ABSTRACT
Since the first-reported case of Severe Acute Respiratory Distress Syndrome-Coronavirus 2 in December 2019, COVID-19 has caused a global pandemic associated with significant morbidity and mortality. After a year of advances in vaccine research and development, three vaccines for the prevention of COVID-19 (manufactured by Pfizer, Moderna and Johnson & Johnson’s Janssen Biotech) are approved for use in the USA. We report the first case of Guillain–Barre Syndrome after receiving the second dose of the Pfizer COVID-19 vaccine, in a 42-year-old woman presenting with progressive ascending weakness and paresthesias. Diagnostic workup demonstrated cytoalbuminologic dissociation on cerebrospinal fluid analysis with confirmatory evidence of early demyelinating electrodiagnostic features on nerve conduction study and an extensive serological workup being negative for other viral or autoimmune disease triggers. Management included administration of intravenous immunoglobulin (total of 2 gm/kg), with frequent monitoring of forced vital capacity and negative inspiratory force. A longitudinal risk profile of neurologic complications caused from COVID-19 vaccines remains limited, and prompt recognition of potential neurological complications from the COVID-19 vaccine is of interest to public health.
KEYWORDS: Guillain Barre syndrome, coronavirus, COVID-19, vaccination, Pfizer
1. Introduction
Severe Acute Respiratory Distress Syndrome Coronavirus-2 (SARS-CoV-2) emerged in Wuhan, China in late 2019, giving rise to global pandemic. The spectrum of coronavirus disease-2019 (COVID-19) ranges from asymptomatic to severe multi-system organ dysfunction and death [1–4]. The rapid rate of transmission, increased virulence, and limited therapeutic options focused efforts on prevention of disease with vaccination. In December 2020, the U.S. Food and Drug Administration (FDA) approved two mRNA vaccines for COVID-19 developed by Pfizer and Moderna, followed by a third adenoviral vector vaccine developed by Johnson & Johnson’s Janssen Biotech. To date, approximately 300 million people worldwide, including 82 million in the USA, have received at least one dose of the vaccine according to the University of Oxford global tracking system[5]. The most common side effects noted were pain at the injection site, fatigue, headache, muscle pain, chills, joint and fever with more people noticing symptoms after the second dose [6,7]. Serious neurologic side effects, however, were noted on rare occasions.
2. Case presentation
A 42-year-old woman with no significant past medical history and a preserved functional baseline presented to the emergency room with symmetric, distal paresthesias in the bilateral upper and lower extremities, accompanied by intractable pruritus in her toes. Over a period of days, she developed ascending numbness to the proximal thighs, new, sharp pains originating in the midline neck with radiation down both arms, and mild, bilateral, diffuse lower extremity weakness and gait instability. One week prior to onset of symptoms, she received the second dose of the Pfizer COVID-19 vaccine, which was administered 21 days after the first dose. She did not endorse recent bowel or bladder incontinence, vision changes, skin rash, or antecedent diarrheal/respiratory illness. She denied any reactions after the first dose, and denied any other symptoms following the second dose. The remainder of her review of systems was unremarkable. She reported no recent exposures, travel, or new medications/herbal supplements. She received no other vaccinations and did not experience any traumatic injury undergo any surgeries during this time. There was no known family history of vasculitis or autoimmune disease.
On presentation, she was mildly hypertensive, with otherwise preserved vitals. Physical examination was remarkable for proximal bilateral lower extremity muscle weakness (4-/5 strength in knee flexion/extension, hip flexion/extension, hip abduction/adduction) as well as distal bilateral upper extremity weakness (4-/5 wrist flexion/extension) with normal muscle tone and bulk throughout. No pronator drift was appreciated. Sensory examination revealed no asymmetry to pinprick but was remarkable for mild vibration loss in the feet, with otherwise preserved proprioception. Sensation to light touch was notably diminished. Deep tendon reflexes were absent throughout. Laboratory evaluation was unremarkable. MRI Brain and Cervical Spine with and without contrast demonstrated no evidence demyelinating lesions, but the presence of multilevel degenerative disc disease with protrusions at C4-C5, C5-C6 and C6-C7 (Figure 1). Lumbar puncture was performed which revealed an elevated CSF protein level of 167 mg/dL (reference range: 15–45 mg/dL) and 0 white blood cells, consistent with cytoalbuminologic dissociation.
Figure 1.
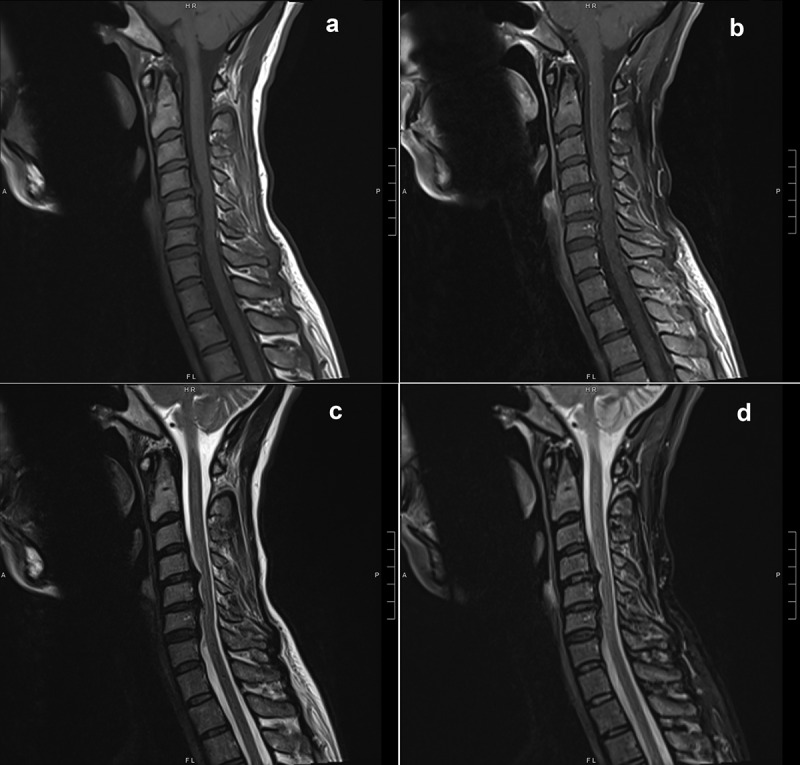
MRI of the cervical spine with and without contrast showing multilevel degenerative disc disease with protrusions at C4-C5, C5-C6 and C6-C7. (A) T1 Turbo Spin Echo (TSE), Sagittal. (B) T1 TSE, Sagittal, Fat Suppressed. (C) T2 TSE, Sagittal. (D) T2 TSE, Short-TI Inversion Recovery, Sagittal
She was given a presumptive diagnosis of Guillain–Barre Syndrome (GBS) and initiated on intravenous immunoglobulin (total of 2 gm/kg in four divided doses), with monitoring of forced vital capacity, negative inspiratory force, and autonomic stability. Clinically, she had slight dysautonomia with intermittent episodes of tachycardia and hypertension. Serological testing for HIV-1/2 Antigen/Antibody, CMV IgM, EBV IgM, Mycoplasma IgM and antiganglioside IgM antibodies was negative. Additional serologies – Lyme disease, rheumatoid factor, Ro/La (SSA/SSB), thiamine, vitamin B6, vitamin B12, homocysteine, anti-neutrophilic cytoplasmic antibody, anti-nuclear antibody, erythrocyte sedimentation rate, C-reactive protein, syphilis (RPR), and cryoglobulins – were negative. Nerve conduction studies (NCS) performed 8 days after symptom onset demonstrated decreased conduction velocity of the right peroneal motor nerve, an absent F-wave in the right peroneal nerve, and prolonged latency (57.44 ms) of left and right tibial F-waves, in addition to sural sparing (Figure 2), consistent with early demyelinating features of GBS. The constellation of clinical findings, CSF cytoalbuminologic dissociation, and early electrodiagnostic findings were highly suggestive of GBS. The patient completed a 4-day course of intravenous immunoglobulin over which time both subjective and objective motor recovery occurred. Her hospital course remained uncomplicated with the exception of mild dysautonomia and a persistent absence of deep tendon reflexes. She was discharged in stable condition with planned outpatient neurology follow-up and repeat NCS in 3 weeks. The repeat NCS, 28 days from symptom onset, revealed sural sparing, as well as persistent borderline slowing of motor nerves in the lower extremities, within demyelinating ranges in the right peroneal motor nerve, and the absence of late responses in bilateral lower extremities, worse on the right. Over the next several weeks, the patient’s symptoms improved overall, with only mild tingling in her fingertips and mild numbness in her lower extremities, and no complaints of muscle weakness, pain, or gait instability.
Figure 2.
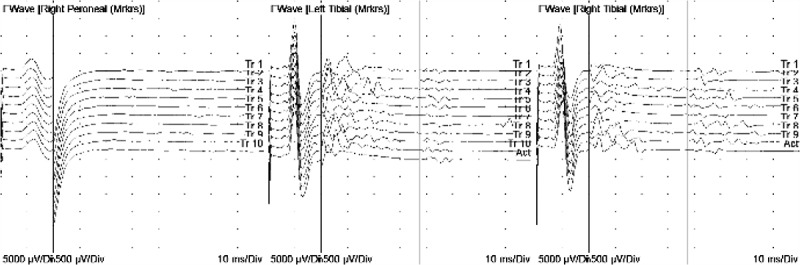
F-wave studies of the right peroneal, left tibial and right tibial nerves showing an ascending pattern of absent, prolonged, and non-reproducible late-response
3. Discussion
GBS is a well-described inflammatory polyradiculoneuropathy often associated with infection. Patients classically present with variable, progressive, ascending muscle weakness, absent deep tendon reflexes, paresthesias, and dysautonomia [8,9]. Indeed, COVID-19 has been reported to be associated with GBS and it has been hypothesized that it shares a similar autoimmune-mediated pathogenic mechanism[10]. Although rare, vaccine-related GBS has been reported with traditional vaccines such as meningococcal, poliovirus, influenza and rabies [11]. To date, the Vaccine Adverse Event Reporting System (VAERS) has reported on 34 such possible cases of GBS associated with COVID-19 vaccination; however, further details are lacking. At least two published reports have highlighted the development of GBS after vaccination with the Janssen and Pfizer vaccines – a 60-year-old woman with horizontal diplopia, headache, back and bilateral proximal lower extremity myalgias 16 days after vaccination [12] and an 82 year-old female with proximal muscle weakness one week following her first vaccination, respectively[13]. In this latter report, diagnosis was based upon physical examination and cytoalbuminologic dissociation on lumbar puncture, with no electrodiagnostic testing performed.
Our patient, in contrast, was noted to have GBS symptoms one week following completion of the two-dose Pfizer COVID-19 vaccine series. Serological investigations were noted to be negative for other causes of GBS including autoimmune conditions, viral infections as well as antiganglioside antibodies which have a known association with Campylobacter jejuni associated GBS[14]. Diagnostic workup demonstrated cytoalbuminologic dissociation with mild evidence of early demyelination noted on NCS. The results of both NCS (performed 8 days and 28 days from symptom onset) showed mild demyelinating features in the lower extremities. The definite electrodiagnostic criteria for acute intermittent demyelinating polyneuropathy [15] were not met; however, the studies were consistent with probable electrodiagnostic criteria [16] proposed by the American Association of Neuromuscular & Electrodiagnostic Medicine. Additionally, the overall picture, including cytoalbuminologic dissociation, clinical presentation, and early electrodiagnostic findings were consistent with the diagnosis.
Although rare, GBS can be associated with COVID-19 infection itself, with a higher reported prevalence in males[9]. Additionally, approximately a third of COVID-19 related GBS patients do not show radiographic or clinical features of pneumonia, suggesting that GBS may develop in asymptomatic COVID-19 infection[9]. Nevertheless, given the larger proportion of reported cases in this context, the risk of GBS associated with COVID-19 infection may be higher than the suspected risk of GBS associated with COVID-19 vaccinations. While the underlying pathophysiologic mechanism remains unknown, it remains plausible that the immune response in the post-vaccination period may be trigger an autoimmune process, leading to the production of autoantibodies against myelin.
4. Conclusion
We report the first case of GBS following receiving both doses of the two-dose Pfizer COVID-19 vaccine series, diagnosed based on clinical presentation, electrodiagnostic and CSF findings. Given the current pandemic and ongoing vaccination efforts, it is essential for physicians worldwide to recognize the development of neurological complications, which may be potentially associated with the vaccination. Nevertheless, such significant adverse events remain rare, and the overall risk of neurological complications remains low.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dhama K, Khan S, Tiwari R, et al. Coronavirus Disease 2019 –COVID-19. Clin Microbiol Rev. 2020;33(4):48. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782. [DOI] [PubMed] [Google Scholar]
- [4].Jorgensen SCJ, Tse CLY, Burry L, et al. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacother J Hum Pharmacol Drug Ther. 2020;40(8):843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Statistics and Research: Coronavirus (COVID-19) Vaccinations . University of oxford: our world in data website. Oxford Martin School, University of Oxford. AccessedMarch52021, https://ourworldindata.org/covid-vaccinations [Google Scholar]
- [6].Pfizer-BioNTech COVID-19 Vaccine . US food & drug administration website. U.S. Food & Drug Administration. 2021Accessed Mar 5. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine [Google Scholar]
- [7].Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caress JB, Castoro RJ, Simmons Z, et al. COVID‐19-associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve. 2020;62(4):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abu-Rumeileh S, Abdelhak A, Foschi M, et al. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020August25. Published online. DOI: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wajih Ullah M, Qaseem A, Amray A.. Post vaccination Guillain Barre syndrome: a case report. Cureus. 2018April20. Published online. DOI: 10.7759/cureus.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].FDA briefing document: Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Published onlineFebruary26, 2021. [cited 2021 Mar 5. https://www.fda.gov/media/146217/download
- [13].Waheed S, Bayas A, Hindi F, et al. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021February18. Published online. DOI: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ogawara K, Kuwabara S, Mori M, et al. Axonal Guillain‐Barré syndrome: relation to anti‐ganglioside antibodies and campylobacter jejuni infection in Japan. Ann Neurol. 2000;48(4):624–631. [PubMed] [Google Scholar]
- [15].Preston DC, Shapiro BE. Electromyography and neuromuscular disorders: clinical-electrophysiologic-ultrasound correlations. Fourth ed. Philadelphia, PA.LElsevier; 2021. [Google Scholar]
- [16].Umapathi T, Lim CSJ, Ng BCJ, et al. Graded, electrodiagnostic criterion for Guillain-Barré syndrome that incorporates sensory nerve conduction studies. Sci Rep. 2019;9(1):7724. [DOI] [PMC free article] [PubMed] [Google Scholar]


