Abstract
The majority of people with prediabetes transition to type 2 diabetes. Research has suggested that persons with type 2 diabetes are likely to discount the future and focus on immediate rewards. This study was designed to assess whether this process of delay discounting (DD) is associated with glycemic regulation, medication adherence and eating and exercise behaviors in adults with prediabetes. Participants included 81 adults with prediabetes who were also prescribed hypertension or dyslipidemia drugs, which is common for people with prediabetes. Participants completed adjusting amount DD $100 and $1000 tasks, as well assessments of glycemic control (Hemoglobin (Hb) A1c), medication adherence, diet quality, and objectively measured physical activity. Relationships between DD and these variables were assessed. Results showed higher rates of DD were related to higher HbA1c; as well as poorer medication adherence, lower diet quality and lower physical activity. Hierarchical regression showed that the association between minority status, a known risk factor for type 2 diabetes, was moderated by DD, as minorities with higher DD had greater HbA1c values. Delay discounting may represent a novel target to prevent progression from prediabetes to type 2 diabetes.
Keywords: Delay discounting, prediabetes, glycemic control, medication adherence, diet quality, physical activity
Introduction
The development of type 2 diabetes has a predictable trajectory from normoglycemia to prediabetes to type 2 diabetes.1 Although changes in health behaviors can prevent or delay the transition from prediabetes to type 2 diabetes in many people,2 type 2 diabetes prevalence continues to rise.1 A majority of individuals with prediabetes also have comorbid diseases, including hypertension and/or dyslipidemia, both of which exacerbate the risk of long-term cardiometabolic disturbances associated with prediabetes.1 Modifying these behaviors requires people to forego the immediate pleasure associated with food or sedentary activities to achieve the healthier, but delayed consequences associated with lower blood sugar values. Persons with type 1 diabetes3 or type 2 diabetes4,5 tend to discount the future, focusing on immediate gratification associated with their current lifestyle, rather than making behavior changes that would improve their eating and exercise behaviors 4 or allow them to adhere consistently to their medications to reduce cardiometabolic risks associated with prediabetes.6
Discounting of the future can be assessed by delay discounting (DD), a behavioral economic trans-disease process7 that measures the extent to which people prefer small immediate rewards over larger, delayed rewards.8 DD has been related to a wide variety of conditions, including drug addiction, gambling, and obesity, as well as health behaviors, such as seat belt and sunscreen use and preventive health checkups.7,9 However, no investigators have studied the relationship between DD and glycemic control in persons with prediabetes; nor the relationships between DD and behaviors that could have a direct influence on glycemic control, such as diet, activity; or medication adherence. The conceptual basis for studying how changes in DD may be related to changes in glycemic control is the experimental medicine approach.10,11 The experimental medicine approach seeks to identify a behavioral target that is related to an important health outcome, then to identify methods to manipulate the target, and finally to demonstrate that manipulate of the target is related to improvement in the health outcome. While research shows that DD is cross-sectionally related to Hemoglobin (Hb) A1c control,4,5 no parallel studies exist that demonstrate that DD is related to prediabetes, which is the first step in an experimental medicine approach to targeting DD to prevent the transition to type 2 diabetes. In support of the cross sectional relationship between DD and HbA1c and related health behaviors is the observation that increases in DD are predictive of changes in HbA1c over a year for those with prediabetes.12
This study provides an opportunity to understand the contribution of behavioral economic decision making on HbA1c and health behaviors by people with prediabetes. We hypothesize that greater discounting of the future as assessed by DD is related to poorer glycemic control, lower medication adherence, lower diet quality and less physical activity.
Methods
Participants and Procedures
Participants were persons who were recruited for a study on the relationship between prediabetes and health behaviors. Participants were recruited from the Buffalo, New York and Roanoke, Virginia communities via physician networks using flyers, media ads, referrals, and direct mailings. Interested participants were screened online or by phone to assess initial eligibility. Participants were invited to a screening appointment if they were at least 18 years of age, were not previously diagnosed with or currently have diabetes, were prescribed medications for hypertension and/or dyslipidemia, were not pregnant, did not have any health factors that influenced their blood glucose, did not use illicit drugs, and were able to adhere to study procedures. Since hypertension and dyslipidemia are common comorbidities for people with prediabetes,1 and one goal of the study was to assess the relationship between DD and medication adherence, having been prescribed medication for hypertension or dyslipidemia were included as eligibility criteria. Four-hundred sixty-two persons were assessed for eligibility for the study, and 211 were brought in for screening. After screening, 84 participants met criteria and began the baseline portion of the study. Reasons for exclusion included non-systematic DD, HbA1c values outside of the prediabetic range (5.7% - 6.4%), not being prescribed a medication for comorbid hypertension or hyperlipidemia, and inability to schedule subsequent visits. Of the people who began the baseline measurements, 81 completed the study.
At screening, participants were provided details about the study procedures, signed consent and medical release forms, and completed DD tasks and questionnaires. Recent major life events were assessed to address barriers to participation. Additional assessments included height, weight, point of care (POC) HbA1c, cardiovascular risk factors to identify people with comorbid hypertension or dyslipidemia, plus urine tests for drug use and pregnancy. Dietary recalls and Actigraph measured activity were collected the week following the collection of the other baseline variables, and if eligible, participants were scheduled for an fMRI scan. Participants who attended the baseline screening for the present sample were provided monetary incentives of $30 for completing each of the baseline screening session and the following measurement session. Participants were also provided with $15 for completion of each of the three dietary recalls ($5 × 3) and $5 per day of Actigraph wear (up to 7 days, 10 hours each day), with an additional $15 bonus for wearing the Actigraph all 7 days, and up to a $25 bonus for completing all of these measurements. Total monetary compensation for this part of the study could be $150.
All procedures were conducted in accordance with guidelines for the ethical conduct of human research outlined by the National Institute of Health and with approval of the University at Buffalo and the Carilion Clinic Institutional Review Boards.
Measures
Demographics
Race/ethnicity, household income, and educational level were assessed using a questionnaire adapted from MacArthur’s network for studies on socio-economic status and health.13
Anthropometrics
Weight was measured to the nearest 0.2 lb using a Tanita (Hong Kong, China) digital scale. Height was measured in centimeters to the nearest millimeter using a SECA (Chino, California) stadiometer. Measurements were used to calculate Body Mass Index (BMI = kg/m2).
Laboratory Measures
HbA1c was measured using the A1CNow+® system (PTS Diagnostics, Sunnyvale, CA), which has been validated in comparison to the National Glycohemoglobin Standardization Program (NGSP) standards.14 Non-fasting lipid profiles (total cholesterol, high density lipoproteins (HDL), low density lipoproteins (LDL), triglycerides, and HDL/total cholesterol ratio) were measured using the Alere Cholestech LDX® system (Alere Inc., Coral Springs, FL), a validated POC lipid measurement device.15 Blood pressure was measured in triplicate on the dominant arm in a sitting posture using an automated Omron HEM 907XL (Kyoto, Japan) validated16 blood pressure device, with pressures taken at one minute intervals. The latter two readings were averaged to assess systolic, diastolic and mean arterial pressure. Participants provided a urine sample to detect recent drug use. Urine was tested using the QuickTox® Drug Screen (Branan Medical Corp., Irvine, California), a reliable and accurate measure of drug use. Females were given a pregnancy test using the SA Scientific™ Ultimate hCG test (San Antonio, TX).
Delay Discounting
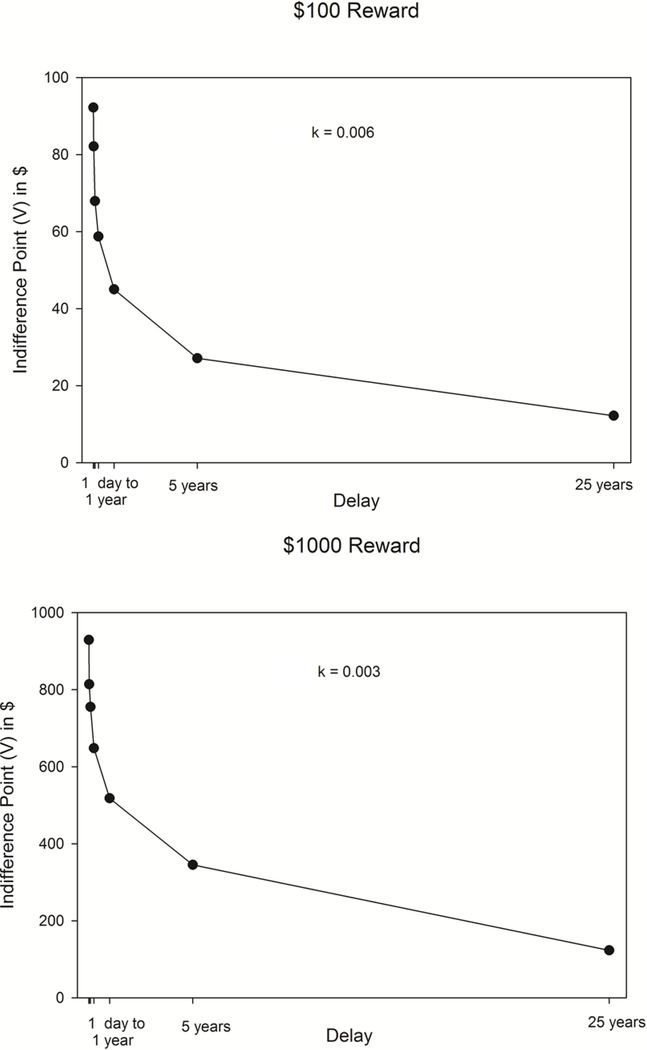
Delay discounting was assessed using an adjusting amount task.17 The adjusting amount task was used to calculate DD for two delayed monetary rewards ($100 and $1000). Two amounts were used because people discount large delayed rewards less than smaller delayed rewards,18 and demonstration of the magnitude effect can be used as evidence of measurement validity in assessments of discounting.19 In an exploratory study we wanted to study discounting across different amounts of delayed rewards. DD was assessed at each of seven delays (i.e., 1 day, 1 week, 1 month, 3 months, 1 year, 5 years, and 25 years), presented in a random order. Depending on the participant’s response, the immediate available amount was adjusted up (if delayed choice selected) or down (if immediate choice selected). Each time delay included six trials with the amount adjusting half that of the previous adjustment.20 For example, a participant might be asked whether they preferred to receive $50 now or $100 in one month. If they chose $50, then the amounts would be changed to $75 now versus $100 in one month. Indifference points were calculated, which represent values for which the participant is indifferent to the choice of the immediate versus delayed reward. For example, for a $100 delayed reward, V = $95 would reflect only 5% discounting of the reward, as opposed to a steeper discounting V = $50, reflects 50% discounting. Instructions to the participants for the task were as follows:
“In the following tasks, you will be asked to choose between receiving different amounts of money at different points in time. For these tasks, you’ll be using these pedals to submit your answer choices. To choose the option on the left side of the screen, press the left pedal, and to choose the option on the right side of the screen, press the right pedal. These are hypothetical choices, but please choose the answers as if they were real. There are no right or wrong answers in this task. Please take your time and answer thoughtfully. We will be monitoring your answers to make sure you are paying attention and you may be discontinued if you are not. While you are completing this task, I’ll be in the next room. The rooms are connected by a microphone. If you have any questions, please ask out loud and I will be able to hear and answer you. Do you have any questions?” Pedals were used as the response manipulandum since selected people also participated in an functional magnetic resonance imaging (fMRI) study,21 and we wanted to keep the conditions of measurement in the lab and in the fMRI environment as similar as possible.
To calculate delay discounting we used a hyperbolic discounting model (Mazur, 1987),
Where V is discounted value, A is reward amount, D is delay, and k is a free parameter that indexes rate of discounting. Higher values of k indicate more rapid devaluation of the delayed reward and greater impulsivity. Patterns of responding in the adjusting amount task were checked to see if systematic responding was observed using two rules: 1) If the subsequent indifference point was 20% less than the preceding indifference point of the larger, later reward. 2) If the last indifference point was not less than the first indifference point by at least a magnitude equal to 10% of the larger later reward 22. Non-systematic participants were excluded from analyses for each respective discounting measure, resulting in 79 participants with valid $100 discounting, and all 81 participants showed valid responding for the $1000 discounting task. Figure 1 presents the hyperbolic discounting curve for both the $100 and $1000 delayed rewards.
Figure 1.
Graph of the indifference points for choosing the $100 and $1000 delayed rewards.
Medication and health behavior adherence
Adherence to anti-hypertensive and/or lipid-lowering therapy was measured using a self-report questionnaire. This questionnaire has been validated for individuals with type 2 diabetes who have comorbid hypertension or dyslipidemia against MEMs, the Medication Event Monitoring System, an automated pill counting system that is recognized as the gold standard for adherence.23 In validation research with persons with Type 2 diabetes, hypertension and dyslipidemia, MEMs recorded 78.9% adherence, while the self-report questionnaire recorded 78.3% adherence for monthly and 82.3% for weekly adherence
Diet Quality
Diet quality, another measure of behavioral adherence, was assessed using randomly scheduled repeated 24-hour food recalls, with two weekdays and one weekend day, collected by the Cincinnati Center for Nutrition Research and Analysis (CCNRA) using the latest version of the Nutrition Data System for Research (NDSR). Interviewers phoned subjects and employed the USDA Automated Multiple Pass Method (AMPM) when conducting 24-hour dietary recalls, a validated method of collecting energy intake.24 If participants were not available, they were recalled to obtain the measures. Overall diet quality was scored using Healthy Eating Index 2010 (HEI)25 which provides indices of healthy eating based on intake of 12 categories of food, including fruits, vegetables, grains, dairy, proteins, seafood and plant proteins, fatty acids, sodium and empty calories. The HEI provides scores ranging from 0 to 100, with higher scores indicating a healthy diet, more in conformance with the 2010 Dietary Guidelines for Americans. 26
Physical activity
Physical activity, the final measure of behavioral adherence, was measured using the Actigraph WGT3X-BT accelerometer (AG; ActiGraph, Pensacola, FL), a validated measure of physical activity.27 Participants were asked to wear the monitor on their non-dominant side for one week, snug to the hip during waking and non-swimming/bathing/showering hours. They recorded time the device was worn. Data were collected at a rate of 30 Hz and were filtered first for non-wear time based on 90 consecutive minutes of non-wear28 and then filtered based on participants’ wear time diaries. The dependent measure was steps per minute.
Analytic Plan
The relationship between DD, HbA1c and health behaviors was explored in four complementary ways. First, we established the correlations between the $100 and $1000 delay discounting measures (natural log k values) and HbA1c, medication adherence and health behaviors using Pearson’s correlation coefficients. Due to incomplete data and participants inconsistent responding on the delay discounting tasks, the sample ranged from 77 to 79 (95% to 97.5% of the sample) participants for each correlation. Pairwise correlations were used to maximize the sample size for each analysis. The Benjami-Hochberg29 False Discovery Rate (FDR) was used to control for multiple correlations on the same dataset. Secondly, following zero order correlations, multiple regressions were used to assess whether sociodemographic variables moderated the relationship between DD and the biological or behavioral outcomes.30,31
Third, mediational analyses30,31 tested whether medication adherence, diet quality or physical activity mediated the relationship between DD and HbA1c. The mediational model used 10,000 bootstrapping simulations to determine the indirect effects of DD on HbA1c change mediated by medication adherence, diet quality or physical activity.30,31 Finally, hierarchical regression was used to assess the incremental effects of DD, BMI, eating, activity, and medication adherence on HbA1c beyond the association of sociodemographic factors (age, sex, education level, minority status). In addition to the correlational analyses, repeated measures ANOVA was used to test differences in k values between smaller ($100) and larger ($1000) adjusting amounts. Data were centered prior to analysis for the moderation analysis.
Results
Characteristics of the sample and delay discounting values are shown in Table 1. Participants’ HEI scores of 46.2 were approximately 10% lower than those of participants in a nationally-representative sample, reflecting a poorer quality diet.32 Participants wore the accelerometer between 36.2 and 122.1 hours, with an average of 90.5 + 17.6 hours. Participants had 6.9 steps/minute, which is approximately 20% lower for baseline levels of activity than persons with Type 2 diabetes who began an intervention program to improve physical activity.33 Consistent with research on reward magnitude, participants discounted $100 delayed rewards more than the $1000 delayed rewards (100$: log k = −5.14, k = 0.104, $1000: log k = −5.61, k = 0.145, F(1,78) = 7.73, p = 0.007).34
Table 1.
Participant characteristics
| Characteristic | |
|---|---|
| Sex (male/female) | 31/50 (38/62%) |
| Minority (minority/non-minority) | 23/58 (28/72%) |
| Asian | 1 |
| Native American | 1 |
| Black / African American | 20 |
| White (Non-Hispanic) | 56 |
| Hispanic | 2 |
| Multi-race | 1 |
| Age (years) | 55.2 ± 11.7 |
| Body Mass Index (kg/m2) | 32.2 ± 8.7 |
| Years of Education | 14.7 ± 2.1 |
| High School Graduate or less | 17 |
| Some college/vocational school | 32 |
| College graduate | 17 |
| Graduate degree | 12 |
| Refused | 3 |
| Annual Household Income ($) | 63,687 ± 47044 |
| < $30,000 | 25 |
| $30,000 – $70,000 | 19 |
| $70,000–$110,000 | 27 |
| $110,000–$140,000 | 4 |
| > $140,000 | 5 |
| Refused | 1 |
| HbA1c (%) | 6.0 ± 0.2 |
| Delay Discounting (log k) | |
| Adjusting Amount $100 | −5.2 ± 2.5 |
| Adjusting Amount $1000 | −5.6 ± 2.6 |
| Healthy Eating Index (HEI) | 46.2 ± 13.0 |
| Accelerometer steps per minute | 6.9 ± 3.3 |
| Medication adherence to hypertension and/or dyslipidemia medication | 90.7 ± 21.7 |
Table 2 shows the relationships between all discounting tasks and demographics, HbA1c and medication adherence, diet and exercise behaviors. Even after correcting for the False Discovery Rate,29 results across the $100 and $1000 tasks showed DD was associated with minority status, BMI, years of education, income, HEI, and adherence to hypertension and/or dyslipidemia medication. Minorities, participants with greater BMI, lower education, lower income, lower diet quality, HEI scores and lower adherence to hypertension/dyslipidemia medication discounted the future more. In addition, $1000 discounting was related to sex, HbA1c, and physical activity, with women, and participants with higher HbA1c, and lower physical activity discounting more.
Table 2.
Relationships (pearson r) between delay discounting and HbA1c, medication adherence and general preventive health behaviors.
| Delay discounting measures | ||||||
|---|---|---|---|---|---|---|
| $100 | p | FDR p | $1000 | p | FDR p | |
| Sex (male/female) | 0.06 | 0.628 | 0.698 | 0.25 | 0.022* | 0.037§ |
| Minority (minority/non-minority) | 0.38 | 0.001* | 0.003§ | 0.36 | 0.001* | 0.004§ |
| Age (years) | −0.05 | 0.659 | 0.659 | −0.03 | 0.779 | 0.779 |
| Body Mass Index (BMI) | 0.25 | 0.027* | 0.055§ | 0.23 | 0.042* | 0.046§ |
| Years of Education | −0.28 | 0.014* | 0.036§ | −0.33 | 0.004* | 0.012§ |
| Annual Household Income ($) | −0.41 | <0.001* | 0.002§ | −0.52 | <0.001* | <0.001§ |
| HbA1c (%) | 0.17 | 0.125 | 0.156 | 0.24 | 0.028* | 0.040§ |
| Healthy Eating Index (HEI) | −0.24 | 0.031* | 0.052§ | −0.31 | 0.005* | 0.014§ |
| Accelerometer steps per minute | −0.22 | 0.058 | 0.103 | −0.27 | 0.016* | 0.048§ |
| Adherence to hypertension and/or dyslipidemia medication | −0.32 | 0.004* | 0.015§ | −0.28 | 0.012* | 0.024§ |
Unadjusted p <.05
Significant after Benjamini-Hochberg adjusted p with 10% False Discovery Rate (FDR).
Moderation analyses, shown in Table 3, showed that minority status moderated the association between DD and HbA1c. Breakdown of minority status and 1 standard deviation above and below mean DD to define low and high DD showed that HbA1c values were similar for non-minority participants across both low and high DD grouping (5.95 vs 5.92), while HbA1c values were much higher for high DD (6.11) versus low DD (5.81) minority participants. Mediational analysis, shown in Table 4, did not show that diet quality, physical activity or medication adherence (p’s > 0.05) mediated the relationship between DD and HbA1c.
Table 3.
Moderation estimates testing whether sex, education, household income, or minority status moderates the relationship between delay discounting and HbA1c for the $100 and $1000 rewards. Predictors were centered to aid in interpretation of main effects.
| β | S.E. | T | P | β | S.E. | T | P | |
|---|---|---|---|---|---|---|---|---|
| $100 | $1000 | |||||||
| Sex | ||||||||
| Constant | 5.97 | 0.02 | 263.50 | <0.001 | 5.97 | 0.02 | 263.18 | <0.001 |
| Sex | 0.08 | 0.05 | 1.72 | 0.09 | 0.05 | 0.05 | 1.15 | 0.26 |
| DD | 0.01 | 0.01 | 1.36 | 0.18 | 0.02 | 0.01 | 1.81 | 0.07 |
| Sex * DD | −0.01 | 0.02 | −0.46 | 0.64 | −0.01 | 0.02 | −0.59 | 0.56 |
| Education | ||||||||
| Constant | 5.96 | 0.02 | 251.29 | <0.001 | 5.96 | 0.02 | 252.82 | <0.001 |
| Education | −0.00 | 0.01 | −0.18 | 0.86 | 0.00 | 0.01 | 0.08 | 0.94 |
| DD | 0.01 | 0.01 | 1.40 | 0.17 | 0.02 | 0.01 | 2.19 | 0.03 |
| Education * DD | −0.00 | 0.00 | −0.06 | 0.96 | −0.00 | 0.00 | −0.10 | 0.92 |
| Income | ||||||||
| Constant | 5.98 | 0.02 | 243.60 | <0.001 | 5.99 | 0.03 | 236.39 | <0.001 |
| Income | −0.00 | 0.00 | −0.45 | 0.65 | 0.00 | 0.00 | 0.30 | 0.77 |
| DD | 0.01 | 0.01 | 1.41 | 0.16 | 0.02 | 0.01 | 2.27 | 0.03 |
| Income * DD | 0.00 | 0.00 | 1.55 | 0.13 | 0.00 | 0.00 | 1.66 | 0.10 |
| Minority | ||||||||
| Constant | 5.94 | 0.02 | 251.02 | <0.001 | 5.96 | 0.02 | 257.11 | <0.001 |
| Minority | 0.03 | 0.06 | 0.56 | 0.58 | 0.07 | 0.05 | 1.26 | 0.21 |
| DD | 0.01 | 0.01 | 1.26 | 0.21 | 0.014 | 0.01 | 1.54 | 0.13 |
| Minority * DD | 0.07 | 0.02 | 2.74 | 0.01 | 0.029 | 0.02 | 1.40 | 0.17 |
Table 4.
Summary of simple mediation models assessing the indirect effect of healthy eating, physical activity or medication adherence on the relationship between delay discounting and HbA1c.
| Bca 95% CIc | |||||||
|---|---|---|---|---|---|---|---|
| Mediator | Effect of DD on HbA1c | Effect of DD on mediator | Effect of Mediator on HbA1c | DD on HbA1c through mediator | Lower | Upper | Effect Ratiod |
| (Direct effect C’)a | (Path A)a | (Path B)a | (Indirect effect)b | ||||
| Healthy Eating Index | 0.017*(0.01) | −1.54 (0.06) | 0.00 (0.79) | −0.0007 | −0.007 | +0.005 | −0.0438 |
| Physical Activity | 0.015 (0.08) | −0.311* (0.04) | −0.002 (0.72) | 0.0007 | −0.002 | +0.007 | −0.0462 |
| Medication Adherence | 0.022* (0.02) | −2.32* (0.01) | 0.0012 (0.26) | −0.0028 | −0.001 | +0.002 | −0.1468 |
Standardized regression coefficients, p values in ().
The magnitude of the indirect effect is estimated by the product of the regression coefficients of the predictive variables from Path A (DD to mediator) and Path B (mediator to HbA1c).
BCa = bias-corrected and accelerated 95% confidence intervals obtained from 10,000 bootstrap resamples. Confidence intervals not containing zero suggest that the indirect effect is significant at the 0.95 level.
Effect ratio = indirect effect/total effect. The effect ratio quantifies the proportion of the total effect explained by the indirect effect.
Hierarchical regression showed that participant age was the only sociodemographic variable that was associated with HbA1c for step one of the regression model. DD and the interaction of DD x minority status significantly increased variance accounted for in step 2 by 8.8%, and medication adherence increased variance accounted for beyond sociodemographic variables, DD, BMI, eating and physical activity by 4.5%.
Discussion
The results of this study show that both adjusting amount measures of DD predict concurrent HbA1c values in a sample of adults with prediabetes, and DD was related to medication adherence, eating, and activity behaviors. DD is related to a wide variety of preventive health behaviors, ranging from drug addiction to gambling as well as health behaviors such as preventive health checkups, seat belt and sun screen use,7 and is also related to obesity.9 As such, the finding that those people who discount the future may be less likely to engage in important health care behaviors should not be surprising,4,5 Nor should the observation that those who discount more would engage in eating and activity behaviors that lead to diabetes, similar to relationship of DD to obesity.9 The cross-sectional relationship between DD and glycemic control represents a basic step in the experimental medicine approach,10,11 suggesting that DD can be a target for intervention, which may improve glucose regulation. This relationship was observed for a sample that by self-report was generally adherent to most of the health behaviors studied, with participants reporting over 90% adherence to their medications. Thus, even given a very restricted range of behavior, DD still was related to adherence and preventive health behaviors. To strengthen this hypothesis, we have recently observed that increases in DD predict increases in HbA1c values over one year.12
Clearly to prevent a disease from occurring, such as preventing the transition from prediabetes to type 2 diabetes, one must focus on the future benefits of health behaviors. Eating high energy dense, palatable foods or engaging in sedentary behaviors may be more reinforcing for many people than eating healthy foods and being physically active.35–37 Participants in this study were generally obese, and had previously diagnosed hypertension and/or dyslipidemia. People with current diseases that could be prevented would seem to be motivated to engage in behaviors that have long-term value and could prevent the transition to type 2 diabetes. However, our mediational analyses did not show that diet quality or physical activity, which were related to delay discounting, could be considered as mechanisms for the relationship between DD and HbA1c values. Several factors may account for this. One may be that the HEI does not capture important aspects of the diet that may influence glycemic control, such as the glycemic index of foods. Glycemic index of foods refers to the changes in blood glucose that occur after eating food, and a diet that is low in high glycemic foods may be more important than other aspects of the diet for those with prediabetes.38 Another possibility was the low level of activity in the sample of relatively inactive participants was not adequate to influence glycemic control. Higher levels, or more variability in activity levels, may be needed to show the influence of activity on glycemic control. Also the cross sectional nature of the design may limit our ability to argue that a history of unhealthy eating or inactivity are related to current HbA1c values. The ideal model to argue mediation would be to collect these data prior to measurement of HbA1c. Prospective research is needed to better understand what variables account for the relationship between DD and HbA1c values.
In this sample of middle-income individuals with post high-school education, discounting of the future was strongly related to income (r’s > 0.50), which may be relevant to understand the relationship between delay discounting and health behaviors and HbA1c. Understandably people with limited income need to put their resources toward immediate concerns, and may not allocate monetary or time resources toward future goals.39 If you are worried about paying the rent, you may not be allocating cognitive resources towards changing your health behaviors. In fact, we have shown that manipulating hypothetical monetary scarcity can increase discounting of the future.40,41 Greater discounting for people experiencing monetary scarcity may help explain the disproportionate loading of diabetes in those with lower income or education.42
Moderation analysis showed that the relationship between DD and HbA1c levels was influenced by minority status, and this interaction was significant even when controlling for relevant sociodemographic variables such as age, sex, and education. Discounting of the future was a more important predictor of HbA1c for minority participants. This is important because minorities are two to six times more likely to have diabetes than non-minority persons,43 and minorities have many misconceptions about the disease trajectory and need for medication adherence and self-care if they have diabetes.44 Adherence to medications for diseases that are often comorbid with diabetes also showed a significant association with HbA1c values after controlling for sociodemographic variables, which may relate to those who do not take their medications also do not engage in other aspects of self-care that are relevant for glycemic control.45
Given the potential for modifying delay discounting to improve glycemic control, research has shown a number of different approaches to reliably reduce discounting of the future and improve delay discounting choices.46,47 The most researched approach to modifying delay discounting is episodic future thinking, that reduces delay discounting by having people vividly imagine positive future events.48–52 Episodic future thinking has been shown to reduce energy intake in obese persons49 improve food purchasing behavior 53 and improve success with weight loss,51 which may be useful in regulating body weight and glycemic control. Importantly, given that low income people discounted more in this study, and they are more likely to have diabetes,42 EFT can modify DD even in persons with low income on government assistance.54 Incorporating episodic future thinking into existing therapies47 with standard diet and exercise approaches to influence glycemic control would be an interesting extension of this prior research.
Despite the significant relationships between delay discounting and glycemic control, limitations of this study should be considered in interpreting the results. Note that the definition of prediabetes involves a relatively narrow range of HbA1c values of 0.7%, from 5.7% to 6.4%. Statistically, regression analyses attempting to predict a variable with limited variability may result in smaller estimates of a relationship than if you were attempting to predict the relationship between delay discounting and HbA1c in a sample that included a wide range of HbA1c values, including people with normoglycemia, prediabetes, and type 2 diabetes. As previously noted, the sample was very adherent by self-report to their hypertension or dyslipidemia medications. While the adherence measure had been validated against MEMs,6 an objective measure of medication adherence, the level of adherence reported is likely to be an overestimate of actual adherence. Increasing the variability in adherence would likely increase the magnitude of an already significant relationship. The delay discounting task is a monetary discounting task that asks people to choose among smaller immediate amounts of money versus larger amounts of money provided at a later date. While this is an analog to a wide variety of temporal decisions, perhaps developing intertemporal tasks that assess real life decisions that people with prediabetes have to make would show stronger relationships. These could be the choice of eating a preferred high energy dense food now versus losing weight later, or watching television versus not showing fitness improvements later. HbA1c was measured using a point of care (POC) device. While this device has been validated in comparison to the National Glycohemoglobin Standardization Program (NGSP) standards,14 some variation in HbA1c values in comparison to venous blood samples may be possible, but without a systemic bias that could significantly change the pattern of results. Finally, the cross-sectional nature of the data limit the ability to test whether eating behaviors, exercise behaviors or medication adherence mediate the relationships between delay discounting and HbA1c.
The results of this study provide one step in an experimental medicine approach10,11 to identify delay discounting as a behavioral target to modify the transition from prediabetes to type 2 diabetes. The cross-sectional relationship identified in this paper, along with the prospective relationship between changes in delay discounting and HbA1c,12 provide observational data that delay discounting is related to glycemic control. Ongoing research on using episodic future thinking to modify delay discounting, followed by research to examine whether episodic future thinking can be useful to prevent type 2 diabetes will provide the other data needed to confirm the importance of delay discounting as an important target for diabetes prevention.
Conclusions
This study adds to the growing body of research on the relationship between delay discounting and glycemic control in those with type 1 diabetes,3 type 2 diabetes,4,5 and now in those with prediabetes. This study suggests that the same aspect of behavioral decision making may be related to diet and exercise behaviors, preventive health behaviors, and HbA1c. Research is needed that studies the effect of modifying delay discounting on prevention of diabetes.
Table 5.
Hierarchical regression model predicting HbA1c values.
| Step | Predictors | β Coeff | SE | Std β Coeff | t | p | R2 | ΔR2 |
|---|---|---|---|---|---|---|---|---|
| 1 | Constant | 5.731 | 0.189 | 0.00 | 30.30 | <0.001 | 0.137 | |
| Age | 0.004 | 0.002 | 0.253 | 2.22 | 0.03 | |||
| Sex | 0.073 | 0.045 | 0.183 | 1.63 | 0.11 | |||
| Minority | 0.094 | 0.052 | 0.212 | 1.82 | 0.07 | |||
| Education | −0.005 | 0.011 | −0.051 | 0.45 | 0.66 | |||
| 2 | DD | −0.006 | 0.011 | −0.073 | 0.54 | 0.59 | 0.225 | 0.088 |
| DD x Minority | ||||||||
| F test for incremental variance: F(2) = 3.80, p = 0.027 | ||||||||
| 3 | BMI | 0.003 | 0.003 | 0.154 | 1.28 | 0.42 | 0.244 | 0.019 |
| F test for incremental variance: F(1) = 1.66, p = 0.202 | ||||||||
| 4 | HEI | −0.002 | 0.002 | −0.136 | 1.122 | 0.27 | 0.270 | 0.026 |
| Physical Activity | 0.008 | 0.007 | 0.149 | 1.218 | 0.228 | |||
| F test for incremental variance: F(2) = 1.14, p = 0.326 | ||||||||
| 5 | Med Adherence | 0.002 | 0.001 | 0.249 | 2.034 | 0.046 | 0.315 | 0.045 |
| F test for incremental variance: F(1) = 4.14, p = 0.046 | ||||||||
Note – HEI is Healthy Eating Index, Med Adherence is Medication Adherence, Coefficent represents the B coefficient, SE is the standard error of the B coefficient, Std B coefficent is the standardized beta coefficient, R2 is the amount of variance cumulative amount of variance accounted for in the model, and ΔR2 is the incremental amount of variance accounted for in the model.
Acknowledgments
This research was funded in part by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Institute of Diabetes and Digestive and Kidney Diseases (1UH2DK109543), awarded to Drs. Epstein and Bickel.
Footnotes
Conflict of Interest: Dr. Epstein was a consultant and had equity in Daltri when the study was implemented. Dr. Bickel is a consultant or has equity in HealthSim LLC, NotifiUs LLC, Sober Grid Inc., DxRx, Prophase LLC, Teva Branded Pharmaceuticals, General Genetic Corporation. The other authors do not declare any conflict of interest with respect to the authorship or publication of this article.
Compliance with ethical standards:
Ethical approval: All procedures performed were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration and its later amendment of comparable ethical standards.
Informed consent: Informed consent was obtained from all participants included in the study.
References
- 1.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansing AH, Stanger C, Crochiere R, Carracher A, Budney A. Delay discounting and parental monitoring in adolescents with poorly controlled type 1 diabetes. J Behav Med. 2017;40:864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reach G, Michault A, Bihan H, Paulino C, Cohen R, Le Clesiau H. Patients’ impatience is an independent determinant of poor diabetes control. Diabetes Metab. 2011;37:497–504. [DOI] [PubMed] [Google Scholar]
- 5.Lebeau G, Consoli SM, Le Bouc R, Sola-Gazagnes A, Hartemann A, Simon D, Reach G, Altman JJ, Pessiglione M, Limosin F, Lemogne C. Delay discounting of gains and losses, glycemic control and therapeutic adherence in type 2 diabetes. Behav Process. 2016;132:42–48. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez JS, Schneider HE, Wexler DJ, Psaros C, Delahanty LM, Cagliero E, Safren SA. Validity of medication adherence self-reports in adults with Type 2 Diabetes. Diabetes Care. 2013;36:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickel WK, Stein JS, Moody LN, Snider SE, Mellis AM, Quisenberry AJ. Toward narrative theory: Interventions for reinforcer pathology in health behavior. In: Stevens JR, ed. Impulsivity: How time and risk ifnluence decision making. New York, NY: Springer; 2017:227–267. [PubMed] [Google Scholar]
- 8.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. [DOI] [PubMed] [Google Scholar]
- 9.Amlung M, Petker T, Jackson J, Balodis I, MacKillop J. Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychol Med. 2016;46:2423–2434. [DOI] [PubMed] [Google Scholar]
- 10.Bernard C An introduction to the study of experimental medicine. North Chelmsford, MA: Courier Corporation; 1927. [Google Scholar]
- 11.Riddle M, Science of Behavior Change Working Group. News from the NIH: using an experimental medicine approach to facilitate translational research. Translat Behav Med. 2015;5:486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein LH, Paluch RA, Stein JS, Mellis AM, Quattrin T, Mastrandrea LD, Bree KA, Greenawald MH, Bickel WK. Role of delay discounting in predicting change in HBA1c for individuals with prediabetes. J Behav Med In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychol. 2000;19:586–592. [DOI] [PubMed] [Google Scholar]
- 14.Bode BW, Irvin BR, Pierce JA, Allen M, Clark AL. Advances in hemoglobin A1C point of care technology. J Diabetes Sci Tech. 2007;1:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey M, Markham C, Gaffney P, Boran G, Maher V. Validation of a point of care lipid analyser using a hospital based reference laboratory. Irish J Med Sci. 2006;175:30–35. [DOI] [PubMed] [Google Scholar]
- 16.White WB, Anwar YA. Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit. 2001;6:107–110. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koffarnus MN, Bickel WK. A 5-trial adjusting delay discounting task: Accurate discount rates in less than one minute. Exp Clin Psychopharm. 2014;22:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du WJ, Green L, Myerson J. Cross-cultural comparisons of discounting delayed and probabilistic rewards. Psychol Rec. 2002;52:479–492. [Google Scholar]
- 21.Deshpande HU, Mellis AM, Lisinski JM, Stein JS, Koffarnus MN, Paluch R, Schweser F, Zivadinov R, LaConte SM, Epstein LH, Bickel WK. Reinforcer pathology: Common neural substrates for delay discounting and snack purchasing in prediabetics. Brain Cogn. 2019;132:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Exp Clin Psychopharm. 2008;16:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. [DOI] [PubMed] [Google Scholar]
- 24.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, Staples RC, Cleveland LE. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–332. [DOI] [PubMed] [Google Scholar]
- 25.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HAB, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition,. Washington, DC U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 2011;14:411–416. [DOI] [PubMed] [Google Scholar]
- 28.Choi L, Liu ZW, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sport Exer. 2011;43:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 30.Hayes AF. An introduction to mediation, moderation, and conditional process modeling: A regression-based approach. New York, New York: Guilford Press; 2013. [Google Scholar]
- 31.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. [DOI] [PubMed] [Google Scholar]
- 32.Guenther PM, Kirkpatrick SI, Reedy J, Krebs-Smith SM, Buckman DW, Dodd KW, Casavale KO, Carroll RJ. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yates T, Edwardson CL, Henson J, Gray LJ, Ashra NB, Troughton J, Khunti K, Davies MJ. Walking Away from Type 2 diabetes: a cluster randomized controlled trial. Diabetic Med. 2017;34:698–707. [DOI] [PubMed] [Google Scholar]
- 34.Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Mem Cogn. 1997;25:715–723. [DOI] [PubMed] [Google Scholar]
- 35.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychol Bull. 2007;133:884–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein LH, Saelens BE. Behavioral economics of obesity: Food intake and energy expenditure. In: Bickel WK, Vuchinich RE, eds. Reframing health behavior change with behavioral economics. Mahwah, N.J: Lawrence Erlbaum; 2000:293–311. [Google Scholar]
- 37.Epstein LH, Roemmich JN. Reducing sedentary behavior: Role in modifying physical activity. Exer Sport Sci Rev. 2001;29:103–108. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig DS. The glycemic index - Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. [DOI] [PubMed] [Google Scholar]
- 39.Epstein LH, Jankowiak N, Lin H, Paluch R, Koffarnus MN, Bickel WK. No food for thought: moderating effects of delay discounting and future time perspective on the relation between income and food insecurity. Am J Clin Nutr. 2014;100:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bickel WK, Wilson AG, Chen C, Koffarnus MN, Franck CT. Stuck in time: Negative income shock constricts the temporal window of valuation spanning the future and the past. PloS one. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sze YY, Stein JS, Bickel WK, Paluch RA, Epstein LH. Bleak present, bright future: Online episodic future thinking, Scarcity, delay discounting, and food demand. Clin Psychol Sci. 2017;5:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maty SC, Everson-Rose SA, Haan MN, Raghunathan TE, Kaplan GA. Education, income, occupation, and the 34-year incidence (1965–99) of Type 2 diabetes in the Alameda County Study. Int J Epidemiol. 2005;34:1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125:221–232. [DOI] [PubMed] [Google Scholar]
- 44.Mann DM, Ponieman D, Leventhal H, Halm EA. Misconceptions about diabetes and its management among low-income minorities With diabetes. Diabetes Care. 2009;32:591–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quisel T, Foschini L, Zbikowski SM, Juusola JL. The association between medication adherence for chronic conditions and digital health activity tracking: Retrospective analysis. J Med Internet Res. 2019;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: A review. J Exp Anal Behav. 2013;99:32–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rung JM, Madden GJ. Experimental reductions of delay discounting and impulsive choice: A systematic review and meta-analysis. J Exp Psychol Gen. 2018;147:1349–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniel TO, Sawyer A, Dong YL, Bickel WK, Epstein LH. Remembering versus imagining: When does episodic retrospection and episodic prospection aid decision making? J Appl Res Mem Cogn. 2016;5:352–358. [Google Scholar]
- 49.Daniel TO, Stanton CM, Epstein LH. The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychol Sci. 2013;24:2339–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daniel TO, Stanton CM, Epstein LH. The future is now: comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appetite. 2013;71:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sze YY, Daniel TO, Kilanowski CK, Collins RL, Epstein LH. Web-based and mobile delivery of an Episodic Future Thinking intervention for overweight and obese families: a feasibility study. JMIR mHealth uHealth. 2015;16:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stein JS, Wilson AG, Koffarnus MN, Daniel TO, Epstein LH, Bickel WK. Unstuck in time: episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology. 2016;233:3771–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schacter DL, Benoit RG, Szpunar KK. Episodic future thinking: mechanisms and functions. Curr Opin Behav Sci. 2017;17:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Donnell S, Daniel TO, Koroschetz J, Kilanowski C, Otiminshi A, Bickel WK, Epstein LH. Do process simulations during episodic future thinking enhance the reduction of delay discounting for middle income participants and those living in poverty? J Behav Decis Mak. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]