Abstract
Inflammatory bowel disease (IBD) is a chronic intestinal disease with painful clinical manifestations and high risks of cancerization. With no curative therapy for IBD at present, the development of effective therapeutics is highly advocated. Drug delivery systems have been extensively studied to transmit therapeutics to inflamed colon sites through the enhanced permeability and retention (EPR) effect caused by the inflammation. However, the drug still could not achieve effective concentration value that merely utilized on EPR effect and display better therapeutic efficacy in the inflamed region because of nontargeted drug release. Substantial researches have shown that some specific receptors and cell adhesion molecules highly expresses on the surface of colonic endothelial and/or immune cells when IBD occurs, ligand-modified drug delivery systems targeting such receptors and cell adhesion molecules can specifically deliver drug into inflamed sites and obtain great curative effects. This review introduces the overexpressed receptors and cell adhesion molecules in inflamed colon sites and retrospects the drug delivery systems functionalized by related ligands. Finally, challenges and future directions in this field are presented to advance the development of the receptor-mediated targeted drug delivery systems for the therapy of IBD.
KEY WORDS: Receptor-mediated target, Inflammatory bowel disease, Crohn's disease, Ulcerative colitis, Drug delivery, Cell adhesion molecule, Active target, Targeted therapy
Abbreviations: ACQ, aggregation-caused quenching; ADR, adverse drug reaction; AIE, aggregation-induced emission; BSA, bovine serum albumin; CAM, cell adhesion molecule; CD, Crohn's disease; CRD, cysteine-rich domain; CS, chondroitin sulfate; CT, computed tomography; CTLD, c-type lectin-like domain; DCs, dendritic cells; DSS, dextran sulfate sodium salt; EGF, epidermal growth factor; EPR, enhanced permeability and retention; FNII, fibronectin type II domain; FR, folate receptor; FRET, fluorescence resonance energy transfer; GIT, gastrointestinal tract; HA, hyaluronic acid; HUVEC, human umbilical vein endothelial cells; IBD, inflammatory bowel disease; ICAM, intercellular adhesion molecule; LMWC, low molecular weight chitosan; LPS, lipopolysaccharide; MAP4K4, mitogen-activated protein kinase kinase kinase kinase 4; MGL, macrophage galactose lectin; MPO, myeloperoxidase; MR, mannose receptor; MRI, magnetic resonance imaging; MPS, mononuclear phagocyte system; PAMAM, poly(amidoamine); PepT1, peptide transporter 1; PEI, polyethylenimine; PSGL-1, P-selectin glycoprotein ligand-1; QDs, quantum dots; RES, reticuloendothelial system; TfR, transferrin receptor; UC, ulcerative colitis; VCAM, vascular cell adhesion molecule
Graphical abstract
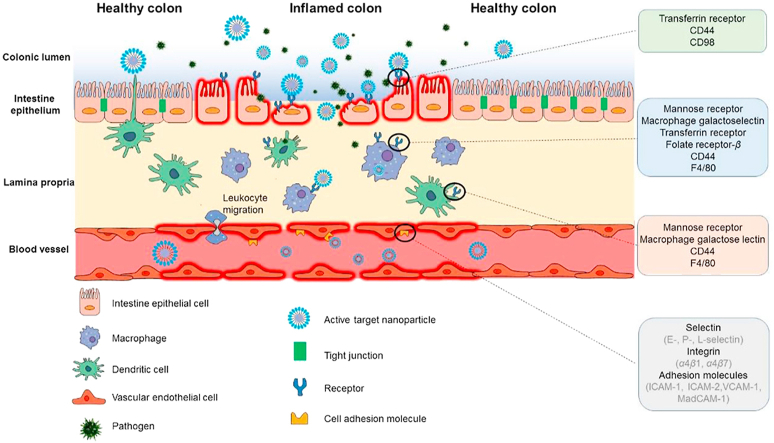
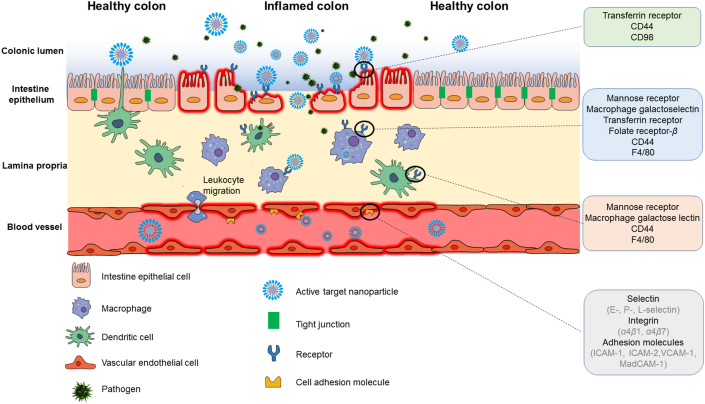
When inflammatory bowel disease occurs, some specific receptors and cell adhesion molecules, like mannose receptor, CD98, CD44 and ICAM-1, are overexpressed on the colonic epithelial cells and/or immune cells. Drug delivery systems superficially modified with related ligands can specifically bind to receptors on inflamed cells and deliver drug into target area.
1. Introduction
Inflammatory bowel disease (IBD), comprised of Crohn's disease (CD) and ulcerative colitis (UC), is characterized by chronic inflammation in the intestine1. Patients with IBD may suffer from abdominal pain, rectal bleeding, serious diarrhea, fever, weight loss, anemia and 2-fold higher risk of fatal colorectal cancer relative to healthy populations2,3. IBD possesses the highest prevalence with 0.3% in Western counties in 2017, and its incidence is increasing year by year worldwide4,5. To date, the exact etiology of IBD still remains unknown, however, it is well-accepted that environmental factors, genetic predeposition, gut microbiota, and immune function dysregulation participate in the occurrence and development of IBD6. Conventional medications, including antibiotics, aminosalicylates, corticosteroids, immunomodulators, and monoclonal antibodies, have been applied for achieving and prolonging clinical remission, reducing surgical intervention and improving the quality of life7. But prolonged and systemic administration of such therapeutics could lead to heavy economic burden and/or serious adverse drug reaction (ADR), like superinfection, anaphylaxis, myelosuppression, gastrointestinal reaction, and osteoporosis7.
One of the principal challenges in the therapeutic strategies of IBD is delivering therapeutics specifically to colonic inflammation regions. Conventional colon targeted drug delivery systems are generally designed to release drugs based on the traditional cognition about gastrointestinal pH, transit time, enzyme, and microbiome in the colon. However, the changes of gastrointestinal pH, transit time, enzyme and microbiome are unclear in the development of IBD, which results in aimless drug release, unsatisfactory therapeutic efficacy and enhanced ADR8. In recent years, novel nanocarriers, like liposome9, nanoparticle10, hydrogel11, dentrimer12, have received substantial attention and investigation due to their excellent physicochemical properties. The tissue permeability is increased in the inflamed colon, due to destroyed intestinal tight junction, the loss of cellular integrity and the infiltration of immune cells caused by inflammatory cytokines. Exploiting the leaky intestine, nanocarriers can passively deliver drugs to the inflamed colon sites based on enhanced permeability and retention (EPR) effect13,14. In addition, nanocarriers can also improve the solubility and stability of drugs. However, the therapeutics still could not achieve effective concentration in the inflamed region because of nontargeted drug release. Therefore, more strategies are needed to realize the targeted drug delivery in the inflamed colon sites.
When colonic inflammation occurs, some specific receptors and cell adhesion molecules, like mannose receptor, macrophage galactose lectin, transferrin receptor, folate receptor, CD98, CD44, PepT1 and F4/80, are overexpressed on the colonic epithelia and/or immune cells15,16. Inspired by the leukocyte-endothelial biochemistry, which mediates the recruitment of leukocytes to the site of inflammation, the ligand–receptor interaction has been widely applied into the design and development of inflammation-related drug delivery systems. Receptor-mediated targeted drug delivery systems exploit ligand-modified drug carriers as “missiles” to deliver drugs to the target area, because specific ligands are linked to the receptors on target cells so it avoids drug release in other parts of the body (Fig. 1). This review briefly introduces the overexpressed receptors and cell adhesion molecules in inflamed colon sites and retrospects the relevant ligand-modified delivery systems that have been applied to improve the therapeutic efficacy of IBD. Then, challenges and future directions in this field are presented to advance the development of the receptor-mediated targeted drug delivery systems for the therapy of IBD.
Figure 1.
Schematic illustration of receptor-mediated targeted drug delivery for treatment of IBD.
2. General considerations of IBD targeted therapy
Usually, UC is restricted to diffuse superficial mucosal inflammation in the colon, extending proximally from the rectum. Unlike UC, CD manifests segmental transmural inflammation almost in the entire gastrointestinal tract (GIT), commonly in colon and terminal ileum17. Something similar between two diseases is that mucosal integrity and tight junction are destroyed, substantial immune cells infiltrate, certain receptors and cell adhesion molecules overexpress when inflammation occurs18. Targeted therapy exploits physiological or pathological characteristics of IBD patients to deliver drug to inflamed colon sites. The consideration for IBD targeted therapy primarily focuses on the in vivo fate of drug delivery systems via different administration routes and targeting strategies.
Generally, the administration routes of therapeutics for IBD include systemic, oral and rectal delivery. Different administration routes present different requirements on preparation. Systemic administration possesses high bioavailability, but it puts rather strict requirements on drug carriers' preparation. During systemic circulation before targeting to inflamed colon, intravenously administrated drug carriers would encounter many physiological barriers in vivo19. Drug delivery systems undergo opsonin adsorption and subsequent uptake by mononuclear phagocyte and reticuloendothelial system, which leads to non-target accumulation in liver and spleen. Small drug delivery systems (<10 nm) may undergo renal clearance, while large ones around 110 nm are more easily absorbed into inflamed sites compared to those >180 or <50 nm, probably due to EPR limitation, mononuclear phagocyte system (MPS) and reticuloendothelial system's (RES) attack (>200 nm)20,21. Drug delivery systems may undergo other barriers such as cellular endocytosis, endosomal degradation and efflux pumps. Endocytosis mechanisms (e.g., pinocytosis, phagocytosis and receptor-mediated endocytosis) and intracellular fates can be affected by several factors such as shape, size and superficial modification, of which ligands modification could prompt receptor-mediated endocytosis of drug delivery systems. Enzymes and acidic environments in lysosome may degrade drugs loaded in nanoparticles, especially polypeptide and genetic drugs, efflux pumps may transport drug out of cells due to drug resistance19. Oral administration is the most acceptable option for IBD patients. The physiological barriers of oral dosage forms are extreme pH gradient (1.0–8.3), substantial digestive enzymes, variable transit time and gut bacterial enzymes22. It requires the drug carriers should be stable before they reach the inflamed colon sites. The protective coating with specific functional materials, such as pH resistant or sensitive, enzyme responsive molecules, could address this requirement. Chitosan, alginate and PLGA are commonly used in surface coating due to their stability in upper GIT23. Drug delivery systems that enter the systemic circulation via intestinal epithelial cells' absorption may encounter the barriers as intravenous delivery systems meet. Unabsorbed drug delivery systems will be excreted with the feces. Rectal administration can achieve high drug concentration topically and reduce the systemic circulation of drugs. From the perspective of anatomy, there is less intestinal liquid and absorption surface area in rectum and rectosigmoid, it requires high drug solubility and low viscosity of drug carrier via rectal administration24. In conclusion, drug delivery systems are required to bind to inflammatory cells specifically via different administration routes. To develop such inflammation specific delivery system, attention could be paid to the pathophysiological changes at inflamed sites during IBD.
Conventional pharmaceutical strategies utilize gastrointestinal pH gradients, transit time and gut bacterial enzyme to target inflamed colon, as shown in Table 125, 26, 27. pH-Dependent drug delivery systems exploit the highest pH values (7.2–8.3) of terminal ileum and colon to passively target colon. The common way is to coat the drug with biocompatible polymer which dissolves under alkaline environment, such as Eudragit28. The transit time of small intestine is 2–4 h29. Based on this, time-dependent delivery system is designed to control the drug release sites by coating immediate-release drug core with sustained-release materials, like ethylcellulose30. Substantial bacterial enzymes exist in terminal ileum and colon, like azoreductases, polysaccharidases, nitroreductase31,32. Such enzymes are used to design enzyme-degradation drug delivery system. Some azoreductase-mediated prodrugs have been approved for IBD treatment, such as sulphasalazine, olsalazine and balsalazine25. However, the pH, transit time and gut bacterial enzyme are closely related to the individual differences and pathological changes of IBD patients33. It is difficult for conventional passive drug delivery systems to deliver drugs into inflamed sites accurately and exhibit consistent therapeutic effects, even a dual targeting strategy combining pH- and time-dependent or enzyme-degradation, because they didn't combine the pathophysiological changes in GIT of IBD. The pathophysiological changes, like overexpressed receptors and cell adhesion molecules, should be taken into consideration when designing the delivery strategies.
Table 1.
Representative approved delivery systems for the treatment of IBD.
| Mechanism of targeting | Drug | Brand name | Company | Administration route | Formulation | Indication |
|---|---|---|---|---|---|---|
| pH-Responsive | Aminosalicylate | Asacol HD | Allergan (USA) | Oral | Tablet coated with Eudragit S | Moderate UC |
| Aminosalicylate | Salofalk | Dr. Falk Pharma GmbH (Germany) | Oral | Tablet coated with Eudragit L | Mild to moderate UC | |
| Budesonide | Entocor | Tillots Pharma (Japan) | Oral | Capsule coated with Eudragit S | Induction and maintenance of remission of mild-to-moderate CD | |
| Beclomethasone | Clipper | Chiesi Pharma (Italy) | Oral | Tablet coated with Eudragit L 100/55 | Mild-to-moderate | |
| Time-dependent | Aminosalicylate | Pentasa | Kyorin (Japan) | Oral | Microgranule coated with ethylcellulose | Mild-to-moderate UC |
| pH-Responsive and time-dependent | Aminosalicylate | Lialda | Shire (USA) | Oral | A multi matrix core comprised of amphiphilic and lipophilic excipients outer coated with Eudragit S | Mild-to-moderate UC |
| Budesonide | Uceris | Salix (USA) | Oral | A multi matrix core comprised of amphiphilic and lipophilic excipients outer coated with Eudragit S | Active or mild-to-moderate UC | |
| Enzyme-degradation | Olsalazine | Dipentum | Alaven (USA) | Oral | Olsalazine is synthesized by two molecules of aminosalicylate via azo bond | Induction and maintenance of remission of mild-to-severe UC |
| Sulfasalazine | Azulfidine | Pfizer (USA) | Oral | Sulfasalazine is synthesized by aminosalicylate and sulfapyridine via azo bond | Introduction of remission of UC | |
| Balsalazide | Colazide | Salix (USA) | Oral | Balsalazide is synthesized by aminosalicylate and 4-aminobenzoyl-β-alanine via azo bond | UC & CD | |
| Enzyme-degradation and pH-responsive | Aminosalicylate | CODESTM | Yamanouchi Pharmaceuticals (Japan) | Oral | A tablet core coated with lactulose and acid-soluble materials | IBD |
| Anti-α4β7 integrin | Anti-α4β7 integrin mAb | Entyvio (Vedolizumab) | Takeda (Japan) | i.v. infusion | Each single-dose vial contains vedolizumab (300 mg), l-histidine (23 mg), l-arginine hydrochloride (131.7 mg), polysorbate 80 (3 mg), l-histidine monohydrochloride (21.4 mg), and sucrose (500 mg) | Moderate-to-severe CD & UC |
| Anti-α4 integrin | Anti-α4 integrin mAb | Tysabri (Natalizumab) | Elan (Ireland)/ Biogen Idec (USA) | i.v. infusion | Each single-dose vial contains: natalizumab (300 mg), sodium chloride (123 mg), phosphate (17 mg), monohydrate (17 mg), monobasic (17 mg), sodium phosphate (7.24 mg), heptahydrate (7.24 mg), dibasic (7.24 mg), polysorbate 80 (3 mg) | Induction and maintenance of remission of moderate -to-severe CD |
| Anti-TNF-α | Anti-TNF-α mAb | Remicade (Infliximab) | Johnson & Johnson (USA)/ Merck & Co. (USA) | i.v. infusion | Each single-dose vial contains: infliximab (100 mg), dibasic sodium phosphate (6.1 mg), dihydrate (6.1 mg), monobasic sodium phosphate (2.2 mg), monohydrate (2.2 mg), polysorbate 80 (0.5 mg), and sucrose (500 mg). No preservatives are present | Induction and maintenance of remission of moderate -to-severe CD & UC |
| Anti-IL-12 and IL-23 | Anti-IL-12 and IL-23 mAb | Stelara (Ustekinumab) | Johnson & Johnson (USA) | i.v. infusion | Each single-dose vial contains: ustekinumab (130 mg), edetate disodium (0.52 mg), histidine (20 mg), histidine monohydrochloride monohydrate (27 mg), methionine (10.4 mg), polysorbate 80 (10.4 mg), Sucrose (2210 mg) | Moderate-to-severe UC |
CD, Crohn's disease; IBD, inflammatory bowel disease; i.v., intravenous; UC, ulcerative colitis.
3. Receptors and cell adhesion molecules for active targeting
When colonic inflammation occurs, some specific receptors and cell adhesion molecules, like mannose receptor, macrophage galactose lectin, transferrin receptor, folate receptor, CD98, CD44, PepT1, F4/80, are overexpressed on the colonic epithelial cells and/or immune cells, as summarized in Table 2. Drug delivery systems superficially modified with related ligands can specifically bind to receptors on inflamed cells, deliver drug into target area and achieve great curative effect. Based on the ligand–receptor interaction, receptor-mediated active targeted drug delivery systems could be a promising therapeutic strategy for IBD, as listed in Table 334, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57.
Table 2.
IBD-related cell types and highly expressive receptors or cell adhesion molecules.
| Cell type | Receptor and cell adhesion molecule |
|---|---|
| Intestine epithelial cell | Transferrin receptor, CD44, CD98 |
| Macrophage | Mannose receptor, macrophage galactose lectin, transferrin receptor, folate receptor-β, CD44, F4/80 |
| Dendritic cell | Mannose receptor, macrophage galactose lectin, CD44, F4/80 |
| Vascular endothelial cell | Selectin (E-, P-, L-selectin), integrin (α4β1, α4β7), adhesion molecule (ICAM-1, ICAM-2, VCAM-1, MadCAM-1) |
CAM, cell adhesion molecules; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule.
Table 3.
Representative drug delivery systems targeting receptors for the treatment of IBD.
| Receptor | Ligand | Carrier | Loading cargo | Delivery route | Characterization | Experimental model | Principal finding | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mannose receptor | Mannose | Nanoparticle: PLGA–PEG | Ovalbumin | Ex vivo | S: 212.0 ± 8.0 Z: −7.0 ± 2.0 |
Proinflammatory cytokines treated Caco-2 cell, DSS-induced female C57BL/6 mice colitis | Mannose modification enhanced the endocytosis by Caco-2 cells and accumulation in inflamed colon | 34 |
| Mannose | Nanoparticle: TPP/poly (CBA-bPEI)-PEG | siTNF-α | Ex vivo | S:275.00 Z: ‒ |
Raw264.7 macrophage, DSS-induced male FVB mice colitis | Introduction of mannose demonstrated a significant increase in intracellular uptake, gene silencing in vitro and ex vivo | 35 | |
| Mannose | Nanoparticle: lipid phase: Compritol 888 ATO/Labrafac WL 1349/stearylamine/Span 80 aqueous phase: Poloxamer 188/ Polysorbate 80 | Budesonide | Oral | S: 301.7 ± 2.88 Z: +7.51 ± 0.71 |
Oxazolone-induced Wistar rat colitis | Surface conjugation of mannose led to superior effects in reduction of MPO, inflammatory cytokines (TNF-α, IL-1β) in vivo | 36 | |
| Mannose | Nanoparticle: trimethyl chitosan | Mir-146b | Oral | S: 213.6 ± 16.6 Z: +28.3 ± 6.3 |
Bone marrow-derived macrophage, DSS-induced C57BL/6 mice colitis | Mannosylated nanoparticles could be efficiently recognized and endocytosized by activated intestine macrophages | 37 | |
| Mannose | Nanoparticle: trimethyl chitosan | siTNF-α | Oral | S: 143.3 ± 1.1 Z: +18.7 ± 0.6 |
Raw264.7 macrophage, DSS-induced C57BL/6 mice colitis | Different densities of mannose on nanoparticle displayed different effects on the intracellular uptake and therapeutic efficacy in vitro and in vivo | 38 | |
| Macrophage galactose lectin | Lactobionic acid | Nanoparticle: chitosan-PLGA | siTNF-α | Oral | S: 245.60 ± 0.33 Z: +13.03 ± 0.65 |
Raw264.7 macrophage, DSS-induced male C57BL/6 mice colitis | Galactosylation remarkedly improved the targeted delivery into macrophage and TNF-α silencing effect in vitro and in vivo | 39 |
| Lactobionic acid | Nanoparticle: trimethyl chitosan-cysteine | siMap4k4 | Oral | S: 147.2 ± 7.8 Z: +26.2 ± 2.0 |
LPS-stimulated Raw264.7 macrophage, DSS-induced C57/BL6 mice colitis | The endocytosis kinetics and gene silencing effect of galactosylated nanoparticle outperformed those nanoparticles without galactosylation | 40 | |
| Lactobionic acid | Nanoparticle: PLGA/PVA/chitosan | siTNF-α & IL-22 | Oral | S: 261.3 ± 5.6 Z: −6.3 ± 1.4 |
Raw264.7 macrophage, DSS-induced male FVB mice colitis | Co-delivery of siTNF-α and recombinant human IL-22 could significantly inhibit the infiltration of mucosal neutrophils, promoted the colon epithelia regeneration and mucosal integrity | 41 | |
| Lactobionic acid | Nanoparticle: LMWC |
TNF-α antisense oligonucleotide |
Oral | S: ‒ Z: ‒ |
TNBS-induced mice colitis, CD45RBhi transferred mice colitis | Activated macrophages in the colon lamina propria of colitis mice efficiently absorbed galactosylated TNF-α antisense oligonucleotide nanoparticles | 42 | |
| Lactobionic acid | Nanoparticle: LMWC | Mir-16 | Rectal | S: ‒ Z: ‒ |
TNBS-induced BALB/c mice colitis | Galactosylated nanoparticle significantly accumulated in inflamed colon and delivers Mir-16 into colonic macrophages rather than T cells and colonic epithelial cells | 43 | |
| CD44 | HA | Nanoparticle: BSA-KPV/PLGA/PVA-chitosan | KPV | Oral | S: 272.3 Z: −5.3 |
LPS-stimulated Raw264.7 macrophage, DSS-induced FVB mice colitis | Colon-26 cells and Raw264.7 macrophages selectively absorbed HA modified nanoparticles | 44 |
| HA | Nanoparticle: spermidine/PLGA/chitosan | Curcumin& siCD98 | Oral | S: 246.2 ± 7.8 Z: −13.7 ± 4.1 |
LPS-stimulated Raw264.7 macrophage, DSS-induced FVB mice colitis | Surface grafting with HA enhanced the nanoparticles binding to colitis tissues and to be endocytosed by colonic macrophages | 45 | |
| HA | Copolymer: HA-bilirubin | Bilirubin | Oral | S:171 ± 30 Z: −39.1 ± 0.7 |
M0/M1/M2 J774A.1 macrophage, DSS-induced C57BL/6 mice colitis | HA-bilirubin copolymer could accumulate in inflamed colon epithelia and be internalized into M1 macrophages | 46 | |
| CS | Nanoparticle: silk fibroin | Curcumin | Oral or I.V. injection | S: 180.8 Z: −30 |
LPS-stimulated Raw264.7 macrophage, DSS-induced mice colitis | CS functionalization facilitated nanoparticle to be internalized into macrophage via CD44-mediated endocytosis | 47 | |
| HA | Hydrogel: methylcellulose/HA | BSA | Rectal | S: ‒ Z: ‒ |
Caco-2 monolayer | HA/ Methylcellulose hydrogel could rapidly permeate across Caco-2 monolayer | 48 | |
| Folate receptor | Folate | Nanoparticle: PLGA/PLA–PEG | 6-shogaol | Oral | S: 249.60 ± 1.30 Z: −24.17 ± 0.41 |
Raw264.7 macrophage, DSS-induced FVB/NJ mice colitis | Efficacy of 6-shogaol increased significantly after loading into folate modified nanoparticle, compared to drug suspension | 49 |
| Folate | Dendrimer: PAMAM-PEG/acetic anhydride | – | Tail vein injection | S: ‒ Z: ‒ |
Raw264.7 macrophage, DSS-induced C57BL6 mice colitis | Folate conjugation facilitated dendrimers binding to macrophages and accumulating in inflamed colon sites after tail vein injection | 50 | |
| Folate | Liposome: DSPC/cholesterol/PEG3400-DSPE | Betamethasone | Tail vein injection | S:100 ± 10 Z: ‒ |
Raw264.7 and peritoneal macrophage, DSS-induced C57/BL6 mice colitis | Folate grafting directed the liposomes to significantly accumulate in the inflamed colon and bind to peritoneal macrophages | 51 | |
| CD98 | CD98 Fab’ | Nanoparticle: PLA/bPEI/PVA/Mal-PEG | Quantum dots | Ex vivo | S: 458 Z: +19 |
Colon-26 cell and Raw264.7 macrophage, DSS-induced FVB mice colitis | CD98 Fab’ dramatically promoted the nanoparticle transporting into the CD98 glycoprotein overexpressed cells and inflamed colon. | 52 |
| Single-chain CD98 Ab | Nanoparticle: PEI/UAC- PEG-scCD98 | siCD98 | Oral | S: 210 Z: +15 |
Raw264.7 macrophage, colon-26 cells, DSS-induced C57/BL6 mice colitis, CD4+ CD45RBhigh T cell transferred RAG–/– mice colitis | Antibody conjugation endowed the nanoparticle with excellent biometrics capacity to bind CD98 glycoprotein on activated macrophage and polarized colon epithelia | 53 | |
| Transferrin receptor | TfR antibody | Liposome: HSPC/HSPG/DSPE-PEG-NHS | – | Ex vivo | S:105.60 ± 7.00 Z: −14.10 ± 3.30 |
Proinflammatory cytokines co-incubated Caco-2 cell, DNBS-induced rat colitis | Surface modification with TfR antibody resulted in more endocytosis by Caco-2 cells and accumulation in inflamed colon sites ex vivo | 54 |
| Seven peptides | Nanoparticle: PEG-b-PCL | Coumarin 6 | In vitro | S: 35.94 ± 2.76 Z: −3.10 ± 0.84 |
Caco-2 cell | Specific 7 peptides conjugation exhibited faster absorption rate in live cells | 55 | |
| Peptide transporter 1 | KPV | Nanoparticle: PLGA/montmorillonite/chitosan | Cyclosporine A | Oral | S:185.7 ± 3.0 Z:30 |
DSS-induced mice colitis | Surface modification with KPV promote nanoparticle to accumulate in inflamed colon. | 56 |
| F4/80 | F4/80 Ab Fab’ | Nanoparticle: PLA–PEG | siTNF-α | Oral | S: 609 ± 37 Z: ‒ |
LPS-stimulated Raw264.7 macrophage, DSS-induced mice colitis | F4/80 Ab Fab’ introduction increased the phagocytosis of nanoparticles by intestinal macrophages in mice | 57 |
‒, not applicable; BSA, bovine serum albumin; CS, chondroitin sulfate; DSS, dextran dulfate sodium salt; HA, hyaluronic acid; I.V., intravenous; LMWC, low molecular weight chitosan; LPS, lipopolysaccharide; MPO, myeloperoxidase; KPV, lysine-proline-valine tripeptide; S, size (nm); Z, zeta potential (mV).
3.1. Mannose receptor
Mannose receptor (MR), also named as cluster of differentiation 206 (CD206), is an effective endocytic carbohydrate-binding receptor58,59. MR is overexpressed on the surface of macrophages and dendritic cells (DCs) under inflammation conditions36,60. It's a transmembrane receptor contains C-type lectin-like domains (CTLD), fibronectin type II domains (FNII), R-type cysteine-rich domains (CRD)61,62. Each domain has different affinity to few special carbohydrates, in which CRD highly binds to 3′(or 4′)-sulfated galactose or N-acetyl-d-galactosamine63,64, FNII highly binds to collagens I‒IV65, and CTLD highly binds to mannose, fucose and N-acetyl-d-glucosamine59,66. Macrophages are responsible for pathogen phagocytosis, antigen processing and presentation, and mediator secretion. DCs receive and integrate signals from surroundings; they both significantly accelerate the pathological progress of IBD and proliferate in quantities during IBD67,68. Targeting MR on macrophages and DCs is gradually becoming a promising drug delivery strategy for IBD. Mannose is the most common ligand of MR. Mannose-modified delivery systems exhibit superior inflamed colon targeting via ex vivo and oral routes.
Coco et al.34 fabricated three types of nanoparticles, Eudragit S100 coated ovalbumin NPs, N,N,N-trimethylchitosan chloride coated ovalbumin NPs, mannose modified ovalbumin NPs, respectively, to compare their ability to reach the inflamed colon sites. Only mannosylated ovalbumin NPs could cross the Caco-2 intestinal barrier through endocytosis, and then significantly accumulated in inflamed colon ex vivo. Another mannose conjugated polycation (PPM) is synthesized through N,N′-cystamine bisacrylamide (CBA) and branched polyethylenimine (bPEI). TPP-crosslinked PPM kept the sustained release of siTNF-α in a reductive cellular environment. Importantly, TPP-PPM/siTNF-α was efficiently uptaken by Raw264.7 cells, dramatically silenced TNF-α expression in Raw264.7 cells and colitis colon tissues ex vivo compared to polycation without mannose conjugation35.
Ex vivo researches above proved the superior targeting properties of mannose modification. However, nanoparticles may be destroyed by gastrointestinal pH gradient (1.0–8.3) via oral administration (discussed in Section 2). Inert or pH-responsive materials could be used as protective outer layers. Chitosan is acid and alkali resistant and widely used in colon target delivery systems. Mannosylated trimethylchitosan (MTC) could keep the structure intact before reaching the colon, specifically bind to macrophages in inflamed intestine, primarily distribute in the intestine and show a long-term of 48 h intestinal retention without any significant cytotoxicity37. Mir-146b loaded in MTC remarkedly prompted the anti-inflammatory IL-10 secretion and intestinal epithelia proliferation via STAT3 signaling pathway, and downregulated pro-inflammatory iNOS, TNF-α and IL-1β gene through TLR4 signaling pathway in colitis tissues. Budesonide is a novel glucocorticoid that significantly and nonspecifically inhibits inflammation via nongenomic corticosteroid effects69. Since budesonide has hepatic first pass effect, oral route is suggested for administration70. Mannosylated nanoparticles with Eudragit coating could deliver budesonide to inflamed colon sites via oral route, exhibited noncytotoxic properties and significantly inhibited the increase of colon/body weight ratio, clinical activity index, histopathological score, TNF-α and IL-1β level in oxazolone-induced colitis tissue compared to drug suspension and non-mannosylated group36.
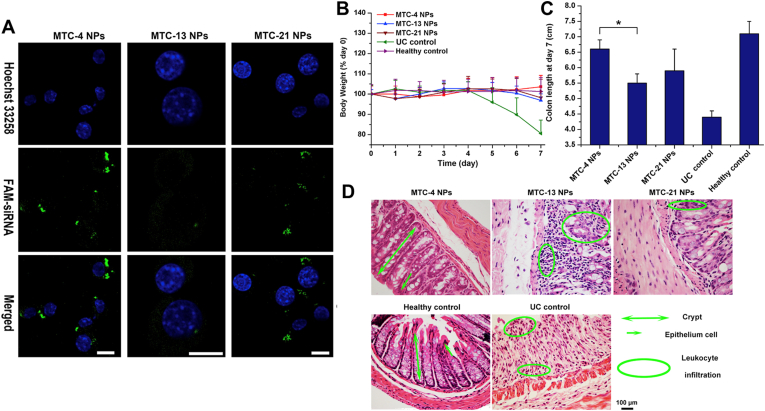
The cellular endocytosis efficiency is not always proportional to the densities of ligands71. 21% densities mannose coverage of siTNF-α-loaded trimethylchitosan cysteine nanoparticle (21-TMC) exhibited the maximum cellular uptake and gene silencing capability in vitro compared with 4% (4-TMC) and 13% (13-TMC) densities. Surprisingly, the highest level of 4-TMC was found in vivo after oral administration. The siRNA loading dose of 4-TMC was 2–3 orders of magnitude less than the published siTNF-α vectors to inflamed colon, it still significantly reduced the TNF-α level in vivo compared with 13-TMC and 21-TMC (Fig. 2)38. Consequently, to achieve high performance and low toxicity, the ligand density screening is a crucial procedure in the development of drug delivery systems.
Figure 2.
MR-mediated targeted therapy for IBD. (A) The endocytosis of FAM-siRNA loaded MTC NPs by Raw264.7 macrophages after 4 h incubation. Green represents the labeling of FAM, blue represents the fluorescence of Hoechst 33258. Scale bar represents 10 μm. (B) Changes of mice body weight over time. Data are presented as mean ± standard deviation (SD, n = 6). (C) Changes of mice colon length over time. Data are presented as mean ± SD (n = 6). ∗P < 0.05. (D) H&E staining of mice colon sections. Scale bar represents 100 μm. Reprinted with permission from Ref. 38. Copyright © 2015, Elsevier.
Unlike protein ligands, mannose is a small molecule compound with low immunogenicity and low cost, and thus derivatives of mannose are widely exploited for surface modification. MR is overexpressed on macrophages and DCs, it is noteworthy that MR is highly expressed on anti-inflammatory M2 macrophage, rarely expressed on pro-inflammatory M1 macrophage. Considering this, mannosylated anti-inflammatory drug-loaded delivery systems are more likely to target M2 macrophage, not M1 macrophage, and then therapeutic effects of mannosylated drug delivery system is controversial. Although substantial studies demonstrated that mannosylated drug delivery systems could target inflamed or cancerous sites and exert significant therapeutic effects, rare studies revealed the interaction between mannosylated delivery systems and cells, as well as the progress of macrophage phenotype transformation. Besides, since MR is also highly expressed in the liver, oral or rectal routes are suggested for mannosylated drug delivery systems to avoid hepatic endocytosis72.
3.2. Macrophage galactose lectin
Macrophage galactose lectin (MGL), also named as cluster of differentiation 301 (CD301), is highly expressed on the surface of activated macrophages and dendritic cells73. Unlike MR, MGL is a transmembrane type II C-type lectin composed of N-terminal cytoplasmic domains, transmembrane domains, extracellular stalk domains, and C-type CRD that only specifically binds to galactose and N-acetylgalactosamine74,75. Based on efficient carbohydrate–protein interaction, compounds with galactose residue have been widely used in drug delivery system decoration to improve the targeting ability. In the current researches, there are two routes for galactosylated nanoparticles to target CD301 in inflamed colon: oral and rectal routes.
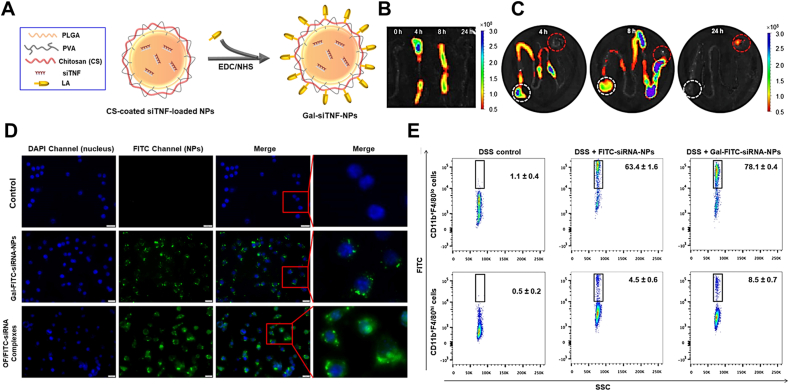
Chitosan derivatives and PLGA are the most common used matrices for oral dosage forms of IBD due to their stability in upper GIT. TNF-α is a critical inflammatory cascade reaction cytokine that highly participates in the deterioration of IBD, anti-TNF-α is an effective strategy for IBD therapy76. TNF-α antisense oligonucleotide loaded in galactosylated low molecular weight chitosan (LMWC) nanoparticles could be efficiently uptaken by activated macrophages in inflamed colon, markedly decrease colonic TNF-α mRNA level and some other inflammatory cytokines, and significantly ameliorate the acute or chronic clinical symptom of TNBS-induced or CD45RBhi-transfered colitis, respectively42. Galactosylated siTNF-α-loaded PLGA nanoparticle (GPN) could promote the recognition and uptake of nanoparticles by Raw264.7 cells compared with galactose-negative control, significantly inhibiting the TNF-α mRNA transcription and translation in vitro. The GPN could resist the acid and alkali in GIT, keep the siTNF-α intact before reaching the inflamed colon, ameliorate the serous clinical symptoms of colitis, and display superior efficacy in colonic TNF-α gene silencing than galactose-negative group39. Mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4) can activate the expression of TNF-α and IL-1β simultaneously without passing through JNK1/2, ERK1/2, P38 and NF-κB pathways, which indicates that it’s a potential therapeutic target for IBD77. Galactosylated trimethylchitosan–cysteine (GTC/TPP) nanoparticles could keep siMap4k4 intact in serum and intestinal fluids, allowed more siMap4k4 to accumulate in inflamed colon tissue rather than being absorbed into systemic circulation, significantly decreased TNF-α level and relieved clinical symptom of colitis40. IL-22 could promote the recovery of IBD by accelerating colonic epithelia proliferations and enhancing mucosal barrier78. However, the colonic IL-22 level decreases accordingly after anti-TNF-α antibody treatment, like Infliximab41. Encapsulating siTNF-α and recombinant IL-22 into galactosylated chitosan/PLGA NPs could precisely target macrophages in inflamed colon, chitosan/PLGA kept RNA structure integrity during delivery process. Co-delivery of siTNF-α and IL-22 significantly inhibited the TNF-α level and infiltration of mucosal neutrophils, promote the colon epithelia regeneration (Fig. 3).
Figure 3.
MGL-mediated targeted therapy for IBD. (A) Schematic illustrations of fabrication of Gal-siTNF-NPs. The colonic accumulation of Gal-FITC-siRNA-NPs in mice colon sites (B) and other sections of GIT (C) at 4, 8 and 24 h after oral administration. White circle and red circle represent stomach and cecum, respectively. (D) Cellular uptake of Gal-FITC-siRNA-NPs (green) by macrophages. Scale bar represents 10 μm. (E) FCS analysis of colonic macrophages in mice after 12 h of FITC-siRNA-NPs or Gal-FITC-siRNA-NPs administration. Data are presented as mean ± SD (n = 3). Reprinted with permission from Ref. 41. Copyright © 2018, Elsevier.
Galactosylated drug carriers still could target CD301 via the rectal route. Mir-16, a small noncoding RNA, can lead to breakage of TNF-α mRNA and IL-12p40 mRNA after specifically binding to their respective 3′-untranslated AU-rich regions43,79. Since Mir-16 can regulate multiple genes, systemic absorption of Mir-16 may cause cellular dysfunction and impede its therapeutic efficacy. Galactosylated LMWC (Gal-C) could deliver Mir-16 to activated colonic macrophage rather than colon epithelia and T cells in TNBS-induced colitis mice through rectal administration, significantly ameliorate the weight loss, colon shortening and increased myeloperoxidase (MPO) level, and exhibit long-term therapeutic effect which still remarkedly inhibited the expression and production of TNF-α and IL-12p40 in colon tissues up to 3 days.
Lactobionic acid, consisted of galactose residue and carboxyl group, was commonly used for functional modification to target CD301. However, the asialoglycoprotein receptor, which highly expressed on hepatocytes, could also efficiently recognize and internalize galactose and N-acetylgalactosamine80. Galactosylated drug delivery system administrated by injection would be absorbed by the liver. To achieve desirable therapeutic effects, oral administration is suggested for such delivery systems when treated to patients with IBD.
3.3. CD44
CD44 is a multifunctional transmembrane receptor responsible for cell adhesion, cellular uptake or degradation of hyaluronic acid (HA), immune activation81. CD44 almost presents on all cells, whereas highly presents on cancer cells and inflammatory cells. Colon epithelia and macrophages in colitis tissues possess abundant CD44 on their surface, which makes CD44 as an effective drug delivery target in the treatment of IBD82. The ligand of CD44, like HA, laminin, collagen and chondroitin sulfate, has been widely used in superficial modification83. CD44 ligand-modified nanoparticles can target the inflamed colon via different routes, including oral, systemic and rectal routes.
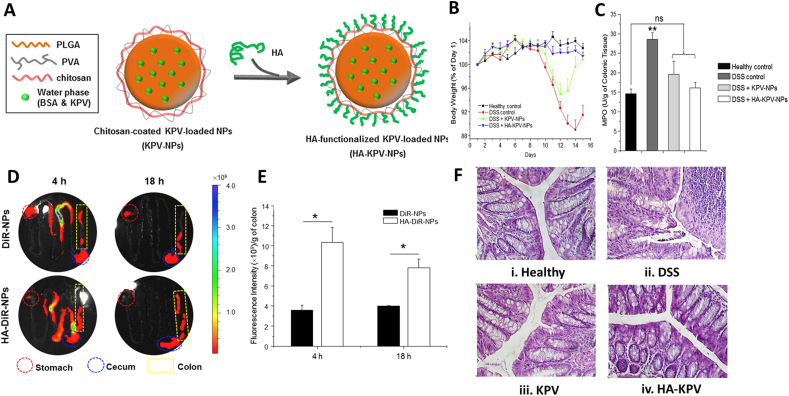
Partial nanoparticles administered by oral routes take chitosan and/or PLGA as protective matrices. Lysine-proline-valine tripeptide (KPV), the dissociation products of α-melanocyte-stimulating hormone, manifests stronger anti-inflammatory effect inside the cells by blocking inflammatory pathway84,85. Free KPV could significantly inhibit colonic TNF-α level, MPO activity and the severity of dextran dulfate sodium salt (DSS)-induced or TNBS-induced colitis via oral administration, but 12,000-fold lower concentration than free KPV could achieve identical therapeutic efficacy after loading into HA modified PLGA/chitosan nanoparticles (Fig. 4)44,86. As described in CD98 chapter, topical interfering the CD98 expression could attenuate colitis. Curcumin can attenuate colitis via blocking multiple signaling pathways, like NF-κB and Toll-like receptor signaling pathways87. Combination therapy of curcumin and siCD98 displayed stronger efficacy in downregulation of CD98 and TNF-α level compared to curcumin or siCD98 treatment alone45. HA does not only act as ligand of CD44, it could also be applied as formulation matrix. HA with different molecular weights vary in pro- or anti-inflammation regulatory effects88. Compared to 10 or 700 kDa, 100 kDa HA-bilirubin copolymer could self-assemble into spherical nanoparticle with desirable diameter, possesses notable efficacy in inhibiting pro-inflammatory cytokines, increasing anti-inflammatory cytokines, and regulating gut microbiota and its efficacy outperformed HA, bilirubin and HA & bilirubin46. Maybe due to the specific covalent binding, chemical structure HA-bilirubin structure is not destroyed by gastrointestinal acid-base and enzyme, copolymer still possesses anti-inflammatory effects when reaching inflamed colon via oral routes.
Figure 4.
CD44-mediated targeted therapy for IBD. (A) Schematic illustrations of preparation of HA-KPV-NPs. (B) Changes of mice body weight over time. Data are presented as mean ± SD (n = 5). (C) Colonic MPO level in different mice groups. Data are presented as mean ± SD (n = 5). ∗∗P < 0.01. ns, not significant. (D) The accumulation of KPV-NPs and HA-KPV-NPs in GIT at 4 or 8 h after oral administration, red circle and blue circle represent stomach and cecum, respectively. (E) Colonic quantification of the fluorescence intensity. Data are presented as mean ± SD (n = 5). ∗P < 0.05. (F) H&E staining of mice colon sections. The scale bar represents 100 μm. Reprinted with permission from Ref. 44. Copyright © 2015, Elsevier.
Whether by oral or systemic routes, chondroitin sulfate-functionalized silk fibroin nanoparticles (CS-SFs) dramatically guided curcumin to accumulate in the colitis tissues compared to carboxymethyl cellulose or plain functionalization. Due to the long circulatory, maximum curcumin concentrated at inflamed colon via systemic route exhibited the best therapeutic efficacy through downregulating TNF-α, IL-6 and IL-12 level, upregulating IL-10 level and improving gut microbiota composition47.
A novel thermoresponsive hydrogel administrated by rectal route is formed by the cross-linking of HA and methylcellulose at a ratio of 0.225% or 3.5%. The resultant hydrogel is liquid-like with good flowability at 20 °C, but it seems freezing at 37 °C. The model drug, bovine serum albumin (BSA), can be released from the gel rapidly in the presence of sodium dodecyl sulfate and permeated across Caco-2 monolayer, which suggested that the HA-methylcellulose hydrogel could be a potential rectal drug carrier for IBD48.
wSince CD44 can present on all cells, delivery system targeting CD44 may not be able to deliver the drug specifically to the inflamed colon, which could result in aimless drug release and systemic ADR. There are some other ligands of CD44, including osteopontin, matrix metalloproteases, collagens and antidody89, which have not been used in targeted modification of drug delivery systems for IBD yet, at least to our best knowledge. It would be very interesting to investigate them in practice.
3.4. Folate receptor
Folate receptor (FR), the collective name of four isoforms (FR-α, -β, -γ, and -δ), could highly bind to folate and efficiently transport them into cytoplasm through receptor-mediated endocytosis90. Since folate is essential for one-carbon metabolism, protein metabolism and DNA synthesis, FR (except for FR-γ) is usually overexpressed on the surface of rapid proliferating cells91, of which FR-α mainly exists on apical sides of epithelia, like lung and kidney92, FR-γ only exists on hematologic normal tissue or malignancies, like spleen and bone marrow93, FR-δ is merely found on regulatory T cells and oocytes94, FR-β exists on activated macrophages at an extremely high level but cannot be detected on resting/quiescent macrophages or any other normal cells51. Studies elucidated that substantial FR-β-positive macrophages infiltrated into human inflamed colon during IBD95. Due to the accurate expression pattern, targeting FR-β provides a novel way for targeted therapy of IBD. Folate-functionalized nanoparticles could target FR-β on macrophages in inflamed colon via systemic and oral routes.
Chitosan/alginate (3:7) hydrogel is commonly used in the encapsulating of drug delivery systems administrated by oral routes. Zhang et al.49 fabricated folate modified PLGA/PLA–PEG nanoparticles (PPNs). Folate modification could improve the biocompatibility of PPNs, the viability of Raw264.7 cells isn't altered during 48 h incubation with FA-PPNs up to 1 mg/mL and no toxicological or pathological changes were found in blood biochemistry and major organs during the 7 days' treatment with FA-PPNs. 6-Shogaol, a component in ginger, possesses significant antioxidant and anti-inflammatory effects96. 6-Shogaol/FA-PPNs encapsulating into chitosan/alginate (3:7) hydrogel exhibited superior effect by significantly decreasing the level of lipocalin-2, inhibiting the expression of TNF-α, IL-6, IL-1β and iNOS, facilitating the healing gene expression of Nrf-2 and HO-1 compared to 6-shogaol suspension49.
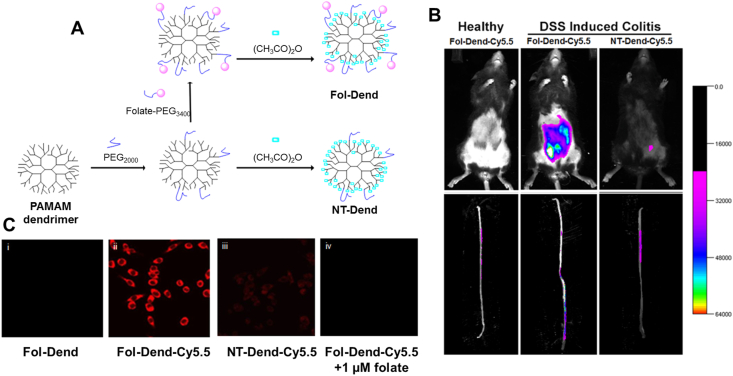
Folate functionalized drug carriers still exhibit great targeting properties when administrated via the systemic route. The fluorescence intensity of folate functionalized poly(amidoamine) (PAMAM) dendrimers in macrophage was significantly higher than that of non-functionalized group, but when co-incubation with free folate, all dendrimers displayed the same negative results. In vivo imaging experiments demonstrated that folate-dendrimer preferred to accumulate in inflamed colon rather than healthy colon after tail vein injection50 (Fig. 5). This excellent FR targeted accumulation capacity was consistent with Scott Poh's findings51, that only 0.1% folate could statistically direct DSPC/cholesterol/DSPE (56:40:4) liposome to concentrate in inflamed colon and adhere for 4-times longer than healthy colon after intravenous administration. Betamethasone is a kind of glucocorticoid (the anti-inflammatory mechanism of glucocorticoid is introduced in Section 3.1). Betamethasone loaded in folate-conjugated liposome possessed a superior effect in decreasing colon thickness compared to blank-conjugated liposome51.
Figure 5.
FR-mediated targeted therapy for IBD. (A) Schematic illustration of fabrication of folat-modified PAMAM dendrimers. (B) Fluorescence image of Cy5.5-labled dendrimer in vivo or ex vivo 12 h after 1 mg/kg Fol-Dend or NT-Dend administration through tail vein injection (n = 4). Colitis mice were induced by drinking 3% DSS water for 6 d λex = 625 nm, λem = 700 nm. (C) The endocytosis of dendrimers by Raw264.7 macrophages after 2 h incubation at 37 °C. λex=670 nm, λem = 700 nm. Reprinted with permission from Ref. 50. Copyright © 2017, American Chemical Society.
Tissue or organism may express different FR isoforms simultaneously, simple folate cannot distinguish the difference, and thus folate conjugated carrier may not completely deliver drugs into inflamed sites. N5,N10-dimethyl tetrahydrofolate, a synthetic folate derivative, could evade other FR's recognition and only bind to FR-α97. But as for FR-β, we haven't retrieved any correlative reports. There is an extremely urgent need for FR-β special ligand because of so many prevalent macrophage-related inflammatory diseases, like UC, CD, rheumatoid arthritis, atherosclerosis, psoriasis, and diabetes51. A meta-analysis revealed that IBD patients are intimately related to serious serum folate deficiency98, which promote the deterioration progress of colitis to colitis-related cancer99, appropriate folate supplement may be an auxiliary method for the treatment of IBD98. Considering this, maybe folate-conjugated drug delivery system will display special advantages.
3.5. CD98
CD98 is a transmembrane glycoprotein heterodimer linked by disulfide bond between CD98 heavy chain (hc) and CD98 light subunit52,53. CD98, responsible for protein transport100, can express on the surface of all normal cells except platelets at a balanced level, but substantial CD98 glycoprotein is found on colon epithelia in apical side when the integrity of intestinal epithelial barrier is broken and pathogenic microorganisms invade101. Meanwhile, overexpressed CD98 glycoprotein was detected in colonic tissues from mice and patients with colitis, which highly promoted the intestinal inflammation initiation, development, and cancerization of colitis102, 103, 104. Hence, CD98 provides a novel approach for IBD targeted therapy.
Nanoparticles modified by CD98 ligands can bind to CD98 on epithelia of inflamed colon via ex vivo and oral route. Quantum dots (QDs) is a promising imaging agent but with cytotoxicity, encapsulating with biocompatible polymers could decrease the cytotoxicity of QDs105. To target the CD98 and track the intracellular progress, Xiao et al.52 digested the CD98 antibody with pepsin to obtain Fab’ fragment, and then grafted it onto the surface of QDs-loaded polymer. As expected, the Raw264.7 and Colon-26 cells rapidly endocytosed CD98 Fab’-QDs NPs into cytoplasm in a concentration-dependent manner, but the uptake efficiency decreased 65.0% and 87.4%, respectively, after blocking down the CD98 expression. The CD98 Fab’ accelerated QDs' NPs penetrating the inflamed colon ex vivo.
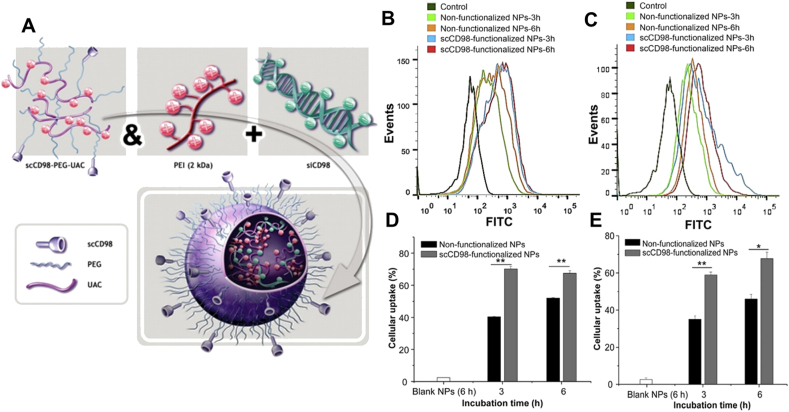
To investigate the potential of CD98 in vivo, single-chain CD98 antibody-conjugated siCD98-loaded nanoparticles (ssCD98 NPs) were administrated via oral route (Fig. 6)53. Nanoparticles were encapsulated in chitosan/alginate hydrogel to ensure the integrity of nanoparticles during upper GIT. Severe clinical manifestation of acute colitis had been significantly ameliorated after ssCD98 NPs treatment, including weight loss, enhanced MPO activity, upreguated CD98, TNF-α, IL-6 and IL-12 level, and aberrant histology. The same excellent therapeutic effect was observed in chronic RAG–/– mice colitis induced by CD4+CD45RBhigh T cells. Even though it showed excellent efficacy in vivo, flow cytometry revealed that only 24.1% of colon macrophage and 9.6% colonic epithelia isolated from inflamed colon tissue internalized the ssCD98 NPs during 12 h post-administration, which is consistent with the research result that medicine could manifest effective effect in vivo with cellular absorptivity ranging from 5% to 20%106.
Figure 6.
CD98-mediated targeted therapy for IBD. (A) Schematic illustration of self-assembly procedure of scCD98′-siCD98 loaded nanoparticles. FCS analysis about cellular uptake of FITC-siRNA loaded NPs by Colon-26 cells (B) and Raw264.7 macrophages (C) at 3 or 6 h. And related percentage analysis of FITC positive Colon-26 cells (D) and Raw264.7 macrophages (E), data are expressed as mean ± SEM (standard error of mean, n = 3); ∗P < 0.05, ∗∗P < 0.01. Reprinted with permission from Ref. 53. Copyright © 2014, Elsevier.
CD98 highly promotes the intestinal inflammation initiation, development, and cancerization of colitis94. Blocking the CD98 expression of experimental colitis mice has achieved good therapeutic effects. It indicates that CD98 is also a good target for the treatment of IBD45,107. Meanwhile, CD98 glycoprotein is a translocation protein that could be a target for drug delivery into cytoplasm. However, CD98 can express on the surface of all normal cells except platelets. Therefore, necessary measures should be employed to avoid the binding of nanoparticles with other cells when designing delivery systems targeting CD98.
3.6. Transferrin receptor
Transferrin receptor (TfR), a transmembrane glycoprotein, could efficiently uptake transferrin through receptor-mediated endocytosis to maintain intracellular iron homeostasis108. TfR almost presents on all normal cell types at low level, but highly exists on rapidly proliferating cells like activated macrophages, lymphocytes and some cancer cells109. Transferrin derivatives and some special ligands have been extensively used in the targeted modification of drug delivery systems. Modified nanoparticles have exhibited great therapeutic effects on cancer therapy and other inflammatory diseases110,111. The drug delivery systems targeting TfR have been gradually applied to the researches of IBD.
Specific polypeptides and antibodies are used as ligands for functional modification of drug delivery systems. Septapeptide (His-Ala-Ile-Tyr-Pro-Arg-His) conjugation could facilitate Caco-2 cells to recognize and endocytose coumarin-6-loaded PEG-b-PCL copolymer, further transporting more copolymer across cell monolayer55. Pro-inflammatory cytokines co-incubated Caco-2 cells also possess a high TfR level and internalized more TfR antibody modified immunoliposomes than liposomes without antibody54. Unlike healthy colonic tissue, enterocytes of murine or human inflamed colon overexpress TfR both in apical and basolateral domains54,112. The everted gut-sac experiments showed that more than 4.5-fold of TfR antibody modified liposome prefer accumulating in DNBS-induced colitis colon sites rather than healthy colon ex vivo.
Nevertheless, current strategies targeting TfR are limited in in vitro and ex vivo models. It is noteworthy that the pharmacological efficacy of these delivery systems in IBD animal models has not been reported. When administered orally or intravenously, necessary measures must be taken to avoid the possible degradation of ligands by acid, base, hydrolase, phagocytosis by reticuloendothelial system, especially for antibody and polypeptides. Since the excellent serum stability, transferrin conjugation could shield drug from renal filtration and concentrate at the inflamed colon sites through receptor–ligand interaction and EPR effect. Studies pointed out rice-derived recombinant transferrin could be a substitute of human serum transferrin, which indicates that transferrin would be a promising and economic ligand for IBD targeted therapy113.
3.7. Peptide transporter 1
Peptide transporter 1 (PepT1) is a small intestinal receptor responsible for the absorption of oligopeptides and peptidomimetic drugs from the diet114. Normally, PepT1 does not exist in colon tissues but is highly expressed on inflammatory colonic epithelial cells and macrophages when IBD occurs115,116. Lysine-proline-valine tripeptide (KPV) possesses a high affinity to PepT1, and it has been used in superficial modification to target PepT185. A novel KPV-conjugated fluorescent probe for ulcerative colitis real-time monitoring has been reported previously117. KPV–PepT1 interaction is also used in the design of drug delivery system for IBD.
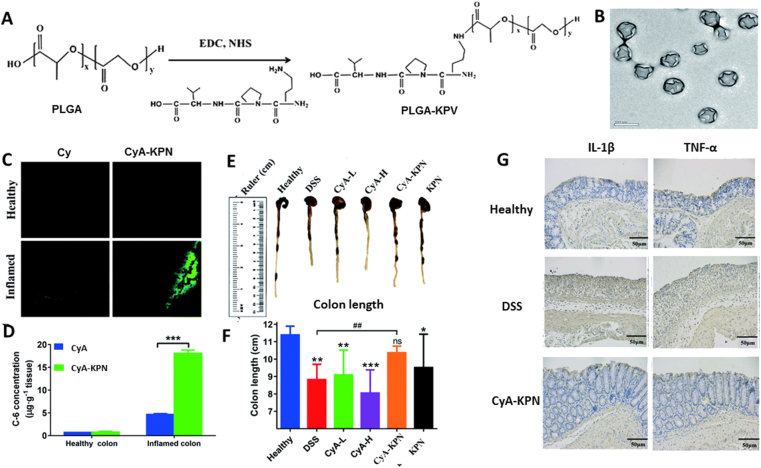
KPV-conjugated PLGA nanoparticles (KPNs) exhibited great PepT1 targeting properties via oral route. Due to the excellent stability in upper GIT, montmorillonite/chitosan nanocomposites were employed to coat KPNs as a protective layer. KPV conjugation promoted the accumulation of nanoparticles in inflamed colon and prolonged the retention time of nanoparticle up to 24 h. As a immunosuppressant, cyclosporine A (CyA) can specifically inhibit the activation of T cell and expression of pro-inflammatory cytokine118. Loading CyA into KPNs increased the concentration of CyA in inflamed colon by 23-fold. CyA KPNs significantly reduced DSS-induced mice mortality, colon atrophy, weight loss, oxidative stress, and elevated TNF-α and IL-1β level compared with free CyA (Fig. 7)56.
Figure 7.
PepT1-mediated targeted therapy for IBD. (A) Schematic illustration of KPNs synthesis. (B) Morphology of KPNs characterized by TEM. (C) The distribution of KPNs in colon. (D) Quantitative analysis of KPNs distribution in colon, data are expressed as mean ± SD (n = 3); ∗∗∗P < 0.001. The Visual expression (E) and quantitative analysis (F) of colon length from different groups, data are expressed as mean ± SD (n = 4–9); ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ##P < 0.01. ns, not significant. (G) The immunohistochemistry analysis of TNF-α and IL-1β. The scale bar represents 50 μm. Reprinted with permission from Ref. 56. Copyright © 2019, the Royal Society of Chemistry.
A report revealed that 5-aminosalicylate could inhibit the transportation of PepT1, hence it had better not deliver 5-aminosalicylate and its derivatives with PepT1 ligand-modified drug delivery system119.
3.8. F4/80
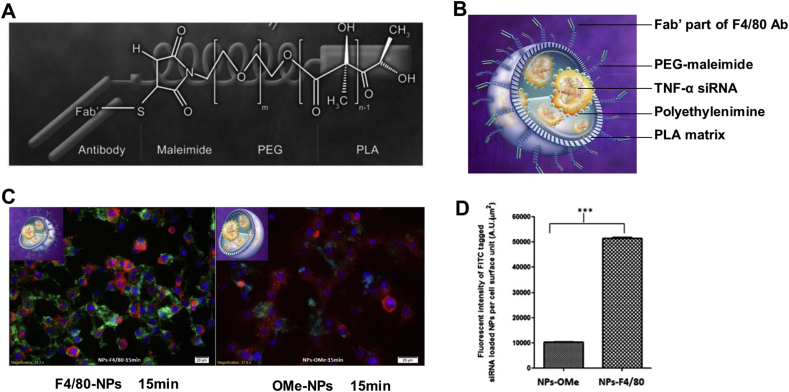
F4/80, also named as EMR1 and ADGRE1, is a transmembrane glycoprotein composed of 7 transmembrane motifs and some epidermal growth factor-like domains (EGFs) outside the cells120. F4/80 only exists on eosinophils in human and monocytes, macrophages, myeloid dendritic cells, eosinophils in mouse121, it is highly expressed when IBD occurs122. F4/80 is an essential marker of macrophage, and its antibody has been used for surface modification.
Laroui et al.57 covalently linked the F4/80 Ab Fab’ to PLA–PEG-maleimide polymer through amino–thiol reaction. The resultant copolymers didn't aggregate and could self-assemble into uniform spherical nanoparticles (F4/80 Fab’ NPs) with good colloidal stability. Besides, siTNF-α-loaded F4/80 Fab’ NPs could be efficiently endocytosed by Raw264.7 cells and significantly dampen the sharply elevated TNF-α level induced by LPS in vitro (Fig. 8). siTNF-α-loaded F4/80 Fab’ NPs encapsulated in chitosan/alginate (3:7) hydrogel exerted superior curative effect than F4/80 Fab’-deficient NPs in weight loss, MPO activity, TNF-α overproduction, IKβα accumulation and aberrant histopathology of DSS-induced colitis mice after oral administration.
Figure 8.
F4/80-mediated targeted therapy for IBD. (A) Semi-developed formula of basic unit of nanoparticles. (B) Tridimensional schematic of F4/80 Fab’ TNF-α siRNA/PEI-loaded nanoparticles. (C) Cellular uptake fluorescent microscopy of 500 μg/mL F4/80 Fab’- FITC-tagged NPs (green) and OMe F4/80 Fab’- FITC-tagged (green) NPs by MPs after 15 min incubation. Red represents cell cytosol, purple represents cell nucleus. The scale bar represents 20 μm. (D) Quantification of the fluorescent intensity. Reprinted with permission from Ref. 57. Copyright © 2014, Elsevier.
Since F4/80 is only expressed on eosinophils which plays a key role in IBD deterioration in human123, it indicates that F4/80 would be a potential delivery target. However, the ligand of F4/80 is merely monoclonal antibody so far, which brings huge costs on preparation. Design and screening of novel cheaper ligands based on computer-aided drug design technology would help a lot. Even though the surface modification of F4/80 antibody has achieved effective efficacy in mice, it still needs to be carefully studied when it's applied to human with IBD.
3.9. Cell adhesion molecules
When gut inflammation occurs, mucosal microvasculature endothelia overexpress cell adhesion molecules (CAMs) in the luminal sides to recruit leukocytes from blood, and subsequently trigger a more severe inflammatory cascade124. Some CAMs, comprised of selectin (E-, P-, and L-selectin), integrin (α4β1 and α4β7), adhesion molecules (ICAM-1, ICAM-2, VCAM-1 and MadCAM-1), participate in the leukocytes recruitment process125. Such CAMs are expressed on most cells normally, but they are expressed on inflamed colonic epithelia or tissues at a high level during IBD. Substantial researches in vitro and in vivo demonstrated that CAMs are promising targets for drug delivery of IBD, as listed in Table 4126, 127, 128, 129, 130.
Table 4.
Representative drug delivery systems targeting cell adhesion molecules for the treatment of IBD.
| Cell adhesion molecules | Ligand | Carrier | Loading cargo | Delivery route | Characterization | Experimental model | Principal finding | Ref. |
|---|---|---|---|---|---|---|---|---|
| ICAM-1 | ICAM-1 Ab | Nanoparticle: polystyrene bead | α-Galactosidase | In vitro | S: 258.5 ± 11.3 Z: −12.9 ± 0.4 |
TNF-α-activated Caco-2 cell | 62.4 ± 6.2-fold higher ICAM-1 Ab coated NPs bound to Caco-2 cell compared to mice IgG coating | 126 |
| P-Selectin | Sialyl Lewisx | Nanoparticle: PLGA | Diclofenac sodium salt | In vitro | S: 4,630 Z: ‒ |
Slides coated with P-selectin | The binding capacity of PLGA microsphere to selectin increased with sLex density | 127 |
| ICAM-1VCAM-1E-selectin | ICAM-1VCAM-1E-selectin Ab |
Nanoparticle: PLA–PEG | – | In vitro | S: ‒ Z: ‒ |
TNF-α or IL-1β-treated HUVEC | Ligand conjugation promoted nanoparticle to adhere to inflamed epithelia in a density-dependent manner | 128 |
| P-Selectin | P-selectin glycoprotein ligand-1 | Nanoparticle: PLA–PEG | – | i.v. injection | S: ‒ Z: ‒ |
Trauma-induced mice endothelium inflammation | PSGL-1 conjugation led to 10-fold higher adhesion to inflamed endothelium | 128 |
| E-Selectin | E-Selectin Ab | Nanoparticle: PLA–PEG | – | i.v. injection | S: ‒ Z: ‒ |
TNF-α-induced mice endothelium inflammation | E-selectin mAb conjugation led to 6-fold more adhesion to inflamed endothelium | 128 |
| ICAM-1 | ICAM-1 Ab | Nanoparticle: polystyrene bead | – | Oral | S: 269.8 ± 6.3 Z: −7.1 ± 0.2 |
Normal C57BL/6 mice | ICAM-1 Ab coating significantly prolonged the nanoparticles retention in GIT compared to mice IgG |
129 |
| Integrin β7 | Integrin β7 Ab | Liposome: PC:DPPE:Chol (3:1:1) | SiCyclin D1 | i.v. injection | S: 114 ± 7 Z: +13.5 ± 1.2 |
DSS-induced C57BL/6 mice colitis | Integrin β7 liposomes specifically and efficiently bound to leukocyte and internalized into cytoplasm immediately | 130 |
‒, not applicable; CAM, cell adhesion molecules; GIT, gastrointestinal tract; ICAM, intercellular adhesion molecule; I.V., intravenous; S, size (nm); VCAM, vascular cell adhesion molecule; Z, zeta potential (mV).
Usually, the ligands of CAMs are antibodies and some specific peptides131,132. Nanoparticles modified by related ligands could effectively target CAMs in vitro. ICAM-1+ Caco-2 cells could rapidly recognize ICAM-1 Ab coated polystyrene beads and internalize it into cytoplasm compared to mice IgG coating. The significant internalization could be dramatically reversed by the inhibitor of CAM-endocytic pathways, amiloride and EIPA126. Sialyl Lewisx (sLex), a sialylated and fucosylated carbohydrate, is the ligand of P-selectin. Laminar flow assay revealed that sLex conjugated PLGA microsphere could bind to P-selectin through leukocytes–endothelial interaction, and the binding intensity was positively correlated with the density of sLex127. Sakhalkar et al.128 explored the adhesion property of PEG–PLA NPs which were grafted with different endothelial CAMs ligands (PSGL-1, ICAM-1 mAb, VCAM-1 mAb, and E-selectin mAb) to inflamed endothelia in vitro, and ligands modified nanoparticles displayed significant targeting properties.
When administrated to mice via oral route, ICAM-1 Ab modified nanoparticles without any protective outer layer encountered destructive enzyme-dependent degradation, negligible nanoparticles with integral structure arrived at colon sites129. Cyclin D1 (CyD1) is the vital cell cycle regulating molecule that participated in the pathogenesis of IBD130. Peer et al.130 encapsulated siCyd1 into integrin β7 Ab functionalized liposomes (β7-LPs), and then administrated β7-LPs to mice via systemic route. 10% β7-LPs accumulated in inflamed colon, 3.5-fold higher than that of healthy group. The mRNA of CyD1 level and cell proliferation decreased remarkedly in mononuclear leukocytes of inflamed colon. Moreover, severe weight loss, tissue damage, abnormally high IL-10 and IL-12 level were significantly inhibited by siCyd1-loaded β7-LPs. As for IgG-LPs, it was almost undetectable in the colon and no any therapeutic effect.
Since the ligands of CAMs are antibodies and some specific peptides, additional protective coating must be taken into account when such ligands were applied to IBD targeted therapy131,132. CAMs usually express on vasculature endothelial cells in the apical side when inflammation occurs. Therefore, systemic administration is a desirable route for CAMs ligand-modified nanoparticles to target inflamed sites.
4. Discussion and perspectives
Receptor-mediated targeted drug delivery strategy through ligand–receptor interaction does achieve excellent curative effects in the treatment of IBD. Since the pathogenesis of IBD is still unclear, the recommended prevention measures for IBD are healthy lifestyles and balanced nutrition supplement5. IBD has a long disease cycle, high relapse and cancerization rate. To prevent disease relapse and colitis-induced cancerization, long-term maintenance chemotherapy is needed. Common chemoprevention strategy is administration of nonsteroidal anti-inflammatory drugs, ursodeoxycholic acid, statins and folic acid133. Long-term non-targeting chemotherapy could lead to serious ADR, and receptor-mediated targeted drug delivery system can reduce the drug dosage and systemic ADR, improving the topic drug concentration and efficacy. Actually, there are lots of known receptors or cell adhesion molecules highly upregulated in colon tissues of IBD patients, like lipocalin-2 receptor134, human H+-coupled oligopeptide transporter85, fractalkine125, and G protein-coupled bile acid receptor135. They are still not applied in the targeted delivery studies, and their specific ligands remain unknown. In addition, there are lots of unknown molecules involved in the pathological process of IBD, so more advanced techniques, like molecular docking, proteomics, transcriptomics and high throughput screening, should be introduced to design, screen and confirm more IBD-related specific receptors, ligands or biomarkers, for instance, N5,N10-dimethyl tetrahydrofolate, a synthetic folate derivative, could evade other FR subnets' recognition and only bind to FR-α97. It will be extremely meaningful to understand the pathogenesis of IBD and develop more effective targeted drug delivery systems for IBD diagnosis and therapy.
Interestingly, some peptides and biologically derived exosomes preferentially interact with inflammatory cells and have been investigated in colitis model. TKPR is a serum-stable peptide that could specifically interacts with macrophages, polymersomes superficially functionalized by TKPR could significantly accumulate at inflamed colon sites and showed superior therapeutic effects136. Maybe due to the presence of CAMs on surface, TGF-β1 gene-edited bone marrow-derived dendritic cells-derived exosomes could be recruited to the inflamed colon sites, activate CD4+Foxp3+ T-regs in mesentery lymph nodes lymphocytes, and significantly ameliorate the colitis symptoms even only containing 150 pg TGF-β1. However, 4500 pg TGF-β1 cytokines injection worked slightly137. Exosomes derived from mesenchymal stem cells138, and M2b macrophages139 could specifically home to inflamed colon tissues, and significantly inhibit the severity of colitis. Exosomes isolated from edible ginger possessed excellent stability in stomach and intestine simulated solution, and preferentially accumulated in inflamed colon140. Meanwhile, siCD98-loaded ginger-derived exosomes effectively targeted inflamed colon and inhibited the expression of CD98107. Grapefruit derived exosomes (GDEs) also possessed high affinity to intestinal macrophages, methotrexate loading into GDEs presented lower toxicity and higher therapeutic effects141. In addition, some biomimetic nanoparticles exploited proteolipid from human leukocytes142, and neutrophil143 could also specifically target the inflamed vasculatures in vivo. Biomimetic strategy is widely used in diagnosis and targeted therapy due to its great biocompatibility, prolonged systemic circulation and desirable targeting ability14. It provides an additional option for the development of active targeted drug delivery system for IBD.
Receptor-mediated targeted delivery systems had been applied in IBD diagnosis and imaging studies. Current diagnostic approaches, such as colonoscopy, tissue biopsy, computed tomography (CT) and magnetic resonance imaging (MRI), are invasive, low sensitivity, false positive or non-targeted imaging144,145. A PepT1-targeted fluorescent probe could accurately bind to inflamed colon sites and distinguish between chronic and acute colitis, it provided guidance for IBD chemotherapy117. Ligand-conjugated contrast agents overcome the problem of non-targeted imaging, and it had been used for in vivo targeted diagnosis and obtained superior imaging effects, like folic acid modified Gd146, folic acid-cysteamine modified Au147, transferrin conjugated Fe3O4148. Furthermore, cathepsins B and matrix metalloproteinase are universal inflammatory biomarkers that have been applied in IBD diagnosis researches149. But if IBD-specific biomarkers are found and applied in IBD diagnosis combined with receptor-mediated targeted technology, it will revolutionize the diagnosis of IBD.
Substantial receptor-mediated targeted delivery systems had been published recent years, only few of them had entered clinical trials, such as MCC-465150, SGT-53151, BIND-014152. There are many challenges in the clinical transformation of nanomedicine, including the researches of in vivo fate, the design of nanomedicine, the evaluation of biological properties and industrial production. Few researches were conducted on the in vivo fate of drug delivery system for the treatment of IBD, but it's essential for the pharmacokinetics exploration and clinical transformation of nanomedicine. Detecting the signal of isotope or fluorescent probe labeled delivery system is a common method to study in vivo fate. However, isotope and fluorescence probes still produce signals after dissociating from the delivery system, which can interfere with the experimental results. Environmental-responsive fluorescent dyes can avoid this problem by distinguishing the signals of free probe from probe-labeled delivery system. It can be classified into three categories, namely fluorescence resonance energy transfer (FRET), aggregation-induced emission (AIE) and aggregation-caused quenching (ACQ)153,154. Because of high sensitivity, small interference and strong universality, ACQ dyes have been used in tracing nanoparticles in vivo via different administration routes155,156. So ACQ dyes are suggested for IBD researches. Optimal design is helpful to overcome physiological barriers (as mentioned in Section 2.1), imprecise targeting and aim-less drug release of nanomedicines in vivo27,157,158. Surface functionalization can address such problems, for instance, PEG modification could inhibit reticuloendothelial attack and prolong systemic circulation159, chitosan-coating could restrain acidic or alkaline degradation in upper GIT160, and ligands conjugation could evade the intervention of immune systems and prompt cellular endocytosis161. The shape, size and surface potential could also affect endocytosis and targeting of nanoparticles. For instance, neutrophils are more likely to uptake negatively-charged, cube-shaped, >100 nm sized nanoparticles162. Different delivery systems present different requirements for the design, and thus it needs to be carefully optimized during the preclinical study. Biological evaluation comprises cytological evaluation in vitro and zoological evaluation in vivo. Cytological evaluation could preliminarily demonstrate the biocompatibility and the interaction between nanomedicine and cells, but cell culture plate can't fully simulate the complicated GIT environment, physiological barrier and inflamed colon tissues. Zoological evaluation could reveal biocompatibility, pharmacokinetics, biodistribution and targeting properties of nanomedicine in vivo. However, the passive targeting properties through EPR effect is weaker in human, and widely used colitis model induced by DSS and TNBS can't fully reflect pathological changes in IBD patients163. And some receptors' expression pattern in mice is different from that in human, like F4/80. The lack of animal experimental models that can accurately reflect human diseases is one of the obstacles that leads to the discrepancy between the results of preclinical studies and clinical trials164. Novel animal models could be introduced into IBD preclinical researches, like humanized mouse model165, genetic engineering mouse model166, or large mammal (dog, pig and monkey) models. Industrial production is another challenge to clinical transformation. Complex preparation process and harsh preparation conditions will increase the difficulty of industrialization. The simplification of formulation design and preparation process is more conducive to large-scale production. Although the clinical transformation progress is slow, receptor-mediated active targeted strategy still possesses great promise in revolutionizing the diagnosis and treatment of IBD.
Acknowledgments
This work was funded by the Science and Technology Development Fund, Macao S.A.R (Grant No. 0023/2019/A and SKL-QRCM(UM)-2020-2022, China), National Key Research and Development Program of China (Grant No. 2017YFE0191500), and the Research Fund of the University of Macau, Macao S.A.R. (Grant No. MYRG2019-00143-ICMS, China).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Shengpeng Wang, Email: swang@um.edu.mo.
Yitao Wang, Email: ytwang@um.edu.mo.
Author contributions
Peng Liu and Caifang Gao performed the literature search and wrote the manuscript. Hongguo Chen, Chi Teng Vong, Xu Wu and Xudong Tang reviewed the manuscript. Shengpeng Wang and Yitao Wang designed the study and revised the manuscript. All of the authors have read and approved the final manuscript.
Conflict of interests
The authors have no conflicts of interest to declare.
References
- 1.Gao C., Liu L., Zhou Y., Bian Z., Wang S., Wang Y. Novel drug delivery systems of Chinese medicine for the treatment of inflammatory bowel disease. Chin Med. 2019;14:23. doi: 10.1186/s13020-019-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart D.C., Sandborn W.J. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 3.Pohl C., Hombach A., Kruis W. Chronic inflammatory bowel disease and cancer. Hepato-Gastroenterol. 2000;47:57–70. [PubMed] [Google Scholar]
- 4.Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan G.G., Ng S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321. doi: 10.1053/j.gastro.2016.10.020. e2. [DOI] [PubMed] [Google Scholar]
- 6.Sartor R.B. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139:1816–1819. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Abraham B.P., Ahmed T., Ali T. Inflammatory bowel disease: pathophysiology and current therapeutic approaches. Handb Exp Pharmacol. 2017;239:115–146. doi: 10.1007/164_2016_122. [DOI] [PubMed] [Google Scholar]
- 8.Hua S., Marks E., Schneider J.J., Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11:1117–1132. doi: 10.1016/j.nano.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki M., Muraki Y., Nishimoto Y., Murakawa Y., Matsuo T. Fluorescence-labeled liposome accumulation in injured colon of a mouse model of T-cell transfer-mediated inflammatory bowel disease. Biochem Biophys Res Commun. 2017;494:188–193. doi: 10.1016/j.bbrc.2017.10.058. [DOI] [PubMed] [Google Scholar]
- 10.Lee A., De Mei C., Fereira M., Marotta R., Yoon H.Y., Kim K. Dexamethasone-loaded polymeric nanoconstructs for monitoring and treating inflammatory bowel disease. Theranostics. 2017;7:3653–3666. doi: 10.7150/thno.18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J.K., Tam M.F., Samaei S., Lerouge S., Barralet J., Stevenson M.M. Mucoadhesive chitosan hydrogels as rectal drug delivery vessels to treat ulcerative colitis. Acta Biomater. 2017;48:247–257. doi: 10.1016/j.actbio.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Shen W., Shi X., Fu F., Fan Y., Shen W. Alpha-tocopheryl succinate-conjugated G5 PAMAM dendrimer enables effective inhibition of ulcerative colitis. Adv Healthc Mater. 2017;6:1700276. doi: 10.1002/adhm.201700276. [DOI] [PubMed] [Google Scholar]
- 13.Barua S., Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9:223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C., Wang J., Wang Y., Gao H., Wei G., Huang Y. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hua S. Orally administered liposomal formulations for colon targeted drug delivery. Front Pharmacol. 2014;5:138. doi: 10.3389/fphar.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua S. Targeting sites of inflammation: intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front Pharmacol. 2013;4:127. doi: 10.3389/fphar.2013.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 18.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238. doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe A., Tanaka H., Sakurai Y., Tange K., Nakai Y., Ohkawara T. Effect of particle size on their accumulation in an inflammatory lesion in a dextran sulfate sodium (DSS)-induced colitis model. Int J Pharm. 2016;509:118–122. doi: 10.1016/j.ijpharm.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 21.Chomoucka J., Drbohlavova J., Huska D., Adam V., Kizek R., Hubalek J. Magnetic nanoparticles and targeted drug delivering. Pharmacol Res. 2010;62:144–149. doi: 10.1016/j.phrs.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S., Langer R., Traverso G. Nanoparticulate drug delivery systems targeting inflammation for treatment of inflammatory bowel disease. Nano Today. 2017;16:82–96. doi: 10.1016/j.nantod.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran P.H.L., Tran T.T.D. Current film coating designs for colon-targeted oral delivery. Curr Med Chem. 2021;28:1957–1969. doi: 10.2174/0929867327666200604170048. [DOI] [PubMed] [Google Scholar]
- 24.Nyman-Pantelidis M., Nilsson A., Wagner Z.G., Borga O. Pharmacokinetics and retrograde colonic spread of budesonide enemas in patients with distal ulcerative colitis. Aliment Pharmacol Ther. 1994;8:617–622. doi: 10.1111/j.1365-2036.1994.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 25.Roldo M., Barbu E., Brown J.F., Laight D.W., Smart J.D., Tsibouklis J. Azo compounds in colon-specific drug delivery. Expet Opin Drug Deliv. 2007;4:547–560. doi: 10.1517/17425247.4.5.547. [DOI] [PubMed] [Google Scholar]
- 26.Patel M.M. Cutting-edge technologies in colon-targeted drug delivery systems. Expet Opin Drug Deliv. 2011;8:1247–1258. doi: 10.1517/17425247.2011.597739. [DOI] [PubMed] [Google Scholar]
- 27.Bak A., Ashford M., Brayden D.J. Local delivery of macromolecules to treat diseases associated with the colon. Adv Drug Deliv Rev. 2018;136–137:2–27. doi: 10.1016/j.addr.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Monteleone G., Neurath M.F., Ardizzone S., Di Sabatino A., Fantini M.C., Castiglione F. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn's disease. N Engl J Med. 2015;372:1104–1113. doi: 10.1056/NEJMoa1407250. [DOI] [PubMed] [Google Scholar]
- 29.Davis S.S., Hardy J.G., Fara J.W. Transit of pharmaceutical dosage forms through the small-intestine. Gut. 1986;27:886–892. doi: 10.1136/gut.27.8.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie H.J., Gan Y., Ma S.W., Gan L., Chen Q.H. Optimization and evaluation of time-dependent tablets comprising an immediate and sustained release profile using artificial neural network. Drug Dev Ind Pharm. 2008;34:363–372. doi: 10.1080/03639040701657701. [DOI] [PubMed] [Google Scholar]
- 31.Scheline R.R. Metabolism of foreign compounds by gastrointestinal microorganisms. Pharmacol Rev. 1973;25:451–523. [PubMed] [Google Scholar]
- 32.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nugent S.G., Kumar D., Rampton D.S., Evans D.F. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48:571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coco R., Plapied L., Pourcelle V., Jerome C., Brayden D.J., Schneider Y.J. Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int J Pharm. 2013;440:3–12. doi: 10.1016/j.ijpharm.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Xiao B., Laroui H., Ayyadurai S., Viennois E., Charania M.A., Zhang Y. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-alpha RNA interference for IBD therapy. Biomaterials. 2013;34:7471–7482. doi: 10.1016/j.biomaterials.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinhmar G.K., Shah N.N., Rawal S.U., Chokshi N.V., Khatri H.N., Patel B.M. Surface engineered lipid nanoparticle-mediated site-specific drug delivery system for the treatment of inflammatory bowel disease. Artif Cells Nanomed Biotechnol. 2018;46:565–578. doi: 10.1080/21691401.2018.1463232. [DOI] [PubMed] [Google Scholar]
- 37.Deng F., He S., Cui S., Shi Y., Tan Y., Li Z. A molecular targeted immunotherapeutic strategy for ulcerative colitis via dual-targeting nanoparticles delivering miR-146b to intestinal macrophages. J Crohns Colitis. 2019;13:482–494. doi: 10.1093/ecco-jcc/jjy181. [DOI] [PubMed] [Google Scholar]
- 38.Chu S., Tang C., Yin C. Effects of mannose density on in vitro and in vivo cellular uptake and RNAi efficiency of polymeric nanoparticles. Biomaterials. 2015;52:229–239. doi: 10.1016/j.biomaterials.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 39.Huang Y., Guo J., Gui S. Orally targeted galactosylated chitosan poly(lactic-co-glycolic acid) nanoparticles loaded with TNF-α siRNA provide a novel strategy for the experimental treatment of ulcerative colitis. Eur J Pharm Sci. 2018;125:232–243. doi: 10.1016/j.ejps.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Tang C., Yin C. Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials. 2013;34:3667–3677. doi: 10.1016/j.biomaterials.2013.01.079. [DOI] [PubMed] [Google Scholar]
- 41.Xiao B., Chen Q., Zhang Z., Wang L., Kang Y., Denning T. TNFalpha gene silencing mediated by orally targeted nanoparticles combined with interleukin-22 for synergistic combination therapy of ulcerative colitis. J Control Release. 2018;287:235–246. doi: 10.1016/j.jconrel.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuo L.S., Huang Z., Dong L., Xu L.Q., Zhu Y., Zeng K. Targeting delivery of anti-TNF alpha oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut. 2010;59:470–479. doi: 10.1136/gut.2009.184556. [DOI] [PubMed] [Google Scholar]
- 43.Huang Z., Ma J.T., Chen M.J., Jiang H.Y., Fu Y., Gan J.J. Dual TNF-alpha/IL-12p40 interference as a strategy to protect against colitis based on miR-16 precursors with macrophage targeting vectors. Mol Ther. 2015;23:1611–1621. doi: 10.1038/mt.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao B., Xu Z., Viennois E., Zhang Y., Zhang Z., Zhang M. Orally Targeted delivery of tripeptide KPV via hyaluronic acid-functionalized nanoparticles efficiently alleviates ulcerative colitis. Mol Ther. 2017;25:1628–1640. doi: 10.1016/j.ymthe.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao B., Zhang Z., Viennois E., Kang Y., Zhang M., Han M.K. Combination therapy for ulcerative colitis: orally targeted nanoparticles prevent mucosal damage and relieve inflammation. Theranostics. 2016;6:2250–2266. doi: 10.7150/thno.15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee Y., Sugihara K., Gillilland M.G., 3rd, Jon S., Kamada N., Moon J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2020;19:118–126. doi: 10.1038/s41563-019-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gou S., Huang Y., Wan Y., Ma Y., Zhou X., Tong X. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials. 2019;212:39–54. doi: 10.1016/j.biomaterials.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Aprodu A., Mantaj J., Raimi-Abraham B., Vllasaliu D. Evaluation of a methylcellulose and hyaluronic acid hydrogel as a vehicle for rectal delivery of biologics. Pharmaceutics. 2019;11:127. doi: 10.3390/pharmaceutics11030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang M., Xu C., Liu D., Han M.K., Wang L., Merlin D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J Crohns Colitis. 2018;12:217–229. doi: 10.1093/ecco-jcc/jjx115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poh S., Putt K.S., Low P.S. Folate-targeted dendrimers selectively accumulate at sites of inflammation in mouse models of ulcerative colitis and atherosclerosis. Biomacromolecules. 2017;18:3082–3088. doi: 10.1021/acs.biomac.7b00728. [DOI] [PubMed] [Google Scholar]
- 51.Poh S., Chelvam V., Ayala-Lopez W., Putt K.S., Low P.S. Selective liposome targeting of folate receptor positive immune cells in inflammatory diseases. Nanomedicine. 2018;14:1033–1043. doi: 10.1016/j.nano.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 52.Xiao B., Yang Y., Viennois E., Zhang Y., Ayyadurai S., Baker M. Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticle. J Mater Chem B. 2014;2:1499–1508. doi: 10.1039/C3TB21564D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao B., Laroui H., Viennois E., Ayyadurai S., Charania M.A., Zhang Y. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology. 2014;146:1289–12300. doi: 10.1053/j.gastro.2014.01.056. e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harel E., Rubinstein A., Nissan A., Khazanov E., Nadler Milbauer M., Barenholz Y. Enhanced transferrin receptor expression by proinflammatory cytokines in enterocytes as a means for local delivery of drugs to inflamed gut mucosa. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du W., Fan Y., Zheng N., He B., Yuan L., Zhang H. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials. 2013;34:794–806. doi: 10.1016/j.biomaterials.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Wu Y., Sun M., Wang D., Li G., Huang J., Tan S. A PepT1 mediated medicinal nano-system for targeted delivery of cyclosporine A to alleviate acute severe ulcerative colitis. Biomater Sci. 2019;7:4299–4309. doi: 10.1039/c9bm00925f. [DOI] [PubMed] [Google Scholar]
- 57.Laroui H., Viennois E., Xiao B., Canup B.S., Geem D., Denning T.L. Fab'-bearing siRNA TNFalpha-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J Control Release. 2014;186:41–53. doi: 10.1016/j.jconrel.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weis W.I., Taylor M.E., Drickamer K. The C-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. doi: 10.1111/j.1600-065x.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 59.Taylor P.R., Gordon S., Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Groger M., Holnthoner W., Maurer D., Lechleitner S., Wolff K., Mayr B.B. Dermal microvascular endothelial cells express the 180-kDa macrophage mannose receptor in situ and in vitro. J Immunol. 2000;165:5428–5434. doi: 10.4049/jimmunol.165.10.5428. [DOI] [PubMed] [Google Scholar]
- 61.East L., Isacke C.M. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 62.East L., Isacke CMJBeBA -G.S. The mannose receptor family. Biochim Biophys Acta (BBA)-General Sub. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y., Chirino A.J., Misulovin Z., Leteux C., Feizi T., Nussenzweig M.C. Crystal structure of the cysteine-rich domain of mannose receptor complexed with a sulfated carbohydrate ligand. J Exp Med. 2000;191:1105–1116. doi: 10.1084/jem.191.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leteux C., Chai W., Loveless R.W., Yuen C.T., Uhlin-Hansen L., Combarnous Y. The cysteine-rich domain of the macrophage mannose receptor is a multispecific lectin that recognizes chondroitin sulfates A and B and sulfated oligosaccharides of blood group Lewis(a) and Lewis(x) types in addition to the sulfated N-glycans of lutropin. J Exp Med. 2000;191:1117–1126. doi: 10.1084/jem.191.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Pomares L., Wienke D., Stillion R., McKenzie E.J., Arnold J.N., Harris J. Carbohydrate-independent recognition of collagens by the macrophage mannose receptor. Eur J Immunol. 2006;36:1074–1082. doi: 10.1002/eji.200535685. [DOI] [PubMed] [Google Scholar]
- 66.Taylor M.E., Bezouska K., Drickamer K. Contribution to ligand binding by multiple carbohydrate-recognition domains in the macrophage mannose receptor. J Biolog Chem. 1992;267:1719–1726. [PubMed] [Google Scholar]
- 67.Gren S.T., Grip O. Role of monocytes and intestinal macrophages in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:1992–1998. doi: 10.1097/MIB.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Y., Tao H., Wang A., Zhong Z., Wu X., Wang M. Chinese herb pair Paeoniae Radix Alba and Atractylodis Macrocephalae Rhizoma suppresses LPS-induced inflammatory response through inhibiting MAPK and NF-kappaB pathway. Chin Med. 2019;14:2. doi: 10.1186/s13020-019-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panettieri R.A., Schaafsma D., Amrani Y., Koziol-White C., Ostrom R., Tliba O. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci. 2019;40:38–49. doi: 10.1016/j.tips.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ford A.C., Bernstein C.N., Khan K.J., Abreu M.T., Marshall J.K., Talley N.J. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590–599. doi: 10.1038/ajg.2011.70. [DOI] [PubMed] [Google Scholar]
- 71.D'Addio S.M., Baldassano S., Shi L., Cheung L.L., Adamson D.H., Bruzek M. Optimization of cell receptor-specific targeting through multivalent surface decoration of polymeric nanocarriers. J Control Release. 2013;168:41–49. doi: 10.1016/j.jconrel.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi K., Donovan M.J., Rogers R.A., Ezekowitz R.A.B. Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res. 1998;292:311–323. doi: 10.1007/s004410051062. [DOI] [PubMed] [Google Scholar]
- 73.Sano Y., Usami K., Izawa R., Denda-Nagai K., Higashi N., Kimura T. Properties of blocking and non-blocking monoclonal antibodies specific for human macrophage galactose-type C-type lectin (MGL/ClecSF10A/CD301) J Biochem. 2007;141:127–136. doi: 10.1093/jb/mvm017. [DOI] [PubMed] [Google Scholar]
- 74.Zizzari I.G., Napoletano C., Battisti F., Rahimi H., Caponnetto S., Pierelli L. MGL receptor and immunity: when the ligand can make the difference. J Immunol Res. 2015;2015:450695. doi: 10.1155/2015/450695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ng W.C., Liong S., Tate M.D., Irimura T., Denda-Nagai K., Brooks A.G. The macrophage galactose-type lectin can function as an attachment and entry receptor for influenza virus. J Virol. 2014;88:1659–1672. doi: 10.1128/JVI.02014-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vulliemoz M., Brand S., Juillerat P., Mottet C., Ben-Horin S., Michetti P. TNF-alpha blockers in inflammatory bowel diseases: practical recommendations and a user's guide: an update. Digestion. 2020;101(suppl 1):1–11. doi: 10.1159/000506898. [DOI] [PubMed] [Google Scholar]
- 77.Aouadi M., Tesz G.J., Nicoloro S.M., Wang M., Chouinard M., Soto E. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature. 2009;458:1180–1184. doi: 10.1038/nature07774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mizoguchi A., Yano A., Himuro H., Ezaki Y., Sadanaga T., Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465–474. doi: 10.1007/s00535-017-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jing Q., Huang S., Guth S., Zarubin T., Motoyama A., Chen J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 80.D'Souza A.A., Devarajan P.V. Asialoglycoprotein receptor mediated hepatocyte targeting - strategies and applications. J Control Release. 2015;203:126–139. doi: 10.1016/j.jconrel.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 81.Sneath R.J., Mangham D.C. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 1998;51:191–200. doi: 10.1136/mp.51.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hankard G.F., Cezard J.P., Aigrain Y., Navarro J., Peuchmaur M. CD44 variant expression in inflammatory colonic mucosa is not disease specific but associated with increased crypt cell proliferation. Histopathology. 1998;32:317–321. doi: 10.1046/j.1365-2559.1998.00404.x. [DOI] [PubMed] [Google Scholar]
- 83.Morath I., Hartmann T.N., Orian-Rousseau V. CD44: more than a mere stem cell marker. Int J Biochem Cell Biol. 2016;81:166–173. doi: 10.1016/j.biocel.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 84.Konnengiesser K., Maaser C., Heidemann J., Luegering A., Ross M., Brzoska T. Melanocortin-derived tripeptide KPV has anti-inflammatory potential in murine models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:324–331. doi: 10.1002/ibd.20334. [DOI] [PubMed] [Google Scholar]
- 85.Dalmasso G., Charrier-Hisamuddin L., Nguyen H.T., Yan Y., Sitaraman S., Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134:166–178. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laroui H., Dalmasso G., Nguyen H.T., Yan Y., Sitaraman S.V., Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138:843–853. doi: 10.1053/j.gastro.2009.11.003. e1–2. [DOI] [PubMed] [Google Scholar]
- 87.Lubbad A., Oriowo M.A., Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322:127–135. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- 88.Petrey A.C., de la Motte C.A. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Senbanjo L.T., Chellaiah M.A. CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol. 2017;5:18. doi: 10.3389/fcell.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheung A., Bax H.J., Josephs D.H., Ilieva K.M., Pellizzari G., Opzoomer J. Targeting folate receptor alpha for cancer treatment. Oncotarget. 2016;7:52553–52574. doi: 10.18632/oncotarget.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Locasale J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Shannessy D.J., Yu G., Smale R., Fu Y.S., Singhal S., Thiel R.P. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–425. doi: 10.18632/oncotarget.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shen F., Wu M., Ross J.F., Miller D., Ratnam M. Folate receptor-type-gamma is primarily a secretory protein due to lack of an efficient signal for glycosylphosphatidylinositol modification—protein characterization and cell-type specificity. Biochemistry-US. 1995;34:5660–5665. doi: 10.1021/bi00016a042. [DOI] [PubMed] [Google Scholar]
- 94.Tian Y., Wu G., Xing J.C., Tang J., Zhang Y., Huang Z.M. A novel splice variant of folate receptor 4 predominantly expressed in regulatory T cells. BMC Immunol. 2012;13:30. doi: 10.1186/1471-2172-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu Y., Wang B., Shen J., Low S.A., Putt K.S., Niessen H.W.M. Depletion of activated macrophages with a folate receptor-beta-specific antibody improves symptoms in mouse models of rheumatoid arthritis. Arthritis Res Ther. 2019;21:143. doi: 10.1186/s13075-019-1912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shim S., Kim S., Choi D.S., Kwon Y.B., Kwon J. Anti-inflammatory effects of [6]-shogaol: potential roles of HDAC inhibition and HSP70 induction. Food Chem Toxicol. 2011;49:2734–2740. doi: 10.1016/j.fct.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 97.Vaitilingam B., Chelvam V., Kularatne S.A., Poh S., Ayala-Lopez W., Low P.S. A folate receptor-alpha-specific ligand that targets cancer tissue and not sites of inflammation. J Nucl Med. 2012;53:1127–1134. doi: 10.2967/jnumed.111.099390. [DOI] [PubMed] [Google Scholar]
- 98.Pan Y., Liu Y., Guo H., Jabir M.S., Liu X., Cui W. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. doi: 10.3390/nu9040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chapkin R.S., Kamen B.A., Callaway E.S., Davidson L.A., George N.I., Wang N. Use of a novel genetic mouse model to investigate the role of folate in colitis-associated colon cancer. J Nutr Biochem. 2009;20:649–655. doi: 10.1016/j.jnutbio.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan Y., Vasudevan S., Nguyen H.T., Merlin D. Intestinal epithelial CD98: an oligomeric and multifunctional protein. Biochim Biophys Acta. 2008;1780:1087–1092. doi: 10.1016/j.bbagen.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Charania M.A., Laroui H., Liu H., Viennois E., Ayyadurai S., Xiao B. Intestinal epithelial CD98 directly modulates the innate host response to enteric bacterial pathogens. Infect Immun. 2013;81:923–934. doi: 10.1128/IAI.01388-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen H.T., Dalmasso G., Yan Y., Laroui H., Dahan S., Mayer L. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem. 2010;285:1479–1489. doi: 10.1074/jbc.M109.057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen H.T., Merlin D. Homeostatic and innate immune responses: role of the transmembrane glycoprotein CD98. Cell Mol Life Sci. 2012;69:3015–3026. doi: 10.1007/s00018-012-0963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nguyen H.T., Dalmasso G., Torkvist L., Halfvarson J., Yan Y., Laroui H. CD98 expression modulates intestinal homeostasis, inflammation, and colitis-associated cancer in mice. J Clin Invest. 2011;121:1733–1747. doi: 10.1172/JCI44631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matea C.T., Mocan T., Tabaran F., Pop T., Mosteanu O., Puia C. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomed. 2017;12:5421–5431. doi: 10.2147/IJN.S138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu Q., Talton J., Zhang G., Cunningham T., Wang Z., Waters R.C. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med. 2012;18:1291–1296. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang M.Z., Wang X.Y., Han M.K., Collins J.F., Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12:1927–1943. doi: 10.2217/nnm-2017-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gammella E., Buratti P., Cairo G., Recalcati S. The transferrin receptor: the cellular iron gate. Metallomics. 2017;9:1367–1375. doi: 10.1039/c7mt00143f. [DOI] [PubMed] [Google Scholar]
- 109.Pallone F., Fais S., Squarcia O., Biancone L., Pozzilli P., Boirivant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987;28:745–753. doi: 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou J., Li M., Lim W.Q., Luo Z., Phua S.Z.F., Huo R. A transferrin-conjugated hollow nanoplatform for redox-controlled and targeted chemotherapy of tumor with reduced inflammatory reactions. Theranostics. 2018;8:518–532. doi: 10.7150/thno.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu L.P., Ahmadvand D., Su J., Hall A., Tan X., Farhangrazi Z.S. Crossing the blood–brain-barrier with nanoligand drug carriers self-assembled from a phage display peptide. Nat Commun. 2019;10:4635. doi: 10.1038/s41467-019-12554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tirosh B., Khatib N., Barenholz Y., Nissan A., Rubinstein A. Transferrin as a luminal target for negatively charged liposomes in the inflamed colonic mucosa. Mol Pharm. 2009;6:1083–1091. doi: 10.1021/mp9000926. [DOI] [PubMed] [Google Scholar]
- 113.Zhang D., Lee H.F., Pettit S.C., Zaro J.L., Huang N., Shen W.C. Characterization of transferrin receptor-mediated endocytosis and cellular iron delivery of recombinant human serum transferrin from rice (Oryza sativa L.) BMC Biotechnol. 2012;12:92. doi: 10.1186/1472-6750-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merlin D., Si-Tahar M., Sitaraman S.V., Eastburn K., Williams I., Liu X. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. 2001;120:1666–1679. doi: 10.1053/gast.2001.24845. [DOI] [PubMed] [Google Scholar]
- 115.Adibi S.A. The oligopeptide transporter (Pept-1) in human intestine: biology and function. Gastroenterology. 1997;113:332–340. doi: 10.1016/s0016-5085(97)70112-4. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y., Hu Y., Li P., Weng Y., Kamada N., Jiang H. Expression and regulation of proton-coupled oligopeptide transporters in colonic tissue and immune cells of mice. Biochem Pharmacol. 2018;148:163–173. doi: 10.1016/j.bcp.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng M.Y., Shao A.D., Li H., Tang Y., Li Q.A., Guo Z.Q. Peptide receptor-targeted fluorescent probe: visualization and discrimination between chronic and acute ulcerative colitis. Acs Appl Mater Inter. 2017;9:13029–13036. doi: 10.1021/acsami.7b00936. [DOI] [PubMed] [Google Scholar]
- 118.Liddicoat A.M., Lavelle E.C. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol. 2019;163:472–480. doi: 10.1016/j.bcp.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 119.Miyake M., Fujishima M., Nakai D. Inhibitory potency of marketed drugs for ulcerative colitis and Crohn's disease on PEPT1. Biol Pharm Bull. 2017;40:1572–1575. doi: 10.1248/bpb.b17-00181. [DOI] [PubMed] [Google Scholar]
- 120.Cassado A.D. F4/80 as a major macrophage marker: the case of the peritoneum and spleen. Results Probl Cell Differ. 2017;62:161–179. doi: 10.1007/978-3-319-54090-0_7. [DOI] [PubMed] [Google Scholar]
- 121.Gordon S., Hamann J., Lin H.H., Stacey M. F4/80 and the related adhesion-GPCRs. Eur J Immunol. 2011;41:2472–2476. doi: 10.1002/eji.201141715. [DOI] [PubMed] [Google Scholar]
- 122.Liu G.H., Liu H.M., Chen Y.S., Lee T.Y. Effect of electroacupuncture in mice with dextran sulfate sodium-induced colitis and the influence of gut microbiota. Evid Based Complement Alternat Med. 2020;2020:2087903. doi: 10.1155/2020/2087903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim H.J., Jung Y.J. The Emerging role of eosinophils as multifunctional leukocytes in health and dsisease. Immune Netw. 2020;20 doi: 10.4110/in.2020.20.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 125.Danese S., Semeraro S., Marini M., Roberto I., Armuzzi A., Papa A. Adhesion molecules in inflammatory bowel disease: therapeutic implications for gut inflammation. Dig Liver Dis. 2005;37:811–818. doi: 10.1016/j.dld.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 126.Ghaffarian R., Bhowmick T., Muro S. Transport of nanocarriers across gastrointestinal epithelial cells by a new transcellular route induced by targeting ICAM-1. J Control Release. 2012;163:25–33. doi: 10.1016/j.jconrel.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eniola A.O., Rodgers S.D., Hammer D.A. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 2002;23:2167–2177. doi: 10.1016/s0142-9612(01)00349-0. [DOI] [PubMed] [Google Scholar]
- 128.Sakhalkar H.S., Dalal M.K., Salem A.K., Ansari R., Fu J., Kiani M.F. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2003;100:15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mane V., Muro S. Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice. Int J Nanomed. 2012;7:4223–4237. doi: 10.2147/IJN.S34105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peer D., Park E.J., Morishita Y., Carman C.V., Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zimmerman T., Blanco F.J. Inhibitors targeting the LFA-1/ICAM-1 cell-adhesion interaction: design and mechanism of action. Curr Pharmaceut Des. 2008;14:2128–2139. doi: 10.2174/138161208785740225. [DOI] [PubMed] [Google Scholar]
- 132.Villanueva F.S., Jankowski R.J., Klibanov S., Pina M.L., Alber S.M., Watkins S.C. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998;98:1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- 133.Triantafillidis J.K., Nasioulas G., Kosmidis P.A. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. [PubMed] [Google Scholar]
- 134.Langelueddecke C., Roussa E., Fenton R.A., Thevenod F. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Biagioli M., Carino A., Cipriani S., Francisci D., Marchiano S., Scarpelli P. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J Immunol. 2017;199:718–733. doi: 10.4049/jimmunol.1700183. [DOI] [PubMed] [Google Scholar]
- 136.Xu X., Yang W.J., Liang Q.J., Shi Y.A., Zhang W.X., Wang X. Efficient and targeted drug/siRNA co-delivery mediated by reversibly crosslinked polymersomes toward anti-inflammatory treatment of ulcerative colitis (UC) Nano Res. 2019;12:659–667. [Google Scholar]
- 137.Cai Z., Zhang W., Yang F., Yu L., Yu Z., Pan J. Immunosuppressive exosomes from TGF-beta1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2012;22:607–610. doi: 10.1038/cr.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mao F., Wu Y., Tang X., Kang J., Zhang B., Yan Y. Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. BioMed Res Int. 2017;2017:5356760. doi: 10.1155/2017/5356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang R., Liao Y., Wang L., He P., Hu Y., Yuan D. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi: 10.3389/fimmu.2019.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang M., Viennois E., Prasad M., Zhang Y., Wang L., Zhang Z. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang B., Zhuang X., Deng Z.B., Jiang H., Mu J., Wang Q. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22:522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Molinaro R., Corbo C., Martinez J.O., Taraballi F., Evangelopoulos M., Minardi S. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15:1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Norling L.V., Spite M., Yang R., Flower R.J., Perretti M., Serhan C.N. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu Y., Briley K., Tao X. Nanoparticle-based imaging of inflammatory bowel disease. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:300–315. doi: 10.1002/wnan.1357. [DOI] [PubMed] [Google Scholar]
- 145.Tontini G.E., Vecchi M., Pastorelli L., Neurath M.F., Neumann H. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J Gastroenterol. 2015;21:21–46. doi: 10.3748/wjg.v21.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ye M., Qian Y., Tang J., Hu H., Sui M., Shen Y. Targeted biodegradable dendritic MRI contrast agent for enhanced tumor imaging. J Control Release. 2013;169:239–245. doi: 10.1016/j.jconrel.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 147.Khademi S., Sarkar S., Shakeri-Zadeh A., Attaran N., Kharrazi S., Ay M.R. Folic acid–cysteamine modified gold nanoparticle as a nanoprobe for targeted computed tomography imaging of cancer cells. Mat Sci Eng C-Mater. 2018;89:182–193. doi: 10.1016/j.msec.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 148.Wang J., Zhang B.L., Yang G., Su L.C., Wang L., Gao F.B. Transferrin-conjugated superparamagnetic iron oxide nanoparticles as in vivomagnetic resonance imaging contrast agents. J Nanosci Nanotechnol. 2020;20:2018–2024. doi: 10.1166/jnn.2020.17311. [DOI] [PubMed] [Google Scholar]
- 149.Finnberg N.K., Liu Y., El-Deiry W.S. Detection of DSS-induced gastrointestinal mucositis in mice by non-invasive optical near-infrared (NIR) imaging of cathepsin activity. Cancer Biol Ther. 2013;14:736–741. doi: 10.4161/cbt.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Matsumura Y., Gotoh M., Muro K., Yamada Y., Shirao K., Shimada Y. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004;15:517–525. doi: 10.1093/annonc/mdh092. [DOI] [PubMed] [Google Scholar]
- 151.Pirollo K.F., Nemunaitis J., Leung P.K., Nunan R., Adams J., Chang E.H. Safety and efficacy in advanced solid tumors of a targeted nanocomplex carrying the p53 gene used in combination with docetaxel: a phase 1b study. Mol Ther. 2016;24:1697–1706. doi: 10.1038/mt.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Von Hoff D.D., Mita M.M., Ramanathan R.K., Weiss G.J., Mita A.C., LoRusso P.M. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22:3157–3163. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 153.Chen M., Chen R., Shi Y., Wang J.G., Cheng Y.H., Li Y. Malonitrile-functionalized tetraphenylpyrazine: aggregation-induced emission, ratiometric detection of hydrogen sulfide, and mechanochromism. Adv Funct Mater. 2018;28:1704689. [Google Scholar]
- 154.Sen E., Meral K., Atilgan S. From dark to light to fluorescence resonance energy transfer (FRET): polarity-sensitive aggregation-induced emission (AIE)-active tetraphenylethene-fused BODIPY dyes with a very large pseudo-Stokes shift. Chem Eur J. 2016;22:736–745. doi: 10.1002/chem.201503457. [DOI] [PubMed] [Google Scholar]
- 155.Xie Y., Shi B., Xia F., Qi J., Dong X., Zhao W. Epithelia transmembrane transport of orally administered ultrafine drug particles evidenced by environment sensitive fluorophores in cellular and animal studies. J Control Release. 2018;270:65–75. doi: 10.1016/j.jconrel.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 156.Wang T., Qi J., Ding N., Dong X., Zhao W., Lu Y. Tracking translocation of self-discriminating curcumin hybrid nanocrystals following intravenous delivery. Int J Pharm. 2018;546:10–19. doi: 10.1016/j.ijpharm.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 157.Alexis F., Pridgen E., Molnar L.K., Farokhzad O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perrault S.D., Walkey C., Jennings T., Fischer H.C., Chan W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9:1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 159.Avgoustakis K. Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: preparation, properties and possible applications in drug delivery. Curr Drug Deliv. 2004;1:321–333. doi: 10.2174/1567201043334605. [DOI] [PubMed] [Google Scholar]
- 160.Patel M.P., Patel R.R., Patel J.K. Chitosan mediated targeted drug delivery system: a review. J Pharm Pharmaceut Sci. 2010;13:536–557. doi: 10.18433/j3jc7c. [DOI] [PubMed] [Google Scholar]
- 161.Muhammad Q., Jang Y., Kang S.H., Moon J., Kim W.J., Park H. Modulation of immune responses with nanoparticles and reduction of their immunotoxicity. Biomater Sci-UK. 2020;8:1490–1501. doi: 10.1039/c9bm01643k. [DOI] [PubMed] [Google Scholar]
- 162.Lin Q., Fathi P., Chen X. Nanoparticle delivery in vivo: a fresh look from intravital imaging. EBioMedicine. 2020;59:102958. doi: 10.1016/j.ebiom.2020.102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bertrand N., Wu J., Xu X.Y., Kamaly N., Farokhzad O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choi S.Y.C., Lin D., Gout P.W., Collins C.C., Xu Y., Wang Y.Z. Lessons from patient-derived xenografts for better in vitro modeling of human cancer. Adv Drug Deliv Rev. 2014;79–80:222–237. doi: 10.1016/j.addr.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 165.Goettel J.A., Kotlarz D., Emani R., Canavan J.B., Konnikova L., Illig D. Low-dose interleukin-2 ameliorates colitis in a preclinical humanized mouse model. Cell Mol Gastroenter. 2019;8:193–195. doi: 10.1016/j.jcmgh.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mizoguchi A., Takeuchi T., Himuro H., Okada T., Mizoguchi E. Genetically engineered mouse models for studying inflammatory bowel disease. J Pathol. 2016;238:205–219. doi: 10.1002/path.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]