Abstract
Synthetic lethality is a proven effective antitumor strategy that has attracted great attention. Large-scale screening has revealed many synthetic lethal genetic phenotypes, and relevant small-molecule drugs have also been implemented in clinical practice. Increasing evidence suggests that CDKs, constituting a kinase family predominantly involved in cell cycle control, are synthetic lethal factors when combined with certain oncogenes, such as MYC, TP53, and RAS, which facilitate numerous antitumor treatment options based on CDK-related synthetic lethality. In this review, we focus on the synthetic lethal phenotype and mechanism related to CDKs and summarize the preclinical and clinical discoveries of CDK inhibitors to explore the prospect of CDK inhibitors as antitumor compounds for strategic synthesis lethality in the future.
KEY WORDS: Synthetic lethality, Cyclin-dependent kinase, Antitumor therapy, Oncogenes, MYC, P53, RAS, PARP
Graphical abstract
This review summarizes the CDKs-related synthetic lethality cases and the mechanisms, giving a guidance for drug combination antitumor strategies and tumor prediction by specific biomarkers.

1. Introduction
Chemotherapy and target therapy can effectively reduce the symptoms and prolong the survival time of advanced cancer patients1. However, traditional cytotoxic chemotherapeutic drugs are limited by safety and specificity concerns, driving people to seek other antitumor strategies. Since the creative antitumor strategy proposed by Hartwell in 19972, synthetic lethality has gradually entered the field of tumor treatment. The earliest concept of synthetic lethality was proposed based on gene–gene interactions in drosophila3, 4, 5, and it is defined as the mutation of either gene A or gene B is viable in cell while mutations of both gene A and B are lethal6. The concept of synthetic lethality nowadays has been expanded in a broad sense (Fig. 1). For instance, synthetic dosage lethality is caused by gene overexpression combined with another mutated gene7,8; and conditional synthetic lethality is based on genetic mutations or loss of function under certain cellular microenvironmental conditions (such as hypoxia) or genetic signal pathway deregulation9,10. ‘Oncogene addiction’ is a relatively common phenomenon, the tumor is driven by oncogenes and the pathways relevant for its excessive activation and exacerbation, making genotype-target chemotherapy feasible11,12. However, some types of tumors still have no targetable oncogenes or are generated from mutations in cancer suppressors, which are difficult to treat by oncogene-targeted chemotherapy. In these tumors, synthetic lethality shows enormous therapeutic potential for antitumor target identification and drug discovery12.
Figure 1.
The principle of synthetic lethality. When single mutation occurs, the cell can survive as normal. Synthetic lethality results from the interference to two genes that will lead to cell death. From the broad sense, the interference of genes comprises not only mutation or inhibition, but also overexpression and condition stress.
Poly(ADP-ribose) polymerase (PARP) is one of the widely recognized synthetic lethal targets, for which the inhibitor olaparib has received the approval of the FDA and become a treatment of BRCA-mutated triple-negative breast cancer (TNBC) and ovarian cancer13,14. In terms of mechanism, olaparib can inhibit the function of PARPs and inhibit DNA single-strand break (SSB) repair, which can subsequently accumulate DNA double-strand breaks (DSBs) in cancer cells with BRCA1/2 mutations. When BRCA1/2-defective cells cannot repair DSBs via homologous recombination (HR), chromosome deletions, translocations, and death ultimately follow14. However, PARP inhibitors (PARPi) in the treatment of breast cancer and ovarian cancer often are limited by drug resistance caused by the upregulation of PDL1, and the drug combination of a PARPi and an anti-PDL1 is undergoing clinical trials and reported beneficial results15. In addition, PARPi is a potential breast and ovarian cancer therapy in combination with CDKs, PI3K and epigenetic inhibitors16,17. In addition, ATR, a kinase that is a downstream molecule of replication protein A (RPA), can protect cells from replication stress18. Inhibition of ATR causes the accumulation of DNA damage, which requires the ATM/CHK2/P53 signaling pathway to repair19. According to this relation, ATR inhibitors are currently undergoing preclinical trials for treatment of multiple malignancies with ATM/P53 defects20. From this perspective, a synthetic lethal anticancer strategy shows great potential for safety and effectiveness because of its specificity in multigene phenotypes.
Cyclin-dependent kinases (CDKs) constitute a crucial protein family in cell cycle control and are closely associated with tumor occurrence. To date, approximately 20 homologous members are characterized as CDK proteins21, and they consist of several conserved structures, including a catalytic core combined with an activated T-loop motif, a PSTAIRE-like cyclin-binding domain and an ATP-binding pocket, structures that determine the functions of each CDK22. CDKs form protein complexes combined with cyclin (CCN) to precisely regulate the progression of the cell cycle and transcription. For example, the transition through the G1/S phase is regulated by the CDK2–CCNE complex23, and CDK1–CCNB is essential to the G2/M phase transition24. Recently, CDK proteins such as CDK7/8/9/12/13 have also been found to play important roles in transcriptional regulation. CDK7 is crucial in forming the RNA polymerase complex to initiate transcription, which is followed by RNA strand elongation driven by CDK9-cyclin T125. CDK12/13 can phosphorylate the C-terminal domain (CTD) of RNA polymerase II to regulate transcription26, which indicates that CDKs participate in the DNA damage response by controlling relevant protein expression.
To date, many studies have indicated that the deregulation of CDKs can lead to tumorigenesis in certain types of cancer. The following examples will illustrate the point. CDK2 inhibits the differentiation of myeloid cells by activating PRDX2, while inhibition of CDK2 drives differentiation in the five major subtypes of acute myelocytic leukemia (AML)27. CDK4/6-retinoblastoma (Rb) pathway regulates G1/S checkpoints in the cell cycle, and it's a general phenomenon that excessive activation in this pathway leads to booming cell proliferation in various cancers28. As for transcription related CDKs, CDK7 can drive oncogene transcriptional addiction, and inhibiting CDK7 leads to genome instability and activates antitumor immunity in cancer cells29,30. CDK9 inhibition reduces the phosphorylation of BRG1, which contributes to epigenetically silenced genes reactivation, leading to tumor suppressor genes expression and tumor elimination31. To prevent the overactivation of CDKs, CDK protein inhibitors (CKIs), such as P16 and P21, are needed. As they are regarded as tumor suppressor factors, mutations that cause functional CDK inactivation can also promote tumor formation32,33. Therefore, members of the CDK protein family may be promising targets for tumor therapy, especially under conditions of kinase malfunction.
In this review, we summarize several interactive phenotypes and mechanisms between some cancer-related synthetic lethal targets and CDKs. By analyzing data from preclinical and clinical trials of CDK inhibitors, MYC, TP53, RAS, and PARP are treated as potential synthetic lethal partners of CDKs during the process of DNA damage response, apoptosis signal transmission and so on. We hope that the exploration of the strategic use of the potential synthetic lethality of CDKs will offer new directions for the application of CDK inhibitors, especially in tumor therapy.
2. Synthetic lethal pathway associated with CDKs
2.1. MYC and CDKs
MYC is a crucial transcription factor in cell proliferation, cell differentiation, cell cycle control, and apoptosis34. MYC is categorized into three subtypes, C-MYC, N-MYC and L-MYC, and it can promote tumorigenesis by transcriptional regulation. For example, overexpression of MYC can stimulate the G1/S phase transition and cause abnormal proliferation of lung cancer cells35. In addition, N-MYC is an essential actuator for advanced paediatric neuroblastomas, which are mediated by aberrant regulatory elements such as focally amplified distal enhancers and chromosomal translocation due to enhancer hijacking36. However, MYC is difficult to directly target by small-molecule drugs because of the pattern of activation via the bromodomain37. Therefore, researchers have attempted to make use of synthetic lethality to treat tumors with MYC overexpression.
The CDK pan-inhibitor roscovitine inhibited the proliferation of the IMR32 and SHEP-21N neuroblastoma cell lines with N-MYC overexpression (with LC50 levels of 3.0 and 7.5 μmol/L, respectively). Mechanistic studies have shown that P53 and its target gene are involved in apoptosis signaling (TRAIL-R2, FDXR) and are upregulated after CDK2 is inhibited38. The inhibition of CDK1 in tumors overexpressing MYC is lethal. It has been confirmed that roscovitine and purvalanol have better antitumor effects in vivo, as both can inhibit CDK1, in the treatment of MYC-dependent lymphomas and hepatoblastoma tumors39.
The synthetic lethality of MYC and CDK also has tremendous therapeutic potential in breast cancer treatment. The CDK inhibitor dinaciclib in an i.p. dose of 50 mg/kg induced 50% tumor regression in triple-negative breast cancer (TNBC) with MYC overexpression in a xenograft model40. Subsequently, it was determined that only the inhibition of CDK1 could selectively upregulate the pro-apoptotic protein BIM and subsequently cause MYC-dependent synthetic lethality in triple-negative breast cancer cells41. Generally, MYC has played a significant role in CDK1/2-inhibited antitumor treatment in preclinical trials, and RNAi-mediated MYC silencing reduced the rate of CDK1/2 inhibition-dependent cell death from approximately 60%–20%40. The process of inhibiting CDK1/2 in MYC-dependent tumor cells usually involves the apoptosis-inducing signaling pathway, and recently, research has shown that the interaction between CDK2 and MYC can prevent the apoptosis of cancer cells42. However, the mechanism of CDK1 inhibitors in MYC-dependent tumor cells remains ambiguous, and the correlation between MYC, CDK, and apoptosis-inducing factors is worthy of further exploration.
Additionally, transcriptional control by CDKs also has a MYC-dependent synthetic lethal effect. CDK9 is an active kinase for positive transcription elongation factor b (P-TEFb). C-MYC increases P-TEFb transcription and elongation via the recruitment of the CDK9/P-TEFb complex specifically to the promoter, which enables the inhibition of CDK9 to suppress the proliferation of B-cell lymphoma and liver cancer cells with MYC overexpression43,44. This finding, showing the inhibition of MYC expression by a CDK9 inhibitor, provides a strategy by which other MYC-activated targets can be synergized with a CDK9 inhibitor.
2.2. P53 and CDKs
P53 is a tumor suppressor protein that regulates apoptosis, genome stability, and angiogenesis45,46. Approximately 50% of solid tumors carry mutated P53, and this high percentage has drawn attention to P53-related synthetic lethal strategies47. An important basic finding is that silencing or inhibiting CDK2 can disrupt the apoptosis signal of immortalized epithelial cells (HaCaT) and cause the death of P53-deficient HaCaT cells. On a deeper level, when CDK2 is inhibited, a decrease in the phosphorylation at the AKT Ser-473/474 site in the S/G2 phase is observed, which indicates the downregulation of AKT/mTOR pathway activity. In addition, BCL2-associated agonist of cell death (BAD) reduces Ser-155 phosphorylation, which implies that BAD can effectively form a dimer with BCL-xL and thus induce apoptosis. This discovery reveals that CDK2 is correlated with the PI3K/AKT/mTOR signaling pathway and shows additional potential for CDK inhibitors in P53-independent apoptosis48. Furthermore, CDK1/2 and PI3K are a pair of powerful synthetic lethality targets in the treatment of malignant glioma, and the cooperation of CDK1/2 and PI3K inhibitors leads to the depletion of the antiapoptotic protein survivin and shows clinical therapeutic potential in glioma xenografts49.
CDK inhibition is also lethal to P53-mutant cancer cells by disrupting the DNA damage response (DDR). The administration of the pan-CDK inhibitor roscovitine had a synthetic lethal effect on P53-mutated TNBC cells before doxorubicin treatment. Mechanistic research revealed that the inhibition of CDK1 exacerbated DNA double-strand breaks (DSBs) and suppressed the recruitment of homologous recombination (HR) proteins to repair TNBC cells, which arrested P53-mutant TNBC cells at the G2/M checkpoint, resulting in increased sensitivity to cytotoxic doxorubicin. This therapeutic regimen is more effective and less toxic than the use of doxorubicin alone50.
However, in contrast to their synthetic lethality in P53-mutated cells, CDK7 inhibitors usually depend on P53 to exert activity. Cell death due to the inhibition of CDK7 can lead to P53 overexpression and reduce the expression of anti-apoptotic genes such as MCL-1, survivin, and XIAP in tumor cells and not arrest the cell cycle directly51. In addition, increasing evidence has indicated that CDK7 mainly functions via transcriptional regulation52. In the P53-wild-type HCT116 colorectal cancer cell line, the inhibition of CDK7 and activation of P53 were shown to cause synthetic lethality. Pretreatment with P53 agonist (5-FU/nutlin-3) followed by application of CDK7 inhibitor (THZ1 or YKL-1-116) can lead to cell death, and 1 μmol/L 5-FU can change the IC50 of YKL-1-116 from 0.8 to 0.1 μmol/L. Mechanistic studies have revealed that CDK7 inhibition results in decreased expression of MDM2 and P21 proteins, while the DR5 (death receptor 5) and FAS pathways are activated53. Thus, synthetic lethality strategies for P53-dependent tumors can be based on CDK7 inhibitors, while strategies for P53-defective tumors may be made more efficient via CDK1/2 inhibition.
2.3. RAS and CDKs
The RAS protein is a molecular switch encoded by the RAS genes, which is active when binding with guanosine triphosphate (GTP), and becomes inactive when binding with guanosine diphosphate (GDP)54. RAS mutations are common in human cancers, and tumors generated from K-RAS mutation cause approximately one million deaths worldwide each year, quantitatively similar as malaria and tuberculosis quantitatively55. Therefore, the therapeutic regimen based on RAS mutation phenotype has elicited extensive attention, and the application of synthetic lethal has become a potential breakthrough for poor responsiveness. According to this therapeutic strategy, the proliferation of non-small cell lung cancer (NSCLC) with K-RASG12V mutation can be restrained forcefully by the CDK4 knockout or the CDK4/6 inhibitor palbociclib both in cell and xenograft models56. However, the association between the K-RASG12V mutation and CDK4/6 has not been revealed. The K-RAS mutation can activate MEK/ERK and PI3K/AKT signaling pathways57,58; therefore, it has been proposed that the activation of these two signaling pathways may upregulate the CDK4/6–cyclin D1 complex. Apart from K-RAS, the loss of von Hippel-Lindau (VHL) in renal epithelial cells can increase the expression of cyclin D1, and the functional inhibition of CDK6 and MEK1 in VHL-deficient cells can lead to clear cell renal carcinoma cell death59. Therefore, it is reasonable that K-RAS and CDK4/6 are connected by the MEK/ERK and PI3K/AKT signaling pathways.
Although the mechanism is still ambiguous, the synthetic lethality study of RAS and CDK4/6 has laid an important foundation for the development of CDK4/6 inhibitors. To date, CDK4/6 and RAS-related tumor treatments have been widely used in melanoma, glioblastoma, NSCLC, colorectal, breast, and thyroid cancer60, 61, 62, 63, 64, 65 (Table 138, 39, 40, 41,43,44,48, 49, 50,53,56,59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70). Among these cases, the interaction of CDK4/6-RAS/MAPK pathways and CDK4/6-PI3K/AKT/mTOR pathways also provides a new perspective on cancer therapy. In human hepatic carcinoma, MAPK/ERK activity is positively related to cyclin D1 protein expression, while the CDK4/6–cyclin D1 complex is the upstream regulator of RB phosphorylation and promotes the cell cycle process71,72, which indicates that cyclin D1 may be a key protein that explains the synthetic lethality of CDK4/6 and RAS73. In addition, CDK1, in combination with K-RAS, is also a target for synthetic lethality. Knockdown of CDK1 can inhibit K-RAS-mutated colorectal cancer cells, and subsequently, a strong inhibitory effect has been obtained in 26 types of colorectal and pancreatic tumor cell lines. This phenomenon may be attributed to the reduction in Rb phosphorylation caused by CDK1 inhibition, followed by cell cycle arrest in the G1/S phase66.
Table 1.
Synthesis lethality research related to CDK.
| CDK | Synthetic lethality factor | Synthetic lethality object | Ref. |
|---|---|---|---|
| CDK1 | MYC | Lymphomas and hepatoblastoma | 39 |
| CDK1 | MYC | TNBC | 40,41 |
| CDK1 | P53 mutation | TNBC | 50 |
| CDK1 | K-RAS mutation | K-RAS mutated colorectal cancer cells | 66 |
| CDK1 | PARPs | TNBC | 67,68 |
| CDK2 | N-MYC | Neuroblastoma cell-line IMR32 and SHEP-21N | 38 |
| CDK2 | P53-deficient | HaCaT | 48 |
| CDK1/2 | PI3K | Glioma | 49 |
| CDK4/6 | K-RASG12V mutation | NSCLC | 56 |
| CDK4/6 | MEK1 | VHL-deficiency clear cell renal cell carcinomas | 59 |
| CDK4/6 | MEK | K-RAS mutant colorectal cancer cell | 60 |
| CDK4/6 | MEK | NSCLC with K-RAS expression | 61 |
| CDK4/6 | mTOR | Glioblastoma (GBM) | 62 |
| CDK4/6 | RAF | K-RAS, N-RAS or BRAF mutant tumor | 63 |
| CDK4/6 | MEK1/2 | N-RAS mutant melanoma | 64 |
| CDK4/6 | H-RAS | Anaplastic thyroid carcinomas (ATCs) | 65 |
| CDK7 | P53 agonist | Colorectal cancer cell HCT116 | 53 |
| CDK9 | C-MYC | B-cell lymphoma and liver cancer cells | 43,44 |
| CDK12 | PARPs | EWS/FLI-mutations in Ewing sarcoma | 69 |
| CDK12 | PARPs | TNBC | 70 |
2.4. PARPs and CDKs
Poly(ADP-ribose) polymerase (PARP) is significant in DNA replication and DNA damage repair74,75. When DNA single-strand breaks (SSBs) occur, PARP1/2, as a constituent of the base excision repair (BER) complex, binds with DNA ligase III, DNA polymerase beta, and XRCC1 to repair broken DNA single-strands76. Excessive activation of PARPs is usually followed by depletion of NAD+, alteration of mitochondrial membrane permeability, release of AIF and cytochrome c, and ultimately apoptosis77,78. PARP inhibitors have shown effects in tumor therapy when applied alone or combined with cytotoxic drugs79,80. However, PARP1/2 inhibitors usually aim at tumors with BRCA1/2 mutations, which make homologous recombination (HR) deficient in repairing DNA double-strand breaks (DSBs)81. Only when both SSB and DSB repair fail can synthetic lethality be leveraged, and this requirement has prevented greater widespread use of PARP inhibitors in tumor therapy.
In the past decade, scientists have discovered that CDK and PARPs are synthetically lethal in BRCA-wide type tumor cells and that PARP inhibitors have great potential in tumor treatment. CDK1 is essential in many processes of HR repair; therefore, inhibiting CDK1 can achieve the effect of BRCA1 mutation and increase the sensitivity of TNBC cells to PARP inhibitors by more than 100-fold67,68. The combination of the CDK pan-inhibitor dinaciclib with the PARP1/2 inhibitor ABT-888 is useful for treating melanoma (MM). Dinaciclib was been proven to decrease the protein levels of RAD51 and impair the phosphorylation of BRCA1, which means that dinaciclib directly blocks the HR repair of chromosomal DSBs82. Moreover, the combination of the CDK12 inhibitor THZ531/THZ1 and the PARP inhibitor olaparib has exhibited an excellent curative effect on Ewing's sarcoma. On the one hand, the occurrence of EWS/FLI mutations in Ewing sarcoma results in DNA damage repair defects, leading to sensitivity to the DNA damage response (DDR) inhibitor. On the other hand, the CDK12 inhibitor THZ531 was confirmed to be effective in impairing HR and preventing damaged DNA from recruiting RAD5169. The CDK12 inhibitor SR-4835 can induce a ‘BRCAness’ phenotype that is similar as a BRCA mutation phenotype. At the molecular level, the expression of ATR, ATM, RAD51, and other cell cycle checkpoint proteins is decreased, while at the cellular level, HR repair cannot be completed, and TNBC is lethal when SR-4835 is used with olaparib70. However, the mechanism of how homologous recombination repair defects are caused by CDK inhibition needs to be further elucidated. The latest research has revealed that CDK12 inhibits the premature cleavage of poly A and affects the extension of long-chain (>45 kb) mRNA, resulting in the abnormal expression of HR repair-related genes83.
CDK1/12 inhibitors can be used in a potential strategy to induce the ‘BRCAness’ phenotype, and combining CDK1/12 inhibitors and PARP inhibitors can be used in the treatment of malignant tumors. However, the side effects of the ‘BRCAness’ phenotype induced by CDK12 inhibitors in vivo remain unclear, and the tolerance of normal cells needs to be examined when CDK1/12 inhibitors are combined with PARP inhibitors. Although CDK12/13 inhibitors combined with PARP inhibitors have great prospects for synthetic lethality, they have not yet entered clinical trials. However, their subsequent development deserves continuous attention (Table 1).
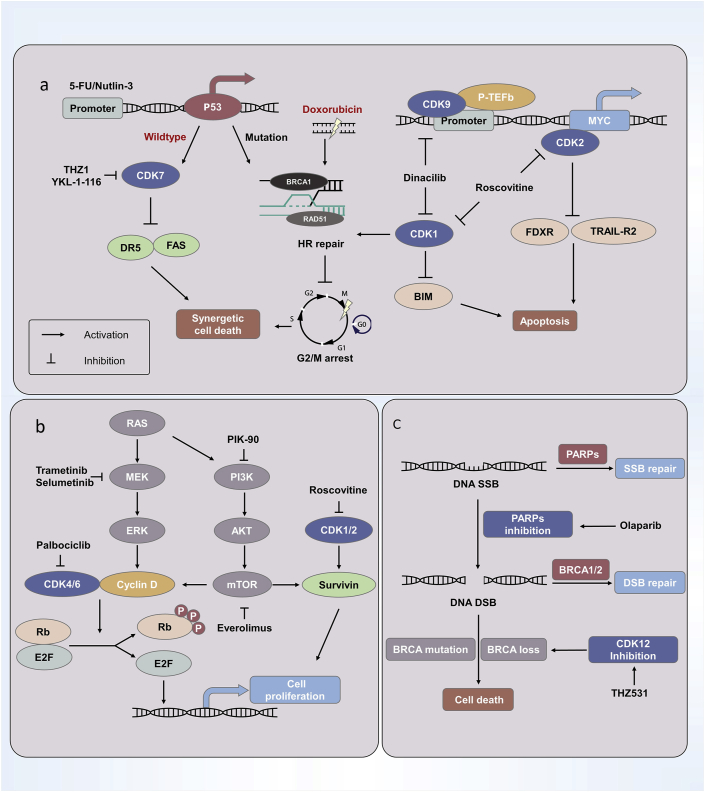
Fig. 2 illustrates the mechanism of CDK relevant synthetic lethality.
Figure 2.
The mechanism of CDK relevant synthetic lethality. a) The inhibition of CDK1/2/9 in cancer cells with MYC amplification can induce the apoptosis pathway and lead to synthetic lethality. Meanwhile, there are some synergistic strategies based on the synthetic lethality, such as CDK7 inhibitors plus 5-FU/Nutlin-3 in P53-wildtype cells, and CDK1 inhibitors combining with doxorubicin in P53-mutation cells. b) RAS regulates the expression of cyclin D via the MAPK and PI3K signal pathway, and it is a synthetic lethal factor in combination with CDK4/6 or with CDK1/2. c) PARPs are the proteins responsible for DNA single-strand break (SSB) repair, and BRCA1/2 are known as DNA double-strand break (DSB) homologous recombination (HR) repair factors. CDK12 inhibition can result in the BRCA-loss phenotype, which makes PARPs and CDK12 inhibitors be able to be a valid synthetic lethality anti-tumor strategy in the future.
3. Clinical trials of CDK inhibitors
Considering that a large number of CDKs have indispensable roles in tumor progression and that strategies based on synthetic lethality induced by CDKs such as CDK4/6 and CDK1/2/5/9 have been clearly verified, clinical trials of CDK inhibitors have been carried out on a large scale. CDK-related synthetic lethality provides hints for possible clinical drug combinations. Here, we summarize the remarkable completed and ongoing clinical trials.
3.1. CDK inhibitors with low specificity
Dinaciclib exerts effects on the treatment of blood cancer. Dinaciclib is a novel selective inhibitor of CDK1/2/5/9 (IC50 < 5 nmol/L), and clinical trials show that dinaciclib is well tolerated at a 50 mg/m2 intravenous (i.v.) dose, with adverse events consisting of myelosuppression and gastrointestinal toxicities84. However, the significant antitumor activity of dinaciclib was observed only in the treatment of chronic lymphocytic leukaemia (CLL)85, and not in the treatment of NSCLC or breast cancer84,86. It is inspiring that dinaciclib shows promising anti-leukaemia activity, compared to ofatumumab, in relapsed/refractory CLL. For patients receiving dinaciclib, the median PFS/OS equals 13.7/21.2 months, respectively, which exceeds the median PFS/OS of 5.9/16.7 months for patients receiving ofatumumab, although the sample size in this study was limited to only 44 patients87. Dinaciclib has been undergoing further optimization through other drug delivery modes and synergistic strategies88 (Table 2).
Table 2.
Clinical trials on CDK inhibitors with synthetic lethality potential.
| Inhibitor | Inhibition of CDK | Clinical applicability | Status | Ref. |
|---|---|---|---|---|
| Palbociclib (combination with letrozole, an aromatase inhibitor) | CDK4/6 | ER+ and HER– breast cancer | FDA approved | 89,90 |
| Palbociclib (combination with cetuximab, an inhibitor of EGFR) | CDK4/6 | Head and neck squamous-cell carcinomas (HNSCCs) | Phase II | 91 |
| Palbociclib (combination with cetuximab, an inhibitor of EGFR) | CDK4/6 | K-RAS, N-RAS and BRAF wild-type metastatic colorectal cancer (CRC) | Phase II | 92 |
| Palbociclib (combination with MEK162, an inhibitor of MEK) | CDK4/6 | K-RAS and N-RAS mutant metastatic colorectal cancers (CRC) | Phase II | 93 |
| Palbociclib (combination with gedatolisib, a mTOR inhibitor) | CDK4/6 | Lung cancer squamous cell, cancers of head, neck and pancreas | Phase I | 94 |
| Dinaciclib (MK-7965) (combination with MK2206, an AKT inhibitor) | CDK1/2/5/9 | Pancreatic cancer | Phase I | 95 |
| Dinaciclib (combination with pembrolizumab, a monoclonal anti-PD-L1) | CDK1/2/5/9 | Hematologic malignancies | Phase I | 96 |
| Dinaciclib (combination with ofatumumab, a monoclonal anti-CD20) | CDK1/2/5/9 | Relapsed/refractory CLL | Phase III | 87 |
| Alvocidib (combination with venetoclax, a BCL-2 inhibitor) | CDK1/2/4/9 | AML | Phase I | 97,98 |
Alvocidib, also known as flavopiridol, is a traditional CDK1/2/4/9 inhibitor that shows limited antitumor activity when used alone. Alvocidib in combination with cytarabine and mitoxantrone is an emerging treatment for CLL and AML and shows encouraging effects in clinical trials99, 100, 101. In addition, a combination scheme of alvocidib and BCL-2 inhibitors is undergoing phase I clinical trials and may be a potential synthetic lethality strategy for AML (Table 2). However, not all CDK inhibitors have yielded ideal clinical results. Roscovitine, as an inhibitor of CDK1/2/5/7/9, has an impact on the fertilization in mice and exhibits bone marrow toxicity102, and roscovitine alone has not shown obvious antitumor effects on NSCLC in clinical trials103, which suggests that the indication for the use of roscovitine needs further exploration.
We acknowledge that, while synthetic lethality is easy to conceptually define, the clinical application of this strategy may not have a very clear boundary, especially inhibitors with low specificity. CDK inhibitors with low specificity may have many potential inhibiting targets in theory104. As a result, they are usually detrimental in vivo and not suitable for synthetic lethality antitumor therapy in clinical practice unless the preclinical trial has determined the specific synthetic lethality target. Recent studies have made great efforts to improve the selectivity of CDK inhibitors, and fortunately, some of the inhibitors with higher selectivity and pan-inhibitors have been used in optimized drug combination schemes. For creating inhibitors with higher selectivity, switching the target to those with better targeting feasibility and lower off-target probability is one of the solutions. The alternative inhibitor targets, such as CDK7, CDK9, and CDK12/13, are more closely related to transcriptional regulation and DDR105, 106, 107, and these CDKs are the targets that generate less impact on the normal cell cycle and show enormous potential in antitumor chemotherapy and target therapy based on synthetic lethality.
3.2. CDK inhibitors with high specificity
With regard to the optimization of drug combinations, mounting efforts have led to great breakthroughs. As the CDK4/6 inhibitor palbociclib has been approved by the FDA for TNBC treatment, a synthetic lethal strategy based on palbociclib was tried in the treatment of various types of cancer in clinical trials (as shown in Table 2).
The selective CDK4/6 inhibitor palbociclib plus letrozole or fulvestrant successfully became the first-line target therapy for oestrogen receptor-positive (ER+) breast cancer89,90. As shown in previous research, the application of letrozole does not have a strong therapeutic effect against advanced breast cancer and might lead to poor cost-effectiveness ratios because of the drug resistance resulting from involvement of the cyclin D1-RB signaling pathway108,109. In addition, in a phase II study group of ER+ breast cancer with amplification of cyclin D1, palbociclib plus letrozole was a safe and efficient treatment, as the median progression-free survival was 26.1 months, much higher than that based on the use of letrozole alone (5.7 months)90. This finding indicates that the development of CDK inhibitors has led to significant progress. However, the CDK4/6 inhibitor resistance in ER+ breast cancer has attracted researchers’ attention, and therefore it might be resolved through combination therapy with PI3K/AKT/mTOR signaling inhibitors and CDK4/6 inhibitors108.
Moreover, the CDK4/6 inhibitor palbociclib has also recently been confirmed to induce the upregulation of PD-L1 in breast cancer xenograft models. Mechanistic analysis showed that the inhibition of the CDK4–cyclin D interaction reduces the phosphorylation of SPOP and ubiquitin-dependent degradation of PD-L1, resulting in the upregulation of PD-L1 and drug resistance in breast cancer110. Therefore, a strategy of synthetic lethality with CDK4 and PD-L1 inhibition can also be considered to address the problem of drug resistance in breast cancer. In addition, because compensatory enhancement of the cyclin D1-RB-E2F signaling pathway results from the inhibition of EGFR and may lead to drug resistance to EGFR inhibitors, the administration of CDK4/6 and EGFR is synergistic111. To prove the therapeutic effect of this strategy, Adkins et al.91 examined the combination of the CDK4/6 inhibitor palbociclib and the EGFR inhibitor cetuximab in the treatment of platinum resistance in head and neck squamous-cell carcinomas (HNSCCs) in phase I/II clinical trials. In one trial, patients were categories into two groups: patients who were resistant to platinum and were sensitive to cetuximab (group 1) and patients who were resistant to cetuximab (group 2). The results indicated that the proportion of patients with objective response was encouraging and higher than that of patients treated with cetuximab. The adverse reactions were similar as those caused by monotherapy and were generally tolerable, although the reaction duration was short (group 1/2 equals 4.0/6.0 months), remaining for further study91.
Apart from palbociclib, many other CDK inhibitors are combined with PI3K/AKT/mTOR signaling inhibitors or PD-L1 monoclonal antibodies in clinical studies, and their synergistic effects can also be explained from the perspective of synthetic lethality. As described above, the inhibition of CDK1/2 and PI3K in malignant glioma is lethal because of the pro-apoptotic effects activated in malignant glioma49, which implies a relationship between CDK and the PI3K/AKT/mTOR signaling pathway. Using combination tumor immunotherapy, recent studies have shown that C-MYC and CDK9 are interaction partners of BRD4 and PD-L1. C-MYC regulates the expression of PD-L1, and CDK9 is one of the regulating factors of MYC112, which is the key to the effects of a combination of CDK9 and a PD-L1 monoclonal antibody. Hence, the interaction between CDK and PD-L1 provides a new direction for applications of synthetic lethality.
4. Conclusions and prospects
With the development of RNAi, CRISPR, statistical genetics, and bioinformatics10,113, synthetic lethality has been exploited to develop anticancer therapeutics, and CDK has been confirmed to have synthetic lethal effects with many cancer-related genes, such as MYC, TP53, RAS, and PARP. As a result, the status of CDK in cancer treatment has been continuously enhanced, which has attracted increasing attention for the development of CDK inhibitors. However, notably, many mechanisms of synthetic lethality related to CDK have not been fully elucidated, and many potential synthetic lethal targets related to CDK have not been discovered, which requires further exploration and effort. In the future, CDK antitumor strategy research based synthetic lethality will mainly focus on aspects that include the exploration of targets with potential synthesis lethality, CDK-related synthetic lethal mechanism clarification, and strategic application of synthetic lethality using more-selective CDK inhibitors.
Notably, traditional synthetic lethal discovery depends on molecular biology methodology at the cellular level to screen genes with cell viability and cell death indicators. Although the association of synthetic lethal genes was readily established, the overall impact of the microenvironment on tumor tissue has been ignored, which results in differences in screening outcomes of components with synthetic lethality in vitro and clinical trials. Moreover, it is difficult to determine the conditional synthetic lethality caused by the tumor microenvironment or immune response, which adds additional requirements for the selection of the models and biomarkers of synthetic lethality. Recently, researchers used chick embryo models to investigate the function of the CDK inhibitors palbociclib and RO-3306 in regulating cell differentiation, tumor progression, and metastasis in neuroblastoma. When SK-N-AS and BE(2)C cells were transplanted into the chorioallantoic membrane of chick embryos and treated with CDK inhibitors, tumor cell proliferation was reduced, and hypoxic preconditioning-driven metastasis was reduced by 60%114.
As described above, CDK inhibitors have recently been confirmed to show significant effects on the regulation of tumor immunity. For instance, Goel et al.115 demonstrated that the selective CDK4/6 inhibitor abemaciclib can promote the secretion of IFN to enhance the antigen-presenting function of tumor cells and inhibit the proliferation of immune-suppressive Treg cells to overcome tumor immune escape. In addition, the inhibition of CDK4/6 promotes the activation of NFAT family histones and IL2 expression, which are two key factors that activate T cells and launch tumor immunity116,117. The inhibition of CDK4-cyclin D decreases ubiquitination-dependent degradation of PD-L1, which leads to drug resistance of CDK4 inhibitors in tumor therapy110. These findings provide a strong theoretical basis for expanding the application of CDK to promote antitumor immunity, such as the combination of CDK inhibitors and PD-L1 monoclonal antibody drugs. In the future, we should explore synthetic lethality in the field of tumor immunity and use biomarkers of immune cells and tumor cells as indicators to screen for conditional synthetic lethality factors related to tumor immunity, thereby overcoming the limitations of traditional screening for synthetic lethality. Hence, it is worth looking forward to the day when a strategy for the use of CDK inhibitor synthetic lethality is applied to clinical practice.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81872885 to Ji Cao), Leading Talent of “Ten Thousand Plan”-National High-Level Talents Special Support Plan, and the Zhejiang Provincial Natural Science Foundation of China (No. LY15H160009 to Wen Meng).
Author contributions
Chengliang Zhu and Ji Cao conceived, designed the conception of review article. Kailin Li, Jieqiong You, Qian Wu and Wen Meng collected the related research articles and conducted the paper. Qiaojun He, Bo Yang, Chengliang Zhu and Ji Cao made the amendments of the paper.
Conflicts of interest
No potential conflicts of interest were disclosed.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Chengliang Zhu, Email: chengliangzhu@zju.edu.cn.
Ji Cao, Email: caoji88@zju.edu.cn.
References
- 1.Jacob S., Hovey E., Ng W., Vinod S., Delaney G.P., Barton M.B. Estimation of an optimal chemotherapy utilisation rate for lung cancer: an evidence-based benchmark for cancer care. Lung Cancer. 2010;69:307–314. doi: 10.1016/j.lungcan.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell L.H., Szankasi P., Roberts C.J., Murray A.W., Friend S.H. Integrating genetic approaches into the discovery of anticancer drugs. Science. 1997;278:1064–1068. doi: 10.1126/science.278.5340.1064. [DOI] [PubMed] [Google Scholar]
- 3.Dobzhansky T. Genetics of natural populations.13. Recombination and variability in populations of Drosophila pseudoobscura. Genetics. 1946;31:269–290. doi: 10.1093/genetics/31.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sturtevant A.H. A Highly specific complementary lethal system in Drosophila melanogaster. Genetics. 1956;41:118–123. doi: 10.1093/genetics/41.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucchesi J.C. Synthetic lethality and semi-lethality among functionally related mutants of Drosophila melanogaster. Genetics. 1968;59:37–44. doi: 10.1093/genetics/59.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaelin W.G. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 7.Kroll E.S., Hyland K.M., Hieter P., Li J.J. Establishing genetic interactions by a synthetic dosage lethality phenotype. Genetics. 1996;143:95–102. doi: 10.1093/genetics/143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Measday V., Hieter P. Synthetic dosage lethality. Methods Enzymol. 2002;350:316–326. doi: 10.1016/s0076-6879(02)50971-x. [DOI] [PubMed] [Google Scholar]
- 9.Chan N., Bristow R.G. “Contextual” synthetic lethality and/or loss of heterozygosity: tumor hypoxia and modification of DNA repair. Clin Cancer Res. 2010;16:4553–4560. doi: 10.1158/1078-0432.CCR-10-0527. [DOI] [PubMed] [Google Scholar]
- 10.O'Neil N.J., Bailey M.L., Hieter P. Synthetic lethality and cancer. Nat Rev Genet. 2017;18:613–623. doi: 10.1038/nrg.2017.47. [DOI] [PubMed] [Google Scholar]
- 11.Pagliarini R., Shao W., Sellers W.R. Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep. 2015;16:280–296. doi: 10.15252/embr.201439949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang A., Garraway L.A., Ashworth A., Weber B. Synthetic lethality as an engine for cancer drug target discovery. Nat Rev Drug Discov. 2020;19:23–38. doi: 10.1038/s41573-019-0046-z. [DOI] [PubMed] [Google Scholar]
- 13.Moore K., Colombo N., Scambia G., Kim B.G., Oaknin A., Friedlander M. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 14.Lyons T.G. Targeted therapies for triple-negative breast cancer. Curr Treat Options Oncol. 2019;20:82. doi: 10.1007/s11864-019-0682-x. [DOI] [PubMed] [Google Scholar]
- 15.Lord C.J., Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey J.P.W., Karakas C., Bui T.Y., Chen X., Vijayaraghavan S., Zhao Y. Synthetic lethality of PARP inhibitors in combination with MYC blockade is independent of brca status in triple-negative breast cancer. Cancer Res. 2018;78:742–757. doi: 10.1158/0008-5472.CAN-17-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philip C.A., Laskov I., Beauchamp M.C., Marques M., Amin O., Bitharas J. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer. 2017;17:638. doi: 10.1186/s12885-017-3639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menolfi D., Jiang W., Lee B.J., Moiseeva T., Shao Z., Estes V. Kinase-dead ATR differs from ATR loss by limiting the dynamic exchange of ATR and RPA. Nat Commun. 2018;9:5351. doi: 10.1038/s41467-018-07798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantidze O.L., Velichko A.K., Luzhin A.V., Petrova N.V., Razin S.V. Synthetically lethal interactions of ATM, ATR, and DNA-PKcs. Trends Cancer. 2018;4:755–768. doi: 10.1016/j.trecan.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Ashworth A., Lord C.J. Synthetic lethal therapies for cancer: what's next after PARP inhibitors?. Nat Rev Clin Oncol. 2018;15:564–576. doi: 10.1038/s41571-018-0055-6. [DOI] [PubMed] [Google Scholar]
- 21.Kalra S., Joshi G., Munshi A., Kumar R. Structural insights of cyclin dependent kinases: implications in design of selective inhibitors. Eur J Med Chem. 2017;142:424–458. doi: 10.1016/j.ejmech.2017.08.071. [DOI] [PubMed] [Google Scholar]
- 22.Lim S.H., Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsubo M., Theodoras A.M., Schumacher J., Roberts J.M., Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varadarajan R., Ayeni J., Jin Z.G., Homola E., Campbell S.D. Myt1 inhibition of cyclin A/Cdk1 is essential for fusome integrity and premeiotic centriole engagement in Drosophila spermatocytes. Mol Biol Cell. 2016;27:2051–2063. doi: 10.1091/mbc.E16-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parua P.K., Fisher R.P. Dissecting the Pol II transcription cycle and derailing cancer with CDK inhibitors. Nat Chem Biol. 2020;16:716–724. doi: 10.1038/s41589-020-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenleaf A.L. Human CDK12 and CDK13, multi-tasking CTD kinases for the new millennium. Transcription. 2019;10:91–110. doi: 10.1080/21541264.2018.1535211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying M., Shao X., Jing H., Liu Y., Qi X., Cao J. Ubiquitin-dependent degradation of CDK2 drives the therapeutic differentiation of AML by targeting PRDX2. Blood. 2018;131:2698–2711. doi: 10.1182/blood-2017-10-813139. [DOI] [PubMed] [Google Scholar]
- 28.Yuan K., Wang X., Dong H., Min W., Hao H., Yang P. Selective inhibition of CDK4/6: a safe and effective strategy for developing anticancer drugs. Acta Pharm Sin B. 2021;11:30–54. doi: 10.1016/j.apsb.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Zhang T., Kwiatkowski N., Abraham B.J., Lee T.I., Xie S. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163:174–186. doi: 10.1016/j.cell.2015.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H., Christensen C.L., Dries R., Oser M.G., Deng J., Diskin B. CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell. 2020;37:37–54.e9. doi: 10.1016/j.ccell.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H., Pandey S., Travers M., Sun H., Morton G., Madzo J. Targeting CDK9 reactivates epigenetically silenced genes in cancer. Cell. 2018;175:1244–1258.e26. doi: 10.1016/j.cell.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy M.J., Synnott N.C., Crown J. Mutant p53 as a target for cancer treatment. Eur J Cancer. 2017;83:258–265. doi: 10.1016/j.ejca.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Karimian A., Ahmadi Y., Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016;42:63–71. doi: 10.1016/j.dnarep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Bretones G., Delgado M.D., Leon J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849:506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Zajac-Kaye M. Myc oncogene: a key component in cell cycle regulation and its implication for lung cancer. Lung Cancer. 2001;34 Suppl 2:S43–S46. doi: 10.1016/s0169-5002(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman M.W., Liu Y., He S.N., Durbin A.D., Abraham B.J., Easton J. MYC drives a subset of high-risk pediatric neuroblastomas and is activated through mechanisms including enhancer Hijacking and focal enhancer amplification. Cancer Discov. 2018;8:320–335. doi: 10.1158/2159-8290.CD-17-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dang C.V. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molenaar J.J., Ebus M.E., Geerts D., Koster J., Lamers F., Valentijn L.J. Inactivation of CDK2 is synthetically lethal to MYCN over-expressing cancer cells. Proc Natl Acad Sci U S A. 2009;106:12968–12973. doi: 10.1073/pnas.0901418106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goga A., Yang D., Tward A.D., Morgan D.O., Bishop J.M. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med. 2007;13:820–827. doi: 10.1038/nm1606. [DOI] [PubMed] [Google Scholar]
- 40.Horiuchi D., Kusdra L., Huskey N.E., Chandriani S., Lenburg M.E., Gonzalez-Angulo A.M. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J Exp Med. 2012;209:679–696. doi: 10.1084/jem.20111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang J., Sergio C.M., Sutherland R.L., Musgrove E.A. Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer. 2014;14:32. doi: 10.1186/1471-2407-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hydbring P., Castell A., Larsson L.G. MYC Modulation around the CDK2/p27/SKP2 axis. Genes. 2017;8:174. doi: 10.3390/genes8070174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory G.P., Hogg S.J., Kats L.M., Vidacs E., Baker A.J., Gilan O. CDK9 inhibition by dinaciclib potently suppresses Mcl-1 to induce durable apoptotic responses in aggressive MYC-driven B-cell lymphoma in vivo. Leukemia. 2015;29:1437–1441. doi: 10.1038/leu.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang C.H., Lujambio A., Zuber J., Tschaharganeh D.F., Doran M.G., Evans M.J. CDK9-mediated transcription elongation is required for MYC addiction in hepatocellular carcinoma. Gene Dev. 2014;28:1800–1814. doi: 10.1101/gad.244368.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfaff M.J., Mukhopadhyay S., Hoofnagle M., Chabasse C., Sarkar R. Tumor suppressor protein p53 negatively regulates ischemia-induced angiogenesis and arteriogenesis. J Vasc Surg. 2018;68 doi: 10.1016/j.jvs.2018.02.055. 222S-33S.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eischen C.M. Genome stability requires p53. Cold Spring Harb Perspect Med. 2016;6:a026096. doi: 10.1101/cshperspect.a026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ladds M., Lain S. Small molecule activators of the p53 response. J Mol Cell Biol. 2019;11:245–254. doi: 10.1093/jmcb/mjz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nekova T.S., Kneitz S., Einsele H., Bargou R., Stuhler G. Silencing of CDK2, but not CDK1, separates mitogenic from anti-apoptotic signaling, sensitizing p53 defective cells for synthetic lethality. Cell Cycle. 2016;15:3203–3209. doi: 10.1080/15384101.2016.1241915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng C.K., Gustafson W.C., Charron E., Houseman B.T., Zunder E., Goga A. Dual blockade of lipid and cyclin-dependent kinases induces synthetic lethality in malignant glioma. Proc Natl Acad Sci U S A. 2012;109:12722–12727. doi: 10.1073/pnas.1202492109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jabbour-Leung N.A., Chen X., Bui T., Jiang Y., Yang D., Vijayaraghavan S. Sequential combination therapy of CDK inhibition and doxorubicin is synthetically lethal in p53-mutant triple-negative breast cancer. Mol Cancer Therapeut. 2016;15:593–607. doi: 10.1158/1535-7163.MCT-15-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong L., Yang S., Jia Y., Lei K. Inhibition of cyclin-dependent kinase 7 suppresses human hepatocellular carcinoma by inducing apoptosis. J Cell Biochem. 2018;119:9742–9751. doi: 10.1002/jcb.27292. [DOI] [PubMed] [Google Scholar]
- 52.Lu P., Geng J., Zhang L., Wang Y., Niu N., Fang Y. THZ1 reveals CDK7-dependent transcriptional addictions in pancreatic cancer. Oncogene. 2019;38:3932–3945. doi: 10.1038/s41388-019-0701-1. [DOI] [PubMed] [Google Scholar]
- 53.Kalan S., Amat R., Schachter M.M., Kwiatkowski N., Abraham B.J., Liang Y. Activation of the p53 transcriptional program sensitizes cancer cells to Cdk7 inhibitors. Cell Rep. 2017;21:467–481. doi: 10.1016/j.celrep.2017.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu P., Wang Y., Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9:871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simanshu D.K., Nissley D.V., McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Puyol M., Martin A., Dubus P., Mulero F., Pizcueta P., Khan G. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18:63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z.L., Yin M.C., Chu P.L., Lou M.Q. STAT3 inhibitor sensitized KRAS-mutant lung cancers to RAF innioitor by activating MEK/ERK signaling pathway. Aging. 2019;11:7187–7196. doi: 10.18632/aging.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alamo P., Gallardo A., Di Nicolantonio F., Pavon M.A., Casanova I., Trias M. Higher metastatic efficiency of KRas G12V than KRas G13D in a colorectal cancer model. FASEB J. 2015;29:464–476. doi: 10.1096/fj.14-262303. [DOI] [PubMed] [Google Scholar]
- 59.Bommi-Reddy A., Almeciga I., Sawyer J., Geisen C., Li W.L., Harlow E. Kinase requirements in human cells: III. Altered kinase requirements in VHL–/– cancer cells detected in a pilot synthetic lethal screen. Proc Natl Acad Sci U S A. 2008;105:16484–16489. doi: 10.1073/pnas.0806574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M.S., Helms T.L., Feng N., Gay J., Chang Q.E., Tian F. Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget. 2016;7:39595–39608. doi: 10.18632/oncotarget.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J., Zhang S., Chen X., Zheng X., Yao Y., Lu G. Palbociclib, a selective CDK4/6 inhibitor, enhances the effect of selumetinib in RAS-driven non-small cell lung cancer. Cancer Lett. 2017;408:130–137. doi: 10.1016/j.canlet.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 62.Olmez I., Brenneman B., Xiao A.Z., Serbulea V., Benamar M., Zhang Y. Combined CDK4/6 and mTOR inhibition is synergistic against glioblastoma via multiple mechanisms. Clin Cancer Res. 2017;23:6958–6968. doi: 10.1158/1078-0432.CCR-17-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen S.H., Gong X., Zhang Y., Van Horn R.D., Yin T., Huber L. RAF inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6 inhibition by abemaciclib via superior inhibition of phospho-RB and suppression of cyclin D1. Oncogene. 2018;37:821–832. doi: 10.1038/onc.2017.384. [DOI] [PubMed] [Google Scholar]
- 64.Hayes T.K., Luo F., Cohen O., Goodale A.B., Lee Y., Pantel S. A Functional landscape of resistance to MEK1/2 and CDK4/6 inhibition in NRAS-mutant melanoma. Cancer Res. 2019;79:2352–2366. doi: 10.1158/0008-5472.CAN-18-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopes-Ventura S., Pojo M., Matias A.T., Moura M.M., Marques I.J., Leite V. The efficacy of HRAS and CDK4/6 inhibitors in anaplastic thyroid cancer cell lines. J Endocrinol Invest. 2019;42:527–540. doi: 10.1007/s40618-018-0947-4. [DOI] [PubMed] [Google Scholar]
- 66.Costa-Cabral S., Brough R., Konde A., Aarts M., Campbell J., Marinari E. CDK1 is a synthetic lethal target for KRAS mutant tumours. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson N., Li Y.C., Walton Z.E., Cheng K.A., Li D.A., Rodig S.J. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med. 2011;17:875–882. doi: 10.1038/nm.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia Q., Cai Y.C., Peng R.J., Wu G.S., Shi Y.X., Jiang W.Q. The CDK1 inhibitor RO3306 improves the response of BRCA-proficient breast cancer cells to PARP inhibition. Int J Oncol. 2014;44:735–744. doi: 10.3892/ijo.2013.2240. [DOI] [PubMed] [Google Scholar]
- 69.Iniguez A.B., Stolte B., Wang E.J., Conway A.S., Alexe G., Dharia N.V. EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in ewing sarcoma. Cancer Cell. 2018;33:202–216. doi: 10.1016/j.ccell.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quereda V., Bayle S., Vena F., Frydman S.M., Monastyrskyi A., Roush W.R. Therapeutic targeting of CDK12/CDK13 in triple-negative breast cancer. Cancer Cell. 2019;36:545–558. doi: 10.1016/j.ccell.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Delire B., Starkel P. The Ras/MAPK pathway and hepatocarcinoma: pathogenesis and therapeutic implications. Eur J Clin Invest. 2015;45:609–623. doi: 10.1111/eci.12441. [DOI] [PubMed] [Google Scholar]
- 72.Asati V., Mahapatra D.K., Bharti S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314–341. doi: 10.1016/j.ejmech.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 73.Kim J., Guan K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat Cell Biol. 2019;21:63–71. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 74.Hanzlikova H., Kalasova I., Demin A.A., Pennicott L.E., Cihlarova Z., Caldecott K.W. The importance of poly(ADP-ribose) polymerase as a sensor of unligated Okazaki fragments during DNA replication. Mol Cell. 2018;71:319–331. doi: 10.1016/j.molcel.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dantzer F., Ame J.C., Schreiber V., Nakamura J., Menissier-de Murcia J., de Murcia G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006;409:493–510. doi: 10.1016/S0076-6879(05)09029-4. [DOI] [PubMed] [Google Scholar]
- 76.Caldecott K.W., Aoufouchi S., Johnson P., Shall S. XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly(ADP-ribose) polymerase, and DNA ligase III is a novel molecular ‘nick-sensor’ in vitro. Nucleic Acids Res. 1996;24:4387–4394. doi: 10.1093/nar/24.22.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berger N.A., Berger S.J., Catino D.M., Petzold S.J., Robins R.K. Modulation of nicotinamide adenine-dinucleotide and poly(adenosine diphosphoribose) metabolism by the synthetic C-nucleoside analogs, tiazofurin and selenazofurin—a new strategy for cancer-chemotherapy. J Clin Invest. 1985;75:702–709. doi: 10.1172/JCI111750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cipriani G., Rapizzi E., Vannacci A., Rizzuto R., Moroni F., Chiarugi A. Nuclear poly(ADP-ribose) polymerase-1 rapidly triggers mitochondrial dysfunction. J Biol Chem. 2005;280:17227–17234. doi: 10.1074/jbc.M414526200. [DOI] [PubMed] [Google Scholar]
- 79.Norris R.E., Adamson P.C., Nguyen V.T., Fox E. Preclinical evaluation of the PARP inhibitor, olaparib, in combination with cytotoxic chemotherapy in pediatric solid tumors. Pediatr Blood Cancer. 2014;61:145–150. doi: 10.1002/pbc.24697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Audeh M.W., Carmichael J., Penson R.T., Friedlander M., Powell B., Bell-McGuinn K.M. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 81.Lord C.J., Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 82.Alagpulinsa D.A., Ayyadevara S., Yaccoby S., Reis R.J.S. A cyclin-dependent kinase inhibitor, dinaciclib, impairs homologous recombination and sensitizes multiple myeloma cells to PARP inhibition. Mol Cancer Therapeut. 2016;15:241–250. doi: 10.1158/1535-7163.MCT-15-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krajewska M., Dries R., Grassetti A.V., Dust S., Gao Y., Huang H. CDK12 loss in cancer cells affects DNA damage response genes through premature cleavage and polyadenylation. Nat Commun. 2019;10:1757. doi: 10.1038/s41467-019-09703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stephenson J.J., Nemunaitis J., Joy A.A., Martin J.C., Jou Y.M., Zhang D. Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer. Lung Cancer. 2014;83:219–223. doi: 10.1016/j.lungcan.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 85.Flynn J., Jones J., Johnson A.J., Andritsos L., Maddocks K., Jaglowski S. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia. 2015;29:1524–1529. doi: 10.1038/leu.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mita M.M., Joy A.A., Mita A., Sankhala K., Jou Y.M., Zhang D. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer. 2014;14:169–176. doi: 10.1016/j.clbc.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Ghia P., Scarfo L., Perez S., Pathiraja K., Derosier M., Small K. Efficacy and safety of dinaciclib vs ofatumumab in patients with relapsed/refractory chronic lymphocytic leukemia. Blood. 2017;129:1876–1878. doi: 10.1182/blood-2016-10-748210. [DOI] [PubMed] [Google Scholar]
- 88.Mita M.M., Mita A.C., Moseley J.L., Poon J., Small K.A., Jou Y.M. Phase 1 safety, pharmacokinetic and pharmacodynamic study of the cyclin-dependent kinase inhibitor dinaciclib administered every three weeks in patients with advanced malignancies. Br J Cancer. 2017;117:1258–1268. doi: 10.1038/bjc.2017.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhillon S. Palbociclib: first global approval. Drugs. 2015;75:543–551. doi: 10.1007/s40265-015-0379-9. [DOI] [PubMed] [Google Scholar]
- 90.Finn R.S., Crown J.P., Lang I., Boer K., Bondarenko I.M., Kulyk S.O. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 91.Adkins D., Ley J., Neupane P., Worden F., Sacco A.G., Palka K. Palbociclib and cetuximab in platinum-resistant and in cetuximab-resistant human papillomavirus-unrelated head and neck cancer: a multicentre, multigroup, phase 2 trial. Lancet Oncol. 2019;20:1295–1305. doi: 10.1016/S1470-2045(19)30405-X. [DOI] [PubMed] [Google Scholar]
- 92.U.S. National Library of Medicine . 2018. Palbociclib and cetuximab in metastatic colorectal cancer.https://clinicaltrials.gov/ct2/show/NCT03446157?term=Palbociclib&recrs=abdefi&draw=3&rank=20 clinicaltrials.gov [Internet] Available from: [Google Scholar]
- 93.U.S. National Library of Medicine . 2019. Combination of MEK inhibitor binimetinib and CDK4/6 inhibitor palbociclib in KRAS and NRAS mutant metastatic colorectal cancers.https://clinicaltrials.gov/ct2/show/record/NCT03981614?term=Palbociclib+and+MEK162&draw=2&rank=2 clinicaltrials.gov [Internet] Available from: [Google Scholar]
- 94.U.S. National Library of Medicine . 2017. Phase I study of the CDK4/6 inhibitor palbociclib (PD-0332991) in combination with the PI3K/mTOR inhibitor gedatolisib (PF-05212384) for patients with advanced squamous cell lung, pancreatic, head & neck and other solid tumors.https://clinicaltrials.gov/ct2/show/record/NCT03065062?term=palbociclib+everolimus&draw=2&rank=3 clinicaltrials.gov [Internet] Available from: [Google Scholar]
- 95.U.S. National Library of Medicine . 2017. A phase I trial of dinaciclib (SCH727965) and MK-2206 in metastatic pancreatic cancer with an expansion cohort in advanced pancreatic cancer.https://clinicaltrials.gov/ct2/show/record/NCT01783171?term=dinaciclib&draw=2&rank=6 clinicaltrials.gov [Internet] Available from: [Google Scholar]
- 96.U.S. National Library of Medicine . 2016. Phase Ib trial of pembrolizumab (MK-3475) in combination with dinaciclib (MK-7965) in subjects with hematologic malignancies.https://clinicaltrials.gov/ct2/show/record/NCT02684617?term=dinaciclib&draw=2&rank=2 clinicaltrials.gov [Internet] Available from: [Google Scholar]
- 97.U.S. National Library of Medicine . 2018. Phase 1b study of venetoclax and alvocidib in patients with relapsed/refractory acute myeloid leukemia.https://clinicaltrials.gov/ct2/show/record/NCT03441555?term=Venetocax+and+Alvocidib&draw=2&rank=1 clinicaltrials.gov [Internet] Available from: [Google Scholar]
- 98.Bogenberger J., Whatcott C., Hansen N., Delman D., Shi C.X., Kim W. Combined venetoclax and alvocidib in acute myeloid leukemia. Oncotarget. 2017;8:107206–107222. doi: 10.18632/oncotarget.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karp J.E., Garrett-Mayer E., Estey E.H., Rudek M.A., Smith B.D., Greer J.M. Randomized phase II study of two schedules of flavopiridol given as timed sequential therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica. 2012;97:1736–1742. doi: 10.3324/haematol.2012.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.LaCerte C., Ivaturi V., Gobburu J., Greer J.M., Doyle L.A., Wright J.J. Exposure-response analysis of Alvocidib (flavopiridol) treatment by bolus or hybrid administration in newly diagnosed or relapsed/refractory acute leukemia patients. Clin Cancer Res. 2017;23:3592–3600. doi: 10.1158/1078-0432.CCR-16-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Litzow M.R., Wang X.V., Carroll M.P., Karp J.E., Ketterling R.P., Zhang Y. A randomized trial of three novel regimens for recurrent acute myeloid leukemia demonstrates the continuing challenge of treating this difficult disease. Am J Hematol. 2019;94:111–117. doi: 10.1002/ajh.25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yin X., Qi Y., Ren M., Wang S.Y., Jiang H.Q., Feng H.L. Roscovitine treatment caused impairment of fertilizing ability in mice. Toxicol Lett. 2015;237:200–209. doi: 10.1016/j.toxlet.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 103.Cicenas J., Kalyan K., Sorokinas A., Stankunas E., Levy J., Meskinyte I. Roscovitine in cancer and other diseases. Ann Transl Med. 2015;3:135. doi: 10.3978/j.issn.2305-5839.2015.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Asghar U., Witkiewicz A.K., Turner N.C., Knudsen E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang T.H., Kwiatkowski N., Olson C.M., Dixon-Clarke S.E., Abraham B.J., Greifenberg A.K. Covalent targeting of remote cysteine residues to develop CDK12 and CDK13 inhibitors. Nat Chem Biol. 2016;12:876–884. doi: 10.1038/nchembio.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nilson K.A., Guo J.N., Turek M.E., Brogie J.E., Delaney E., Luse D.S. THZ1 reveals roles for Cdk7 in co-transcriptional capping and pausing. Mol Cell. 2015;59:576–587. doi: 10.1016/j.molcel.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Squires M.S., Feltell R.E., Wallis N.G., Lewis E.J., Smith D.M., Cross D.M. Biological characterization of AT7519, a small-molecule inhibitor of cyclin-dependent kinases, in human tumor cell lines. Mol Cancer Therapeut. 2009;8:324–332. doi: 10.1158/1535-7163.MCT-08-0890. [DOI] [PubMed] [Google Scholar]
- 108.Portman N., Alexandrou S., Carson E., Wang S.D., Lim E., Caldon C.E. Overcoming CDK4/6 inhibitor resistance in ER-positive breast cancer. Endocr-Relat Cancer. 2019;26:R15–R30. doi: 10.1530/ERC-18-0317. [DOI] [PubMed] [Google Scholar]
- 109.Mamiya H., Tahara R.K., Tolaney S.M., Choudhry N.K., Najafzadeh M. Cost-effectiveness of palbociclib in hormone receptor-positive advanced breast cancer. Ann Oncol. 2017;28:1825–1831. doi: 10.1093/annonc/mdx201. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J., Bu X., Wang H., Zhu Y., Geng Y., Nihira N.T. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beck T.N., Georgopoulos R., Shagisultanova E.I., Sarcu D., Handorf E.A., Dubyk C. EGFR and RB1 as dual biomarkers in HPV-negative head and neck cancer. Mol Cancer Therapeut. 2016;15:2486–2497. doi: 10.1158/1535-7163.MCT-16-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao L., Li P., Zhao L., Wang M., Tong D., Meng Z. Expression and clinical value of PD-L1 which is regulated by BRD4 in tongue squamous cell carcinoma. J Cell Biochem. 2020;121:1855–1869. doi: 10.1002/jcb.29420. [DOI] [PubMed] [Google Scholar]
- 113.Zimmermann C., Garcia I., Omerzu M., Chymkowitch P., Zhang B., Enserink J.M. Mapping the synthetic dosage lethality network of CDK1/CDC28. G3 (Bethesda) 2017;7:1753–1766. doi: 10.1534/g3.117.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swadi R.R., Sampat K., Herrmann A., Losty P.D., See V., Moss D.J. CDK inhibitors reduce cell proliferation and reverse hypoxia-induced metastasis of neuroblastoma tumours in a chick embryo model. Sci Rep. 2019;9:9136. doi: 10.1038/s41598-019-45571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goel S., DeCristo M.J., Watt A.C., BrinJones H., Sceneay J., Li B.B. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Deng J., Wang E.S., Jenkins R.W., Li S., Dries R., Yates K. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schaer D.A., Beckmann R.P., Dempsey J.A., Huber L., Forest A., Amaladas N. The CDK4/6 inhibitor abemaciclib induces a t cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep. 2018;22:2978–2994. doi: 10.1016/j.celrep.2018.02.053. [DOI] [PubMed] [Google Scholar]