whether flavonoid-mediated auxin inhibition is a necessary step for nodule primordium initiation in soybean, it is necessary to closely examine GmPIN1 distribution and auxin gradient in weak alleles of isoflavone synthase (IFS) or chalcone synthase (CHS) mutagenized soybean plants within nodule primordium cells. Despite flavonoids being the best candidates to prime the auxin signal, an obvious question remains unresolved. Soybean nodules are initiated at the outer cortex cells, while L. japonicus nodules are initiated at the inner cortex cells and the M. truncatula nodule begins from the pericycle, endodermis, and cortex cells (Gauthier-Coles et al., 2018). So, how does the deposition of flavonoids in different cell layers in different kinds of legume plants modulate PINs and auxin transport?
In the present study, we demonstrate that a PIN-dependent auxin transport module participates in soybean nodule primordium formation. GmPIN1b, c, d target to the founder cells of nodule primordia, reallocating the auxin stream toward the nodule primordium and flows away from the primordium. A proposed mathematic model postulates that nodule formation is associated with the reduction in PIN function and an increase in auxin concentration in all root cell layers (Xiao et al., 2014). Nevertheless, this auxin-involved nodulation module is based on specific auxin accumulation from the inner to outer cell layers; hence, the proliferation of cortex cells should start from the interior layers and proceed outwards (Xiao et al., 2014). Yet, the determinate nodule exhibits dual directions of cell proliferation, in that it begins from cortex cells and proceeds outwards as well as inwards. Therefore, only the initial steps of nodule primordium development (proceed outward) fit with the proposed model (Xiao et al., 2014).
NPA disrupts auxin gradient formation during nodule primordium formation. In Stage I, auxin accumulates with its maximum at the nodule primordium apex, compared with a bulk deposition of auxin along with the dividing cortical cells in NPA-treated group; in Stages II and III, auxin response gradually reduces from the nodule primordium apex, compared with an ectopic auxin accumulation in and around the dividing cortical cells (Figure 1B). Together, the three-fold increase in small nodules in NPA-treated roots indicates that disruption of auxin efflux by NPA causes ectopic nodule primordium formation. Similar to the NPA treatment, loss of GmPIN1ABC impairs PAT, generating clusters of abnormal dividing cells and defective nodule primordia after continuous rhizobial inoculation (Figure 5E). Hence, the establishment of an appropriate auxin gradient is a prerequisite to control nodule primordia positioning. Whereas, nodule number was promoted in NPA-treated roots, which was opposite with the consequence of decreased nodules in Gmpin1abc mutants. NPA directly associates with PINs, and it inhibits PINs activity by stabilizing PIN1, PIN2, PIN3, PIN4, and PIN7 homo‐ and heteromers in Arabidopsis (Abas et al., 2021; Teale et al., 2021). Apparently, NPA not only inhibits GmPIN1 activity but also functions on other GmPINs. Hence, NPA-induced nodules might be caused by the additive effects of NPA on all GmPIN proteins.
Auxin supply is involved in nodule enlargement
Despite acropetal auxin transport capacity and auxin concentration being severely decreased in the root and nodule of the Gmpin9d single mutant, it did not display significant defects in nodulation. A plausible explanation for this is that GmPIN9d functions at later stages of nodulation where it is highly redundant with other PINs, including GmPIN1, GmPIN2, and GmPIN3. Mutagenesis of multiple GmPINs recently became possible via CRISPR/Cas9. After nodule primordia are formed, auxin maxima are rapidly concentrated within nodule vascular bundles, suggesting an additive effect of other auxin transporters for auxin canalization between the primordium and vascular bundles. Therefore, the nodule-expressed GmPIN2 and GmPIN3 might also participate in later stages of nodule development. The fundamental effect of auxin has been well known to stimulate cell expansion (Du et al., 2020). Hence, auxin stream in vascular bundles could be a source of auxin to maintain auxin supply within the developing nodule, modulating nodule enlargement.
Materials and methods
Plant growth and phenotype analysis
Soybean (Glycine max) cultivar Williams 82 and Huachun6, and A. thaliana Columbia-0 were used as WT. Seeds of A. thaliana were sown on 0.8% agar containing 1/2 Murashige and Skoog (MS) media at 22°C under 16-h light/8-h dark photoperiod. Soybean seeds were germinated in vermiculite. To analyze nodulation phenotype, 7-day-old plants were inoculated with Bradyrhizobium sp. (BXYD3) which was re-suspended in low-nitrogen buffer (530 µM N, including KNO3, NH4NO3, Ca(NO3)2•4H2O, and (NH4)2SO4(15:4:12:3), and 1.2 mM CaCl2, 1.05 mM K2SO4, 0.5 mM MgSO4•7H2O, 25 µM MgCl2, 2.5 µM NaB4O7•10H2O, 0.5 µM MnSO4•H2O, 1.5 µM ZnSO4•7H2O, 0.5 µM CuSO4•5H2O, 0.15 µM (NH4)6Mo7O24•4H2O, 40 µM Fe-Na-EDTA, 250 µM KH2PO4) to Optial Density (OD)600 = 0.15 for 2 h, then these inoculated plants were transferred to soil (vermiculite and irrigated with low-nitrogen buffer) for an additional 14 days (25°C, 14-h light/10-h dark photoperiod). Light growth condition for soybean plants is white light added with blue and red LEDs, the light intensity is 13,000 LUX (measured by an HR-350 Light Meter; Hipoint). The resultant plants were collected for nodule number and density quantification by ImageJ.
For NPA treatment, soybean seeds were germinated in vermiculite. The 7-day-old plants were inoculated with BXYD3 (OD600 ≧ 1.0) that was re-suspended in a low-nitrogen buffer for 2 h, then the inoculated plants were transferred to the low-nitrogen hydroponic solution with or without NPA (10−7 M) for additional 21 days. Nodules were harvested and analyzed.
For DHF, genistein, or 6-BA chemical treatments, DHF and genistein were dissolved in DMSO, and 6-BA was dissolved in NaOH as stock solutions. The 7-day-old seedlings were irrigated by a chemical-containing low-nitrogen solution.
RNA extraction and RT-qPCR analysis
RNA was extracted using the TransZol Up Plus RNA kit (TransGen Biotech), and the first-strand cDNA was synthesized using TransScript All-in-One SuperMix (TransGen Biotech). qRT-PCR was performed using a Bio-Rad CFX96 real-time system (Bio-Rad) with 20 µL volumes containing 2 µL of 1:5 diluted reverse transcription product, 0.8 µL of specific primers, 7.2 µL of ddH2O, and 10 µL of 2×TransStart Green qPCR SuperMix (TransGen Biotech). The housekeeping gene GmActin-11 was used as a reference and relative expression levels of each gene were calculated using the 2−ΔΔCT method. All primers used in this study are listed in Supplemental Table S1.
Phylogenetics and motif analysis
Arabidopsis thaliana PIN genes were obtained from TAIR (http://www.arabidopsis.org). Soybean PIN genes were obtained from Phytozome (https://phytozome.jgi.doe.gov). Klebsormidium flaccidum PIN (KfPIN) was obtained from Genbank (https://www.ncbi.nlm.nih.gov). PIN genes were translated into protein sequences and aligned with Clustal X (Larkin et al., 2007). Phylogenetic analysis was constructed using MEGA X with neighbor-joining (NJ) criteria and verified using the maximum likelihood (ML) method (Kumar et al., 2018), and 1,000 bootstrap replicates were performed based on the multiple alignments of the protein sequences encoded by PIN genes. NJ analysis was performed using the protein Poisson distances and the pairwise deletion of gap sites. The default parameters were used for ML analysis. Moreover, Phylogenetics conserved motifs were detected in PIN members using the motif analysis tool Multiple Em for Motif Elicitation (MEME) (http://meme-suite.org/tools/meme) (Bailey et al., 2009) with the default parameters except for a maximum number of motifs, 25. The PIN sequence alignment is provided as Supplemental File S1 and a machine-readable alignment is provided as Supplemental File S2.
Used primers, vectors, and cloning strategy
Promoter fragments of GmPIN9d (2,003 bp), GmPIN1a (2,132 bp), GmPIN1b (2,043 bp), GmPIN1c (1,917 bp), GmPIN1d (1,809 bp), and GmPIN1e (1,476 bp) were amplified from Williams 82 genomic DNA. The fragments were cloned into pDONR221 using Gateway BP Clonase II enzyme mix and recombined into the pGWB633 Gateway destination vector by LR reaction to create pGmPIN:GUS (primers are provided in Supplemental Table S1). To create pAtPIN1:GmPIN1a-e-GFP, pGmPIN1a-e:GmPIN1a-e-GFP, pGmPIN9d:GmPIN9d-GFP, pAtPIN2:GmPIN2b-GFP, and pAtPIN2:GmPIN9d-GFP, the insertion site of GFP was described in previous studies (Benkova et al., 2003; Xu and Scheres, 2005; Wisniewska et al., 2006). GFP was inserted into the genomic fragments at position 1,646 (after ATG) in GmPIN9d, at position 1,680 in GmPIN2b, at position 1,396 in GmPIN1a, at position 1,547 in GmPIN1b, at position 1,407 in GmPIN1c, at position 1,914 in GmPIN1d, and at position 1,904 in GmPIN1e. Genomic DNA of GmPINs and GFP were cloned into pBGWK by infusion clone technology.
To generate Gmpin1abc triple mutant and GmPIN9d single mutant, sgRNA was designed according to the prediction from CRISPR-GE (http://skl.scau.edu.cn/home/). sgRNA cloning approach was performed as described previously (Bai et al., 2020). To generate the constructs with modified dTomato-tag, dTomato fragment was amplified using the primers of pGmEF1A-dTomato F2 and R2, which was driven by the promoter of pGmEF1a; Tnos fragment was amplified using the primers of pGmEF1A-dTomato F3 and R3. Then, pGmEF1a, dTomato, and Tnos were assembled by Goldengate assembly kit using BsaI and T4 ligase. Finally, pGmEF1a-dTomato fragments were assembled with other fragments to create destination vectors via Goldengate cloning methods. All strategies of vectors and cloning used in this study are listed in Supplemental Table S2. Locus numbers of GmPIN genes are listed in Supplemental Table S3. Amino acid information of GmPIN proteins is listed in Supplemental Table S4.
Rhizogenes-mediated hairy root transformation
Rhizogenes-mediated hairy root transformation was carried out as described before (Kereszt et al., 2007). Agrobacterium strain K599 carrying the constructs was directly injected into the hypocotyl proximal to the cotyledon of the 5-day-old healthy soybean seedlings. The infected plants were grown under high humidity condition until hairy roots emerged in high-nitrogen hydroponic solution (contains 5.3 mM nitrogen including KNO3, NH4NO3, Ca(NO3)2•4H2O, (15:4:12), and 0.3 mM K2SO4, 0.5 mM MgSO4•7H2O, 25 µM MgCl2, 2.5 µM NaB4O7•10H2O, 0.5 µM MnSO4•H2O, 1.5 µM ZnSO4•7H2O, 0.5 µM CuSO4•5H2O, 0.15 µM (NH4)6Mo7O24•4H2O, 40 µM Fe-Na-EDTA, 250 µM KH2PO4). After the primary roots were removed, the plants with the hairy roots were transferred into fresh wet vermiculite with irrigation of low-nitrogen buffer, and further inoculated with rhizobium strain BXYD3 for additional 14 days of growth. The positive hairy roots with dTomato fluorescence were selected by a hand-held lamp with a 555 nm excitation laser.
Rhizobial spot inoculation
The 5-day-old soybean seedlings were used for spot inoculation in the low-nitrogen hydroponic system. The 0.5-cm root segments below the root–shoot junction were covered by humid foam with BXYD3 (OD600 ≧ 1.0) by continuous spot inoculation for 48 h (equipped as shown in Figure 5D). Above the inoculation site, 3-cm foam (keep humid with low-nitrogen buffer) was wrapped to keep roots humid. Auxin concentration was measured in the above (U) and below (L) root segments close to the inoculated site, and auxin transport was measured across the spot inoculation site at 48 hpi.
Generation of stable transgenic soybean plants
For soybean stable plant transformation, the established constructs were transformed into the Agrobacterium tumefaciens strain EHA101 or GV3101. Gmpin9d mutant was generated in Williams 82 ecotype, Gmpin1abc mutant and 35s:GmPIN1 lines were generated in Huachun6 ecotype. Transformants were established and screened by PCR amplification using primers for basta and the corresponding genes (Supplemental Table S1).
Histochemical GUS staining
GUS staining solution contains 50 mM sodium phosphate buffer (pH 7.0), 0.1% (v/v) Triton X-100, 0.1 mM K3Fe(CN)6, 0.1 mM K4[Fe(CN)6]•3H2O, 1 mg/mL X-Gluc, and 1% (v/v) dimethylformamide. Samples were stained for 1–4 h at 37°C. Chlorophyll was removed by sequential incubations in 50% ethanol, 100% ethanol, and 50% ethanol for several hours at each step. After rehydration, samples were transferred to glass slides with 50% glycerol mounted.
Endogenous hormone measurement
Phytohormone extraction and measurement were performed using HPLC as described previously (Yang et al., 2019) with modifications. Briefly, root or nodules were ground to powder in liquid nitrogen. Approximately 100 mg powder with the addition of a mixture of internal standards were extracted with 1 mL of ethyl acetate by vortexed for 10 min and sonicated for 20 min at 4°C, respectively. After centrifugation at 12,000 rpm for 3 min at 4°C, the supernatant was obtained and then evaporated until dry using a vacuum concentrator (Eppendorf, Germany) at 30°C. The dried residues were diluted in 500 µL of 70% methanol and then filtered through a 0.22-mm cellulose acetate filter.
Auxin transport measurements
The 5-day-old seedlings were used to measure the capacity of auxin transport as described previously (Lewis and Muday, 2009) with modifications. The 2-cm root segments above the root tip were collected (Figure 8D). For the measurement of acropetal or basipetal auxin transport, the bottom or top of segmented roots was inserted into the tube that contains a consistent volume of small [3H]-IAA agar block, for 3-h incubation in the dark. The 0.5-cm root segments just above the inoculation site were excised and washed by 1/2 MS liquid for 15 min. Then, these segments were placed into scintillation vials containing 2 mL of scintillation fluid for 16–24 h. Radioactivity was measured as described previously (Lewis and Muday, 2009), and auxin transport efficiency was calculated according to the method described previously (Lewis and Muday, 2009).
For auxin transport measurement in spot inoculation system, 2.5-cm root segments below root–shoot junction area were collected (Figure 5D), the top of segmented roots (U) was inserted into the tube that contains a consistent amount of [3H]-IAA solution, for 3-h incubation in the dark. The 0.5-cm root segments in L position were collected for radioactivity in scintillation vials containing 1 mL of scintillation fluid for 16–24 h.
Tissue slicing and microscopy observation
For resin embedding and sectioning, the different stages of nodules were collected and fixed in Formalin solution, and then dehydrated in a graded alcohol series. The fixed materials were subsequently embedded in Technovit 7100 (Kulzer GmbH) according to the manufacturer’s protocol.
For pPIN:GUS and PIN-GFP analysis in soybean plants, root segments were collected and embedded within 7%–8% agarose. After solidification, the samples were sliced using a Leica RM2255 microtome in transversal or longitudinal directions of 80-μm thickness. For GFP samples, the root/nodule median sections were selected under dissecting microscope with obvious vasculature, and the fluorescence was further observed by confocal microscopy under the same setting. For GUS samples, the slices were further stained with GUS staining solution for 4–8 h or with 0.001% Toluidine Blue O staining for 10–20 s. All samples in a single experiment were stained with consistent time.
For fluorescence observation, images were taken by either Zeiss LSM 880 (with Airyscan) or Leica SP8 confocal microscopes. The settings of excitation and detection were: GFP: 488 nm, 505–550 nm; FM4-64: 535 nm, 610 nm. All images in a single experiment were captured with the same setting.
For nodule primordium assay, 5 dpi seedlings were used to quantify the density of nodule primordium. The 1-cm root segments were consistently collected from lateral roots in root–shoot junction areas. Five to 10 individual root segments were collected from each plant, and at least eight plants were analyzed. For nodule primordium assay in spot inoculation system, root segments were collected from the foam-covered inoculated site. Root sections with 80-μm thickness were produced using a vibratome (Leica). The root sections were then stained with 0.25% potassium permanganate for 10–30 s and soaked into Schiff’s fuchsin-sulfite solution. Nodule primordium was observed by Nikon Ti-E light microscope and the density was quantified by ImageJ.
Statistical analysis, image analysis, and figure preparation
Statistical data were analyzed in Graphpad Prism 7 (GraphPad Software, La Jolla, California USA, www.graphpad.com). Statistical images were generated by Graphpad Prism 7. Camera and confocal images were prepared with ImageJ (http://imagej.nih.gov/ij/). All the experiments were carried out at least in triplicate.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under soybean gene accession numbers: GmPIN1a (Glyma.08G054700), GmPIN1b (Glyma.07G102500), GmPIN1c (Glyma.09G176300), GmPIN1d (Glyma.03G126000), GmPIN1e (Glyma.19G128800), GmPIN2a (Glyma.13G101900), GmPIN2b (Glyma.17G057300), GmPIN3a (Glyma.07G217900), GmPIN3b (Glyma.20G014300), GmPIN3c (Glyma.07G164600), GmPIN3d (Glyma.09G117900), GmPIN5a (Glyma.09G251600), GmPIN5b (Glyma.18G241000), GmPIN6a (Glyma.13G038300), GmPIN6b (Glyma.14G120900), GmPIN8a (Glyma.05G109800), GmPIN8b (Glyma.17G157300), GmPIN8c (Glyma.09G240500), GmPIN8d (Glyma.18G255800), GmPIN9a (Glyma.09G061800), GmPIN9b (Glyma.15G168100), GmPIN9c (Glyma.09G097300), GmPIN9d (Glyma.15G208600). Arabidopsis PIN accession numbers:AtPIN1(AT1G73590), AtPIN2(AT5G57090), AtPIN3(AT1G70940), AtPIN4(AT2G01420), AtPIN5(AT5G16530), AtPIN6(AT1G77110), AtPIN7(AT1G23080), AtPIN8(AT5G15100). KfPIN:(KJ466099).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . NPA promotes small nodule formation.
Supplemental Figure S2 . Structure of GmPINs.
Supplemental Figure S3 . Expression pattern of GmPINs.
Supplemental Figure S4 . Auto-fluorescence in GFP channel in WT roots and nodules.
Supplemental Figure S5 . GmPIN1b is polarly localized toward nodule primordium apex.
Supplemental Figure S6 . Phenotype analysis of Gmpin1abc triple mutant.
Supplemental Figure S7 . Phenotype analysis of GmPIN1 overexpression lines.
Supplemental Figure S8 . Flavonoids influence GmPIN1b and auxin distribution.
Supplemental Figure S9 . Cytokinin treatment increases nodule density.
Supplemental Figure S10 . Conjugated IAA is not influenced in Gmpin9d mutants.
Supplemental Figure S11 . GmPIN9d has a dual cellular localization in both ER and PM.
Supplemental Figure S12 . dTomato-based hairy root selection system.
Supplemental Table S1 . Primers used for genotyping and RT-qPCR analysis.
Supplemental Table S2 . Cloning Strategy.
Supplemental Table S3 . GmPIN gene locus number.
Supplemental Table S4 . Amino acid information.
Supplemental File S1 . PIN sequence alignments.
Supplemental File S2 . Readable tree file.
Supplementary Material
Acknowledgments
We thank Hong Liao for the donation of soybean seeds of Williams 82, Huachun6, and rhizobia Bradyrhizobium sp., Le Gao for technical help of soybean transformation, Yonghong Wang for the help of auxin transport measurement, Xiaomin Yu for the technical help of auxin content measurement, Steffen Vanneste for critical reading and language modification, and Zhongquan Lin for microscopy assistance.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFD0100705 and 2017YFA0506100), the Fok Ying Tung Education Foundation (161027), and Training Program for Excellent Young Scholars of Fujian Agriculture and Forestry University (KSYLX011) (to X.C.); National Natural Science Foundation of China (31900213), Natural Science Foundation of Fujian (2019J01420), and Distinguished Young Scholar Program of Fujian Agriculture and Forestry University (xjq201921) to (Z.G.); National Natural Science Foundation of China (32000190) (to D.H.); MEYS project (CZ.02.1.01/0.0/0.0/16_019/0000738) (to J.P.).
Conflict of interest statement. None declared.
Contributor Information
Zhen Gao, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Zhiwei Chen, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Yuanyuan Cui, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Meiyu Ke, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; College of Life Science, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Huifang Xu, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; College of Life Science, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Qinzhen Xu, Photosynthesis Research Center, Key Laboratory of Photobiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Advanced Agricultural Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Jiaomei Chen, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Yang Li, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China.
Laimei Huang, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Hong Zhao, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Dingquan Huang, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Siyuan Mai, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; College of Life Science, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Tao Xu, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China; College of Life Science, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Xiao Liu, Photosynthesis Research Center, Key Laboratory of Photobiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Advanced Agricultural Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Shujia Li, State Key Laboratory of Plant Genomics and National Center for Plant Gene Research, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, China.
Yuefeng Guan, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
Wenqiang Yang, Photosynthesis Research Center, Key Laboratory of Photobiology, Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China; College of Advanced Agricultural Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Jiří Friml, Institute of Science and Technology Austria (IST Austria), Am Campus 1, 3400 Klosterneuburg, Austria.
Jan Petrášek, Department of Experimental Plant Biology, Faculty of Science, Charles University, Viničná 5, 128 43 Prague 2, Czech Republic; The Czech Academy of Sciences, Institute of Experimental Botany, Rozvojová 263, 165 02 Prague 6, Czech Republic.
Jing Zhang, State Key Laboratory of Plant Physiology and Biochemistry, College of Biological Sciences, China Agricultural University, Beijing 100193, China.
Xu Chen, Haixia Institute of Science and Technology, Horticultural Plant Biology and Metabolomics Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China.
†Senior author.
These authors contributed equally (Z.G., Z.C., Y.C., M.K.)
Z.G. and X.C.: designed research; Z.G., Z.C., Y.C., M.K., H.X., Q.X., and S.M.: performed the research; Z.G.: contributed new analytic tools; Y.L., H.Z., D.H., T.X., and X.L.: helped the manuscript interpretation and analyzed data; L.H. and J.C.: generated the transgenic soybean plants; Y.G.: contributed soybean CRISPR/Cas9 vector; S.L., W.Y., J.F., J.P., and J.Z.: contributed the technical help and helpful discussion; Z.G. and X.C.: wrote the paper.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Xu Chen (chenxu@fafu.edu.cn).
References
- Abas L, Kolb M, Stadlmann J, Janacek DP, Lukic K, Schwechheimer C, Sazanov LA, Mach L, Friml J, Hammes UZ (2021) Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proc Natl Acad Sci U S A 118: e2102232118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamowski M, Friml J (2015) PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27: 20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Yuan J, Kuang H, Gong P, Li S, Zhang Z, Liu B, Sun J, Yang M, Yang L, et al. (2020) Generation of a multiplex mutagenesis population via pooled CRISPR-Cas9 in soya bean. Plant Biotechnol J 18: 721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37: W202–W208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkova E, Bielach A (2010) Lateral root organogenesis - from cell to organ. Curr Opin Plant Biol 13: 677–683 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bozsoki Z, Gysel K, Hansen SB, Lironi D, Kronauer C, Feng F, de Jong N, Vinther M, Kamble M, Thygesen MB, et al. (2020) Ligand-recognizing motifs in plant LysM receptors are major determinants of specificity. Science 369: 663–670 [DOI] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Kordbacheh F, Truong TT, Hocart CH, Djordjevic MA (2013) Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 238: 171–189 [DOI] [PubMed] [Google Scholar]
- Cai Z, Wang Y, Zhu L, Tian Y, Chen L, Sun Z, Ullah I, Li X (2017) GmTIR1/GmAFB3-based auxin perception regulated by miR393 modulates soybean nodulation. New Phytol 215: 672–686 [DOI] [PubMed] [Google Scholar]
- Calvert HE, Pence MK, Pierce M, Malik NSA, Bauer WD (1984) Anatomical analysis of the development and distribution of Rhizobium infections in soybean roots. Canad J Bot 62: 2375–2384 [Google Scholar]
- Chen X, Irani NG, Friml J (2011) Clathrin-mediated endocytosis: the gateway into plant cells. Curr Opin Plant Biol 14: 674–682 [DOI] [PubMed] [Google Scholar]
- Du M, Spalding EP, Gray WM (2020) Rapid auxin-mediated cell expansion. Annu Rev Plant Biol 71: 379–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Friml J (2008) PIN polar targeting. Plant Physiol 147: 1553–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Gauthier-Coles C, White RG, Mathesius U (2018) Nodulating legumes are distinguished by a sensitivity to cytokinin in the root cortex leading to pseudonodule development. Front Plant Sci 9: 1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, ers N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jurgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Bhuvaneswari TV, Torrey JG, Bisseling T (1989) Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci U S A 86: 1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip H, D'Aoust F, Begum AA, Zhang H, Smith DL, Driscoll BT, Charles TC (2001) Bradyrhizobium japonicum mutants with enhanced sensitivity to genistein resulting in altered nod gene regulation. Mol Plant Microbe Interact 14: 1404–1410 [DOI] [PubMed] [Google Scholar]
- Kereszt A, Li D, Indrasumunar A, Nguyen CD, Nontachaiyapoom S, Kinkema M, Gresshoff PM (2007) Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat Protoc 2: 948–952 [DOI] [PubMed] [Google Scholar]
- Kohlen W, Ng JLP, Deinum EE, Mathesius U (2018) Auxin transport, metabolism, and signalling during nodule initiation: indeterminate and determinate nodules. J Exp Bot 69: 229–244 [DOI] [PubMed] [Google Scholar]
- Kuhn BM, Nodzynski T, Errafi S, Bucher R, Gupta S, Aryal B, Dobrev P, Bigler L, Geisler M, Zazimalova E, et al. (2017) Flavonol-induced changes in PIN2 polarity and auxin transport in the Arabidopsis thaliana rol1-2 mutant require phosphatase activity. Sci Rep 7: 41906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J, Shull TE, Smalle JA (2019) Antagonistic activity of auxin and cytokinin in shoot and root organs. Plant Direct 3: e00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont C, Ivanovici A, Gautrat P, Brault M, Djordjevic MA, Frugier F (2020) The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat Commun 11: 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K, Lindemann A, Hauser F, Gottfert M (2008) The genistein stimulon of Bradyrhizobium japonicum. Mol Genet Genom 279: 203–211 [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Muday GK (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protoc 4: 437–451 [DOI] [PubMed] [Google Scholar]
- Liao CY, Smet W, Brunoud G, Yoshida S, Vernoux T, Weijers D (2015) Reporters for sensitive and quantitative measurement of auxin response. Nat Methods 12: 207–210, 202 p following 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CW, Murray JD (2016) The role of flavonoids in nodulation host-range specificity: an update. Plants (Basel) 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D, Tuong T, Nogueira M, Sinclair T (2019) Three-dimensional reconstruction of soybean nodules provides an update on vascular structure. Am J Bot 106: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhavy P, Duclercq J, Weller B, Feraru E, Bielach A, Offringa R, Friml J, Schwechheimer C, Murphy A, Benkova E (2014) Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr Biol 24: 1031–1037 [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A 108: 18512–18517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink HP, Of Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14: 23–34 [DOI] [PubMed] [Google Scholar]
- Mravec J, Kubes M, Bielach A, Gaykova V, Petrasek J, Skupa P, Chand S, Benkova E, Zazimalova E, Friml J (2008) Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135: 3345–3354 [DOI] [PubMed] [Google Scholar]
- Muller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K (2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315: 101–104 [DOI] [PubMed] [Google Scholar]
- Ng JL, Hassan S, Truong TT, Hocart CH, Laffont C, Frugier F, Mathesius U (2015) Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 27: 2210–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JLP, Mathesius U (2018) Acropetal auxin transport inhibition is involved in indeterminate but not determinate nodule formation. Front Plant Sci 9: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stages of arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacios-Bras C, Schlaman HR, Boot K, Admiraal P, Langerak JM, Stougaard J, Spaink HP (2003) Auxin distribution in Lotus japonicus during root nodule development. Plant Mol Biol 52: 1169–1180 [DOI] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, et al. (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312: 914–918 [DOI] [PubMed] [Google Scholar]
- Pierre-Jerome E, Drapek C, Benfey PN (2018) Regulation of division and differentiation of plant stem cells. Annu Rev Cell Dev Biol 34: 289–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp C, Ott T (2011) Regulation of signal transduction and bacterial infection during root nodule symbiosis. Curr Opin Plant Biol 14: 458–467 [DOI] [PubMed] [Google Scholar]
- Qi J, Wang Y, Yu T, Cunha A, Wu B, Vernoux T, Meyerowitz E, Jiao Y (2014) Auxin depletion from leaf primordia contributes to organ patterning. Proc Natl Acad Sci U S A 111: 18769–18774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D, Nadzieja M, Novak O, Heckmann AB, Sandal N, Stougaard J (2017) Cytokinin biosynthesis promotes cortical cell responses during nodule development. Plant Physiol 175: 361–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rightmyer AP, Long SR (2011) Pseudonodule formation by wild-type and symbiotic mutant Medicago truncatula in response to auxin transport inhibitors. Mol Plant Micr Inter 24: 1372–1384 [DOI] [PubMed] [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M, Martinoia E (2008) Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J Biol Chem 283: 31218–31226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Bishopp A, Kieber JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27: 44–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiessl K, Lilley JLS, Lee T, Tamvakis I, Kohlen W, Bailey PC, Thomas A, Luptak J, Ramakrishnan K, Carpenter MD, et al. (2019) Nodule inception recruits the lateral root developmental program for symbiotic nodule organogenesis in Medicago truncatula. Curr Biol 29: 3657–3668 e3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23: 3961–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O (2006) Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J 48: 261–273 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O (2007) Distinct, crucial roles of flavonoids during legume nodulation. Trends Plant Sci 12: 282–285 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Kawaguchi M (2014) Root nodulation: a developmental program involving cell fate conversion triggered by symbiotic bacterial infection. Curr Opin Plant Biol 21: 16–22 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M (2012) Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139: 3997–4006 [DOI] [PubMed] [Google Scholar]
- Teale WD, Pasternak T, Dal Bosco C, Dovzhenko A, Kratzat K, Bildl W, Schworer M, Falk T, Ruperti B, Schaefer JV, et al. (2021) Flavonol-mediated stabilization of PIN efflux complexes regulates polar auxin transport. EMBO J 40: e104416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Nizampatnam NR, Baron M, Coppin S, Damodaran S, Adhikari S, Arunachalam SP, Yu O, Subramanian S (2013) Ectopic expression of miR160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiol 162: 2042–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden GE, Kerim T, Goffard N, Wiblin R, Pellerone FI, Rolfe BG, Mathesius U (2007) Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol 144: 1115–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene T, Delwiche CF, Rensing SA, Friml J (2013) Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci 18: 5–10 [DOI] [PubMed] [Google Scholar]
- Wang Y, Chai C, Valliyodan B, Maupin C, Annen B, Nguyen HT (2015a) Genome-wide analysis and expression profiling of the PIN auxin transporter gene family in soybean (Glycine max). BMC Genomics 16: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang W, Zuo Y, Zhu L, Hastwell AH, Chen L, Tian Y, Su C, Ferguson BJ, Li X (2019) GmYUC2a mediates auxin biosynthesis during root development and nodulation in soybean. J Exp Bot 70: 3165–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li K, Chen L, Zou Y, Liu H, Tian Y, Li D, Wang R, Zhao F, Ferguson BJ, et al. (2015b) MicroRNA167-directed regulation of the auxin response factors GmARF8a and GmARF8b is required for soybean nodulation and lateral root development. Plant Physiol 168: 984–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Sauer M, Meurette O, Friml J, Ljung K, Sandberg G, Hooykaas P, Offringa R (2005) Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17: 2517–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312: 883. [DOI] [PubMed] [Google Scholar]
- Wu C, Dickstein R, Cary AJ, Norris JH (1996) The auxin transport inhibitor N-(1-naphthyl)phthalamic acid elicits pseudonodules on nonnodulating mutants of white sweetclover. Plant Physiol 110: 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, Yamaguchi N, Xiao J, Bargmann B, Estelle M, Sang Y, Wagner D (2015) Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. Elife 4: e09269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao TT, Schilderink S, Moling S, Deinum EE, Kondorosi E, Franssen H, Kulikova O, Niebel A, Bisseling T (2014) Fate map of Medicago truncatula root nodules. Development 141: 3517–3528 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Jiao Y (2019) The diverse roles of auxin in regulating leaf development. Plants (Basel) 8: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-ribosylation factor 1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Wu MF, Winter CM, Berns MC, Nole-Wilson S, Yamaguchi A, Coupland G, Krizek BA, Wagner D (2013) A molecular framework for auxin-mediated initiation of flower primordia. Dev Cell 24: 271–282 [DOI] [PubMed] [Google Scholar]
- Yang H, Wang Y, Li L, Li F, He Y, Wu J, Wei C (2019) Transcriptomic and phytochemical analyses reveal root-mediated resource-based defense response to leaf herbivory by ectropis oblique in tea plant (Camellia sinensis) J Agric Food Chem 67: 5465–5476 [DOI] [PubMed] [Google Scholar]
- Zhang J, Subramanian S, Stacey G, Yu O (2009) Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 57: 171–183 [DOI] [PubMed] [Google Scholar]
- Zhang J, Nodzynski T, Pencik A, Rolcik J, Friml J (2010) PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc Natl Acad Sci U S A 107: 918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Huang X, Li Y, Tao F, Zhao Q, Li W (2021) Polar auxin transport may be responsive to specific features of flavonoid structure. Phytochemistry 185: 112702. [DOI] [PubMed] [Google Scholar]
- Zhao Y (2018) Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol 69: 417–435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.