Abstract
Background
Reduced-dose pneumococcal conjugate vaccine (PCV) schedules are under consideration in countries where children are recommended to receive 3 doses. Whereas PCV-derived protection against vaccine-serotype colonization is responsible for herd effects of vaccination, dose-specific PCV effectiveness against colonization endpoints is not known. We aimed to assess the performance of differing PCV schedules against vaccine-serotype colonization in children.
Methods
From 2009–2016, we monitored pneumococcal carriage in southern Israel, where children should receive PCV at ages 2 months, 4 months, and 12 months (2 primary [p] +1 booster [b] schedule). We analyzed nasopharyngeal swabs and vaccination histories from 5928 children aged 0–59 months without symptoms of diseases potentially attributable to pneumococci. Matching individuals on age, sex, ethnicity, visit timing, and recent antibiotic receipt, we measured schedule-specific 7-valent PCV (PCV7) and 13-valent PCV (PCV13) effectiveness against vaccine-serotype colonization in a modified case-control framework. We sampled from the distribution of all possible case-control match assignments for statistical analyses.
Results
Receiving 2 primary-series PCV13 doses conferred 53% (95% confidence interval [CI], 32–67%) protection against PCV13-serotype colonization at ages ≤12 months; 1 primary-series dose was not protective. A 2p+1b PCV13 series conferred 40% (95% CI, 4–67%) and 62% (95% CI, 33–83%) protection against PCV13-serotype colonization at ages 13–24 months and 25–59 months, respectively. Estimates suggested greater PCV13-conferred protection against PCV7-targeted serotypes than the 6 PCV13-only serotypes. As compared to children receiving 2p+1b PCV13 dosing, those receiving 1p+1b and 2p+0b schedules experienced 2.05-fold (95% CI, 1.12–5.00) and 3.33-fold (95% CI, 2.28–4.93) greater odds, respectively, of vaccine-serotype pneumococcal colonization at ages 13–24 months.
Conclusions
Our results demonstrate real-world effectiveness of 2p+1b PCV dosing against vaccine-serotype colonization. Reduced-dose schedules may confer lower protection against vaccine-serotype carriage during and beyond the first year of life.
Keywords: Streptococcus pneumoniae, pneumococcal conjugate vaccine, PCV, vaccine effectiveness, vaccine schedule
A single, primary pneumococcal conjugate vaccine (PCV) dose is not strongly protective against colonization at ages ≤12 months. Relative to 3 doses, 2-dose PCV schedules showed reduced effectiveness against vaccine-serotype colonization during and beyond the second year of life.
Commensal carriage of Streptococcus pneumoniae is the source of transmission and a precursor to pneumococcal diseases [1]. Pneumococcal conjugate vaccines (PCVs) targeting 7–13 of the 96+ pneumococcal serotypes have been introduced in 147 countries worldwide [2], reducing the incidence of invasive pneumococcal disease (IPD) caused by vaccine-serotype pneumococci among vaccinated children, as well as unvaccinated children and adults experiencing indirect protection [3]. However, costs of PCVs strain the budgets of national health-care programs [4]. Recently, this expense has drawn the continuation of 10-valent PCV (PCV10) and 13-valent PCV (PCV13) programs in certain lower middle-income countries into question as they phase out full support for PCVs from charities [5].
Most countries implement 2- or 3-dose primary PCV series in the first year of life, with or without a booster dose at age 12 months (3 primary [p] +1 booster [b], 2p+1b, and 3p+0b schedules). A recent trial demonstrated noninferior immunogenicity of a 1p+1b PCV13 series at age 13 months, relative to the 2p+1b series currently implemented [6]. Whether reduced-dose schedules will sustain the population-wide impacts achieved to date by PCV programs depends on their effectiveness against vaccine-serotype colonization [7]. To date, there have been no assessments of the protection against vaccine-serotype colonization conferred by reduced-dose schedules, nor have postlicenvsure studies measured the real-world effectiveness of the standard 2p+1b PCV schedule against vaccine-serotype colonization [3, 8, 9].
Because many children do not receive PCVs according to recommended schedules, observational studies comparing children with differing vaccination histories present an opportunity to assess alternative dosing schemes [10]. Long-term population-based surveillance in southern Israel has afforded a view of pneumococcal colonization among children in this setting amid 7-valent PCV (PCV7) and PCV13 implementation [11]. We used data from an ongoing study to estimate the dose-specific effects of PCV against vaccine-serotype colonization.
METHODS
Setting
Prospective surveillance of pneumococcal carriage is undertaken at Soroka University Medical Center (SUMC) in Be’er Sheva, Israel. As the only medical center in Southern Israel (the Negev District), SUMC is where >90% of births and >90% of pediatric emergency room visits and hospitalizations of children <5 years old occur. The surrounding region is inhabited by socioeconomically distinct Jewish and Bedouin (Muslim) populations. Despite their proximity, Jewish and Bedouin children have limited contact. The Bedouin population is transitioning from a semi-nomadic lifestyle to permanent settlements; in comparison to Jewish communities, Bedouin communities are distinguished by lower incomes, higher birth rates, larger family sizes, and poorer health status, despite receiving care at the same facilities [12]. Before PCV7/13 implementation, the region’s Bedouin and Jewish populations resembled those of lower- and higher-income countries, respectively, in pneumococcal epidemiology [13].
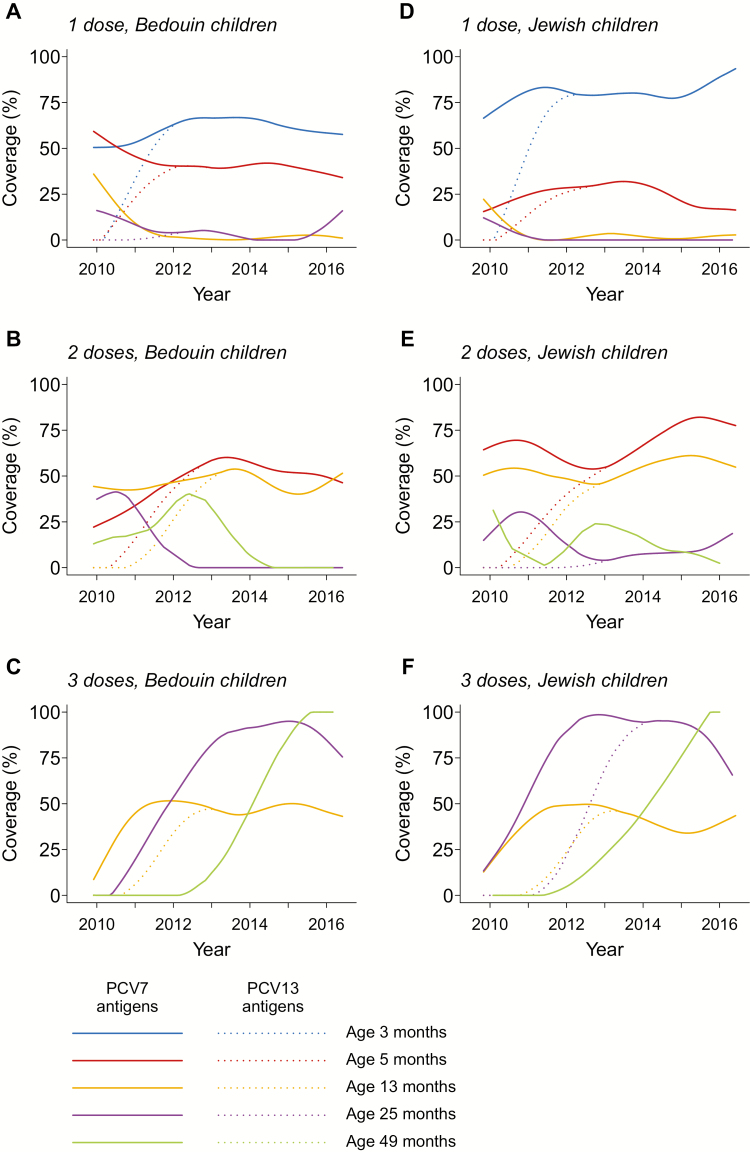
The uptake of PCVs in southern Israel has been described previously [11]. A 2p+1b PCV7 schedule (with doses at ages 2 months, 4 months, and 12 months) was introduced in July 2009, with a catch-up campaign among children aged <2 years. Beginning in November 2010, PCV13 replaced PCV7 without additional catch-up. We illustrate PCV7/13 coverage trends in Figure 1.
Figure 1.
Vaccine coverage in the study population. We illustrate trends in age-specific coverage of immunization against PCV7-type (solid lines) and PVC13-type (dotted lines) antigens in the Bedouin and Jewish populations, among all children enrolled in the study. Israel added PCV7 to the pediatric vaccination schedule in late 2009, using a 2 + 1 dosing scheme, with a catch-up campaign for children under 2 years old. The substitution of PCV13 for PCV7 began in November 2010. We fitted trends to data using smoothing splines with 5 knots. Abbreviations: PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
Surveillance Study
Surveillance of pneumococcal carriage among Jewish and Bedouin children has been undertaken at SUMC since PCV7 introduction; each working day, a nasopharyngeal swab is obtained from the first 4 Jewish and first 4 Bedouin children aged 0–59 months presenting to the pediatric emergency department who are residents of the Negev region and whose parents provide informed consent. Previous pneumococcal vaccination data (number and timing of PCV7 and PCV13 doses) were ascertained from the children’s medical records [11].
Nasopharyngeal samples were obtained via dacron-tipped swabs and placed in MW173 Amies transport medium (Transwab, Medical Wire & Equipment, Potley, UK) before being plated, within 16 hours, on 5% sheep blood/5.0µg gentamicin agar media, followed by 48-hour incubation at 35°C. Pneumococcal identification was based on alpha-hemolysis and optochin inhibition and confirmed by slide agglutination. We selected 1 colony per plate for serotyping by the Quellung reaction (Statens Seruminstitut, Copenhagen, Denmark), as described previously [11].
Case-control Framework
While previous studies of pneumococcal vaccine effectiveness have defined individuals with nonvaccine-serotype infection as controls [14, 15], PCV increases individuals’ risk of nonvaccine-serotype carriage indirectly by preventing vaccine-serotype carriage [16–19]; thus, the odds ratio of vaccination among vaccine-serotype carriers versus nonvaccine-serotype carriers is not equal to the direct effect of PCV against vaccine-serotype carriage [20]. Likewise, children without pneumococcal carriage may present an inappropriate control group, as PCV7/13 receipt has been associated with the absence of carriage in southern Israel [21] and certain other settings [22, 23]. To ensure that our analysis met the requirement that control status be independent of vaccination (enabling an unbiased estimation of vaccine direct effects in case-control studies [24, 25]), we measured vaccine effectiveness in a modified case-control framework. Specifically, our analysis compared the odds of prior PCV receipt among case children experiencing vaccine-serotype colonization versus matched control children sampled from the eligible study population at random, irrespective of colonization status. We demonstrate formally that our approach provides unbiased estimates, and we detail problems arising with various alternative case-control designs, in the Supplementary Information. We matched 3 controls to each case for all analyses and, where possible, included additional eligible controls, as available; up to 6 controls were available per case.
To counteract potential confounding, we matched children on ethnicity (Jewish/Bedouin), visit timing (≤90 days from the case child’s visit), recent antibiotic receipt (≤30 days before the visit), sex, and age. We defined age-eligible controls to be ≤30 days, ≤60 days, and ≤120 days from the case child’s age for the 0–12 month, 13–24 month, and 25–59 month age groups, respectively. To ensure analyses addressed PCV effects on carriage and not disease progression, we excluded children diagnosed with otitis media, pneumonia, influenza, lower or upper respiratory infection, conjunctivitis, bacteremia/sepsis, or meningitis.
Vaccine Effectiveness Estimation
We estimated protection against vaccine-serotype carriage endpoints associated with several vaccine exposures: (1) PCV7/13-conferred protection against carriage of serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F (PCV7 serotypes); (2) PCV7-conferred protection against carriage of PCV7 serotypes, in analyses excluding children who received PCV13; (3) PCV13-conferred protection against carriage of PCV7 serotypes, in analyses excluding children who received PCV7; and (4) PCV13-conferred protection against carriage of all PCV13-targeted serotypes and against the 6 serotypes contained only in PCV13 (1, 3, 5, 6A, 7F, 19A; +6PCV13 serotypes), in analyses excluding children who received PCV7.
Our primary analyses addressed the effectiveness of 1 and 2 primary-series doses (received at ages ≤7 months) against vaccine-serotype colonization at ages ≤12 months; and the effectiveness of a 2p+1b series (2 doses at ages ≤7 months and 1 dose at ages ≥10 months) against vaccine-serotype colonization at ages 13–24 months and 25–59 months. Because the matched odds ratio (mOR) is constructed from discordant sets, children who were not age-eligible for vaccination were excluded by design. We estimated vaccine effectiveness as 1 minus the mOR of receipt of each vaccine sequence, versus receipt of 0 PCV doses, among case and control children.
In additional analyses, we assessed differences in vaccine-serotype carriage among children who received differing sequences of PCV doses. For children aged ≤12 months, we estimated the mOR for receipt of 1 versus 2 primary-series doses among case and control children. For children aged 13–24 months and 25–59 months, we assessed schedules resembling a 2p+0b series (2 doses at ages ≤7 months), a 1p+1b series (1 dose at ages ≤7 months and 1 dose at ages ≥10 months), and a 0p+2b series (2 doses at ages ≥10 months). We estimated the mOR for receipt of each schedule, relative to the standard 2p+1b series, among case and control children.
We conducted statistical analyses by sampling from the distribution of all possible assignments of matched cases and controls (Supplementary Information). At each of 5000 iterations, we matched eligible case and control children according to a random sequence, and computed the mOR for receipt of various schedules via conditional logistic regression, accounting for matching strata. We obtained point estimates and 95% confidence limits for the mOR from the distribution of estimates generated across all 5000 independent repetitions of this procedure.
Secondary Analyses
We also assessed differences in protection against serotype 3, the remaining +6PCV13 serotypes, and PCV7 serotypes [15]; differences in protection among Bedouin and Jewish children; and differences in protection during the first half of the study (up to June 2013) and the second half of the study (July 2013 onward) amid changes in the composition of circulating serotypes. Because dose-specific analyses within these strata were underpowered, we compared the association of each carriage endpoint with the total PCV doses received, defined as a continuous variable. We estimated the mOR using conditional logistic regression, consistent with the primary analyses described above (Supplementary Information).
RESULTS
Enrollment
Between November 2009 and June 2016, we enrolled 5780 Bedouin and 4386 Jewish children aged 0–59 months; 3425 Bedouin and 2503 Jewish children were not diagnosed with diseases potentially caused by pneumococci and had complete records. Data were incomplete for 52 eligible Bedouin children (1.5% of 3477) and 21 eligible Jewish children (0.8% of 2524; Supplementary Table S1), who were excluded from the analyses. Eligible children generally did not differ from those experiencing diseases potentially caused by pneumococcus, but were less likely to be hospitalized or to have received antimicrobial treatment in the preceding month (Supplementary Table S2).
Of those eligible, 1639 Bedouin (47.8% of 3425) and 980 Jewish (39.2% of 2503) children carried pneumococci (Table 1). All children aged 0–23 months at the beginning of the study were eligible for routine or catch-up PCV7 doses (Figure 1); coverage with PCV13 increased over time. Among the 2776 vaccinated children aged ≥13 months, 1728 (62.2%) received doses resembling a 2p+1b schedule (Supplementary Table S4), while 226 (8.1%) received 2p+0b dosing, 57 (2.1%) received 1p+1b dosing, and 201 (7.2%) received 0p+2b dosing.
Table 1.
Enrollment of Eligible Children by Age, Pneumococcal Carriage, and Total Doses Received
| Age, Months | Doses | Bedouin Children | Jewish Children | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | PCV7-Type Carriage | +6PCV13-Type Carriage | NVT Carriage | No Carriage | Total | PCV-Type Carriage | +6PCV13-Type Carriage | NVT Carriage | No Carriage | ||
| n = 3425 | n = 242 | n = 204 | n = 1190 | n = 1789 | n = 2503 | n = 92 | n = 92 | n = 796 | n = 1523 | ||
| 0–1 | 0 PCV7 | 307 | 13 | 15 | 72 | 207 | 116 | 1 | 2 | 12 | 101 |
| ≥1 PCV7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 0 + 6PCV13 | 307 | 13 | 15 | 72 | 207 | 116 | 1 | 2 | 12 | 101 | |
| ≥1 + 6PCV13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total for age | 307 | 13 | 15 | 72 | 207 | 116 | 1 | 2 | 12 | 101 | |
| 2–4 | 0 PCV7 | 167 | 11 | 12 | 63 | 81 | 56 | 2 | 2 | 12 | 40 |
| 1 PCV7 | 261 | 13 | 21 | 91 | 136 | 164 | 7 | 3 | 45 | 109 | |
| 2 PCV7 | 33 | 2 | 0 | 12 | 19 | 32 | 1 | 0 | 6 | 25 | |
| 0 + 6PCV13 | 208 | 18 | 16 | 75 | 99 | 87 | 7 | 3 | 18 | 59 | |
| 1 + 6PCV13 | 223 | 7 | 17 | 80 | 119 | 142 | 3 | 2 | 39 | 98 | |
| 2 + 6PCV13 | 30 | 1 | 0 | 11 | 18 | 23 | 0 | 0 | 6 | 17 | |
| Total for age | 461 | 26 | 33 | 166 | 236 | 252 | 10 | 5 | 63 | 174 | |
| 5–12 | 0 PCV7 | 37 | 5 | 4 | 13 | 15 | 14 | 3 | 2 | 4 | 5 |
| 1 PCV7 | 165 | 22 | 18 | 56 | 69 | 38 | 3 | 3 | 17 | 15 | |
| 2 PCV7 | 761 | 58 | 44 | 267 | 392 | 545 | 16 | 12 | 160 | 357 | |
| 3 PCV7 | 32 | 2 | 3 | 14 | 13 | 26 | 0 | 2 | 3 | 21 | |
| 0 + 6PCV13 | 240 | 46 | 23 | 72 | 99 | 140 | 10 | 11 | 34 | 85 | |
| 1 + 6PCV13 | 133 | 16 | 14 | 47 | 56 | 44 | 1 | 2 | 18 | 23 | |
| 2 + 6PCV13 | 599 | 24 | 31 | 220 | 324 | 427 | 11 | 6 | 131 | 279 | |
| 3 + 6PCV13 | 23 | 1 | 1 | 11 | 10 | 12 | 0 | 0 | 1 | 11 | |
| Total for age | 995 | 87 | 69 | 350 | 489 | 623 | 22 | 19 | 184 | 398 | |
| 13–24 | 0 PCV7 | 34 | 7 | 1 | 12 | 14 | 35 | 5 | 1 | 14 | 15 |
| 1 PCV7 | 41 | 5 | 4 | 10 | 22 | 20 | 1 | 2 | 6 | 11 | |
| 2 PCV7 | 164 | 25 | 13 | 59 | 67 | 132 | 4 | 12 | 40 | 76 | |
| 3 PCV7 | 517 | 16 | 27 | 190 | 284 | 469 | 13 | 12 | 192 | 252 | |
| 4 PCV7 | 19 | 0 | 1 | 8 | 10 | 27 | 1 | 0 | 8 | 18 | |
| 0 + 6PCV13 | 215 | 30 | 20 | 78 | 87 | 164 | 12 | 17 | 52 | 83 | |
| 1 + 6PCV13 | 66 | 3 | 6 | 23 | 34 | 49 | 1 | 4 | 18 | 26 | |
| 2 + 6PCV13 | 91 | 9 | 6 | 31 | 45 | 95 | 1 | 4 | 31 | 59 | |
| 3 + 6PCV13 | 393 | 11 | 14 | 141 | 227 | 364 | 10 | 2 | 157 | 195 | |
| 4 + 6PCV13 | 10 | 0 | 0 | 6 | 4 | 11 | 0 | 0 | 2 | 9 | |
| Total for age | 775 | 53 | 46 | 279 | 397 | 683 | 24 | 27 | 260 | 372 | |
| 25–59 | 0 PCV7 | 220 | 32 | 17 | 64 | 107 | 191 | 22 | 19 | 41 | 109 |
| 1 PCV7 | 28 | 1 | 3 | 10 | 14 | 17 | 0 | 1 | 5 | 11 | |
| 2 PCV7 | 127 | 9 | 7 | 60 | 51 | 94 | 2 | 7 | 33 | 52 | |
| 3 PCV7 | 492 | 21 | 13 | 181 | 277 | 472 | 11 | 9 | 183 | 269 | |
| 4 PCV7 | 20 | 0 | 1 | 8 | 11 | 55 | 0 | 3 | 15 | 37 | |
| 0 + 6PCV13 | 420 | 45 | 34 | 138 | 203 | 384 | 25 | 28 | 106 | 225 | |
| 1 + 6PCV13 | 94 | 2 | 2 | 47 | 43 | 54 | 4 | 0 | 18 | 32 | |
| 2 + 6PCV13 | 39 | 1 | 0 | 16 | 22 | 44 | 2 | 1 | 18 | 23 | |
| 3 + 6PCV13 | 321 | 15 | 4 | 117 | 185 | 327 | 4 | 8 | 129 | 186 | |
| 4 + 6PCV13 | 13 | 0 | 1 | 5 | 7 | 20 | 0 | 2 | 6 | 12 | |
| Total for age | 887 | 63 | 41 | 323 | 460 | 829 | 35 | 39 | 277 | 478 |
Data indicate the number of children enrolled who were not diagnosed with diseases potentially caused by pneumococcus, with complete data available, as detailed in Supplementary Table S1. We present the numbers of children receiving the specific dosing schedules assessed in Supplementary Table S3.
Abbreviations: +6PCV13, the 6 serotypes contained in PCV13 and not PCV7; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; NVT, nonvaccine type (serotypes not included in PCV13).
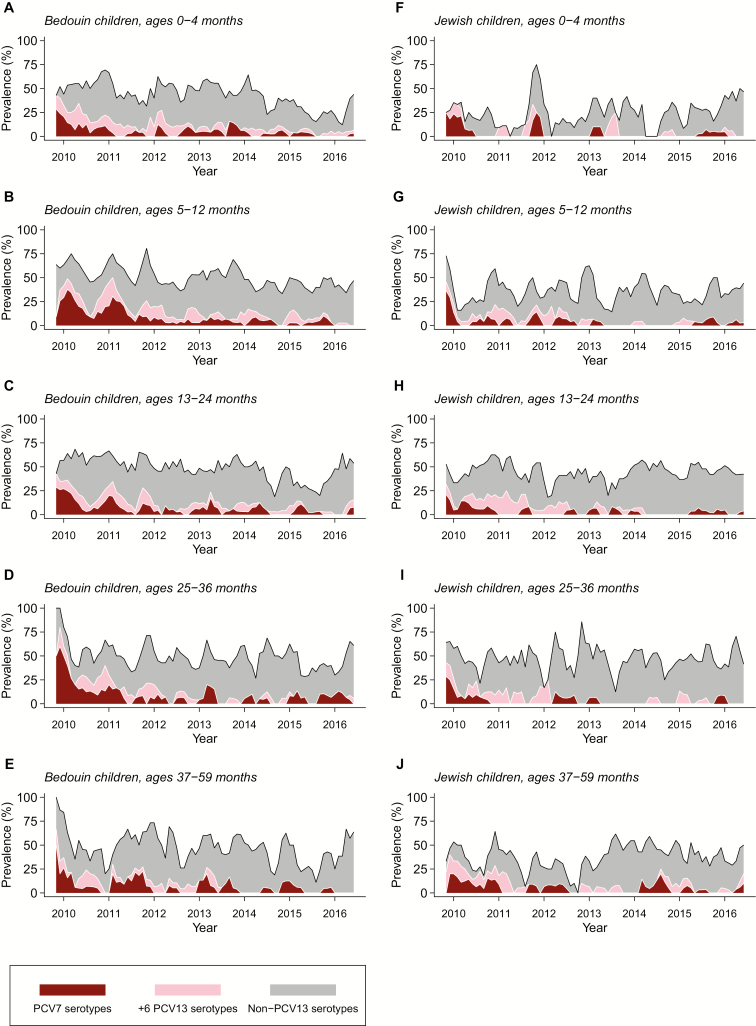
While PCV7 and PCV13 serotypes originally comprised >50% of pneumococcal carriage, nonvaccine serotypes comprised an increasing proportion of carried pneumococci over time (Figure 2; Supplementary Figure S1). Vaccine-serotype carriers did not differ markedly from other children in the measured variables, but were more likely to have received antibiotics in the preceding month (173/630, 26.1%) than nonvaccine-serotype carriers (325/1986, 16.4%; Supplementary Table S3).
Figure 2.
Pneumococcal carriage in the study population. We illustrate pneumococcal carriage prevalence by age, ethnicity, vaccine type, and over time (calculated as a 3-month moving average within each age group). Although non-PCV13 serotype carriage largely offset reductions in the carriage of vaccine-targeted serotypes, the carriage of serotypes targeted by PCV13 persisted as of 2016 in all age groups. Abbreviations: PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
Vaccine Effectiveness
We provide the total numbers of children eligible for each comparison in Table 2. At ages ≤12 months, 2 primary-series PCV13 doses conferred 53% (95% confidence interval [CI], 32–67%) protection against PCV13-serotype carriage (Table 3), including 64% (95% CI, 43–78%) protection against PCV7-serotype carriage and 42% (95% CI, 7–63%) protection against +6PCV13-serotype carriage. We estimated lower protection against PCV7-serotype carriage (35%; 95% CI, 8–55%) in analyses that included children who received PCV7 or PCV13 doses; we did not find clear evidence of protection among those who received PCV7 only. We also did not identify strong evidence that a single primary dose was protective. Compared to children receiving 2 primary-series PCV13 doses, those receiving 1 dose experienced 1.83-fold (95% CI, 1.28–2.62) greater odds of carrying PCV13-serotype pneumococci, including 1.46-fold (95% CI, 1.03–2.16) and 2.45-fold (95% CI, 1.50–4.05) greater odds of carrying +6PCV13 serotypes and PCV7-targeted serotypes, respectively (Table 3; Supplementary Table S5).
Table 2.
Match-Eligible Population
| Age, Months | Carriage Endpoint | Vaccine | Dosing Compared | Bedouin Children | Jewish Children | ||
|---|---|---|---|---|---|---|---|
| Exposure vs Reference | Eligible Case Population | Match-Eligible Non-case Population | Eligible Case Population | Match-Eligible Non-case Population | |||
| n | n | n | n | ||||
| ≤12 | PCV7 serotypes | PCV7/13 | 1p vs 0p | 62 | 535 | 15 | 83 |
| 2p vs 0p | 119 | 985 | 30 | 210 | |||
| 1p vs 2p | 47 | 379 | 14 | 99 | |||
| PCV7 | 1p vs 0p | 47 | 375 | 11 | 47 | ||
| 2p vs 0p | 54 | 409 | 11 | 73 | |||
| 1p vs 2p | 43 | 192 | 10 | 49 | |||
| PCV13 | 1p vs 0p | 44 | 453 | 10 | 68 | ||
| 2p vs 0p | 54 | 494 | 16 | 97 | |||
| 1p vs 2p | 40 | 362 | 14 | 99 | |||
| PCV13 serotypes | PCV13 | 1p vs 0p | 101 | 637 | 19 | 131 | |
| 2p vs 0p | 113 | 742 | 28 | 172 | |||
| 1p vs 2p | 94 | 622 | 23 | 164 | |||
| +6PCV13 serotypes | PCV13 | 1p vs 0p | 57 | 552 | 9 | 71 | |
| 2p vs 0p | 59 | 548 | 12 | 78 | |||
| 1p vs 2p | 54 | 435 | 9 | 73 | |||
| 13–25 | PCV7 serotypes | PCV7/13 | 0p+0p vs 2p+1b | 23 | 112 | 17 | 121 |
| 2p+0b vs 2p+1b | 31 | 213 | 13 | 137 | |||
| 1p+1b vs 2p+1b | 19 | 112 | 13 | 116 | |||
| 0p+2b vs 2p+1b | 21 | 115 | 14 | 124 | |||
| PCV7 | 0p+0p vs 2p+1b | 10 | 23 | 6 | 12 | ||
| 2p+0b vs 2p+1b | 9 | 36 | 1 | 6 | |||
| 1p+1b vs 2p+1b | 5 | 21 | 2 | 7 | |||
| 0p+2b vs 2p+1b | 8 | 33 | 3 | 16 | |||
| PCV13 | 0p+0p vs 2p+1b | 18 | 90 | 15 | 100 | ||
| 2p+0b vs 2p+1b | 18 | 141 | 11 | 115 | |||
| 1p+1b vs 2p+1b | 12 | 84 | 10 | 94 | |||
| 0p+2b vs 2p+1b | 11 | 75 | 10 | 94 | |||
| PCV13 serotypes | PCV13 | 0p+0p vs 2p+1b | 31 | 163 | 18 | 114 | |
| 2p+0b vs 2p+1b | 33 | 218 | 15 | 141 | |||
| 1p+1b vs 2p+1b | 24 | 147 | 12 | 103 | |||
| 0p+2b vs 2p+1b | 23 | 143 | 13 | 108 | |||
| +6PCV13 serotypes | PCV13 | 0p+0p vs 2p+1b | 13 | 96 | 3 | 14 | |
| 2p+0b vs 2p+1b | 15 | 120 | 4 | 26 | |||
| 1p+1b vs 2p+1b | 12 | 89 | 2 | 9 | |||
| 0p+2b vs 2p+1b | 12 | 87 | 3 | 19 | |||
| 25–59 | PCV7 serotypes | PCV7/13 | 0p+0p vs 2p+1b | 49 | 272 | 31 | 177 |
| 2p+0b vs 2p+1b | 18 | 148 | 10 | 86 | |||
| 1p+1b vs 2p+1b | 17 | 144 | 9 | 79 | |||
| 0p+2b vs 2p+1b | 21 | 155 | 10 | 81 | |||
| PCV7 | 0p+0p vs 2p+1b | 35 | 146 | 23 | 104 | ||
| 2p+0b vs 2p+1b | 4 | 11 | 1 | 4 | |||
| 1p+1b vs 2p+1b | 2 | 6 | 1 | 3 | |||
| 0p+2b vs 2p+1b | 6 | 20 | 2 | 7 | |||
| PCV13 | 0p+0p vs 2p+1b | 46 | 246 | 26 | 151 | ||
| 2p+0b vs 2p+1b | 13 | 111 | 5 | 52 | |||
| 1p+1b vs 2p+1b | 13 | 109 | 4 | 47 | |||
| 0p+2b vs 2p+1b | 14 | 110 | 4 | 45 | |||
| PCV13 serotypes | PCV13 | 0p+0p vs 2p+1b | 66 | 288 | 52 | 220 | |
| 2p+0b vs 2p+1b | 16 | 138 | 13 | 100 | |||
| 1p+1b vs 2p+1b | 16 | 135 | 11 | 95 | |||
| 0p+2b vs 2p+1b | 17 | 136 | 11 | 91 | |||
| +6PCV13 serotypes | PCV13 | 0p+0p vs 2p+1b | 20 | 148 | 26 | 156 | |
| 2p+0b vs 2p+1b | 3 | 40 | 8 | 71 | |||
| 1p+1b vs 2p+1b | 3 | 38 | 7 | 67 | |||
| 0p+2b vs 2p+1b | 3 | 37 | 7 | 64 |
Data indicate numbers of all children available for each comparison (including children eligible for inclusion as cases for whom matched controls, defined irrespective of colonization status, could be identified). As controls could include children carrying vaccine-serotype pneumococci (here listed as “case-eligible” children), the numbers of cases and controls in each analysis iteration do not necessarily correspond to the column entries.
Abbreviations: b, booster; p, primary; +6PCV13, the 6 serotypes contained in PCV13 and not PCV7; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
Table 3.
Effectiveness Primary-series Pneumococcal Conjugate Vaccine Doses Among Children Aged ≤12 Months
| Vaccine Exposure | Carriage Endpoint | Dosing Comparisons | ||||
|---|---|---|---|---|---|---|
| 1p ref. 0p | 2p ref. 0p | 1p ref. 2p | ||||
| mOR (95% CI) | VE (95% CI), % | mOR (95% CI) | VE (95% CI), % | mOR (95% CI) | ||
| PCV7/13 doses | PCV7 serotypes | 1.07 (.80–1.43) | −7 (−43 to 20) | 0.65 (.45–.92) | 35 (8–55) | 1.65 (1.16–2.35) |
| PCV13 doses only | All PCV13 serotypes | 0.86 (.64–1.13) | 14 (−13 to 36) | 0.47 (.63–.68) | 53 (32–67) | 1.83 (1.28–2.62) |
| +6PCV13 serotypes | 0.85 (.59–1.23) | 15 (−23 to 41) | 0.58 (.37–.93) | 42 (7–63) | 1.46 (1.03–2.16) | |
| PCV7 serotypes | 0.89 (.64–1.22) | 11 (−22 to 36) | 0.36 (.22–.57) | 64 (43–78) | 2.45 (1.50–4.05) | |
| PCV7 doses only | PCV7 serotypes | 1.23 (.74–2.11) | −23 (−111 to 26) | 1.00 (.59–1.72) | 0 (−72 to 41) | 1.24 (.74–2.05) |
Vaccine effectiveness estimates are calculated as 1-mOR times 100%. The mORs are calculated from the relative odds of receipt of each vaccination series among case children versus matched controls. Comparisons are structured as “1p ref. 0p”, “2p ref. 0p”, and “1p ref. 2p”. Here, “1p ref. 0p” and “2p ref. 0p” present the effectiveness of 1 and 2 primary doses, respectively, in comparison to 0 primary doses; “1p ref. 2p” presents the effectiveness of a single primary dose, in comparison to 2 primary doses.
Abbreviations: +6PCV13, the 6 serotypes contained in PCV13 and not PCV7; CI, confidence interval; mOR, matched odds ratio; p, primary dose; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; VE, vaccine effectiveness.
At ages 13–24 months, we estimated that 2p+1b PCV13 dosing conferred 40% (95% CI, 4–67%) protection against PCV13-serotype carriage (Table 4); we estimated 62% (95% CI, 33–83%) protection at ages 25–59 months. We estimated 71% (95% CI, 50–83%) effectiveness of 2p+1b dosing with PCV13 against PCV7-serotype carriage at ages 13–24 months, and similar levels of protection against PCV7-serotype carriage at ages 13–24 months and 25–59 months, associated with receipt of PCV7/13.
Table 4.
Effectiveness of Pneumococcal Conjugate Vaccine Doses Among Children Aged ≥13 Months
| Age, Months | Vaccine Exposure | Carriage Endpoint | Dosing Comparisons | ||||
|---|---|---|---|---|---|---|---|
| 2p+1b ref. 0p+0b | 2p+0b ref. 2p+1b | 1p+1b ref. 2p+1b | 0p+2b ref. 2p+1b | ||||
| mOR (95% CI) | VE (95% CI), % | mOR (95% CI) | mOR (95% CI) | mOR (95% CI) | |||
| 13–24 | PCV7/13 doses | PCV7 serotypes | .38 (.25–.56) | 62 (44–75) | 2.83 (1.87–4.34) | 1.94 (1.06–4.40) | 1.05 (.48–2.28) |
| PCV13 doses only | All PCV13 serotypes | .60 (.33–.96) | 40 (4–67) | 3.33 (2.28–3.93) | 2.05 (1.12–5.00) | 4.60 (2.34–6.00) | |
| +6PCV13 serotypes | … | … | 3.73 (2.58–6.12) | … | … | ||
| PCV7 serotypes | .29 (.17–.50) | 71 (50–83) | 3.16 (1.94–5.31) | 2.96 (1.67–5.00) | … | ||
| PCV7 doses only | PCV7 serotypes | … | … | 1.17 (.89–1.77) | 1.48 (.73–3.31) | 1.29 (.63–1.28) | |
| 25–59 | PCV7/13 doses | PCV7 serotypes | .36 (.16–1.91) | 64 (−91–84) | 1.79 (1.01–3.65) | 1.68 (1.00–3.00) | … |
| PCV13 doses only | All PCV13 serotypes | .38 (.17–.67) | 62 (33–83) | 0.95 (.53–2.09) | … | … | |
| +6PCV13 serotypes | … | … | 1.99 (1.00–6.00) | … | … | ||
| PCV7 serotypes | … | … | 1.13 (.76–2.34) | … | … | ||
| PCV7 doses only | PCV7 serotypes | .37 (.25–.41) | 63 (59–75) | 1.39 (1.00–3.00) | … | … |
Vaccine effectiveness estimates are calculated as 1-mOR times 100%. Matched odds ratios are calculated from the relative odds of receipt of each vaccination series among case children versus matched controls. Comparisons are structured as “2p + 1b ref. 0p + 0b”, “2p + 0b ref. 2p + 1b”, etc., to present the effectiveness of alternative vaccination schedules. Here, “2p + 1b ref 0p + 0b” presents the effectiveness of 2p + 1b dosing, compared to receipt of zero primary series or booster doses. “2p + 0b ref. 2p + 1b” presents the effectiveness of 2p + 0b dosing, compared to receipt of the standard 2p + 1b schedule.
Abbreviations: +6PCV13, the 6 serotypes contained in PCV13 and not PCV7; b, booster dose; CI, confidence interval; mOR, matched odds ratio; OR, odds ratio; p, primary dose; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; VE, vaccine effectiveness.
Compared to children receiving PCV13 under 2p+1b dosing, children receiving PCV13 under 2p+0b and 1p+1b schedules experienced 3.33-fold (95% CI, 2.28–3.93) and 2.05-fold (95% CI, 1.12–5.00) greater odds, respectively, of carrying PCV13-targeted serotypes at ages 13–24 months (Table 4; Supplementary Table S5). While fewer match lists could be constructed for comparisons at ages 25–59 months, we estimated that children who received PCV7/13 according to 2p+0b and 1p+1b schedules had 1.79-fold (95% CI, 1.01–3.65) and 1.68-fold (95% CI, 1.00–3.00) greater odds, respectively, of carrying PCV7-targeted serotypes than children receiving doses according to a 2p+1b schedule. We also estimated elevated odds of PCV7-serotype carriage among children who received PCV7 under 2p+0b versus 2p+1b dosing in this age group (Table 4; Supplementary Table S5).
Secondary Analyses
On average, each additional PCV7/13 dose received was associated with 30% (95% CI, 22–37%) lower odds of carrying PCV7-targeted serotypes (Table 5). While this did not differ greatly from our estimate of the association between PCV13 doses received and carriage of serotypes 1, 5, 6A, 7F, and 19A, receipt of additional PCV13 doses was associated with greater odds of serotype 3 carriage.
Table 5.
Differential Protection Against Vaccine-Serotype Carriage Endpoints
| Serotype | Vaccine | Reduction, Per Dose Received | DR and MR Per Dose Received, % | |||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | Reference serotypes 1, 5, 6A, 7F, and 19A | Reference serotype 3 | ||||
| DR (95% CI)a | MR (95% CI)b | DR (95% CI)a | MR (95% CI)b | |||
| PCV7 serotypes | PCV7/13 | 30 (22–37) | 10 (−5 to 26) | 12 (−7 to 28) | 84 (35–144) | 53 (33–68) |
| Serotypes 1, 5, 6A, 7F, 19A | PCV13 only | 19 (5–32) | … | … | 74 (23–135) | 46 (22–64) |
| Serotype 3 | PCV13 only | −55 (−115 to −5) | −74 (−135 to −23) | −93 (−179 to −28) | … | … |
Vaccine effectiveness estimates are calculated as 1-mOR times 100%. The mORs are calculated from the relative odds of receipt of vaccine doses (defined as a continuous variable) among case children versus matched controls.
Abbreviations: CI, confidence interval; DR, difference in reduction; mOR, matched odds ratio; MR, marginal reduction; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
aDR, per dose received, was estimated as for a comparison of the magnitude of protection against endpoints i and j.
bMR, per dose received, was estimated as to quantify the additional protection conferred against endpoint i beyond protection conferred against endpoint j.
We did not identify clear differences in protection against carriage of PCV7-targeted or all PCV13-targeted serotypes among Jewish and Bedouin children (Supplementary Table S6). Whereas the total number of PCV13 doses received predicted greater protection against +6PCV13-serotype carriage among Jewish children, we identified no association between the number of PCV13 doses received and carriage of +6PCV13 serotypes among Bedouin children. Notably, we estimated increases in odds of serotype 3 carriage among Bedouin children with additional PCV13 doses.
On average, each additional PCV7/13 dose was associated with 31% (95% CI, 22–39%) and 33% (95% CI, 21–43%) lower odds of carrying PCV7-targeted serotypes before and after 1 July 2013, respectively, while each additional PCV13 dose was associated with 25% (95% CI, 9–37%) and 22% (95% CI, 7–33%) lower odds, respectively, of PCV13-serotype carriage over the same periods (Supplementary Table S7).
DISCUSSION
Using data from a large carriage study, we report the first estimates of PCV effectiveness against vaccine-serotype pneumococcal colonization. For children aged ≤12 months, we did not identify protection against vaccine-serotype colonization after 1 primary dose, whereas a 2-dose PCV13 primary series confers moderate (95% CI, 42–64%) protection. We estimate that 2p+1b PCV13 dosing confers roughly 40% and 71% protection against carriage of PCV13-targeted and PCV7-targeted serotypes at ages 13–24 months, respectively. Comparable levels of protection persist at ages 25–59 months. Comparing 2p+0b, 1p+1b, and 0p+2b PCV13 recipients to those who received 2p+1b dosing suggests that children receiving reduced-dose schedules may experience a greater risk of vaccine-serotype pneumococcal carriage beyond the first year of life.
Few studies have assessed dose-specific PCV-conferred protection against vaccine-serotype colonization [8, 19], and none have addressed postimplementation effectiveness. Consistent with our findings, no studies have identified a protective effect of a single PCV dose against vaccine-serotype colonization at ages ≤12 months [26]. In 1 randomized trial [27], 3 primary-series doses were estimated to confer 54% (95% CI, −59 to 87%) and 25% (95% CI, −98 to 71%) greater protection than a single dose at various follow-up intervals, resembling our findings of enhanced protection at ages ≤12 months after receipt of additional primary-series doses [17, 28]. Elsewhere, 3p+0b and 2p+1b schedules have been estimated to confer 60–69% protection against vaccine-serotype carriage at ages ≥13 months [29, 30]. These findings are roughly consistent with our estimates of 40–71% effectiveness of a 2p+1b PCV schedule against vaccine-serotype carriage at ages 13–24 months, and closely resemble our estimates of 62–64% effectiveness at ages 25–59 months.
Comparing 2-dose schedules at ages ≥13 months to a 2p+1b series offers insights into their relative effectiveness. Our finding of greater protection against vaccine-serotype carriage among children receiving 2p+1b dosing, as compared to 1p+1b dosing, suggests the effect of the booster dose is enhanced by a 2-dose primary series. Although we could not compare 1p+1b and 2p+0b schedules head-to-head, we estimate greater elevations in children’s odds of carrying vaccine serotypes under 2p+0b dosing than 1p+1b dosing, relative to 2p+1b. Maintaining the booster dose is likely of importance in settings that implement reduced-dose schedules, consistent with data from immunogenicity studies [5, 7].
Epidemiologic surveillance reveals that vaccine serotypes, and especially serotypes 3, 14, 19A, and 19F, have not been successfully eliminated from countries using PCV13 [31–33]. Because a single primary dose is unlikely to provide strong protection against carriage acquisition, and offers limited protection against disease [10], cases of vaccine-serotype IPD among infants may increase under reduced-dose schedules. Ongoing surveillance is needed to monitor this possibility, to confirm the differences in protection suggested by our study, and to assess whether schedule changes lead to circulation of PCV-targeted pneumococcal serotypes. Emerging evidence that indirect protection among adults depends upon the elimination of vaccine-serotype pneumococci among toddlers and school-aged children [34] underscores the need to maximize protection among older children.
Unexpectedly, we estimated differential protection against PCV7-targeted serotypes among children who received PCV7 and PCV13. Notably, PCV7 implementation included a catch-up campaign. Thus, booster doses of PCV7 tended to be administered at older ages than booster doses of PCV13 (Supplementary Table S8; Supplementary Figure S2). In addition, unvaccinated children not reached by the PCV7 catch-up campaign may have differed from children not receiving PCV13 under the routine schedule. Cohorts receiving PCV7 also experienced greater PCV7-serotype circulation before and after vaccination than cohorts receiving PCV13; differential naturally acquired immunity in vaccinated and unvaccinated children may complicate comparisons of protection among cohorts eligible for PCV7 and PCV13 doses.
Although we estimate negative effectiveness of PCV13 against serotype 3 carriage, this finding does not necessarily indicate that vaccination directly increases susceptibility to serotype 3 carriage. Unvaccinated or under-vaccinated children may be more likely to carry vaccine-targeted serotypes, wielding competitive advantages against serotype 3 in the nasopharynx. In a previous study, serotype 3 showed heightened susceptibility to competition relative to serotypes 6A, 6B, 9V, 14, 19F, and 23F, among others [35]. Our point estimate of 40% protection conferred by 2p+1b dosing with PCV13 at ages 13–24 months is lower than our point estimates of protection in other strata. This difference may owe to age-specific confounding from variables not included in our matching procedure, age specificity in exposure to vaccine-type and nonvaccine-type pneumococci that compete for the nasopharynx, or random chance.
Our study has several limitations. Nonrandomized vaccination is the most important obstacle to causal interpretation of our results. However, our ability to match children on age, sex, visit timing, prior antibiotic receipt, and ethnicity helps to ensure cases and controls encountered similar exposures; in addition, sampling both cases and controls in a clinical setting reduces confounding of vaccination with health care–seeking behavior and health status [36]. Statistical power was limited in our primary estimates of vaccine effectiveness, as there were few unvaccinated children; however, our estimates of differences in carriage among children receiving PCV according to alternative dosing schemes convey the information most relevant to policymaking. Our analysis addressed the effects of PCVs on the prevalence of vaccine-serotype carriage, which may differ from protection against acquisition of vaccine-serotype pneumococci [37]. Last, our study does not address how doses are spaced within the primary series, or between the primary and booster series [38].
Randomized assessments should determine the efficacy of alternative PCV schedules against vaccine-serotype colonization as an increasing number of countries consider schedule changes [5]. Cluster-randomized trials may reveal how reduced-dose schedules affect herd protection. Our direct-effect estimates may inform transmission-dynamic models, helping to illustrate the potential long-term consequences of reduced-dose schedules [7, 39, 40]. Such evidence can help anticipate the impact of schedule changes on the public health benefits of PCV programs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This study was funded in part by a grant from Pfizer (grant number 0887X1-4603 to R. D.).
Potential conflicts of interest. J. A. L. received support from a Robert Austrian Young Investigator award from the International Symposium on Pneumococci and Pneumococcal Diseases; has received research grants from Pfizer to Harvard University and to the University of California, Berkeley; and has received consulting fees from Pfizer. R. D. has received a research grant, consulting fees, and speaker fees from Pfizer; a research grant and consulting fees from Merck Sharp & Dohme; and consulting fees from MeMed. N. G.-L. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004; 4:144–54. [DOI] [PubMed] [Google Scholar]
- 2.View-hub. Pneumococcal conjugate vaccine introduction: universal vaccine introduction over time. Available at: https://view-hub.org/viz/. Accessed 24 September 2019.
- 3.Loo JD, Conklin L, Fleming-Dutra KE, et al. Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr Infect Dis J 2014; 33(Suppl 2):S161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavi, the Vaccine Alliance. Pneumococcal vaccine supply and procurement roadmap. Available at: http://linkinghub.elsevier.com/retrieve/pii/S1068607X00000706. Accessed 4 November 2019.
- 5.O’Brien KL. When less is more: how many doses of PCV are enough? Lancet Infect Dis 2018; 18:127–8. [DOI] [PubMed] [Google Scholar]
- 6.Goldblatt D, Southern J, Andrews NJ, et al. Pneumococcal conjugate vaccine 13 delivered as one primary and one booster dose (1 + 1) compared with two primary doses and a booster (2 + 1) in UK infants: a multicentre, parallel group randomised controlled trial. Lancet Infect Dis 2018; 18:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flasche S, Van Hoek AJ, Goldblatt D, et al. The potential for reducing the number of pneumococcal conjugate vaccine doses while sustaining herd immunity in high-income countries. PLOS Med 2015; 12:e1001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitney CG, Goldblatt D, O’Brien KL. Dosing schedules for pneumococcal conjugate vaccine: considerations for policy makers. Pediatr Infect Dis J 2014; 33(Suppl 2):S172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrejko K, Hosangadi D, Cohen O, et al. WHO technical expert consultation report on optimization of PCV impact: review of evidence and programmatic considerations to inform policy department of immunizations, vaccines, and biologics. Available at: https://bit.ly/2UpZHzj. Accessed 1 September 2019.
- 10.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 2006; 368:1495–502. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Shimol S, Givon-Lavi N, Greenberg D, Dagan R. Pneumococcal nasopharyngeal carriage in children <5 years of age visiting the pediatric emergency room in relation to PCV7 and PCV13 introduction in southern Israel. Hum Vaccin Immunother 2016; 12:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israel Central Bureau of Statistics. Statistical abstract of Israel. 2016; 67. [Google Scholar]
- 13.Ben-Shimol S, Greenberg D, Givon-Lavi N, et al. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: an active prospective nationwide surveillance. Vaccine 2014; 32:3452–9. [DOI] [PubMed] [Google Scholar]
- 14.Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med 1980; 303:549–52. [DOI] [PubMed] [Google Scholar]
- 15.Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014; 14:839–46. [DOI] [PubMed] [Google Scholar]
- 16.Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis 2002; 185:927–36. [DOI] [PubMed] [Google Scholar]
- 17.Lewnard JA, Givon-Lavi N, Huppert A, et al. Epidemiological markers for interactions among Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in upper respiratory tract carriage. J Infect Dis 2016; 213:1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gils EJ, Veenhoven RH, Hak E, et al. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA 2009; 302:159–67. [DOI] [PubMed] [Google Scholar]
- 19.Fleming-Dutra KE, Conklin L, Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J 2014; 33(Suppl 2):S152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews N, Waight PA, Ladhani S, et al. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLOS ONE 2011; 6:e28435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewnard JA, Givon-Lavi N, Weinberger DM, Lipsitch M, Dagan R. Pan-serotype reduction in progression of Streptococcus pneumoniae to otitis media after rollout of pneumococcal conjugate vaccines. Clin Infect Dis 2017; 65:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricketson LJ, Wood ML, Vanderkooi OG, et al. ; Calgary Streptococcus pneumoniae Epidemiology Research (CASPER) Investigators. Trends in asymptomatic nasopharyngeal colonization with Streptococcus pneumoniae after introduction of the 13-valent pneumococcal conjugate vaccine in Calgary, Canada. Pediatr Infect Dis J 2014; 33:724–30. [DOI] [PubMed] [Google Scholar]
- 23.Steens A, Caugant DA, Aaberge IS, Vestrheim DF. Decreased carriage and genetic shifts in the Streptococcus pneumoniae population after changing the seven-valent to the thirteen-valent pneumococcal vaccine in Norway. Pediatr Infect Dis J 2015; 34:875–83. [DOI] [PubMed] [Google Scholar]
- 24.Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epidemiol 2016; 45:2060–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. II. Types of controls. Am J Epidemiol 1992; 135:1029–41. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls TR, Leach AJ, Morris PS. The short-term impact of each primary dose of pneumococcal conjugate vaccine on nasopharyngeal carriage: systematic review and meta-analyses of randomised controlled trials. Vaccine 2016; 34:703–13. [DOI] [PubMed] [Google Scholar]
- 27.Russell FM, Carapetis JR, Satzke C, et al. Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster. Clin Vaccine Immunol 2010; 17:1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dagan R, Givon-Lavi N, Porat N, Greenberg D. The effect of an alternative reduced-dose infant schedule and a second year catch-up schedule with 7-valent pneumococcal conjugate vaccine on pneumococcal carriage: a randomized controlled trial. Vaccine 2012; 30:5132–40. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis 2007; 196:1211–20. [DOI] [PubMed] [Google Scholar]
- 30.Lourenco J, Obolski U, Swarthout TD, et al. Determinants of high residual post-PCV13 pneumococcal vaccine type carriage in Blantyre, Malawi: a modelling study. BMC Med 2019; 17:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glikman D, Dagan R, Barkai G, et al. ; Israel Bacteremia and Meningitis Active Surveillance Group . Dynamics of severe and non-severe invasive pneumococcal disease in young children in Israel following PCV7/PCV13 introduction. Pediatr Infect Dis J 2018; 37:1048–53. [DOI] [PubMed] [Google Scholar]
- 33.Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis 2018; 18:441–51. [DOI] [PubMed] [Google Scholar]
- 34.Weinberger DM, Pitzer VE, Regev-Yochay G, Givon-Lavi N, Dagan R. Association between the decline in pneumococcal disease in unimmunized adults and vaccine-derived protection against colonization in toddlers and preschool-aged children. Am J Epidemiol 2019; 188:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipsitch M, Abdullahi O, DʼAmour A, et al. Estimating rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kenya with a Markov transition model. Epidemiology 2012; 23:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewnard JA, Tedijanto C, Cowling BJ, Lipsitch M. Measurement of vaccine direct effects under the test-negative design. Am J Epidemiol 2018; 187:2686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auranen K, Rinta-Kokko H, Goldblatt D, et al. ; Pneumococcal Carriage Group (PneumoCarr) . Colonisation endpoints in Streptococcus pneumoniae vaccine trials. Vaccine 2013; 32:153–8. [DOI] [PubMed] [Google Scholar]
- 38.Spijkerman J, Veenhoven RH, Wijmenga-Monsuur AJ, et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine administered according to 4 different primary immunization schedules in infants: a randomized clinical trial. JAMA 2013; 310:930–7. [DOI] [PubMed] [Google Scholar]
- 39.Yang A, Cai F, Lipsitch M. Herd immunity alters the conditions for performing dose schedule comparisons: an individual-based model of pneumococcal carriage. BMC Infect Dis 2019; 19:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi YH, Andrews N, Miller E. Estimated impact of revising the 13-valent pneumococcal conjugate vaccine schedule from 2 + 1 to 1 + 1 in England and Wales: A modelling study. PLOS Med 2019; 16:e1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.