Abstract
Use of non-therapeutic broad spectrum antimicrobial agents triclosan (TCS) and benzalkonium chloride (BC) can contribute to bacterial resistance to clinically-relevant antibiotics. Antimicrobial-resistant bacteria within wastewater may reflect the resistance burden within the human microbiome, as antibiotics and pathogens in wastewater can track with clinically relevant parameters during perturbations to the community. In this study, we monitored culturable resistant wastewater bacteria and cross-resistance to clinically-relevant antibiotics to gauge the impact of each antimicrobial and identify factors influencing cross-resistance profiles. Bacteria resistant to TCS and BC were isolated from wastewater influent over 21 months, and cross-resistance, taxonomy, and monthly changes were characterized under both antimicrobial selection regimes. Cross-resistance profiles from each antimicrobial differed within and between taxa. BC-isolated bacteria had significantly higher prevalence of resistance to “last-resort antibiotic” colistin, while isolates resistant to TCS exhibited higher rates of multidrug resistance. Prevalence of culturable TCS-resistant bacteria decreased over time following FDA TCS bans. Cross-resistance patterns varied according to sampling date, including among the most clinically-important antibiotics. Correlations between strain-specific resistance profiles were largely influenced by taxonomy, with some variation associated with sampling date. The results reveal that time, taxonomy, and selection by TCS and BC impact features of cross-resistance patterns among diverse wastewater microorganisms, which could reflect the variety of factors influencing resistance patterns relevant to a community microbiome.
Graphical Abstract
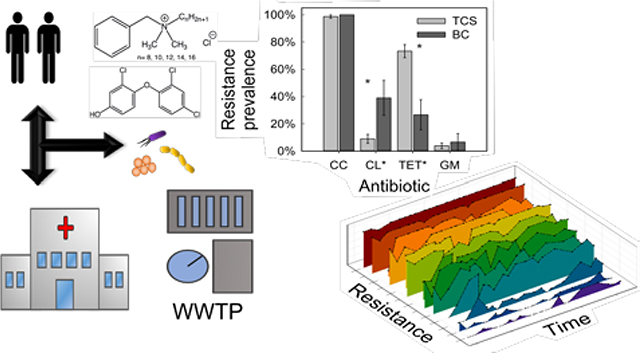
Introduction/Background
Widespread use of broad-spectrum antimicrobial compounds has contributed to the antimicrobial resistance (AMR) crisis1, with antimicrobial resistant bacteria found in hospitals2–4, households5,6, human and animal guts7, wastewater8 and environmental waters9. Two commonly used non-therapeutic antimicrobial compounds, triclosan (TCS) and benzalkonium chloride (BC), are ubiquitous in personal care products, hospital materials including sutures and sheets, plastics, toys, fabrics and other consumer and clinical products10. Widespread use results in measurable concentrations of these antimicrobials in surface waters world-wide11. Partially motivated by the alarming rise of antibiotic resistance potentially linked to long-term uses of these antibacterial products12, the Food and Drug Administration (FDA) issued a ban on consumer hand-washes and soaps containing triclosan in September 2016 with a one-year window to achieve compliance. A second ban on triclosan-based soaps and antiseptics in healthcare settings was instituted in December 201712,13. The ban applies to 24 antiseptics and antimicrobials, but the FDA deferred a ruling on BC as of June 202014. The European Union enacted similar bans on triclosan for use in human hygiene biocidal products15 and the Canadian Government restricts the use of triclosan in consumer products, like cosmetics and mouthwashes16. Although there has been some push to regulate BC in consumer products17, the use of BC has experienced an upsurge in the last 5 years18. Of note, companies including Henkel, producer of Dial soaps and other personal care products, have replaced TCS with BC in consumer soaps in response to the ban19 suggesting a shift in antimicrobial pressure.
Antimicrobial products exert selective pressure, driving enrichment of resistant microorganisms. In consumer products, TCS concentrations have been reported from 7 ppm to >3000 ppm, compared to microbial sensitivity measured as low as 0.01 ppm in some Gram-negative organisms20. TCS loads in wastewater come from direct disposal and human excretion, as TCS was detected in 75% of human urine samples in a 2008 US study21. In the USA, measured influent concentrations of TCS in wastewater treatment plants ranged from 2.70 –26.8 mg/L22–27. While evidence suggests wastewater TCS concentrations in have decreased since the implement of the FDA’s triclosan bans28, it is unclear whether this decrease resulted in a concomitant decrease in resistant microorganisms. Despite decreasing triclosan use, quaternary ammonium compounds (QACs) like BC are still present in hospital antiseptics and disinfectants, consumer cleaning products, fabrics, industrial processes, wood surfaces and more, at concentrations ranging from 60 ppm to over 2,000 ppm29. BC has been reported in concentrations exceeding 0.11 ppm in Austrian wastewater influent30 and up to 21 mg/g in US estuarine sediments31.
Studies have established a relationship between bacterial resistance to TCS and BC and multidrug resistance (MDR)32–41 due to overlapping mechanisms of resistance, despite varied mechanisms of action32. TCS can specifically target lipid synthesis in cells, effectively inhibiting normal cell growth33,34,42, while other cell components can also be targeted depending on concentration43. BC and other QACs disrupt the phospholipid bilayer, resulting in membrane leakage35,36,44. Bacteria achieve reduced susceptibility to these antimicrobials via general mechanisms, such as efflux pumps and strengthened cell walls or outer membranes10,35–40,45–47 that also impart resistance to other antibiotic compounds. Thus, selective pressure on bacterial communities through TCS and BC use can concurrently select for resistance to clinically relevant antibiotics48–50.
Sewage-based surveillance of antimicrobial resistance may hold the key to understanding resistance dynamics in a human population51, and this reservoir of resistance can result in further human exposure. Substantial antimicrobial loads in consumer products and wastewater contribute to an extensive bacterial “resistome,” or resistant microbiome, which exists even in the absence of additional antimicrobials. Fluctuations in prescription practices, with increased use in fall and winter months, likely due to seasonal illness52, are accompanied by similar shifts among clinical organisms53. Changes in antimicrobial use can impact wastewater, as the wastewater resistome may reflect clinical resistance trends on a geographic gradient54. One study demonstrated that Klebsiella pneumonia isolated downstream of hospital effluent shared characteristics with clinical isolates55, showing environmental resistance can reflect population-based resistance. Wastewater contains known and well-characterized clinically-important resistance genes along with novel resistance genes not seen outside of wastewater56–60, and lateral gene transfer occurs both inter- and intra-species in activated sludge61,62. Wastewater functions as both a proxy for resistance in the human population and a reservoir of resistance that can spread directly into human and natural systems63,64.
Given the potential changes in use brought on by recent regulation regarding antimicrobials, we set out to determine the impact of these changes on antimicrobial and antibiotic resistance in wastewater as a human-relevant microbiome. We investigated whether changes in regulation are reflected in the number of TCS resistant bacteria in primary effluent over time. We also compared the differences in selected taxa and resistance profiles selected by TCS or BC to anticipate public health concerns that might arise from replacement of one antimicrobial (i.e. TCS) by another (i.e. BC) in response to regulation. Although many studies of resistance employ species-specific isolation, known gene targets, or particular phenotypes for screening, this study used broad-spectrum antimicrobial agents TCS and BC to select for both intrinsic, taxonomic-related resistance and acquired resistance. Diverse bacterial isolates were recovered using a non-species-specific isolation method to look for overarching patterns both within and between taxonomic groups. Using selection by two antimicrobials, patterns of cross-resistance were assessed specific to TCS and BC resistance to understand the importance of taxonomy, collection date and selection on phenotypic resistance. Both emerging and opportunistic pathogens were captured enabling exploration of important cross-species resistance patterns related to broad-spectrum antimicrobial resistance.
Methods
Site characterization
Two U.S. wastewater treatment plants were selected for this study, the first (WWTP1) serving a large urban center with multiple hospitals and the second (WWTP2) serving a suburban/rural community receiving septic tank waste, direct residential wastewater, and industrial food-processing waste. These plants represent two common types of wastewater plants in the United States and reflect urban and suburban/rural populations. The plants receive approximately 200 million and 25 million gallons day−1 and serve nearly 1.5 million and 200,000 residents, respectively. These WWTPs do not directly receive and treat stormwater, as sewer and stormwater systems are separate. Primary effluent (PE) was collected from July through September 2016 and from January 2017 through April 2018 from WWTP1. One-gallon grab samples of PE were collected from WWTP1 at 23 discrete sampling events over this time period. Grab samples reduce potential changes in the microbial community associated with long incubations within collection bottles associated with composite samples and allow for a shorter overall period between removal from the wastewater and processing. To confirm that the isolated taxa and observed resistance from WWTP1 were typical for wastewater, 100-mL grab samples of PE were collected from WWTP2 during two sampling events, which were paired with sampling at WWTP1. Samples were collected at one foot-depth in a triple rinsed single use or sterilized container, collected at the same time of day (10 am for WWTP1; mid-morning- WWTP2) and stored at room temperature until processing in the lab. Samples were retrieved and processed from the plants on the same day.
Microbial cultivation
Prior to sampling, non-selective and antimicrobial-containing medias were prepared. Mueller-Hinton Agar (Thermo Scientific™ Oxoid™ dehydrated media, Waltham, MA) was prepared according to manufacturer instructions. Powdered Irgasan (triclosan) (Sigma Aldrich 97.0% HPLC, St. Louis, MO) was dissolved in HPLC-grade absolute methanol for a stock concentration of 20 g/L. TCS stock was added to cooled liquid media to achieve a concentration of 50 mg/L TCS, at which point the media became visibly cloudy with triclosan precipitate to guarantee selection of bacteria highly resistant to TCS. BC (Acros Organics, Benzalkonium chloride alkyl distribution C8H17 to C16H33, Morris Plains, NJ) was dissolved in room-temperature Milli-Q water (Millipore) for a stock concentration of 20 g/L. BC stock was added to cooled liquid Mueller-Hinton agar for final concentrations of 250 mg/L and 500 mg/L to achieve concentrations exceeding those in consumer products. Cross-resistance profiles did not vary significantly between the BC concentrations used for isolation, so all BC data were consolidated and the concentration of antimicrobial in the selection media was not investigated as a variable in further analysis. LB agar (Fischer BioReagents™, Miller dehydrated media, Hampton, NH) was prepared according to manufacturer instructions.
PE samples were vigorously shaken and allowed to settle for five minutes before a 1-mL aliquot was drawn from the top 2-cm of the bulk water sample. Ten-fold serial dilutions from 1:101 to 1:107 were performed in sterile phosphate buffered saline, and 0.1 mL of each dilution was plated on LB agar and antimicrobial-containing media. This process was performed in duplicate beginning from bulk sampling. From February 2017 through January 2018, only TCS-containing media was used to select resistant organisms. In January 2017, and from February 2018 through April 2018, both TCS- and BC-containing media was used to select resistant organisms.
Quantification of total anti-microbial resistance over time and antimicrobial resistant strain isolation
Plates were incubated at room temperature for 48 hours prior to colony selection. Plates were rechecked for additional growth after five days. Colonies from plated serial dilutions as described above were counted after 48 hours for all media types in duplicate to evaluate total culturable colony forming units (CFUs) in WWTP1 PE. Colony counts below twenty and above 300 per plate were discarded, as per United States Pharmacopeia biological indicators for resistance performance testing specifications65. Up to 20 colonies per time period, per antimicrobial were picked based on unique morphology and re-streaked for isolation and characterization.
Multidrug non-susceptibility analysis
All isolates were cultured in LB broth, incubated at 25°C for 24 hours, or up to 72 hours until visibly turbid. Cultures were plate-spread for Kirby-Bauer disc diffusion susceptibility testing (BD BBL™ Sensi-Disc™ Susceptibility Test Discs, Franklin Lakes, NJ) on Mueller-Hinton agar for the following antibiotics: amoxicillin/clavulanic acid, ampicillin, azithromycin, cefotaxime, chloramphenicol, ciprofloxacin, clindamycin, colistin, erythromycin, gentamicin, nitrofurantoin, tetracycline, and trimethoprim. After 48 hours of incubation at 25°C, zones of inhibition were measured, and resistance interpretations determined with species-specific criteria after species classification (see below). Four reference strains of E. coli with known resistance profiles were assessed with the disc test according to EUCAST protocols. Sensi-Discs used to identify colistin-resistant Pseudomonas have been reported to have 93.6% sensitivity and 94.0% specificity66. Due to the uncertainty surrounding disc-based colistin resistance testing, isolates were only considered colistin resistant if no zone of inhibition was observed.
Molecular identification of bacteria
All isolates were re-streaked to purity and resuspended in liquid culture. DNA was extracted from 0.75 mL of liquid culture using the Invitrogen™ PureLink™ Genomic DNA Mini Kit (Carlsbad, CA) according to manufacturer instructions for sequencing and quantified with the Qubit High Sensitivity kit (Invitrogen, Carlsbad, CA). Extracted DNA was prepared for sequencing using the Illumina NexteraXT Library Preparation Kit (San Diego, CA). Three-hundred base-pair paired-end shotgun whole-genome sequencing was performed on an Illumina MiSeq instrument at the Johns Hopkins Applied Physics Laboratory. The sequence data was deposited in the GenBank Short Read Archive under BioProject accession number PRJNA664939.
Filtering and trimming of low-quality and primer sequence were done using TrimGalore!67 and “bmtagger”68. Reads passing quality filtering were assembled with MEGAHIT (version 1.1.3), using the default options69,70. 16S rRNA genes were extracted from assemblies using Barrnap71 16S rRNA gene sequences were aligned with Clustal Omega and a distance matrix was created with ClustalW2. For taxonomic classification, Mash Screen (version 2.0)72 was used to match reads to GenBank reference strains. The defaults for k-mer sample size (1000) and length (21 base pairs) were used and matches were output using the “winner-take-all” strategy. The “winner-take-all” strategy in Mash allows sampled k-mers to be matched to a reference only once and selects between multiple possible matches by picking reference that matches the greatest fraction of k-mers in a sample. This functions to remove redundancy from classification results. A minimum threshold of 600 of 1000 shared k-mers between an isolate and a GenBank reference genome was used for reference assignment.
Assemblies with conflicting genus-level classifications were discarded due to suspected contamination likely from incomplete strain purification. Sterile water was used as a negative control during extraction, library processing and sequencing. Negative controls did not yield positive results, suggesting contamination was not likely introduced during the sequencing pipeline. All assemblies were submitted to the Microbial Genome Atlas to confirm classification via average amino-acid identity to RefSeq genomes73. If isolates from the same date were classified as the same GenBank reference strain by >60% of reads, they were considered possibly clonal. The 60% cut-off was chosen to conservatively identify clones and corresponds with the similarity threshold used in taxonomic classification. If resistance profiles between clonal isolates from the same date were identical, only one representative was used in the analysis. All isolates eliminated via this method were removed from all analyses. An overview of the isolation process is presented in Fig. S1.
Statistical analyses
All statistical analysis was performed in Stata 13.0 (StataCorp, College Station, TX) and R (version 3.5.1). The categorical cross-resistance profile for each isolate was converted to numerical data. Isolates with incomplete resistance profiles were removed, and the remaining data were clustered by Manhattan distance and average agglomeration (UPGMA) with the “cluster” package in R74,75. The resulting dendrogram was plotted with average linkage using the packages “ggplot2”76 and “dendextend”77 in R. Comparisons between isolation media and cross-resistance, as well as multidrug nonsusceptibility, were performed in Stata using a t-test with unequal variance. Logistic regression was used for comparative odds-ratios. Pearson correlation with the R packages “corrplot” and “Hmisc” was performed between resistance over the sampling period. Variance partitioning was completed with the R package “vegan”. An overview of the statistical analysis is presented in Fig. S2.
Results and Discussion
Response of culturable and resistant-bacteria to changes in antimicrobial regulation
We measured culturable TCS- and BC-resistant bacteria over time in wastewater from one WWTP to determine if changes in the wastewater resistome could be identified in response to regulatory changes. Quantification of TCS-resistant culturable bacteria in primary effluent (PE) began after the first ban in February 2017, and continued until April 2018, after the second ban (Fig. 1). While the corresponding antimicrobial concentrations were not measured in this study, previous work demonstrated that the concentration of triclosan decreased in wastewater influent from 2012–2017 corresponding to changes to federal regulations28, even though concentrations did not reach zero likely to previously produced products. The incidence of bacterial TCS resistance in wastewater over this time period decreased significantly as compared to total culturable bacteria (Fig. 1). The average number of culturable bacteria on LB agar was 1.4×106 ± 1.3×106 CFUs/mL PE, with a non-significant change over time (n=20, p = 0.337), which could be related to seasonal dynamics that a longer-term study might reveal. For TCS-resistant culturable bacteria, counts ranged from 2.3×105 – 1.8×103 CFUs/mL PE, which accounts for as much as 17% of the culturable community. Simple linear regression revealed a slight decreasing trend over time (n=20, p = 0.050), although the fit was poor (R2=0.198). The change in TCS CFUs over time when normalized by the total CFUs is not significant (Fig. S3). We also observed a slight positive, but non-significant (n=4, p=0.397), trend in total BC resistance during the subset of the dates for which BC isolates were measured (Fig. S4). For BC-isolated culturable bacteria, counts averaged 3.8×103 ± 2.1×103 CFUs/mL PE, which accounts for at most 0.2% of the culturable community.
Figure 1.
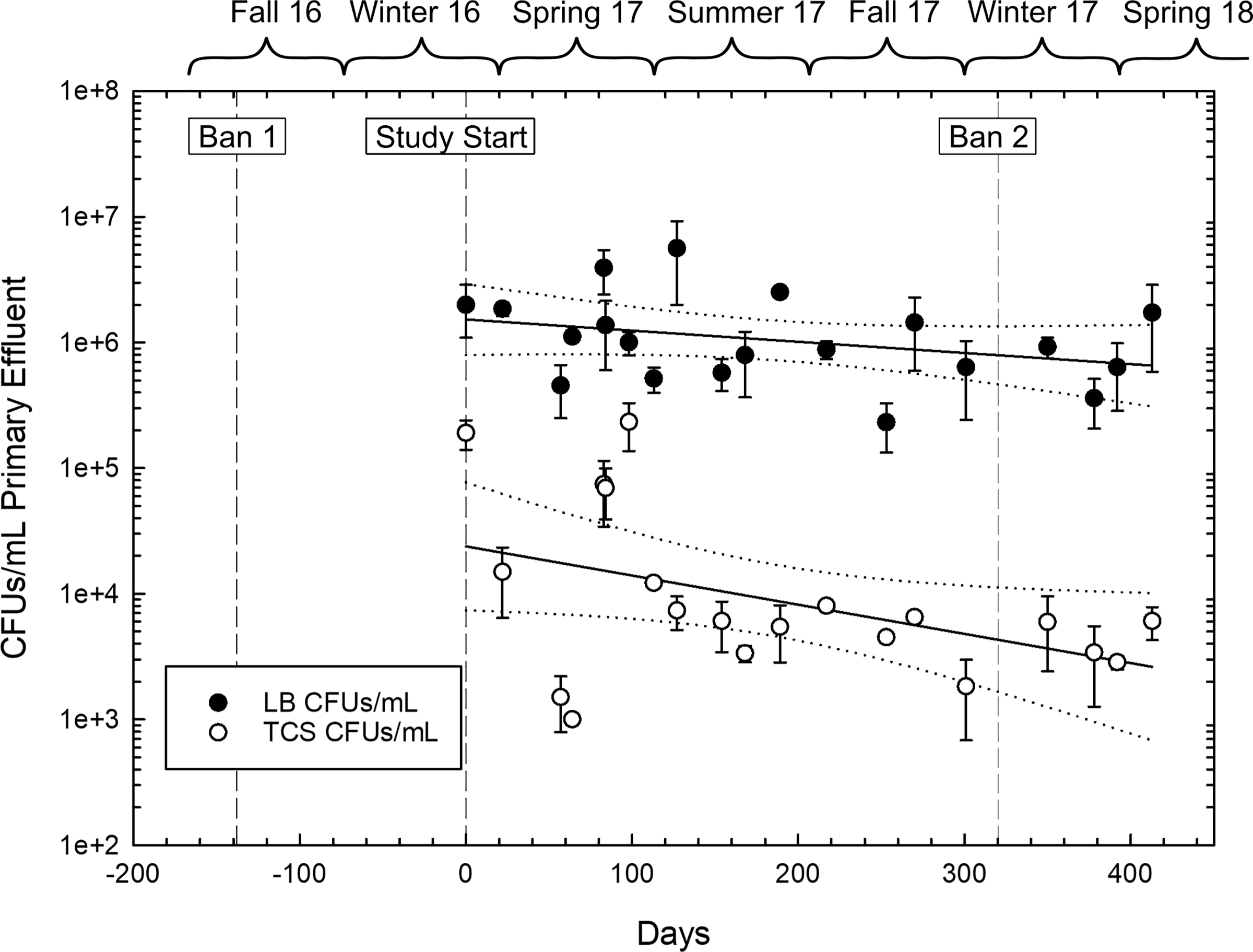
Total culturable bacteria as colony-forming units (CFU) on non-selective media (LB) and triclosan (TCS)-isolated bacterial CFUs from primary effluent from one wastewater treatment plant (WWTP1) over time. Error bars represent ± standard deviation. Black lines represent the linear regression and dotted lines indicate 95% confidence intervals. Days are relative to Feb 16, 2017. TCS: n=20, linear regression, y=−223.75x + 74693.25, R2=0.198, p=0.05. LB: n=20, linear regression y=−2175.8x+1.71×106, R2=0.05, p=0.337.
Although total culturable bacteria resistant to TCS and BC displayed the expected trends, these trends were weak, demonstrating a possibly slow response of associated resistance in wastewater microbes to changes in use driven by regulation. Total use within the sewershed may not have decreased due to the continued use of TCS in hospitals until the second ban in December 2017. Products remaining in inventory purchased pre-ban could have been used post-ban. TCS or BC concentrations in the wastewater might reflect changes in use and were not measured at the time of collection. Total culturable bacteria counts from LB plates were roughly two orders of magnitude greater than those from TCS-containing plates (n=20, p=3.3×10−4) and three orders of magnitude greater than those from BC-containing plates (n=4, p=2.6×10−4), demonstrating the high occurrence of culturable TCS-resistance compared with BC-resistance (n=4, p=0.05). Longer-term studies are needed to reveal whether changes in regulation cause substantial shifts in the wastewater resistome.
Gammaproteobacteria, including potential pathogens like Pseudomonas aeruginosa, are the most common wastewater isolates selected by TCS and BC
Bacterial resistance in wastewater is of public health interest due to the diversity of the bacterial wastewater community, relevance to the human microbiome, and presence of pathogens, both frank and opportunistic. In order to evaluate the potential clinical relevance of TCS- and BC-resistant isolates, sequencing was performed to classify isolates. Over the nineteen-month sampling period from both WWTPs, 242 isolates with full resistance profiles passed quality control. An additional 32 isolates were included which had partial or incomplete resistance profiles because one or more tests did not pass our quality controls. Of all 274 isolates, 18.2% were isolated on BC-media and 81.8% on TCS-media. The majority of isolates were classified as Pseudomonadaceae (53.3%±14.6% of sequenced BC isolates and 80.3%±5.2% of sequenced TCS isolates), followed by Enterobacteriaceae (20.5% and 5.7% respectively), and Yersiniaceae (15.4% and 3.1% respectively). Other taxonomic families comprised 10.3% of BC isolates and 7.8% of TCS isolates (Table S1). The taxonomic diversity of bacteria isolated with BC was typically higher that the diversity of bacteria isolated with TCS (Fig. S5). In total, 10 taxonomic families and 45 species or sub-genus designations were identified, along with 94 isolates (38.1% of sequenced isolates) uncharacterized at the species level. The majority of isolates were classified within Pseudomonas genus (207 total; Table S2), which harbors numerous intrinsic AMR phenotypes due to efflux complexes and restrictive porins78. Of Pseudomonas isolates, 29 (11.7%) were classified as P. aeruginosa, all isolated from TCS, with other classifications including P. putida, P. fluorescens, P. mosselii, P. fulva and undefined Pseudomonas species from both BC and TCS isolation.
P. aeruginosa can express both the TCS target, fabI, as well as the non-target proxy fabV, in addition to the mexAB-oprM efflux system capable of expelling TCS79. This efflux complex, along with a less permeable membrane, imparts P. aeruginosa with reported intrinsic resistance to ampicillin, erythromycin, and clindamycin80,81. Reported TCS MICs in P. aeruginosa strains range from 1 mg/L to 2,125 mg/L due to their intrinsic resistance to TCS in many strains40, 79, 82. Center for Disease Control and Prevention lists multidrug-resistant (MDR) P. aeruginosa as one of the top AMR threats to public health, responsible for the deaths of over 400 people annually in the US83. The use of specific antimicrobials in healthcare may preferentially impact the prevalence of MDR in P. aeruginosa in clinical settings.
TCS resistance in non-aeruginosa Pseudomonas species, such as P. putida and P. fluorescens, has been reported in the literature at lower levels than P. aeruginosa, with MICs ranging from 0.50 mg/L to 40.0 mg/L84, 85. TCS resistance has not been well-characterized in other Pseudomonas species. Previous studies have demonstrated high rates of resistance to penicillin, tetracycline, erythromycin, cefotaxime, nitrofurantoin and trimethoprim in environmental- and wastewater-isolated Pseudomonas spp86,87. To our knowledge, resistance to gentamicin has not been reported in environmental, uncharacterized Pseudomonas spp.87,88, yet ten of the twelve gentamicin resistant isolates in this study were Pseudomonas spp.
Many of the isolates are of clinical relevance, including genera of multidrug-resistant Gram-negative bacilli (MDR-GNB), considered a high-priority clinical dilemma78. These organisms include Aeromonas spp., Serratia spp., Burkholderia spp., and Klebsiella spp. Among the isolates, 54.8% are species of clinical relevance, with infections reported from both environmental and nosocomial sources (Table S3). The use of broad-spectrum antimicrobials may select for MDR-GNB, possibly enabling the spread of previously-unencountered nosocomial infections, particularly among uncharacterized Pseudomonas spp. Given the resistance of Pseudomonas spp. to disinfection in wastewater treatment systems89,90, special focus should be given to ensure complete removal in treated wastewater of these MDR bacteria that can arise from antimicrobial exposure and selection.
Rates of resistance to multiple antibiotics including last-resort antibiotic colistin differ between BC- isolates and TCS-isolated bacteria, while isolation date and taxonomy modify the effect size
Cross resistance patterns among TCS- and BC-resistant isolates were evaluated to identify the potential of TCS and BC to select for resistance to clinically-relevant antimicrobials. Overall, high rates of resistance to clinically-important antibiotics were observed among all antimicrobial-resistant bacterial isolates (Table S4). Isolates were tested for resistance to 13 antibiotics. The minimum cross-resistance was to 2 antibiotics tested (0.7%), the maximum to 13 (0.7%), and median to 9 antibiotics (19.7%). Resistance to clindamycin, a lincosamide antibiotic, and ampicillin, a beta-lactam antibiotic, was observed in nearly all tested isolates (98.9% and 97.4% respectively; Fig. 2). Resistance to ampicillin may be partly due to widespread use of beta-lactams, with 63.2 million prescriptions in the US in 2016, though rates of lincosamide prescriptions are substantially lower91. The mechanisms of action of TCS, BC, ampicillin (transpeptidase inhibitor), and clindamycin (50S subunit inhibitor) all differ92, indicating multiple or overlapping resistance mechanisms like efflux or reduced permeability, whether intrinsic or acquired. Over 50% of isolates from both media types were resistant to erythromycin, trimethoprim, azithromycin, nitrofurantoin, and amoxicillin/clavulanic acid, representing four distinct antibiotic classes. Previous work demonstrates cross-resistances to ampicillin, cefotaxime and sulfamethoxazole are commonly identified in species that develop resistance BC or TCS among Gram-negative bacteria93.
Figure 2.
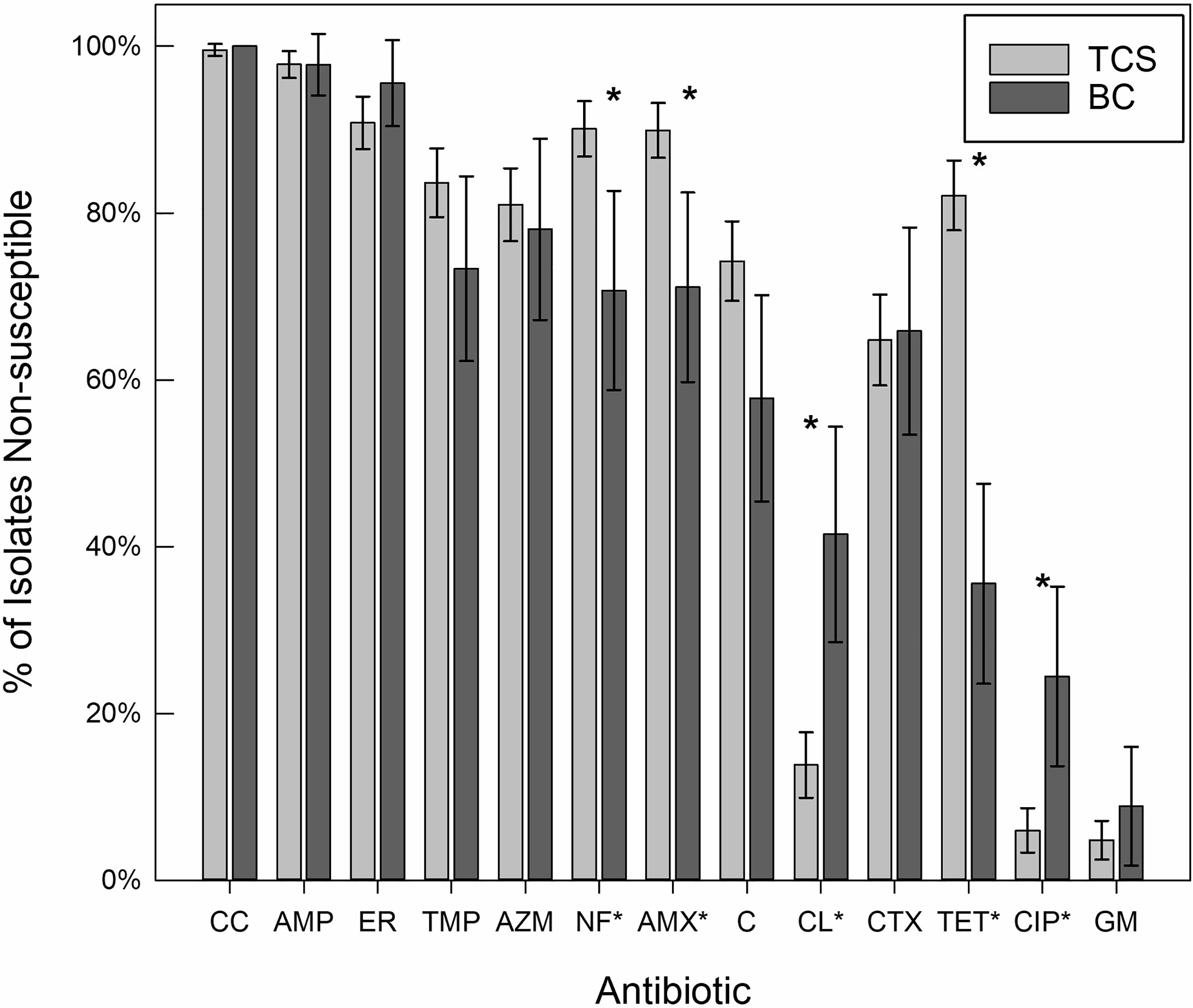
The percent of isolates from triclosan-containing media (TCS) and benzalkonium-chloride-containing media (BC) displaying non-susceptibility to tested antibiotics. Only antibiotics tested on the majority of isolates are shown. Error bars represent a 95% Confidence Interval, and asterisks (*) are assigned to all antibiotics (labels, x-axis) with a statistically significant difference in prevalence of non-susceptibility between the different isolation media. CC: clindamycin (n=274); AMP: ampicillin (n=273); ER: erythromycin (n=274); TMP: trimethoprim (n=265); AZM: azithromycin (n=262); NF: nitrofurantoin (n=263); AMX: amoxicillin/clavulanic acid (n=273); C: chloramphenicol (n=274); CL: colistin (n=251); CTX: cefotaxime (n=251); TET: tetracycline (n=274); CIP: ciprofloxacin (n=262); GM: gentamicin (n=274).
Levels of cross-resistance among both isolate groups were lowest for gentamicin, a rarely used and sometimes toxic antibiotic94. Prevalence of gentamicin resistance was 6.7% ± 6.2% among BC-isolated organisms and 3.9% ± 2.1% among TCS-isolated organisms. A low rate of resistance to ciprofloxacin, a fluoroquinolone antibiotic, was observed among the isolates, at 15.6% ± 9.1% for BC-isolated organisms and 4.1% ± 2.4% for TCS-isolated organisms, despite 29.7 million fluoroquinolone prescriptions in the United States in 201691. Odds ratios (OR) of gentamicin and ciprofloxacin resistance were higher among BC-isolated organisms (ORs: 1.39, 5.08; p=0.574, p<0.001, respectively), adding to the need for studies exploring the impact of BC on MDR. Some existing studies show changes in cross-resistance among species highly resistant to BC41, but other studies show increased rates of ciprofloxacin resistance in Pseudomonas aeruginosa with exposure to biocidal agents95.
Overall, cross-resistance rates for the two selective media types were statistically significantly different for four antibiotics (Table S4): amoxicillin/clavulanic acid (OR 0.28, pvalue=0.001), colistin (OR 4.42, pvalue<0.0001), nitrofurantoin (OR 0.27, pvalue=0.001), and tetracycline (OR 0.11, pvalue<0.0001). BC selected for higher colistin resistance than TCS, with 39.0% ± 12.8% of BC isolates displaying resistance compared to only 9.1% ± 3.3% of TCS isolates. TCS preferentially selected for resistance to nitrofurantoin (88.7%± 3.5% in TCS isolates and 65.9%± 12.4% for BC isolates) and tetracycline (73.4% ± 4.8% of TCS isolates compared to only 26.7% ± 11.1% resistance among BC isolates). Odds-ratios (Table S4) of the likelihood of non-susceptibility to each antibiotic among TCS-isolated organisms as compared to BC-isolated organisms mirror these results, with the addition of significantly increased non-susceptibility to ciprofloxacin among BC-isolated organisms (OR: 5.08, p<0.005).
Both differential selection of taxonomic groups and unequal sample collection contribute to the observed differences in cross-resistance between antimicrobials. To control for taxonomic differences, odd-ratios were calculated for Pseudomonas spp., the largest genus represented. The discrepancy in non-susceptibility between TCS and BC-selection remained significant (p<0.05) for amoxicillin/clavulanic acid, colistin, and tetracycline. Among the P. aeruginosa isolates, rates of non-susceptibility were similar to the average rates among total isolates for all antibiotics except for decreased non-susceptibility to chloramphenicol and erythromycin (OR=0.422, p=0.020 and OR=0.486, p=0.055 respectively). Non-susceptibility was decreased in P. aeruginosa compared to all Pseudomonas spp. for chloramphenicol, erythromycin and trimethoprim (OR=0.222, p<0.001, OR=0.351, p=0.009 and OR=0.188, p=0.003 respectively).
Given the high rate of colistin resistance among total isolates (13.9% among all isolates), we compared observed colistin resistance to reported clinical levels, especially among pathogenic P. aeruginosa. Reported colistin resistance prevalence in clinical multidrug-resistant P. aeruginosa occurred at 2% in an Iranian hospital96, 3% in a cystic fibrosis patient cohort in the UK97, 1.4% in a tertiary care facility in South Korea98, and 0.9% in a major university hospital in Brazil99, compared to 3.4% in this study. As wastewater in this study receives hospital effluent, a similar rate of resistance to those encountered in the clinical setting is expected and demonstrates the potential of wastewater to reflect the healthcare associated community resistome87, 100.
Colistin is used to treat extensively drug-resistant infections, particularly among Gram-negative bacteria including P. aeruginosa and K. pneumoniae101–103. Mechanisms of action of colistin and BC can differ, via beta-lactamase inhibition and disruption of the lipid bilayer, respectively29,34,35. Colistin, a cationic polypeptide, can have a detergent-like effect on cell membranes104. Similarly, BC and other quaternary ammonium ions are cationic surfactants, acting by cell-membrane disruption. When considering sampling dates when isolates were taken from both selection media (winter 2017 and spring 2018), the effect size of discrepancies between the two media decreased (OR= 2.60, p=0.088 from OR=4.42, p=<0.001). Factoring in either a taxonomic or temporal effect, colistin resistance remained higher in BC-isolated bacteria. The high rates of colistin resistance among BC-isolated organisms suggest the potential for a mechanistic overlap. Given the increase in BC-containing products corresponding to the phasing-out of TCS, potential for selection of colistin resistance by BC will require further inquiry, given the gaps in scientific knowledge regarding BC in wastewater and the environment.
Multidrug non-susceptibility is extensive, while clinically-important non-susceptibility displayed a date-dependence
Nearly all isolates in this study were non-susceptible to at least three antibiotics. Thresholds were established for multidrug non-susceptibility: resistance or intermediate resistance to four or more, five or more, and six or more antibiotics. TCS-isolated organisms were somewhat more likely to be non-susceptible to four or more antibiotic classes (OR=0.327, p=0.056). For five or more and six or more antibiotics, TCS-isolated organisms were more likely to express multidrug non-susceptibility (OR= 0.371 and 0.492, respectively), although these results were not significant (p=0.119 and 0.114, respectively). On average, TCS-isolates were non-susceptible to 8.66 antibiotics and BC-isolates to 7.98 antibiotics. Multiple antibiotic resistance index (MARI) was assessed by dividing the number of antibiotics to which as isolate was resistant by the number of antibiotics tested. The average MARI for TCS-isolated organisms exceeded that of BC-isolated organisms (0.611 vs. 0.527, n=242, p=0.013 paired t-test). The mean non-susceptibility counts do not vary significantly (p>0.05) season to season, with a median of non-susceptibility to 9 antibiotics for all seasons except autumn, indicating there was no seasonal increase in overall resistance. Variations in flow conditions or wastewater quality at the time of sampling could contribute to the lack of a significant seasonal trend in resistance.
In order to assess the overall clinical relevance of resistance among the isolates, a scale of clinical importance (CI) was assigned to each isolate based on non-susceptibility to gentamicin, ciprofloxacin and colistin, used to treat MDR Pseudomonas infections. Multivariate logistic regression was performed to determine the influence of taxonomy, isolation date, and selection media on CI resistance thresholds. For non-susceptibility to at least one CI antibiotic, three isolation dates were the most significant predictors, May 2017 (p<0.01), June 2017 (p<0.01), and September 2017 (p<0.05). Interaction between media and time was non-significant. When the threshold was increased to susceptibility to at least two CI antibiotics, no variables were significant. When time was considered linear rather than discrete, the time variable was non-significant. When colistin was considered alone, as discussed above, the selection antimicrobial had a strong influence on the prevalence of resistance. However, when multiple antibiotics of clinical concerns were considered, time became a significant variable. This finding may illustrate the importance of considering all clinically-essential antibiotics when accounting for resistance trends. Variability in this group had date-dependence, which could better reflect changes in community disease or antibiotic usage as a response. In contrast, the above overall MDR rates may be more influenced by taxonomy or intrinsic resistance factors given the high rates of resistance to certain antibiotics among these isolates.
Correlation between observed resistance profiles across isolation date and method reveals multiple contributors to resistance profiles
The correlation between resistance to TCS or BC and clinically-relevant antibiotics may indicate overlapping mechanisms of resistance or co-location of genes (e.g. on plasmids) which warrants concern about the overuse of non-specific antiseptic compounds. When cross-resistance profiles were broken down into prevalence trends at each sampling date for individual antibiotics (Fig. 3a), concurrent cross-resistance trends become visible for some antibiotics, with corresponding dips and peaks in resistance prevalence over time.
Figure 3.
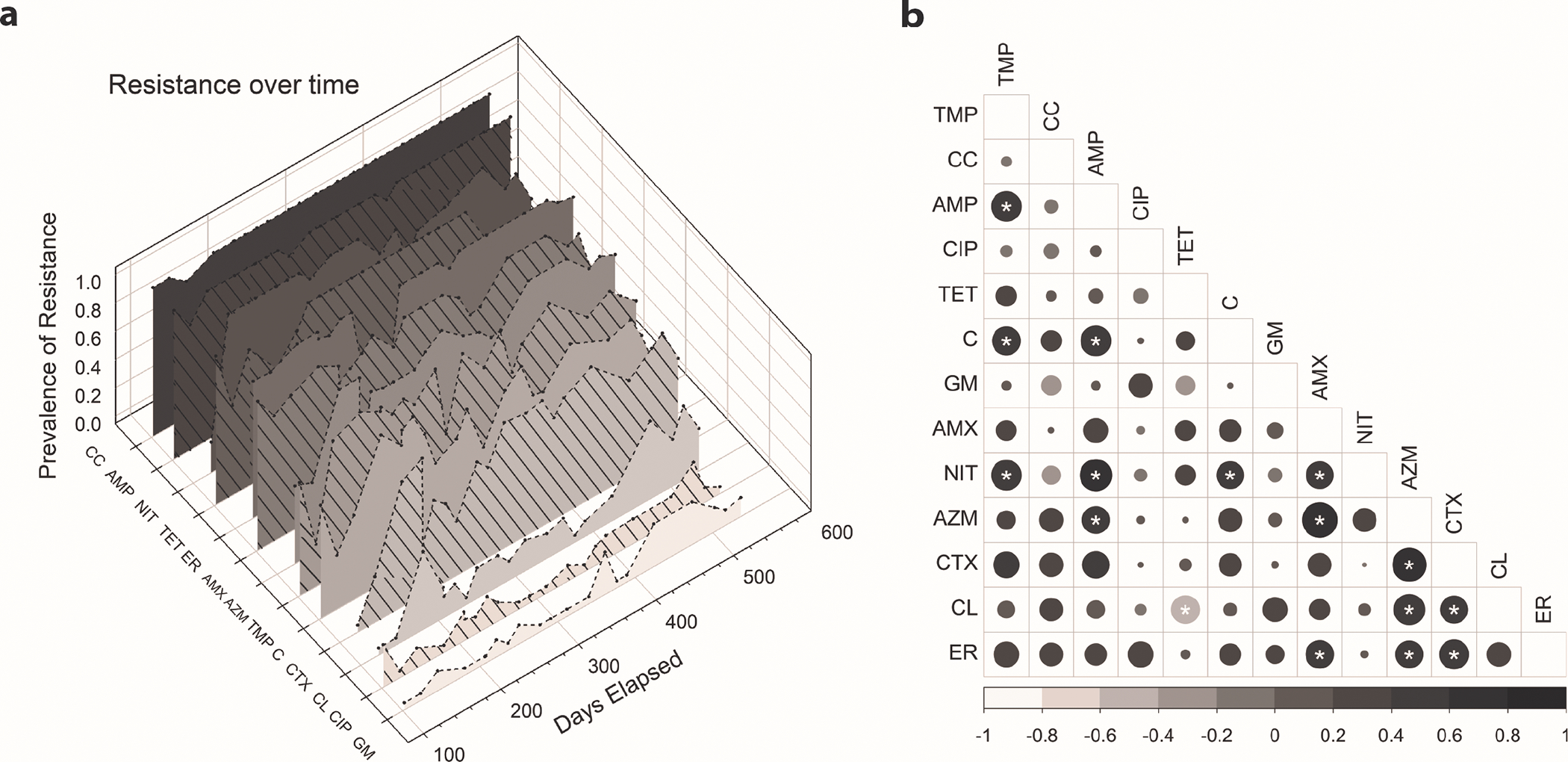
(a). Prevalence of resistance over time among all isolates (n=273) collected on each sample date. An increase in resistance to colistin and gentamicin can be observed after in the introduction of BC testing, introduced at 371 days elapsed. (b). Pearson correlation of resistance prevalence by sample date. Asterisks (*) represent significant relationships (n=20, p<0.05), from strong negative correlation in light grey to strong positive correlation in black, with circle size indicating strength of the relationship. Self-correlations (diagonals) were removed for clarity and exact correlations can be found in Table S5. CC: clindamycin; AMP: ampicillin; ER: erythromycin; TMP: trimethoprim; AZM: azithromycin; NF: nitrofurantoin; AMX: amoxicillin/clavulanic acid; C: chloramphenicol; CL: colistin; CTX: cefotaxime; TET: tetracycline; CIP: ciprofloxacin; GM: gentamicin
There was a significant (p<0.05) positive correlation over time between the prevalence of some of the observed antibiotic resistance phenotypes (Fig. 3b). The most highly correlated phenotypes include resistance to i) nitrofurantoin, trimethoprim and ampicillin, ii) chloramphenicol and trimethoprim, and iii) azithromycin and amoxicillin/clavulanic acid resistance. Only one significant negative correlation was identified between tetracycline and colistin resistance. Restricting the analysis to only TCS-isolates (Fig. S6), significant positive correlations between trimethoprim, chloramphenicol, ampicillin and nitrofurantoin remained, although ampicillin resistance occurs at nearly 100% prevalence among the isolates in this study. Because these represent unique classes of antibiotics, these patterns may correspond with overall changes in MDR in the community. More work is needed to determine the mechanism driving these correlations.
Differences between isolate resistance profiles associated with sampling date and taxonomy
Differences between resistance phenotype patterns of isolates, referred to as a resistance profile, may be related to isolation period, isolation media, or isolate taxonomy. For example, some microorganisms have intrinsic resistance, so taxonomically similar isolates could have several common resistances phenotypes based on their intrinsic resistance. However, the isolation media could select for resistance to phenotypes that share a common mechanism, and the selection pressures experienced over time could change the resistance within the community. To determine the factors contributing the most to differences in observed resistance profiles, 150 unique resistance profiles, as combinations of resistant, susceptible and intermediate, from isolates with complete datasets from both WWTPs combined were compared. Of these, 27 profiles were observed multiple times. On average, repeated profiles were observed in 4.41 isolates.
One profile was observed 27 times, characterized by resistance to ampicillin, amoxicillin/clavulanic acid, azithromycin, chloramphenicol, clindamycin, erythromycin, nitrofurantoin, tetracycline, and trimethoprim, susceptibility to ciprofloxacin, colistin and gentamicin, and intermediate susceptibility to cefotaxime. Nearly all of the isolates in this group were Pseudomonas, including 7 distinct Pseudomonas species and 14 uncharacterized Pseudomonas. While universal intrinsic resistance in Pseudomonas spp. may be difficult to define, intrinsic resistance to ampicillin and clindamycin are well-reported in characterized species (Table S3). To determine whether repeated resistance profiles display temporal patterns, we compared pairwise average weeks apart for repeated and non-repeated resistance profiles. An average of 10.2 weeks separated isolates with repeated resistance patterns, while non-identical resistance patterns were separated by 22.5 weeks, suggesting that identical resistance profiles are more likely to occur closer in time than the overall average (1-way Anova: p<0.001).
To better assess factors influencing differences in observed resistance profiles, isolates were clustered based on phenotypic resistance profile to explore patterns in resistance (Fig. 4; Table S6). Twenty-nine significant clusters, designated by blue boxes, were established with 95% confidence. Only two clusters contained exclusively BC isolates, while 11 clusters contained a mix of BC and TCS isolates. The high proportion of Pseudomonas spp. among the isolates is visible, although clusters 5 through 7 and 25 through 28 contain no Pseudomonas spp. Taxonomic distance and temporal separation were assessed for each significant cluster, using days separation or genetic distance based on isolate 16S rRNA gene sequence distance. Total phylogenetic distance, both within and between of clusters, ranged from 0.03 to 0.59. Temporal distance ranged from 0 weeks to 51.4 weeks apart. Nineteen of the clusters exhibited closer average temporal distance than non-clustered isolates, while eighteen of the clusters exhibited closer average phylogenetic distance than non-clustered isolates.
Figure 4.
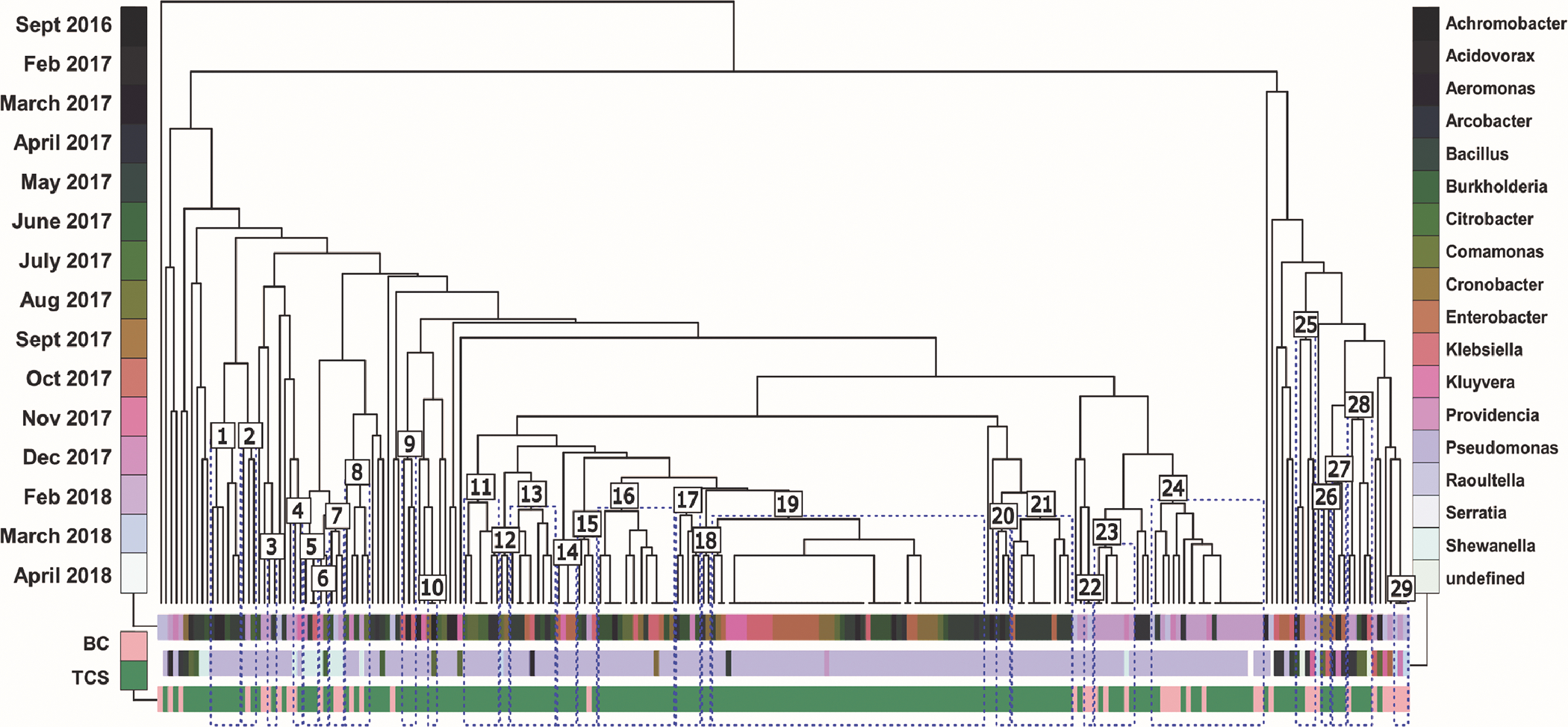
Isolates are clustered by resistance profile using Manhattan distance. Color bars categorize each terminal leaf (unique isolate) by isolation date (row 1), genus (row 2), and isolation media (row 3). Divergent coloring in row 1 indicates increased temporal separation, while similar coloring indicates temporal nearness. Statistically significant clusters (p<0.05) are outlined in blue and labeled numbers 1 through 29.
The largest cluster (19), containing 53 isolates, contained higher-than average rates of resistance to all antibiotics except for the three antibiotics of greatest clinical concern, ciprofloxacin, colistin and gentamicin. This cluster also exhibited closer temporal (13.5 weeks) and phylogenetic distance (0.27) than both un-clustered isolates and all isolates (25.0 weeks, 0.35; and 21.2 weeks, 0.32). Clusters 3 and 4 contained isolates exhibiting complete resistance to two of three clinically-important antibiotics. The relationships depicted within the clusters are indicative of the complexity dictating resistance profiles among these diverse isolates. Some clusters exhibited taxonomic diversity and temporal variability, with clustering associated with atypical resistance profiles, while other clusters maintain low diversity and more temporal similarity.
Due to this variability, variance partitioning was employed to evaluate the variance associated with resistance profile distance for all isolates. Alone, isolation media contributed to none of the variance, while sampling date alone contributed to 11% and taxonomy, as a function of genus and species, to 37% of variance. Overall, 4% of variance was concurrently observed in all three features, and another 7% of variance could be attributed to two of three features (total constrained variance=59%). When limiting analysis to only Pseudomonas spp. isolates, species-level taxonomy accounted for 7% of the variance, while sampling date alone contributed to 19% (total constrained variance=32%). Even among bacteria with high intrinsic resistance like Pseudomonas species, examining overall temporal patterns in wastewater isolates may represent larger trends within a community.
When taxonomy, and therefore potential intrinsic resistance is accounted for, antibiotic and antimicrobial usage patterns may dictate the prevailing resistance profiles within wastewater. Changes like those in antibiotic prescription rates in the United States, with an average of 25% more prescriptions in winter than in summer, with the largest increase of penicillin and macrolide prescriptions52 may impact the wastewater resistome. Seasonal variability in antimicrobial usage can introduce new genes or resistance patterns into the bacterial community that may be amplified due to gene transfer, and development of extreme resistance in non-pathogenic organisms may contribute to resistance in pathogens due to gene exchange. These differences could drive observed seasonal differences in antibiotic resistance and antibiotic gene resistance previously observed in wastewater communities105–107. This indicates that phenotypes, particularly MDR phenotypes associated in part with broad-spectrum antimicrobial resistance, vary temporally across bacterial populations even within overarching resistance patterns.
This work demonstrates that antimicrobial resistance in wastewater may be responsive to changes in regulation, and that cross-resistance to clinically-important antibiotics in bacteria selected by non-therapeutic antimicrobials varies with selective antimicrobial, taxonomy, and date. Among the isolates, numerous bacteria of clinical concern were identified, such as opportunistic pathogens that can cause MDR infections. Future resistance gene analysis may shed light on the predominant mechanisms of resistance among poorly-characterized Pseudomonas spp., but this work suggests that overlapping mechanisms may be responsible for the prevalence of resistance to numerous classes of antibiotics among the isolates. Although this paper does not investigate or compare the resistance patterns in microbial communities in the absence of any selective pressure to those found through selection on antimicrobials, it does highlight differences in the taxa and cross-resistance patterns selected by different antimicrobials in a human microbiome-relevant community. Microorganisms resistant to BC currently make up a small percent of the culturable community in these samples, but widespread use of BC could result in a larger fraction of the community becoming resistant. This work shows a clear relationship between BC-isolation and colistin resistance that demands additional investigation. As BC replaces TCS in many consumer products, effects of BC-use on the dissemination of resistance to clinically-relevant antibiotics must be understood, especially concerning last-resort antibiotic cross-resistance.
Supplementary Material
ACKNOWLEDGMENTS
The work is dedicated to the memory of Dr. Edward Bouwer, a man defined by his devotion to science and to his students, and by his profound kindness. He is deeply missed. This work was supported by the Johns Hopkins E2SHI seed grant, the Johns Hopkins Department of Environmental Health and Engineering seed grant, the NSF Integrative Graduate Education and Research Traineeship (Water, Climate, and Health: Grant 1069213), and the NSF Graduate Research Fellowship Program. The authors would like to thank Shivani Pandey, Meghan Perez, Ryan Peters, Bavisha Kalyan, Louisa Kishton, and Eleanor Baran for their assistance in the laboratory, and Kathleen Veratti at the Johns Hopkins Applied Physics Laboratory.
Footnotes
The authors declare no competing financial interests.
ASSOCIATED CONTENT
Supporting Information.
The Supporting Information is available free of charge at http://pubs.acs.org.
REFERENCES
- 1.Croft AC; D’Antoni AV; Terzulli SL, Update on the antibacterial resistance crisis. Medical science monitor : international medical journal of experimental and clinical research 2007, 13, (6), RA103. [PubMed] [Google Scholar]
- 2.Roy TE; Collins AM; Craig G; Duncan IB, A survey of the incidence of resistance to antibiotics in bacteria isolated in a children’s hospital. Canadian Medical Association journal 1957, 77, (9), 844–850. [PMC free article] [PubMed] [Google Scholar]
- 3.Bret AJ; Coupe C, Epidemiology of the staphylococcal and mycotic infection of the newborn in the maternity ward. Antibiotic resistance of bacteria of maternal origin and bacteria of hospital origin. Revue francaise de gynecologie et d’obstetrique; 1960, 55, 255. [PubMed] [Google Scholar]
- 4.Levinson DC; Griffith GC; Pearson HE, Antibiotics in management of staphylococcal endocarditis; with special reference to increasing bacterial resistance. California medicine 1951, 74, (3), 167. [PMC free article] [PubMed] [Google Scholar]
- 5.Rodman MJ, The status of the antibiotics. RN 1960, 23, (8), 51. [Google Scholar]
- 6.Levy SB, Antibacterial household products: cause for concern. Emerging infectious diseases 2001, 7, (3), 512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells DM; James OB, Transmission of Infectious Drug Resistance from Animals to Man. The Journal of hygiene 1973, 71, (1), 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kralikova K; Krcmery V, Transferable resistance to gentamicin and other antibiotics in Enterobacteriaceae isolates from municipal wastewater. Journal of hygiene, epidemiology, microbiology, and immunology 1984, 28, (2), 161–166. [PubMed] [Google Scholar]
- 9.Morgan RC; Guerry P; Colwell RR, Antibiotic Resistant Bacteria in Chesapeake Bay. Chesapeake Science 1976, 17, (3), 216–219. [Google Scholar]
- 10.Halden RU, On the Need and Speed of Regulating Triclosan and Triclocarban in the United States. Environmental science & technology 2014, 48, (7), 3603–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brausch JM; Rand GM, A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, (11), 1518–1532. [DOI] [PubMed] [Google Scholar]
- 12.FDA FDA issues final rule on safety and effectiveness of consumer hand sanitizers. http://www.fda.gov/news-events/press-announcements/fda-issues-final-rule-safety-and-effectiveness-consumer-hand-sanitizers (October 12, 2019) [Google Scholar]
- 13.FDA Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. https://www.federalregister.gov/documents/2017/12/20/2017-27317/safety-and-effectiveness-of-health-care-antiseptics-topical-antimicrobial-drug-products-for (October. 29, 2020) [PubMed] [Google Scholar]
- 14.Johnson SR FDA gives manufacturers a year to remove triclosan from healthcare antiseptics. https://www.modernhealthcare.com/article/20171220/NEWS/171229988/fda-gives-manufacturers-a-year-to-remove-triclosan-from-healthcare-antiseptics (October. 29, 2020) [Google Scholar]
- 15.Halden RU; Lindeman AE; Aiello AE; Andrews D; Arnold WA; Fair P; Fuoco RE; Geer LA; Johnson PI; Lohmann R; McNeill K; Sacks VP; Schettler T; Weber R; Zoeller RT; Blum A, The Florence Statement on Triclosan and Triclocarban. Environmental Health Perspectives 2017, 125, (6), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canada Cosmetic Ingredient Hotlist: Prohibited and Restricted Ingredients https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients.html-shr-pg0 (October. 29, 2020) [Google Scholar]
- 17.Merchel Piovesan Pereira B; Tagkopoulos I, Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Applied and Environmental Microbiology 2019, 85, (13), e00377–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy V Global benzalkonium chloride market demand witnessing escalation due to recent approvals by FDA for its application. https://www.industryarc.com/PressRelease/1826/Benzalkonium-Chloride-Market-Research.html (October. 29, 2020) [Google Scholar]
- 19.Lovell T Companies replace triclosan with ‘unproven chemicals’. https://chemicalwatch.com/50272/companies-replace-triclosan-with-unproven-chemicals (October. 18, 2019) [Google Scholar]
- 20.Triclosan - Priority Existing Chemical Assessment Report No. 30. In Ageing A. G. D. o. H. a., Ed. National Industrial Chemicals Notification and Assessment Scheme: Sydney, Australia, 2009. [Google Scholar]
- 21.Calafat AM; Ye X; Wong L-Y; Reidy JA; Needham LL, Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environmental Health Perspectives 2008, 116, (3), 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesan AK; Pycke BFG; Barber LB; Lee KE; Halden RU, Occurrence of triclosan, triclocarban, and its lesser chlorinated congeners in Minnesota freshwater sediments collected near wastewater treatment plants. Journal of hazardous materials 2012, 229–230, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAvoy DC; Schatowitz B; Jacob M; Hauk A; Eckhoff WS, Measurement of triclosan in wastewater treatment systems. Environmental Toxicology and Chemistry 2002, 21, (7), 1323–1329. [PubMed] [Google Scholar]
- 24.Waltman EL; Venables BJ; Waller WZ, Triclosan in a North Texas wastewater treatment plant and the influent and effluent of an experimental constructed wetland. Environmental Toxicology and Chemistry 2006, 25, (2), 367–372. [DOI] [PubMed] [Google Scholar]
- 25.Reiss R; Mackay N; Habig C; Griffin J, An ecological risk assessment for triclosan in lotic systems following discharge from wastewater treatment plants in the United States. Environmental Toxicology and Chemistry 2002, 21, (11), 2483–2492. [PubMed] [Google Scholar]
- 26.Heidler J; Halden RU, Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 2007, 66, (2), 362–369. [DOI] [PubMed] [Google Scholar]
- 27.Thompson A; Griffin P; Stuetz R; Cartmell E, The fate and removal of triclosan during wastewater treatment. Water Environ Res 2005, 77, (1), 63–67. [DOI] [PubMed] [Google Scholar]
- 28.Brose DA; Kumar K; Liao AN; Hundal LS; Tian GL; Cox A; Zhang H; Podczerwinski EW, A reduction in triclosan and triclocarban in water resource recovery facilities’ influent, effluent, and biosolids following the US Food and Drug Administration’s 2013 proposed rulemaking on antibacterial products. Water Environ Res 2019, 91, (8), 715–721. [DOI] [PubMed] [Google Scholar]
- 29.Kampf G, Benzalkonium Chloride. In Antiseptic Stewardship: Biocide Resistance and Clinical Implications, Kampf G, Ed. Springer International Publishing: Cham, Switzerland, 2018; pp 259–370. [Google Scholar]
- 30.Clara M; Scharf S; Scheffknecht C; Gans O, Occurrence of selected surfactants in untreated and treated sewage. Water Res 2007, 41, (19), 4339–4348. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C; Cui F; Zeng GM; Jiang M; Yang ZZ; Yu ZG; Zhu MY; Shen LQ, Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci Total Environ 2015, 518, 352–362. [DOI] [PubMed] [Google Scholar]
- 32.Carey DE; McNamara PJ, The impact of triclosan on the spread of antibiotic resistance in the environment. Frontiers in Microbiology 2015, 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMurry LM; Oethinger M; Levy SB, Triclosan targets lipid synthesis. Nature 1998, 394, (6693), 531–532. [DOI] [PubMed] [Google Scholar]
- 34.Heath RJ; Rubin JR; Holland DR; Zhang E; Snow ME; Rock CO, Mechanism of Triclosan Inhibition of Bacterial Fatty Acid Synthesis. Journal of Biological Chemistry 1999, 274, (16), 11110–11114. [DOI] [PubMed] [Google Scholar]
- 35.McDonnell G; Russell AD, Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev 1999, 12, (1), 147-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman JS, Biocide resistance mechanisms. Int Biodeter Biodegr 2003, 51, (2), 133–138. [Google Scholar]
- 37.Russell AD, Mechanisms of bacterial resistance to biocides. Int Biodeter Biodegr 1995, 36, (3–4), 247–265. [Google Scholar]
- 38.Lupo A; Coyne S; Berendonk TU, Origin and evolution of antibiotic resistance: the common mechanisms of emergence and spread in water bodies. Antimicrobials, Resistance and Chemotherapy 2012, 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XZ; Barre N; Poole K, Influence of the MexA-MexB-OprM multidrug efflux system on expression of the MexC-MexD-OprJ and MexE-MexF-OprN multidrug efflux systems in Pseudomonas aeruginosa. J Antimicrob Chemoth 2000, 46, (6), 885–893. [DOI] [PubMed] [Google Scholar]
- 40.Chuanchuen R; Beinlich K; Hoang TT; Becher A; Karkhoff-Schweizer R; Schweizer HP, Cross-Resistance between Triclosan and Antibiotics inPseudomonas aeruginosa Is Mediated by Multidrug Efflux Pumps: Exposure of a Susceptible Mutant Strain to Triclosan Selects nfxB Mutants Overexpressing MexCD-OprJ. Antimicrobial Agents and Chemotherapy 2001, 45, (2), 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kampf G, Adaptive microbial response to low-level benzalkonium chloride exposure. J Hosp Infect 2018, 100, (3), E1–E22. [DOI] [PubMed] [Google Scholar]
- 42.Kampf G, Triclosan. In Antiseptic Stewardship: Biocide Resistance and Clinical Implications, Kampf G, Ed. Springer International Publishing: Cham, Switzerland, 2018; pp 211–258. [Google Scholar]
- 43.Gilbert P; Allison DG; McBain AJ, Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? Journal of Applied Microbiology 2002, 92, (s1), 98S–110S. [PubMed] [Google Scholar]
- 44.Russell AD, Biocides - Mechanisms of Action and Microbial Resistance. World J Microb Biot 1992, 8, 58–59. [DOI] [PubMed] [Google Scholar]
- 45.Li XZ; Livermore DM; Nikaido H, Role of Efflux Pump(S) in Intrinsic Resistance of Pseudomonas-Aeruginosa - Resistance to Tetracycline, Chloramphenicol, and Norfloxacin. Antimicrobial Agents and Chemotherapy 1994, 38, (8), 1732–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole K, Efflux-mediated antimicrobial resistance. J Antimicrob Chemoth 2005, 56, (1), 20–51. [DOI] [PubMed] [Google Scholar]
- 47.Russell AD; Day MJ, Antibiotic and biocide resistance in bacteria. Microbios 1996, 85, (342), 45–65. [PubMed] [Google Scholar]
- 48.Venter H; Henningsen ML; Begg SL, Antimicrobial resistance in healthcare, agriculture and the environment: the biochemistry behind the headlines. Essays Biochem 2017, 61, (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell AD, Bacterial adaptation and resistance to antiseptics, disinfectants and preservatives is not a new phenomenon. J Hosp Infect 2004, 57, (2), 97–104. [DOI] [PubMed] [Google Scholar]
- 50.Schweizer HP, Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiology Letters 2001, 202, (1), 1–7. [DOI] [PubMed] [Google Scholar]
- 51.Aarestrup FM; Woolhouse MEJ, Using sewage for surveillance of antimicrobial resistance. Science 2020, 367, (6478), 630. [DOI] [PubMed] [Google Scholar]
- 52.Suda KJ; Hicks LA; Roberts RM; Hunkler RJ; Taylor TH, Trends and Seasonal Variation in Outpatient Antibiotic Prescription Rates in the United States, 2006 to 2010. Antimicrobial Agents and Chemotherapy 2014, 58, (5), 2763–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez EP; Cepeda M; Jovanoska M; Bramer WM; Schoufour J; Glisic M; Verbon A; Franco OH, Seasonality of antimicrobial resistance rates in respiratory bacteria: A systematic review and meta-analysis. Plos One 2019, 14, (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parnanen KMM; Narciso-da-Rocha C; Kneis D; Berendonk TU; Cacace D; Do TT; Elpers C; Fatta-Kassinos D; Henriques I; Jaeger T; Karkman A; Martinez JL; Michael SG; Michael-Kordatou I; O’Sullivan K; Rodriguez-Mozaz S; Schwartz T; Sheng HJ; Sorum H; Stedtfeld RD; Tiedje JM; Della Giustina SV; Walsh F; Vaz-Moreira I; Virta M; Manaia CM, Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci Adv 2019, 5, (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lepuschitz S; Schill S; Stoeger A; Pekard-Amenitsch S; Huhulescu S; Inreiter N; Hartl R; Kerschner H; Sorschag S; Springer B; Brisse S; Allerberger F; Mach RL; Ruppitsch W, Whole genome sequencing reveals resemblance between ESBL-producing and carbapenem resistant Klebsiella pneumoniae isolates from Austrian rivers and clinical isolates from hospitals. Sci Total Environ 2019, 662, 227–235. [DOI] [PubMed] [Google Scholar]
- 56.Munck C; Albertsen M; Telke A; Ellabaan M; Nielsen PH; Sommer MOA, Limited dissemination of the wastewater treatment plant core resistome. Nat Commun 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo Y; Yang F; Mathieu J; Mao D; Wang Q; Alvarez PJJ, Proliferation of Multidrug-Resistant New Delhi Metallo-β-lactamase Genes in Municipal Wastewater Treatment Plants in Northern China. Environmental Science & Technology Letters 2014, 1, (1), 26–30. [Google Scholar]
- 58.Mao D; Yu S; Rysz M; Luo Y; Yang F; Li F; Hou J; Mu Q; Alvarez PJJ, Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res 2015, 85, 458–466. [DOI] [PubMed] [Google Scholar]
- 59.Berglund B, Environmental dissemination of antibiotic resistance genes and correlation to anthropogenic contamination with antibiotics. Infection Ecology & Epidemiology 2015, 5, (1), 28564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizzo L; Manaia C; Merlin C; Schwartz T; Dagot C; Ploy MC; Michael I; Fatta-Kassinos D, Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci Total Environ 2013, 447, 345–360. [DOI] [PubMed] [Google Scholar]
- 61.Geisenberger O; Ammendola A; Christensen BB; Molin S; Schleifer KH; Eberl L, Monitoring the conjugal transfer of plasmid RP4 in activated sludge and in situ identification of the transconjugants. Fems Microbiology Letters 1999, 174, (1), 9–17. [DOI] [PubMed] [Google Scholar]
- 62.Merlin C; Bonot S; Courtois S; Block JC, Persistence and dissemination of the multiple-antibiotic-resistance plasmid pB10 in the microbial communities of wastewater sludge microcosms. Water Res 2011, 45, (9), 2897–2905. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y; Li B; Zou SC; Fang HHP; Zhang T, Fate of antibiotic resistance genes in sewage treatment plant revealed by metagenomic approach. Water Res 2014, 62, 97–106. [DOI] [PubMed] [Google Scholar]
- 64.Szczepanowski R; Linke B; Krahn I; Gartemann KH; Gutzkow T; Eichler W; Puhler A; Schluter A, Detection of 140 clinically relevant antibiotic-resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiol-Sgm 2009, 155, 2306–2319. [DOI] [PubMed] [Google Scholar]
- 65.Tirumalai RS, Biological Indicators-Resistance Performance Tests. In United States Pharmacopoeia: USP29, United States Pharmacopeial Convention: p 2501. [Google Scholar]
- 66.Javed M; Ueltzhoeffer V; Heinrich M; Siegrist HJ; Wildermuth R; Lorenz FR; Neher RA; Willmann M, Colistin susceptibility test evaluation of multiple-resistance-level Pseudomonas aeruginosa isolates generated in a morbidostat device. J Antimicrob Chemoth 2018, 73, (12), 3368–3374. [DOI] [PubMed] [Google Scholar]
- 67.Krueger F, Trim Galore!: A wrapper tool around Cutadapt and FastQC to consistently apply quality and adapter trimming to FastQ files. 2015.
- 68.Rotmistrovsky K; Agarwala R, BMTagger: Best Match Tagger for removing human reads from metagenomics datasets. 2011.
- 69.Li DH; Luo RB; Liu CM; Leung CM; Ting HF; Sadakane K; Yamashita H; Lam TW, MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [DOI] [PubMed] [Google Scholar]
- 70.Li DH; Liu CM; Luo RB; Sadakane K; Lam TW, MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, (10), 1674–1676. [DOI] [PubMed] [Google Scholar]
- 71.Seemann T barrnap 0.9 : rapid ribosomal RNA prediction. https://github.com/tseemann/barrnap (January. 01, 2019) [Google Scholar]
- 72.Ondov BD; Starrett GJ; Sappington A; Kostic A; Koren S; Buck CB; Phillippy AM, Mash Screen: high-throughput sequence containment estimation for genome discovery. Genome Biol 2019, 20, (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-R LM; Gunturu S; Harvey WT; Rossello-Mora R; Tiedje JM; Cole JR; Konstantinidis KT, The Microbial Genomes Atlas (MiGA) webserver: taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res 2018, 46, (W1), W282–W288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Team RC, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- 75.Maechler M; Rousseeuw P; Struyf A; Hubert M; Hornik K, cluster: Cluster Analysis Basics and Extensions. 2019.
- 76.Wickham H, ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag; New York: 2016. [Google Scholar]
- 77.Galili T, dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, (22), 3718–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryan KJ; Ray CG, Sherris medical microbiology, 6e. In Sixth edition. ed.; McGraw-Hill Education Medical: New York, NY, 2014. [Google Scholar]
- 79.Huang YH; Lin JS; Ma JC; Wang HH, Functional Characterization of Triclosan-Resistant Enoyl-acyl-carrier Protein Reductase (FabV) in Pseudomonas aeruginosa. Frontiers in Microbiology 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pang Z; Raudonis R; Glick BR; Lin T-J; Cheng Z, Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnology Advances 2019, 37, (1), 177–192. [DOI] [PubMed] [Google Scholar]
- 81.Reygaert WC, An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 2018, 4, (3), 482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D’Arezzo S; Lanini S; Puro V; Ippolito G; Visca P, High-level tolerance to triclosan may play a role in Pseudomonas aeruginosa antibiotic resistance in immunocompromised hosts: evidence from outbreak investigation. BMC Res Notes 2012, 5, 43–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biggest Threats and Data: 2019 AR Threats Report. In Prevention C. f. D. C. a., Ed. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP): 2019. [Google Scholar]
- 84.Aiello AE; Marshall B; Levy SB; Della-Latta P; Larson E, Relationship between triclosan and susceptibilities of bacteria isolated from hands in the community. Antimicrobial Agents and Chemotherapy 2004, 48, (8), 2973–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meade MJ; Waddell RL; Callahan TM, Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp denitrificans inactivate triclosan in liquid and solid substrates. Fems Microbiology Letters 2001, 204, (1), 45–48. [DOI] [PubMed] [Google Scholar]
- 86.Luczkiewicz A; Kotlarska E; Artichowicz W; Tarasewicz K; Fudala-Ksiazek S, Antimicrobial resistance of Pseudomonas spp. isolated from wastewater and wastewater-impacted marine coastal zone. Environ. Sci. Pollut. Res 2015, 22, (24), 19823–19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Igbinosa IH; Nwodo UU; Sosa A; Tom M; Okoh AI, Commensal Pseudomonas Species Isolated from Wastewater and Freshwater Milieus in the Eastern Cape Province, South Africa, as Reservoir of Antibiotic Resistant Determinants. Int J Env Res Pub He 2012, 9, (7), 2537–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Welsch TT; Gillock ET, Triclosan-resistant bacteria isolated from feedlot and residential soils. J Environ Sci Heal A 2011, 46, (4), 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Becerra-Castro C; Macedo G; Silva AMT; Manaia CM; Nunes OC, Proteobacteria become predominant during regrowth after water disinfection. Sci Total Environ 2016, 573, 313–323. [DOI] [PubMed] [Google Scholar]
- 90.Alexander J; Knopp G; Dötsch A; Wieland A; Schwartz T, Ozone treatment of conditioned wastewater selects antibiotic resistance genes, opportunistic bacteria, and induce strong population shifts. (1879–1026 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 91.Durkin MJ; Jafarzadeh SR; Hsueh K; Sallah YH; Munshi KD; Henderson RR; Fraser VJ, Outpatient Antibiotic Prescription Trends in the United States: A National Cohort Study. Infection control and hospital epidemiology 2018, 39, (5), 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kapoor G; Saigal S; Elongavan A, Action and resistance mechanisms of antibiotics: A guide for clinicians. J Anaesthesiol Clin Pharmacol 2017, 33, (3), 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kampf G, Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics-Basel 2018, 7, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kushner B; Allen PD; Crane BT, Frequency and Demographics of Gentamicin Use. Otol Neurotol 2016, 37, (2), 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mc Cay PH; Ocampo-Sosa AA; Fleming GT, Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology 2010, 156, (Pt 1), 30–8. [DOI] [PubMed] [Google Scholar]
- 96.Goli HR; Nahaei MR; Rezaee MA; Hasani A; Kafil HS; Aghazadeh M, Emergence of colistin resistant Pseudomonas aeruginosa at Tabriz hospitals, Iran. Iran J Microbiol 2016, 8, (1), 62–69. [PMC free article] [PubMed] [Google Scholar]
- 97.Pitt TL; Sparrow M; Warner M; Stefanidou M, Survey of resistance of Pseudomonas aeruginosa from UK patients with cystic fibrosis to six commonly prescribed antimicrobial agents. Thorax 2003, 58, (9), 794–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee JY; Song JH; Ko KS, Identification of Nonclonal Pseudomonas aeruginosa Isolates with Reduced Colistin Susceptibility in Korea. Microb Drug Resist 2011, 17, (2), 299–304. [DOI] [PubMed] [Google Scholar]
- 99.Rossi F; Girardello R; Cury AP; Di Gioia TSR; de Almeida JN; Duarte AJD, Emergence of colistin resistance in the largest university hospital complex of Sao Paulo, Brazil, over five years. Braz J Infect Dis 2017, 21, (1), 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Slekovec C; Plantin J; Cholley P; Thouverez M; Talon D; Bertrand X; Hocquet D, Tracking Down Antibiotic-Resistant Pseudomonas aeruginosa Isolates in a Wastewater Network. Plos One 2012, 7, (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Granata G; Petrosillo N, Resistance to colistin in Klebsiella pneumoniae: a 4.0 strain? Infect Dis Rep 2017, 9, (2), 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Apostolakos I; Piccirillo A, A review on the current situation and challenges of colistin resistance in poultry production. Avian Pathol 2018, 47, (6), 546–558. [DOI] [PubMed] [Google Scholar]
- 103.Dosselmann B; Willmann M; Steglich M; Bunk B; Nubel U; Peter S; Neher RA, Rapid and Consistent Evolution of Colistin Resistance in Extensively Drug-Resistant Pseudomonas aeruginosa during Morbidostat Culture. Antimicrobial Agents and Chemotherapy 2017, 61, (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Information N. C.f. B. Polymixin E (colistin) PubChem CID 5311054. https://pubchem.ncbi.nlm.nih.gov/compound/5311054 (March 24, 2019) [Google Scholar]
- 105.Schages L; Wichern F; Kalscheuer R; Bockmuhl D, Winter is coming - Impact of temperature on the variation of beta-lactamase and mcr genes in a wastewater treatment plant. Sci Total Environ 2020, 712, 11. [DOI] [PubMed] [Google Scholar]
- 106.Harnisz M; Kiedrzynska E; Kiedrzynski M; Korzeniewska E; Czatzkowska M; Koniuszewska I; Jozwik A; Szklarek S; Niestepski S; Zalewski M, The impact of WWTP size and sampling season on the prevalence of antibiotic resistance genes in wastewater and the river system. Sci Total Environ 2020, 741, 14. [DOI] [PubMed] [Google Scholar]
- 107.Hubeny J; Buta M; Zielinski W; Harnisz M; Korzeniewska E; Nowrotek M; Plaza G, The Prevalence of tet(A) and tet(M) Tetracycline Resistance Genes in Municipal Wastewater. J. Ecol. Eng 2019, 20, (10), 1–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


