ABSTRACT
Background: Many countries are experiencing outbreaks of the second wave of COVID-19 infection. With these outbreaks, the severity of the disease is still ambiguously projected. Certain inflammatory markers are known to be associated with the severity of the disease and regular monitoring of these biomarkers in intensive care unit admissions is paramount to improve clinical outcomes.Objectives: This study was aimed to compare the severity markers of the patients infected during the first wave versus the second wave in an intensive care unit.Methods: We conducted a retrospective study obtaining patient’s data from hospital records, admitted during the first wave in March-May 2020, and compared the data with those COVID-19 patients admitted during the second wave from October–November 2020. A descriptive comparison was done among the patients admitted to intensive care unit (ICU) during both waves of the pandemic.Results: 92 patients from first wave and 68 patients from second wave were included in the analysis, all admitted to ICU with equal gender distribution. Increased age and length of ICU stay was observed during the first wave. BMI, in-hospital mortality and invasive ventilation were statistically indifferent between both the waves. There was significantly higher APACHE-II during first wave (p = 0.007), but SOFA at day 1 (p = 0.213) and day 7 of ICU stay remain indifferent (p = 0.119). Inflammatory markers were less severe during second wave while only neutrophils and lymphocytes were found to peak higher.Conclusion: Most of the severity markers were less intense during the early analysis of second wave.
KEYWORDS: COVID-19, severity, waves, pandemic, mortality, markers
1. Introduction
The first case of the coronavirus disease-19 (COVID-19) pandemic was documented in the city of Wuhan, Hubei province, China in December 2019, following which an additional 24.7 million cases inclusive of 830,000 deaths have been reported globally by the World Health Organization (WHO) till August 2020 [1]. A handful of countries have witnessed a two-wave pattern of announced cases, called ‘the second wave following the first wave’ [2]. The infectivity rate of COVID-19 has decreased after the first wave, which amounted to an epidemic threshold of 1.0 in March and April 2020 [1]. The crude fatality rate (CFR) predicted for the first wave of COVID-19 in China was 5.65%, while in critically ill patients it rose to a drastic 3.6 times higher than the above-mentioned value [3]. Death incidence increased at the 15th to 16th week of the first wave [4]. A prominent rise has been reported in COVID-19-associated deaths portraying definitive affinity towards the male gender, increasing age, black race, poor socioeconomic status, a household with more than four members, presence of comorbidities, early discharge from hospital, and transmission of disease from asymptomatic health care workers [4].
During the 8th week of the global pandemic, the casualty rate was (25%) over the expected percentage at that particular time of the year [4]. The maximum peak of global disease occurred in the province of Daegu and Gyeongbuk during February and March, with a predicted doubling time of 2.8 days for Daegu and 3.6 days for Gyeongbuk, respectively [1]. The second wave in contrast to the first wave made itself strongly evident in newly documented cases, yet there was no observed tangible rise in death tolls [5]. In countries like Germany and Spain, the peak of the second wave was expected to yield 2–3 million infections along with a mortality count in thousands [6]. The death rate was reported to be diminished during the second wave as compared to the first wave in 43 out of 53 countries, accounting for no rise in fatality rate around the globe [2]. The declined casualty ratio in the second wave as compared to the first wave can be due to an increased demise of the elderly population and those with comorbidities especially in countries with peaking infection rates [2]. A decrease in CFR during the second wave of COVID-19 is a positive manifestation indicating a decline in transmission of the viral illness [2].
Outbreak of second wave of COVID-19 infection was reported in late days of October 2020 by majority of countries comprising of Pakistan, Belgium, France, Germany, Spain, Ireland and Czech Republic with a significant rise in new cases recorded thus attaining their highest number of total cases [7,8]. Japanese population suffered second wave of COVID-19 in late March and early May with sudden rise in new cases [7]. With these outbreaks, the severity of the disease is still ambiguously projected. Apart from circulating cytokines levels, there are certain inflammatory markers known to be associated with the severity of the disease such as C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, procalcitonin (PCT), and D-dimer [9]. Some of them are non-specific markers of sepsis/inflammation while others are consistent with cytokine releasing syndrome (CRS), hence been elevated with the severity of the disease [10]. Intensive care unit (ICU) mortality is well predicted by certain score systems, Acute Physiology And Chronic Health Evaluation II (APACHE-II) and Sequential Organ Failure Assessment (SOFA) score [11,12]. Regular monitoring of these biomarkers/scores in intensive care unit admissions with COVID-19 is paramount to improve clinical outcomes.
This study was aimed to compare the severity markers of the patients infected during the first wave versus the second wave in an intensive care unit at a tertiary care hospital of a developing country.
2. Methods
We conducted a retrospective study obtaining patient’s data from hospital records, admitted during the first wave in March 2020, and compared the data with those COVID-19 patients admitted during the second wave from October 2020 onwards. Only the severity markers of patients admitted to the intensive care unit (ICU) were compared. This study excluded those patients who were managed in isolation wards and discharged without experiencing any severe course of the disease. The study variables primarily included the laboratory parameters liable for predicting the severity of COVID-19 infection like C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, procalcitonin (PCT), D-dimer, serum urea and creatinine levels, total leukocyte count (TLC), neutrophils, lymphocytes, and platelet counts. The admitting values of Day 1 were recorded, followed by the peak values (highest rise) during the ICU stay along with the day of reaching peak value in each parameter were recorded. Lymphocyte and platelet counts tend to decrease with severity hence their lowest levels and days to attain the lowest levels were recorded during the ICU stay. APACHE-II and SOFA scores were also calculated at admission in ICU.
After collecting the severity parameters, a descriptive comparison was performed among the patients admitted during both waves of the pandemic. All the data was assembled in SPSS version 25.0 (IBM Corp., Armonk, NY). Data were presented as either median and interquartile range for continuous, or frequency and percentages for categorical variables. The normality of the data was checked through the Shapiro-Wilk test. Non-parametric test i.e, Mann-Whitney U test was used to compare differences between two independent groups when the data was not normally distributed. Chi-square test or Fisher’s exact test was applied to frequency distribution accordingly. Box plots were also used to show the distribution of numerical data and skewness among the two waves of the COVID-19 pandemic.
3. Results
A total of 160 patients were included in the analysis, all admitted to ICU with 92 patients from the first wave in March-May 2020, and 68 patients from the second wave in October-November 2020. Equal gender distribution was observed in both sets of patients (p = 0.869), however, increased age was evident during the second wave (p < 0.001). The mean length of ICU stay was increased during the first wave (p < 0.001). BMI, in-hospital mortality and invasive ventilation were statistically indifferent between both the waves. There was significantly higher APACHE-II score during first wave (p = 0.007), but SOFA at day 1 (p = 0.213) and day 7 of ICU stay remain indifferent (p = 0.219). The comparative frequencies of multisystem involvement and in-hospital events are shown in Table 1.
Table 1.
Baseline, demographic characteristics along with in-hospital events and severity indices of the study population (n = 160)
| Variables | First wave | Second wave | p-value |
|---|---|---|---|
| BMI (kg/m2) | 25.00 (4.50) | 25.50 (4.00) | 0.301* |
| Male gender | 58 (63.0%) | 42 (61.8%) | 0.869** |
| Age (in years) | 56.00 (19.00) | 65.00 (18.50) | <0.001* |
| In-hospital mortality | 23 (25.0%) | 13 (19.1%) | 0.378** |
| Invasive ventilation | 25 (27.1%) | 11 (16.1%) | 0.100** |
| Non-invasive ventilation | 28 (30.4%) | 23 (33.8%) | 0.649* |
| APACHE-II | 15.00 (7.50) | 13.00 (5.50) | 0.007* |
| SOFA score at Day 1 of ICU stay | 4.00 (2.50) | 3.00 (2.00) | 0.213* |
| SOFA score at Day 7 of ICU stay | 3.50 (2.00) | 2.50 (2.00) | 0.119* |
| Systolic blood pressure (mmHg) | 124.00 (20.00) | 125.50 (20.75) | 0.862* |
| Diastolic blood pressure (mmHg) | 74.50 (12.00) | 76.25 (12.50) | 0.342* |
| Acute kidney injury | 21 (22.8%) | 12 (17.6%) | 0.423* |
| ARDS | 16 (17.4%) | 10 (14.7%) | 0.649* |
| Mild ARDS | 3 (3.2%) | 1 (1.4%) | 0.864† |
| Moderate ARDS | 9 (9.8%) | 7 (10.2%) | |
| Severe ARDS | 4 (4.3%) | 2 (2.9%) | |
| MODS | 9 (9.8%) | 4 (5.9%) | 0.372** |
| Thrombotic events | 11 (11.9%) | 6 (8.8%) | 0.525** |
| Bilateral lung involvement | 42 (45.6%) | 26 (38.2%) | 0.348** |
| Cardiac event | 6 (6.5%) | 3 (4.4%) | 0.734† |
| Hemodialysis support | 14 (15.2%) | 8 (11.7%) | 0.531** |
| Vasopressor support | 15 (16.3%) | 7 (10.2%) | 0.275** |
* Mann Whitney U-test, ** Chi-square test, †Fisher Exact test.
Data presented as median (IQR) or frequency (percentage).
Abbreviation: BMI: body mass index; APACHE: Acute Physiology And Chronic Health Evaluation II; SOFA: Sequential Organ Failure Assessment; ARDS: Acute respiratory distress syndrome; MODS: Multiple Organ Dysfunction Syndrome; ICU: intensive care unit.
Concerning the laboratory markers, total leukocyte count was equally affected during both waves, but peak levels were attained early during the second wave (p = 0.017). Neutrophil count was high on day 1 of the second wave (p = 0.001), and higher peak levels were observed in the second wave (p < 0.001). However, mean days to reach peak levels were statistically insignificant (p = 0.981). Similarly, lymphocytes were lower on day 1 with persistently lower levels were observed during the second wave (p < 0.001), but with no difference in duration to reach lowest levels (p = 0.834). Similar platelet counts were observed between both the waves, except for early attainment of lowest levels during the second wave (p = 0.018) as shown in Table 2.
Table 2.
Comparison of serial biochemical markers among the first and second waves of COVID-19 (n = 160)
| Variables | First wave (n = 92) | Second wave (n = 68) | p-value |
|---|---|---|---|
| Length of stay in hospital | 12.00 (6.50) | 9.00 (4.50) | <0.001* |
| Length of ICU stay (in days) | 8.00 (5.75) | 5.00 (4.00) | <0.001* |
| Total leukocyte count (x109/uL) | |||
| Peak on Day 1 of ICU stay | 10.00 (5.78) | 10.00 (6.85) | 0.587* |
| Peak levels during ICU stay | 16.10 (8.58) | 16.90 (10.28) | 0.403* |
| Days to reach peak levels during ICU stay | 6.00 (5.00) | 3.00 (4.00) | 0.017* |
| Neutrophil count (%) | |||
| Peak on Day 1 of ICU stay | 79.50 (13.75) | 86.00 (13.50) | 0.001* |
| Peak levels during ICU stay | 85.00 (10.00) | 92.00 (7.00) | <0.001* |
| Days to reach peak levels during ICU stay | 4.00 (5.50) | 4.00 (5.00) | 0.981* |
| Lymphocyte count (%) | |||
| † Peak on Day 1 of ICU stay | 16.00 (10.75) | 8.00 (10.00) | <0.001* |
| Lowest count during ICU stay | 10.00 (10.00) | 4.00 (4.00) | <0.001* |
| Days to reach lowest count during ICU stay | 4.00 (5.00) | 4.00 (5.00) | 0.834* |
| Platelet count (x109/uL) | |||
| † Peak on Day 1 of ICU stay | 220.00 (112.00) | 204.00 (100.50) | 0.221* |
| Lowest count during ICU stay | 172.00 (114.00) | 152.00 (109.50) | 0.293* |
| Days to reach lowest count during ICU stay | 3.50 (7.00) | 2.00 (3.00) | 0.018* |
| Serum urea (mg/dL) | |||
| Peak on Day 1 of ICU stay | 38.50 (42.75) | 53.50 (47.00) | 0.079* |
| Peak levels during ICU stay | 84.00 (112.00) | 83.00 (81.95) | 0.947* |
| Days to reach peak levels during ICU stay | 6.00 (7.00) | 3.00 (3.00) | 0.040* |
| Serum creatinine (mg/dL) | |||
| Peak on Day 1 of ICU stay | 1.05 (1.03) | 1.04 (0.60) | 0.840* |
| Peak levels during ICU stay | 1.46 (1.87) | 1.37 (1.41) | 0.601* |
| Days to reach peak levels during ICU stay | 4.00 (7.00) | 2.00 (3.00) | 0.256* |
| C-reactive protein (mg/L) | |||
| Peak on Day 1 of ICU stay | 168.75 (221.18) | 112.00 (140.50) | 0.016* |
| Peak levels during ICU stay | 210.00 (170.95) | 141.00 (140.83) | 0.006* |
| Days to reach peak levels during ICU stay | 1.00 (2.00) | 1.00 (1.75) | 0.522* |
| Serum Ferritin (ng/mL) | |||
| Peak on Day 1 of ICU stay | 949.50 (1127.75) | 767.50 (723.75) | 0.035* |
| Peak levels during ICU stay | 1528.00 (1872.00) | 999.00 (831.50) | <0.001* |
| Days to reach peak levels during ICU stay | 4.00 (6.00) | 2.00 (2.00) | 0.006* |
| Serum Lactate dehydrogenase (U/L) | |||
| Peak on Day 1 of ICU stay | 513.50 (303.25) | 534.00 (337.00) | 0.846* |
| Peak levels during ICU stay | 765.00 (528.75) | 577.00 (443.75) | <0.001* |
| Days to reach peak levels during ICU stay | 4.00 (4.00) | 2.00 (3.00) | <0.001* |
| Procalcitonin (ng/mL) | |||
| Peak on Day 1 of ICU stay | 0.26 (1.23) | 0.24 (0.93) | 0.343* |
| Peak levels during ICU stay | 1.11 (18.69) | 0.23 (1.94) | 0.004* |
| Days to reach peak levels during ICU stay | 8.00 (9.00) | 3.00 (2.00) | 0.002* |
| D-dimer (mcg/mL) | |||
| Peak on Day 1 of ICU stay | 2.57 (5.46) | 1.11 (1.04) | 0.004* |
| Peak levels during ICU stay | 8.88 (19.75) | 3.90 (6.20) | <0.001* |
| Days to reach peak levels during ICU stay | 4.00 (5.00) | 4.00 (3.00) | 0.500* |
* Mann–Whitney U-test
†Peak for lymphocytes and platelets are taken as lowest count.
Data presented as median (interquartile range).
Abbreviation: ICU: intensive care unit; COVID-19: coronavirus disease 19.
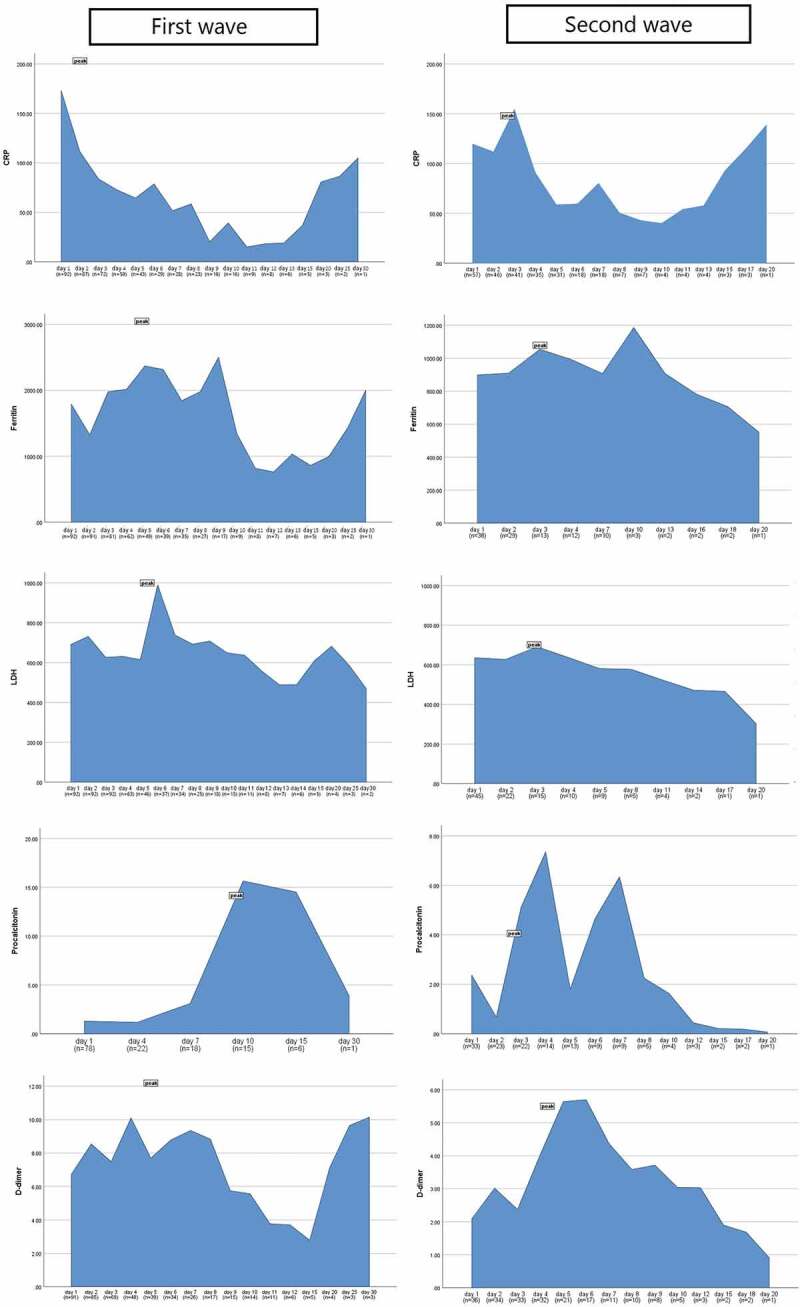
Similar rise in serum urea levels was observed during both waves, while the day of peak was attained early in the second wave (p = 0.040) as shown in Figure 1(a). Although, creatinine was deranged equally during both waves. CRP usually peaked after day 1 of ICU stay in both the waves with higher peak levels were observed during the first wave (p = 0.006). Similarly, serum ferritin prominently peaked during the first wave (p < 0.001), usually on day 4 of ICU stay (p = 0.006). Serum LDH also peaked higher during the first wave (p < 0.001), but peak levels were reached earlier during the second wave (p < 0.001). Higher peak procalcitonin levels were observed during the first wave (p = 0.004,) on day 8 of the ICU stay (p = 0.002). D-dimer was higher on day 1 (p = 0.004) as well as a prominent peak was observed during the first wave (p < 0.001), however the peak was usually reported on day 4 during both the waves (p = 0.500) as shown in Figure 1(b). Figure 2 shows the box plots to show the distribution of laboratory markers and skewness among the two waves of the COVID-19 patients. Figure 3 demonstrates the cross compactors of each marker with different clinical outcomes. Higher peaking inflammatory markers were observed during the first wave correlating with mortality and ventilatory support but during the second wave, neutrophil and lymphocyte countsbetter correlated with the in-hospital events.
Figure 1.
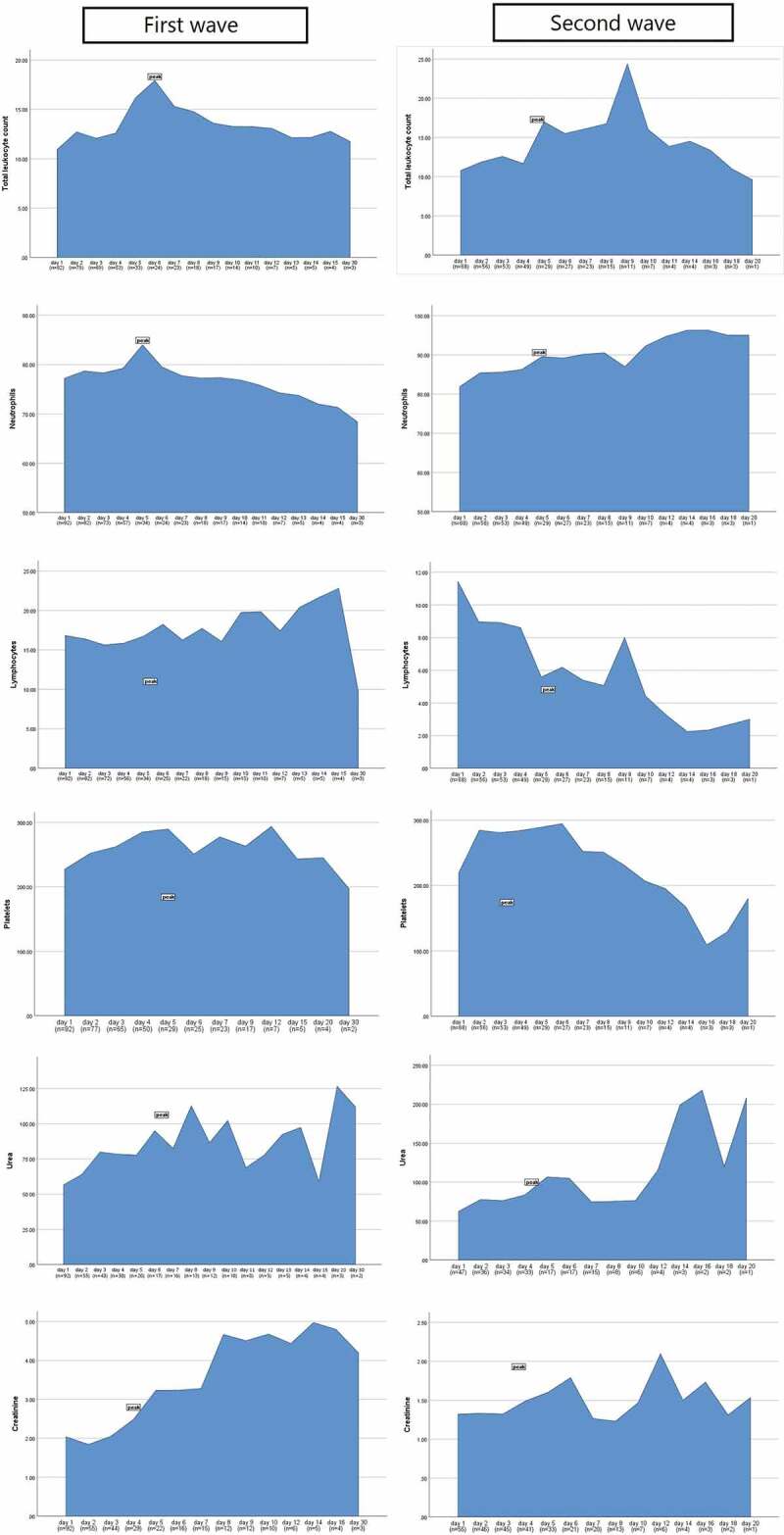
(a) Comparison of peaks of laboratory markers among the first and second waves of COVID-19. (b) Comparison of peaks of inflammatory markers among the first and second waves of COVID-19
Figure 1b.
(countined)
Figure 2.
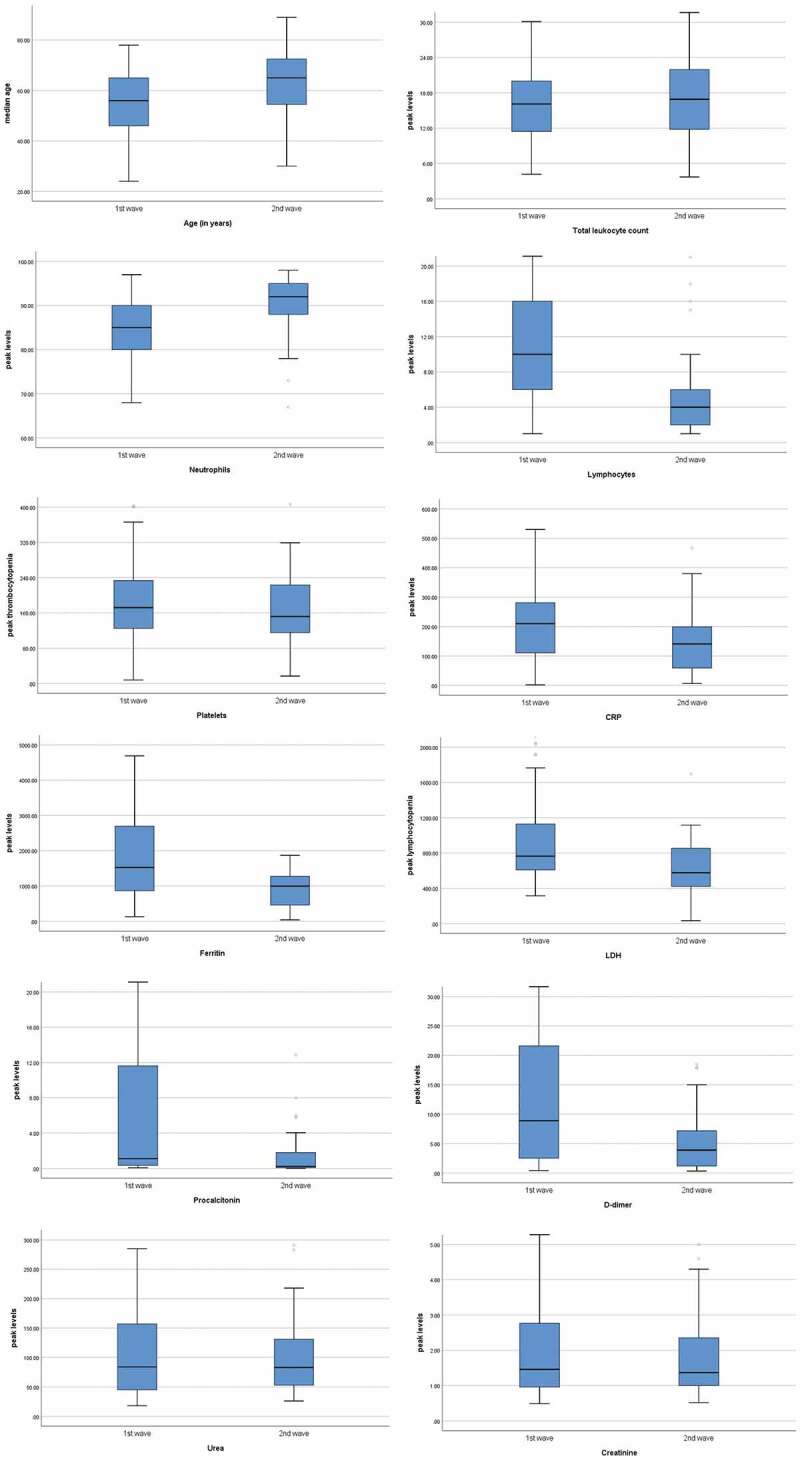
Box plots representing the distribution of laboratory markers and skewness among the two waves of the COVID-19 patients
Figure 3.
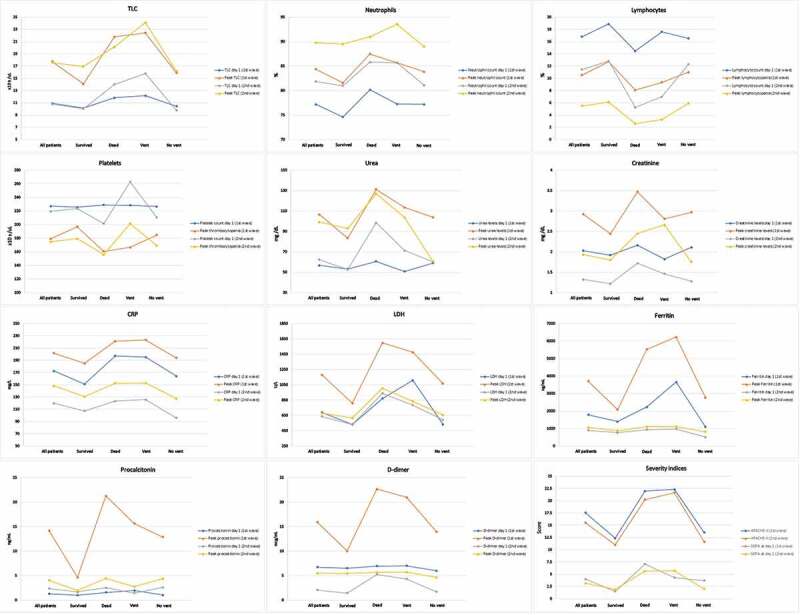
Cross compactor of biochemical markers in each wave with different patient clinical outcomes
4. Discussion
In a study conducted in Wuhan China, peak levels of severity markers were assessed between survivors and non-survivors [13]. Total leukocyte count peaked on the 15th to 17th day of infection with values above 14.0 x109/µL in non-survivors and 9.0 x109/µL in survivors, respectively. Peak absolute neutrophil count was 13000 cell/µL in non-survivors while 6000 cells/µL in survivors with maximum levels reached by day 17. Peak lymphocytopenia was evident on day 5 and day 17 among non-surviving patients while patients that survived had a peak on day 19 of the infection. Peak D-dimer levels were attained on day 13 among critically ill patients with values above 1000 mg/L. Deranged blood urea nitrogen along with serum creatinine peaked at day 17th among non-surviving patients, while onset of abnormal range was attained after day 11. Another study conducted on hematological parameters of COVID-19 patients’ revealed a lymphocyte count ranging from 500 to 1000 cells/µL in non-surviving, in comparison to surviving patients having counts of more than 2000 cells/µL. The same study showed an absolute neutrophil count ranging from 10,000 to 15,000 cell/µL, along with a lower hemoglobin and platelet levels in non-surviving patients. The lowest platelet counts were slightly above 200,000 in non-surviving and 250,000 in surviving patients, respectively [14]. Another study conducted in invasively ventilated patients of COVID-19 showed a 7-day follow-up of laboratory markers [15]. Total leukocyte count was highest on day 7 for non-surviving patients, conversely, it declined to a minimum value on day 7 for surviving patients (14 vs 10 x109/µL). Peak lymphocytopenia was evident on day 6 of the post-intubation period with a count of 400 cell/uL. The neutrophil count was highest on post-intubation day 1, meanwhile, non-survivors had shown a decline on day 7 of post-intubation. Persistent declining thrombocyte count was a feature in the non-surviving patients from admission till post intubation period. BUN was highest on day 7 and creatinine on day 5 post-intubation. Peak CRP level of 125 mg/dL was noticed on day 4 of non-surviving intubated patients, contrary to this surviving patients had levels below 50 mg/dL on the same day followed by a declining pattern [15].
Lowest lymphocyte count of 400 cells/µL, peak CRP levels of 214.7 mg/L, peak procalcitonin 0.4 ng/mL, peak dimer 2.02 mcg/mL, peak LDH 475 U/L, peak ferritin 1442 ng/mL, and peak thrombocytopenia of 166 x109/µL were observed according to one study [16]. Another similar study compared these markers among survivors and non-survivors showed that lymphocyte count decreased significantly in both survivors (from 0.94 to 0.51 × 109 cells/L) and non-survivors (from 1.24 to 0.61 × 109 cells/L), respectively. Among survivors, the increase in CRP level was from 158.0 to 178.7 mg/L while among non-survivors, the increase in CRP level was from 166.8 to 207.7 mg/L [17]. There was an equal rise in serum ferritin among the survivors (1321.13 to 2141.18 ng/mL) and non-survivors (1227.01 to 1662.7 ng/mL). The rise in serum LDH levels in survivors was 829.59 to 1018.6 U/L while in non-survivors it was 816.2 to 1056.61 U/L. D-dimer levels increased significantly in both survivors and non-survivors (from 7.2 to 28.8 µg/mL, and from 8.75 to 29.52 µg/mL, respectively) [17]. Similarly, another study concluded peak D-dimer levels of 42.2 µg/ml on day 22 of admission in non-survivors, peak lymphocytopenia on day 7 for survivors (0.91 x 109 cells/L), and day 22 for non-surviving patients (0.42 x 109 cells/L). Surviving patients exhibited peak ferritin levels of 635 µg/L on day 13, while non-surviving patients had peak levels of more than 2000 µg/L from day 16 to day 19 [18]. LDH levels were highest from day 10 to day 13 in surviving patients (302 U/L) while non-survivors evinced a constant incremental pattern from day 16 to a peak value of 590 U/L on day 25 [18].
One study compared severity markers with four stages of illness and found slightly rising total leucocyte count in a severely affected group of patients. Granulocyte counts portrayed a proportional increase in both severely and non-severely affected groups of patients. Lymphocyte count was significantly depreciated in the severely affected group, consequently, granulocyte to lymphocyte ratio depicted a persistent rise in the same group. CRP showed a significantly higher pattern in stage 1 to 3 illness of severe group, but declined and became indifferent to the mild group in stage 4 illness [19]. Another study compared the mean values of CRP and d-dimer levels on day 1 and day 7 of hospitalization and found that CRP levels in deceased patients rose from 125 to 225 mg/L while no significant difference was observed with upgrading patients to ICU, performing dialysis, or being on a ventilator. Similarly, D-dimer levels of non-surviving patients were 4.0 mcg/mL at day 1 and 6.5 mcg/mL at day 7, respectively. Compared to patients not requiring additional treatment modalities, D-dimer levels rose from 2.5 to 7.0 mcg/mL in ICU admitted patients, 4.5 to 7.0 mcg/mL in ventilator acquiring patients and 2.5 to 4.0 mcg/mL in patients requiring dialysis, respectively [20]. A couple of studies documented the severity markers after using tocilizumab on severely affected patients showed peak CRP levels of 74.3 mg/L declining to 38.6 mg/L after 3 days of therapy [21], with no significant difference among survivors and non-survivors. Another extended study documented effects of Tocilizumab on different severity markers among survivors and non-survivors. CRP levels showed a decline from 190 to 40 mg/L on day 10 in non-surviving patients, while a reduction of 138 to 11 mg/L between days 7–15 was observed in survivors. Serum ferritin showed a declining pattern from 1923 to 554 ng/mL on day 15 in non-survivors and from 1066 to 382 ng/mL in surviving patients, respectively. LDH levels reduced slightly from 431 to 342 U/L over the period of 15 days post Tocilizumab use. An initial rise of 485 to 621 U/L occurred in non-surviving patients till 7th day with a subsequent decline up to 490 U/L on day 15 [22]. D-dimer levels showed an initial rise up to day 4 post Tocilizumab use in (0.51 to 1.59 mcg/mL) non-surviving and (0.29 to 0.79 mcg/mL) surviving patients, respectively. Subsequently a drop in both sets of patients became comparable on day 10 (0.42 vs 0.38 mcg/mL). However, an increment of (0.67 mcg/mL) was recorded in non-surviving patients compared to a progressive decline in survivors (0.26 mcg/mL) on day 15 [22].
Lastly, platelet to lymphocyte ratio (PLR) was monitored in one study revealing peak levels of 262 in non-severe and 626 in severely affected patients. The corresponding peak platelet counts were 301 and 392 x109/µL, respectively [23]. Another study conducted on hematological parameters of COVID-19 patients showed a mean PLR of 152 in non-severe and 257 in severely affected patients, respectively [24]. Another study conducted previously on a similar population revealed follow-ups of severe laboratory markers in recovered and non-surviving COVID-19 patients, with most of the trends were similar to our study [25]. An analysis conducted on 154 ICU admissions of COVID-19 showed APACHE-II score of 15 and SOFA score of 2.62 with significantly higher scores were predictive of mortality [26]. Another study of 45 patients including 20 intubated patients had APACHE-II score of 14 and SOFA score of 4 showing a higher risk for intubation. Additionally, SOFA score correlated with lymphocytopenia and elevated LDH [27]. Similarly, APACHE-II >15 and SOFA >4 were predictive of ICU mortality from a study conducted in Wuhan, China [28]. Out of 59 studied COVID-19 infected patients, the mortality was observed high (in 41 of them) [28].
The limitations of this study are single-center, retrospective design, and a limited number of patients. The patients included from the second wave were admitted early during the outbreak, and might have been less severe in the disease course as compared to the patients selected from the peak time of first wave.
5. Conclusion
Most of the inflammatory markers were less severe during the early analysis of second wave, while only neutrophils and lymphocytes were observed to reach higher peak levels during this time. Severity of the disease was also more predictive from the latter during the second wave, which might be due to the dampening of inflammatory response from early use of immunosuppressants, antibiotics/antivirals and anticoagulants as guided by the recent research which was not available during the first wave. The second wave is still on peak in Pakistan with daily rising number of cases and mortality. We hope that the emerging wave proves less fatal as observed through the severity markers in our results, and the crude fatality rate might fall as compared to the first wave.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Funding Statement
This work is not supported by any sponsors. No funding required in this study.
Data availability statement
Data will be made available on reasonable request from the corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethical approval statement
Ethical approval was taken in this study from the institutional review board, and consent to participate was not required due to retrospective nature of the study.
References
- [1].Shim E, Tariq A, Chowell G.. Spatial variability in reproduction number and doubling time across two waves of the COVID-19 pandemic in South Korea, February to July 2020. Inter J Infect Dis. 2020;102:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan G, Yang Z, Lin Q, et al. Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2020;00:1–3. [DOI] [PubMed] [Google Scholar]
- [3].Deng X, Yang J, Wang XL.. Case fatality risk of the first pandemic wave of novel coronavirus disease 2019 (COVID-19) in China. Clin Infect Dis. 2020;ciaa57. DOI: 10.1093/cid/ciaa578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lusignan SD, Joy M, Oke J, et al. Disparities in the excess risk of mortality in the first wave of COVID-19: cross sectional study of the English sentinel network. J Infect. 2020;81(5):785–792. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Linden M, Dehning J, Mohr SB, et al. The foreshadow of a second wave: an analysis of current COVID-19 fatalities in Germany. [Preprint]. arXiv:2010.05850 [q-bio.PE]. 2020. [Google Scholar]
- [6].Castro FD. Modelling of the second (and subsequent) waves of the coronavirus epidemic. Spain and Germany as case studies. MedRxiv. 2020. [Preprint]. DOI: 10.1101/2020.06.12.20129429. [DOI] [Google Scholar]
- [7].Ali A, Bai J, Ma Z. Second wave of Covid-19 in Pakistan; are more episodes down the road? BMJ. 2020;371. DOI: 10.1136/bmj.m4113. [DOI] [Google Scholar]
- [8].Bassino JP, Ladmiral G. Excess mortality during the first and second waves of COVID-19 spread in Japan (December 2019-May 2020); evidence from municipality level data. SSRN. 2020. [Preprint]. DOI: 10.2139/ssrn.3651968. [DOI] [Google Scholar]
- [9].Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Inter J Infect Dis. 2020;95:304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Knaus WA, Draper EA, Wagner DP, et al. a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- [12].Vincent J, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. [DOI] [PubMed] [Google Scholar]
- [13].Wang D, Hu B, Chang H, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mousavi SA, Rad S, Rostami T, et al. Hematological predictors of mortality in hospitalized patients with COVID-19: a comparative study. Hematology. 2020;25(1):383–388. [DOI] [PubMed] [Google Scholar]
- [15].Zhao S, Lin Y, Zhou C, et al. Short-term outcomes of patients with COVID-19 undergoing invasive mechanical ventilation: a retrospective observational study from Wuhan, China. Front Med. 2020;7(571542):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lenka J, Chabbria MS, Sharma N, et al. Clinical characteristics and outcomes of critically ill patients with COVID-19 in a tertiary community hospital in upstate New York. J Community Hosp Intern Med Perspect. 2020;10(6):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bahadur S, Rahim F, Amin S, et al. Do laboratory biomarkers predict survival in severe COVID-19? A cross-sectional study. Res Square. 2020. [Preprint]. DOI: 10.21203/rs3.rs-67563/v1. [DOI] [PubMed] [Google Scholar]
- [18].Zhou F, Du R, Fan G, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tan C, Huang Y, Shi F, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ullah W, Thalambedu N, Haq S, et al. Predictability of CRP and D-dimer levels for in hospital outcomes and mortality of COVID-19. J Community Hosp Intern Med Perspect. 2020;10(5):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Masood A, Farooq O, Waris S, et al. Analysis of serial C-reactive protein levels in critically ill COVID-19 patients receiving Tocilizumab. Biomedica. 2020;36(2):171–176. . [Google Scholar]
- [22].Morrison AR, Johnson MJ, Griebe KM, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving Tocilizumab. J Autoimmun. 2020;114:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qu R, Ling Y, Zhang YHZ, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;92(9):1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Asghar MS, Khan NA, Kazmi SJH, et al. Hematological parameters predicting severity and mortality in COVID-19 patients of Pakistan: a retrospective comparative analysis. J Community Hosp Intern Med Perspect. 2020;10(6):514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Asghar MS, Haider Kazmi SJ, Khan NA, et al. Role of biochemical markers in invasive ventilation of coronavirus disease 2019 patients: multinomial regression and survival analysis. Cureus. 2020;12(8):e10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zou X, Li S, Fang M, et al. Acute physiology and chronic health evaluation II score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med. 2020;48(8):e657–e665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu Y, Xu Z, Liu X, et al. Clinical findings of COVID-19 patients admitted to intensive care units in Guangdong Province, China: a multicenter, retrospective, observational study. Front Med (Lausanne). 2020;7:576457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang ZH, Shu C, Ran X, et al. Critically Ill patients with coronavirus disease 2019 in a designated ICU: clinical features and predictors for mortality. Risk Manag Healthc Policy. 2020;13:833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request from the corresponding author.


