Abstract
Background
Tacrolimus may be administered during hospitalization as an IV formulation or oral suspension. However, literature suggesting appropriate ratios for conversion from these formulations to capsules is limited.
Objective
To evaluate conversion ratios after a switch in formulation of tacrolimus for solid-organ transplant recipients.
Methods
This single-centre observational longitudinal study involved hospitalized patients who underwent a switch in formulation of tacrolimus according to 1 of 3 possible scenarios: IV to oral suspension, IV to capsule, or oral suspension to capsule. Data were collected from the earliest accessible electronic file (January 2009) to January 1, 2019. Conversion ratios were calculated for each of the 3 groups using data for blood concentrations and doses before and after the switch. The calculated ratios were then compared with recommended conversion ratios: 1:5 (i.e., 1 mg of IV tacrolimus is converted to 5 mg of oral tacrolimus, expressed as “5”) for either of the switches involving an IV formulation and 1:1 (i.e., same amount, expressed as “1”) for the switch from oral formulation to capsules.
Results
For the group who underwent switching from the IV formulation to oral suspension, the mean calculated conversion ratio was 3.04, which was significantly different from the recommended ratio of 5. For the group who underwent switching from the IV formulation to capsules, the calculated conversion ratio was 5.18, which was not significantly different from the recommended ratio of 5. For the group who underwent switching from oral suspension to capsules, the calculated conversion ratio was 1.17, which was not significantly different from the recommended ratio of 1.
Conclusion
In this small retrospective study of tacrolimus therapy, the calculated conversion ratio was significantly different from the recommended ratio for patients who were switched from IV administration to oral suspension, but not for those switched from IV administration or oral suspension to capsules. Therapeutic drug monitoring therefore appears indispensable, regardless of conversion ratios.
Keywords: conversion ratio, formulation, solid-organ transplant, tacrolimus, therapeutic drug monitoring
RÉSUMÉ
Contexte
Le tacrolimus peut être administré par IV ou sous forme de suspension orale pendant une hospitalisation. Cependant, il existe peu de documents qui proposent des ratios appropriés pour convertir ces formulations en capsules.
Objectif
Évaluer les ratios de conversion après un changement de formulation du tacrolimus pour les bénéficiaires de greffes d’organes solides.
Méthodes
Cette étude observationnelle longitudinale unicentrique impliquait des patients hospitalisés, pour qui la formulation de tacrolimus changeait en fonction de chacun des trois scénarios possibles: passage de l’administration par IV à la suspension orale, passage de l’administration par IV aux capsules ou passage de l’administration par suspension aux capsules. Le recueil des données a été effectué à partir du plus ancien dossier électronique accessible (janvier 2009) jusqu’au 1er janvier 2019. Les ratios de conversion ont été calculés pour chacun des trois groupes à l’aide de données pour les concentrations de sang et des doses avant et après le changement. Les ratios calculés ont ensuite été comparés avec les ratios de conversion recommandés: 1:5 (c.-à-d., 1 mg de tacrolimus administré par IV est converti en 5 mg de tacrolimus par voie orale, conversion exprimée par le nombre « 5 ») pour chacun des changements impliquant une formulation IV et 1:1 (c.-à-d. même quantité, conversion exprimée par le nombre « 1 ») pour le passage de la formulation orale aux capsules.
Résultats
Dans le groupe dont l’administration par IV est passée à une suspension orale, le ratio de conversion moyen calculé était de 3,04, ce qui était significativement différent par rapport au ratio recommandé de 5. Pour le groupe dont l’administration par IV est passée à des capsules, le ratio de conversion moyen calculé était de 5,18, ce qui n’était pas significativement différent par rapport au ratio recommandé de 5. Pour le groupe dont l’administration est passée de la suspension orale aux capsules, le ratio de conversion moyen calculé était de 1,17, ce qui n’était pas significativement différent par rapport au ratio recommandé de 1.
Conclusion
Dans cette petite étude rétrospective de la thérapie à l’aide du tacrolimus, le ratio de conversion calculé était significativement différent du ratio recommandé pour les patients qui passaient d’une administration IV à une suspension orale, mais pas pour ceux qui passaient d’une administration par IV ou d’une suspension orale à des capsules. La surveillance thérapeutique des médicaments semble donc indispensable, quels que soient les ratios de conversion.
Mots-clés: ratio de conversion, formulation, greffe d’organe solide, tacrolimus, surveillance thérapeutique des médicaments
INTRODUCTION
Calcineurin inhibitors represent the cornerstone of immunosuppressive maintenance therapy in solid-organ transplantation.1,2 Indeed, their introduction dramatically changed patients’ outcomes, with an increase in 1-year post-transplant survival rate from 50% with azathioprine to 70%–80% with cyclosporine, the first calcineurin inhibitor on the market.1,3 Despite their apparent benefits, these drugs are associated with multiple complications, most of which are concentration-dependent, such as nephrotoxicity, neurotoxicity, and hyperglycemia; as such, therapeutic drug monitoring is justified, to optimize efficacy and limit toxicity.1,3,4
Tacrolimus is one of the calcineurin inhibitors now in use. Because of its narrow therapeutic range and large inter- and intra-individual pharmacokinetic variability, therapeutic drug monitoring remains an important aspect of tacrolimus therapy. For example, various studies in patients with renal transplant have shown a strong correlation between low concentrations of drug and transplant rejection, and between high concentrations and nephrotoxicity.5 The whole-blood concentration of tacrolimus varies widely among individuals, mainly because of its complex pharmacokinetic properties.2 Indeed, the sources of pharmacokinetic variability can be numerous, whether external (such as drug–drug interactions) or internal (such as hepatic function and hematocrit).2
One source of pharmacokinetic variability is the formulation or route of administration.2 Pharmacokinetic studies have shown that the bioavailability of oral tacrolimus is roughly 20% to 25%.6–8 These results explain why the conversion ratio for IV to oral administration recommended by the International Society for Heart and Lung Transplantation (ISHLT) is 1:5 (i.e., 1 mg of IV tacrolimus converted to 5 mg of oral tacrolimus, expressed in this article as “5”).9 However, this recommended ratio does not seem to have been thoroughly validated through randomized controlled trials. An observational study involving patients who underwent stem-cell grafting reported a ratio of 5, but it remains questionable whether this conversion ratio is adequate for patients who have undergone solid organ transplants.10 In addition, the question remains as to what conversion ratio would be adequate for a switch from oral suspension to capsules. In a phase 1 single-dose study, Undre and Dickinson11 found that the bioavailability of prolonged-release tacrolimus suspension prepared from opened capsules seemed equivalent to that of the intact capsules. However, the conversion ratio recommended by the ISHLT might not be adequate for converting from IV doses to oral suspension doses. Moreover, the question remains as to whether a ratio of 1 is adequate for switching between the 2 oral formulations, even if their bioavailabilities seem equivalent.11
In the early post-transplant period, patients are usually in the intensive care unit and may be unable to take oral medications. Others may present months or years after the transplant with a complication that prevents oral administration of medication. These patients are started on tacrolimus by IV administration until the oral route is feasible. In addition, an oral suspension is often used as a bridge between the IV formulation and capsules or for patients who are unable to swallow capsules. The pharmacists in our transplant centre have long noticed that despite using the recommended conversion ratio of 5 when switching patients from IV to oral administration of tacrolimus, the trough concentration always seems to fluctuate greatly, and it is difficult to determine the proper dose adjustment. Therefore, the primary objective of this study was to calculate the conversion ratio for switches in tacrolimus formulation in solid organ transplant recipients.
METHODS
Study Design and Setting
We conducted a single-centre, observational, descriptive, cross-sectional, longitudinal study in a university-affiliated tertiary hospital. The design and conduct of this study were reviewed and approved by the Ethics Committee of the Institut universitaire de cardiologie et de pneumologie de Québec – Université Laval, which waived the need for written informed consent. Participant selection was performed retrospectively using pharmacy database software, from which we identified all patients who underwent a switch of tacrolimus formulation during the period from January 1, 2009 (earliest accessible electronic file) to January 1, 2019. Patients were included if they met the following inclusion criteria: at least one switch in formulation of tacrolimus during the hospital stay, specifically by 1 of 3 possible scenarios (IV to capsules [immediate release], IV to oral suspension, or oral suspension to capsules [immediate release]); age 18 years or older; and administration of each formulation for a minimum of 3 days. A given patient could be included multiples times if there were multiple switches of formulation during the same hospital stay.
Data Collection
Data were collected from electronic medical records and pharmacy database software.
Primary Outcome Measures
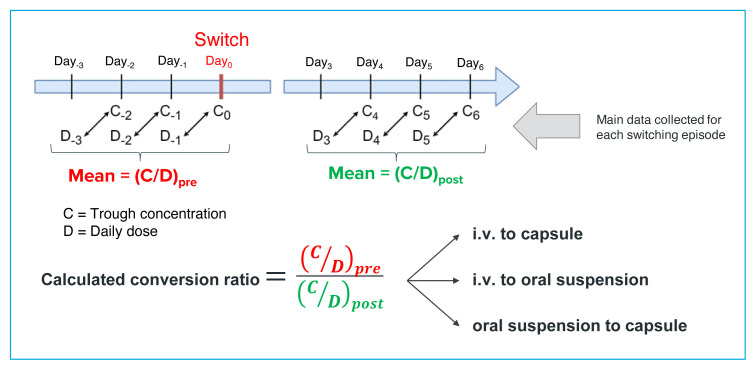
The main data collected were doses of tacrolimus received and trough concentrations of tacrolimus measured in the blood. At the study site, samples for determination of tacrolimus trough concentrations (measured by liquid chromatography coupled with tandem mass spectroscopy) were obtained at 0600, 60 minutes before the first dose of the day (at 0700), reflecting the daily dose received the previous day. We collected the trough concentrations associated with the 3 doses before the switch in formulation and the trough concentrations associated with doses on days 3, 4, and 5 after the switch (Figure 1). For included patients, the dose of tacrolimus, but not the formulation, could have changed in the period from Day−3 to Day−1 or in the period from Day+3 to Day+5; patients with a change in formulation during either of the data collection periods were excluded. The concentration of the drug at steady state referred to here was therefore a formulation-related, rather than a dose-related, steady state concentration. In this study, interindividual variability in metabolism and elimination was attenuated by using the concentration/dose (C/D) ratio (Figure 1), while intraindividual variability was attenuated by considering a 3-day average. Using ratios and averages allows drug monitoring without reliance on absolute doses and target concentrations. We also considered that collecting the concentration data at days 4 to 6 after the switch would yield values more likely to be at equilibrium, which would further reduce any remaining influence of the initial formulation.6
FIGURE 1.
Calculation of the conversion ratio. For reporting purposes, the ratio is presented as a single value, according to the calculation shown.
Secondary Outcome Measures
Other data collected were related to confounding variables: the transplanted organ, hematocrit, alanine aminotransferase (ALT), creatinine, estimated glomerular filtration rate, age, potentially interacting drugs (mainly inhibitors or inducers of cytochrome P450 3A4/5 [CYP3A4/5] isozymes and/or P-glycoprotein), and plasma albumin.2,7,12–17
Concerning potentially interacting medications, we considered only the presence of medications with a course of usage that overlapped with the observation period. The following medications were considered: any corticosteroids, any azole antifungals, nondihydropyridine calcium-channel blockers (verapamil, diltiazem), antibacterials (clarithromycin, erythromycin, rifampin), antiepileptic drugs (phenytoin, carbamazepine, primidone, phenobarbital), and protease inhibitors. For corticosteroids, we collected the doses received a week before the switch, on the day of the switch, and on day 5 after the switch, because it is known that the CYP3A4/5 induction effect of corticosteroids is dose-dependent.18 To standardize any potential impact of corticosteroids, the dose data were all converted to equivalent prednisone doses. For the azoles, the starting date, dose, and ending date were collected, given that the degree of CYP3A4 inhibition seems to be dose-dependent for certain antifungals.16,19 Clinical data, such as hematocrit and ALT, were collected before and after the switch.
The confounding variables were also used for subgroup analyses because of their potential effects on tacrolimus concentrations. To alleviate the effects of drug interactions, hematocrit fluctuations, and elevated ALT, we performed subgroup analyses in which patients were excluded if they had used azole antifungals, if they had a significant change in their corticosteroid dose, or if they had a change in ALT above 3 times the upper limit of normal. Other subgroup analyses were performed for patients with normalized hematocrit and for heart transplant recipients only.
Statistical Analysis
General demographic information was collected. Using the tacrolimus concentrations and doses from patients’ medical records, we calculated C/D ratios, whereby tacrolimus trough concentrations were divided by the total daily dose received the previous day.17 Mean C/D ratios were therefore calculated for the 3 days before the switch (denoted C/Dpre) and for days 4 to 6 after the switch (denoted C/Dpost), as shown in Figure 1. The conversion ratio was defined as C/Dpre divided by C/Dpost. For each of the 3 possible scenarios, a mean conversion ratio was then calculated across all patients. The Student t test was then performed to compare the mean calculated conversion ratio with the recommended ratio. For switching from IV to oral formulations (capsule or suspension), the recommended conversion ratio was 1:5 (i.e., oral dose 5 times higher than IV dose), and for switching from capsules to suspension, the recommended conversion ratio was 1:1. A p value of less than 0.05 was considered to represent a statistically significant difference between the recommended and calculated conversion ratios.
For the subgroup analysis considering hematocrit, all C/D ratios were normalized to a hematocrit (Hct) value of 45% using the following equation: (C/D) × (Hct/0.45).2,20 For the subgroup analysis considering the use of corticosteroids, we defined a relevant change in corticosteroid dose as being a modification of at least 25% from the previously recorded dose.
RESULTS
In total, 41 episodes of formulation switching were identified, distributed among 37 patients: 8 episodes (in 8 patients) of switching from IV to oral suspension, 14 episodes (in 14 patients) of switching from IV to capsules, and 19 episodes (in 15 patients) of switching from oral suspension to capsules. The mean age of the cohort was 52.5 (standard deviation [SD] 11.7) years, with women representing 13 (32%) of all episodes (Table 1). For most episodes (n = 36, 88%), the patients were heart transplant recipients. For most episodes, the patient was receiving enteral feeding on the day of the switch (n = 31, 76%), and none had any history of gastrointestinal disease. For most episodes (n = 26, 63%), the patients had undergone surgery (of any type) less then 30 days before the switch. Regarding the use of potentially interacting drugs, there were 38 episodes (93%) in which corticosteroids were used during the observation period. In contrast, there were only 5 episodes (12%) in which azoles were used during the observation period. No other interacting drugs from the prespecified list were used during the period of data collection. Mean hematocrit was 0.284 (SD 0.042) before the switch and 0.266 (SD 0.031) afterward.
TABLE 1.
Demographic and Clinical Variables of 3 Scenarios for Switching Tacrolimus Formulations
| Variable | Scenario; No. (%) of Episodes or Mean ± SD | ||
|---|---|---|---|
|
| |||
| IV to Oral Suspension (n = 8) | IV to Capsule (n = 14) | Oral Suspension to Capsule (n = 19) | |
| Sex, female | 3 (38) | 5 (36) | 5 (26) |
|
| |||
| Age (years) | 56.4 ± 9.0 | 54.5 ± 12.6 | 50.2 ± 12.0 |
|
| |||
| Weight (kg) | 77 ± 14.1 | 79.2 ± 15.6 | 78.25 ± 20.2 |
|
| |||
| Time since transplanta (months) | 39.4 ± 108.4 | 6.3 ± 8.2 | 22.9 ± 53.6 |
|
| |||
| Enteral feeding | 8 (100) | 7 (50) | 16 (84) |
|
| |||
| Recent surgeryb | 8 (100) | 8 (57) | 10 (53) |
|
| |||
| Type of transplanted organ | |||
| Heart | 7 (88) | 12 (86) | 17 (89) |
| Kidney | 1 (12) | 1 (7) | 1 (5) |
| Lung | 0 (0) | 1 (7) | 1 (5) |
|
| |||
| Interacting drugs | |||
| Azoles | 1 (12) | 0 (0) | 4 (21) |
| Corticosteroid | 8 (100) | 12 (86) | 18 (95) |
| Dose 7 days before switch (mg) | 53.8 ± 25.9 | 161.2 ± 335.7 | 39.7 ± 54.3 |
| Dose on day of switch (mg) | 42.2 ± 18.3 | 29.5 ± 20.5 | 22.1 ± 12.1 |
| Dose 5 days after switch (mg) | 37.7 ± 25.1 | 22.7 ± 15.1 | 20.9 ± 11.1 |
|
| |||
| Clinical lab results before switch | |||
| ALT (IU/L) | 87.3 ± 101.6 | 63.3 ± 85.2 | 44 ± 57.7 |
| eGFR (mL/min/1.73 m2) | 61.8 ± 31.4 | 59.1 ± 33.6 | 57.6 ± 35.1 |
| Hematocrit | 0.311 ± 0.053 | 0.272 ± 0.033 | 0.283 ± 0.044 |
| Albumin (g/L) | 33.8 ± 8.7 | 32.8 ± 4.4 | 30.9 ± 4.4 |
|
| |||
| Clinical lab results after switch | |||
| ALT (IU/L) | 71.8 ± 89.4 | 40.1 ± 24.1 | 62.5 ± 112.3 |
| eGFR (mL/min/1.73 m2) | 49.9 ± 36.5 | 53.4 ± 27.2 | 55.9 ± 35.8 |
| Hematocrit | 0.266 ± 0.014 | 0.273 ± 0.055 | 0.263 ± 0.021 |
| Albumin (g/L) | 28.7 ± 6.4 | 32.7 ± 3.8 | 29.8 ± 5.0 |
ALT = alanine aminotransferase, eGFR = estimated glomerular filtration rate, SD = standard deviation.
Time since transplant at the moment of switch in formulation.
Surgery of any type that occurred within 30 days of the switch in formulation.
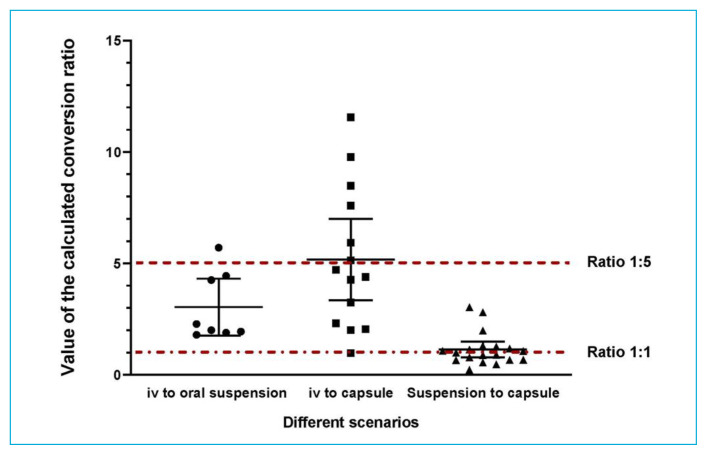
For the patients who underwent switching from IV to oral suspension, we calculated a conversion ratio of 3.04 (SD 1.53), with a 95% confidence interval (CI) of 1.77–4.32 (p = 0.008), which was significantly different from the recommended conversion ratio of 5 (oral dose 5 times higher than IV dose). For those who underwent switching from IV to capsules, the calculated conversion ratio was 5.18 (SD 3.17) (95% CI 3.35–7.00, p = 0.84) which was not significantly different from the recommended ratio of 5. For those who underwent switching from oral suspension to capsules, the calculated conversion ratio was 1.17 (SD 0.74) (95% CI 0.82–1.58, p = 0.32), which was not significantly different from the recommended ratio of 1. Figure 2 and Table 2 illustrate these findings.
FIGURE 2.
Calculated conversion ratios according to the various scenarios. Data points for individual patients are shown, along with the mean (longer horizontal line) and standard deviation (vertical line with shorter horizontal end lines) for each scenario.
TABLE 2.
Calculated Conversion Ratios for Each Switching Scenario and Subsequent Subgroup Analyses
| Scenario and Subgroup Analysis | Calculated Conversion Ratio (Mean ± SD) | p Value |
|---|---|---|
| IV to oral suspension (n = 8 switching episodes) | 3.04 ± 1.53 (95% CI 1.77–4.32) | 0.008 |
| Excluding azoles (n = 7) | 3.22 ± 1.56 | 0.023 |
| Excluding significant CS dose change (n = 3) | 3.30 ± 2.10 | 0.30 |
| Hematocrit normalized (n = 7) | 2.76 ± 1.24 | 0.003 |
| Excluding 3 × ULN ALT (n = 7) | 2.66 ± 1.17 | 0.002 |
| Heart transplant only (n = 7) | 3.20 ± 1.58 | 0.023 |
|
| ||
| IV to capsule (n = 14 switching episodes) | 5.18 ± 3.17 (95% CI 3.35–7.00) | 0.84 |
| Excluding azoles (n = 0) | NA | NA |
| Excluding significant CS dose change (n = 5) | 6.10 ± 3.13 | 0.48 |
| Hematocrit normalized (n = 14) | 4.87 ± 2.65 | 0.85 |
| Excluding 3 × ULN ALT (n = 9) | 4.32 ± 2.89 | 0.50 |
| Heart transplant only (n = 12) | 5.06 ± 3.24 | 0.95 |
|
| ||
| Oral suspension to capsule (n = 19 switching episodes) | 1.17 ± 0.74 (95% CI 0.82–1.58) | 0.32 |
| Excluding azoles (n = 15) | 1.10 ± 0.62 | 0.51 |
| Excluding significant CS dose change (n = 13) | 1.05 ± 0.61 | 0.79 |
| Hematocrit normalized (n = 16) | 1.11 ± 0.79 | 0.60 |
| Excluding 3 × ULN ALT (n = 13) | 1.20 ± 0.85 | 0.42 |
| Heart transplant only (n = 17) | 1.00 ± 0.56 | 0.97 |
ALT = alanine aminotransferase, CI = confidence interval, CS = corticosteroid, NA = not applicable, SD = standard deviation, ULN = upper limit of normal.
We were also interested in evaluating, through subgroup analysis, switching episodes that were not biased by drug–drug interactions. In the group that switched from IV to oral suspension, excluding the single episode involving a patient who was using an azole did not affect the overall result: the calculated conversion ratio remained significantly different from the recommended ratio of 5. However, in the same group, excluding episodes for patients with relevant changes in corticosteroid doses (n = 5 episodes excluded) did affect the result, with the difference between calculated and recommended conversion ratios becoming nonsignificant; this may have been related to the small number of episodes in this subgroup analysis (n = 3). For the group with switching from IV to capsules, only utilization of corticosteroids was present; excluding episodes with relevant changes in corticosteroid doses (n = 9 episodes excluded) yielded a calculated conversion ratio that was nonsignificantly different from the recommended ratio of 5. In the group with switching from oral suspension to capsules, excluding the 4 episodes involving patients who were using an azole yielded a calculated conversion ratio that was nonsignificantly different from the recommended ratio of 1. In the same group, excluding episodes involving patients with relevant changes in corticosteroid dose (n = 6 episodes excluded) also yielded a nonsignificant difference between the calculated and recommended conversion ratios. In general, the results for these subgroup analyses were not substantially different from the results for the main analysis.
We also tried to alleviate the effect of abnormal physiological functions known to affect the pharmacokinetics of tacrolimus (low hematocrit or elevated ALT). To address hematocrit variation, we calculated normalized conversion ratios; the results for all 3 switching scenarios remained consistent with the results in the main analysis. Similarly, when we excluded episodes in which the ALT was elevated (at least 3 times the upper limit of normal), the results remained consistent with the main analysis. No significant changes in serum albumin were observed after the switch in formulation (relative to before the switch), with any of the 3 switching scenarios.
We were also interested in alleviating the potential impact of enteral feeding on the calculated conversion ratios. For the group switched from IV to oral suspension, all patients were receiving enteral feeding and it was therefore impossible to alleviate the impact of this factor. When patients with enteral feeding were eliminated from the group with switching from IV to capsules, the mean calculated conversion ratio was 5.93 (SD 2.96; p = 0.44), while for the group with switching from oral suspension to capsules, the ratio was 0.93 (SD 0.34; p = 0.74), both of which were not significantly different from the respective recommended ratios.
Given that the Institut universitaire de cardiologie et de pneumologie de Québec – Université Laval is a centre that specializes in heart transplants, there was a special interest in assessing results for this specific population. When patients with non-heart transplants were excluded from the analysis, the results for all 3 switching scenarios remained consistent with the original analysis, from a statistical standpoint (details shown in Table 2).
DISCUSSION
This study aimed to evaluate dose-conversion ratios for switches in formulation of tacrolimus in solid organ transplant recipients, in relation to the ISHLT’s recommended ratios of 5 for conversion from IV to oral administration and 1 for conversion from oral suspension to capsules. For the conversion from IV to capsules, the calculated conversion ratio was 5.18 (SD 3.17), which is not significantly different from the recommended ratio of 5. This finding is in agreement with another study, which showed a similar ratio for allograft patients.10 Moreover, in various subgroup analyses for the IV-to-capsule scenario, the calculated conversion ratios remained nonsignificantly different from the recommended ratios. Interestingly, for the conversion from IV to oral suspension, we found a conversion ratio of 3.04 (SD 1.53), which was significantly different from the recommended ratio of 5. In contrast, the conversion from oral suspension to capsules yielded a calculated conversion ratio that was not significantly different from the recommended ratio of 1.
From a pharmacokinetic standpoint, these results suggest that different formulations likely have different bioavailabilities.2,6 Of note, a European phase 1 study involving 20 healthy men aimed to evaluate the relative bioavailability of tacrolimus administered orally or via nasogastric tube using either capsules or an oral suspension. In that study, when the drug was given orally, bioavailability was similar for the capsules and the oral suspension.11 In fact, in the current study, the difference in results for conversion from IV administration to oral formulations (significantly different from recommended ratio for oral suspension, but not significantly different for oral capsules) was surprising. Indeed, given that the oral formulations did not yield any significant difference when compared with each other, these differing results for conversion from IV administration were unexpected. The statistically significant difference observed for the conversion from IV to oral suspension is intriguing yet convincing, given that it was observed with the smallest sample size of the 3 possible scenarios (n = 8 switching episodes).
This observed discrepancy in the ratio for conversion from IV to oral suspension might be due to administration of tacrolimus through the enteral feeding tube. In fact, we had no means to evaluate the adequacy and consistency of this method of administration.21,22 Nevertheless, given that most of the switching episodes from IV to oral suspension occurred in patients with enteral feeding, this result could suggest, from a pharmacokinetic perspective, increased absorption of the drug. Indeed, enteral feeding might have allowed more tacrolimus to reach lower parts of the intestine, where there are fewer CYP3A4 gut enzymes or efflux pumps such as P-glycoprotein, thus enabling greater absorption of the drug.2 Of note, neither the duration or flow of enteral feeding nor the presence of diarrhea were evaluated in this study. Greater enteral feeding flow or presence of diarrhea would likely push the medication further into the digestive tract, thus increasing absorption.2,23 Furthermore, the use of enteral feeding might suggest the possible presence of digestive tract and intestinal malfunction, which would affect tacrolimus absorption in the case of switching from IV to oral suspension.24 Conversely, a switch from oral suspension to capsule would generally suggest clinical improvement, particularly in the gastrointestinal tract, possibly signifying more “normal” gut functions and absorption. This “normalization” might explain the similar pharmacokinetics of oral suspension and capsules and the ratio of 1 that we observed.
As for the subgroup analyses, although the calculated ratios varied a little, the observed tendencies between calculated and recommended ratios remained consistent with those of the main analysis. Concerning potential drug-drug interactions, the results remained consistent in all groups after exclusion of the few episodes involving use of azoles. However, it remains difficult to completely eliminate the possibility of drug interactions, given that only known major CYP3A4 inhibitors or inducers were considered.2,15,17 There is also the possibility that substrate-substrate interactions occurred but were not accounted for.2,15,17 Potential induction by corticosteroid is known to be a dose-dependent effect, but there does not seem to be a cut-off dose highlighted in the literature.2,17,18 Thus, we empirically chose to exclude switching episodes associated with corticosteroid dose changes of 25% or more; however, the calculated conversion ratios cannot be taken at face value because the sample size was considerably reduced in these subgroup analyses. It was also difficult to evaluate whether a change of dose within such a short period of time could really affect the metabolism of tacrolimus. In a study by van Duijnhoven and others,25 a 2-week tapering period followed by a single corticosteroid-free week led to an increase in tacrolimus exposure. In our case, the observation period was a little longer than a week. Nevertheless, the results remained consistent for all 3 scenarios relative to the main analysis.
The results also remained generally consistent in subgroup analyses accounting for physiological markers, such as hematocrit and ALT. Normalizing the hematocrit to 45% enabled us to alleviate the effect of variable red blood cell linkage to tacrolimus before and after the switch in formulation.2,20 A reduction in hematocrit can potentially increase the unbound fraction of tacrolimus, thus increasing its hepatic clearance and lowering its total concentration.2,13 For ALT, we considered 3 times the upper limit of normal as a sign of potential liver dysfunction, but excluding such episodes nonetheless yielded the same results. Because albumin is known to bind tacrolimus, we considered potential changes in serum albumin before and after the switches that might have accounted for variation in tacrolimus concentration in all 3 scenarios. No significant changes were observed.
When the raw data points (Figure 2) are examined, it is important to also notice the range of results (represented by SD). The calculated conversion ratios had large CIs for the IV to capsules scenario, but a much narrower range for the IV to oral suspension scenario, which yielded a statistically significant difference between the calculated and recommended ratios. The data points contributing to the calculated ratio clustered around 2 and 4, with a single higher value (5.71), which pulled the mean to a higher value (3.04). These data suggest that strong interindividual variability does exist, and can hardly be alleviated, despite attempts to reduce both external and internal confounding factors. There could also be one or more unidentified confounding variables not accounted for in the present study. Either way, these results further reinforce the importance of therapeutic drug monitoring, despite any recommended conversion ratio.
Other potential confounding factors that might have been considered for evaluation include sex, age, ethnicity, and genetic polymorphisms. It is still not clear whether sex has any significant effect on dose requirements for tacrolimus. Indeed, several pharmacokinetic studies have shown tacrolimus clearance and dose requirements to be higher in women,17,20,26 whereas others have not.27–29 Moreover, when midazolam was used as a drug probe, intestinal and hepatic CYP3A4 activity displayed only small differences between the 2 sexes.30 In the current study, most of the patients were men (proportions ranging from 63% to 75% of each group). However, given the small sample sizes (including n = 8 in the IV to oral suspension group), any interpretation of the influence of sex would be risky.
Age is another potential modulator of tacrolimus dose requirements. It has well-characterized effects on the disposition of numerous drugs, especially relevant for geriatric (>75 years) and frail patients. For instance, it has been suggested, though not consistently proven, that as adults age, their tacrolimus dose requirement declines steadily.2 Indeed, it was demonstrated more than 30 years ago that elderly patients have reduced total body water and lean body mass, and thus a relative increase in body fat,31 providing a larger volume of distribution for hydrophobic drugs such as tacrolimus. Modulation of gastric pH and intestinal transit, as well as decreased liver volume and hepatic blood flow, have also been observed in elderly people.32 Interestingly, no significant differences in either hepatic or combined hepatic and intestinal CYP3A activity have been demonstrated between young adults and elderly people (although the studies involved mostly healthy volunteers who had not undergone transplant).33–35 Moreover, numerous population-based pharmacokinetic studies (with only limited numbers of elderly patients) showed no significant effect of aging on tacrolimus disposition.36–41 In contrast, a much larger study, analyzing 2205 patients included in the DeKAF study, showed lower dosing requirements in elderly people.42 In the current study, mean age was similar across groups and fell within a narrow range of 50 to 56 years, with no geriatric patients, which prevented any analysis of the effect of age on tacrolimus dosing.
Other potential contributors to variability in tacrolimus dosing requirements are ethnicity and genetic polymorphisms, which are unequally distributed among different ethnic populations worldwide. For instance, African Americans were shown to require higher doses of tacrolimus than whites, which is mainly attributable to 20% to 50% lower bioavailability of the drug.42–46 In today’s era of pharmacogenetics, it is known that observed differences in the disposition of tacrolimus are mostly determined by ethnic variability in common polymorphisms for genes encoding drug-metabolizing enzymes and drug transporters. Of particular interest in the context of tacrolimus disposition is the CYP3A5*1 allele, found in 45% to 73% of African Americans, 5% to 15% of whites, 15% to 35% of Asians, and 25% of Mexicans.2 Carrying a CYP3A5*1 allele was shown to produce an average 30% increase in the oral clearance of tacrolimus, resulting in a 50% higher dose requirement.47 However, it is still unclear why African American noncarriers of the CYP3A5*1 allele had dose requirements similar to those of white noncarriers.2
Other genetic differences that could be involved in ethnicity-related variability in tacrolimus disposition are an increased frequency of inactivating alleles of CYP3A5*6 and CYP3A5*7 in African Americans, along with CYP3A4*1B and ABCB1 (Pgp) 3435CC variants, although the effect of these latter 2 on tacrolimus disposition appears to be of limited importance.48–50 Again, in the present study, the small number of patients involved and the fact that all were white suggest limited impact of ethnicity and associated genetic polymorphisms on tacrolimus disposition. Moreover, as mentioned previously, the use of C/D ratios further attenuated interindividual variability.
This study had some limitations. One of our main concerns was not being able to ascertain the adequacy or consistency of drug administration through enteral feeding tubes. As such, adherence of drug molecules to the tube wall or interactions with food might have affected the absorption of tacrolimus,21,22 especially for the scenario involving the oral suspension formulation. Furthermore, it was not possible to ascertain for all patients whether drugs were indeed administered through the tube, nor did we know the duration of feeding, the flow rate of enteral feeding, or the presence of diarrhea. As such, we cannot exclude the possibility of an effect of enteral feeding on tacrolimus administration, when dealing with the oral suspension, as it was shown to affect absorption of the drug.51 Moreover, as with any retrospective study, complete detailed pathophysiological data were not available in the electronic patient records. In addition, the study sample consisted mostly of heart transplant recipients, so the generalizability of our results to the context of other solid-organ transplants is unknown.
This study was only a stepping stone to understanding the pharmacokinetic implications of switching formulations of tacrolimus. The sample sizes were quite small, and hence it is difficult to draw any firm conclusions. Larger studies are needed to determine consistent and adequate conversion ratios for switching between tacrolimus formulations. As it stands, our findings seem to suggest that switching from IV to oral suspension might require a different conversion ratio than the 1:5 recommended by the ISHLT, while the ratios currently used for the other 2 scenarios are likely adequate. Ultimately, however, considering the wide variability in trough concentrations of tacrolimus (as indicated by wide CIs and large SDs in our data), it is evident that therapeutic drug monitoring remains crucial, no matter which conversion ratio is being used.
CONCLUSION
In this small retrospective, cross-sectional, longitudinal study, a change in formulation of tacrolimus from IV to oral suspension yielded a conversion ratio different from the 1:5 ratio recommended by the ISHLT, whereas conversion ratios calculated for switches from IV to oral capsules and from oral suspension to capsules did not differ from the recommended ratios (5 and 1, respectively). Thorough therapeutic drug monitoring should remain the gold standard, no matter which conversion ratio is used.
Acknowledgements
The authors thank Sylvie Pilote, MSc, for her technical assistance with figure preparation and Serge Simard, MSc, for the statistical analysis.
Footnotes
Competing interests: None declared.
Funding: None received.
References
- 1.Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care. 2015;21(1 Suppl):S12–S23. [PubMed] [Google Scholar]
- 2.Vanhove T, Annaert P, Kuypers DR. Clinical determinants of calcineurin inhibitor disposition: a mechanistic review. Drug Metab Rev. 2016;48(1):88–112. doi: 10.3109/03602532.2016.1151037. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56(1):23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol. 2007;2(2):374–84. doi: 10.2215/CJN.03791106. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadpour N, Elyasi S, Vahdati N, Mohammadpour AH, Shamsara J. A review on therapeutic drug monitoring of immunosuppressant drugs. Iran J Basic Med Sci. 2011;14(6):485–98. [PMC free article] [PubMed] [Google Scholar]
- 6.Wallemacq PE, Verbeeck RK. Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. Clin Pharmacokinet. 2001;40(4):283–95. doi: 10.2165/00003088-200140040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29(6):404–30. doi: 10.2165/00003088-199529060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Spencer CM, Goa KL, Gillis JC. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs. 1997;54(6):925–75. doi: 10.2165/00003495-199754060-00009. [DOI] [PubMed] [Google Scholar]
- 9.Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Shasank D, et al. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29(8):914–56. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Suetsugu K, Ikesue H, Miyamoto T, Shiratsuchi M, Yamamoto-Taguchi N, Tsuchiya Y, et al. Analysis of the variable factors influencing tacrolimus blood concentration during the switch from continuous intravenous infusion to oral administration after allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2017;105(3):361–8. doi: 10.1007/s12185-016-2135-7. [DOI] [PubMed] [Google Scholar]
- 11.Undre N, Dickinson J. Relative bioavailability of single doses of prolonged-release tacrolimus administered as a suspension, orally or via a nasogastric tube, compared with intact capsules: a phase 1 study in healthy participants. BMJ Open. 2017;7(4):e012252. doi: 10.1136/bmjopen-2016-012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coto E, Tavira B, Suárez-Álvarez B, López-Larrea C, Díaz-Corte C, Ortega F, et al. Pharmacogenetics of tacrolimus: ready for clinical translation? Kidney Int Suppl. 2011;1(2):58–62. doi: 10.1038/kisup.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limsrichamrern S, Chanapul C, Mahawithitwong P, Sirivatanauksorn Y, Kositamongkol P, Asavakarn S, et al. Correlation of hematocrit and tacrolimus level in liver transplant recipients. Transplant Proc. 2016;48(4):1176–8. doi: 10.1016/j.transproceed.2015.12.096. [DOI] [PubMed] [Google Scholar]
- 14.Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23(10):1631–4. doi: 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rancic NK, Vavic NN, Kovacevic AM, Mikov MM, Dragojević-Simić VM. Drug-drug interactions of tacrolimus. Hosp Pharmacol. 2015;2(3):291–6. [Google Scholar]
- 16.Gubbins PO, Heldenbrand S. Clinically relevant drug interactions of current antifungal agents. Mycoses. 2010;53(2):95–113. doi: 10.1111/j.1439-0507.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- 17.Stratta P, Quaglia M, Cena T, Antoniotti R, Fenoglio R, Ferrante D, et al. The interactions of age, sex, body mass index, genetics, and steroid weight-based doses on tacrolimus dosing requirement after adult kidney transplantation. Eur J Clin Pharmacol. 2012;68(5):671–80. doi: 10.1007/s00228-011-1150-0. [DOI] [PubMed] [Google Scholar]
- 18.El-Sankary W, Plant NJ, Gibson GG, Moore DJ. Regulation of the CYP3A4 gene by hydrocortisone and xenobiotics: role of the glucocorticoid and pregnane X receptors. Drug Metab Dispos. 2000;28(5):493–6. [PubMed] [Google Scholar]
- 19.La Delfa I, Xia YF, Blaschke TF. Dose-dependent inhibition of cyclosporine metabolism in mice by fluconazole. Can J Physiol Pharmacol. 1990;68(1):89–93. doi: 10.1139/y90-013. [DOI] [PubMed] [Google Scholar]
- 20.Størset E, Holford N, Midtvedt K, Bremer S, Bergan S, Asberg A, et al. Importance of hematocrit for a tacrolimus target concentration strategy. Eur J Clin Pharmacol. 2014;70(1):65–77. doi: 10.1007/s00228-013-1584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnuson BL, Clifford TM, Hoskins LA, Bernard AC. Enteral nutrition and drug administration, interactions, and complications. Nutr Clin Pract. 2005;20(6):618–24. doi: 10.1177/0115426505020006618. [DOI] [PubMed] [Google Scholar]
- 22.Grissinger M. Preventing errors when drugs are given via enteral feeding tubes. P T. 2013;38(10):575–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Bowling TE. Diarrhoea in the enterally fed patient. Frontline Gastroenterol. 2010;1(3):140–3. doi: 10.1136/fg.2009.000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd DA, Powell-Tuck J. Artificial nutrition: principles and practice of enteral feeding. Clin Colon Rectal Surg. 2004;17(2):107–18. doi: 10.1055/s-2004-828657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Duijnhoven EM, Boots JMM, Christiaans MHL, Stolk LML, Undre NA, van Hooff JP. Increase in tacrolimus trough levels after steroid withdrawal. Transpl Int. 2003;16(10):721–5. doi: 10.1007/s00147-003-0615-1. [DOI] [PubMed] [Google Scholar]
- 26.Kuypers DR, Claes K, Evenepoel P, Maes B, Coosemans W, Pirenne J, et al. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet. 2004;43(11):741–62. doi: 10.2165/00003088-200443110-00005. [DOI] [PubMed] [Google Scholar]
- 27.de Jonge H, Vanhove T, de Loor H, Verbeke K, Kuypers DR. Progressive decline in tacrolimus clearance after renal transplantation is partially explained by decreasing CYP3A4 activity and increasing haematocrit. Br J Clin Pharmacol. 2015;80(3):548–59. doi: 10.1111/bcp.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzsimmons WE, Bekersky I, Dressler D, Raye K, Hodosh E, Mekki Q. Demographic considerations in tacrolimus pharmacokinetics. Transplant Proc. 1998;30(4):1359–64. doi: 10.1016/S0041-1345(98)00275-9. [DOI] [PubMed] [Google Scholar]
- 29.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–53. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Ma L, Drusano GL, Bertino JS, Jr, Nafziger AN. Sex differences in CYP3A activity using intravenous and oral midazolam. Clin Pharmacol Ther. 2006;80(5):531–8. doi: 10.1016/j.clpt.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Fülöp T, Jr, Wórum I, Csongor J, Fóris G, Leövey A. Body composition in elderly people. I. Determination of body composition by multiisotope method and the elimination kinetics of these isotopes in healthy elderly subjects. Gerontology. 1985;31(1):6–14. doi: 10.1159/000212676. [DOI] [PubMed] [Google Scholar]
- 32.Woodhouse KW, Wynne HA. Age-related changes in liver size and hepatic blood flow. The influence on drug metabolism in the elderly. Clin Pharmacokinet. 1988;15(5):287–94. doi: 10.2165/00003088-198815050-00002. [DOI] [PubMed] [Google Scholar]
- 33.Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44(1):33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Gorski JC, Vannaprasaht S, Hamman MA, Ambrosius WT, Bruce MA, Haehner-Daniels B, et al. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther. 2003;74(3):275–87. doi: 10.1016/S0009-9236(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 35.Hunt CM, Westerkam WR, Stave GM, Wilson JA. Hepatic cytochrome P-4503A (CYP3A) activity in the elderly. Mech Ageing Dev. 1992;64(1–2):189–99. doi: 10.1016/0047-6374(92)90106-N. [DOI] [PubMed] [Google Scholar]
- 36.Bergmann TK, Hennig S, Barraclough KA, Isbel NM, Staatz CE. Population pharmacokinetics of tacrolimus in adult kidney transplant patients: impact of CYP3A5 genotype on starting dose. Ther Drug Monit. 2014;36(1):62–70. doi: 10.1097/FTD.0b013e31829f1ab8. [DOI] [PubMed] [Google Scholar]
- 37.Op den Buijsch RA, Christiaans MHL, Stolk LML, de Vries JE, Cheung CY, Undre NA, et al. Tacrolimus pharmacokinetics and pharmacogenetics: influence of adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome (CYP) 3A polymorphisms. Fundam Clin Pharmacol. 2007;21(4):427–35. doi: 10.1111/j.1472-8206.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 38.Miura M, Satoh S, Kagaya H, Saito M, Inoue T, Tsuchiya N, et al. No impact of age on dose-adjusted pharmacokinetics of tacrolimus, mycophenolic acid and prednisolone 1 month after renal transplantation. Eur J Clin Pharmacol. 2009;65(10):1047–53. doi: 10.1007/s00228-009-0721-9. [DOI] [PubMed] [Google Scholar]
- 39.Staatz CE, Tett SE. Pharmacokinetic considerations relating to tacrolimus dosing in the elderly. Drugs Aging. 2005;22(7):541–57. doi: 10.2165/00002512-200522070-00001. [DOI] [PubMed] [Google Scholar]
- 40.Wei-lin W, Jing J, Shu-sen Z, Li-hua W, Ting-bo L, Song-feng Y, et al. Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl. 2006;12(5):775–80. doi: 10.1002/lt.20709. [DOI] [PubMed] [Google Scholar]
- 41.Zhu L, Yang J, Zhang Y, Jing Y, Zhang Y, Li G. Effects of CYP3A5 genotypes, ABCB1 C3435T and G2677T/A polymorphism on pharmacokinetics of tacrolimus in Chinese adult liver transplant patients. Xenobiotica. 2015;45(9):840–6. doi: 10.3109/00498254.2015.1021733. [DOI] [PubMed] [Google Scholar]
- 42.Jacobson PA, Schladt D, Oetting WS, Leduc R, Guan W, Matas AJ, et al. Lower calcineurin inhibitor doses in older compared to younger kidney transplant recipients yield similar troughs. Am J Transplant. 2012;12(12):3326–36. doi: 10.1111/j.1600-6143.2012.04232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson PA, Schladt D, Israni A, Oetting WS, Lin YC, Leduc R, et al. Genetic and clinical determinants of early, acute calcineurin inhibitor-related nephrotoxicity: results from a kidney transplant consortium. Transplantation. 2012;93(6):624–31. doi: 10.1097/TP.0b013e3182461288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancinelli LM, Frassetto L, Floren LC, Dressler D, Carrier S, Bekersky I, et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin Pharmacol Ther. 2001;69(1):24–31. doi: 10.1067/mcp.2001.113183. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan M, Pankewycz O, El-Ghoroury M, Shihab F, Wiland A, McCague K, et al. Outcomes in African American kidney transplant patients receiving tacrolimus and mycophenolic acid immunosuppression. Transplantation. 2013;95(4):566–72. doi: 10.1097/TP.0b013e318277438f. [DOI] [PubMed] [Google Scholar]
- 46.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation. 1998;65(4):515–23. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 47.Terrazzino S, Quaglia M, Stratta P, Canonico PL, Genazzani AA. The effect of CYP3A5 6986A>G and ABCB1 3435C>T on tacrolimus dose-adjusted trough levels and acute rejection rates in renal transplant patients: a systematic review and meta-analysis. Pharmacogenet Genomics. 2012;22(8):642–5. doi: 10.1097/FPC.0b013e3283557c74. [DOI] [PubMed] [Google Scholar]
- 48.Roy JN, Lajoie J, Zijenah LS, Barama A, Poirier C, Ward BJ, et al. CYP3A5 genetic polymorphisms in different ethnic populations. Drug Metab Dispos. 2005;33(7):884–7. doi: 10.1124/dmd.105.003822. [DOI] [PubMed] [Google Scholar]
- 49.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49(3):141–75. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 50.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet. 2010;49(4):207–21. doi: 10.2165/11317550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Silva RME, Portela RDP, da Costa IHF, de Oliveira AB, Woods DJ, de Oliveira CLCG, et al. Immunosuppressives and enteral feeding tubes: an integrative review. J Clin Pharm Ther. 2020;45(3):408–18. doi: 10.1111/jcpt.13093. [DOI] [PubMed] [Google Scholar]