Abstract
Background:
Despite advances in development of pharmacotherapy for alcohol use disorder (AUD), the need for medication treatment that can be administered to actively drinking outpatients that promotes reduction in harmful alcohol consumption remains. The primary aim of this pilot study was to determine whether high-dose gabapentin (3600 mg/daily) is more effective than placebo in reducing harmful alcohol consumption in outpatients with alcohol use disorder.
Methods:
Women and men (n = 40) who met DSM-IV-TR criteria for alcohol dependence and reporting at least 4 heavy drinking days (HDD) per week were recruited at a single site. Participants were actively-drinking at study entry and received double-blind gabapentin (3600 mg/day) or placebo for 8-weeks. Study medication was titrated over 5 days and administered in three divided doses (1200 mg three times per day). The proportion of HDD (primary outcome) and percent days abstinent (PDA) (secondary outcome) were analyzed using generalized longitudinal mixed models with predictors study arm, week, study arm by week interaction, and baseline outcome.
Results:
A significant interaction between study arm and week was found (F7,215=3.33, p=.002) for proportion of HDD per week. A significant interaction between study arm and week was found (F7,215=3.11, p=0.004) for PDA per week. The overall retention rate was 67.5% with no significant difference in time-to-dropout between treatment groups. There were no serious adverse events. No participants were removed from the trial for the development of moderate-to-severe alcohol withdrawal (CIWA ≥ 13).
Conclusions:
Gabapentin treatment rapidly titrated to a dose of 3600 mg per day is associated with a reduction in the proportion of HDD per week and an increase in PDA per week in actively drinking outpatients with AUD. High-dose gabapentin is potentially a feasible approach to treating AUD and deserving of further study.
Keywords: gabapentin, pharmacotherapy, alcohol use disorder
1. Introduction
Alcohol Use Disorder (AUD) continues to be the most common substance use disorder in the US (SAMHSA 2020) responsible for substantial morbidity and mortality worldwide (Whiteford et al., 2013). Three FDA-approved pharmacotherapies are available (disulfiram, naltrexone, and acamprosate), yet these treatments are used for a small minority of patients in treatment (Walker et al., 2019, Rubinsky et al., 2015). The current FDA-approved pharmacotherapuetic options (naltrexone, disulfiram, acamprosate) for AUD are lacking in that no single agent has proven to be a safe and effective outpatient treatment for alleviating withdrawal symptoms, reducing harmful drinking, and promoting abstinence. Evidence-based non-FDA approved AUD pharmacotherapy (Kranzler and Soyka, 2018), including gabapentin (Kranzler et al., 2019, Leung et al., 2015), topiramate (Blodgett et al., 2014), and baclofen (Pierce et al., 2018), is available, but not frequently prescribed in the community.
Anticonvulsant agents, most notably topiramate and gabapentin, have been shown to be effective for the treatment of AUD. Topiramate has been shown to be effective in treating AUD in actively-drinking outpatients (Johnson et al., 2003, Johnson et al., 2007, Blodgett et al., 2014) in doses up to 300 mg/day. Gabapentin has been shown to be effective for AUD in doses up to 1800 mg (Mason et al., 2014) and 1200 mg (Anton et al., 2020), but an extended-release formulation of gabapentin, with lower bioavailability, was not shown to be effective at a dose of 1200 mg/day (Falk et al., 2019). Large-scale retrospective studies of gabapentin effects on alcohol consumption have shown benefit (Rentsch et al., 2019), but also the potential for harms (Rentsch et al., 2020).
Gabapentin was initially synthesized as a structural analogue of the neurotransmitter γ-aminobutryric acid (GABA) (Satzinger, 1994), and while it is not a GABA-mimetic agent (Maneuf et al., 2003), it has been shown to influence GABA (Errante et al., 2002, Petroff et al., 1996) and glutamatergic (Suto et al., 2014) activity. Gabapentin binds to the α2δ−1 and α2δ−2 subunits of voltage-gated calcium channels (Gee et al., 1996, Wang et al., 1999, Marais et al., 2001) and inhibits calcium currents (Stefani et al., 1998, Alden and Garcia, 2001, Sutton et al., 2002, Stahl, 2004), which leads to attenuation of postsynaptic excitability (Sills, 2006, Cheng and Chiou, 2006). Gabapentin is not appreciably metabolized in humans, does not bind to plasma proteins, or induce hepatic enzymes, and is eliminated by renal excretion as an unchanged drug. The safety and tolerability of gabapentin in actively drinking individuals has been studied under double-blind placebo-controlled conditions in both the natural environment and in a bar-lab setting and no differences in subjective effects or objective measures of intoxication were reported (Myrick et al., 2007). In the human laboratory setting, gabapentin does not significantly alter subjective and performance effects of alcohol (Bisaga and Evans, 2006). These data support the safety of administering gabapentin to actively drinking outpatients.
Gabapentin has been found to be effective for the treatment of alcohol withdrawal in open-label (Mariani et al., 2006) and double-blind (Myrick et al., 2009) active-control trials. A three-arm trial (n=57) comparing valproic acid, gabapentin and placebo as add-on therapy to benzodiazepines did not find any advantage of anticonvulsant treatment as compared to placebo (Trevisan et al., 2008). Following detoxification, gabapentin treatment has been shown to reduce relapse and alcohol consumption as compared to placebo in AUD outpatients (Brower et al., 2008, Furieri and Nakamura-Palacios, 2007). There is consistent evidence that gabapentin is an effective treatment for sleep disruption associated with either alcohol use (Bazil et al., 2005), alcohol withdrawal (Malcolm et al., 2007), or during the period of early abstinence from alcohol (Karam-Hage and Brower, 2003, Karam-Hage and Brower, 2000). Post-hoc analysis has shown that higher severity pretreatment alcohol withdrawal symptoms predicts improved withdrawal symptom and alcohol use outcomes when gabapentin was co-administered with flumazenil (Anton et al., 2009). In addition, a history of alcohol withdrawal predicted improved treatment response of co-administered gabapentin and naltrexone (Anton et al., 2011) as compared to naltrexone alone. When studied prospectively (n = 145), higher pre-treatment alcohol withdrawal symptom severity history predicted gabapentin (1200 mg/day) benefit on no heavy drinking days and total abstinence as compared with placebo (Anton et al., 2020).
Gabapentin treatment of AUD (n = 150) in doses of 900 mg and 1800 mg (divided in three doses per day) has been found to significantly improve the rates of abstinence and no heavy drinking as compared to placebo (Mason et al., 2014). In this three-arm study, a linear dose effect was found on rates of no heavy drinking and complete abstinence favoring the 1800 mg/day dose. A multi-site trial (n = 346) found no benefit of gabapentin enacarbil extended-release (GE-XR) 1200 mg per day on alcohol use outcomes or craving (Falk et al., 2019). GE-XR is a prodrug, with different pharmacokinetics than gabapentin, and a dose of gabapentin 1800 mg produces higher serum blood levels and overall exposure (area under the curve) than GE-XR at a dose of 1200 mg (Swearingen et al., 2018), suggesting that higher doses of GE-XR may be needed for treating AUD. A 12-week trial of gabapentin 300 mg daily (n=112) reported an significant effect on drinking days per week as compared to placebo, but the retention rate of 30% limits interpretation of the findings (Chompookham et al., 2018).
Even without titration, gabapentin is well tolerated (Beydoun et al., 1998, McLean et al., 1999) at doses of 3600 mg/day (Bergey et al., 1997). Since gabapentin can be titrated quickly to clinically relevant doses, an immediate or early effect of pharmacotherapy, as suggested by existing animal data (Roberto et al., 2008), should be neuroinhibition. The current pilot study was a double-blind, placebo-controlled randomized clinical trial of treatment with gabapentin on outpatients with AUD using an abstinence initiation model (Swift, 2003), where participants were actively drinking at study entry, and study medication was rapidly titrated to the target dose of 3600 mg per day. Our hypothesis was that gabapentin at doses of 3600 mg per day would be more effective than placebo reducing heavy drinking days, increasing abstinent days, and reducing symptoms of alcohol withdrawal in patients with AUD.
2. Material and methods
2.1. Setting:
The study was conducted at the Substance Treatment and Research Service (STARS) of the New York State Psychiatric Institute and Columbia University Irving Medical Center located in New York City.
2.2. Study Participants:
Participants were recruited through advertising directed at potential participants seeking treatment for an alcohol use problem. Trial enrollment began in August 2010 and all participants completed study participation in December 2012. The Structured Clinical Interview for DSM-IV (SCID)(Patient Version 2.0; First et al., 1994), a clinical psychiatric evaluation, medical history, physical and laboratory examination were conducted.
Participants met DSM-IV-TR criteria for current alcohol dependence; reported drinking a minimum of 5 standard drinks for men or 4 standard drinks for women at least 4 days per week over the past 28 days; were between the ages of 18 and 65; and were able to provide informed consent and comply with study procedures. Participants were excluded if they: had a current Axis I psychiatric disorder as defined by DSM-IV-TR, other than alcohol dependence, that in the investigator’s judgment might require intervention with either pharmacological or non-pharmacological therapy over the course of the study; were receiving psychotropic medication treatment; demonstrated evidence of moderate-to-severe alcohol withdrawal (CIWA-Ar ≥ 13); had a history of alcohol withdrawal seizures or alcohol withdrawal delirium; had a history of allergic reaction to gabapentin; were pregnant, lactating, or failed to agree to use adequate contraceptive methods (females); had an unstable physical disorder which might make participation hazardous; had a current DSM-IV-TR diagnosis of other substance dependence, with the exception of nicotine and caffeine dependence (a diagnosis of substance abuse was not exclusionary, as long as the current primary substance use disorder was alcohol dependence); or were legally mandated to participate in an alcohol use disorder treatment program. The research psychiatrist offered participation to eligible participants and obtained informed consent.
2.3. Study Procedures
Participants were randomly allocated (1:1) to receive gabapentin or an identical-appearing, inert placebo. The randomization was carried out in computer-generated randomized blocks of four (two medication and two placebo assignments per block in randomly permutated order) stratified by gender and the severity of alcohol use (> 35 standard drinks/week for men; >28 standard drinks/week for women) by the study statistician. Treatment assignment was conducted by the NYSPI research pharmacy. All research clinic staff were blinded to the participants intervention assignment. Gabapentin was administered in 400 mg capsules; placebo capsules appeared identical to the gabapentin capsules. Gabapentin was titrated over a 5-day period (Table 1) to the dose target or the maximum tolerated dose. Dose reductions for tolerability were made by the research psychiatrist. Study medication was dispensed weekly. Medication adherence was assessed with a weekly pill count interview. A modest monetary incentive ($10) was provided for returning the prior week’s pill bottle. There were no important changes to the study methods after trial commencement.
Table 1.
Medication Titration Schedule
| Study group | Week 1 Days 1–2 | Week 1 Days 3–4 | Week 1 Days 5–7 | Weeks 2–8 | Week 9 |
|---|---|---|---|---|---|
| Experimental group | Gabapentin 400 mg three times daily | Gabapentin 800 mg three times daily | Gabapentin 1200 mg three times daily | Gabapentin 1200 mg three times daily | Taper over 1 week |
| Placebo control group | Placebo 400 mg three times daily | Placebo 800 mg three times daily | Placebo 1200 mg three times daily | Placebo 1200 mg three times daily | Taper over 1 week |
All participants had a weekly supportive behavioral treatment session with the research psychiatrist using a manual designed for pharmacotherapy trials in subjects with alcohol use disorders (Pettinati et al., 2005). This psychosocial intervention promotes abstinence from alcohol and other substances, encourages mutual-support meeting attendance, and facilitates compliance with study medication and other study procedures. All study physicians were trained in providing Medical Management and refresher training sessions were provided every 6 months. Study physicians completed self-report forms assessing their adherence to the intervention manual.
Study visits occurred daily for the first 4 days of the study period, then approximately every other day for the remainder of week 1, for a total of 5 study visits (study days 1, 2, 3, 4, and 5 or 7). During the second week, study visits continued every other day for a total of 3 study visits (study days 8, 10, and 12). During the remainder of the 8-week study period, study visits occurred twice weekly. There was a final post-taper visit after study medication was discontinued. One visit per week was with the research psychiatrist for a Medical Management session. Participants were tapered from study medication during week 9. Upon either completion of the trial or drop-out, participants were offered clinical referrals in the community.
Study discontinuation criteria included: 1) development of serious psychiatric symptoms as indicated by a CGI improvement score of 6 (much worse than baseline) or greater for 2 consecutive weeks; 2) development of evidence of moderate-to-severe alcohol withdrawal (CIWA-Ar ≥ 13) indicating a need for alcohol withdrawal treatment; 3) continued alcohol use, even if improved from baseline, that placed the participant at risk for self-destructive behavior or other harm as indicated by a CGI improvement score of 6 (much worse than baseline) or greater for 2 consecutive weeks; 4) pregnancy.
A complete blood count, electrolytes, urinalysis and liver function tests were performed during the screening process. Serum pregnancy testing was performed during screening and a urine pregnancy test was performed at study weeks 4 and 8. Urine samples for toxicology were collected under directly observed conditions during screening and study weeks 4 and 8 to detect any substance use. Vital signs were measured at every study visit. The Alcohol Timeline Follow-Back method (TLFB)(Litten and Allen, 1992) was used to gather self-reported alcohol use data for each day during the 28 days prior to study enrollment and each day during the study period. The TLFB was the primary outcome measure of alcohol consumption. The CIWA-Ar (Sullivan et al., 1989), was performed at each study visit to measure alcohol withdrawal symptoms for data collection and safety monitoring purposes, and was the primary outcome measure of alcohol withdrawal. The Systematic Assessment for Treatment and Emergent Events (SAFTEE) modified for the COMBINE study (Johnson et al., 2005) was performed at each study visit to measure adverse effects. Participants earned $5 for travel at each visit and received an additional $10 for each week that they returned their medication bottle with any remaining capsules; participants were not paid for ingesting capsules, only the bottle return. The total compensation that a participant could earn for completion of the screening process and entire study was $205.
2.4. Outcome Measures
The primary outcome was the proportion of heavy drinking days (HDD) per week as a measure of alcohol consumption. HDDs were defined as any day where the reported number of drinks on TLFB was at least 5 for men and at least 4 for women. The secondary outcomes were the percent days abstinent (PDA) and the CIWA-Ar score which is the outcome measure of alcohol withdrawal. Additionally, the SAFTEE was used to measure adverse effects. There were no changes to trial outcomes after the trial commenced.
2.5. Statistical Analyses
The original power analysis was written as “A total sample of 60 will be able to detect as small as 1.6 days difference in HDD per week at Visit 9 between two groups with at least 80% power (SD=2.5). The corresponding standardized effect size is 0.64, a medium effect size.” Due to evolution in statistical methodologies over the past decade, the primary aim analyzed was not the number of HDD per week as written in the original grant application, but the proportion of HDD per week, which is equivalent. Additionally, the acquired sample size of 40 participants (smaller due to budgetary issues) ensures at least 80% power for a two-sided test with level of significance of 5% to detect a large effect size difference (0.84) in the proportion of HDD per week between gabapentin and placebo groups.
The distributions of all continuous or count outcomes were first assessed for normality using histograms and descriptive statistics. The primary outcome, the proportion of HDD per week, was normally distributed. The secondary outcome, proportion of abstinent days per week (PDA), followed a log-normal distribution. The alcohol withdrawal outcome (CIWA-Ar), a count outcome, followed a Poisson distribution. The primary, secondary, and alcohol withdrawal outcomes were analyzed using longitudinal generalized linear mixed effect models that assess the differences between treatment over time using two-way interactions with corresponding main effects. The models also included a random intercept to account for between-subject variances and a GEE structure to account for within-subject correlations over time as an autoregressive (AR1) process. Each longitudinal model used the appropriate link function (log for log-normal distribution and Poisson, identity for normal distribution) to fit the corresponding outcome using SAS® PROC GLIMMIX. Additionally, each outcome was adjusted by its corresponding baseline measure (the primary outcome was adjusted by the baseline proportion of HDD in the four-week period before study initiation, the secondary outcome by baseline proportion of abstinent days). Time was modeled as a categorical variable to assess potentially non-linear associations over time.
Differences in time-to-dropout across treatment groups were analyzed using Kaplan-Meier survival curves and the log-rank test.
Alcohol withdrawal (CIWA-Ar) was analyzed using a longitudinal mixed effects Poisson model with the effect of baseline CIWA-Ar score, study week, treatment, and two-way interaction between study week and treatment.
For all longitudinal models, if the study week by treatment interaction was not significant, a separate model, which did not include a study week by treatment interaction term, was fitted to test if there were differences between groups that did not vary over time.
Fisher’s exact tests were used to analyze differences between treatment groups in the proportion of participants who were abstinent in the last four weeks and the proportion of participants who had no HDD in the last four weeks.
Fisher’s exact tests were used to analyze differences between treatment groups in the proportion of participants with positive urine drug screens for each drug category at baseline, week 4, and week 8.
All analyses were performed using SAS® 9.4, with all tests at a two-sided level of significance of 5%.
3. Results
3.1. Participants
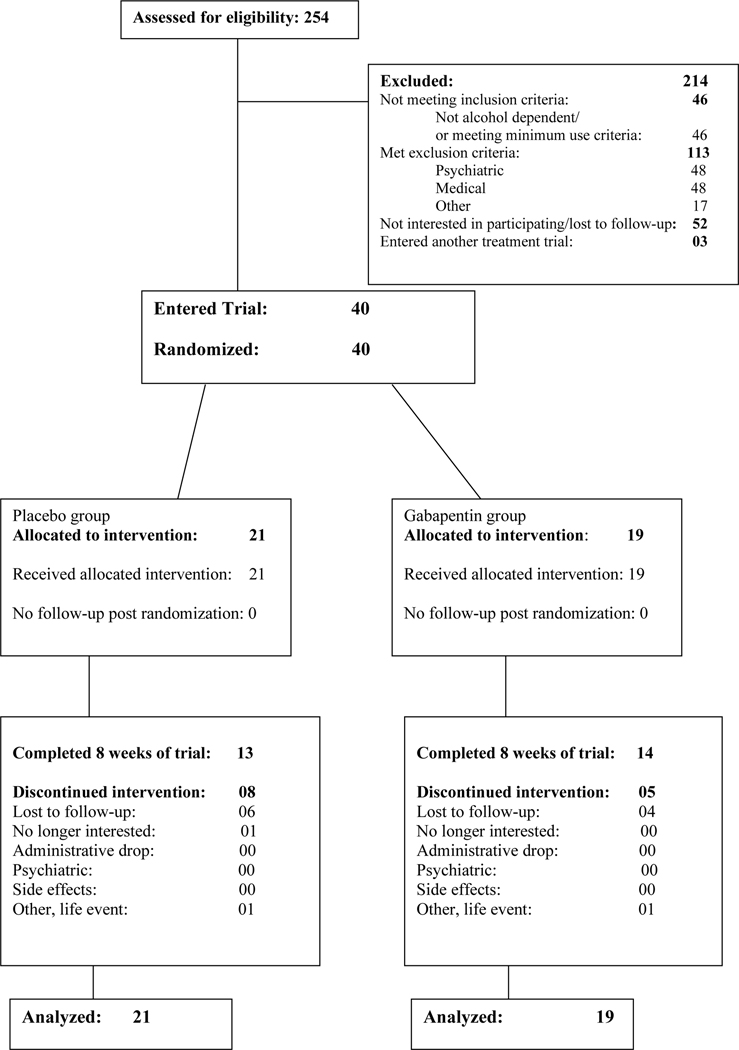
At a single research site 254 individuals were assessed for eligibility and 40 entered the trial. The most common reason for participants who began the screening process failing to enter the trial was meeting an exclusion criterion. The CONSORT diagram shows participant flow (Figure 1).
Figure 1.
CONSORT Diagram
Demographic and baseline clinical characteristics of randomized participants is displayed in Table 2. The majority of the sample was male (67.5%, 27/40), and the most common race/ethnicity was white (55.0%, 22/40) followed by Hispanic (27.5%, 11/40). The mean number of visits attended by participants in the placebo group was 14.9 (SD = 6.0) and 16.9 (SD = 4.1) in the gabapentin group.
Table 2.
Demographic and clinical characteristics by treatment group
| PBO | GABA | |||
|---|---|---|---|---|
| Characteristic | (N=21) | (N=19) | ||
| Demographic Characteristics | Mean | SD | Mean | SD |
| Age (years) | 49.8 | 8.7 | 46.2 | 9.5 |
| n | % | n | % | |
| Male | 14 | 66.7 | 13 | 68.4 |
| Race/Ethnicity | ||||
| Hispanic | 6 | 28.6 | 5 | 26.3 |
| Black | 4 | 19.1 | 3 | 15.8 |
| White | 11 | 52.4 | 11 | 57.9 |
| Education | ||||
| High School/equivalency | 5 | 25.0 | 4 | 21.1 |
| Some College or Post HS Tech training | 6 | 30.0 | 7 | 36.8 |
| Bachelor’s | 5 | 25.0 | 7 | 36.8 |
| Master’s | 4 | 20.0 | 1 | 5.3 |
| Employment Status | ||||
| Full-Time | 8 | 38.1 | 13 | 61.9 |
| Unemployed/Others | 10 | 55.6 | 8 | 44.4 |
| Currently Married | 11 | 52.4 | 9 | 50.0 |
| Clinical Characteristics | n | % | n | % |
| Baseline Alcohol Severity | ||||
| Low | 2 | 9.5 | 1 | 5.3 |
| High | 19 | 90.5 | 18 | 94.7 |
| Baseline DSM IV Other Substance Use Disorder | 2 | 9.5 | 1 | 5.3 |
| Mean | SD | Mean | SD | |
| Baseline DSM IV AUD Criteria | 5.7 | 1.0 | 5.4 | 1.3 |
| Baseline CIWA-Ar Score | 2.5 | 0.7 | 1.8 | 0.5 |
| Baseline Heavy Drinking Days | 24.9 | 4.5 | 24.8 | 3.6 |
| Baseline Drinks Per Day | 8.8 | 4.4 | 7.6 | 2.8 |
| Baseline Drinks Per Drinking Day | 9.5 | 4.3 | 8.0 | 2.9 |
| Baseline Percent Abstinent Days | 0.08 | 0.15 | 0.05 | 0.08 |
| Baseline HAMA Score | 5.1 | 4.1 | 5.6 | 5.3 |
One subject missing married, education, and employment status. Percentages may not sum to 100% due to rounding. Baseline Heavy Drinking Days, Drinks Per Day, Drinks Per Drinking Day, and Percent Abstinent Days calculated over 28 days prior to study entry. High Baseline Alcohol Severity defined as > 35 drinks/week for men and >28 drinks/week for women.
Primary Outcome: Proportion of HDD per week
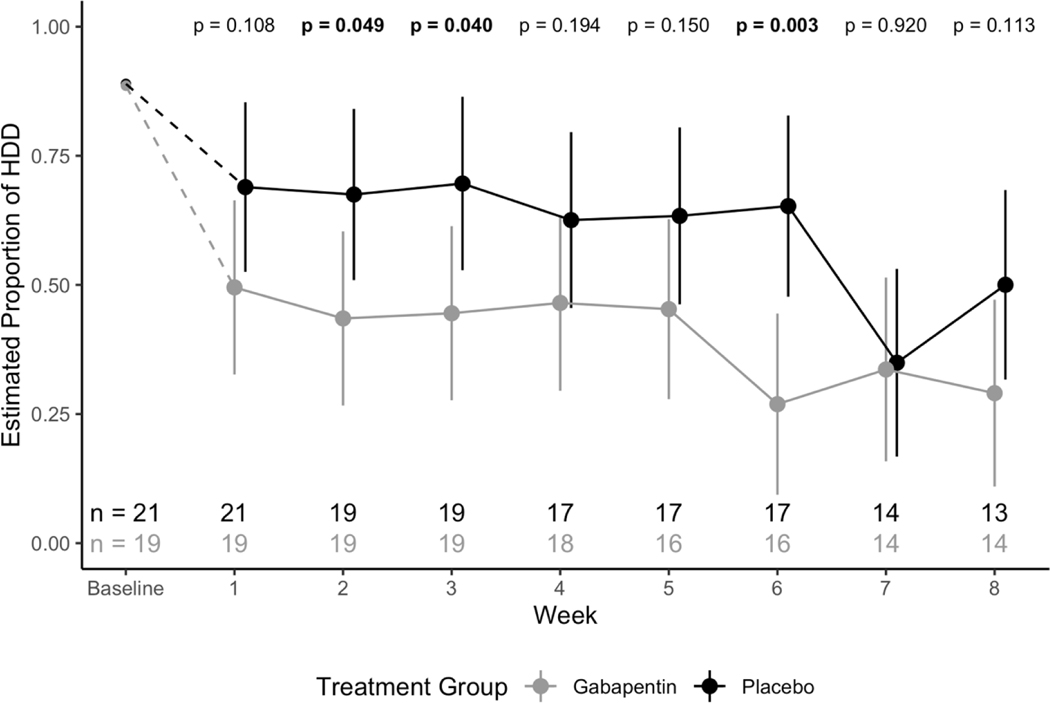
The effect of the two-way interaction between study week and treatment group on the proportion of HDD per week was significant (F7,215=3.33; p=.002), indicating a significant difference in HDD per week over time between gabapentin and placebo groups (See Figure 2). Specifically, subjects in the gabapentin group had a consistently lower estimated proportion of HDD per week than subjects in the placebo group.
Figure 2.
Model estimated proportion of weekly heavy drinking days across treatment groups with 95% confidence intervals. P-values for tests between treatment groups at each week are reported.
Secondary Outcome: Proportion of abstinent days per week
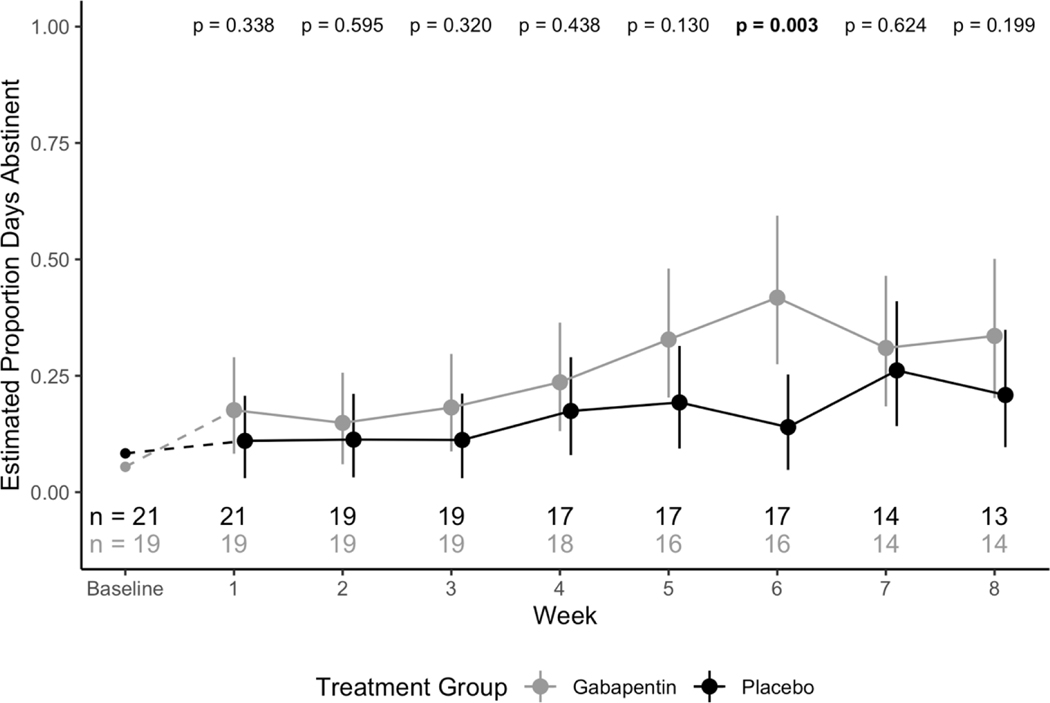
The effect of the two-way interaction between study week and treatment group on the proportion of abstinent days per week was significant (F7,215=3.11; p=.004), indicating a significant difference in proportion of abstinent days per week between gabapentin and placebo groups (See Figure 3). Specifically, subjects in the gabapentin group had a consistently higher estimated proportion of abstinent days per week than subjects in the placebo group.
Figure 3.
Model estimated proportion days abstinent per week across treatment groups with 95% confidence intervals. P-values for tests between treatment groups at each week are reported.
Abstinence and no HDD in the last four weeks
There was no significant difference between treatment groups in the proportion of participants abstinent in the last four weeks of the study (Fisher’s exact p = 0.60). In the placebo group, 4.8% (1/21) of participants were abstinent in the last four weeks, and in the treatment group 10.5% (2/19) of participants were abstinent in the last four weeks. Similarly, there was no significant difference between treatment groups in the proportion of participants with no HDD in the last four weeks of the study (Fisher’s exact p = 0.40). In the placebo group, 9.5% (2/21) of participants had no HDD in the last four weeks, and in the treatment group 21.1% (4/19) of participants had no HDD in the last four weeks.
Time to dropout
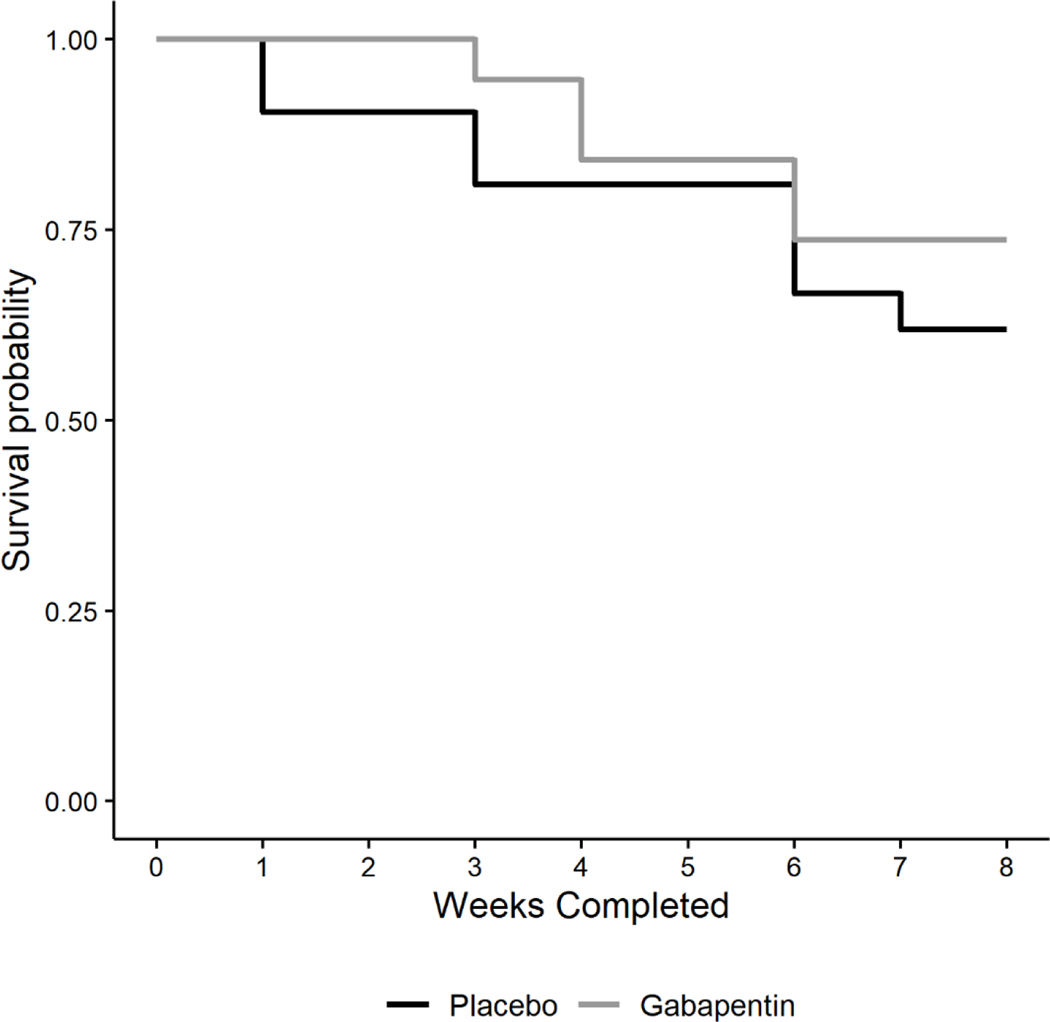
Kaplan-Meier survival curves were generated for each treatment group (See Figure 4). There was no significant difference in time-to-dropout between groups (log-rank =.651; p=.420). The overall observed retention rate was 67.5%. The mean number of weeks retained for participants in the placebo group was 6.5 (SD = 2.4) weeks and for participants in the gabapentin group was 7.1 (SD = 1.7) weeks. The median number of weeks retained in the study was 8 weeks for both treatment groups, with an interquartile range of 6–8 weeks in the placebo group and 7–8 weeks in the gabapentin group.
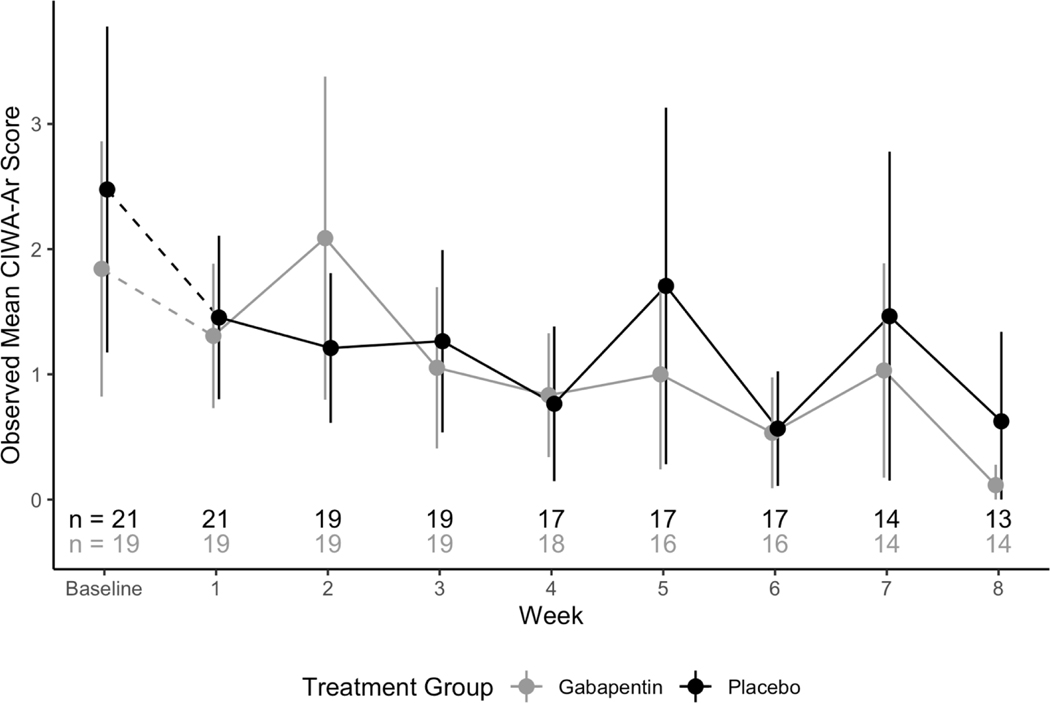
Figure 4.
Observed mean CIWA-Ar score across treatment groups with 95% confidence intervals
Withdrawal
No participants were removed from the trial for the development of moderate-to-severe alcohol withdrawal (CIWA>13). The effect of the two-way interaction between study week and treatment group on the count of CIWA-Ar symptoms was not significant (F7,213 =1.56; p=.150), indicating that there was no significant difference in withdrawal symptoms between treatment groups over time. This non-significant interaction term was removed from the model and withdrawal symptoms were re-analyzed. In this model, there was a significant effect of week (F7,220=4.48; p<.001) but not treatment group (F1,220=0.03; p=.871), indicating that withdrawal symptoms significantly differed from week to week, but did not significantly differ between treatment groups (See Figure 4).
Adverse Effects
The proportion of participants who experienced adverse effects are displayed by treatment group in Table 3a; the proportion of participants who experienced each symptom count is displayed by treatment group in Table 3b. There were no significant differences in the proportion of participants who experienced individual adverse effects or in the proportion of participants with each symptom count between gabapentin and placebo groups. There were no serious adverse events during the trial. One participant in the placebo group discontinued trial participation due to a viral hepatitis infection. One participant in the gabapentin group had complaints of palpitations prior to discontinuing trial participation.
Table 3a.
Individual Adverse Events
| PBO | GABA | ||||
|---|---|---|---|---|---|
| (N=21) | (N=19) | p-value* | |||
| Adverse Effect | n | % | n | % | |
| Abdominal Pain | 1 | 4.8 | 1 | 5.3 | 1.000 |
| Anxiety | 1 | 4.8 | 0 | 0 | 1.000 |
| Appetite Change | 0 | 0 | 1 | 5.3 | 0.475 |
| Constipation | 0 | 0 | 0 | 0 | N/A |
| Cough | 1 | 4.8 | 0 | 0 | 1.000 |
| Diarrhea | 0 | 0 | 1 | 5.3 | 0.475 |
| Dizziness | 1 | 4.8 | 2 | 10.5 | 0.596 |
| Dry Mouth | 4 | 19.1 | 3 | 15.8 | 1.000 |
| Headache | 2 | 9.5 | 2 | 10.5 | 1.000 |
| GI Upset | 1 | 4.8 | 0 | 0 | 1.000 |
| Insomnia | 0 | 0 | 1 | 5.3 | 0.475 |
| Lab Result Changes | 0 | 0 | 0 | 0 | N/A |
| Libido Changes | 1 | 4.8 | 0 | 0 | 1.000 |
| Muscle Pain/Weakness | 1 | 4.8 | 0 | 0 | 1.000 |
| Nasal Congestion | 1 | 4.8 | 0 | 0 | 1.000 |
| Nausea | 2 | 9.5 | 0 | 0 | 0.489 |
| Somnolence | 1 | 4.8 | 2 | 10.5 | 0.596 |
| Fatigue | 5 | 23.8 | 2 | 10.5 | 0.412 |
| Vomiting | 1 | 4.8 | 1 | 5.3 | 1.000 |
| Other | 6 | 28.6 | 8 | 42.1 | 0.370a |
| Any Side Effect | 11 | 52.4 | 15 | 78.9 | 0.079b |
Fishers Exact Test
χ12 = 0.80
χ12 = 3.09
Table 3b.
Number of Adverse Effects
| Table of Number of Patients with Each Symptom Count | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| PBO | 10 (47.6) | 4 (19.1) | 0 (0) | 4 (19.1) | 1 (4.8) | 2 (9.5) |
| GABA | 4 (21.1) | 9 (47.4) | 3 (15.8) | 2 (10.5) | 1 (5.3) | 0 |
| Fisher’s Exact p-value = 0.0511 | ||||||
Medication Adherence
The mean percentage of capsules taken in the gabapentin group was 90.98% (standard deviation (SD) = 12.8) and the mean percentage of capsules taken in the placebo group was 89.44% (SD = 17.6); the mean percentage of capsules taken did not significantly differ between gabapentin and placebo groups (Table 4). In the gabapentin group, 89.5% (17/19) of participants attained a maximum dose of 3600 mg (9 capsules daily), and in the placebo group, 76.2% (16/21) of participants attained a maximum dose (9 capsules daily). In the placebo group, one participant had their dose reduced due to sedation and feeling “lightheaded” and another participant had their dose reduced due to sedation, anxiety, and feeling “foggy”. One participant in the placebo group had their medication discontinued due to palpitations and tachycardia and was evaluated and treated for a respiratory infection. In the gabapentin group, one participant had their dose reduced due to feeling “wobbly” and “off-balance” and another participant had their dose reduced due to fatigue and drowsiness (subsequent laboratory testing detected anemia). In the gabapentin group, one participant had their medication discontinued due to a positive pregnancy test, despite reporting compliance with oral contraception medication.
Table 4.
Compliance by treatment group
| PBO | GABA | ||||
|---|---|---|---|---|---|
| (N=21) | (N=19) | p-value* | |||
| Compliance Measure | Mean | SD | Mean | SD | |
| Percentage of Pills Taken | 90.98 | 12.8 | 89.44 | 17.6 | 0.62a |
Mann-Whitney U-test
U = 362.0
Urine Drug Screens
There were no significant differences between treatment groups in the proportion of positive urine drug screens for any drug category at baseline, week 4, or week 8 (See Table 5).
Table 5.
Urine drug screen results by treatment group at baseline, week 4, and week 8
| Methadone | Opiates | Cocaine | Benzodiazepine | Barbiturate | Amphetamine | Marijuana | Propoxyphene | Phencyclidine | Alcohol Dehydrogenase | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||||
| PBO | 0/18 (0%) | 0/18 (0%) | 1/18 (5.6%) | 1/18 (5.6%) | 0/18 (0%) | 0/18 (0%) | 1/18 (5.6%) | 0/18 (0%) | 0/18 (0%) | 4/18 (22.2%) |
| GABA | 0/17 (0%) | 0/17 (0%) | 1/17 (5.9%) | 0/17 (0%) | 0/17 (0%) | 0/17 (0%) | 4/17 (23.5%) | 0/17 (0%) | 0/17 (0%) | 4/17 (23.5%) |
| p-value* | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.18 | 1.00 | 1.00 | 1.00 |
| Week 4 | ||||||||||
| PBO | 0/12 (0%) | 0/12 (0%) | 1/12 (8.3%) | 1/12 (8.3%) | 0/12 (0%) | 0/12 (0%) | 2/12 (16.7%) | 0/12 (0%) | 0/12 (0%) | 1/12 (8.3%) |
| GABA | 0/14 (0%) | 1/14 (7.1%) | 0/14 (0%) | 0/14 (0%) | 0/14 (0%) | 0/14 (0%) | 2/14 (14.3%) | 0/14 (0%) | 0/14 (0%) | 3/14 (21.4%) |
| p-value* | 1.00 | 1.00 | 0.46 | 0.46 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.60 |
| Week 8 | ||||||||||
| PBO | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) | 1/11 (9.1%) | 0/11 (0%) | 0/11 (0%) | 0/11 (0%) |
| GABA | 0/13 (0%) | 1/13 (7.7%) | 0/13 (0%) | 0/13 (0%) | 0/13 (0%) | 0/13 (0%) | 1/13 (7.7%) | 0/13 (0%) | 0/13 (0%) | 1/13 (7.7%) |
| p-value* | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Fisher’s Exact test
4. Discussion
The results of this pilot study suggest that gabapentin treatment of AUD at 3600 mg per day is associated with a reduction in HDD and an increase in PDA. Study medication was titrated over a five-day period to the target dose and was generally well tolerated. These results are a proof-of-concept that gabapentin can be safely administered to actively drinking outpatients and rapidly titrated to a target dose of 3600 mg daily, which has not been previously reported in an alcohol use disorder treatment trial.
Gabapentin was not found to be associated with a reduction in alcohol withdrawal symptoms as compared to placebo. This outpatient placebo-controlled trial was not designed to primarily measure the effects of gabapentin on alcohol withdrawal. The eligibility criteria were designed to exclude participants at risk of developing moderate-to-severe withdrawal. Abrupt cessation of alcohol was not part of the study design. An exclusion threshold CIWA-Ar score of ≥ 13 was set to protect participants from the consequences of untreated severe alcohol withdrawal symptoms. A potential consequence for this relatively low threshold for CIWA-Ar score may have limited any potential benefit for higher doses of gabapentin.
The benefit on HDD and PDA is consistent with larger trials testing gabapentin for the treatment of AUD (Mason et al., 2014, Anton et al., 2020). A recent meta-analysis found that the percentage of HDD was the outcome most consistently found significant in gabapentin AUD studies conducted to date (Kranzler et al., 2019). In comparing the results of the present study with the negative results of the multi-site GE-XR trial (Falk et al., 2019), it is possible that more aggressive dosing was a factor. While there are a number of gabapentin AUD studies demonstrating therapeutic benefit, the optimal dosing for gabapentin in the treatment of AUD has yet to be defined. A speculative interpretation of the results of the present study is that higher doses of gabapentin may exert a larger, more clinically meaningful effect, as the dose is twice that of the Mason (2014) study and three times that of the Anton (2020) study.
There are novel aspects of the study design that may have influenced the results. First, the decision to enroll actively drinking outpatients, rather than recently detoxified inpatients or outpatients who were able to abstain for several days, was based on the goal of maximizing the generalizability of the results to the community. This approach has been used successfully for studies of topiramate (Johnson et al., 2003, Johnson et al., 2007). The hypothesis for this study was that gabapentin would function as an abstinence-initiation agent (Swift, 2003), where actively drinking individuals receiving gabapentin would find it easier to reduce or stop alcohol consumption. However, it is possible, that the results would have been different if a period of pre-treatment abstinence before starting study medication was required. The largest trials (Anton et al., 2020, Falk et al., 2019) testing gabapentin as a pharmacotherapy for AUD required three days of abstinence prior to randomization. Secondly, the decision to set 3600 mg as the target daily dose of gabapentin was based on our experience with gabapentin in two pilot trials. In an alcohol withdrawal treatment trial, inpatients in acute alcohol withdrawal tolerated gabapentin 2400 mg within the first 24 hours of treatment (Mariani et al., 2006). Outpatients at a methadone maintenance program for opioid use disorder with a concurrent benzodiazepine use disorder, tolerated gabapentin 3600 mg per day after a two week titration (Mariani et al., 2016). Other studies have noted that gabapentin does not appear to add to the intoxicating effects of alcohol (Bisaga and Evans, 2006, Myrick et al., 2007). This preliminary clinical research experience provided a rationale for exploring a higher dose of gabapentin existing studies at the time. While there are published reports of misuse of gabapentinoids (Evoy et al., 2017, Bonnet and Scherbaum, 2017), we did not observe any evidence of misuse during the study.
There are several limitations of the study that may influence interpretation of the results. The study outcomes selected, reduction in HDD (primary), increase in PDA (secondary), and a reduction in withdrawal symptoms (secondary), were intended to detect a signal of efficacy in this small pilot study. However, larger studies have employed more rigorous measures of improvement in alcohol use, including percent no HDD (Falk et al., 2019) and complete abstinence and no heavy drinking (Mason et al., 2014, Anton et al., 2020), which represent a higher bar to demonstrate efficacy. An important limitation of the study is the limited clinical significance of the results; the overall observed retention rate at the end of the 8-week trial was 67.5% and relatively few participants achieved abstinence during the study period. Another limitation of this pilot trial is the brief length of exposure to gabapentin; it is unknown whether a longer exposure to the 3600 mg per day dose would yield different results or whether a gradual dose reduction over time would be beneficial, such as an initial period of 3600 mg per day followed by treatment at a lower dose.
This pilot trial, with a small sample size, is vulnerable to the skewing of results by a relatively small number of participants, and as such, the results should be interpreted conservatively. As a pilot trial, biological markers of medication adherence and improvement in alcohol use outcomes were not included, thereby limiting the validation of self-report measures. The reported rate of adverse effects was in general lower than noted with studies of gabapentin for AUD at lower doses. It is possible that an explanation for this difference is that medication compliance was lower than self-reported. The schedule of study visits during the first two weeks of the trial to monitor for alcohol withdrawal were more frequent than other gabapentin trials for AUD and potentially could confound the comparison of results.
Based on the result of this pilot trial, which demonstrated that gabapentin in doses up to 3600 mg is safe, and possibly efficacious, we would recommend future studies of gabapentin explore higher doses than have been routinely been tested in large scale studies. Mason’s (2014) trial found a dose effect (comparing 900 mg and 1800 mg/day) and it is possible that higher doses of gabapentin will yield more clinically significant effects. However, this pilot study was not designed to directly compare its results to previously conducted gabapentin studies at lower doses and does not prove that higher doses would be superior. The main recommendation from this study is that higher doses of gabapentin can be studied safely.
Future studies also need to examine the effect of pre-trial abstinence or withdrawal symptoms on gabapentin efficacy in an effort to better understand the clinical circumstances gabapentin may be of the most value. The promise of gabapentin for the treatment of AUD is that it can be administered to actively-drinking outpatients to assist them in reducing or ceasing their alcohol use without requiring pre-treatment abstinence. While this study adds to a promising literature, given the small sample, the definitive trial of high-dose gabapentin has not yet been conducted.
Figure 5.
Kaplan-Meier survival curves for time to dropout across treatment groups
ACKNOWLEDGMENTS
This research was supported by NIH grants: R21 AA017691 (Mariani). Authors received additional support from K23-DA021209 (Mariani), P50-DA09236 (Kleber), K24-DA022412 (Nunes), K24 029647 (Levin). Dr. Mariani had full access to all of the data of the study and takes full responsibility for the integrity of the data and for the accuracy of the data analysis. We would like to thank the staff of the Substance Treatment and Research Service (STARS) of the Columbia University Irving Medical Center/New York State Psychiatric Institute for their clinical support and Andrew Glass who provided a preliminary analysis of the data.
Funding Source: Funding for this work was provided by the NIAAA (R21 AA017691)
Declaration of interest
Dr. Mariani has served as a consultant to Indivior and Novartis. Dr. Bisaga received study medication from, and served as an investigator and an unpaid consultant to, Alkermes, Inc. and received study medication from, and served as an unpaid consultant to Go Medical Industries Pty. Dr. Nunes served as unpaid consultant to Alkermes, Braeburn-Camurus and Pear Therapeutics and has received in-kind medication for studies from Reckitt/Indivior, Alkermes, and a therapeutic application from Pear Therapeutics for a study. Dr. Levin receives grant support from the NIDA, SAMHSA and US World Meds and has been an unpaid member of a Scientific Advisory Board for Alkermes, Novartis and US WorldMeds. Drs. Pavlicova and Carpenter reported no biomedical financial interests or potential conflicts of interest. Also, Mr. Basaraba, Ms. Mahony, Mr. Brooks, and Ms. Mamczur-Fuller reported no biomedical financial interests or potential conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT01141049
REFERENCES
- ALDEN KJ & GARCIA J. 2001. Differential effect of gabapentin on neuronal and muscle calcium currents. J Pharmacol Exp Ther, 297, 727–35. [PubMed] [Google Scholar]
- ANTON RF, LATHAM P, VORONIN K, BOOK S, HOFFMAN M, PRISCIANDARO J. & BRISTOL E. 2020. Efficacy of Gabapentin for the Treatment of Alcohol Use Disorder in Patients With Alcohol Withdrawal Symptoms: A Randomized Clinical Trial. JAMA Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTON RF, MYRICK H, BAROS AM, LATHAM PK, RANDALL PK, WRIGHT TM, STEWART SH, WAID R. & MALCOLM R. 2009. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol, 29, 334–42. [DOI] [PubMed] [Google Scholar]
- ANTON RF, MYRICK H, WRIGHT TM, LATHAM PK, BAROS AM, WAID LR & RANDALL PK 2011. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry, 168, 709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAZIL CW, BATTISTA J. & BASNER RC 2005. Gabapentin improves sleep in the presence of alcohol. J Clin Sleep Med, 1, 284–7. [PubMed] [Google Scholar]
- BERGEY GK, MORRIS HH, ROSENFELD W, BLUME WT, PENOVICH PE, MORRELL MJ, LEIDERMAN DB, CROCKATT JG, LAMOREAUX L, GAROFALO E. & PIERCE M. 1997. Gabapentin monotherapy: I. An 8-day, double-blind, dose-controlled, multicenter study in hospitalized patients with refractory complex partial or secondarily generalized seizures. The US Gabapentin Study Group 88/89. Neurology, 49, 739–45. [DOI] [PubMed] [Google Scholar]
- BEYDOUN A, FAKHOURY T, NASREDDINE W. & ABOU-KHALIL B. 1998. Conversion to high dose gabapentin monotherapy in patients with medically refractory partial epilepsy. Epilepsia, 39, 188–93. [DOI] [PubMed] [Google Scholar]
- BISAGA A. & EVANS SM 2006. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend, 83, 25–32. [DOI] [PubMed] [Google Scholar]
- BLODGETT JC, DEL RE AC, MAISEL NC & FINNEY JW 2014. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res, 38, 1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNET U. & SCHERBAUM N. 2017. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol, 27, 1185–1215. [DOI] [PubMed] [Google Scholar]
- BROWER KJ, MYRA KIM H, STROBBE S, KARAM-HAGE MA, CONSENS F. & ZUCKER RA 2008. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res, 32, 1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG JK & CHIOU LC 2006. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci, 100, 471–86. [DOI] [PubMed] [Google Scholar]
- CHOMPOOKHAM P, RUKNGAN W, NILABAN S, SUWANMAJO S, YOOSOM P. & KALAYASIRI R. 2018. A randomized trial of low-dose gabapentin for post hospitalization relapse prevention in a Thai clinical sample of alcohol dependence. Psychiatry Res, 270, 34–40. [DOI] [PubMed] [Google Scholar]
- ERRANTE LD, WILLIAMSON A, SPENCER DD & PETROFF OA 2002. Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Res, 49, 203–10. [DOI] [PubMed] [Google Scholar]
- EVOY KE, MORRISON MD & SAKLAD SR 2017. Abuse and Misuse of Pregabalin and Gabapentin. Drugs, 77, 403–426. [DOI] [PubMed] [Google Scholar]
- FALK DE, RYAN ML, FERTIG JB, DEVINE EG, CRUZ R, BROWN ES, BURNS H, SALLOUM IM, NEWPORT DJ, MENDELSON J, GALLOWAY G, KAMPMAN K, BROOKS C, GREEN AI, BRUNETTE MF, ROSENTHAL RN, DUNN KE, STRAIN EC, RAY L, SHOPTAW S, AIT-DAOUD TIOURIRINE N, GUNDERSON EW, RANSOM J, SCOTT C, LEGGIO L, CARAS S, MASON BJ, LITTEN RZ, NATIONAL INSTITUTE ON ALCOHOL, A. & ALCOHOLISM CLINICAL INVESTIGATIONS GROUP STUDY, G. 2019. Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial Assessing Efficacy and Safety. Alcohol Clin Exp Res, 43, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURIERI FA & NAKAMURA-PALACIOS EM 2007. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry, 68, 1691–700. [DOI] [PubMed] [Google Scholar]
- GEE NS, BROWN JP, DISSANAYAKE VU, OFFORD J, THURLOW R. & WOODRUFF GN 1996. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem, 271, 5768–76. [DOI] [PubMed] [Google Scholar]
- JOHNSON BA, AIT-DAOUD N, BOWDEN CL, DICLEMENTE CC, ROACHE JD, LAWSON K, JAVORS MA & MA JZ 2003. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet, 361, 1677–85. [DOI] [PubMed] [Google Scholar]
- JOHNSON BA, AIT-DAOUD N. & ROACHE JD 2005. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol Suppl, 157–67; discussion 140. [DOI] [PubMed] [Google Scholar]
- JOHNSON BA, ROSENTHAL N, CAPECE JA, WIEGAND F, MAO L, BEYERS K, MCKAY A, AIT-DAOUD N, ANTON RF, CIRAULO DA, KRANZLER HR, MANN K, O’MALLEY SS & SWIFT RM 2007. Topiramate for treating alcohol dependence: a randomized controlled trial. Jama, 298, 1641–51. [DOI] [PubMed] [Google Scholar]
- KARAM-HAGE M. & BROWER KJ 2000. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry, 157, 151. [DOI] [PubMed] [Google Scholar]
- KARAM-HAGE M. & BROWER KJ 2003. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci, 57, 542–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRANZLER HR, FEINN R, MORRIS P. & HARTWELL EE 2019. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction, 114, 1547–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRANZLER HR & SOYKA M. 2018. Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA, 320, 815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG JG, HALL-FLAVIN D, NELSON S, SCHMIDT KA & SCHAK KM 2015. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother, 49, 897–906. [DOI] [PubMed] [Google Scholar]
- LITTEN R. & ALLEN J. 1992. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods, Totowa, NJ, The Humana Press Inc. [Google Scholar]
- MALCOLM R, MYRICK LH, VEATCH LM, BOYLE E. & RANDALL PK 2007. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med, 3, 24–32. [PubMed] [Google Scholar]
- MANEUF YP, GONZALEZ MI, SUTTON KS, CHUNG FZ, PINNOCK RD & LEE K. 2003. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci, 60, 742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARAIS E, KLUGBAUER N. & HOFMANN F. 2001. Calcium channel alpha(2)delta subunits-structure and Gabapentin binding. Mol Pharmacol, 59, 1243–8. [DOI] [PubMed] [Google Scholar]
- MARIANI JJ, MALCOLM RJ, MAMCZUR AK, CHOI JC, BRADY R, NUNES E. & LEVIN FR 2016. Pilot trial of gabapentin for the treatment of benzodiazepine abuse or dependence in methadone maintenance patients. Am J Drug Alcohol Abuse, 42, 333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARIANI JJ, ROSENTHAL RN, TROSS S, SINGH P. & ANAND OP 2006. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict, 15, 76–84. [DOI] [PubMed] [Google Scholar]
- MASON BJ, QUELLO S, GOODELL V, SHADAN F, KYLE M. & BEGOVIC A. 2014. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med, 174, 70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN MJ, MORRELL MJ, WILLMORE LJ, PRIVITERA MD, FAUGHT RE, HOLMES GL, MAGNUS-MILLER L, BERNSTEIN P. & ROSE-LEGATT A. 1999. Safety and tolerability of gabapentin as adjunctive therapy in a large, multicenter study. Epilepsia, 40, 965–72. [DOI] [PubMed] [Google Scholar]
- MYRICK H, ANTON R, VORONIN K, WANG W. & HENDERSON S. 2007. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res, 31, 221–7. [DOI] [PubMed] [Google Scholar]
- MYRICK H, MALCOLM R, RANDALL PK, BOYLE E, ANTON RF, BECKER HC & RANDALL CL 2009. A Double-Blind Trial of Gabapentin Versus Lorazepam in the Treatment of Alcohol Withdrawal. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETROFF OA, ROTHMAN DL, BEHAR KL, LAMOUREUX D. & MATTSON RH 1996. The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Ann Neurol, 39, 95–9. [DOI] [PubMed] [Google Scholar]
- PETTINATI HM, WEISS RD, DUNDON W, MILLER WR, DONOVAN D, ERNST DB & ROUNSAVILLE BJ 2005. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. J Stud Alcohol Suppl, 170–8; discussion 168–9. [DOI] [PubMed] [Google Scholar]
- PIERCE M, SUTTERLAND A, BERAHA EM, MORLEY K. & VAN DEN BRINK W. 2018. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: A systematic review and meta-analysis. Eur Neuropsychopharmacol, 28, 795–806. [DOI] [PubMed] [Google Scholar]
- RENTSCH CT, FIELLIN DA, BRYANT KJ, JUSTICE AC & TATE JP 2019. Association Between Gabapentin Receipt for Any Indication and Alcohol Use Disorders Identification Test-Consumption Scores Among Clinical Subpopulations With and Without Alcohol Use Disorder. Alcohol Clin Exp Res, 43, 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENTSCH CT, MORFORD KL, FIELLIN DA, BRYANT KJ, JUSTICE AC & TATE JP 2020. Safety of Gabapentin Prescribed for Any Indication in a Large Clinical Cohort of 571,718 US Veterans with and without Alcohol Use Disorder. Alcohol Clin Exp Res, 44, 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTO M, GILPIN NW, O’DELL LE, CRUZ MT, MORSE AC, SIGGINS GR & KOOB GF 2008. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci, 28, 5762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINSKY AD, CHEN C, BATKI SL, WILLIAMS EC & HARRIS AH 2015. Comparative utilization of pharmacotherapy for alcohol use disorder and other psychiatric disorders among U.S. Veterans Health Administration patients with dual diagnoses. J Psychiatr Res, 69, 150–7. [DOI] [PubMed] [Google Scholar]
- SATZINGER G. 1994. Antiepileptics from gamma-aminobutyric acid. Arzneimittelforschung, 44, 261–6. [PubMed] [Google Scholar]
- SILLS GJ 2006. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol, 6, 108–13. [DOI] [PubMed] [Google Scholar]
- STAHL SM 2004. Mechanism of action of alpha2delta ligands: voltage sensitive calcium channel (VSCC) modulators. J Clin Psychiatry, 65, 1033–4. [DOI] [PubMed] [Google Scholar]
- STEFANI A, SPADONI F. & BERNARDI G. 1998. Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology, 37, 83–91. [DOI] [PubMed] [Google Scholar]
- SUBSTANCE ABUSE AND MENTAL HEALTH SERVICES ADMINISTRATION. (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20–07-01–001, NSDUH Series H-55). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- SULLIVAN JT, SYKORA K, SCHNEIDERMAN J, NARANJO CA & SELLERS EM 1989. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict, 84, 1353–7. [DOI] [PubMed] [Google Scholar]
- SUTO T, SEVERINO AL, EISENACH JC & HAYASHIDA K. 2014. Gabapentin increases extracellular glutamatergic level in the locus coeruleus via astroglial glutamate transporter-dependent mechanisms. Neuropharmacology, 81, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTON KG, MARTIN DJ, PINNOCK RD, LEE K. & SCOTT RH 2002. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol, 135, 257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEARINGEN D, ARONOFF GM, CIRIC S. & LAL R. 2018. Pharmacokinetics of immediate release, extended release, and gastric retentive gabapentin formulations in healthy adults. Int J Clin Pharmacol Ther, 56, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWIFT RM 2003. Topiramate for the treatment of alcohol dependence: initiating abstinence. The Lancet, 361, 1666–1667. [DOI] [PubMed] [Google Scholar]
- TREVISAN LA, RALEVSKI E, KEEGAN K, OVILE A, VUPPALAPATI D. & GONZALEZ G. 2008. Alcohol detoxification and relapse prevention using valproic acid versus gabapentin in alcohol-dependent patients. Addict Disord Their Treat, 7, 119–28. [Google Scholar]
- WALKER JR, KORTE JE, MCRAE-CLARK AL & HARTWELL KJ 2019. Adherence Across FDA-Approved Medications for Alcohol Use Disorder in a Veterans Administration Population. J Stud Alcohol Drugs, 80, 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG M, OFFORD J, OXENDER DL & SU TZ 1999. Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. Biochem J, 342 ( Pt 2), 313–20. [PMC free article] [PubMed] [Google Scholar]
- WHITEFORD HA, DEGENHARDT L, REHM J, BAXTER AJ, FERRARI AJ, ERSKINE HE, CHARLSON FJ, NORMAN RE, FLAXMAN AD, JOHNS N, BURSTEIN R, MURRAY CJ & VOS T. 2013. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet, 382, 1575–86. [DOI] [PubMed] [Google Scholar]