Abstract
Patient adherence to immunosuppressive medications adherence is crucial to survival of the patient and a transplanted kidney, yet adherence is variable. Using a prospective, descriptive design, immunosuppressive medication adherence of 44 renal transplant recipients was followed for 6 months at a Midwestern transplant center using electronic monitoring. Four medication adherence patterns emerged from a hierarchical cluster analysis: those who took medications on time, those who took medications on time with late/missed doses, those who rarely took medications on time and who were late with morning and/or evening doses, and those who missed doses. This study is a step toward developing and implementing interventions targeted to specific patterns of poor adherence.
Keywords: transplant, drug administration, nursing care, interventions, adherence, compliance, health promotion, weIlness behaviors
Survival of the transplanted kidney is dependent on taking immunosuppressive medications according to the prescriptive plan (De Geest, Abraham, Dunbar-Jacob, & Vanhaecke, 1999). Poor adherence with immunosuppressive medications in renal transplant recipients may lead to rejection, graft loss, return to dialysis, and, in extreme cases, death (De Geest et al., 1995; Douglas, Blixen, & Bartucci, 1996; Hong et al., 1992; Nevins, Kruse, Skeans, & Thomas, 2001; Shoskes, Avelino, Barba, & Sender, 1997).
Medication adherence is defined as the extent to which the patient’s medication-taking behavior corresponds with the recommendations of a health care provider (Sabate, 2003). The prevalence of poor medication adherence in individuals with chronic illnesses averages 20–30% when measured by electronic monitoring (EM; Claxton, Cramer, & Pierce, 2001). Reported rates of poor medication adherence in adult renal transplant recipients average 25% nonadherence, with ranges of 4–55% (De Geest et al., 1995; Denhaerynck et al., 2005; Isaacs, Conners, Nock, Spencer, & Lobo, 1999; Kiley, Lam, & Pollak, 1993). Poor medication adherence may involve not having the prescription filled, taking too much or not enough medication, failure to follow dosing intervals, not taking the medication for the duration of treatment, and taking medications that were not prescribed (Bosworth, 2006).
Understanding patterns of medication adherence is an important preliminary step toward developing interventions and guidelines to improve poor immunosuppressive medication adherence in adult renal transplant recipients. The purpose of this study was to examine immunosuppressive medication adherence patterns in adult renal transplant recipients.
BACKGROUND
Adult renal transplant recipients encounter many factors that are related to poor treatment adherence in the general population (Sabate, 2003). For example, recipients experience complex, long-term medication regimens. Post-transplant discharge instructions commonly include 20 or more different medications. After 3 months, the number of medications is reduced, but immunosuppressants must be taken for the life of the renal transplant. Daily medication dosing frequency for many medications are twice or three times daily (Karch, 2002). Regimens (drug, dosing, and frequency) are adjusted frequently in the first few months after transplantation. Weekly changes in immunosuppression dosing are typical.
Immunosuppressive medications may have to be paid for by the patient, especially in health care systems like the one that exists in the US where these drugs are not fully covered by health insurance. Medication expenses over $1,200 per month are not uncommon.
Finally, the medications often produce distressing symptoms associated with side effects, including fatigue, increased appetite, sleeplessness, trembling hands, brittle skin, difficulties with concentration and sight, bruises, mood swings, pain in the joints and back, muscle weakness, hair loss, impotence, painful menstral flow, sensitivity to light, anxiety, and increased hair growth (Moons, De Geest, Abraham, Van Cleemput, & Vanhaecke, 1998).
Information about patterns of adherence is lacking in the renal transplant medication adherence research literature. Researchers have examined medication adherence by summarizing the number of medication events each day, calculating the percentage of prescribed dosing days with correct intake (adherence rate), percentage of prescribed dose taken, and percentage of drug holidays (one or more days without drug intake). Although these calculations are helpful, they do not allow for exploration of the patterns of taking medications through examining precise timing of drug administration (Vrijens & Goetghebeur, 1997).
A medication can be taken on time, early, late, or not taken. For example, twice-daily doses should be taken 12 ± 3 hours apart for maximum benefit (Karch, 2002). The three monitored drugs, cyclosporine, mycophenolate mofetil, and tacrolimus are metabolized in the liver with half-life variability from 11 to 19 hours (Karch). In addition, setting a timing goal of 25% of the dosing interval is recommended because dosing intervals are determined to maximize drug bioavailability and effectiveness.
Medication adherence patterns more accurately characterize this complex behavior than previously employed medication adherence calculations. Exploring medication adherence patterns through identifying on-time, early, late, or missing patterns for each medication-taking day is a first step in developing interventions that are tailored to an individual’s medication adherence patterns. Self-medication management programs can then be individualized to assist renal transplant recipients in taking their immunosuppressive medications more effectively. For example, if medication-taking patterns indicate that the evening dose is frequently taken late, healthcare providers can explore with their patients cues and reminders that will result in better timing of the evening dose.
Medication adherence has been measured using a variety of methods (Papajcik, Mastroianni, Goormastic, & Flechner, 1999; Siegal, 1993). Researchers have measured medication adherence by self-report, provider and family reports, pill counts, filled prescriptions, medication blood levels, and, in rare instances, EM (Butler, Peveler, Roderick, Horne, & Mason, 2004; De Geest & Vanhaecke, 1999; Nevins et al., 2001). EM is considered to be the closest to a gold standard in medication adherence monitoring (Chaisson et al., 2001; de Klerk, van der Heijde, Landewe, van der Tempel, & van der Linden, 2003; Paterson, Potoski, & Capitano, 2002; Vrijens & Urquhart, 2005).
Study limitations in previous work also include problems with sampling strategies. Except for Weng et al. (2005), researchers examining medication adherence have included only participants who have had their renal transplant for 1 year or longer (De Geest et al., 1995; Kiley et al., 1993; Raiz, Kilty, Henry, & Ferguson, 1999; Weng etal.). Those with poor adherence during their first year of transplant generally have been excluded, which limits the generalizability of the findings. Including transplant recipients who vary in length of time since transplant enhances the generalizability of study results.
The research question to be addressed in this study is “What are the patterns of immunosuppressive medication adherence in adult renal transplant recipients?”
METHODS
We used a prospective, longitudinal design to examine adult transplant recipients’ medication-taking behavior. Immunosuppressive medications were monitored during the study.
Setting and Sample
A sample of 51 renal transplant recipients was recruited from a renal transplant program in the Midwest. Studies using the cluster analysis procedure have included similar sample sizes (Stuifbergen, 1990; Toraldo, Nicolardi, De Nuccio, Lorenzo, & Ambrosino, 2005; Tousignant, Arsenault, Corriveau, & Phillippe, 2000). The variable medication adherence was measured for 6 months for immunosuppressive medications using the Medication Event Monitoring System (MEMS; MEMS Track CapTM; Aprex Corp., Union City, CA). Inclusion criteria were: (a) 18 years of age or older; (b) prescribed at least two immunosuppressive agents administered twice daily; (c) functioning renal transplant (not on dialysis); (d) transplant physician and nephrologist’s assent that recipient is able to participate in the study; (e) able to speak, hear, and understand English, as determined by the ability to participate in and comprehend conversation about potential inclusion in the study; (f) able to open a MEMS cap, as assessed by the research associate (RA) asking if there is any problem with opening pill bottle caps; (g) willing to use the MEMS cap to dispense immunosuppressive medications; and (h) independent medication management. Exclusion criteria were: (a) cognitive impairment as determined by a score of 23 or below on the Mini Mental State Exam (MMSE); (b) other diagnoses that might shorten the life span, such as metastatic cancer, as determined by the transplant physician statement; and (c) post-transplant care provided by another transplant center.
The clinical nurse specialist (CNS) who supervised the care of all adult renal transplant recipients identified recipients meeting study inclusion and exclusion criteria. The CNS then contacted all eligible recipients and asked if they were willing to be contacted by the research team to discuss possible involvement in the project. If recipients agreed to be contacted, the RA invited them to give verbal consent to participate and arranged to meet them in person. At that meeting, all information previously reviewed over the phone was reviewed again. Written consent was obtained by the RA from those participants who agreed to be in the study.
Table 1 profiles the sample. The average age was 51.73 years (SD = 11.39). The most frequent etiology of renal disease was polycystic kidney disease at 30% (n = 13), followed by diabetes mellitus 22% (n = 10), and hypertension 18% (n = 8). A majority of participants had received deceased donor kidney transplants followed by living related kidney transplants. Twenty-three percent of the sample had received a previous kidney transplant (n = 10). The mean time since transplant was 1,443.82 days (SD = 1,198.16; range, 51–4,395), or 3.95 years.
Table 1.
Demographic Characteristics of the Sample (N = 44)
| Demographic factor | Demographic detail | N | Percent |
|---|---|---|---|
| Sex | Female | 24 | 55 |
| Male | 20 | 45 | |
| Education level | High school/some high school | 26 | 59 |
| Some college/college graduate | 18 | 41 | |
| Ethnicity | Caucasian | 39 | 86 |
| African American | 6 | 14 | |
| Marital Status | Married | 27 | 61 |
| Divorced/never married/widowed | 17 | 39 | |
| Employment status | Disabled | 24 | 55 |
| Full time | 11 | 25 | |
| Unemployed/retired | 5 | 11 | |
| Part time | 4 | 9 | |
| Immunosuppressive | Cyclosporine/sandimmune/gengraf/neoral | 22 | 50 |
| Tacrolimus | 18 | 41 | |
| Mycophenolate mofetil | 4 | 9 | |
| Type of transplant | Deceased donor | 38 | 86 |
| Living related donor | 6 | 14 | |
| Time since transplant | M = 1,443.82 days; SD = 1,198.16; Range = 51–4,395 | ||
To decrease the attrition rate, all participants were paid for their contribution to the study. Participants were paid $10.00 at the beginning of the study and an additional $10.00 after successful completion of medication adherence monitoring.
Instruments
Medication event monitoring system.
Medication adherence was measured using the MEMS and MEMS diary. Medication adherence data were retrieved from the MEMS caps. The MEMS V TrackCAP is a medication bottle cap containing microelectronics that record each cap removal and the date and time of the removal (Claxton et al., 2001). The battery life of the MEMS V is 36 months. When retrieved from participants, the caps were connected to a microcomputer communications port. Each participant’s medication record was downloaded from the cap to a personal computer containing MEMS software that decoded the cap monitor data. Because accidental cap openings might have occurred, the MEMS diary was used to document these events. The MEMS cap data were corrected by the principal investigator using the MEMS diary data. After corrections were made, each cap removal was presumed to represent the patient ingesting one dose of the prescribed immunosuppressant medication.
Several limitations must be carefully considered when using MEMS data. One limitation is that the device cannot distinguish between accidental and purposeful openings. To overcome this limitation, multiple openings within a 15-minute interval, which are most likely due to mishandling the cap, were eliminated from the data as Nevins et al. (2001) recommended. In addition, each participant was asked to maintain a MEMS diary. Participants were asked to document the date and time of any accidental or early cap openings. The diary has been shown to be a useful adjunct to MEMS (Oliveri, Matsui, Hermann, & Koren, 1991). An additional limitation is that if the cap is not opened, the assumption is that the medication was not consumed. Participants may not want to carry their pill bottle with them and may remove several doses at once. To address this limitation, participants were aware that medication adherence was being monitored by the MEMS cap (Oliveriet al.) and a MEMS diary was maintained. Another limitation is that MEMS cannot actually confirm consumption of the medication (Choo et al., 2001). Finally, the initial use of the MEMS may have an intervention effect. To offset this, the first month of data was eliminated from the analysis.
Procedure
The study was approved by the principal investigator’s Institutional Review Board. After written consent was obtained, each participant received training from the RA on the use of the MEMS for6 months with each of the two immunosuppressive medications. The RA requested that each participant provide a successful return demonstration on the use of the MEMS prior to beginning the study. For patients using a pillbox to store their medications, a small marker was provided for them to place in the pillbox that reminded them the medication must be removed from the vial with the MEMS cap. The RA also provided each participant with a MEMS diary in which to document any deviations from routine opening of the MEMS cap and medication administration. They were instructed to document the date, time, and circumstances when the MEMS cap was opened and a medication was not administered. The participants were instructed to continue their routine procedure for obtaining medication refills.
Once a refill was obtained, they were instructed to place their refill medications in the MEMS cap medication bottle. Participants were to make a note in the diary if they refilled their MEMS cap medication bottle at a time when they did not open the bottle to take a medication so that the validity of the data could be maintained. In addition, to confirm that participants were comfortable with the device, the RA contacted them by telephone each week during the first month of using the MEMS and asked if they had any questions or concerns about its use or the use of the MEMS diary. After 6 months, the RA met with each participant during which time the participant returned the device to the RA so that data could be retrieved via the MEMS software program.
Data Analysis
The project biostatistician conducted all data analyses using SAS (v9.1, SAS Institute, Inc., Cary, NC). Adherence patterns were determined for each individual in the study. Medication adherence was defined as 16 possible patterns delineated in Table 2. Pattern 0 is defined as self-administering the immunosuppressive medication within a 3-hour prescribed window in the morning and evening. The other 15 patterns are defined as self-administering at least one of the immunosuppressive medication doses outside of this window. The various patterns were set by the following logic. One medication was supposed to be taken at a specific target time in the morning and a second taken at a target time 12 hours later. The target time was the prescribed time the patient was to take the immunosuppressive medication. For example, if the target time for the medications was 8:00 in the morning and 8:00 in the evening, and if the medication was taken at 8:30 in the morning and 10:00 in the evening, it would have been taken on time and late, or pattern number 2. For each medication, one of four possibilities could occur: the medication could be taken early, on time, late, or missed. On time was defined to be within 90 minutes on either side of the target time. The unit of analysis was the day. Because there are four possible outcomes for each medication on a given day, 16 different patterns were possible for each day. The adherence pattern included the proportion of days (vector P1, P2, …P16) that the participant’s medication-taking fell into each of the 16 adherence categories described in Table 2. For example, a participant with proportions in the 16categoriesof(.90,.06,.04,0,0,0,0,0,0,0,0,0, 0, 0, 0, 0) would have taken both medications on time on 90% of the days, would have taken the morning dose on time and the evening dose early on 6% of the days, and would have taken the morning dose on time and the evening dose late on 4% of the days. Simple summary statistics, including the mean, and minimum and maximum values for each component of the patterns, were calculated. For example, in adherence pattern 1, the morning dose of the medication was taken within the prescribed 3-hour time window, while the evening dose of the medication was taken before the 3-hour time window.
Table 2.
Possible Medication Adherence and Nonadherence Patterns and Assigned Scores
| AM dose | PM dose | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Adherence pattern | Early | On time | Late | Early | On time | Late | Adherence score |
| Adherence 0 | х | х | 1.0 | ||||
| Nonadherence 1 | х | х | .75 | ||||
| Nonadherence 2 | х | х | .75 | ||||
| Nonadherence 3 | х | х | .75 | ||||
| Nonadherence 4 | х | х | .75 | ||||
| Nonadherence 5 | х | х | .50 | ||||
| Nonadherence 6 | х | х | .50 | ||||
| Nonadherence 7 | х | х | .50 | ||||
| Nonadherence 8 | х | х | .50 | ||||
| Nonadherence 9 | х | .50 | |||||
| Nonadherence 10 | х | .50 | |||||
| Nonadherence 11 | х | .25 | |||||
| Nonadherence 12 | х | .25 | |||||
| Nonadherence 13 | х | .25 | |||||
| Nonadherence 14 | х | .25 | |||||
| Nonadherence 15 | 0.0 | ||||||
X = presumed administration of immunosuppressive medication dosage.
Determining the patterns of taking medications (early, on time, late, or missed) was one of the primary aims of this study. Having a numeric score or summary number relating to each daily pattern is also important. For this reason, a point system was defined as follows: .5 was assigned if the dose of the immunosuppressive medication was taken within a 3-hour window (±1.5 hours of the prescribed time); .25 was assigned if the dose of the immunosuppressive medication was not within the 3-hour window but was taken within a 12-hour window (±6 hours of the prescribed time), and 0 was assigned if the dose of the immunosuppressive medication was not taken within a 12-hour window (±6 hours of the prescribed time, i.e., if the dose was missed). On each day, an individual could be assigned a score of 0, .25, .50, .75 or 1 points. The medication adherence score for a participant was the average score over all days that in this study, which involved 5 months of MEMS use, was about 150 days. The point system was developed to quantify degrees of departure from the targeted time, with lower points indicating further departure from adherence. This system allowed for more detailed quantification of medication adherence.
Demographic data were analyzed via descriptive statistics. The CLUSTER procedure in SAS was used to complete hierarchical clustering as a way of finding groups of participants with similar adherence patterns. Further exploration of the characteristics of participants in each cluster was done using Wilcoxon Rank Sums and the Kruskal–Wallis test.
FINDINGS
Fifty-one persons consented to participate in the study. Of this group, 44 completed use of the MEMS caps for 6 months (an 86% retention rate). Reasons for not completing the study included: developed psychological issues that made using the MEMS difficult (n = 1), stopped using MEMS due to inconvenience of using at home or work (n = 2), moved and did not return MEMS (n = 1), and death unrelated to the study (n = 1). One participant never returned the MEMS even after repeated follow up, and another provided no reason for not using the MEMS.
Each participant identified the two immunosuppressive medications they were prescribed at the start of the study when asked “What immunosuppressive medications are you taking?” For every person, two immunosuppressive medications each taken twice a day were monitored using the MEMS caps. Results indicated that 93% of the time, MEMS caps openings for the two immunosuppressive medications were within 10 minutes of each other; 80% of the time, the openings were within 5 minutes of each other. Because we found such similar intra-participant adherence between the two drugs, the first drug the patient identified was used for the subsequent analysis. If we had completed the analysis for both drugs, we believe we would have nearly identical findings for each medication.
Medication Adherence Scores
To decrease the possibility that the monthly telephone calls during the first month of EM could have introduced an intervention effect, the first 30 days of EM data were eliminated prior to beginning the analysis. Medication adherence score results for the group of 44 participants showed that 75% of them had a medication adherence score of .90 or better. Further examination of the distribution of the medication adherence scores indicated that they were skewed to the right. Therefore, the median medication adherence score would be more representative of the sample than the mean score. The median score was .75. A score of .75 corresponds to one of the two medication doses being taken on time and the other being taken either late or early (.50+.25 = .75). No correlation was found between medication adherence scores and age (r = 0.17; p = 0.28), gender χ2 (1, N = 44) = 1.62, p =.20), ethnicity χ2 (1, N =44) = 1.20, p = 0.27), marital status χ2 (3, N =44)=2.54, p =.47), employment χ2 (4, N =44)=5.84, p =0.21), or days since transplantation, defined as the time since transplant to the date of beginning MEMS use (r =.0055; p =.97).
Medication Adherence Patterns
The 44 participants monitored for 5 months produced a total of 6,681 monitored days. Of the 16 possible patterns, the most frequently observed medication adherence pattern observed in the combined sample was pattern 0 at 46.64%, indicating that both the morning and evening medication doses were taken on time (n =3,119). Table 3 shows the medication adherence and nonadherence details by frequency.
Table 3.
Medication Adherence and Nonadherence Pattern Detail (N = 6,681)
| Pattern detail | ||||
|---|---|---|---|---|
|
|
||||
| Morning Dose | Evening dose | Pattern | N | Percent |
| On time | On time | 0 | 3119 | 46.64 |
| Missed | Missed | 15 | 1100 | 16.47 |
| On time | Late | 2 | 588 | 8.77 |
| Late | On time | 4 | 496 | 7.47 |
| On time | Missed | 9 | 367 | 5.48 |
| Missed | On time | 10 | 326 | 4.87 |
| Late | Late | 8 | 263 | 3.95 |
| Missed | Late | 14 | 107 | 1.60 |
| On time | Early | 1 | 93 | 1.40 |
| Late | Missed | 12 | 89 | 1.34 |
| Early | On time | 3 | 72 | 1.08 |
| Missed | Early | 13 | 23 | .34 |
| Early | Missed | 11 | 12 | .18 |
| Early | Late | 6 | 10 | .15 |
| Early | Early | 5 | 8 | .12 |
| Late | Early | 7 | 8 | .12 |
Clusters of Medication Adherence Patterns
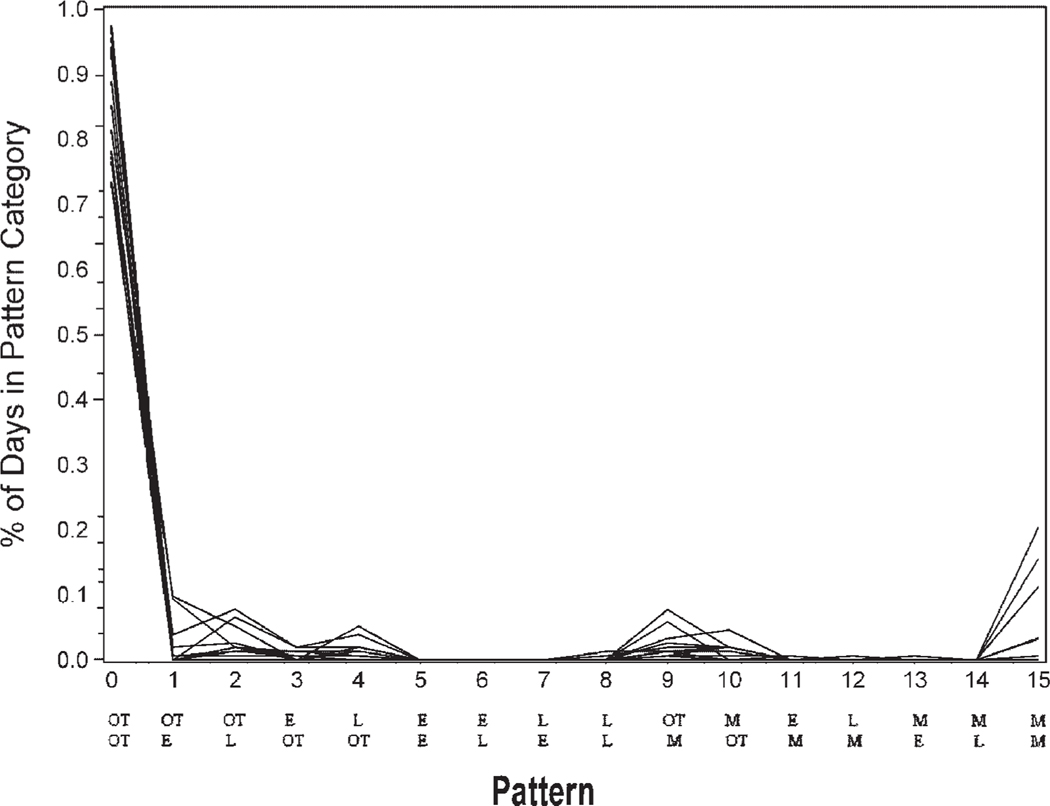
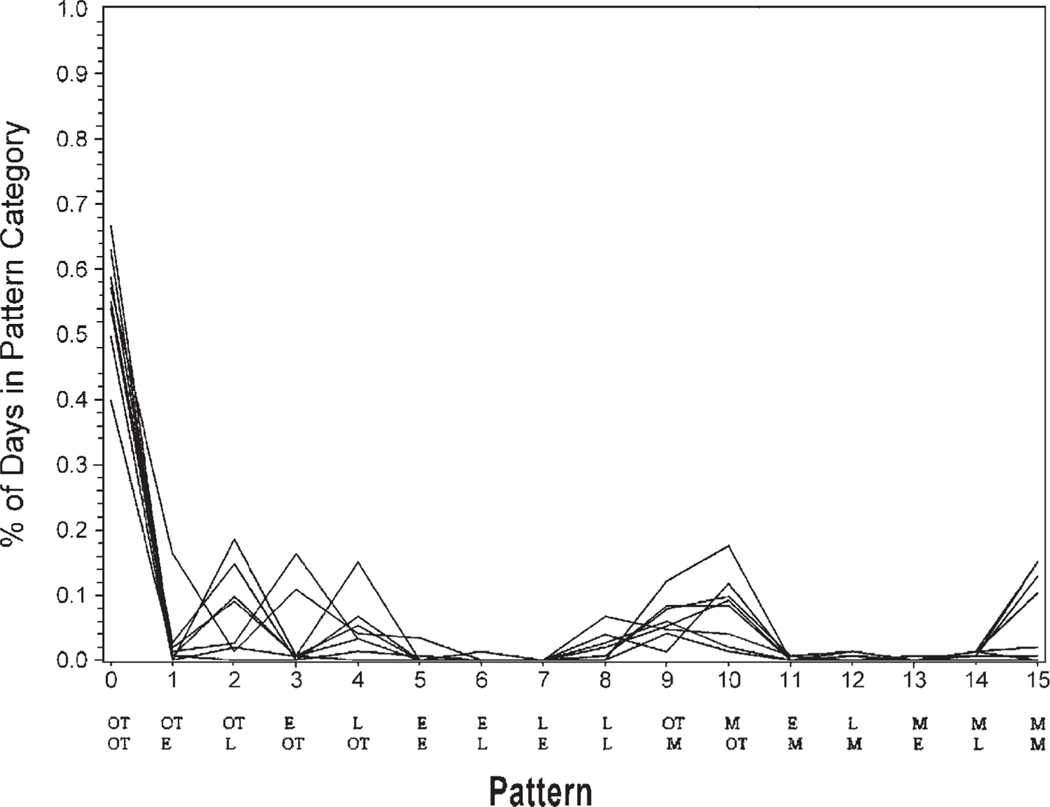
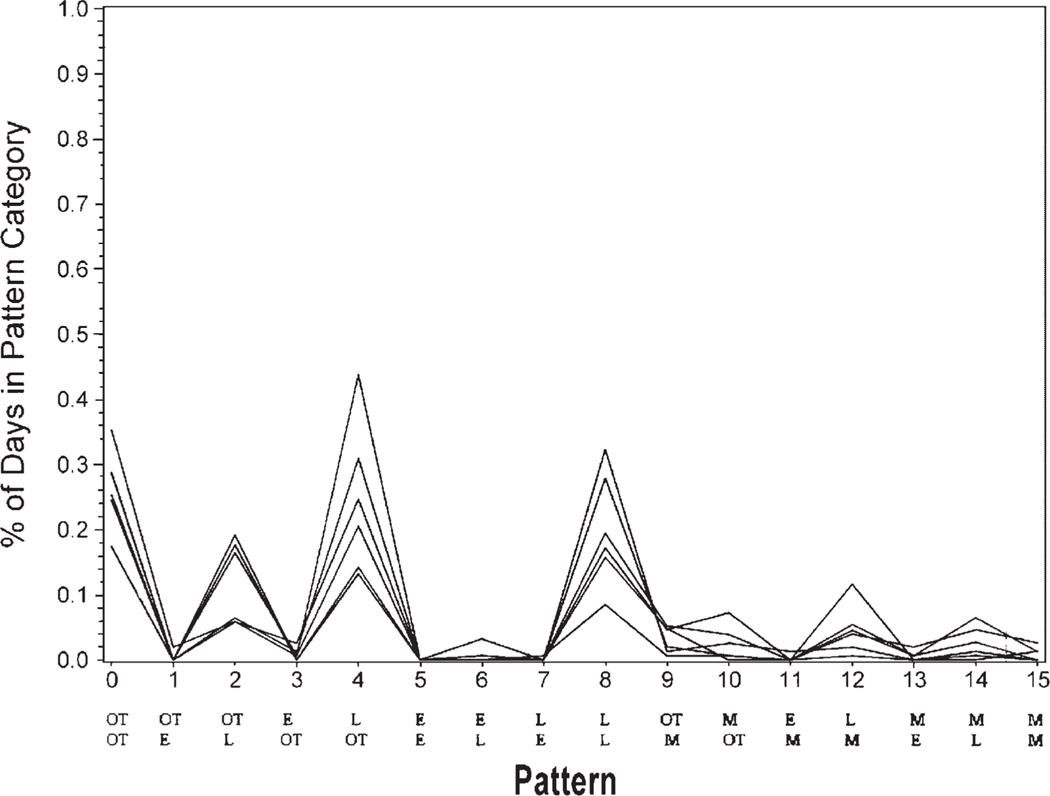
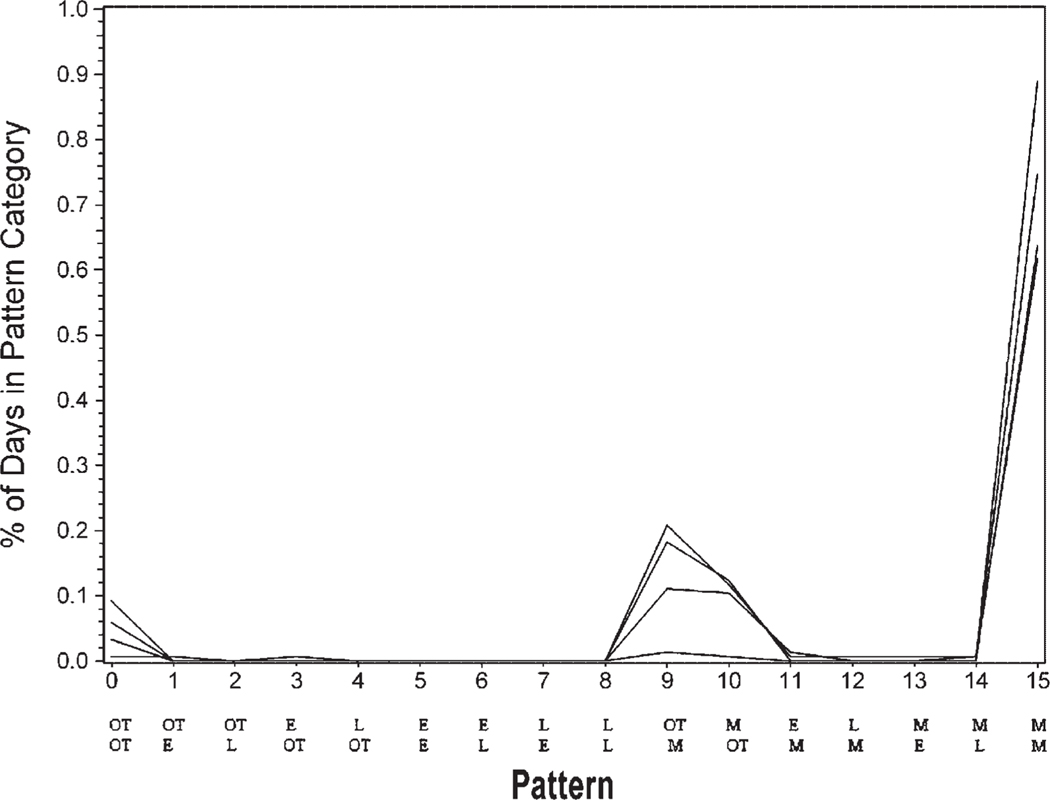
The data initially were reduced to 11 clusters as calculated by the CLUSTER procedure in SAS. Closer examination of these clusters indicated that 7 of the clusters contained only 1–3 participants. None of the 7 clusters had distinct similarities. Yet, 4 of the clusters with 4–14 participants each had distinguishable adherence patterns. Cluster 1 (n =14) included the group that took both morning and evening medications on time a high percentage of time (Fig. 1). Cluster 2 (n =8) included the group that frequently took morning and evening medications on time but were sometimes late or missed medications (Fig. 2). Cluster 3 (n =6) included the group who very rarely took both medications on time and were often late with morning and/or evening medications (Fig. 3). Cluster 4 (n =4) included the group that missed many of the morning and/or evening medications (Fig. 4). The mean adherence score for each cluster was .92, .78, .70, and .16, respectively. The Kruskal–Wallis test indicated significant differences in medication adherence scores between the four groups (χ =24.03, df =3, p < .0001).
FIGURE 1.
Medication adherence patterns cluster 1 (n= 14) Legend: OT is on time. L is late. M is missing. E is early with medication taking. The top row of abbreviations is the morning dose timing. The bottom row is the evening dose timing.
FIGURE 2.
Medication adherence patterns cluster 2 (n = 8) Legend: OT is on time. L is late. M is missing. E is early with medication taking. The top row of abbreviations is the morning dose timing. The bottom row is the evening dose timing.
FIGURE 3.
Medication adherence patterns cluster 3 (n = 6) Legend: OT is on time. L is late. M is missing. E is early with medication taking. The top row of abbreviations is the morning dose timing. The bottom row is the evening dose timing.
FIGURE 4.
Medication adherence patterns cluster 4 (n = 4) Legend: OT is on time. L is late. M is missing. E is early with medication taking. The top row of abbreviations is the morning dose timing. The bottom row is the evening dose timing.
The medication adherence scores for those persons falling outside of the four major clusters were examined. Four of them had deliberately scheduled their medication taking so that there were 14–15 hours between the morning and evening doses.
DISCUSSION
Our study provides several distinct contributions to the body of knowledge regarding medication adherence in adult renal transplant recipients. Previous researchers have used EM to examine medication adherence in adult renal transplant patients, reporting the number of medication events each day by calculating the percentage of prescribed dosing days with correct intake (adherence rate), percentage of prescribed dose taken, and percentage of drug holidays (one or more days without drug intake; Feldman, Hackett, Bilker, & Strom, 1999; Nevins et al., 2001). Such data summaries offer little direction for developing targeted interventions to address medication adherence problems distinctive to individuals. Our study is unique in that patterns of taking a twice-daily dose of immunosuppressive medications early, on time, late, or missed were identified. The large number of clusters initially identified (11) provides an indication of the complexity of possible medication adherence patterns. Of the 11, 4 distinct clusters of medication adherence patterns were identified, which included 32 of the 44 adult renal transplant recipients in this study. Almost no one in any of the clusters took medications early. The most common cluster involved both morning and evening immunosuppressive medications being taken on time. Overall medication adherences scores in this cluster were high with a mean of .92, which represents taking both morning and evening doses on time most days and the other either early or late. Interventions with this adherent group would involve support and encouragement to continue this outstanding pattern of on time medication taking. This support and encouragement could be delivered in a variety of oral or written modes, including traditional mailing, computers, cellular phones, or beepers. Most participants appeared to be compliant with their immunosuppressive medications. Identification of patterns of poor immunosuppressive medication adherence in adult renal transplant recipients will guide identification and treatment of those most at risk for poor outcomes.
Patterns for cluster 2 indicated that both morning and evening medication were generally taken on time, but frequently one of the doses, either morning or evening, was either late or missed. Adherence could be enhanced in this group through interventions encouraging the positive efforts at adherence, while exploring the conditions that result in late or missed medication taking. For example, if participants indicated that medications were taken late due to frequent oversleeping, developing a routine strategy of setting an alarm clock to enhance medication timing might be appropriate. With medications available at the bedside for quick and easy administration, medication adherence could be improved.
Immunosuppressive medication-taking patterns for participants in cluster 3 indicated that medications were available to be taken, but this group may have had routines interrupted or may have had a lack of reminders causing late administration of morning and/or evening doses. These individuals might benefit from interventions focused on cues and reminders. For example, if the evening dose is routinely taken late due to arriving home late from work, the transplant healthcare provider could assist the recipient in problem-solving other options to assist in on time administration of the medications, including keeping extra doses of the medication at work or in the car in an appropriate container. Renal transplant recipients have indicated that planning ahead, organizing, and using cues make taking immunosuppressive medications easier, while changes in routine make this activity more difficult (Russell, Kilburn, Conn, Libbus, & Ashbaugh, 2003). Associating medication taking with routine activities such as brushing teeth or eating meals, or simply keeping medications in plain view of routine activities, have been cited as helpful by adult renal transplant recipients (Russell et al.).
The fourth most prevalent cluster, cluster 4, involved more participants missing both the morning and evening doses (n = 4). The overall medication adherence score for this cluster was the lowest at .16. In addition to exploring the use of cues and reminders, further assessment of medication-taking patterns with these individuals should occur. Specifically, access to medications should be evaluated. Any difficulty in obtaining medication refills should be explored. For example, the healthcare provider could conduct an intensive assessment and subsequent education, support, and follow up for this subgroup. If patients are having difficulty obtaining medication refills from the pharmacy, the healthcare provider could intervene to facilitate this process. The participants in our study resided in a state that provides financial support for immunosuppressive medications. Cost was, therefore, not a factor for them, although it may be for other renal transplant recipients (Sisson, Tripp, Paris, Cooper, & Zuhdi, 1994).
Twelve of the 44 participants did not clearly fall into any of the 4 clusters. Four of them chose to schedule their immunosuppressive medications with 14–15 hours between their morning and evening doses. Although these four persons violated the 12-hour dosing guidelines and may have allowed the trough levels of their immunosuppressive agents to dip low enough to risk rejection, two of the four did appear to administer their medication very close to their self-established 14–15 hour dosing interval. The other two appeared to have taken doses less closely to their self-established dosing intervals. These findings indicate that medication counseling and education are warranted concerning the importance of taking immunosuppressive medications 12 hours apart, the impact of maintaining consistent drug levels, and potential consequences of 14–15 hours between doses.
An additional contribution of this study was inclusion of adult renal transplant recipients at varying times since transplant. Although this group had a transplant for an average of almost 4 years, the range of times since transplant was broad, from 51 days to just over 12 years. In this small group of adult renal transplant recipients, time since transplant was not correlated with medication adherence which is inconsistent with other findings. Greenstein and Siegal (1998) and Siegal (1993), in studies with sample sizes of 1,402 and 519, respectively, discovered that longer time since transplant was significantly associated with poorer adherence in adult renal transplant recipients. In addition, Chisholm et al. (2000) found that in 18 renal transplant recipients, immunosuppressive medication adherence decreased over the first 12 months of renal transplantation. Given the inconsistency of these findings with our findings, this variable warrants continued study.
We considered blood level monitoring for measuring medication adherence. MEMS was selected over blood level monitoring because of its ability continuously to document medication adherence. Blood level monitoring may directly measure medication administration. Yet, it is not a reliable measure to capture medication taking due to medication short half-life and failure to illustrate the dynamic nature of medication taking. In addition, blood level monitoring may not be reliable because patients can improve medication adherence just prior to an office visit to obtain therapeutic blood levels.
There are several important limitations of this project. The inclusion of a small, convenience sample of adult renal transplant recipients from a geographically homogeneous location limits the generalizability of the results. Moreover, an assumption was made that the participants self-administered medication when medication adherence was measured with the MEMS. In addition, the MEMS may have recorded cap removal when the medication was not administered (e.g., with accidental cap removal or deliberate early removal of medications). Use of the MEMS diary offset this limitation. The potential does exist for the use of MEMS to interfere with adherence strategies that the patient has in place. Specifically, if the MEMS was used with a pillbox, the patient may not be able to determine if the medication has been previously administered. Finally, although there are many other important issues surrounding medication taking (e.g., medication safety), the focus of this project was upon medication adherence (Barker, Flynn, Pepper, Bates, & Mikeal, 2002).
CONCLUSIONS
We sought to move beyond previous studies by describing the daily patterns of taking immunesuppressive medications through examining precise timing of daily drug administration. Four major medication adherence patterns emerged from a hierarchical cluster analysis: (a) those who took medications on time; (b) those who took medications on time with late and missed doses; (c) those who rarely took medications on time and who were often late with morning and/or evening doses; (d) and those who missed doses. Almost no medications were taken early. Taking immunosuppressive medications according to the prescribed plan is crucial to survival of the transplanted kidney. This study is an important first step toward gaining the necessary knowledge to guide identification of patients at risk for poor adherence, and to develop and implement interventions targeted to specific patterns of nonadherence.
Acknowledgments
Contract grant sponsor: National Kidney Foundation.
Contract grant sponsor: American Nurses Foundation/Sigma Theta Tau.
We would like to thank Sabina De Geest, PhD, RN, Professor of Nursing and Director Institutional Affiliation: Institute of Nursing Science, University of Basel for her support and guidance on this project.
REFERENCES
- Barker KN, Flynn EA, Pepper GA, Bates DW, & Mikeal RL (2002). Medication errors observed in 36 health care facilities. Archives of Internal Medicine, 162, 1897–1903. [DOI] [PubMed] [Google Scholar]
- Bosworth HB (2006). Medication treatment adherence. In Bosworth HB, Oddone EZ, & Weinberger M. (Eds.), Patient treatment adherence: Concepts, interventions, and measurement (pp. 147–194). Mahwah, NJ: Erlbaum. [Google Scholar]
- Butler JA, Peveler RC, Roderick P, Horne R, & Mason JC (2004). Measuring compliance with drug regimens after renal transplantation: Comparison of self-report and clinician rating with electronic monitoring. Transplantation, 77, 786–789. [DOI] [PubMed] [Google Scholar]
- Chaisson RE, Barnes GL, Hackman J, Watkinson L, Kimbrough L, Metha S, et al. (2001). A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. American Journal of Medicine, 110, 610–615. [DOI] [PubMed] [Google Scholar]
- Choo PW, Rand CS, Inui TS, Lee MT, Canning C, & Platt R. (2001). Derivation of adherence metrics from electronic dosing records. Journal of Clinical Epidemiology, 54, 619–626. [DOI] [PubMed] [Google Scholar]
- Chisholm MA, Vollenweider LJ, Mulloy LL, Jagadeesan M, Wynn JJ, Rogers HE, et al. (2000). Renal transplant patient compliance with free immunosuppressive medications. Transplantation, 70, 1240–1244. [DOI] [PubMed] [Google Scholar]
- Claxton AJ, Cramer J, & Pierce C. (2001). A systematic review of the associations between dose regimens and medication compliance. Clinical Therapeutics, 23, 1296–1310. [DOI] [PubMed] [Google Scholar]
- De Geest S, Abraham I, Dunbar-Jacob J, & Vanhaecke J. (1999). Behavioral strategies for long-term survival of transplant recipients. In: Metry J. & Meyer U. (Eds.), Drug regimen compliance:Issues in clinical trials and patient management (pp.163–180). Chichester, NY: John Wiley & Sons. [Google Scholar]
- De Geest S, Borgermans L, Gemoets H, Abraham I, Vlaminck H, Evers G, et al. (1995). Incidence, determinants, and consequences of subclinical noncompliance with immunosuppressive therapy in renal transplant recipients. Transplantation, 59, 340–347. [PubMed] [Google Scholar]
- De Geest S, & Vanhaecke J. (1999). Methodological issues in transplant compliance research. Transplantation Proceedings, 31, 81S–83S. [DOI] [PubMed] [Google Scholar]
- de Klerk E, van der Heijde D, Landewe R, van der Tempel H, & van der Linden S. (2003). The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: A validation study. Journal of Rheumatology, 30, 2469–2475. [PubMed] [Google Scholar]
- Denhaerynck K, Dobbels F, Cleemput I, Desmyttere A, Schafer-Keller P, Schaub S, & De Geest S. (2005). Prevalance, consequences, and determinants of nonadherence in adult renal transplant patients: A literature review. Transplant International, 18, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Douglas S, Blixen C, & Bartucci R. (1996). Relationship between pretransplant noncompliance and posttransplant outcomes in renal transplant recipients. Journal of Transplant Coordination, 6, 53–58. [DOI] [PubMed] [Google Scholar]
- Feldman HI, Hackett M, Bilker W, & Strom B. (1999). Potential utility of electronic drug compliance monitoring in measures of adverse outcomes assoicated with immunosuppressive agents. Pharmacoepidemiology and Drug Safety, 8, 1–14. [DOI] [PubMed] [Google Scholar]
- Greenstein S, & Siegal B. (1998). Compliance and noncompliance in patients with a functioning renal transplant: A multicenter study. Transplantation, 66, 1718–1726. [DOI] [PubMed] [Google Scholar]
- Hong JH, Sumrani N, Delaney V, Davis R, Dibenedetto A, & Butt KM (1992). Causes of late renal allograft failure in the ciclosporin era. Nephron, 62, 272–279. [DOI] [PubMed] [Google Scholar]
- Isaacs RB, Conners A Jr., Nock S, Spencer C, & Lobo P. (1999). Noncompliance in living-related donor renal transplantation: The United Network of Organ Sharing experience. Transplantation Proceedings, 31, 19S–20S. [DOI] [PubMed] [Google Scholar]
- Karch AM (2002). 2002 Lippincott’s nursing drug guide. Philadelphia: Lippincott. [Google Scholar]
- Kiley DJ, Lam CS, & Pollak R. (1993). A study of treatment compliance following kidney transplantation. Transplantation, 55, 51–56. [DOI] [PubMed] [Google Scholar]
- Moons P, De Geest S, Abraham I, Van Cleemput J, & Vanhaecke J. (1998). Symptom experience associated with maintenance immunosuppression after heart transplantation: patients’ appraisal of side effects. Heart & Lung: Journal of Acute & Critical Care, 27, 315–325. [DOI] [PubMed] [Google Scholar]
- Nevins TE, Kruse L, Skeans MA, & Thomas W. (2001). The natural history of azathioprine compliance after renal transplantation. Kidney International, 60, 1565–1570. [DOI] [PubMed] [Google Scholar]
- Oliveri NF, Matsui D, Hermann C, & Koren G. (1991). Compliance assessed by the Medication Event Monitoring System. Archives of Disease in Childhood, 66, 1399–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papajcik D, Mastroianni B, Goormastic M, & Flechner SM (1999). A tool to identify risk factors for noncompliance in the adult renal transplant recipient. Transplantation Proceedings, 31, 84S–86S. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Potoski B, & Capitano B. (2002). Measurement of adherence to antiretroviral medications. Journal of Acquired Immune Deficiency Syndromes: JAIDS, 31, S103–S106. [DOI] [PubMed] [Google Scholar]
- Raiz LR, Kilty KM, Henry ML, & Ferguson RM (1999). Medication compliance following renal transplantation. Transplantation, 68, 51–55. [DOI] [PubMed] [Google Scholar]
- Russell CL, Kilburn E, Conn VS, Libbus MK, & Ashbaugh C. (2003). Medication taking beliefs of adult renal transplant recipients. Clinical Nurse Specialist, 17, 200–208. [DOI] [PubMed] [Google Scholar]
- Sabate E. (2003). Adherence to long-term therapies: Evidence for action. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Shoskes DA, Avelino L, Barba L, & Sender M. (1997). Patient death or renal graft loss within 3 yr of transplantation in a county hospital: Importance of poor initial graft function. Clinical Transplantation, 11, 618–622. [PubMed] [Google Scholar]
- Siegal BR (1993). Postrenal transplant compliance: Report of 519 responses to a self-report question-naire. Transplantation Proceedings, 25, 2502. [PubMed] [Google Scholar]
- Sisson S, Tripp J, Paris W, Cooper DK, & Zuhdi N. (1994). Medication noncompliance and its relationship to financial factors after heart transplantation [Letter]. Journal of Heart & Lung Transplantation, 13, 930. [PubMed] [Google Scholar]
- Stuifbergen AK (1990). Patterns of functioning in families with a chronically ill parent: An exploratory study. Research in Nursing & Health, 13, 35–44. [DOI] [PubMed] [Google Scholar]
- Toraldo DM, Nicolardi G, De Nuccio F, Lorenzo R, & Ambrosino N. (2005). Pattern of variables describing desaturator COPD patients, as revealed by cluster analysis. Chest, 128, 3828–3837. [DOI] [PubMed] [Google Scholar]
- Tousignant M, Arsenault AB, Corriveau H, & Phillippe P. (2000). Clinical evaluation of patient following stroke: Proposed stroke patient taxonomy based on cluster analysis method. Physiotherapy Theory and Practice, 16, 81–93. [Google Scholar]
- Vrijens B, & Goetghebeur E. (1997). Comparing compliance patterns between randomized treatments. Controlled Clinical Trials, 18, 187–203. [DOI] [PubMed] [Google Scholar]
- Vrijens B, & Urquhart J. (2005). Patient adherence to prescribed antimicrobial drug dosing regimens. Journal of Antimicrobial Chemotherapy, 55, 616–627. [DOI] [PubMed] [Google Scholar]
- Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, et al. ( 2005). Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. Journal of the American Society of Nephrology, 16, 1839–1848. [DOI] [PubMed] [Google Scholar]