Abstract
Hypertrophic scars are unfavorable skin diseases characterized by excessive collagen deposition. Although systemic treatments exist in clinic to manage hypertrophic scars, they pose significant side effects and tend to lose efficacy over prolonged applications. Traditional Chinese medicine (TCM) offers as a promising candidate to treat pathological scars. A large number of TCMs have been studied to show anti-scarring effect, however, the natural barrier of the skin impedes their penetration, lowering its therapeutic efficacy. Herein, we reported the use of dissolvable hyaluronic acid (HA) microneedles (MNs) as a vehicle to aid the transdermal delivery of therapeutic agent, a model TCM called shikonin for the treatment of hypertrophic scars. Here, shikonin was mixed with HA to make MNs with adequate mechanical strength for skin penetration, making its dosage controllable during the fabrication process. The therapeutic effect of the shikonin HA MNs was studied in vitro using HSFs and then further verified with quantitative reverse transcriptase polymerase chain reaction. Our data suggest that the shikonin HA MNs significantly reduce the viability and proliferation of the HSFs and downregulate the fibrotic-related genes (i.e., TGFβ1, FAP-α and COL1A1). Furthermore, we observed a localized therapeutic effect of the shikonin HA MNs that is beneficial for site-specific treatment.
KEY WORDS: Traditional Chinese medicine, Shikonin, Hyaluronic acid, Microneedle, Hypertrophic scarring, Drug delivery, Transdermal delivery, Skin
Graphical abstract
We report the use of dissolvable hyaluronic acid (HA) microneedles (MNs) as a vehicle to facilitate the transdermal delivery of therapeutic agent, a model traditional Chinese medicine (TCM) called Shikonin for the treatment of hypertrophic scars.
1. Introduction
Hypertrophic scars are characterized by excessive collagen deposition by dermal fibroblasts. The underlying causes remain elusive, although ethnicity and genetic predisposition play a role. Current treatments, including corticosteroids, pressure therapy, laser therapy and surgical operation, are not satisfactory and may cause significant side effects over prolonged use1. For instance, intralesional corticosteroid injection is the first-line approach for physicians to prevent and treat the keloids and hypertrophic scars2. While it provides a 50%–100% flattening of the keloids, it requires multiple painful injections, and may cause hypopigmentation and skin atrophy with 9%–50% recurrence rate3. Surgical removal of scars could be temporarily gratifying; however, it is normally followed by more aggressive regrowth of the scar unless other therapies were performed post-surgery. This complicates the treatment and requires exemplary persistence from the patients.
With rich resource and relatively low cost, traditional Chinese medicines (TCMs) have been widely used for thousands of years in prevention and treatment of many diseases including scarring4. Sourced from the nature and typically possessed little side effects, TCMs emphasize on improving the general health of patients. A large amount of TCMs have been studied to display anti-scarring effect. However, the natural skin barrier such as stratum corneum and the compact structure of scar tissues limit the permeation and efficiency of TCM on cutaneous diseases5. Although reformulation through microemulsion or nanoparticles has been found to improve the penetration of TCM, they introduce additional components which alter the original TCM structure and interfere with its functionality and toxicity6. For instance, the microemulsion product of oxymatrine–phospholipid complex (OMT-PLC) consists of not only 10.0% OMT-PLC, but also 8.0% isopropyl myristate, 30.0% cremophor RH40/polyethyleneglycol 400 (1:1) and 52.0% water7. In addition, when TCMs are nanomaterialized, the physicochemical properties of the active ingredient might be altered, diminishing its therapeutic efficacy6.
Microneedle (MN) technology is effective in delivering various drugs intradermally without affecting blood vessels and nerves. It overcomes the protective stratum corneum of the epidermis layer and creates transient micro holes on the skin to aid the permeation of the drugs. Many studies have shown MN's potential to successfully deliver therapeutic agents including oligonucleotide, vaccine, peptide and so on8,9,10.
Thus, we believe the transdermal delivery of TCM formulations utilizing MNs would address the limitations met currently in clinics. As a proof-of-concept, we chose shikonin as a model TCM drug. Shikonin is an active component extracted from Arnebiae Radix (ZiCao) and has been demonstrated to inhibit cell viability, cell proliferation and collagen production in scar-derived fibroblasts, thus offering a promising alternative for the treatment of scars11. It is generally mixed in oil-based creams or ointments before application to human skin, however, achieving high bioavailability is challenging due to its insolubility in water12. The transdermal delivery of shikonin with MN platform offers a promising route to enhance its bioavailability.
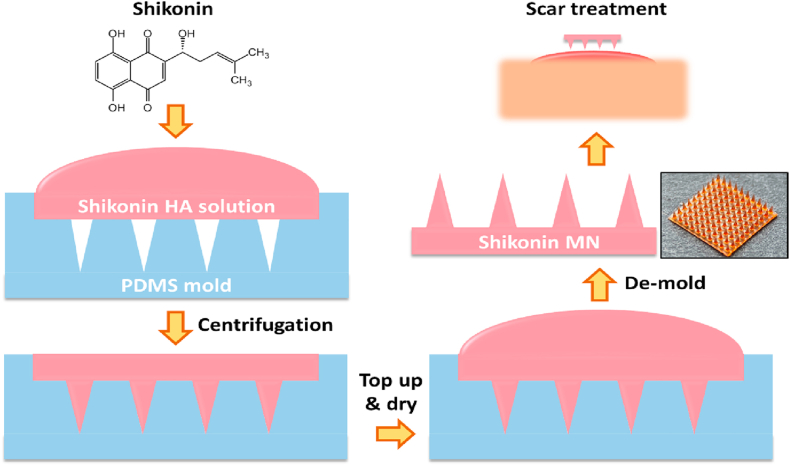
Herein, we incorporate shikonin into MN made from dissolvable hyaluronic acid (HA) to aid the delivery. HA is widely distributed throughout our tissues, offering excellent biocompatibility with minimal side effects13. Shikonin was pre-dissolved in Dimethyl sulfoxide (DMSO) before mixing with HA solution and the mixture solution was then used to make MNs by micro molding (Scheme 1). The amount of shikonin encapsulated in the MN could be easily controlled during the fabrication process by adjusting the pre-dissolved shikonin concentrations. Our previous study indicated that shikonin could inhibit cell proliferation, induce apoptosis of hypertrophic scar derived fibroblast (HSF), and attenuate transdermal growth factor beta 1 (TGFβ1)-induced collagen production14,15. In this study, the efficiency of shikonin in killing the HSF and attenuating scar-related gene expressions was maintained after incorporating into HA MNs. Moreover, shikonin-MNs offers a localized therapeutic effect that is beneficial for site-specific scar treatment.
Scheme 1.
Shikonin hyaluronic acid microneedle for the facile treatment of abnormal wound scarring.
2. Methods
2.1. Materials
All chemicals and reagents were obtained from Sigma–Aldrich (Singapore) except mentioned otherwise. The stainless-steel master mold of MN (300 μm base diameter, 5 μm tip radius, and 1000 μm height) was purchased from Micropoint Technologies Pte Ltd. (Singapore). Hyaluronic acid (HA, 300 kDa) was purchased from Freda Biochem Co., Ltd. (China). Phosphate buffered saline (1 × , PBS), high glucose (4.5 g/L) Dulbecco's modified Eagle's medium (DMEM) were obtained from Lonza. Fetal bovine serum (FBS), Trypsin−EDTA (0.05%), and penicillin−streptomycin (10,000 U/mL) were purchased from Gibco (Singapore). AlarmarBlue cell viability reagent was purchased from Thermofisher (Singapore). TRIzol reagent was obtained from Invitrogen (Singapore). iScript Adv cDNA kit & Universal SYBR® Green Supermix were purchased from Biorad (Singapore).
2.2. Fabrication of the PDMS mold
The fabrication of the PDMS mold followed our previous methodology9. Briefly, the PDMS daughter mold was produced by pouring PDMS into a Petri dish containing stainless steel mother molds. Degassing was performed by vacuum oven and subsequently, curing was performed at 70 °C for 1 h. PDMS molds that reversely replicate the mother molds were obtained by detaching the cured PDMS from the Petri dish.
2.3. Fabrication of the shikonin HA MN
Shikonin was first dissolved in DMSO with a concentration of 50 mg/mL as stock solution for subsequent experiment. HA powder was dissolved in deionized (DI) water and mixed homogeneously before different amounts of shikonin stock were added to a final concentration of 0, 4, 20, 100 and 200 μg/mL. The shikonin‒HA mixtures were then poured into PDMS molds and followed by centrifugation (3200×g, 5 min) to fill the tips of the MN completely and remove air bubbles. More mixtures were then topped up into the PDMS mold to form the MN base. The drying process was conducted in fume hood under room temperature.
2.4. Loading efficiency of shikonin HA MN
Shikonin HA MNs (made from different shikonin mixture concentrations) were thoroughly dissolved in PBS at 37 °C. Subsequently, the solution was mixed gently to ensure homogeneous solution. Shikonin distribution was read under a microplate reader (SpectraMax M5, Molecular Devices) at λ = 520 nm. Solution absorbance was fitted against a standard curve to calculate the concentration of shikonin.
2.5. Compression test of shikonin HA MN
The compression test was done with a Tensile Meter (Instron 5543). HA MNs containing varying amount ofshikonin were placed on the specimen plate with tips facing upwards. The displacement versus the load were measured and recorded until a preset maximum loading force of 80 N was reached.
2.6. Ex vivo penetration test
HA MNs containing the highest shikonin (made from 200 μg/mL shikonin HA solution) was thumb-pressed into an excised porcine skin and hold steadily for 3 min. Subsequently, the treated skin was cryo-sectioned and hematoxylin & eosin (H&E)-stained for histological analysis.
2.7. Fibroblast cell culture
Fibroblasts derived from hypertrophic scar tissue (HSFs) were cultured in high-glucose DMEM with 10% FBS and 1% penicillin−streptomycin in a humidified condition of 37 °C with 5% CO2. Culture medium was replaced every 2 days and cells were sub-cultured when they reached 95% confluency.
2.8. Biocompatibility assessment of HA
The biocompatibility of blank HA MN was investigated using live/dead staining kit which contains calcein-AM and propidium iodide (PI) to stain viable and dead cells, respectively. HA MNs were placed and incubated for 24 h on HSFs when the cells reached 80% confluency in the well plate. The live/dead staining solution were then added as per the manufacturer's instructions and incubated in 37 °C for 15 min. Fluorescence images were taken by a digital microscope (Leica DVM6) at λex = 490 nm and λem = 515 nm for live signal and λex = 535 nm, λem = 617 nm for dead signal.
The biocompatibility of HA solutions (0.125, 0.5, 2 and 4 mg/mL) were investigated using the AlamarBlue assay. In accordance with the manufacturer's protocols, the AlamarBlue reagent was added to cells following 24 h treatment of HA solutions (initial confluency of 60%) and left incubated for 2 h before microplate reading (λex = 560 nm, λem = 590 nm). The normalized metabolism rate was determined by normalizing the fluorescence intensities with that of untreated group.
2.9. Efficacy ofshikonin HA MNs on HSFs
Four groups were investigated in the test including an untreated group, TGFβ1 treated group, TGFβ1 & shikonin solution treated group and TGFβ1 & shikonin HA MN-treated group. The MNs were segmented to ensure the same amount of shikonin were added in the cell culture plate as the shikonin solution group. Above-mentioned treatments were applied to HSFs (80% confluency) for 24 and 48 h. Subsequently, live/dead staining and fluorescence imaging follows as described above. Cell viability rate was then calculated by counting the numbers of live and dead cells in ImageJ. Moreover, cell metabolism rate following 24 h of treatment were assessed with AlamarBlue assay as described above.
2.10. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
To assess the modulation of fibrotic genes on HSFs undergoing shikonin HA MN treatment, cells underwent the treatments mentioned above for 24 h and suspended and lysed with TRIzol. Following RNA isolation through ethanol precipitation, cDNA conversion was performed using the iScript Adv cDNA kit according to the manufacturer's protocol. Primer sequences for TGFβ1, FAP-α, and COL1A1 mRNA were obtained and verified from previous studies16,17 (Supporting Information Table S1). CT values were obtained through qPCR with CFX Connect PCR System (Biorad) and 2−ΔΔCT expression was utilized to calculate the change in fold expression.
2.11. Preparation of 3D in vitro cell model
HSF cell suspension (5 × 106 cells/mL) in culture media was mixed gently with 2.8% of warm agarose solution at 1:1 volume ratio and settled to solidify for 5 min inside the well plate before topping up additional culture media. Cells in agarose hydrogel were incubated for 24 h prior to shikonin HA MN application. The MNs were gently inserted into the hydrogel and the live/dead staining were performed after 24 h of treatment. Following which, 3D fluorescence images were obtained using the Z-stack function of a confocal microscope (Zeiss LSM 800). Viability of the 3D cultured cells was then assessed by quantifying the live/dead signals at 3 different Z-positions.
2.12. Statistical analysis
Data normalization (when applicable) is presented individually in associated figure legend. One-way ANOVA was employed to calculate the significant P values. A suitable post-hoc test was chosen using the IBM SPSS Statistics 22 software (Tukey). In each experiment, values were reported as mean ± standard deviation of 4 samples. Unless stated otherwise, N.S. represents non-significance, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
3. Results and discussion
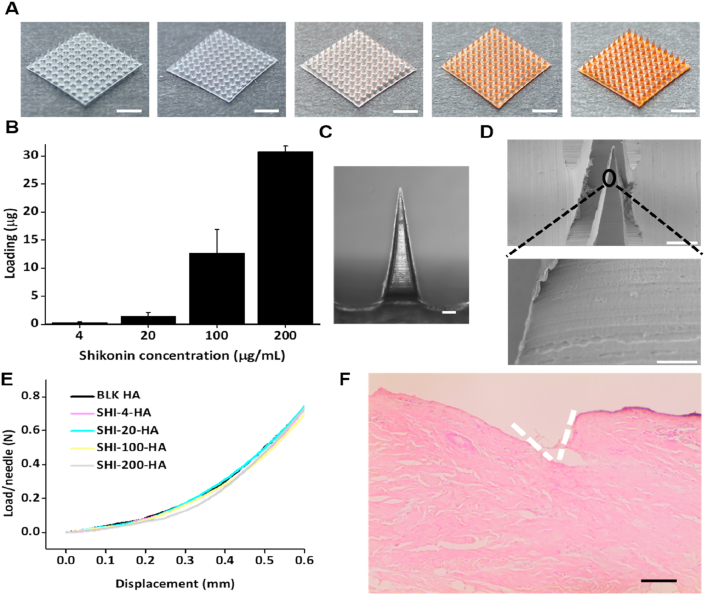
HA (300 KDa) was chosen to make the dissolvable MNs. shikonin was pre-dissolved in DMSO to make a stock solution before mixed with HA solution (50 mg/mL). With this mixture, shikonin HA MNs were made in polydimethylsiloxane (PDMS) mold through a two-step process, where the tips and base of MNs were made consecutively (Scheme 1). The dosage of shikonin loaded in the MNs were reflected by the color of the MN patches (Fig. 1A). While blank MNs are transparent, darker color of the MNs indicates higher dosage of shikonin. The exact dosage of shikonin in the shikonin HA MNs was quantified through MN dissolution and measurement of shikonin absorbance at λ = 520 nm (Fig. 1B). At the highest loading concentration, 30.76 ± 0.98 μg shikonin was carried per MN patch (100 MN tips). The delivery of 1 μg of shikonin every 2 days could effectively treat a burn wound created on porcine with a diameter of 5 cm to remediate hypertrophic scar18. Therefore, we achieved a significantly greater loading if one MN patch is used per dosage. Furthermore, considering the patch size (∼1 cm2) is considerably smaller than a 5 cm wound (area ∼20 cm2), we could simply enlarge the MN size or apply several MN (3–4) to achieve greater shikonin delivery dosage. In overall, we believe that the proposed shikonin MN can meet the dosage requirement for clinical applications.
Figure 1.
Characterization of the Shikonin HA MN patches. (A) Photographic images of the HA MN patches loaded with various amount of Shikonin. From left to right: 0, 4, 20, 100, and 200 μg/mL Shikonin solution was mixed with HA during molding. Scale bar: 2 mm. (B) Quantification of Shikonin loading in each MN patch in (A). (C) Representative microscopic image of a single tip of Shikonin HA MN patch. Scale bar: 100 μm. (D) Scanning electron microscopic image of the MN in (C). Scale bar: top: 100 μm, bottom: 10 μm. (E) Load/needle versus displacement curve of the MNs made from different Shikonin HA solution. (F) H&E staining image of the pig skin after MN (SHI-200-HA) treatment showing micro hole being created by MN. Scale Bar: 0.5 mm.
The shikonin HA MN has a pyramidal shape with the height and base dimension of 1000 and 300 μm, respectively (Fig. 1C and D). Shikonin HA MNs showed the typical elastic deformation under the compression test (Fig. 1E). The minimum force required for MN to penetrate the human skin was proven to be 0.058 N/needle19. As these shikonin HA MNs were strong enough to withstand a force of more than 0.7 N/needle without fracture, we were able to puncture ex vivo porcine skin without any difficulty (Fig. 1F).
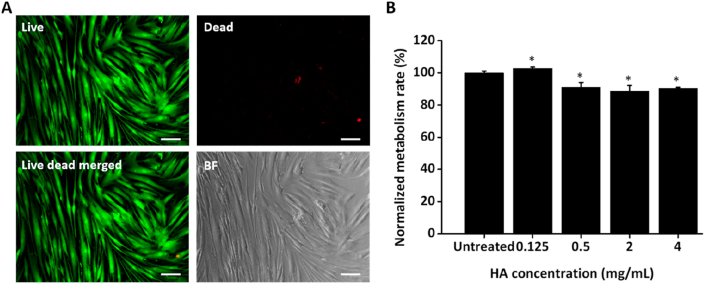
HA is well known for its biocompatibility. Nevertheless, we had to ascertain the overall effect of the blank HA MN (beyond strictly physical or chemical properties20) to distinguish and evaluate the therapeutic effects exerted by the loaded shikonin. In this case, the toxicity of the HA MNs on HSFs were first examined to distinguish the therapeutic efficacy of shikonin. The HA MNs show negligible toxicity to the HSFs as very few dead cells were observed from the live/dead staining (Fig. 2A). HSFs treated with 0.125 mg/mL HA showed slight increase in metabolism rate while the other groups (0.5, 2 and 4 mg/mL HA) showed lower metabolism rate as compared to the untreated group. Nevertheless, the altered metabolism rate on HSFs is minimal, with ∼90% retained even at the highest 4 mg/mL treatment (Fig. 2B), indicating the safety and biocompatibility of HA as an excipient.
Figure 2.
Biocompatibility assessment of blank HA MN. (A) Representative images showing cell morphology and Live/Dead staining (live: green; dead: red) of HSFs treated with blank HA MN. Scale bar: 100 μm. (B) Alamarblue metabolism assay showcase minimal toxicity of HA even up to 4 mg/mL concentration. Error bars indicate the mean ± SD (n = 4). ∗P < 0.05 versus the untreated control. Statistical analysis was performed using one-way ANOVA test.
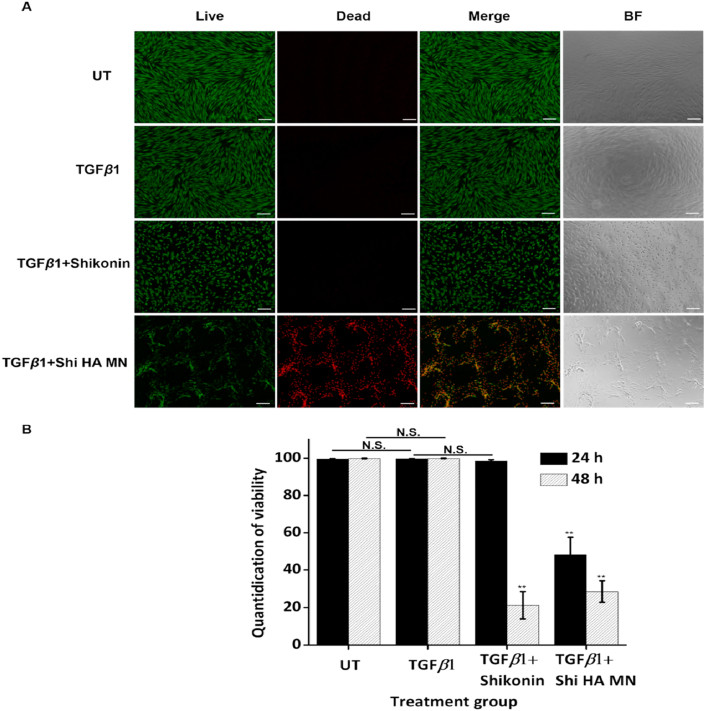
To assess the apoptosis-inducing ability of shikonin HA MN on HSFs, the viability of TGFβ1-stimulated HSFs following shikonin HA MN treatment (TGFβ1 + Shi HA MN) was compared with untreated group (UT), TGFβ1-activated group (TGFβ1) and shikonin solution treated group (TGFβ1 + shikonin). According to our previous study, a concentration of 1 μg/mL shikonin is sufficient for inducing apoptosis on HSFs. Thus, we performed the subsequent cell studies with shikonin HA MN made from 20 μg/mL shikonin solution. Live/dead staining was performed after 24 and 48 h of treatment (Fig. 3A and Supporting Information Fig. S1). After 24 h of treatment, HSFs treated with MNs started to show cell death while those treated by shikonin solution showed significantly altered cell morphology, where the cells partially shrank and change from the original spindle shape to round shape. After 48 h, the viability rate in shikonin solution group is comparable with that in shikonin HA MN group. Notably, compared to the homogenous killing effect observed for the shikonin solution group, shikonin HA MN-treated group showed localized killing of cells in close proximity with the MN. Meanwhile, cells further away from the MNs have good viability even after 48 h of treatment (Supporting Information Fig. S2). Release of shikonin from the MN is associated with the HA tips dissolution. Therefore, cells located in the vicinity of the MNs are exposed to higher amount of shikonin. This is corroborated with more effective killing of cells after 24 h of treatment for shikonin HA MN group, relative to the shikonin solution group in which shikonin was homogeneously distributed throughout the well plate. Such localized cytotoxicity is beneficial for scar treatment, to minimize undesirable side-effects on healthy skin in its vicinity.
Figure 3.
Examination of the Shikonin HA MN killing efficiency on TGFβ1 treated HSF. (A) Microscopic fluorescence (Live/Dead staining) and BF images of HSF treated by different groups after 24 h of incubation. Scale bar: 200 μm. (B)Quantified viability rate following treatment application for 24 & 48 h. Error bars indicate the mean ± SD (n = 3). ∗∗P < 0.01 versus the TGFβ1 group, N.S. means not significant. Statistical analysis was performed using one-way ANOVA test.
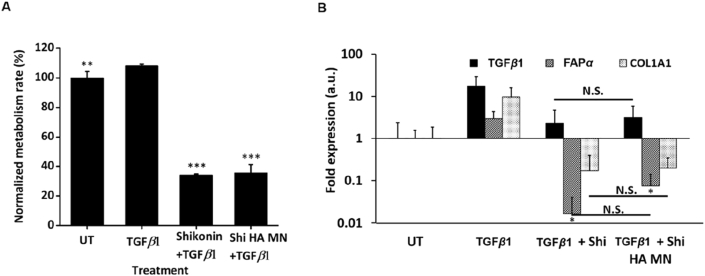
To examine the effect of shikonin HA MNs on the proliferation of TGFβ1 stimulated HSFs, the same groups were tested by AlamarBlue proliferation assay and the results were standardized by the untreated group (UT, Fig. 4A). The addition of TGFβ1 activated and enhanced the proliferation rate of HSFs to 108.27 ± 0.93%. Meanwhile, shikonin solution and shikonin HA MNs significantly reduced the proliferation rate of the TGFβ1-stimulated HSFs to 34.14 ± 0.66% and 35.58 ± 5.57%, respectively. This data indicates that shikonin HA MNs could override the TGFβ1-mediated HSF activation.
Figure 4.
Functionality assay of the Shikonin on TGFβ1 treated HSF. (A) Alamarblue assay demonstrate significant mitigation of cell metabolism from Shikonin-treated groups. Error bars indicate the mean ± SD (n = 4). ∗∗P < 0.01, ∗∗∗P < 0.001 versus the TGFβ1 group. Statistical analysis was performed using one-way ANOVA test. (B) PCR assessment shows downregulated fibrotic genes (i.e., TGFβ1, FAP-α and COL1A1 mRNA) following Shikonin application, reversing the upregulation induced by TGFβ1 addition. Error bars indicate the mean ± SD (n = 3). ∗P < 0.05 versus the TGFβ1 group. N.S. P > 0.05 between groups treated with Shikonin solution and Shikonin HA MNs. Statistical analysis was performed using one-way ANOVA test.
TGFβ1, fibroblast activation protein, alpha (FAP-α) and collagen, type I, alpha 1 (COL1A1) are genes closely related to scar formation. In detail, TGFβ1 improves myofibroblasts differentiation and stimulates the collagen synthesis21, FAP-α is upregulated in scar tissue and most intensively expressed in keloid22, while COL1A1 is the gene encoding collagen type I and found to promote hypertrophic scar formation23. Thus, inhibition of the scar-related gene expression is a potential way to mitigate tissue scarring24. Therefore, we evaluate the modulation of these genes after 24 h of shikonin or shikonin HA MN treatment (Fig. 4B). The results show that TGFβ1 stimulation upregulated the expression of the above-mentioned genes and that shikonin suppresses the TGFβ1-induced upregulations of those genes in HSFs. The gene expressions in the shikonin HA MN-treated group was comparable to that of the shikonin solution treated group, suggesting that the encapsulation of shikonin with HA MNs did not alter the functionality of shikonin in modulating these scar genes.
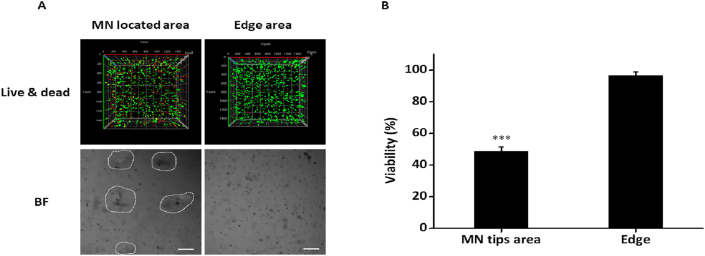
To further clarify the localized efficacy that the MNs endow to shikonin, a 3D cell culture model was established by incorporating HSFs in 1.4% agarose followed by the treatment of shikonin HA MNs. The 3D live/dead staining images show a significantly higher dead signal in area in vicinity of MN as compared to the edge area (Fig. 5A). Further quantification of the fluorescence signals show a significantly lower viability of HSFs in MN tips area than that of the edge area (∼50%; Fig. 5B). This data is consistent with our 2D culture tests and confirm the localized killing effect of shikonin HA MN.
Figure 5.
Examination of the localized killing effect of Shikonin HA MN in 3D HSF culture. (A) Confocal fluorescence (live/dead staining; top) and 2D BF images (bottom) of 3D HSF agarose following Shikonin HA MN treatment. White dashed lines indicate MN tips area. Scale bar: 200 μm. (B) Quantification of the viability rate of 3D HSF culture in (A). Error bars indicate the mean ± SD (n = 3). ∗∗∗P < 0.001. Statistical analysis was performed using one-way ANOVA test.
4. Conclusions
This study aimed to incorporate shikonin into dissolvable HA MN to aid its intradermal delivery for scar treatment. The HA MN showed negligible toxicity to HSFs and different amount of shikonin could be loaded into HA MNs by simply altering the shikonin concentration in the mixture during fabrication. The mechanical performance and surface morphology of the MNs were minimally affected by the addition of shikonin, and the fabricated shikonin HA MNs can withstand a force higher than 0.7 N/needle without fracture and successfully puncture the porcine skin. The fabricated shikonin HA MN can modulate the viability, and metabolism rate of TGFβ1-treated HSFs. Moreover, a localized treatment effect was observed from the shikonin HA MN which is beneficial for the site-specific scar treatment in clinic. In addition, the expression of scar-related genes (TGFβ1, FAP-α and COL1A1) were also attenuated by the treatment of shikonin HA MNs. Taken together, shikonin HA MN possesses great potential for topical scar treatment.
Acknowledgments
Chenjie Xu acknowledges the funding support from Singapore Agency for Science, Technology and Research (A∗STAR) Science and Engineering Research Council Additive Manufacturing for Biological Materials (AMBM) program (A18A8b0059, Singapore), City University of Hong Kong (#9610472, China), General Research Fund (GRF) from University Grant Committee of Hong Kong (UGC) Research Grant Council (RGC) (#9042951, China), and NSFC/RGC Joint Research Scheme (N_CityU118/20, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2021.03.016.
Author contributions
Chenjie Xu and Xiaoyu Ning designed the research. Xiaoyu Ning carried out the experiments and performed data analysis. Christian Wiraja and Sharon Chew Wan Ting participated part of the experiments. Xiaoyu Ning wrote the manuscript. Fan Chen and Christian Wiraja gave comments on the manuscript. Chenjie Xu revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rabello F.B., Souza C.D., Farina Júnior J.A. Update on hypertrophic scar treatment. Clinics. 2014;69:565–573. doi: 10.6061/clinics/2014(08)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juckett G., Hartman-Adams H. Management of keloids and hypertrophic scars. Am Fam Physician. 2009;80:253–260. [PubMed] [Google Scholar]
- 3.Niessen F.B., Spauwen P.H., Schalkwijk J., Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435–1458. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Hou Q., He W.J., Hao H.J., Han Q.W., Chen L., Dong L. The four-herb Chinese medicine ANBP enhances wound healing and inhibits scar formation via bidirectional regulation of transformation growth factor pathway. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Q., Wang S.J., Chen J.Y., Rahman K., Xin H.L., Zhang H. Medicinal plants for the treatment of hypertrophic scars. Evid Based Complement Alternat Med. 2015;2015:101340. doi: 10.1155/2015/101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y., Yan G., Cai B. Advances in studies on nano-technology applied in Chinese Materia Medica. Chin Tradit Herb Drugs. 2011;42:403–408. [Google Scholar]
- 7.Cao F.H., OuYang W.Q., Wang Y.P., Yue P.F., Li S.P. A combination of a microemulsion and a phospholipid complex for topical delivery of oxymatrine. Arch Pharm Res (Seoul) 2011;34:551. doi: 10.1007/s12272-011-0405-8. [DOI] [PubMed] [Google Scholar]
- 8.Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G., Singh M. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 9.Ning X., Wiraja C., Lio D.C.S., Xu C. A Double-layered microneedle platform fabricated through frozen spray-coating. Adv Healthc Mater. 2020;9:2000147. doi: 10.1002/adhm.202000147. [DOI] [PubMed] [Google Scholar]
- 10.Zheng M., Wiraja C., Yeo D.C., Chang H., Lio D.C.S., Shi W. Oligonucleotide molecular sprinkler for intracellular detection and spontaneous regulation of mrna for theranostics of scar fibroblasts. Small. 2018;14:1802546. doi: 10.1002/smll.201802546. [DOI] [PubMed] [Google Scholar]
- 11.Xie Y., Fan C., Dong Y., Lynam E., Leavesley D.I., Li K. Functional and mechanistic investigation of Shikonin in scarring. Chem Biol Interact. 2015;228:18–27. doi: 10.1016/j.cbi.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Albreht A., Vovk I., Simonovska B. Addition of beta-lactoglobulin produces water-soluble shikonin. J Agric Food Chem. 2012;60:10834–10843. doi: 10.1021/jf303153d. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R.C., Lall R., Srivastava A., Sinha A. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci. 2019;6:192. doi: 10.3389/fvets.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan C., Xie Y., Dong Y., Su Y., Upton Z. Investigating the potential of Shikonin as a novel hypertrophic scar treatment. J Biomed Sci. 2015;22:70. doi: 10.1186/s12929-015-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan C., Dong Y., Xie Y., Su Y., Zhang X., Leavesley D. Shikonin reduces TGF-beta 1-induced collagen production and contraction in hypertrophic scar-derived human skin fibroblasts. Int J Mol Med. 2015;36:985–991. doi: 10.3892/ijmm.2015.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo D., Wiraja C., Miao Q., Ning X., Pu K., Xu C. Anti-scarring drug screening with near-infrared molecular probes targeting fibroblast activation protein-α. ACS Appl Bio Mater. 2018;1:2054–2061. doi: 10.1021/acsabm.8b00528. [DOI] [PubMed] [Google Scholar]
- 17.Dabiri G., Tumbarello D.A., Turner C.E., Van de Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta 1 autocrine loop. J Invest Dermatol. 2008;128:2518–2525. doi: 10.1038/jid.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng X., Chen Q., Qiang L., Chi M., Xie N., Wu Y. Development of a porcine full-thickness burn hypertrophic scar model and investigation of the effects of shikonin on hypertrophic scar remediation. Front Pharmacol. 2018;9:590. doi: 10.3389/fphar.2018.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C., Yang H., Kim S., Kim M., Kang H., Kim N. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int J Cosmet Sci. 2016;38:375–381. doi: 10.1111/ics.12299. [DOI] [PubMed] [Google Scholar]
- 20.de Moraes Porto I. IntechOpen Ltd.; London: 2012. Polymer biocompatibility, polymerization. [Google Scholar]
- 21.Penn J.W., Grobbelaar A.O., Rolfe K.J. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 22.Dienus K., Bayat A., Gilmore B.F., Seifert O. Increased expression of fibroblast activation protein-alpha in keloid fibroblasts: implications for development of a novel treatment option. Arch Dermatol Res. 2010;302:725–731. doi: 10.1007/s00403-010-1084-x. [DOI] [PubMed] [Google Scholar]
- 23.Bi S., Chai L., Yuan X., Cao C., Li S. MicroRNA-98 inhibits the cell proliferation of human hypertrophic scar fibroblasts via targeting Col1A1. Biol Res. 2017;50:22. doi: 10.1186/s40659-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan C., El Andaloussi S., Lehto T., Kong K.W., Seow Y. Smad-binding decoy reduces extracellular matrix expression in human hypertrophic scar fibroblasts. Mol Med Rep. 2020;6:4589–4600. doi: 10.3892/mmr.2020.11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.