Abstract
STUDY QUESTION
What is the mechanism of Tim-3+ regulatory T (Treg)-cell accumulation in the decidua during early pregnancy and is its disruption associated with recurrent pregnancy loss (RPL)?
SUMMARY ANSWER
IL-27 and Gal-9 secreted by trophoblasts activate the Tim-3 signaling pathway in CD4+ T cells and Treg cells and so promote accumulation of Tim-3+ Treg cells, the abnormal expression of IL-27 and Gal-9 is associated with impaired immunologic tolerance in RPL patients.
WHAT IS KNOWN ALREADY
Tim-3+ Treg cells are better suppressors of Teff cell proliferation, and display higher proliferative activity than Tim-3− Treg cells. Tim-3+ Treg cells are tissue-specific promoters of T-cell dysfunction in many tumors. These cells express a unique factor that influences and shapes the tumor microenvironment.
STUDY DESIGN, SIZE, DURATION
The animal study included 80 normal pregnant mice. In human study, decidua tissues in the first trimester for flow cytometry analysis were collected from 32 normal pregnant women and 23 RPL patients. Placenta tissues for immunohistochemistry analysis were collected from 15 normal pregnant women. Placenta tissues for western blot analysis were collected from 5 normal pregnant women, 5 RPL patients and 5 women who have experienced one miscarriage. Blood samples for in vitro experiments were collected from 30 normal pregnant women. This study was performed between January 2017 and March 2019.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In this study, we investigated the kinetics of Tim-3+ CD4+ T-cell accumulation, and the proportions of Tim-3+ Treg cells throughout murine pregnancies using flow cytometry. We compared Tim-3 expression on decidual CD4+ T cells and Treg cells during normal pregnancies with expression on the same cell populations in women suffering from RPL. IL-27 and Gal-9 transcription and protein expression in the placenta were determined by RT-PCR and western blot, respectively. An in vitro co-culture model consisting of peripheral CD4+ T cells and primary trophoblasts from early pregnancy was used to mimic the maternal–fetal environment.
MAIN RESULTS AND THE ROLE OF CHANCE
The percentage of Tim-3+ Treg cells present in mouse uteri fluctuates as gestation proceeds but does not change in the spleen. Levels of Tim3+ Treg cells in uteri peaked at pregnancy Day 6.5 (E 6.5), then progressively diminished, and fell to non-pregnant levels by E18.5. In pregnant mice, Tim-3+ Treg cells constituted 40–70% of Treg cells in uteri but were present at much lower abundance in spleens. About 60% of decidual Treg cells were Tim-3 positive at E6.5. Of these decidual Tim3+ Treg cells, nearly 90% were PD-1 positive. However, only about 16% of Tim3− Treg cells expressed PD-1. Blocking the Tim-3 signaling pathway decreased the proportion of Treg cells and led to embryo resorption. Moreover, much lower Tim-3 expression was observed on CD4+ T cells and Treg cells in women who had suffered from RPL at 6–9 gestational weeks compared with those who had normal pregnancies at matched gestations. In a normal pregnancy, Tim-3 expression on decidual CD4+ T cells is induced initially by IL-27. Then Gal-9-Tim-3 interaction promotes differentiation of decidual Tim-3+ CD4+ T cells into Treg cells. IL-27 and Gal-9 cooperatively induced Tim-3+ Treg cells in vitro.
LARGE SCALE DATA
N/A
LIMITATIONS, REASONS FOR CAUTION
We did not investigate the kinetics of human decidual Tim-3+ CD4+ T and Tim-3+ Treg cell populations throughout pregnancy due to limited availability of second and third trimester decidua. In addition, functional suppressive data on the decidual Tim-3+ Treg cells are lacking due to limited and low quantities of these cells in decidua.
WIDER IMPLICATIONS OF THE FINDINGS
These findings might have therapeutic clinical implications in RPL.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by research grants from the National Natural Science Foundation of China (No. 81871186) and National Key Research & Developmental Program of China (2018YFC1003900, 2018YFC1003904). The authors declare no conflict of interest.
Keywords: Tim-3, Treg cells, interleukin 27, galectin-9, PD-1, early pregnancy, recurrent pregnancy loss, trophoblasts, decidua, maternal–fetal interface
Introduction
Establishment of successful pregnancy in both mice and humans requires adequate maternal–fetal immune tolerance. Complex immune regulatory mechanisms at the maternal–fetal interface are activated to keep the mother’s immune system from attacking the fetus. If these mechanisms fail, immune responses are activated, and adverse pregnancy outcomes ensue (PrabhuDas et al., 2015). Regulatory T (Treg) cells are identified as CD4+ T cells expressing the transcription factor forkhead box P3 (FOXP3) (Shevach, 2009). Considerable evidences indicated that Treg cells play a key role to induce immune tolerance and ensure fetal survival within the maternal uterus (Schumacher and Zenclussen, 2014). Depletion of Treg cells in mouse models results in pregnancy loss. In humans, pregnancy complications like recurrent pregnancy loss (RPL) and preeclampsia (PE) are associated with a reduced Treg number and activity (Saito et al., 2010).
T-cell immunoglobulin domain and mucin domain (Tim)-3 was originally defined as a T helper (Th) 1-specific membrane protein that could downregulate Th1 responses by transducing apoptotic signaling via Galectin-9 (Gal-9) activation (Zhu et al., 2005). In an autoimmune disease mouse model of multiple sclerosis, blocking the Tim-3 signaling pathway with specific monoclonal antibodies can lead to an aggravated autoimmune response due to loss of tolerance, which indicates that Tim-3 plays an inhibiting regulatory role (Sánchez-Fueyo et al., 2003). Nowadays, several studies have reported that Tim-3 could be also expressed on Treg cells, natural killer cells (NK cells), monocyte-macrophages and dendritic cells, and regulate their activities (Gupta et al., 2012; Ndhlovu et al., 2012; Han et al., 2013). If Tim-3 expression is dysregulated, it could aggravate or inhibit the inflammatory response and eventually lead to autoimmune diseases, escape of viruses or tumors and pregnancy complications (Das et al., 2017; Hu et al., 2016).
Tim-3 expressed on Treg cells has been shown to enhance the regulatory function of Foxp3+ Treg cells. Indeed, in an in vitro assay, Tim-3+ Treg cells have been demonstrated to have higher proliferative activity and better suppression effects on Teff cell proliferation than Tim-3− Treg cells (Gautron et al., 2014). Tim-3+ Treg cells represent 40% of all graft-infiltrating Treg cells and play a critical role in the maintenance of tolerance to allografts. These graft-infiltrating Tim-3+ Treg cells highly express functional molecules of Treg cells (e.g. CD25, CD39, CD73, CTLA-4, IL-10 and transforming growth factor-β (TGF-β)) and display powerful effector function (Gupta et al., 2012). In tumors, Tim-3+ Treg cells represent the majority of intratumoral Treg cells and are associated with poor cancer progression (Gao et al., 2012; Sakuishi et al., 2013; Yan et al., 2013). The establishment of maternal–fetal immune tolerance is related to the regulatory role of Treg cells during pregnancy. Indeed, a prior study reported that altered number and function of Treg cells are associated with adverse pregnancy outcomes, notably PE and RPL (Saito et al., 2010). More efficient in suppressing Teff cells than Tim-3− Treg cells, Tim-3+ Treg cells might play an important role in promoting fetal tolerance during pregnancy. However, up to now, there are no reports to characterize Tim-3+ Treg cells at the maternal–fetal interface.
In this study, we focus on the characterization of Tim-3+ Treg cells at the maternal–fetal interface and investigate the factors that promote the accumulation of Tim-3+ Treg cells at the maternal–fetal interface and the underlying mechanism.
Materials and methods
Mice
Female C57 mice (12W) and male Balb/c mice (12W) were purchased from the Animal Centre of Tongji Medical College and Center for Disease Control in Hubei province (Wuhan, China). Experimental procedures and animal care protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Virgin C57BL/6 female mice were used as non-pregnant controls, and allogeneically mated (C57BL/6 female × Balb/c male) female mice were used as models of normal pregnancies in our studies (Thuere et al., 2007). All animals were kept in a barrier facility with a 12-h light/12-h dark cycle and were raised in a humidity-controlled room with free access to food and water. Mice were inspected for vaginal plugs every morning. The day of visualization of a plug was designated as Day 0.5 of a pregnancy (Shima et al., 2010).
Blockade of IL-27 in vivo.
Pregnant mice were intraperitoneally injected with 200 µg doses of the anti-IL-27 antibody (clone MM27-7B1, Invitrogen, USA) or PBS on Days 2.5 and 4.5, respectively (Mas et al., 2008). All pregnant mice were monitored at Day 6.5 of pregnancy. The embryo resorption rate, architecture of uterine, phenotype change of decidual immune cells and the size of spleen were evaluated.
Blockade of Tim-3 in vivo.
Pregnant mice were intraperitoneally injected with anti-Tim-3 antibody (clone RMT3-23, Biolegend, USA) or PBS at doses of 250 or 500 µg twice/day on Days 3.5, 5.5, 6.5 and 8.5, respectively (Chabtini et al., 2013). All pregnant mice were monitored at Day 10.5 of pregnancy. The embryo resorption rate, architecture of uterine and placenta, the size of fetus, phenotype change of decidual immune cells and the size of spleen were evaluated.
Preparation of cells from mouse lymphoid organs and decidua
Mice were killed by cervical dislocation and spleens and lymph nodes (LNs) were harvested with sterile instruments into ice-cold PBS. The spleens and LNs were ground up and passed through a 40-μm cell strainer to prepare single-cell suspensions, cells were pelleted by centrifugation (300×g, 10 min, 4°C), and splenic erythrocytes were lysed in red blood cell lysis buffer for 10 min at 4°C. For isolation of decidua leukocytes, the fetal and placental tissues were carefully removed from the uteri of mice on particular gestational days and washed in ice-cold PBS. The uteri were minced with a scalpel, and incubated in RPMI medium 1640 containing 1 mg/ml collagenase IV (Gibco, USA) and 0.1 mg/ml DNaseI (Sigma, USA) for 15 min at 37°C with gentle agitation. The total suspension was filtered through 40 μm cell strainers before staining for flow cytometry analysis.
Human sample collection
Our collection and use of samples were approved by the Huazhong University of Science and Technology Clinical Trial Ethics Committee. Every participant signed a written informed consent form. Peripheral blood samples were obtained from clinically normal pregnant mothers during the first trimester. First-trimester placentae were obtained from voluntary pregnancy terminations (terminated for non-medical reasons at 6–9 gestational weeks), first time spontaneous abortion (diagnosed as the loss of just one pregnancy at 6–9 gestational weeks) and RPL (diagnosed as more than two spontaneous abortions at 6–9 gestational weeks, excluding those resulting from endocrine, anatomic or genetic abnormalities, infection, etc.) (Lee et al., 2012; Wu et al., 2014). The first time spontaneous abortion and RPL we collected are missed abortion. In our practice, both patients with the elective abortion in normal pregnant group and patients with RPL and first time abortion are routinely offered dilatation and curettage. Third-trimester placentae (37–41 weeks of gestation) were obtained from natural deliveries. Second-trimester placentae (between 16 and 27 weeks of gestation) were obtained from pregnant women who underwent early labor due to cervical incompetence (Zhang et al., 2018). After collection from the hospital, all the tissues used for polymerase chain reaction (PCR) and western blotting (WB) analysis were washed by cold PBS and then cryopreserved in −80°C refrigerator. All sample information and applications are presented in Supplementary Tables SI, SII and SIII.
CD4+ T-cell isolation and culture
Peripheral blood was obtained from clinically normal pregnant women with gestational age of 6–9 weeks. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Paque PLUS gradient centrifugation. Total CD4+ T cells were purified from PBMCs by positive selection using CD4+ T-cell isolation reagent from Miltenyi Biotec (Miltenyi Biotec, USA). Enriched CD4+ T cells were stimulated with 2 μg/ml plate-bound anti-CD3 (eBioscience, USA), 1 μg/ml soluble anti-CD28 (eBioscience, USA) and 25 U/ml IL-2 (Peprotech, USA). CD4+ T cells were seeded at 5 × 106 ml/well, and cultured for 1, 3 or 5 days in 24-well round bottom plates in RPMI medium (Gibco, USA).
Co-culture of primary trophoblast cells and CD4+ T cells
Purified CD4+ T cells from human early pregnancy peripheral blood were stimulated with 2 μg/ml plate-bound anti-CD3 antibody (eBioscience, USA) and 1 μg/ml soluble anti-CD28 antibody (eBioscience, USA) for 48 h. The stimulated CD4+ T cells were washed twice using PBS and then added into the culture plates of primary trophoblast cells, which were isolated and cultured as previously described (Hu et al., 2019). In some wells, soluble IL-27 receptor (R&D system, USA), anti-human Tim-3 antibody (Biolegend, USA) and recommend human Gal-9 (Biolegend, USA) were added. After co-culture, the cells were harvested for flow cytometry analysis.
In vitro induction assay
CD4+ T cells sorted from early pregnancy peripheral blood were activated with 2 μg/ml plate-bound anti-CD3 (eBioscience, USA) and 1 μg/ml soluble anti-CD28 (eBioscience, USA) for 2 days and were rested afterwards in the presence of recombination human IL-27 (R&D system, USA) and/or recombination human Gal-9 (Biolegend, USA) or TGF-β (Peprotech, USA) for 7 days.
Flow cytometry analysis
The cell surface of isolated trophoblasts or immune cells was stained with an appropriate fluorescently labeled conjugated mAb (shown in Supplementary Table SIV) for 20 min, away from light. Cells were washed three times with cold phosphate-buffered saline (PBS). Then, cells were fixed and labeled according to the manufacturer’s protocol for intracellular staining. Flow cytometry was performed using a FACScalibur (BD Biosciences, USA). Data were analyzed with FlowJo software (TreeStar, USA).
Quantification of mRNA expression levels by RT-PCR
Total RNA of frozen tissues from normal pregnancies and RPL was isolated by TRIzol extraction (Invitrogen, USA). One microgram of RNA was reverse transcribed using Prime Script RT master mix (Takara Bio, Japan). Relative mRNA levels of IL-27p28, Gal-9 and β-actin were determined by quantitative RT-PCR (qRT-PCR) using SYBR Premix Ex TaqII (Takara Bio, Japan). The primers used for PCR analysis are shown in Supplementary Table SV.
Enzyme-linked immunosorbent assay
Levels of IL-27 and Gal-9 in culture supernatants of primary trophoblasts were quantified using IL-27 and Gal-9 ELISA Kits (USCN Life Science, China), according to the manufacturer’s protocols.
Western blot analysis
Following SDS/PAGE, transfer and blocking, the polyvinylidene difluoride blots were incubated with primary antibodies (shown in Supplementary Table SVI) at 4°C overnight. The membranes were washed and incubated with a secondary antibody (shown in Supplementary Table SVI), followed by enhanced chemiluminescence detection.
Immunohistochemistry
Tissues and cell samples were treated as follows: (i) placental tissue was fixed in 4% paraformaldehyde (w/v), embedded in paraffin and sectioned at a thickness of 5 μm. Sections were deparaffinized in xylene, and rehydrated in graded alcohol solutions. After antigen retrieval, slides were treated with 3% hydrogen peroxide (v/v) for 15 min to suppress endogenous peroxidase activity and blocked with 5% bovine serum albumin (w/v) (Thermo Fisher Scientific, MA, USA) for 30 min, as previously described. (ii) Cultured trophoblasts that had climbed to the carry sheet glass were fixed in methanol at −80°C for 7 min. Treated samples were then incubated with primary antibodies, followed by incubation with a secondary antibody (shown in Supplementary Table SVII).
Statistics
Data are presented as means ± SEM or median (quartiles). Differences were analyzed using Student’s t-test or Mann–Whitney U-test between two groups as applicable, and by one-way ANOVA in multiple groups. All analyses were conducted in Statistical Package for Social Sciences (SPSS) software (Version 18) and GraphPad Prism software (Version 6). Differences were considered significant at P < 0.05.
Results
Tim3+ Treg cells accumulated in the decidua of mice during early pregnancy
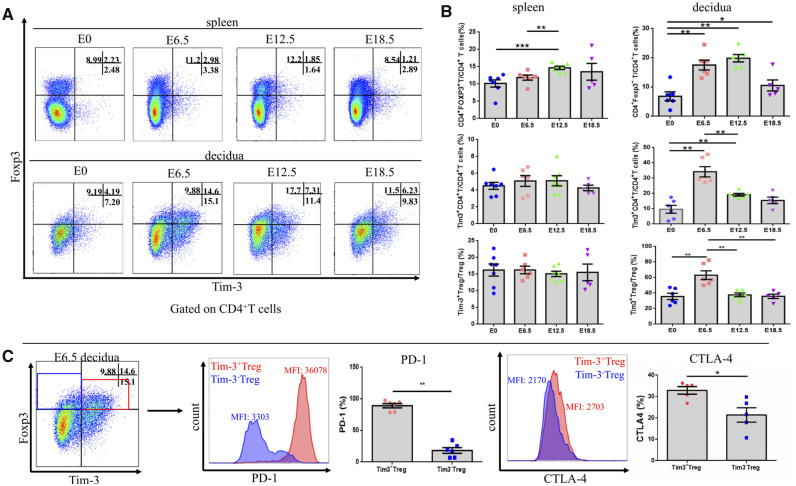
Tim-3+ Treg cells accumulated abundantly in mouse decidua during early stage pregnancy. Tim-3+ Treg cells constituted about 60% of the total Treg cell population in the mouse decidua at E6.5. However, at this time point, only about 16% of spleen Treg cells expressed Tim-3. These results indicate that accumulation of abundant Tim3+ Treg cells in decidua, but not in the periphery, might play a crucial role in establishing early pregnancies in mice. Moreover, the frequency of Tim-3+ Treg cells present in the decidua was subject to fluctuations as gestation proceeded. Tim-3+ Treg cells in the decidua peaked at E6.5, then progressively diminished, and fell to non-pregnant levels by E18.5. In the spleen, Tim-3+ Treg cells were present at very low levels and did not change regardless of stage of pregnancy (Fig. 1A and B).
Figure 1.
Tim3+ Treg cells accumulated in the decidua of mice during early pregnancy. Tim-3 expression on CD4+ T cells and Treg cells in spleen and decidua at time points throughout pregnancy was determined by flow cytometry (n ≥ 6 mice/group). (A) Representative Tim-3 expression on spleen and decidual Treg cells gated on CD4+ T cells. (B) Statistical analysis of Treg cell, Tim-3-expressing CD4+ T cell and Tim-3-expressing Treg cell proportions in the spleen and decidua throughout pregnancy. (C) The frequency and mean fluorescence intensity (MFI) of PD-1 and CTLA-4 expression were assessed and compared between Tim-3+ Treg cells and Tim-3– Treg cells from the decidua on E 6.5. Data are represented as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. E, embryonic day; detection of vaginal plug = E 0.5.
Treg cells play a key role in the establishment and maintenance of maternal-fetal tolerance. The immunosuppressive function of Treg cells seems to be executed mostly in a contact-dependent fashion, and mediated by expression of PD-1, CTLA-4, IL-10 and TGF-β. Here, we surprisingly found that nearly 90% of Tim-3+ Treg cells were PD-1 positive. However, only 18% of Tim-3− Treg cells expressed PD-1. Moreover, the expression of CTLA-4 on Tim-3+ Treg cells was 2-fold higher than that on the Tim-3− Treg cells (Fig. 1C). The observation that a large amount of Treg cell functional molecules were expressed on Tim-3+ Treg cells may indicate that Tim-3+ Treg cells are excellent immune suppressors.
We also compared the proportion of Tim3+ Treg cells in the spleen, uterus-draining LNs and decidua during early pregnancy. Although Tim-3+ Treg cells were present at high proportions only in the decidua, the proportion of Tim-3+ Treg cells in uterine draining LNs was significantly higher than that in the spleen (Supplementary Fig. S1A and B). Moreover, in uterine-draining LNs, compared to Tim-3−CD4+ T and Tim-3− Treg cells, more CCR4 and CCR5 were expressed on Tim-3+ CD4+ T and Tim-3+ Treg cells (Supplementary Fig. S1C and D).
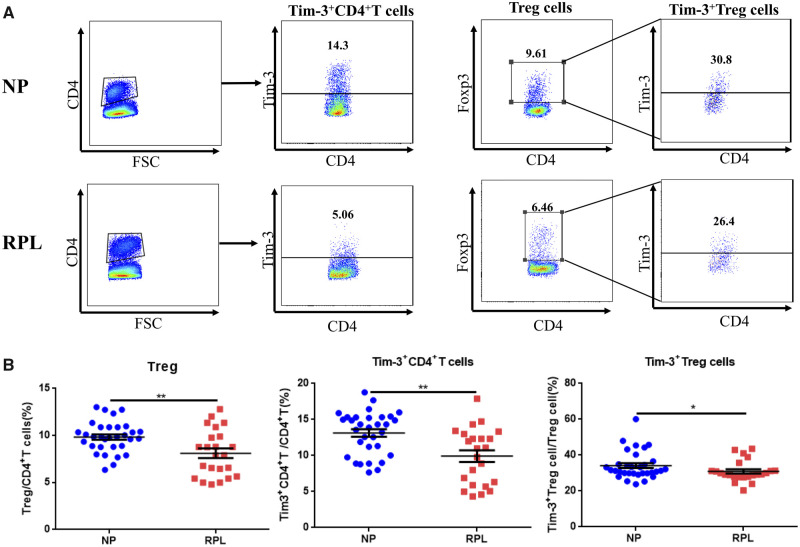
Tim3+ Treg cells constituted the predominant Treg cell population in human decidua during early pregnancy and were decreased in RPL patients
Our results showed that Tim-3 was not expressed at all on the surface of CD4+ T cells from human peripheral blood during early pregnancy (Supplementary Fig. S2A) unless the CD4+ T cells are activated by TCR stimulation in vitro (Supplementary Fig. S2B–F). However, Tim-3 was most expressed on Foxp3− CD4+ T cells after TCR activation. In stark contrast, Tim-3+ Treg cells clearly constitute the predominant Treg cell population in the decidua in a normal early pregnancy. We also assessed whether the proportion of Tim-3+ Treg cells was abnormal in women suffering from RPL. Here, we found that the percentage of decidual Treg cells was decreased in RPL. Moreover, we observed significantly lower Tim-3 expression on CD4+ T cells and Treg cells in RPL compared with those from normal pregnancies (Fig. 2A and B). These data indicated that decreased Tim-3+ CD4+ T cells and Tim-3+ Treg cells might be associated with impaired immunologic tolerance in RPL patients.
Figure 2.
Percentages of Treg cells, Tim-3+ CD4+ T cells and Tim-3+ Treg cells are reduced in decidua from RPL patients. (A) Representative Tim-3 expression on decidual CD4+ T cells and decidual Treg cells from normal pregnant (NP) subjects (n = 32) and patients who have undergone RPL (n = 23). (B) Statistical analysis of proportions of Treg cells, Tim-3-expressing CD4+ T cells and Tim-3-expressing Treg cells. Data are represented as means ± SEM. *P < 0.05, **P < 0.01.
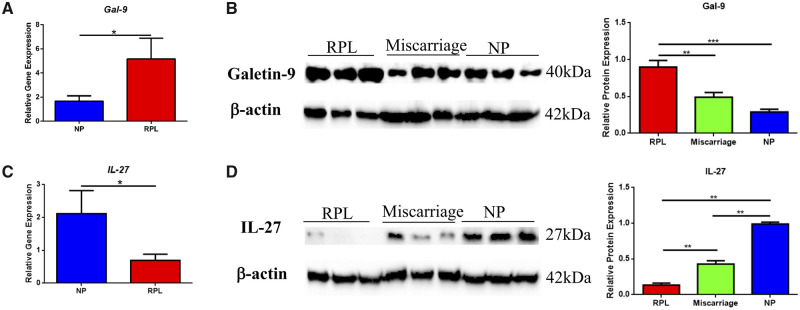
Accumulation of Tim-3+ Treg cells was associated with IL-27 and Gal-9 secreted by trophoblasts
Next, we wanted to know how Tim-3+ Treg cells were accumulating in the decidua. Previous studies have indicated that Tim-3-Gal-9 engagement promotes augmentation of Treg cell populations (Ji et al., 2013). In this study, we found that Gal-9 mRNA and protein expression in RPL patients at 6–9 gestational weeks was significantly higher than that in normal pregnant women (Fig. 3A and B), while the proportion of Treg cells was much lower in PRL patients. Despite obvious Gal-9 upregulation in RPL patients, immune tolerance is still impaired. Insufficient Tim-3 expression and Tim-3-Gal-9 engagement might contribute to this impaired immune tolerance. As IL-27 has been observed to be a potent inducer of Tim-3 in naïve CD4+ T cells in vitro (Zhu et al., 2015), we assessed IL-27 expression in first trimester placentae (between 6 and 9 gestational weeks) from normal pregnancies and RPL patients. As shown in Fig. 3C and D, levels of IL-27 mRNA and protein were much lower in RPL patients than in normal pregnant women. We also investigated the kinetics of IL-27 and Gal-9 protein expression in the placenta at different stages of pregnancy and found that Gal-9 protein expression increased gradually from early pregnancy to late pregnancy, whereas IL-27 protein levels remained stable throughout (Supplementary Fig. S3).
Figure 3.
Abnormal IL-27 and Gal-9 expression in recurrent pregnancy loss (RPL) patients. (A, C) Expression levels of Gal-9 and IL-27 mRNA in placental villi from early pregnancy in patients who have suffered RPL (n = 16) and patients carrying normal pregnancies (NP) (n = 28). (B, D) Protein levels of Gal-9 (40kDa) and IL-27 (27kDa) in early pregnancy villi from RPL (>2 unexplained spontaneous abortions), miscarriage (a single unexplained spontaneous abortion) and normal pregnancy were determined by western blotting normalized to levels of β-actin (42 kDa). Relative band intensities were quantified using ImageJ. Results are shown as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
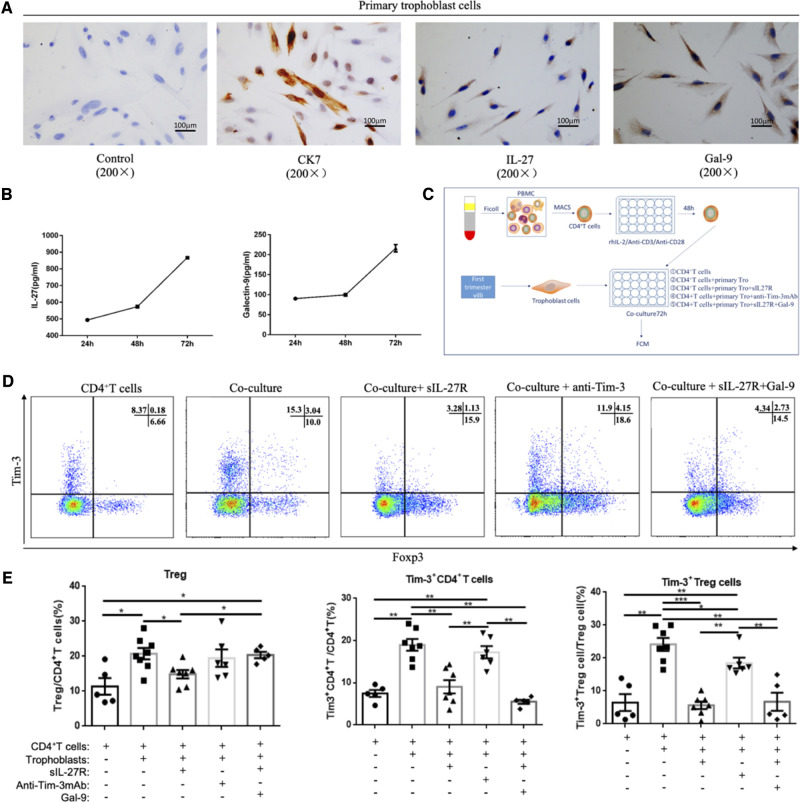
Then, we used an in vitro model containing peripheral blood CD4+ T cells and primary trophoblasts to mimic the maternal–fetal microenvironment to further explore the mechanisms mediating upregulation of Tim-3 expression on decidual CD4+ T cells and Treg cells during early pregnancy. Flow cytometry analyses revealed that primary trophoblasts could significantly promote Tim-3 expression on both CD4+ Foxp3− T cells and CD4+ Foxp3+ Treg cells (Supplementary Fig. S4F–H). Moreover, the proportion of Tim-3+ Treg cells increased gradually with increasing co-cultivation times, with ∼25% of Treg cells expressing Tim-3 on Day 5 (Supplementary Fig. S4I and J).
To determine whether primary trophoblast-mediated induction of Tim-3+ CD4+ T cells and Treg cells in the co-culture system was linked to IL-27 and Gal-9, we first examined their expression in isolated primary trophoblasts by immunohistochemistry. As Fig. 4A shows, IL-27 and Gal-9 were mainly expressed in the primary trophoblasts’ cytoplasm. To confirm that primary trophoblasts produce IL-27 and Gal-9, we measured by ELISA the amount of IL-27 and Gal-9 present in the culture supernatants of primary trophoblasts harvested at various culture times (Fig. 4B). The schema depicting our co-culture system is shown in Fig. 4C. Soluble IL-27 receptor (sIL-27R) or anti-Tim-3 mAb was added into the co-culture system to block IL-27 or Tim-3 signaling pathways. Here, we observed that co-culturing with primary trophoblasts could promote Tim-3 expression, while blockade of IL-27 by sIL27R in the co-culture system significantly inhibited trophoblast-induced Tim-3 upregulation on CD4+ T cells and Treg cells. Moreover, a slight decrease in the Treg cell fraction, albeit not statistically significant, was observed in the presence of sIL-27R in the co-culture system. The proportion of Tim-3+ Treg cells was decreased by blockade of the Tim-3 signaling pathway. However, blockade of Tim-3 signaling by anti-Tim-3mAb did not affect the proportion of Tim-3+ CD4+ T cells and Foxp3+ CD4+ Treg cells. In the co-culture + sIL-27R + Gal-9 group, Tim-3+ Treg cell proportions were not affected and remained at a low level despite sufficient Gal-9 for signaling (Fig. 4D and E). These data indicated that IL-27 was essential to Tim-3 expression induced by co-culture with primary trophoblasts, and that Tim-3 pathway activation might promote Tim-3+ Treg cells accumulation in the decidua.
Figure 4.
Accumulation of Tim-3+ Treg cells is associated with IL-27 and Gal-9 secretion by trophoblasts. (A) IL-27 and Gal-9 expression in primary trophoblast cells from early pregnancy villi were determined by immunohistochemistry. Original magnification: ×200. (B) Levels of IL-27 and Gal-9 secretion by primary trophoblasts were analyzed by ELISA. (C–F) Primary trophoblast cells were co-cultured with CD4+ T cells from early pregnancy peripheral blood for 72 h, while soluble IL-27 receptor (neutralizing IL-27) (100 ng/ml) or anti-Tim-3 mAb (blocking the Tim-3 signaling pathway) (10 µg/ml) or Gal-9 (activating the Tim-3 signal pathway) (1 µg/ml) were added to the co-culture system. Then, Treg cell differentiation and Tim-3 expression on CD4+ T cells and Treg cells were detected by flow cytometry. Before co-culture, CD4+ T cells were activated with 2 μg/ml plate-bound anti-CD3, 1 μg/ml soluble anti-CD28, and 25 U/ml IL-2 for 2 days. A cell culture treatment schematic is shown in C. Representative and statistical results are shown in D and E. CD4+ T cells group: CD4+ T cells alone; Co-culture group: CD4+ T cells co-cultured with primary trophoblast cells; Co-culture + sIL-27R group: CD4+ T cells co-cultured with primary trophoblasts with sIL-27R; Co-culture + anti-Tim-3 group: CD4+ T cells co-cultured with primary trophoblasts with anti-Tim-3mAb; Co-culture + anti-Tim-3mAb + Gal-9 group: CD4+ T cells co-cultured with primary trophoblast cells with anti-Tim-3mAb and Gal-9. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
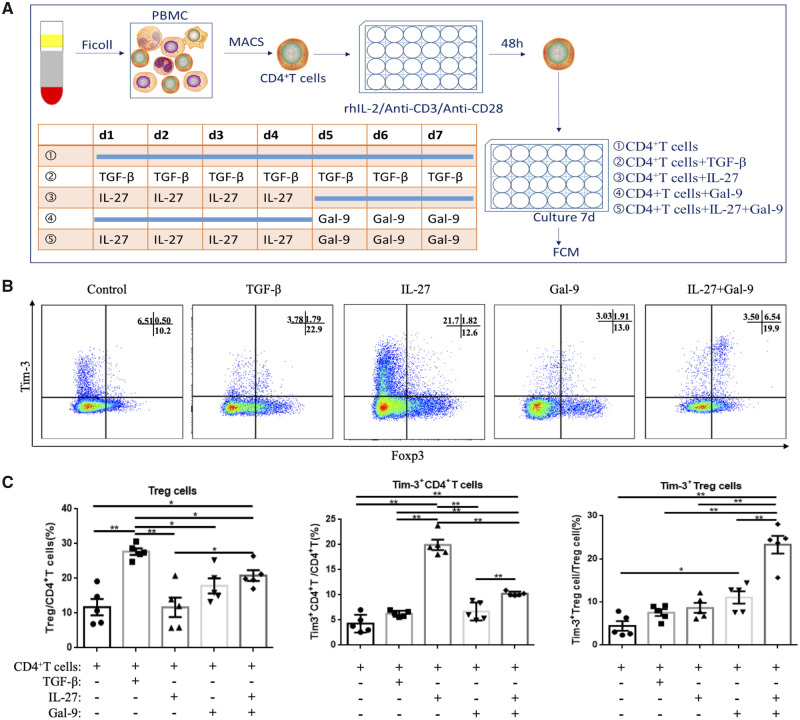
Tim-3+ Treg cells can be cooperatively induced by IL-27 and Gal-9 in vitro
The observation that blockade of either IL-27 or Tim-3 signaling pathways in the co-culture system could decrease the proportion of Tim-3+ Treg cells brought to light an obvious problem: How do Tim-3+ Treg cells accumulate in the decidua? To further confirm the effects of the IL-27 and Tim-3 pathways on Tim-3 expression and Tim-3+ Treg cell accumulation, we cultured isolated human early pregnancy peripheral CD4+ T cells in the presence of recombinant human IL-27 or recombinant human Gal-9. The rhGal-9 was used to activate Tim-3 signaling in this study. The schema depicting this cell culture strategy is shown in Fig. 5A. As Fig. 5B and C show, IL-27-treated CD4+ T cells exhibited high Tim-3 expression. However, most of the Tim-3 protein induced by IL-27 was on Foxp3− T cells. Among these cells, the proportion of Tim-3+ Treg cells was very low. TGF-β was used as a positive control to induce Treg cells differentiation (Chang et al., 2017). Compared to the control group, a slight upregulation of Tim-3 expression was observed in both the TGF-β and Gal-9 groups, albeit neither was statistically significant. Compared to other groups, the percentage of Treg cells and Tim-3+ Treg cells was increased remarkably in the IL-27 + Gal-9 group. Tim-3 could be induced by IL-27 within the first 4 days. Within the following 3 days, Gal-9 activation of the Tim-3 pathway promoted differentiation of Tim-3+ CD4+ T cells into Treg cells. It should be noted that total Tim-3 expression on CD4+ T cells was decreased in the IL-27 + Gal-9 group compared to the IL-27 group, and most Tim-3+ CD4+ T cells also expressed Foxp3.
Figure 5.
IL-27 and Gal-9 cooperatively promoted Tim-3+ Treg cell accumulation in vitro. CD4+ T cells sorted from early pregnancy peripheral blood were activated with plate-bound anti-CD3 (2 μg/ml) and soluble anti-CD28 (1 μg/ml) for 2 days and were rested afterwards in the presence of IL-27 (100 ng/ml) and/or Gal-9 (1 µg/ml) for 7 days. The cell culture treatment schematic is shown in A. CD4+ T cells group: CD4+ T cells alone; IL-27 group: CD4+ T cells treated with rhIL-27 in the first 4 days; Gal-9 group: CD4+ T cells treated with Gal-9 in the last 3 days; IL-27 + Gal-9 group: CD4+ T cells treated with rhIL-27 in the first 4 days, then treated with Gal-9 for 3 days. Representative and statistical results are shown in B and C. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
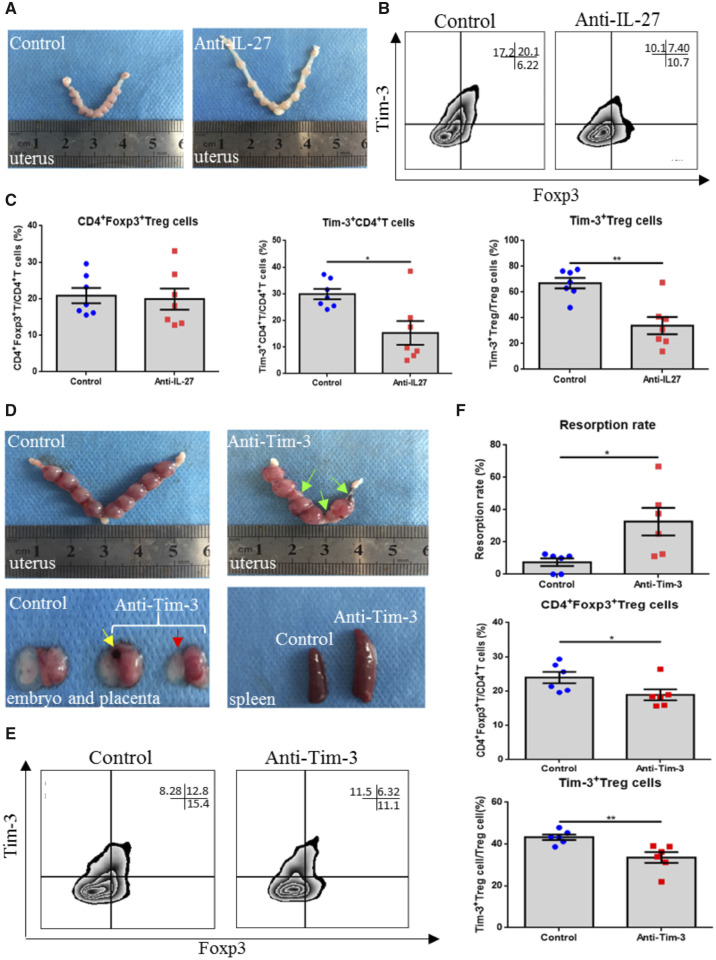
Blocking IL-27 and Tim-3 signaling pathways disrupts immune tolerance and induces pregnancy loss in mice
To verify the role of the IL-27 and Tim-3 signal pathways in vivo, we examined pregnant C57/B6 female mice challenged with an anti-IL-27 mAb or an anti-Tim-3 mAb. In the IL-27 blockade experiment, Tim-3 expression on CD4+ T cells and Treg cells in the decidua was decreased in the IL-27 blockade group. The percentage of Treg cells was not affected by IL-27 blockade (Fig. 6B and C). In addition, there seemed to be no change in the rate of embryo absorption. However, the pregnant mice’ uteri were abnormally stretched, and compared to the control group, the distance between embryos was too large (Fig. 6A). At this time, it is not clear why IL-27 blockade downregulated Tim-3 expression but did not affect the rate of embryo absorption. However, direct blockade of the Tim-3 signaling pathway induced obvious embryo absorption. We found that compared to the control group, the sizes and weights of spleens in the Tim-3 blockade group were much bigger. Moreover, significant embryo absorption was observed in the Tim-3 blockade group, and, the size of the unabsorbed embryos in Tim-3 blockade mice was much smaller than in the control group (Fig. 6D). In addition, obvious bleeding spots could be found in placentae in the Tim-3 blockade group. The percentage of Treg cells and Tim-3+ Treg cells was decreased when the Tim-3 signaling pathway was blocked (Fig. 6E and F).
Figure 6.
Effects of blocking IL-27 or Tim-3 signaling on pregnancy outcome in a mouse model. (A) Representative images of embryos from mice treated with PBS or anti-IL-27 antibody. (B, C) Representative and statistical analysis of Treg cell proportions, Tim-3 expression on CD4+ T cells, and Treg cells following treatment with the anti-IL-27 antibody by flow cytometry. Data are represented as means ± SEM, with seven mice per group. *P < 0.05 and **P < 0.01. (D) Representative images of embryos, placentas and spleens from mice treated with PBS or anti-Tim-3 antibody. Compared with the control group, increased embryo resorption (indicated with the green arrow), placental bleeding (indicated with yellow arrow) and smaller fetus (indicated with red arrow) were observed in the Tim-3 blocking group. (E, F) Representative and statistical analysis of Treg cell proportions, and Tim-3 expression on Treg cells following treatment with the anti-Tim-3 antibody by flow cytometry. Data are represented as means ± SEM of six mice per group. *P < 0.05.
Discussion
Tim3+ Treg cells constitute the predominant Treg population (over 60%) in the mouse decidua during early pregnancy. In stark contrast, the percentage of Tim-3+ Treg cells in the peripheral lymphoid organ and draining LN is much lower. However, the percentage of Tim-3+ CD4+ T cells and Tim-3+ Treg cells in the draining LN is higher than that in the spleen. Previous studies have identified factors in male seminal plasma that are associated with increased paternal antigen-reactive Treg cells in draining LNs (Guerin et al., 2011; Shima et al., 2015). At E3.5, the absolute numbers of Treg cells in the draining LN are about 10-fold greater than those in the uterus. Therefore, draining LN seems to be a primary site of Treg cell production. The higher proportion of Tim-3+ CD4+ T cells and Tim-3+ Treg cells in draining LN suggests that Tim-3 expression might be induced by the paternal HLA or paternal antigens in seminal plasma.
Moreover, much more CCR4 and CCR5 are expressed on Tim-3+CD4+ T cells and Tim-3+ Treg cells in the draining LN. Radiolabeled cells from draining LN have reportedly been recruited into the uterus from the blood during early pregnancy (Johansson et al., 2004). The local expression of chemokines and the chemokines receptors on Treg cells surface is involved in the trafficking and migration of Treg cells into tissues (Curiel et al., 2004; Bono et al., 2007). In the post-mating period, the increased chemokines synthesis in the uterus is associated with the infiltration of a large number of leukocytes, including macrophages, DCs as well as T cells (Robertson et al., 1998). CCL4 (the CCR5 ligand) and CCL17 (the CCR4 ligand) are among these chemokines. They are largely expressed in mice and human placental decidua (Shima et al., 2015). Furthermore, it has been reported that CCR4 and CCR5 are selectively expressed on paternal antigen-specific Treg cells, and CCR4+ Treg cells and CCR5+ Treg cells preferentially accumulate in the gravid uterus (Kallikourdis et al., 2007). CCR4 and CCR5 expressed on Tim3+ CD4+ T cells and Tim-3+ Treg cells might help these cells home to and/or stay within the maternal–fetal interface.
Interestingly, nearly 90% of decidual Tim-3+ Treg cells were PD-1 positive. And the mean fluorescence intensity of PD-1 expression on Tim-3+ Treg cells is 10 times higher than that on Tim-3− Treg cells. Salvany-Celades et al. (Salvany-Celades et al., 2019) reported that the decidual PD-1hi Treg cells which suppress Teff responses by IL-10 is one important subtype of the decidual Treg cells in human pregnancy and the decidual PD-1hi Treg cells could be induced by trophoblast cells. Our observation that PD-1 expression goes hand in hand with Tim-3 on decidual Treg cells suggested that decidual Tim-3+ Treg cells might somewhat resemble PD-1hi Treg cells. The resemblance could be found in two aspects: (i) the proportion of PD-1hi Treg cells in decidua is also much more than that in blood. (ii) Moreover, both Tim-3+ Treg cells and PD-1hi Treg cells is largely expressed CCR5.
We also found that Tim-3+ Treg cells constitute the major decidual Treg population, and that they were specifically present in human decidua in early pregnancy, whereas Tim-3 is not expressed at all in peripheral CD4+ T cells and Treg cells. Tim-3 expression on Treg cells could be detectably induced by co-culturing CD4+ T cells with trophoblasts. During the placentation in humans, the trophoblasts as the most important fetal portion at the maternal–fetal interface have intimate contact to the maternal immune cells. These trophoblasts express lots of soluble and cell surface proteins to educate the maternal immune cells, thus ensure their own survival and that of the fetus (Riley, 2008; Arck and Hecher, 2013).
IL-27 is known to be expressed and secreted by placental cells (Coulomb-L'Hermine et al., 2007). Here, we have demonstrated that IL-27 secreted by trophoblasts could induce Tim-3 expression on CD4+ T cells from peripheral blood during early pregnancy. Subsequently, Gal-9, the Tim-3 ligand, promotes differentiation of Tim-3+CD4+ T cells into Tim-3+ Treg cells. Thus, proportions of Treg cells and Tim-3+ Treg cells are upregulated at the maternal–fetal interface. We also note that in the Tim-3+ Treg cell induction experiment, compared to other groups, in the IL-27 + Gal-9 group, Tim-3+ Treg cells could be detectably induced while total Tim-3 expression on CD4+ T cells was decreased. As Gal-9 could down-regulate CD4+ T-helper1 (Th1) and Th17 responses by triggering apoptotic death of Tim-3+ Teffs (Zhu et al., 2005), we speculate that Gal-9 induces apoptosis in mature differentiated T-cells via Tim-3 in Th1/Th17 cells, and might not, or might to a lesser extent induce apoptosis in Tim-3+ Treg cells. Thus, the proportion of Tim-3+ Treg cells, and total Treg cells could accumulate at the maternal–fetal interface.
Tim-3 expression could be induced during early stage pregnancy in the presence of IL-27. Then, gradually increasing Gal-9 levels activated the Tim-3 signaling pathway. Another study has previously reported the kinetics of IL-27 expression during murine pregnancies. They reported that in pregnant female mice, IL-27 mRNA is highly expressed at E0.5, and gradually decreases to undetectable levels by E6.5 (Mas et al., 2008). This published data is in good accordance with our model. A premature or delayed rise in IL-27 may thus be associated with pathological gestation.
Immune tolerance plays a very important role in maintenance of immune homeostasis. Once immune tolerance is broken, various autoimmune diseases ensue. Gal-9 has been reported to regulate T-cell responses through Tim-3-Gal-9 engagement. Moreover, the promising therapeutic activity of recombinant Gal-9 has been shown in preclinical models of several diseases, including transplant rejection and autoimmunity (Wiersma et al., 2013). The pathogenesis of RPL is somewhat similar to these mentioned diseases. The ultimate goal for treatment of broken immune tolerance diseases is to restore immunological tolerance. Treatment for RPL is no exception. Given that Gal-9 has therapeutic potential for treating autoimmunity by skewing T-cell immunity in preclinical models, using recombinant Gal-9 in RPL patients might be an effective therapeutic strategy for regulating maternal–fetal immune tolerance imbalance. However, here we reported that Gal-9 expression is not insufficient in RPL patients. A mechanism like that of insulin-resistance in type 2 diabetes might explain this phenomenon. Sensitivity to insulin is decreased in type 2 diabetes patients. Under normal conditions, pancreatic islet β-cells increase insulin release sufficiently to overcome the reduced insulin sensitivity, thereby maintaining normal glucose tolerance (Kahn, 1998). Due to ineffective or insufficient Tim-3-Gal-9 engagement, increased Gal-9 expression in RPL patients might be compensatory upregulation. However, even though there is sufficient Gal-9 ligand, Tim-3 receptor expression is decreased. Thus, the contribution of Tim-3/Gal-9 signaling to maternal–fetal immune tolerance is still impaired. Therefore, normal expression of receptor Tim-3 is prerequisite to activation of Tim-3/Gal-9 signaling using recombinant Gal-9.
In this study, there are some limitations. Although many previous studies have reported that Tim-3 expression identifies a subset of Treg cells with increased in vitro suppressor function and we found that decidual Tim-3+ Treg cells displayed some cell surface molecules potentially associated with suppressor function, our study lacks in vitro functional suppressive experiments of decidual Tim-3+ Treg cells. Since Tim-3 is an immune checkpoint receptor and overexpressed Tim-3 on the Teffs lead to the dysfunction or exhaustion of Teffs, it cannot be ruled out that Tim-3+ Treg cells here are just exhausted. Beyond that our study did not investigate the kinetics of human decidual Tim-3+ CD4+ T and Tim-3+ Treg cell populations throughout pregnancy due to limited availability of second and third trimester decidua. These need to be further investigated.
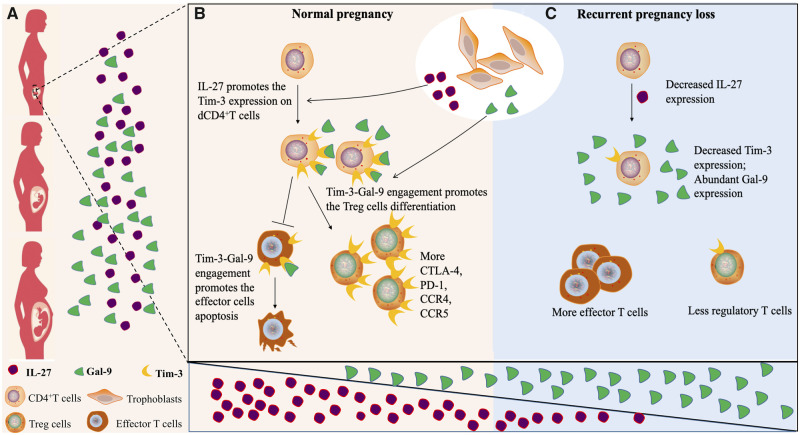
In summary, our study highlights that IL-27 and Gal-9 produced by trophoblasts cooperatively promote expression of Tim-3 and activation of the Tim-3 signaling pathway in CD4+ T cells, contributing to accumulation of Tim-3+ Treg cells and the Treg/Teff balance (Fig. 7). It is conceivable that the strategy of IL-27 and Gal-9 treatment of T cells to induce immunosuppressive Tim-3+ Treg cells might be used therapeutically to attenuate dysregulation of maternal–fetal immune tolerance in RPL, and more generally, in diseases in which the immune balance is perturbed, such as PE, tumors, autoimmunity and transplantation.
Figure 7.
Schematic diagram of IL-27 and Gal-9 promoting Tim-3+ Treg cell accumulation in the decidua. (A and B) IL-27 secreted by trophoblast cells induces Tim-3 expression on decidual CD4+ T cells, then Gal-9 activates the Tim-3 signal pathway promoting Treg cell differentiation. In the presence of Gal-9, effector T cells might be more fragile than Treg cells. In a normal pregnancy, Gal-9 expression gradually increases, while IL-27 expression remains stable from early pregnancy to late pregnancy. (C) In RPL, insufficient Tim-3-Gal-9 engagement due to decreased Tim-3 expression leads to reduced Treg cell proportions.
Authors’ roles
X.H. conceived and carried out experiments and drafted the manuscript. Q.Z. and Y.W. coordinated the sample collection. G.M. provided valuable suggestion about the design of experiment. L.W. and Z.L. helped to optimize our experiment methods. A.L. supervised the study and revised and finalized the manuscript. All authors reviewed the manuscript.
Funding
This study was supported by research grants from the National Natural Science Foundation of China (No. 81871186) and National Key Research & Developmental Program of China (2018YFC1003900, 2018YFC1003904).
Conflict of interest
The authors declare no conflict of interest.
Supplementary Material
References
- Arck PC, Hecher K.Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med 2013;99:548–556. [DOI] [PubMed] [Google Scholar]
- Bono MR, Elgueta R, Sauma D, Pino K, Osorio F, Michea P, Fierro A, Rosemblatt M.The essential role of chemokines in the selective regulation of lymphocyte homing. Cytokine Growth Factor Rev 2007;18:33–43. [DOI] [PubMed] [Google Scholar]
- Chabtini L, Mfarrej B, Mounayar M, Zhu B, Batal I, Dakle PJ, Smith BD, Boenisch O, Najafian N, Akiba H.TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J Immunol 2013;190:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, Wei CY, Zhou WJ, Zhu XY, Shao Jet al. IL-27 triggers IL-10 production in Th17 cells via a c-Maf/RORγt/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis 2017;8:e2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulomb-L'Hermine A, Larousserie F, Pflanz S, Bardel E, Kastelein R, Devergne O.Expression of interleukin-27 by human trophoblast cells. Placenta 2007;28:1133–1140. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow Met al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–949. [DOI] [PubMed] [Google Scholar]
- Das M, Zhu C, Kuchroo VK.Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev 2017;276:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B.TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One 2012;7:e30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron AS, Dominguez-Villar M, Marcken M, Hafler DA.Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. Eur J Immunol 2014;44:2703–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA.Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod 2011;85:397–408. [DOI] [PubMed] [Google Scholar]
- Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, Kuchroo VK, Strom TB.Allograft rejection is restrained by short-lived TIM-3+ PD-1+ Foxp3+ Tregs. J Clin Invest 2012;122:2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Chen G, Shen B, Li Y.Tim-3: an activation marker and activation limiter of innate immune cells. Front Immunol 2013;4:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XH, Tang MX, Mor G, Liao AH.Tim-3: Expression on immune cells and roles at the maternal-fetal interface. J Reprod Immunol 2016;118:92–99. [DOI] [PubMed] [Google Scholar]
- Hu XH, Wang Y, Mor G, Liao AH.Forkhead box P3 is selectively expressed in human trophoblasts and decreased in recurrent pregnancy loss. Placenta 2019;81:1–8. [DOI] [PubMed] [Google Scholar]
- Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M, Moorman JP, Yao ZQ.HCV-infected hepatocytes drive CD4+ CD25+ Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur J Immunol 2013;43:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Bromfield JJ,, Jasper MJ, Robertson SA.Semen activates the female immune response during early pregnancy in mice. Immunology 2004;112:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB.Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell 1998;92:593–596. [DOI] [PubMed] [Google Scholar]
- Kallikourdis M, Andersen KG, Welch KA, Betz AG.Alloantigen-enhanced accumulation of CCR5+ ‘effector’regulatory T cells in the gravid uterus. Proc Natl Acad Sci USA 2007;104:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J.Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol 2012;67:311–318. [DOI] [PubMed] [Google Scholar]
- Mas AE, Petitbarat M, Dubanchet S, Fay S, Ledée N, Chaouat G.Immune regulation at the interface during early steps of murine implantation: involvement of two new cytokines of the IL-12 family (IL-23 and IL-27) and of TWEAK. Am J Reprod Immunol 2008;59:323–338. [DOI] [PubMed] [Google Scholar]
- Ndhlovu LC, Lopez-Vergès S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL.Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012;119:3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM.Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 2015;16:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JK.Trophoblast immune receptors in maternal-fetal tolerance. Immunol Invest 2008;37:395–426. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Allanson M, Mau VJ.Molecular regulation of uterine leukocyte recruitment during early pregnancy in the mouse. Placenta 1998;19:101–119. [Google Scholar]
- Saito S, Nakashima A, Shima T, Ito M.Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 2010;63:601–610. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, Anderson AC.TIM3+ FOXP3+ regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2013;2:e23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvany-Celades M, Van der Zwan A, Benner M, Setrajcic-Dragos V, Bougleux G, Hannah A, Iyer V, Norwitz ER, Strominger JL, Tilburgs T.Three types of functional regulatory T cells control T cell responses at the human maternal-fetal interface. Cell Rep 2019;27:2537–2547. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK.Tim-3 inhibits T helper type 1-mediated auto-and alloimmune responses and promotes immunological tolerance. Nat Immunol 2003;4:1093–1101. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Zenclussen AC.Regulatory T cells: regulators of life. Am J Reprod Immunol 2014;72:158–170. [DOI] [PubMed] [Google Scholar]
- Shevach EM.Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009;30:636–645. [DOI] [PubMed] [Google Scholar]
- Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O, Saito S.Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J Reprod Immunol 2015;108:72–82. [DOI] [PubMed] [Google Scholar]
- Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S.Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol 2010;85:121–129. [DOI] [PubMed] [Google Scholar]
- Thuere C, Zenclussen ML, Schumacher A, Langwisch S, Schulte-Wrede U, Teles A, Paeschke S, Volk HD, Zenclussen AC.Kinetics of regulatory T cells during murine pregnancy. Am J Reprod Immunol 2007;58:514–523. [DOI] [PubMed] [Google Scholar]
- Wiersma VR, Bruyn M, Helfrich W, Bremer E.Therapeutic potential of Galectin-9 in human disease. Med Res Rev 2013;33:102–126. [DOI] [PubMed] [Google Scholar]
- Wu L, Luo LH, Zhang YX, Li Q, Xu B, Zhou GX, Luan HB, Liu YS.Alteration of Th17 and Treg cells in patients with unexplained recurrent spontaneous abortion before and after lymphocyte immunization therapy. Reprod Biol Endocrinol 2014;12:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L.Tim-3 expression defines regulatory T cells in human tumors. PLoS One 2013;8:e58006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Ma LN, Hu XH, Ji JL, Mor G, Liao AH.The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum Reprod 2018;34:25–36. [DOI] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK.The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005;6:1245–1252. [DOI] [PubMed] [Google Scholar]
- Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, Wang C, Tan DJ, Wu C, Rangachari M.An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun 2015;6:6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.