Abstract
Background
The Rel/NF-κB transcription factors have been shown to regulate apoptosis in different cell types, acting as inducers or blockers in a stimuli- and cell type-dependent fashion. One of the Rel/NF-κB subunits, RelA, has been shown to be crucial for normal embryonic development, in which it functions in the embryonic liver as a protector against TNFα-induced physiological apoptosis. This study assesses whether NF-κB may be involved in the embryo's response to teratogens. Fot this, we evaluated how NF-KappaB DNA binding activity in embryonic organs demonstraiting differential sensitivity to a reference teratogen, cyclophosphamide, correlates with dysmorphic events induced by the teratogen at the cellular level (excessive apoptosis) and at the organ level (structural anomalies).
Results
The embryonic brain and liver were used as target organs. We observed that the Cyclophosphamide-induced excessive apoptosis in the brain, followed by the formation of severe craniofacial structural anomalies, was accompanied by suppression of NF-κB DNA-binding activity as well as by a significant and lasting increase in the activity of caspases 3 and 8. However, in the liver, in which cyclophosphamide induced transient apoptosis was not followed by dysmorphogenesis, no suppression of NF-κB DNA-binding activity was registered and the level of active caspases 3 and 8 was significantly lower than in the brain. It has also been observed that both the brain and liver became much more sensitive to the CP-induced teratogenic insult if the embryos were exposed to a combined treatment with the teratogen and sodium salicylate that suppressed NF-κB DNA-binding activity in these organs.
Conclusion
The results of this study demonstrate that suppression of NF-κB DNA-binding activity in embryos responding to the teratogenic insult may be associated with their decreased resistance to this insult. They also suggest that teratogens may suppress NF-κB DNA-binding activity in the embryonic tissues in an organ type- and dose-dependent fashion.
Background
Together with its role in normal embryogenesis [1], apoptosis plays also a role in the formation of anomalies induced by teratogens [2,3]. Many teratogens have the potential to initiate excessive apoptosis, which may lead to structural anomalies in certain but not in other embryonic organs, which are able to resist teratogen-initiated apoptosis and continue normal development.
Apoptosis is a process realized whereby many parallel and converging signal transduction pathways, some of which lead to cell death whereas the others act to provide survival of targeted cells [4]. These transduction cascades comprise molecules that act as activators, effectors and negative regulators of apoptosis. It is conceivable, therefore, that molecules regulating the apoptotic process may be influential in determining the sensitivity or resistance of embryos to teratogens.
The Rel/NF-κB family of transcription factors have recently been demonstrated to play a crucial role in regulating apoptotic cell death acting as inducers or blockers of apoptosis in a stimulus- and cell type-dependent fashion [5,6]. Rel/NF-κB proteins control the expression of target genes by binding to DNA regulatory elements known as κB sites [7-9]. In most cell types, NF-κB exists in an inactive form in the cytoplasm bound to several IκB inhibitor proteins (IκBs) [7-9]. Its activity is regulated primarily by phosphorylation of IκBs. In response to a variety of internal and external stimuli, IκBs are phosphorylated by the IκB kinase (IKK) complex, that is followed by their ubiquitination and degradation leading to the release of and nuclear translocation of the freed NF-κB [10,11].
The Rel/NF-κB family of transcription factors are comprised of several related proteins (subunits) including c-rel, RelA, RelB, p50/p105 and p52/p100 [12]. Experiments in knockout mice addressing the functional role of NF-κB proteins revealed that RelA-/- embryos died on day 15 of gestation from massive hepatocyte apoptosis [13]. Subsequent studies [14-18] have led to the conclusion that RelA acts in the embryonic liver as a protector against TNFα-induced physiological apoptosis. At the same time, in experiments with cyclophosphamide, the teratogenic effect of which is preceded by excessive apoptosis [19,20], we observed [21] that excessive apoptosis registered in the liver of embryos tested 24 but not 48 hours after CP treatment. Furthermore, these embryos exhibited structural craniofacial and limb anomalies, but no dysmorphic events at the organ level were detected in the liver at this time point.
Teratogen-induced apoptotic stimuli inevitably alter the balance between inducers and blockers of physiological apoptosis, and seem to activate caspases, which are the final mediators of the cell death program [4,22]. Since NF-κB itself and certain proteins in the NF-κB-activated signaling pathway may serve as substrates for caspases [23], it is conceivable, that NF-κB may function differently in teratogen-targeted and normal embryonic organs. Nevertheless, the above-mentioned studies in RelA knockout mice and our teratological studies in mice exposed to CP indicate that NF-κB (at least, its RelA subunit) may be involved in regulating not only the physiological but also teratogen-induced apoptosis in some embryonic organs. Hence, NF-κB, while regulating teratogen-induced apoptosis, may also regulate the sensitivity/resistance of the embryo to the teratogenic stress. Therefore, this study was designed to evaluate whether a correlation exists between NF-κB DNA-binding activity in embryonic organs targeted by the teratogenic stress and the ability of these organs to resist a teratogen-induced process of maldevelopment.
As a basic model, we used mouse embryos exposed in vivo to a reference teratogen, cyclophosphamide (CP) [24-27]. To characterize the process of maldevelopment, fetuses were examined for external structural anomalies and such indices as NF-κB DNA-binding activity, the activity of caspase 8 and 3, apoptosis and histopathological changes. These changes were evaluated at different time points in two target organs: the liver, which resists CP-induced teratogenic insult, and the brain, which is very sensitive to the teratogen [21].
An additional set of experiments was performed with embryos of mice exposed to the teratogen in combination with sodium salicylate (NaSAL) that has been shown to be a potent inhibitor of NF-κB [28-30]. The suppressive effect of salicylates on NF-κB DNA-binding activity is primarily associated with their inhibitory effect on IκB kinase-beta (IKKβ), which is essential in the initiation of dissociation of NF-κB from its inhibitor IκB and subsequent translocation of NF-κB to the nucleus [30].
Materials and methods
Animal Models
Six to eight weeks old ICR mice were obtained from the Tel Aviv University animal facility. The animals were maintained on a 14 hr light/10 hr dark cycle with food and tap water ad libitum. Females were caged with males for 3 hr, from 7 to 10 a.m. (dark period) and the presence of a vaginal plug (11 a.m.) was designated as day 1 of pregnancy.
To induce a teratogenic effect, cyclophosphamide (CP) (Sigma, USA) was injected intraperitoneally at 10 a.m. of day 12 of pregnancy at 15 mg/kg or 40 mg/kg (in 0.5 ml saline per 20 g body weight).
Sodium salicylate (NaSAL) (Sigma, USA) was injected intraperitoneally in a dose of 400 mg/kg body weight at 6, 24 and 30 hours after CP treatment. The first injection of NaSAL was timed to match the time of the occurrence of excessive apoptosis in the liver of embryos treated with 40 mg/kg CP (our unpublished data), whereas the second and third injections (24 and 30 hours after CP injection) were timed to coincide with the time in which the liver begins to cease the CP-induced apoptosis [21]. The time of NaSAL injection to intact females matched the time of NaSAL injection to CP-treated females.
Pregnant females injected with saline (0.5 ml per 20 g body weight) were used as a control throughout the study.
Teratological testing
To evaluate the intensity of the CP-induced teratogenic effect, ten to twelve animals of each group, whether treated with 15 or 40 mg/kg CP, NaSAL, or CP+NaSAL, were sacrificed on day 17 of pregnancy. The uteri were removed, the number of implantation sites, resorptions and live embryos was recorded and live fetuses were examined for external anomalies.
Seven-μm paraffin-embedded sections of formalin-fixed embryonic tissues were obtained from 3–4 embryos of different experimental and control groups on day 14 of pregnancy (48 hours after CP treatment). The sections were stained with hematoxylin and eosin and used to characterize dysmorphic events in the liver.
The TUNEL method
Apoptosis was analyzed by Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) in embryos from the various experimental groups, collected 48 hours after CP injection and in age-matched control embryos. Three or four embryos from the control and experimental groups were fixed in formaldehyde, dehydrated in alcohol and xylene and embedded in paraffin. Tissue sections (7-μm) were prepared and the localization of apoptotic cells in the tested organs was visualized by TUNEL as described elsewhere [21].
Evaluation of caspases 3 and 8 activity
Embryonic brains or livers collected from 1–2 litters were pooled and used to prepare a sample for measurement of caspase activity. The caspase activity was tested in embryos collected at 6, 24 or 48 hours after CP injection, age-matched control embryos and embryos exposed to NaSAL alone or NaSAL in combination with CP.
The level of active caspase 8 was measured by a Caspase-8 Fluorometric Assay kit (R&D systems Inc., MN, USA) according to the manufacturer's instructions. Briefly, brain or liver samples were homogenized, centrifuged and resuspended in 1 ml of PBS. Viable cells were counted and 3 × 106 cells were resuspended in 60 μl of cold Cell Lysis Buffer and incubated on ice for 10 minutes.
Caspase-3 activity was evaluated by a FluorAceTM apopain assay kit (Bio-Rad Laboratories, CA, USA) according to the manufacturer's instructions. Briefly, cells from the tested organs were obtained as for caspase-8 activity test. Then, 6 × 106 cells were centrifuged, resuspended in 100 μl of apopain lysis buffer and vortexed gently. The suspensions were frozen and thawed 4–5 times in liquid nitrogen and a 37° C bath, respectively.
Protein concentration was measured using a DC Protein Assay kit (BioRad Laboratories, CA, USA) and a 550 Microplate Reader (Bio-Rad Laboratories, CA, USA) according to the manufacturer's instructions. Samples were frozen in aliquots in liquid nitrogen and stored at -70°C until use.
The measurements of both caspases were performed using a Fluorescence Plate Reader FL-600 (Bio-Tex Instruments, Inc., USA) according to the manufacturer's instructions.
Electrophoretic Mobility Shift Assay (EMSA)
Embryonic brains or livers were collected from 2–3 litters, pooled and used to prepare a sample of nuclear extract. Tissue samples were homogenized mechanically in PBS into single cell suspensions, centrifuged and resuspended in 1 ml PBS. Viable cells were counted and 15 × 106 cells were taken, frozen in liquid nitrogen and stored at -70°C until use. Nuclear extracts were prepared as described by Schreiber et al. [31]. Protein concentration was measured using a DC Protein Assay kit (BioRad Laboratories, CA, USA) and a 550 Microplate Reader (Bio-Rad Laboratories, CA, USA) according to the manufacturer's instructions. Samples were frozen in aliquots in liquid nitrogen and stored at -70°C until use.
A double stranded oligonucleotide, containing the NF-κB site was labeled by [32P] with the Klenow fragment of DNA polymerase 1 (MBI fermentas, Lithuania) and use as a probe. The oligonucleotide's sequence is: 5'GATCCAGAGGGGACTTTTCCGAGAG 3'; 5'GATCCTCTCGGAAAGTCCCCTCTG 3'. (synthesized in the Oligonucleotide and Peptide Synthesis Laboratory in the Weizmann Institute of Science, Rehovot, Israel).
Five μg of nuclear extract were preincubated for 15 minutes at room temperature in 2 μl Gel Shift Binding Buffer (20 mM TrisHCl, pH 7.6; 50 mM KCl; 1 mM MgCl2; 0.2 mM EDTA; 0.01% Triton X-100; 5% Glycerol and 0.5 mM Spermidine in distilled water) × 10, 1 μg poly dI-dC and double distilled water (DDW) in a total volume of 19 μl. One μl of radiolabeled oligonucleotide (20,000 cpm) was added per reaction and samples were incubated for an additional 15 minutes at room temperature. Samples were electrophoresed through a 5.5% acrylamide gel using Tris-Glycine buffer (40 mM TrisHCl and 195 mM Glycine). The NF-κB complexes were identified following autoradiography.
For NF-κB binding specificity, labeled and unlabeled oligonucleotides (100-fold excess of unlabeled NF-κB oligonucleotide or 100-fold excess of unlabeled octamer binding protein (OCT) oligonucleotide) were added after the binding reaction and incubated for 15 minutes at room temperature. The OCT binding sequence probe is:5'TTCAGGGTATGCAAATTATTAGCT3'; 5'TAGGAGCTAATAATTTGCATACCCTGAAGATC 3' (synthesized in the Oligonucleotide and Peptide Synthesis Laboratory in the Weizmann Institute of Science, Rehovot, Israel).
For the supershift assay, 6 μg of specific anti p50 or p65 (RelA) antibodies (#SC-114X and #SC-372X [18], respectively, Santa Cruz Biotechnologies, Inc., CA, USA) were added to the binding reaction and incubated for 15 minutes before adding the DNA probe.
Results of EMSA were reproduced in 3 successive experiments using different samples of liver or brain collected from embryos of the control and experimental groups.
Statistical analysis
Caspase activity
Six to eight samples obtained from the brain or liver of embryos of control and experimental groups were tested for caspase 3 and caspase 8 activity. Fmax-test for homogeneity of variances and ANOVA were used to test whether the data characterizing the activity of a tested caspase in a given organ of control embryos at a given time point matching the time after CP injection (6, 24 and 48 hours) may be pooled and treated as a unified control group [32]. Since this analysis did not reveal statistically significant differences, the results obtained in different control groups were pooled and the average values representing the activity of caspase-3 and caspase-8 in the brain and liver of control embryos were calculated. Caspase activity in an experimental sample was expressed as the ratio of the value registered in a tested sample by the fluorometric reading to the average value representing the activity of a given caspase in a given embryonic organ of control embryos. The GT2-method for multiple comparisons [32] was used for statistical analysis of the data obtained. The two-tailed level of significance of differences was = 0.05.
Teratological testing
Statistical analysis of the teratological data was performed on a litter basis. The proportions of malformed fetuses and resorptions per litter were transformed to arcsine values by Freeman-Turkey's binomial method as described elsewhere [33] and GT2-method for multiple comparisons was used for statistical analysis of the data obtained. The two-tailed level of significance of differences was = 0.05.
Results
Effects induced by CP
External structural anomalies
CP exerted a very strong teratogenic effect at a dose of 40 mg/kg: all fetuses were severely malformed and growth retarded (Table 1 and Fig. 1). The spectrum of anomalies included mainly craniofacial anomalies such as severe microcephaly, agnathia, open eyes, limb reduction anomalies such as phocomelia or amelia, as well as a trunk anomaly, eventration of the abdominal wall, and absence of the tail (Table 1 and Fig. 1). This dose of CP also resulted in a high resorption rate (approximately, 80%).
Table 1.
Effect of cyclophosphamide (CP) and sodium salicylate (NaSAL) on embryonic development
| INDICES | Groups (treatment/ CP dose) | ||||
| CP 40 mg/kg | CP 15 mg/kg | NaSAL | NaSAL+ CP 15 mg/kg | Control | |
| Litters examined | 10 | 11 | 12 | 10 | 10 |
| Implantation sites/litter | 11.3 | 10.9 | 11.5 | 11.6 | 10.8 |
| Live fetuses/litter | 2.5 | 9.7 | 10.3 | 9.8 | 9.8 |
| Resorptions % arcsine (M ± SE) |
77.9* 60.5 ± 3.1a |
10.8 21.8 ± 2.6b |
10.9 21.8 ± 3.2b |
15.5 25.2 ± 3.4b |
9.2 20.6 ± 2.8 b |
| Fetal weight (M ± SE) |
0.26 ± 0.02a |
0.60 ± 0.04 b |
0.74 ± 0.03 c |
0.44 ± 0.03d |
0.75 ± 0.02c |
| Fetuses with external structural anomalies | |||||
| Total examined malformed (%) arcsine (M ± SE) |
25 25(100) 76.9 ± 1.6a |
107 76(71.0) 56.3 ± 4.1 b |
123 0 8.7 ± 0.3 c |
98 98 (100) 81.1 ± 1.3a |
98 0 8.9 ± 0.4c |
| Types of anomalies | |||||
| Craniofacial anomalies (%) arcsine (M ± SE) |
25(100) 76.9 ± 1.6a |
5 (4.7) 13.7 ± 4.2b |
0 8.7 ± 0.3 b |
65 (66.3) 53.7 ± 4.9c |
0 8.9 ± 0.4 b |
| Limb reduction anomalies (%) arcsine (M ± SE) |
25(100) 76.9 ± 1.6a |
5 (4.7) 13.7 ± 4.2b |
0 8.7 ± 0.3 b |
70(71.4) 56.5 ± 5.1c |
0 8.9 ± 0.4 b |
| Digit anomalies (%) arcsine (M ± SE) |
0 13.1 ± 0.6a |
71 (66.3) 53.7 ± 3.6b |
0 8.7 ± 0.3a |
28 (28.6) 33.5 ± 4.5c |
0 8.9 ± 0.4a |
| Tail anomalies (%) arcsine (M ± SE) |
25(100) 76.9 ± 1.6a |
76(71.0) 60.6 ± 3.1b |
0 8.7 ± 0.3 c |
98 (100) 81.1 ± 1.3a |
0 8.9 ± 0.4c |
| Trunk anomalies (%) arcsine (M ± SE) |
25(100) 76.9 ± 1.6a |
3 (2.8) 12.4 ± 2.0 b |
0 8.7 ± 0.3 b |
36 (36.7) 38.0 ± 3.6C |
0 8.9 ± 0.4 b |
The percentage of resorptions and malformed fetuses presented in Table 1 was calculated using the implantation site and the fetus, respectively, as independent variables. Arcsine values were calculated on a litter basis and analyzed statistically by GT-2 method. Means not sharing common superscripts are significantly different. The description of craniofacial, limb reduction, digit, tail and trunk anomalies registered in fetuses of a given group is presented in the text. *- In 4 litters the resorption rate reached 100%.
Figure 1.

External structural anomalies in 17-day fetuses treated with CP alone or in combination with NaSAL. Top row: Left – a control fetus. Right – a fetus treated with NaSAL. No external anomalies were observed in these embryos. Bottom row: Left – a fetus treated with 40 mg/kg CP exhibits severe growth retardation, eventration of the abdominal wall, phocomelia and amelia (hindlimbs), absence of tail, agnathia, open eyes, severe microcephaly. Center: a fetus treated with 15 mg/kg CP exhibits light growth retardation, bowed tail, syndactyly and ectrodactyly. Right: a fetus treated with 15 mg/kg CP and NaSAL exhibits more severe growth retardation than the embryo treated with this dose of CP alone and such anomalies as microcephaly, open eyes, micrognathia, adactyly (forelimbs) and meromelia (hindlimbs), short tail, eventration of the abdominal wall.
The teratogenic effect CP at 15 mg/kg was significantly less prominent: approximately, 30% of fetuses had no external anomalies, whereas 70% exhibited external tail and digit anomalies such as short or crooked tail, syndactyly and ectrodactyly (Table 1 and Fig. 1). Only single fetuses exhibited more severe anomalies such as open eyes, meromelia and eventration of the abdominal wall. The level of resorptions in females treated with this dose of CP did not differ much from that in controls (Table 1).
Apoptosis
CP-induced apoptosis was evaluated in two target organs, the embryonic brain and liver. Our previous studies addressing the role of apoptosis in CP teratogenicity revealed that the temporal pattern of apoptosis induced by CP in these organs is dose- and organ type-dependent. Thus, apoptosis induced CP at 40 mg/kg was registered in the brain and liver of 13 day embryos (E13) by the TUNEL assay and FACS analysis [20,21]. However, if CP was injected at a dose of 15 mg/kg, no apoptosis was registered in the liver of E13 [20]. Apoptosis was also observed in the brain of 14 day embryos (E14) treated with 40 mg/kg CP [21].
In agreement with the above observations, in this study we also observed massive apoptosis in the brain of E14 exposed to 40 mg/kg CP (Fig. 2B). However, no excessive apoptosis was observed in the liver of these embryos (Fig. 3B).
Figure 2.
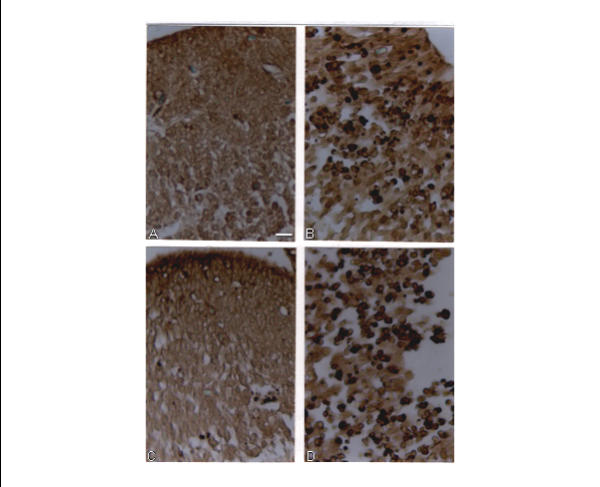
Representative TUNEL-stained sections of the brain of E14. All sections are midsagittal and present a region of the embryonic telencephalon containing the ependyma layer, mantle and marginal layers. A – a control embryo; B – an embryo treated with 40 mg/kg CP; C – an embryo treated with NaSAL; D – an embryo treated with 15 mg/kg CP +NaSAL. Scale bar = 10 μm
Figure 3.
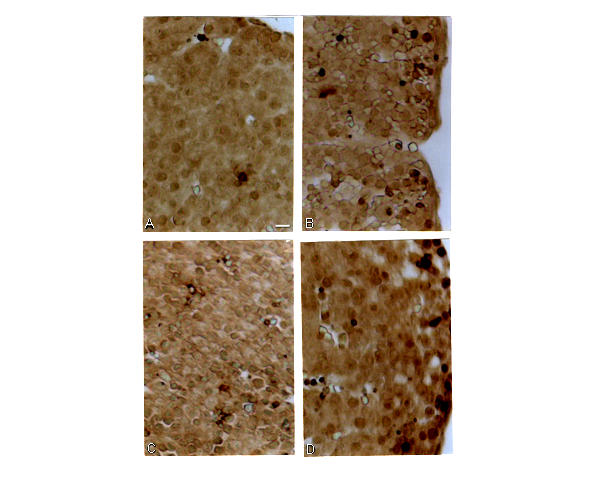
Representative TUNEL-stained sections of the liver of E14. All sections are midsagittal. A – a control embryo; B – an embryo treated with 40 mg/kg CP; C – an embryo treated with NaSAL; D – an embryo treated with 40 mg/kg CP +NaSAL. Scale bar = 10 μm
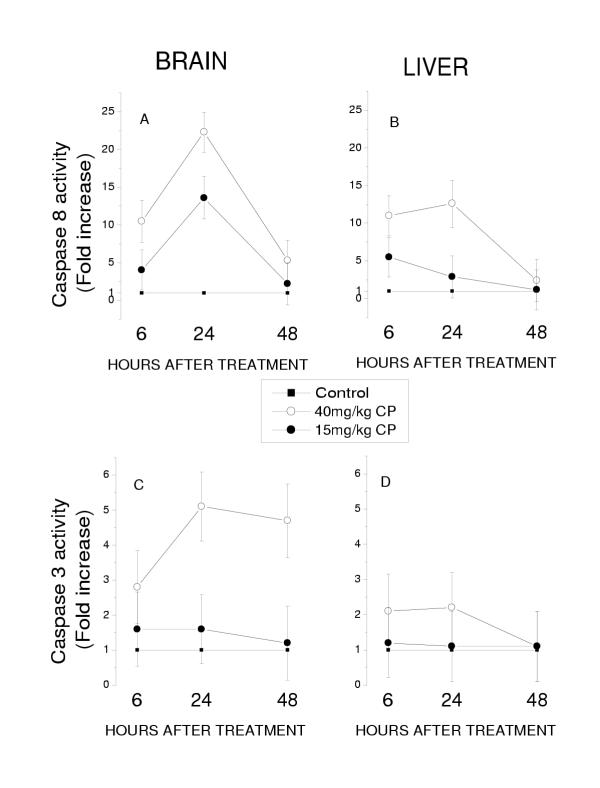
The experiments on temporal pattern of expression of active caspase 3 and caspase 8 in the brain and liver revealed that the extent in which 40 mg/kg CP activated caspase 3 and caspase 8 is different in these organs. A 22-fold increase in caspase 8 activity was observed in the brain 24 hours after exposure to 40 mg/kg CP, whereas in the liver the level of active caspases 8 was significantly lower (Fig. 4A and 4B). We also observed that the extent of CP-induced caspase 3 activation was lesser than that of caspases 8 (Fig. 4C and 4D). Still, caspase 3 activation was more prominent in the brain than in the liver. It is important to emphasize that 48 hours after the teratogenic insult, the level of both active caspases in the brain remained to be significantly higher than that in controls, whereas in the liver it was identical to that in controls (Fig. 4).
Figure 4.
Active caspase 8 and caspase 3 expression in the embryonic brain and liver of control embryos and embryos exposed to CP. Results obtained in experimental groups are presented as 95% limits for the means (fold increase) calculated by GT2-method. Means with limits that do not overlap are significantly different. Means with lower limits < 1 (the level of the activity of the caspases in controls) do not differ significantly from controls.
The low dose of CP increased only the level of active caspase 8 but not caspase 3 in both tested organs (Fig 4). Furthermore, the increased level of active caspase 8 was observed in the brain of embryos tested 24 but not 48 hours after CP injection (Fig. 4A).
NF-κB DNA-binding activity in the brain and liver of CP-treated embryos
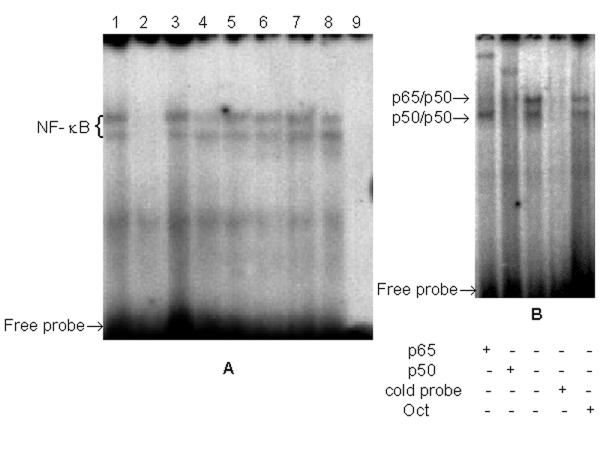
EMSA of nuclear extracts from of control E13 and E14 revealed the existence of active NF-κB complexes in both the brain and liver (Fig. 5A, lanes 1,3,5 and 7). Specificity of NF-κB binding in these and other nuclear extracts presented in Fig. 5A was proven by EMSA of the same nuclear extracts with 100-fold excess of unlabeled NF-κB oligonucleotide or 100-fold excess of OCT oligonucleotide. (Fig. 5B).
Figure 5.
NF-κB complex formation in the embryonic brain and liver.A: Nuclear extracts from the brain (lanes 1–4) and liver (lanes 5–8) of E13 and E 14, control and exposed to 40 mg/kg CP. 1 – E13, control; 2 – E13, CP; 3 – E14, control; 4 – E14, CP; 5 – E13, control; 6 – E13, CP; 7 – E14, control; 8 – E14, CP; 9 – no sample.B: Supershift analysis of -κB-binding proteins presented in nuclear extract from the brain of E13 demonstrating p65/p50 heterodimers (top band) and p50/p50 homodimers (bottom band). Specificity of binding was determined by competition with 100-fold excess of unlabeled NF-κB oligonucleotide or 100-fold excess of OCT oligonucleotide.
Supershift analysis was performed with nuclear extracts from the brain of E13 and E14 as well as from the liver of E13 and E14. Since only p65/p50 heterodimers and p50 homodimers were found to be the active forms of NF-κB in the embryo between days 12 and 14 of pregnancy [18], only anti p65 and anti p50 antibodies were used in the present study. It has been revealed that antibody against p65 inhibited the formation of the top band but not the bottom one, whereas antibody against p50 inhibited formation of both top and bottom bands (Fig. 5B). These results concur with those cited above [18] and suggest that active NF-κB complexes in the embryonic brain and liver primarily composed of p65/p50 heterodimers and p50/p50 homodimers.
Although the pattern of the constitutive expression of active NF-κB complexes in the embryonic liver and brain was identical, NF-κB DNA-binding activity was different in the organs of embryos exposed to the teratogen. Indeed, CP treatment in a dose of 40 mg/kg resulted in the suppression of NF-κB DNA-binding activity in the brain of E13 (Fig. 5A, lane 2) but not in the liver (Fig. 5A, lane 6). Twenty-four hours later, NF-κB DNA-binding activity could be detected in the brain, but it was lower than that found in the brain of control embryos (Fig. 5A, lanes 4 and 3, respectively). At the same time, in the liver of E14, the expression of active NF-κB complexes was similar to controls (Fig. 5A, lanes 8 and 7, respectively).
Thus, the above experiments showed that in the liver, which demonstrates the ability to cease CP-induced apoptosis, the teratogenic insult was not followed by suppression of NF-κB DNA-binding activity. At the same, the suppression of NF-κB DNA-binding activity was observed in the brain, in which the CP-induced excessive apoptosis culminates in structural anomalies.
Effects induced by the combined treatment with CP and NaSAL
To investigate whether the combined treatment with NaSAL and CP modifies sensitivity of the brain to the teratogen, CP was injected in a dose of 15 mg/kg, which induced craniofacial anomalies in less than 5% of fetuses.
We found that a combined treatment significantly increased the sensitivity of embryos to CP. Indeed, in litters of females exposed to CP and NaSAL approximately, 70% of fetuses were severely growth retarded and exhibited brain, craniofacial and limb reduction anomalies such as microcephaly, open eyes, micrognathia, adactyly of the forelimbs and meromelia of hindlimbs, eventration of the abdominal wall (Table 1 and Fig. 1). Fetuses of females exposed to NaSAL alone did not demonstrate external anomalies (Table 1 and Fig. 1).
Combined treatment with this dose of CP and NaSAL was followed by massive apoptosis in the embryonic brain (Fig. 2D), the intensity of which was comparable with that registered in the brain of embryos treated with 40 mg/kg CP (Fig. 2B). No excessive apoptosis was observed in the brain of E14 exposed to NaSAL alone (Fig. 2C) or to 15 mg/kg CP alone (data not shown).
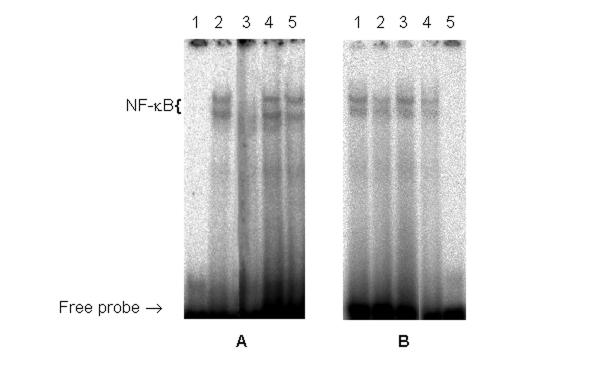
Finally, the level of active NF-κB complexes in nuclear extracts isolated from the brain of embryos exposed to the combined treatment with CP and NaSAL (Fig 6B, lane 2) was lower that that of controls (Fig 6B, lane 3) and similar to that in nuclear extracts from the brain of embryos treated with 40 mg/kg CP (Fig 6B, lane 4). CP alone did not alter the amount of NF-κB binding as compared to controls (Fig. 6B, lanes 1 and 3).
Figure 6.
NF-κB complex formation in the liver and brain of E14 embryos exposed CP in combination with NaSAL.A: Nuclear extracts from the liver: 1 – no sample; 2 – E14, control; 3 – E14, 40 mg/kg CP+NaSAL; 4 – E14, 40 mg/kg CP; 5 – E14, NaSAL.B: Nuclear extracts from the brain: 1 – E14, 15 mg/kg CP; 2 – E14, 15 mg/kg CP + NaSAL; 3 – E14, control; 4 – E14, 40 mg/kg CP; 5 – no sample
While evaluating whether the combined treatment with NaSAL and CP modifies the sensitivity of the liver to the teratogen, we injected CP in a dose of 40 mg/kg. Our previous studies [21] have shown that 14 day embryos of females exposed to this dose of CP were severely growth retarded but the size of their liver did not visually differ from that of age-matching control embryos.
In this study we observed that the size of the liver of E14 exposed to 40 mg/kg CP and NaSAL was obviously smaller than that of embryos exposed to the teratogen alone. The histological examination revealed no histopathological changes in the liver of embryos treated with 40 mg/kg CP but in the liver of embryos exposed to the combined treatment with CP and NaSAL extensive areas of swelling as well as the existence of cells without clear cell borders were observed (data not shown). TUNEL analysis revealed no excessive apoptosis in the liver of E14 exposed to NaSAL alone (Fig 3C). At the same time, some liver areas of embryos exposed to CP and NaSAL demonstrated an increased number of TUNEL-positive nuclei (Fig. 3D).
We also observed that combined treatment resulted in the suppression of NF-κB DNA-binding activity in the liver (Fig. 6A, line 3). It is interesting that NF-κB DNA-binding activity was not altered in the liver of embryos exposed to NaSAL alone (Fig. 6A, lane 5) and the liver of these embryos neither visually nor histologically differed from the liver of controls (data not presented).
Thus, these experiments have showed that the combined treatment with CP and NaSAL increases the sensitivity of both tested organs to the teratogen and this increase is accompanied by suppression of NF-κB DNA-binding activity in these embryonic organs.
Discussion
NF-κB has been detected in practically all murine embryonic tissues, from approximately day 12 of pregnancy onwards [34]. Gene targeting experiments suggest that one of the Rel/NF-κB subunits, RelA, functions as a negative regulator of TNFα-induced physiological apoptosis in hepatocytes of E13 – E15 [35]. These findings gave an impetus to investigate NF-κB DNA-binding activity in embryos responding to the teratogenic insult, which is followed by excessive apoptosis.
The main observations in this study may be summarized as follows.
In the brain, teratogen-induced excessive apoptosis that culminated in fetal anencephaly was accompanied by the suppression of NF-κB DNA-binding activity. However, in the liver, demonstrating the ability to stop apoptosis following this teratogenic insult without signs of dysmorphogenesis, suppression of NF-κB DNA-binding activity was not observed.
In the light of evidence demonstrating that RelA-/- embryos die from massive hepatocyte apoptosis induced by TNFα [14-18], the above observations seem to be expectable and may serve as a basis to suggest that NF-κB might be among mechanisms negatively regulating CP-induced apoptosis in the tested organs and, hence, protecting the embryo from the teratogen.
The results of experiments in mice treated with the teratogen in combination with NaSAL support the concept. Indeed, suppression of NF-kB DNA-binding activity in the liver was associated with a prominent decrease in liver size and histopathological changes in this organ or, in other words, with dysmorphic events suggesting an increase in the sensitivity of the liver to CP-induced teratogenic insult.
Surprisingly, we observed that these dysmorphic events were accompanied by an increased number of TUNEL-positive nuclei only in some liver areas. Since no excessive apoptosis was found at this time point in the liver of embryos exposed to the teratogen alone, this finding does suggest that the ability of the liver to resist CP-induced apoptosis was impaired in embryos exposed to CP and NaSAL. Anyway, the intensity of apoptosis registered in the liver of these embryos was significantly lower than that in RelA-knockout embryos demonstrating massive liver apoptosis [13,16,18].
The reasons for this discrepancy remain unclear. The results of recent studies suggest that NF-κB may be essential for liver regeneration in adult mice [36,37], whereas in the embryonic liver RelA serves a vital protective function against TNFα apoptotic signaling but it is not critical for liver development [18]. These findings suggest that in embryonic liver cells surviving a CP-induced cytotoxic insult, NF-κB begins to act not only as a negative regulator of apoptosis but also as an inducer of cell proliferation, thus possibly explaining differences in the patterns of liver degeneration demonstrated by RelA-knockout embryos and those exposed to the teratogen in combination with NaSAL, and additional studies are planned to test this possibility.
The other explanation may be due to salicylates not only exert inhibitory effects on NF-κB activity but also of alter the expression of a number of other molecules, e.g. those operating in mitogen-activated protein (MAP) kinase signaling pathways [38-40]. Three MAP kinases, the extracellular signal-regulated kinase (ERK), the c-Jun N-terminal kinase (JNK), the stress-activated protein kinase, p38 as well as different components of these three signaling pathways are expressed during mouse embryogenesis [41-43]. Hence, some molecules of MAP kinase signaling pathways may, along with NF-κB, be involved in mediating dysmorphic events induced by the teratogen in combination with NaSAL.
Combined treatment with NaSAL and the low dose of CP that resulted in the suppression of NF-κB DNA-binding activity in the brain was also followed by the occurrence of craniofacial structural anomalies, which lacked in embryos exposed to CP alone. Moreover, these embryos also demonstrated an increase in severity of limb reduction anomalies (adactyly and meromelia vs. digit anomalies). Since NF-κB is ubiquitously expressed in embryonic tissues at this stage of development and the embryonic limbs are, at least, as sensitive as the brain to the teratogen [21], these observations may support the argument for the role of NF-κB in mediating the teratogenic response to CP. In this context, it is interesting to note studies demonstrating that acrolein, one of the teratogenic metabolites of CP [24] induces a dose-dependent inhibition of NF-κB activation in human lung adenocarcinoma (A549) cells [44] and human alveolar macrophages [45].
It is also worth mentioning that NaSAL suppressed NF-κB DNA-binding activity only in the liver and brain of embryos pretreated with CP. This finding raises the question on whether mechanisms regulating the constitutive NF-kB DNA-binding activity differ from those regulating NF-kB DNA-binding activity in cells responding to the cytotoxic insult.
Finally, some results of this study seem to provide some hints into mechanisms through which NF-κB might be involved in regulating the teratogenic response.
Indeed, one of these mechanisms whereby NF-κB serves the antiapoptotic function is presently associated with the interaction between NF-κB and molecules, which act as positive regulators of apoptosis [5]. Caspase 3 and caspase 8 are such molecules and it has been reported that negative feedback mechanisms regulating caspase activation and NF-κB activation exist [5,6,46]. These caspases are involved in transducing and executing both cellular stress- and death receptor-initiated apoptotic stimuli [4,22]. DNA-damaging agents, such as CP, are cellular stress inducers [22] and have also the potential to induce apoptosis via death receptor-mediated signal transduction pathways [47]. Caspase 3 has recently been reported to be involved in CP-induced apoptosis in an organ type-dependent manner [27]. Results of our study confirm the above observation and suggest that caspase 8 may also be involved in mediating CP-induced apoptosis.
The possibility, that "caspase – NF-κB interaction" might be one of the mechanisms predetermining the response of the embryo to CP-induced teratogenic insult is suggested by the comparative analysis of the activation patterns of both these caspases and NF-κB DNA-binding activity in the brain and liver. Thus, one can see, that 6 hours after CP treatment the levels of active caspase 8 as well as caspase 3 were almost equal in the brain and liver. However, 24 hours after CP treatment, the level of both caspases was significantly increased in the brain but it remained the same in the liver. No active NF-κB complexes were observed in the brain at this time point, whereas in the liver the level of the active NF-κB expression did not differ from that in controls.
These findings suggest that "caspase – NF-κB interaction" might occur in the tested organs at the time interval between 6 and 24 hours after CP treatment and result in the suppression of NF-κB DNA-binding activity in the brain but prevention of excessive activation of caspases in the liver. This may explain the resistance of the liver and the sensitivity of the brain to the teratogen and, hence, allows hypothesizing on the involvement of NF-κB in the teratogenic response through "NF-κB – caspases" negative feedback mechanisms acting in an organ type-dependent fashion.
Conclusions
The results of this study demonstrate that teratogenic susceptibility of embryonic tissues to CP correlates inversely with local NF-κB DNA-binding activity prior to target organ dysmorphogenesis. They also suggest that teratogens may suppress NF-κB DNA-binding activity in the embryonic tissues in an organ type- and dose-dependent fashion. Together, the phenomena described in this work may serve as a basis for further studies investigating the role of NF-κB in teratogenesis.
Acknowledgments
Acknowledgments
This work was supported by The Israel Science Foundation (grant #541/00) and The Israel Ministry of Health.
Contributor Information
Arkady Torchinsky, Email: arkadyt@post.tau.ac.il.
Lucy Lishanski, Email: lisha@techunix.technion.ac.il.
Orit Wolstein, Email: nf9542@wicc.weizmann.ac.il.
Jeanne Shepshelovich, Email: jeannes@post.tau.ac.il.
Hasida Orenstein, Email: amosfein@post.tau.ac.il.
Shoshana Savion, Email: shoshans@post.tau.ac.il.
Zeev Zaslavsky, Email: zaslavsk@post.tau.ac.il.
Howard Carp, Email: carp@netvision.net.il.
Alexander Brill, Email: alex_brill@hotmail.com.
Rivka Dikstein, Email: bcrivka@wiccmail.weizmann.ac.il.
Vladimir Toder, Email: toder@post.tau.ac.il.
Amos Fein, Email: amosfein@post.tau.ac.il.
References
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Sadler TW, Hunter ES., III . Principles of abnormal development. Past, present and future. In: Kimmel CA, Buelke-Sam J, editor. Developmental Toxicology. New York, Raven Press; 1994. pp. 53–63. [Google Scholar]
- Knudsen TB. Cell death. In: Kavlock RJ, Daston GP, editor. Drug Toxicity in Embryonic Development I. Berlin, Heidelberg, Springer-Verlag; 1997. pp. 211–244. [Google Scholar]
- Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- M. Barkett, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj/onc/1203238. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB. Apoptosis and nuclear factor-kappaB: a tale of association and dissociation. Biochem Pharmacol. 2000;6:1033–1039. doi: 10.1016/S0006-2952(00)00393-2. [DOI] [PubMed] [Google Scholar]
- Liou HC, Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993;3:477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Baeuerle PA. Multi-step activation of NF-kappa B/Rel transcription factors. Immunobiology. 1995;193:116–127. doi: 10.1016/s0171-2985(11)80534-6. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/ NF-kB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj/onc/1203239. [DOI] [PubMed] [Google Scholar]
- Kami M. Minireview. The beginning of the end: IkB kinase (IKK) and NF-kB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-κB transcription factors: structural views. Oncogene. 1999;18:6845–6852. doi: 10.1038/sj/onc/1203224. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Jonson R, Kami M. The IKKp subunit of ikb kinase (IKK) is essential for Nuclear Factor kb activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Dot TS, Marino MW, Takahashi T, Yoshida T, Sakakura T, Old LJ, Obata Y. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci USA. 1999;96:2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Embryonic lethality, liver degeneration, and impaired NF-kappa B activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am J Pathol. 2000;56:997–1007. doi: 10.1016/S0002-9440(10)64967-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Cyr DG, Hales BF. Role of apoptosis in mediating phosphoramide mustard-induced rat embryo malformations in vitro. Teratology. 1994;50:1–12. doi: 10.1002/tera.1420500102. [DOI] [PubMed] [Google Scholar]
- Torchinsky A, Savion S, Gorivodsky M, Shepsheiovich J, Zaslavsky Z, Fein A, Toder V. Cyclophosphamide-induced teratogenesis in ICR mice: The role of apoptosis. Teratogen Carcinogen Mutagen. 1995;15:179–190. doi: 10.1002/tcm.1770150404. [DOI] [PubMed] [Google Scholar]
- Torchinsky A, Ivnitsky I, Savion S, Shepshelovich J, Gorivodsky M, Fein A, Carp H, Schwartz D, Frankel J, Rotter V, Toder V. Cellular events and the pattern of p53 protein expression following cyclophosphamide-initiated cell death in various organs of developing embryo. Teratog Carcinog Mutagen. 1999;19:353–367. doi: 10.1002/(SICI)1520-6866(1999)19:5<353::AID-TCM5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1067–1074. doi: 10.1038/sj/cdd/4400601. [DOI] [PubMed] [Google Scholar]
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj/cdd/4400598. [DOI] [PubMed] [Google Scholar]
- Mirkes PE. Cyclophosphamide teratogenesis: a review. Teratogen Carcinogen Mutagen. 1985;5:75–88. doi: 10.1002/tcm.1770050202. [DOI] [PubMed] [Google Scholar]
- Chernoff NJ, Rogers M, Alles AJ, Zucker RM, Elstein KH, Massaro EJ, Sulik KK. Cell cycle alterations and cell death in cyclophosphamide teratogenesis. Teratogen Carcinogen Mutagen. 1989;9:199–209. doi: 10.1002/tcm.1770090403. [DOI] [PubMed] [Google Scholar]
- Francis BM, Rogers JM, Sulik KK, Alles AJ, Elstein KH, Zucker RM, Massaro EJ, Rosen MB, Chernoff N. Cyclophosphamide teratogenesis: Evidence for compensatory responses to induced cellular toxicity. Teratology. 1990;42:473–482. doi: 10.1002/tera.1420420504. [DOI] [PubMed] [Google Scholar]
- Mirkes PE, Little SA. Teratogen-induced cell death in postimplantation mouse embryos: differential tissue sensitivity and hallmarks of apoptosis. Cell Death Differ. 1998;5:592–600. doi: 10.1038/sj/cdd/4400390. [DOI] [PubMed] [Google Scholar]
- Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Frantz B, O'Neill EA. The effect of sodium salicylate and aspirin on NF-kappa B. Science. 1995;270:2017–2019. doi: 10.1126/science.270.5244.2017. [DOI] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding-proteins with mini-extracts, prepared from a small number of cells. Nuc Acid Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. New York, Freeman and Company. 3 1995. Biometry, [Google Scholar]
- Torchinsky A, Fein A, Toder V. Immunoteratology: I. MHC involvement in the embryo response to teratogens in mice. Am J Reprod Immunol. 1995;34:288–298. doi: 10.1111/j.1600-0897.1995.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122:2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Gerondakis S, Grossmann M, Nakamura Y, Pohl T, Grumont R. Genetic approaches in mice to understand Rel/NF-kappaB and IkappaB function: transgenics and knockouts. Oncogene. 1999;18:6888–6895. doi: 10.1038/sj/onc/1203236. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Effect of ethanol on tumor necrosis factor signaling during liver regeneration. Clin Biochem. 1999;32:571–578. doi: 10.1016/S0009-9120(99)00057-0. [DOI] [PubMed] [Google Scholar]
- Plumpe J, Maiek NP, Bock CT, Rakemann TMP, Trautwein C. NF-kappaB determines between apoptosis and proliferation in hepatocytes during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2000;278:G173–183. doi: 10.1152/ajpgi.2000.278.1.G173. [DOI] [PubMed] [Google Scholar]
- Schwenger P, Skolnik EY, Vilcek J. Inhibition of tumor necrosis factor-induced p42/p44 mitogen-activated protein kinase activation by sodium salicylate. J Biol Chem. 1996;271:8089–8094. doi: 10.1074/jbc.271.14.8089. [DOI] [PubMed] [Google Scholar]
- Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik EY, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci USA. 1997;94:2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Huang C, Brown RE, Ma WY. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J Biol Chem. 1997;272:9962–9970. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandrini A, Brott BK, Erikson RL. Differential expression of MEK1 and MEK2 during mouse development. Cell Growth Differ. 1997;8:505–511. [PubMed] [Google Scholar]
- Lee JK, Hwang WS, Lee YD, Han PL. Dynamic expression of SEK1 suggests multiple roles of the gene during embryogenesis and in adult brain of mice. Mol Brain Res. 1999;66:133–140. doi: 10.1016/S0169-328X(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Mirkes PE, Wilson KL, Cornel LM. Teratogen-induced activation of ERK, JNK, and p38 MAP kinases in early postimplantation murine embryos. Teratology. 2000;62:14–25. doi: 10.1002/1096-9926(200007)62:1<14::AID-TERA6>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Horton ND, Biswal SS, Corrigan LL, Bratta J, Kehrer JP. Acrolein causes inhibitor KB-independent decreases in nuclear factor kb activation in human lung adenocarcinoma (A546) cells. J Biol Chem. 1999;274:9200–9206. doi: 10.1074/jbc.274.14.9200. [DOI] [PubMed] [Google Scholar]
- Li L, Hamilton RF, Jr, Holian A. Effect of acrolein on human alveolar macrophage NF-κB activity. Am J Physiol. 1999;277:L550–L557. doi: 10.1152/ajplung.1999.277.3.L550. [DOI] [PubMed] [Google Scholar]
- Mayo MW, Baldwin AS. The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–62. doi: 10.1016/S0304-419X(00)00002-0. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Fornace AJ., Jr Death and decoy receptors and p53-mediated apoptosis. Leukemia. 2000;14:1509–1513. doi: 10.1038/sj/leu/2401865. [DOI] [PubMed] [Google Scholar]