Abstract
Our understanding of the pathophysiology of celiac disease has progressed greatly over the last 25 years, yet some fallacies about the clinical characteristics and management persist. Worldwide epidemiologic data are now available showing that celiac disease is ubiquitous. An elevated body mass index is common at the time of the diagnosis. The gluten-free diet is an imperfect treatment for celiac disease; not all individuals show a response. This diet is widely used by people without celiac disease, and symptomatic improvement on a gluten-free diet is not sufficient for diagnosis. Finally, the gluten-free diet is burdensome, difficult to achieve and thus has an incomplete efficacy, opening exciting opportunities for novel, non-dietary, treatments.
Keywords: Celiac disease, gluten-free diet, treatment, non-responsive celiac disease
Introduction
Our conception of celiac disease has expanded and become more sophisticated in concert with the tools available for its investigation (such as anti-tissue transglutaminase antibodies, anti-deamidated gliadin peptides antibodies, HLA typing, videocapsule endoscopy). Along the way, we have learned (and relearned) that celiac disease is different from the disease we thought we knew. In the 1950s, celiac disease was considered exclusively as a pediatric disease and North American adults with steatorrhea and malabsorption were diagnosed with “nontropical sprue” and treated with a diet poor in fat and residue but rich in proteins and simple carbohydrates, sometimes in combination with oral steroids (1). Over the turn of the century, we got new insights about global prevalence and atypical clinical presentations including obesity. Moreover, other “gluten-responsive” conditions, such as non-celiac gluten wheat sensitivity and irritable bowel syndrome have been identified. Contrary to conventional wisdom, there are severe limitations to a gluten-free diet (GFD) as an effective treatment for celiac disease. No medication is as yet approved for celiac disease, but many opportunities for non-dietary treatments are under active study. This commentary is a summary of an invited presentation by Dr Ciaran P. Kelly at the American College of Gastroenterology 2019 Annual Meeting. After writing this article, we learned of a previous publication on “celiac myths” by Erica Boettcher and Sheila Crowe in 2014(1) and we acknowledge their prior work on the topic.
Fallacy #1 – Celiac disease occurs mainly in Europeans and those of European descent
The earliest record of celiac disease is attributed to Aretaeus of Cappadochia who described a chronic malabsorption syndrome in the second century. While the role of diet was recognized by both American (Sydney Haas) and British (Samuel Gee) physicians, it was Wilhelm Dicke’s observations of the effects of rationing on Dutch children with celiac disease during the Second World War that led to identification of the toxicity of the gliadin fraction of wheat(2). Throughout most of the twentieth century, celiac disease was believed to affect primarily Europeans and those of European descent. Diagnosis was based upon clinical suspicion and, later, small intestinal histology. Identification of serum antibodies associated with celiac disease and tissue transglutaminase as the target antigen facilitated development of non-invasive serologic tests and wide-spread screening (3). Epidemiologic data is now available for every continent except Antarctica. Our meta-analysis of this data revealed that while there is some geographic variation, celiac disease is remarkably ubiquitous. Globally, the pooled seroprevalence of celiac disease is 1.4% (95% CI 1.1%-1.7%) and the prevalence of biopsy-confirmed celiac disease is 0.7% (95% CI 0.5%-0.9%) (Figure 1) (4). A study in the US found that the ethnic group with the highest prevalence of villous atrophy suggestive of celiac disease is not of European origin but from Punjab (3.08% versus 1.8% overall in North America)(5).
Figure 1:
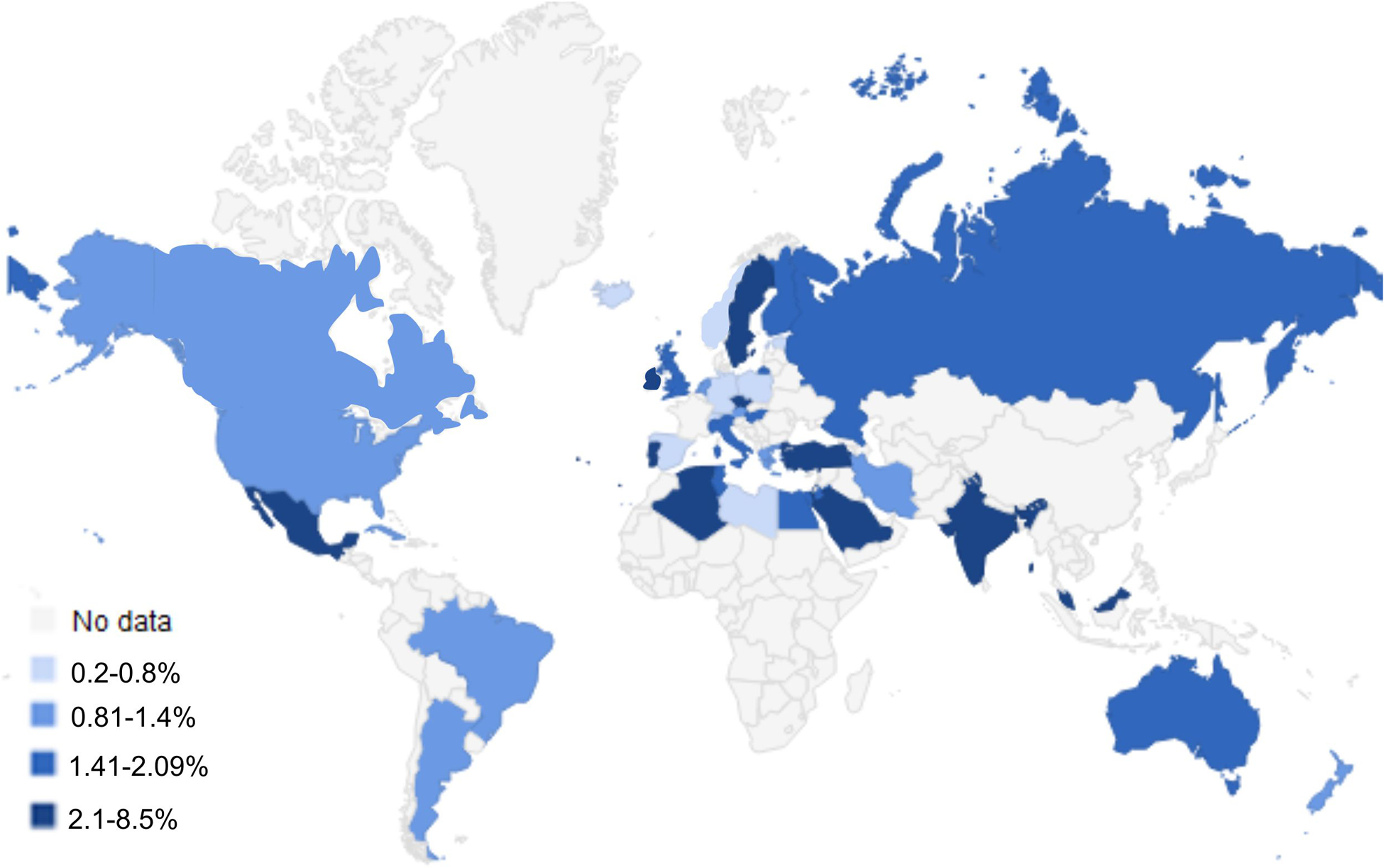
Worldwide Seroprevalence of Celiac Disease
Adapted from Singh P, Arora A, Strand TA, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:823–836 e2. with permission from Elsevier (4, 62–64)
Less is known about the prevalence of celiac disease in Africa. The seroprevalence in North African countries such as Morocco, Algeria, Tunisia is similar to the rest of the world (1.1%), with also similar rates of HLA DQ2 and DQ8 haplotypes carriers in the Moroccan population (6). Studies unrelated to celiac disease found lower prevalence of DQ2 (11%) and DQ8 (6%) in Cameroon, Congo and Gabon (6), allowing us to hypothesize that celiac disease may be less prevalent in these populations. We anecdotally know that celiac disease may occur in Ethiopians, sometimes with a delayed presentation considering the staple diet that is naturally gluten-free. In South Africa, an observational study of children with T1D revealed a prevalence of biopsy-confirmed celiac disease of 2.5% (7). Epidemiologic data is also scant in parts of Asia. Most data are from Israel, Turkey, Iran, India, Jordan, Saudi Arabia and Malaysia (8). Interestingly, there is considerable variation in the rate of HLA DQ2 carrier frequency among Asian countries, ranging from 0.3% in Japan(9), less than 5% in Korea and Indonesia, 5–20% in China, Mongolia, Singapore, Taiwan, Thailand and Vietnam, to > 20% in Australia, Pakistan, Israel and Iran (10). In contrast, HLA DQ8 carrier frequency in Japan (8–10%) was similar to the Western World (11). As such, estimates of seroprevalence of celiac disease among asymptomatic Japanese populations are low, ranging from 0.05 and 0.19% (12, 13). Epidemiologic data is also particularly scant in China, where there could be some geographical / ethnic variations within the country. A study examining rural areas from Northern China showed a seroprevalence of 1.27% with a HLA DQ2 carrier frequency similar to Western populations (14).
While genetic susceptibility, particularly the rate of homozygosity for HLA DQ2.5, influences the prevalence of CeD in a population , other environmental factors, such as the type of staple diet(15), enteric pathogens (16) and antibiotic use (17) are also important. This is exemplified by the striking difference in the prevalence of celiac disease in the Finnish and Russian areas of Karelia whose populations share similar genetic background, but different lifestyle (18).
Fallacy #2 – Individuals with celiac disease are not obese
Paradoxically, many patients with celiac disease are overweight or obese. Classically, children who developed celiac disease shortly after weaning presented with malnutrition secondary to malabsorption that was rapidly reversed by gluten withdrawal. Serologic testing facilitated the recognition that less dramatic presentations are common and many may be “asymptomatic”. In the Western World, it is estimated that between 15–31% of individuals with celiac disease are overweight at the time of the diagnosis and 6.8–13% are obese (19–22). In one US cohort 5% of children were obese at diagnosis(23). In contrast, most patients among a cohort in India were either underweight or normal weight at the time of celiac disease diagnosis, with only 6.2% being overweight and 2.9% obese (24). Among a cohort of 679 adults with celiac disease who we followed for a mean of 39.5 months, one-third had a high BMI at diagnosis (21% overweight plus 12% obese)(25). Overall, BMI increased significantly on a GFD (mean 24.0 to 24.6, P <0.001), and 22% of those with a normal or high BMI at diagnosis increased their BMI substantially (by > 2 points). The degree of BMI increase was proportionate to GFD duration suggesting that weight maintenance counselling is an important aspect of celiac disease follow-up care and dietary education(26). Others have reported that BMI may decrease on a GFD for some overweight or obese individuals (19, 22). This may relate to individual food choices as processed and manufactured gluten-free foods tend to be higher in calories and fat than naturally gluten-free alternatives.
Fallacy #3 – Serum TTG-IgA tests are not useful to diagnose or exclude celiac disease in patients with low serum IgA
Anti-tissue transglutaminase (TTG) IgA antibodies are the recommended initial screening test for celiac disease in all age groups (26, 27). As these tests, and the earlier anti-gliadin IgA and anti-endomysial (EMA) IgA tests, were used clinically it was quickly recognized that they may fail to detect celiac disease in those who are IgA deficient (28). Thus, it is recommended that negative serum TTG IgA testing be followed by determination of total serum IgA. In those who are IgA deficient, TTG IgG and EMA IgG appear to have similar sensitivity and specificity to TTG IgA-based tests in those who are IgA sufficient (28–30). Notably, selective IgA deficiency (total serum IgA <0.07 g/L) is relatively rare compared to partial IgA deficiency (total serum IgA <2 standard deviations below the mean for age). When we evaluated 1000 consecutive patients screened for CeD at our center, TTG IgA was highly sensitive (100%) for CeD in those with partial IgA deficiency(31). Similar findings have been reported in children (32).
Fallacy #4 - All individuals with celiac disease respond to a GFD
For many years, the importance of improving and increasing diagnosis of celiac disease has been emphasized. Now, as the population with diagnosed celiac disease who are following a GFD expands, it is apparent that many individuals with celiac disease do not respond to a GFD. Greater than 15% of adults have persistent or frequent symptoms despite an apparently strict GFD, also called “non-responsive celiac disease” (NRCD)(33). It is important to evaluate these patients because although gluten ingestion is the most common cause of NRCD, it is not the only cause and some causes call for very different management approaches, such as microscopic colitis, other food intolerances, small intestinal bacterial overgrowth and irritable bowel syndrome (Figure 2). Relatively few (0.04–1.5%) have refractory celiac disease, which is defined as persisting symptoms and villous atrophy despite a GFD (34, 35). However, other conditions may still be associated with persisting villous atrophy, including hidden gluten exposure, small intestinal bacterial overgrowth, autoimmune enteropathy, or common variable immunodeficiency. Other factors associated with symptomatic persistent villous atrophy include age > 70 years old, and use of proton-pump inhibitors, non-steroidal anti-inflammatory drugs, or selective serotonin reuptake inhibitors (36).
Figure 2. Diagnostic algorithm for non-responsive celiac disease.
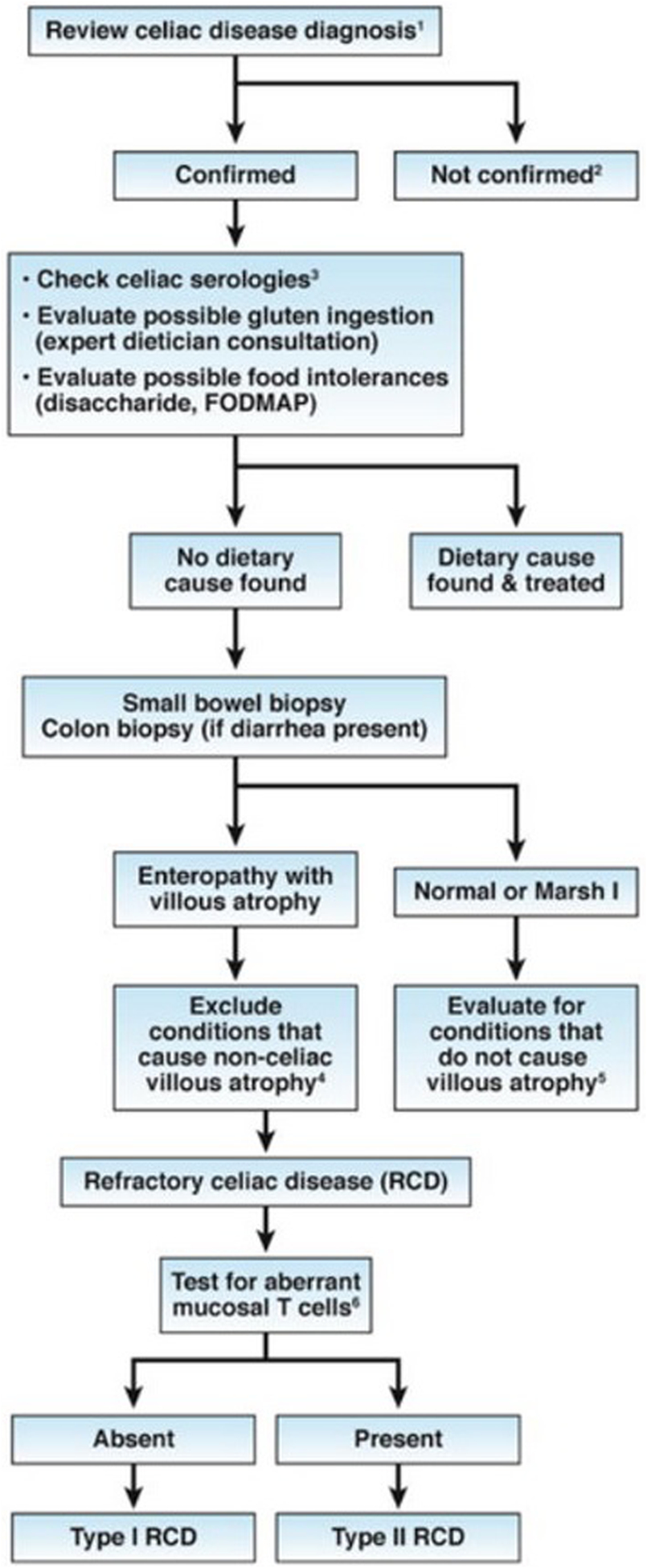
- Confirm the diagnosis of celiac disease by reviewing findings from serologic tests (not anti-gliadin antibody tests) and small bowel histology findings. If patients tested negative for tTG and EMA antibodies, perform HLA DQ2 DQ8 typing.
- Investigate other possible etiologies for clinical presentation and/or abnormal histology findings.
- Increased serum levels of IgA against tTG indciate continued gluten ingestion as a cause
- Non-celiac villous atrophy can be caused by intestinal infections (eg, giardiasis, small intestinal bacterial overgrowth, and viral enteritis, including HIV enteropathy), autoimmune enteropathy, hypogammaglobulinemia, as well as combined variable immunodeficiency, tropical sprue, Crohn’ s disease, peptic duodenitis, or collagenous sprue.
- Conditions that present as NRCD without villous atrophy include irritable bowel syndrome, microscopic colitis, food intolerances, small intestinal bacterial overgrowth, Crohn’s disease, and microscopic colitis.
- Aberrant small intestinal mucosal and intraepithelial lymphocytes in patients with RCD Type II can be identified by immunohistochemistry or flow cytometry (an excess of CD3+ cells without CD4 or CD8 surface proteins) or by T-cell receptor gene rearrangement analysis showing clonal expansion.
Reprinted from Kelly CP, Bai JC, Liu E, et al. Advances in diagnosis and management of celiac disease. Gastroenterology 2015;148:1175–86. with permission from Elsevier(65)
Moreover, mucosal recovery on a GFD is also not universal among those who respond clinically. Overall, only 1/3 of adults have normal villous architecture (a healthy, healed intestine) after 2 years on a GFD, and 2/3 after 5 years on a GFD(36, 37). This is only based on evaluation of the duodenum, so the proportion of celiac disease patients achieving complete mucosal recovery of the entire small intestine remains unknown. Nevertheless, the rate of persistent villous atrophy decreases with time on the GFD, so the majority of individuals with celiac disease may eventually have mucosal recovery(38). Diagnosis during childhood and less severe histologic damage at diagnosis have been associated with mucosal recovery (39).
Fallacy#5 – The GFD is mainly used to treat celiac disease
An increasing number of individuals adopt a gluten-free or gluten-reduced diet for multiple reasons. Some feel relief of gastro-intestinal or extra-intestinal symptoms, either because they have non-celiac gluten sensitivity or IBS (often with fructan intolerance and not an intolerance to all gluten-containing grains) (40–42). Others avoid gluten as part of a fad that is maintained by some athletes and public figures. It was estimated in 2012 that even though at least 2 million people were following a GFD in the US, only 300,000 (15%) actually had celiac disease (43). Nevertheless, a recent analysis of the NHANES cohort showed that even though the prevalence of people avoiding gluten is increasing in the US, the prevalence of diagnosed celiac disease on a GFD is also increasing (from 0.1% in 2009–2010 to 0.4% in 2013–2014). However, in 2013–2014, there were still 0.28% with undiagnosed celiac disease whereas at least 1.7% of the population were avoiding gluten without a diagnosis of celiac disease (44).
Fallacy#6 – A clinical response to a GFD indicates a diagnosis of celiac disease
One consequence of increased awareness of GFD is that self-treatment with a GFD prior to medical consultation is increasingly common. Serologic and histologic findings of celiac disease normalize on a GFD making subsequent diagnosis more challenging. Moreover, IBS and so-called “non-celiac gluten sensitivity” (NCGS) may respond to a GFD (40, 42).
Differentiating between celiac disease and other conditions is clinically important because only celiac disease requires a lifelong strict GFD, carries risk for significant health complications, and is associated with a risk of disease in children and other relatives. By some definitions, elevated TTG IgA excludes NCGS. The conventional diagnosis protocol for NCGS includes following a regular gluten-containing diet for at least 6 weeks, followed by a GFD for at least 6 weeks. Responders should have a subsequent gluten challenge, preferably as a cross-over with a placebo challenge (45). This methodology has identified some individuals with gluten sensitivity among populations with IBS and functional dyspepsia (46, 47). Interestingly, in a recent randomized double-blind placebo-controlled crossover study involving challenges with gluten, fructans and placebo, fructans were associated with significantly higher symptoms scores than gluten in this patient population (40).
Among those in our clinic with a clinical response to a GFD who were evaluated for celiac disease, ever having TTG IgA or DGP IgG/IgA >2x upper limit of normal was associated with a positive likelihood ratio of celiac disease of 130 (95% CI 18.5–918.3)(48). Those with celiac disease were also significantly more likely to have a nutrient deficiency, another autoimmune condition or a family history of celiac disease (Figure 3 and Table 1). HLADQ2/DQ8 genotyping may be useful when there is diagnostic uncertainty, such as when the patient is already on a GFD, there is villous atrophy with normal serology, or to assess if relatives are at risk (34). The negative predictive value of HLA-DQ2/DQ8 is very high (~99%); however, the positive predictive value is much lower(49). Thus, for the 40% of the population who are HLADQ2 and/or DQ8 carriers, prolonged gluten challenge remains the clinical tool of choice to confirm (or exclude) celiac disease (26, 34).
Figure 3:
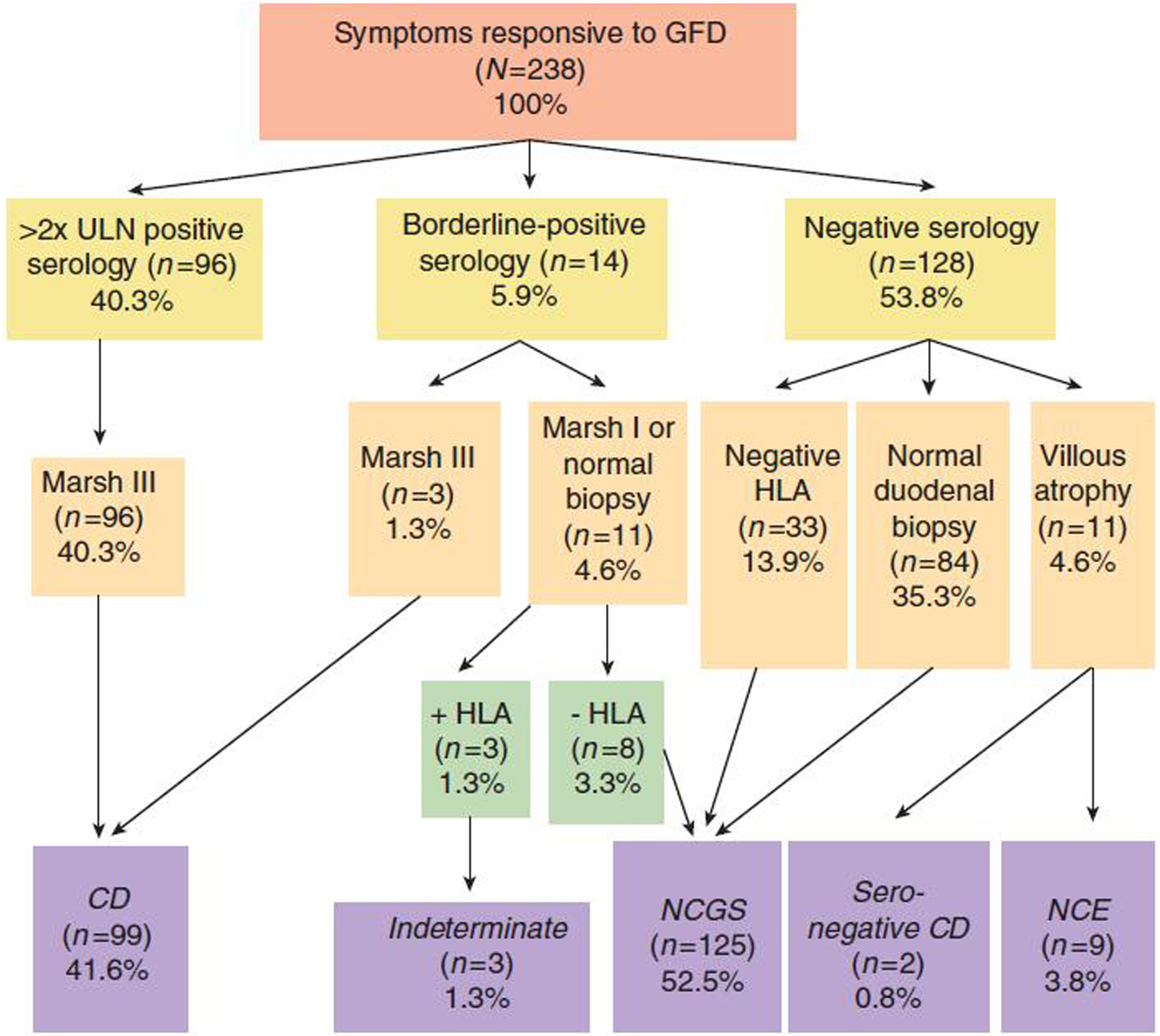
Serological and histological characteristics of individuals with response to a GFD
Reproduced with permission from Kabbani TA, Vanga RR, Leffler DA, et al. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol 2014;109:741–6 (48)
Table 1:
Clinical and demographic differences between celiac disease and non-celiac gluten sensitivity
| Celiac disease (n=101) | Non-celiac gluten sensitive (n=125) | P value | |
|---|---|---|---|
| Age of symptoms onset (year) | 42.2 | 38.0 | 0.03 |
| Female gender (%) | 76.2 | 78.4 | 0.8 |
| Typical celiac symptoms (diarrhea and weight loss) (%) | 67.3 | 24.8 | <0.0001 |
| Family history of celiac disease (%) | 28.7 | 12.8 | 0.004 |
| Personal history of autoimmune disease (%) | 28.7 | 12 | 0.002 |
| Nutrient deficiencya (%) | 57.4 | 18.4 | <0.0001 |
| Mild-to-moderate vitamin D deficiencyb (n) | 20 | 19 | 0.4 |
| Severe vitamin D deficiencyc (n) | 30 | 1 | <0.0001 |
| Iron deficiency anemia (n) | 20 | 3 | <0.0001 |
| Vitamin B12 deficiency (n) | 5 | 1 | 0.1 |
| Zinc deficiency (n) | 3 | 0 | 0.09 |
| Subjects with two or more deficiencies (n) | 20 | 1 | <0.0001 |
CD, celiac disease; NCGS, non-celiac gluten sensitivity.
Nutrient deficiency is defined as vitamin D, iron deficiency anemia, vitamin B12, or zinc deficiency.
Vitamin D levels between 10 and 30 ng/dl
Vitamin D levels less than 10ng/dl
Reproduced with permission from Kabbani TA, Vanga RR, Leffler DA, et al. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol 2014;109:741–6 (48)
Fallacy #7 – The Gluten-Free Diet has solved the problem of Celiac Disease
Notwithstanding persistent symptoms and ongoing villous atrophy, a GFD is an imperfect therapy and the treatment burden is high. Among patients at our hospital, following a GFD for treatment of celiac disease was reportedly more burdensome than treatments for type 1 diabetes, IBS, IBD and congestive heart failure. Those with end-stage renal disease on hemodialysis were the only group to report a higher treatment burden than those with celiac disease(50). A strict GFD is difficult to maintain, particularly when eating food prepared by others outside the home, in restaurants or cafeterias, when travelling, or at social events. Groups who particularly struggle with a GFD include the elderly, the illiterate, those with mental or psychological impairment and those with limited financial means. Naturally gluten-free grains may have gluten-containing grains introduced during planting, harvesting or processing(51). Food preparation details also matter. Dusting meat with flour before grilling, using stock to cook rice or steaming vegetables in the pasta water is not disclosed on menus. Gluten may also be found in some vitamins and supplements and products not intended for consumption such as glues, lipsticks, and play-doh.
Fallacy#8 – An “almost” gluten-free diet is adequate
Adhering to an absolutely strict 100% GFD is a tremendous challenge, thus our patients often ask if a less strict diet is sufficient. Carlo Catassi and colleagues attempted to answer this question in a double-blind microchallenge study(52). Patients with biopsy-confirmed celiac disease who had normal duodenal villous architecture after being on a strict GFD for two years or longer were randomized to take 10 mg gluten, 50 mg gluten or cornstarch placebo daily for three months while maintaining their usual strict GFD. The 50 mg daily gluten exposure was a low dose roughly equivalent to one fortieth of a slice of bread. Villous height crypt depth ratio (Vh:Cd) was similar in all three groups at baseline; however, there was a significant decrease in Vh:Cd in the 50 mg group after 3 months compared to placebo. Importantly, individual sensitivity is likely highly variable. One subject in the 10 mg group dropped out of the study after a month when they exhibited signs of relapse (vomiting, diarrhea, abdominal distension). The investigators included a run-in period, since some individuals participating in a research study may suddenly become more strict with their gluten-free diet and this could bias the effects observed from the intervention; nevertheless, 19/39 participants (including 2 in the 50 mg group) had an increase/improvement in their Vh:Cd over the course of the trial. To put this into perspective, a regular diet is about 5–15g of gluten/day in the Western World, which is at least 100 times the amount of gluten that is considered harmful (53). A prior study among children compared the effects of a daily intake of 100 mg and 500 mg of gluten. Both group had a worsening of the Vh:Cd and frequencies of IELs, except for one child in the 100 mg group who had a slight improvement of the Vh:Cd ratio (54). These studies are difficult to perform because they are necessarily conducted on a background of a “gluten-free” diet that contains low levels of gluten (see below). On the other end, recent studies using gluten immunogenic peptides tests in urine and stools still detect gluten exposures among those with Marsh 0–1 histology (55–57). It is fair to conclude that individual responses to gluten exposure are highly variable, but a chronic gluten exposure of at least 50 mg for more than a month will likely induce intestinal damage.
Fallacy#9 – Most patients with known celiac disease follow a GFD
In a survey of adults diagnosed with celiac disease in England, 40% reported intentional gluten exposure within the last 6 months and an additional 30% reported unintentional gluten exposure during the same period(58). Recently, our group completed the Determination of Gluten Grams Ingested and Excreted By Adults eating Gluten-free (DOGGIEBAG) study (57). Eighteen adults with biopsy-confirmed celiac disease who had been on a GFD for 24 months collected food (25% portions in a ‘doggie bag’), urine and stool samples over a 10-day period. Although no intentional gluten exposures were reported, two-thirds had at least one sample that tested positive for gluten immunogenic peptides. Eliminating all dietary gluten may be an aspirational goal that is difficult to attain even for highly motivated patients. This has been implicitly acknowledged for years as the definition of “gluten-free” is not absolute but allows for 20 parts per million gluten in foods.
Fallacy#10 – A GFD is sufficient therapy for celiac disease
All guidelines for management of celiac disease recommend lifelong adherence to a strict GFD. However, as previously mentioned, the treatment burden is high on a GFD and it is an imperfect treatment for celiac disease (Table 2). As such, this condition is poised for drug development, being a commonly encountered disorder needing a lifelong therapy, with many steps in celiac disease pathogenesis being well elucidated. Surveys suggest that a majority of celiac disease patients would be interested in a medical therapy(59). The potential target celiac disease populations have also evolved, indications for therapies being initially as adjuncts to the GFD for people with RCD or NRCD. We are now aiming for the ultimate goal – to achieve “tolerance” to allow those with celiac disease to consume gluten safely; either in small amounts or ultimately in the amounts found in a normal diet. Examples of therapeutic agents in the pipeline include glutenases (latiglutenase NCT03585478, TAK062 NCT03701555), tight junction regulator (larazotide NCT03569007) and nanoparticles inducing tolerance to gliadin (TAK-101 NCT04530123, KAN-101 NCT04248855).
Table 2:
50 years of real world clinical experience with a gluten-free diet
| “It was the best of times, It was the worst of times” *Charles Dickens | |
|---|---|
| Millions have been helped | Gluten elimination not achievable for many/most patients |
| Safe | Adverse effects common – weight gain, nutritional deficiencies (unfortified grains), social effects/isolation/implications |
| Efficacious | Limited effectiveness – especially on an “intent to treat” basis |
| Non-pharmacologic | A GFD is more costly and the cost is borne by the patient in most countries |
| Self-administered | Minimal medical support and high treatment burden |
| The “only” treatment for celiac disease | Research and development stifled – NO approved treatments |
As with many other conditions, diagnosis of celiac disease is limited by our not remembering to include it as a diagnostic consideration. Failure to consider celiac disease continues to be a common contributor to diagnostic delays (60, 61). Celiac disease has been reported in every continent except Antarctica, although epidemiologic data is still missing in several African and Asian countries (4). It does present among individuals who are overweight and obese and the effects of the gluten-free diet on body habitus are variable (22). Differentiating celiac disease from non-celiac gluten sensitivity is also crucial because the importance to adhere to a strict for celiac disease patients is currently essential, but very burdensome (50). For many, symptoms persist, involuntary exposures are frequent(57) and mucosal recovery is not universal (36). Inducing tolerance to gluten in celiac disease could be a “game changer” for celiac disease patients, but also for other auto-immune disorders with less well-defined disease pathogenesis and antigenic triggers.
Funding:
Research reported in this publication was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number K23 DK119584. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Not related to current work; CPK has acted as a scientific advisor to companies attempting to develop new diagnostic and management approaches for Celiac disease including Cour Pharma, Glutenostics, Innovate, ImmunogenX and Takeda. He also acts as Principal Investigator on a research grant on Celiac disease supported by Aptalis. Jocelyn A. Silvester has received consulting fees from Takeda Pharmaceuticals International Co, and research support from Cour Pharmaceuticals, Biomedal SL and Glutenostics LLC. She was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number K23 DK119584. AT was supported by Douglas G Kinnear Award from the Association des Gastroenterologues du Quebec and FRQS Phase II award for clinician-scientist training
References
- 1.Green PA, Wollaeger EE. The clinical behavior of sprue in the United States. Gastroenterology 1960;38:399–418. [PubMed] [Google Scholar]
- 2.Van De Kamer JH, Weijers HA, Dicke WK. Coeliac disease. IV. An investigation into the injurious constituents of wheat in connection with their action on patients with coeliac disease. Acta Paediatr 1953;42:223–31. [DOI] [PubMed] [Google Scholar]
- 3.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797–801. [DOI] [PubMed] [Google Scholar]
- 4.Singh P, Arora A, Strand TA, et al. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2018;16:823–836 e2. [DOI] [PubMed] [Google Scholar]
- 5.Krigel A, Turner KO, Makharia GK, et al. Ethnic Variations in Duodenal Villous Atrophy Consistent With Celiac Disease in the United States. Clin Gastroenterol Hepatol 2016;14:1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piancatelli D, Ben El Barhdadi I, Oumhani K, et al. HLA Typing and Celiac Disease in Moroccans. Med Sci (Basel) 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paruk IM, Naidoo VG, Pirie FJ, et al. Prevalence and characteristics of celiac disease in South African patients with type 1 diabetes mellitus: Results from the Durban Diabetes and Celiac Disease Study. J Gastroenterol Hepatol 2019;34:673–678. [DOI] [PubMed] [Google Scholar]
- 8.Singh P, Arora S, Singh A, et al. Prevalence of celiac disease in Asia: A systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:1095–101. [DOI] [PubMed] [Google Scholar]
- 9.Saito S, Ota S, Yamada E, et al. Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens 2000;56:522–9. [DOI] [PubMed] [Google Scholar]
- 10.Cummins AG, Roberts-Thomson IC. Prevalence of celiac disease in the Asia-Pacific region. J Gastroenterol Hepatol 2009;24:1347–51. [DOI] [PubMed] [Google Scholar]
- 11.Lionetti E, Catassi C. Co-localization of gluten consumption and HLA-DQ2 and -DQ8 genotypes, a clue to the history of celiac disease. Dig Liver Dis 2014;46:1057–63. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga M, Ishimura N, Abe T, et al. Serological screening for celiac disease in adults in Japan: Shimane CoHRE study. JGH Open 2020;4:558–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukunaga M, Ishimura N, Fukuyama C, et al. Celiac disease in non-clinical populations of Japan. J Gastroenterol 2018;53:208–214. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Gao F, Gao J, et al. Prevalence of coeliac disease in Northwest China: heterogeneity across Northern Silk road ethnic populations. Aliment Pharmacol Ther 2020;51:1116–1129. [DOI] [PubMed] [Google Scholar]
- 15.Lionetti E, Castellaneta S, Francavilla R, et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N Engl J Med 2014;371:1295–303. [DOI] [PubMed] [Google Scholar]
- 16.Kemppainen KM, Lynch KF, Liu E, et al. Factors That Increase Risk of Celiac Disease Autoimmunity After a Gastrointestinal Infection in Early Life. Clin Gastroenterol Hepatol 2017;15:694–702 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dydensborg Sander S, Nybo Andersen AM, Murray JA, et al. Association Between Antibiotics in the First Year of Life and Celiac Disease. Gastroenterology 2019;156:2217–2229. [DOI] [PubMed] [Google Scholar]
- 18.Kondrashova A, Mustalahti K, Kaukinen K, et al. Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med 2008;40:223–31. [DOI] [PubMed] [Google Scholar]
- 19.Ukkola A, Maki M, Kurppa K, et al. Changes in body mass index on a gluten-free diet in coeliac disease: a nationwide study. Eur J Intern Med 2012;23:384–8. [DOI] [PubMed] [Google Scholar]
- 20.Dickey W, Kearney N. Overweight in celiac disease: prevalence, clinical characteristics, and effect of a gluten-free diet. Am J Gastroenterol 2006;101:2356–9. [DOI] [PubMed] [Google Scholar]
- 21.Tucker E, Rostami K, Prabhakaran S, et al. Patients with coeliac disease are increasingly overweight or obese on presentation. J Gastrointestin Liver Dis 2012;21:11–5. [PubMed] [Google Scholar]
- 22.Cheng J, Brar PS, Lee AR, et al. Body mass index in celiac disease: beneficial effect of a gluten-free diet. J Clin Gastroenterol 2010;44:267–71. [DOI] [PubMed] [Google Scholar]
- 23.Venkatasubramani N, Telega G, Werlin SL. Obesity in pediatric celiac disease. J Pediatr Gastroenterol Nutr 2010;51:295–7. [DOI] [PubMed] [Google Scholar]
- 24.Singh I, Agnihotri A, Sharma A, et al. Patients with celiac disease may have normal weight or may even be overweight. Indian J Gastroenterol 2016;35:20–4. [DOI] [PubMed] [Google Scholar]
- 25.Kabbani TA, Goldberg A, Kelly CP, et al. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment Pharmacol Ther 2012;35:723–9. [DOI] [PubMed] [Google Scholar]
- 26.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–76; quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–60. [DOI] [PubMed] [Google Scholar]
- 28.Cataldo F, Marino V, Ventura A, et al. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut 1998;42:362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang N, Truedsson L, Elvin K, et al. Serological assessment for celiac disease in IgA deficient adults. PLoS One 2014;9:e93180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korponay-Szabo IR, Dahlbom I, Laurila K, et al. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut 2003;52:1567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallav K, Xu H, Leffler DA, et al. Immunoglobulin A deficiency in celiac disease in the United States. J Gastroenterol Hepatol 2016;31:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan MR, Silvester JA, Sparks B, et al. The Utility of IgA-Based Serologic Markers in Diagnosing Celiac Disease in Children 24 Months of Age or Younger. J Pediatr 2020;224:158–161 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leffler DA, Dennis M, Hyett B, et al. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol 2007;5:445–50. [DOI] [PubMed] [Google Scholar]
- 34.Al-Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019;7:583–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Wanrooij RL, Bouma G, Bontkes HJ, et al. Outcome of Referrals for Non-Responsive Celiac Disease in a Tertiary Center: Low Incidence of Refractory Celiac Disease in the Netherlands. Clin Transl Gastroenterol 2017;8:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahadev S, Murray JA, Wu TT, et al. Factors associated with villus atrophy in symptomatic coeliac disease patients on a gluten-free diet. Aliment Pharmacol Ther 2017;45:1084–1093. [DOI] [PubMed] [Google Scholar]
- 37.Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol 2002;118:459–63. [DOI] [PubMed] [Google Scholar]
- 38.Lebwohl B, Murray JA, Rubio-Tapia A, et al. Predictors of persistent villous atrophy in coeliac disease: a population-based study. Aliment Pharmacol Ther 2014;39:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szakacs Z, Matrai P, Hegyi P, et al. Younger age at diagnosis predisposes to mucosal recovery in celiac disease on a gluten-free diet: A meta-analysis. PLoS One 2017;12:e0187526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skodje GI, Sarna VK, Minelle IH, et al. Fructan, Rather Than Gluten, Induces Symptoms in Patients With Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018;154:529–539 e2. [DOI] [PubMed] [Google Scholar]
- 41.Potter MDE, Walker MM, Jones MP, et al. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-based Study: Association Between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am J Gastroenterol 2018;113:1036–1044. [DOI] [PubMed] [Google Scholar]
- 42.Catassi C, Alaedini A, Bojarski C, et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012;107:1538–44; quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 44.Choung RS, Unalp-Arida A, Ruhl CE, et al. Less Hidden Celiac Disease But Increased Gluten Avoidance Without a Diagnosis in the United States: Findings From the National Health and Nutrition Examination Surveys From 2009 to 2014. Mayo Clin Proc 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catassi C, Elli L, Bonaz B, et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015;7:4966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elli L, Tomba C, Branchi F, et al. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients 2016;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barone M, Gemello E, Viggiani MT, et al. Evaluation of Non-Celiac Gluten Sensitivity in Patients with Previous Diagnosis of Irritable Bowel Syndrome: A Randomized Double-Blind Placebo-Controlled Crossover Trial. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabbani TA, Vanga RR, Leffler DA, et al. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol 2014;109:741–6; quiz 747. [DOI] [PubMed] [Google Scholar]
- 49.Brown NK, Guandalini S, Semrad C, et al. A Clinician’s Guide to Celiac Disease HLA Genetics. Am J Gastroenterol 2019;114:1587–1592. [DOI] [PubMed] [Google Scholar]
- 50.Shah S, Akbari M, Vanga R, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am J Gastroenterol 2014;109:1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson T, Lee AR, Grace T. Gluten contamination of grains, seeds, and flours in the United States: a pilot study. J Am Diet Assoc 2010;110:937–40. [DOI] [PubMed] [Google Scholar]
- 52.Catassi C, Fabiani E, Iacono G, et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am J Clin Nutr 2007;85:160–6. [DOI] [PubMed] [Google Scholar]
- 53.Hoppe C, Gobel R, Kristensen M, et al. Intake and sources of gluten in 20- to 75-year-old Danish adults: a national dietary survey. Eur J Nutr 2017;56:107–117. [DOI] [PubMed] [Google Scholar]
- 54.Catassi C, Rossini M, Ratsch IM, et al. Dose dependent effects of protracted ingestion of small amounts of gliadin in coeliac disease children: a clinical and jejunal morphometric study. Gut 1993;34:1515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Carnicer A, Garzon-Benavides M, Fombuena B, et al. Negative predictive value of the repeated absence of gluten immunogenic peptides in the urine of treated celiac patients in predicting mucosal healing: new proposals for follow-up in celiac disease. Am J Clin Nutr 2020. [DOI] [PubMed] [Google Scholar]
- 56.Moreno ML, Cebolla A, Munoz-Suano A, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017;66:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silvester JA, Comino I, Kelly CP, et al. Most Patients With Celiac Disease on Gluten-Free Diets Consume Measurable Amounts of Gluten. Gastroenterology 2020;158:1497–1499 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hall NJ, Rubin GP, Charnock A. Intentional and inadvertent non-adherence in adult coeliac disease. A cross-sectional survey. Appetite 2013;68:56–62. [DOI] [PubMed] [Google Scholar]
- 59.Tennyson CA, Simpson S, Lebwohl B, et al. Interest in medical therapy for celiac disease. Therap Adv Gastroenterol 2013;6:358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paez MA, Gramelspacher AM, Sinacore J, et al. Delay in Diagnosis of Celiac Disease in Patients Without Gastrointestinal Complaints. Am J Med 2017;130:1318–1323. [DOI] [PubMed] [Google Scholar]
- 61.Pulido O, Zarkadas M, Dubois S, et al. Clinical features and symptom recovery on a gluten-free diet in Canadian adults with celiac disease. Can J Gastroenterol 2013;27:449–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jamnik JV CR, Dhir SB, Jenkins DJA, El-Sohemy A Prevalence of positive coeliac disease serology and HLA risk genotypes in a multiethnic population of adults in Canada: a cross-sectional study. BMJ Open 2017;7. [Google Scholar]
- 63.Johnston SD, Watson RG, McMillan SA, et al. Prevalence of coeliac disease in Northern Ireland. Lancet 1997;350:1370. [DOI] [PubMed] [Google Scholar]
- 64.Hovdenak N, Hovlid E, Aksnes L, et al. High prevalence of asymptomatic coeliac disease in Norway: a study of blood donors. Eur J Gastroenterol Hepatol 1999;11:185–7. [DOI] [PubMed] [Google Scholar]
- 65.Kelly CP, Bai JC, Liu E, et al. Advances in diagnosis and management of celiac disease. Gastroenterology 2015;148:1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


