Abstract
Background
Since July 2019, Pakistan and Afghanistan have been facing an outbreak of serotype-2 circulating vaccine derived poliovirus (cVDPV2) in addition to continued transmission of serotype-1 wild poliovirus (WPV1) and SARS-CoV-2 in 2020. Understanding the risks of cVDPV2 transmission due to pause of global vaccination efforts and the impact of potential vaccination response strategies in the current context of COVID-19 mitigation measures is critical.
Methods
We developed a stochastic, geographically structured mathematical model of cVDPV2 transmission which captures both mucosal and humoral immunity separately and allows for reversion of serotype-2 oral polio vaccine (OPV2) virus to cVDPV2 following vaccine administration. The model includes geographic heterogeneities in vaccination coverage, population immunity and population movement. The model was fitted to historic cVDPV2 cases in Pakistan and Afghanistan between January 2010-April 2016 and July 2019-March 2020 using iterated particle filtering. The model was used to simulate spread of cVDPV2 infection from July 2019 to explore impact of various proposed vaccination responses on stopping transmission and risk of spread of reverted Sabin-2 under varying assumptions of impacts from COVID-19 lockdown measures on movement patterns as well as declines in vaccination coverage.
Results
Simulated monthly incidence of cVDPV2 from the best-fit model demonstrated general spatio-temporal alignment with observed cVDPV2 cases. The model predicted substantial spread of cVDPV2 infection, with widespread transmission through 2020 in the absence of any vaccination activities. Vaccination responses were predicted to substantially reduce transmission and case burden, with a greater impact from earlier responses and those with larger geographic scope. While the greatest risk of seeding reverted Sabin-2 was predicted in areas targeted with OPV2, subsequent spread was greatest in areas with no or delayed response. The proposed vaccination strategy demonstrated ability to stop the cVDPV2 outbreak (with low risk of reverted Sabin-2 spread) by February 2021.
Conclusion
Outbreak response vaccination campaigns against cVDPV2 will be challenging throughout the COVID-19 pandemic but must be implemented urgently when feasible to stop transmission of cVDPV2.
1. Background
The COVID-19 pandemic is causing not only devastating consequences on the health and livelihoods of populations but having substantial negative impacts on global health programmes, including poliovirus eradication. In recent years, the Global Polio Eradication Initiative (GPEI) has been facing significant challenges in interrupting continued transmission of serotype-1 wild poliovirus (WPV1) in the only remaining WPV1- endemic countries, Pakistan and Afghanistan and emerging multi-country outbreaks of circulating serotype-2 vaccine-derived poliovirus (cVDPV2). In 2019, 173 WPV1 cases were reported in Pakistan and Afghanistan (compared to 33 in 2018). In the same year, 366 cVDPV2 cases were reported globally (compared to 71 in 2018), putting the trajectory of polio eradication off course. The cVDPV2 outbreak in Pakistan and Afghanistan that began in July 2019 has resulted in 103 cases (as of 04 Aug 2020). These challenges have been exacerbated by the global pause in vaccination activities due to the COVID-19 pandemic [1].
While eradication of all WPV1 cases remains critical, stopping outbreaks of cVDPV2 is becoming an increasing priority. Serotype-2 oral poliovirus vaccine (OPV) (OPV2) was globally withdrawn from use in April 2016 (switch from trivalent OPV [tOPV] to bivalent OPV (containing types 1 and 3)). Since May 2016, there have been 47 distinct cVDPV2 outbreaks in 23 countries, resulting in 766 cVDPV2 cases [2] (as of 04 Aug 2020); the majority (∼88%) of which have most likely been seeded from monovalent OPV2 (mOPV2) use as part of outbreak response [3]. In 2019 alone, 357 cVDPV2 cases were reported from 16 countries (compared to only 17 cVDPV2 cases from 4 countries during May 2016-April 2017). This substantial increase in cVDPV2 case burden and transmission reflects the accumulation of susceptible birth cohorts with increasing time since OPV2 withdrawal. Although the trivalent Inactivated Poliovirus Vaccine (IPV) was introduced globally into routine immunization (RI) in 2015, which results in humoral immunity and individual protection from paralysis, IPV does not directly induce mucosal protection required to stop person-to-person transmission. In addition, RI coverage (including for both OPV and IPV) is insufficient in most countries currently reporting cVDPV2 outbreaks, and low IPV immunity is a prominent risk factor for high cVDPV2 case burden [4]. These challenges have been compounded by the low case to infection ratio of cVDPV2 and incomplete surveillance in many high-risk countries resulting in extensive silent transmission.
In order to stop cVDPV2 outbreaks, vaccination responses with OPV2 (most commonly through Sabin mOPV2 but increasingly through trivalent OPV, particularly in Pakistan and Afghanistan) are currently required; however, the inherent risk of its use in seeding more cVDPV2 necessitates strategic and appropriate vaccination response strategies [5]. The spatial scale of response must be sufficient to stop cVDPV2 transmission but no larger given the risk of OPV2 reverting to virulence and spreading vaccine-derived virus. Despite the importance of strategic OPV2 use, the quality of many OPV2 responses has been low and a great deal of uncertainty around mitigating risk of cVDPV2 remains. The complexity of responding to cVDPV2 outbreaks in 2020 has been exacerbated by the COVID-19 pandemic [6], [7], [8] (which led to a 4-month ban on all vaccination campaigns between Mar-Jul 2020, despite ongoing outbreaks [9]). In this current context, opportunities to vaccinate in a large-scale are limited, given i) national imposed lockdowns, ii) the risk to vaccinators traveling house to house, iii) re-focused polio resources to support in-country COVID-19 strategies and iv) potential declines in polio routine surveillance (partly due to decreased mobilisation of polio staff in the communities for active case search and campaigns, and competing demands of lab capacity for SARS-CoV-2 testing). Therefore, appropriate and strategic vaccination response plans against cVDVP2 are particularly important.
In addition to endemic transmission of WPV1, Pakistan and Afghanistan are currently facing a cVDPV2 outbreak (since July 2019, likely seeded from inadvertent trivalent OPV use [3]). This coupled with an increasingly complex challenge of SARS-CoV-2 transmission is posing a major threat to these two countries. Given the need to ensure appropriate vaccination response against cVDPV2 (while keeping in mind competing challenges, including substantial areas remaining inaccessible to vaccinating children in Afghanistan [10]), we have developed and fitted a stochastic spatially-structured metapopulation mathematical model of cVDPV2 transmission in Pakistan and Afghanistan to explore cVDPV2 risk and outbreak response strategies. We have validated the model to all historic cVDPV2 daily incidence data reported since 2010, and calibrated it to the recent outbreak originating in Diamir, Pakistan in July 2019 (up to 29 February 2020). This work was performed in June 2020 to inform the outbreak response strategy following the end of the suspension of vaccination activities due to the COVID-19 pandemic. The model captures geographic heterogeneity in serotype 2 population immunity and population movement patterns [11], [12]. Moreover, the model allows for OPV2 to revert to cVDPV2 following vaccine administration and tracks reverted Sabin-2 transmission separately from the initial cVDPV2 outbreak. Mucosal and humoral immunity are modelled separately, capturing the differential impact of oral versus inactivated vaccines, respectively. In this work, we consider varying assumptions of impacts from COVID-19 lockdown measures on movement patterns as well as declines in RI coverage and explore the impact of various response strategies on stopping cVDPV2 transmission between June 2020 and February 2021. Finally, we provide the response adopted by the country programmes that were informed by this modelling work.
2. Methods
2.1. Serotype-2 circulating vaccine-derived poliovirus (cVDPV2) data
All poliomyelitis cases are confirmed through isolation and sequencing of poliovirus from stool collected from notified cases of all-cause Acute Flaccid Paralysis (AFP) – described as sudden onset of flaccid paralysis in one or more limbs. AFP is not specific to poliovirus infection and is characteristic of many aetiologies, including Guillain-Barre syndrome, trauma and non-polio enterovirus infections [3]. Stool samples are collected from AFP cases and tested for poliovirus [13] (negative stool samples are referred to as non-polio AFP). Here, reported cVDPV2 poliomyelitis cases with clinical onset between 1 January 2010 and 1 March 2020 (as of 23 April 2020). No cVDPV2 cases were reported in Pakistan or Afghanistan prior to 2010.
2.2. Underlying cVDPV2 transmission model
To capture cVDPV2 transmission dynamics, we developed a spatiotemporal stochastic model of poliovirus transmission in Pakistan and Afghanistan amongst children < 36 months old (given that this cohort contributes most to transmission [14]) based on the susceptible-exposed-infected-recovered (SEIR) compartmental framework. In brief, the model includes a district-specified geographic structure, whereby the dynamics of infection in each district depends on the local transmission rate within the district as well as importation rate of virus from other districts. The model assumes homogeneous mixing within districts. We assumed the latent and infectious periods for poliovirus to be a mean of 4 and 14 days, respectively [15], [16]. Additionally, birth and death rate, μ, are assumed to be constant and the duration in the cohort was assumed to be a median of three years. Data on actual number of births at the district level were not available.
In addition to the SEIR compartments, the transmission model was modified to the cVDPV2 context to by incorporating additional states, including: i) newly seeded cVDPV2 infection that is generated from the outbreak response with Sabin-2 vaccine (compartment J); ii) infection with Sabin-2 vaccine virus (i.e. OPV2) (compartment O); and iii) incorporating individuals with waned intestinal mucosal immunity to serotype-2 (compartment W); see supplemental materials. Distinguishing cVDPV2 infections resulting from recently reverted Sabin-2 virus used in the response from those attributed to the initial cVDPV2 outbreak is important to enable assessment of OPV2 response strategies; specifically to determine the amount by which cVDPV2 seeded from the OPV2 response to the outbreak contributed to the total amount of cVDPV2 infection. Additional details are provided in S1 Text. Model input parameters are presented in Table S2.
2.3. Force of infection
The per district (i) force of infection λ determines the rate at which a susceptible individual is infected with cVDPV2 at time t + 1:
| (1) |
where Ii,t and Ij,t refer to the number of the original cVDPV2 infected children in district i and j at time t, Ni,t and Nj,t refer to the total number of children < 36 months of age in district i and j at time t, βl and βb refer to the local and between-district transmission coefficients respectively. Local transmission in district i is based on the local transmission coefficient βl and the proportion of infections in the district at time t. Transmission between districts is based on the between-district transmission coefficient βb and the summation of infection coming into district i from all other districts. The probability of importation of infection from each external district is based upon the radiation model [17] and denoted in equation (1) by the probability of people on average who move from district j to i, (where j ≠ i) given by qj,i, which we have previously shown to sufficiently capture the spatial transmission of poliovirus in Pakistan and Afghanistan [18]. Given that the model includes three infectious polioviruses i.e., 1) original cVDPV2 (Ii), 2) newly reverted cVDPV2 from Sabin-2 (Ji), and 3) Sabin-2 poliovirus (Oi), there are three distinct force of infections, indexed as λ1, λ2 and λ3, respectively (see S1 Text for definitions). Additionally, we allowed transmission both locally and between districts to be seasonal (see S1 Text).
2.4. Vaccination with OPV2 and IPV
The model includes routine immunisation with three tOPV doses until April 2016, after which one IPV dose is administered for type 2 humoral immunity instead. Births immunised with tOPV enter the R compartment (mucosal and humoral immunity) whilst births immunised with IPV enter a new compartment V which represents humoral immunity only.
The model also captures vaccination through supplementary immunisation activities (SIA) at specific points in time. Susceptible children (S), children with waned mucosal immunity (W) or children with humoral immunity (V) can be immunised with OPV2 (tOPV or mOPV2) and the majority of them (1- κ) enter O compartment (OPV2 infection) for a mean duration of 14 days after which they progress to full mucosal immunity (R). However, we assume OPV2 virus will instantaneously revert to create a new cVDPV2 in a small proportion of immunised children (κ) and these children enter state J (whereby J is equally as infectious as I). Susceptible children or children with waned mucosal immunity can also be immunised with IPV after which they enter the V compartment (humoral immunity) or R compartment (boosting of waned mucosal immunity to full mucosal immunity), respectively.
District-specific RI and SIA coverage were estimated from non-polio AFP data in 6-month time intervals between 2010 and 2016 and spatially and temporally smoothed, as described in [12], [18], (S1 Text). The Jan-Jun 2010 and Jan-Jun 2016 estimates were used for the model as periods prior to and following OPV2 withdrawal respectively. Both RI and SIA vaccination coverage of IPV is assumed to be the same as for that of OPV (IPV doses have not historically been reported in AFP data and therefore cannot be estimated), which is supported by evidence in Pakistan demonstrating comparable coverage between IPV and OPV SIAs [18]. The number and timing of SIA campaigns with OPV2 or IPV were extracted from the WHO SIA calendar (Fig S4).
2.5. Observation model
While infection with poliovirus is typically asymptomatic, few studies have estimated the case to infection ratio, particularly for serotype-2. Although there is consensus that a lower proportion of infections result in paralysis for serotype 2 compared to serotype 1 (with the latter having a case-to-infection ratio of ∼ 1:150–1:200 [19], [20], [21]), the estimates for serotype-2 have been variable [21], [22], [23], [24] and as low as 1:2000. We explored the model fit under assumptions ranging from 1:400 to 1:2000 and the assumption of 1:400 provided the best fit to the data (Table S2). Therefore, we assume an average of 1 poliomyelitis case in every 400 cVDPV2 infections; and that only fully susceptible children that are subsequently infected can develop poliomyelitis (i.e. children with waned mucosal immunity could be re-infected but could not develop disease [25]). Sensitivity analysis exploring the results under the assumption 1:2000 is presented in S1 Text. Moreover, surveillance sensitivity of cases is variable between countries and may be higher in endemic countries, such as Pakistan and Afghanistan [26] meaning the case to infection ratio may be underestimated in areas if surveillance quality is poor.
2.6. Defining the process model
We used the R package POMP [27], which enables simulating and fitting partially-observed Markov process models (i.e., state-space models) to time series. The model was fitted to the data using iterated particle filtering, which computes the probability of the data Yt given the states Xt and is proportional to the likelihood function. At each day for a given spatial unit the binomial likelihood of the observed number of poliomyelitis cases was computed given the reporting fraction τ and the simulated number of people completing the incubation period on that day (see S1 Text for further details).
2.7. Model validation
The transmission model was fitted to historic daily incidence of cVDPV2 from 2010 to 2016; it was then modified for forward simulation following OPV2 withdrawal (April 2016) by stopping routine immunisation with tOPV, incorporating SIAs with mOPV2 and incorporating an additional state representing immunisation with IPV only (V) through RI and SIAs. We estimated three model parameters by fitting the model to the incidence of cVDPV2 cases with clinical onset between January 2010 and April 2016: These parameters were i) local transmission coefficient (i.e. βl); ii) scaling factor for between-district transmission (i.e. βb = βlf, whereby f is the estimated parameter); and iii) probability of Sabin-2 reversion (i.e. κ). The parameter f was constrained to be ≤ 1 (i.e. assuming that local transmission will always be greater than between district transmission, given the increased probability of local contacts compared to between-district contacts).
The model was then simulated under from April 2016 through June 2019 with implemented vaccination campaigns to estimate mucosal and humoral immunity prior to the start of the Diamir, Pakistan outbreak. The model was then calibrated to daily incidence data of this outbreak, incorporating the SIA calendar, and was used to explore transmission patterns and impact of vaccination responses. The outbreak was seeded with 400 infections in Diamir in July 2019 since the origin of this virus was not known and was not directly linked to any formal OPV2 use in Pakistan. The details for all initial conditions are presented in S1 Text. The local transmission coefficient and scaling factor for between-district transmission were re-estimated. Due to the limited number of cases and OPV2 responses, the reversion probability was fixed based on the 2010–2016 validation.
2.8. Simulating the spread of the cVDPV2 outbreak originating in Diamir, Pakistan
The model was used to simulate spread of cVDPV2 infection from Diamir from July 2019 through February 2021. Based on 100 simulations, we determined the median proportion of districts infected with > 400 cVDPV2 infections (i.e., sufficient transmission to result in clinical cVDPV2 cases) and the median cumulative number of cVDPV2 cases over time.
In addition to considering cVDPV2 transmission under typical movement patterns, we adjusted the poliovirus transmission rate to account for lockdowns due to COVID-19 between 15 March to 30 June 2020. It is unknown how the reductions in movement affected poliovirus transmission and so we simulated the model under a variety of assumptions. Data from mobile devices using Google services indicated transit related movement decreased by ∼ 50% during this time period [28]. In all scenarios of movement, we assumed a 50% reduction in estimated between-district transmission. We also examined a range of reductions to within-district transmission (i.e., local R0) between 15 March and 30 June 2020, including reducing the within-district reproduction number from the estimated value by 50% (assuming baseline R0 = 1) as well as assuming transmission is continuing to grow at a substantially reduced rate (i.e. R0 local = 1.1) or is brought below the threshold at which an outbreak is expected to grow (R0 local = 0.9). These adjustments are hereafter referred to as ‘modest reduction’, ‘substantial reduction’ and ‘complete halt’ in poliovirus transmission, respectively. For all scenarios, we also considered the impact of the lockdown on RI through assuming a 50% reduction in estimated district-specific IPV RI coverage between 15 March and 30 June 2020 as related to cVDPV2 case burden.
2.9. Simulating the impact of vaccination responses on the Diamir outbreak
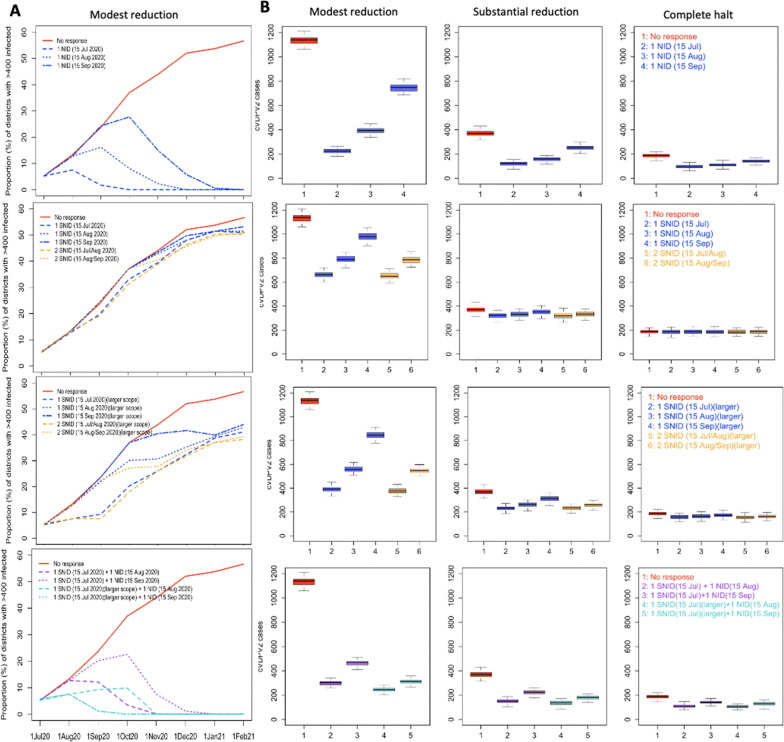
The impact of a range of OPV2 vaccination responses was explored, considering the impact of timing, geographic scale (i.e. from targeted Sub-National Immunization Days (SNIDs) to National Immunization Days (NIDs)) and coverage of response on cVDPV2 transmission. We specifically considered the differential impact of NIDs with increasing monthly delays in implementation (i.e., 15 Jul, 15 Aug and 15 Sep 2020) and compared with SNIDs only targeting districts with substantial cVDPV2 transmission. Districts with substantial cVDPV2 transmission were determined based on districts with > 400 infections 15 days prior to the SNID. The number of districts targeted and the total target population < 5 years of age was estimated for each SNID. Moreover, 2 versus 1 SNIDs were compared along with a combined approach of one early SNID (i.e. 15 Jul) followed by one NID 1–2 months later (i.e., 15 Aug/Sep). For the SNID, in addition to considering targeting districts with > 400 infections 15 days prior to the SNID, a larger target population was also considered (i.e., districts with > 100 infections).
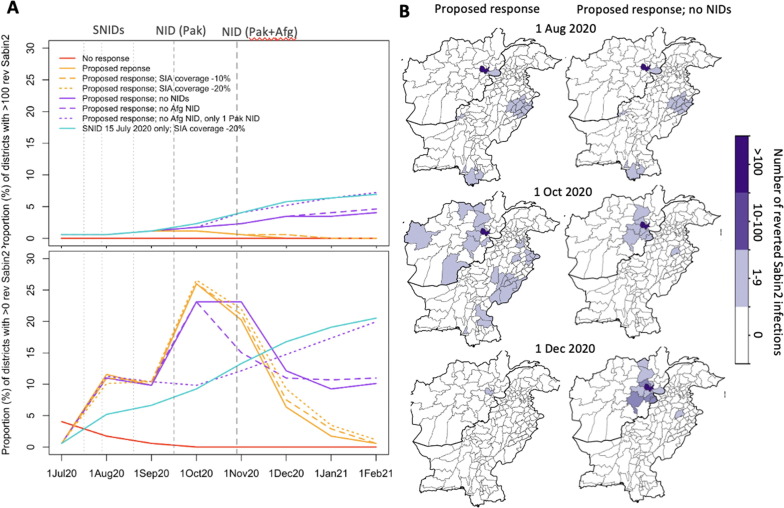
In consultation with the Pakistan and Afghanistan country polio programmes, a proposed response was considered to incorporate the context complexities and current operational challenges. In Pakistan, this planned response included two SNID mOPV2 rounds (Jul-Aug 2020) (plus an initial round termed ‘round 0′ incorporated for three highest risk districts – Karachi, Faisalabad and Quetta – to be conducted urgently) followed by 2 NIDs (Sep-Oct 2020). In Afghanistan, 2 mOPV2 SNIDs in July targeting 3 provinces (Nangarhar, Kunar and Laghman), potentially followed by one NID in September. Given the challenges in successfully implementing these SIAs, this option was explored under assumptions of reduced SIA coverage of 10% and 20% absolute declines as well as under assumption of no NIDs or only NIDs in Pakistan. Moreover, due to the risk of problems with the first round in July resulting in the inability to conduct any subsequent rounds, a scenario was explored with only the July SNID at a 20% reduced SIA coverage. Finally, the risk of seeding new cVDPV2 transmission was evaluated by considering the proportion of districts with any or larger numbers (i.e., >100 infections) of reverted Sabin-2 infections over time.
3. Results
3.1. Reported cVDPV2 cases
Between January 2010 and June 2016, a total of 87 cVDPV2 cases were reported in Pakistan and 18 cases were reported in Afghanistan. Following OPV2 withdrawal, in December 2016 there was 1 cVDPV2 case reported in Quetta. In July 2019, a cVDPV2 outbreak originated in Diamir, Pakistan which resulted in 59 cases in Pakistan and Afghanistan by 29 February 2020 (Fig. 1 C,D). The first cVDPV2 case in Afghanistan was reported in February 2020 in Nangarhar province. An additional 44 cases have been reported in Pakistan and Afghanistan since 1 March 2020 (as of 07 Aug 2020).
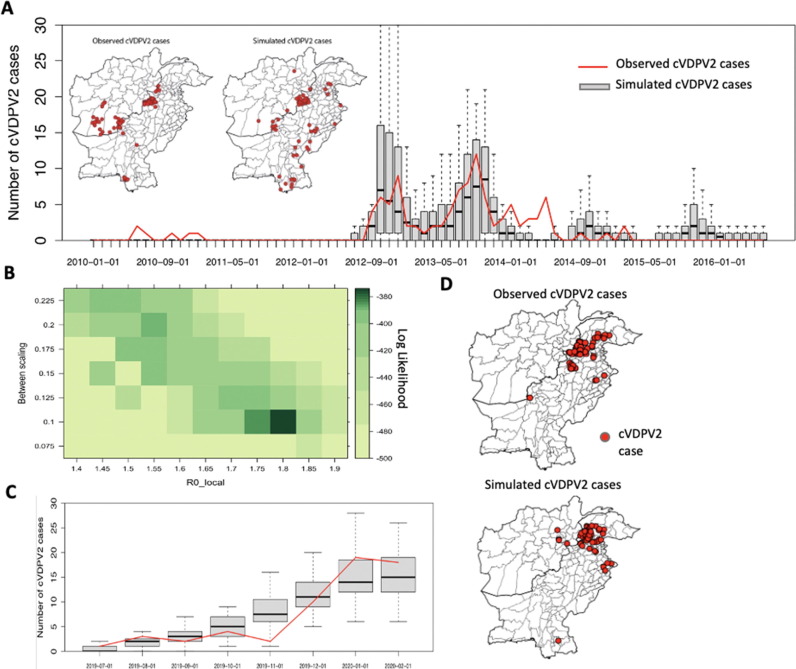
Fig. 1.
Model validation. (A) Monthly incidence of cVDPV2 observed cases (red line) in Pakistan and Afghanistan between 2010 and 2016 and simulated cases (boxplots, across 100 simulations). Inset maps display spatial distribution of observed and simulated cumulative cVDPV2 cases between 2010 and 2016. (B-D) Model calibration of Diamir, Pakistan cVDPV2 outbreak (between 01 July 2019 to 29 February 2020), including (B) log likelihood profile for R0 local and the factor determining the relative between- district coefficient compared to within-district transmission. (C) Monthly observed and simulated cumulative cVDPV2 cases and (D) spatial distribution of observed and simulated cases.
3.2. Vaccination
Both RI and SIA coverage is spatially heterogeneous across Pakistan and Afghanistan and fairly consistent over time between 2010 and 2016 (Fig S5- FigS6). In Jan-Jun 2016, vaccination coverage through RI and SIAs was 70% (IQR: 45%-83%) and 74% (64%-83%), respectively (Fig. 3A and Fig. 5A). In Pakistan and Afghanistan, the lowest RI coverage was in Balochistan (34%) and Hilmand (26%); and highest in Punjab (88%) and Kabul (87%). SIA coverage was highest in Punjab (82%) and Jawzjan (94%) and lowest in Balochistan (63%) and Kandahar (34%).
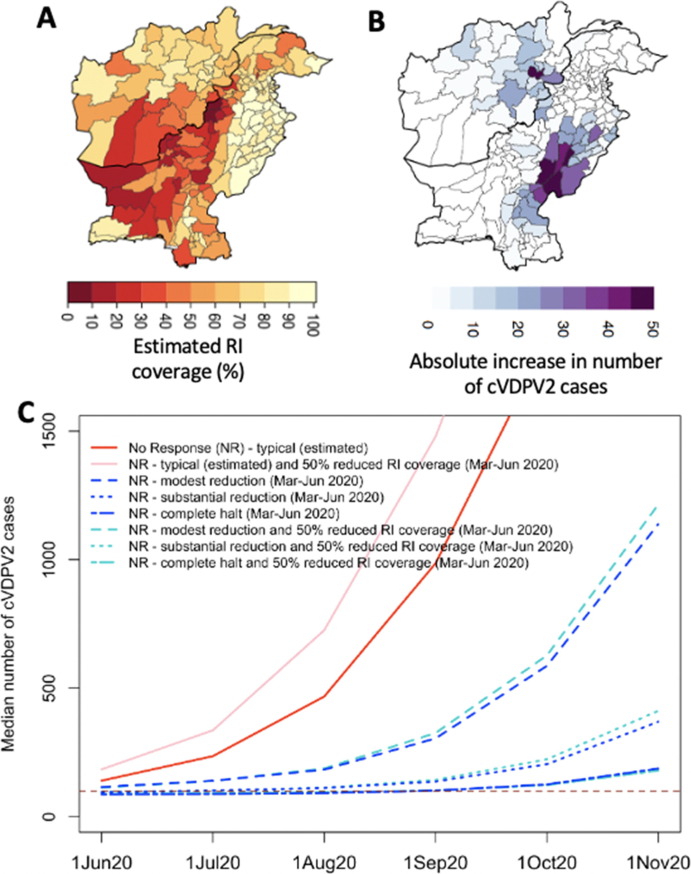
Fig. 3.
Forward simulation of cVDPV2 outbreak originating in Diamir, Pakistan, assuming no vaccination response (since March 2020) based on 50% reduction in routine immunization (RI) coverage (between March-June 2020) due to COVID-19 lockdown. (A) Estimated RI coverage. (B) Absolute increase in median cumulative number of cVDPV2 cases by 1 November 2020 due to 50% reduced RI during March–June 2020 compared to normal RI coverage (assuming typical poliovirus transmission). (C) Cumulative number of cVDPV2 cases from March 2020 across the assumptions of changes in poliovirus transmission and with or without 50% reduction in RI coverage.
Fig. 5.
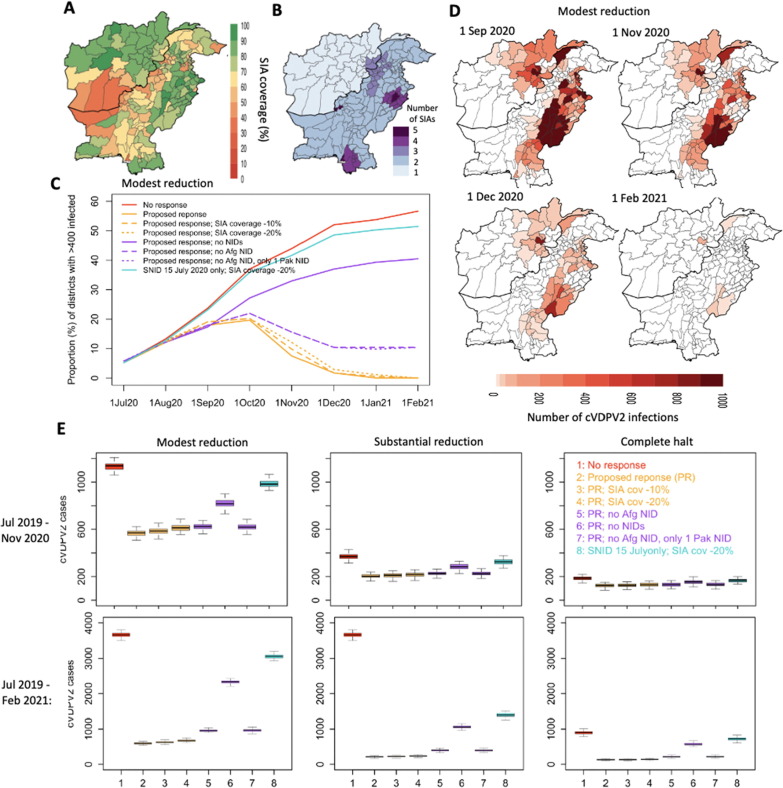
Forward simulation of cVDPV2 outbreak originating in Diamir, Pakistan, considering impact of proposed vaccination response. (A) Estimated SIA coverage. (B) Geographic scope and number of proposed SIAs. (C) Impact of proposed SIAs in proportion of districts with > 400 cVDPV2 infections under-estimated and reduced SIA coverage and considering responses with and without NIDs. (D) Maps displaying the number of cVDPV2 infections over time under the proposed response and modest reduction in transmission between March-June 2020. (E) Distribution of cumulative number of cVDPV2 cases between July 2019 to November 2020 (top panel) and July 2019 to February 2021 (bottom panel) based on the different assumptions of movement and vaccination strategies.
3.3. Parameter estimation
For the model validation between Jan 2010 to Apr 2016, the local and overall R0 were estimated to be 2.35 and 3.58, respectively (Fig S7). Between-district transmission was estimated to be 0.52 * qji times that of local transmission. The Sabin-2 reversion probability was estimated to be 1.24 × 10-4. The model calibration for Jul 2019 to Mar 2020, resulted in a modestly lower local and overall R0 of 1.8 and 1.98, respectively, and between-district transmission was estimated to be 0.1 * qji times that of local transmission.
3.4. Model fit
The monthly number of cVDPV2 cases simulated from the model (based on 100 simulations) from January 2010 to April 2016 generally align with observed cVDPV2 cases (Fig. 1A). The model captures peak incidence of cVDPV2 in July-December 2012 and July-December 2013, albeit failing to capture the incidence in mid-2014. The spatial distribution of total cVDPV2 cases simulated from the model between Jan 2010 to Jun 2016 is similar to observed cVDPV2 cases (Fig. 1A). The model underestimates the number of cVDPV2 cases in Southern Afghanistan and overestimates the number of cVDPV2 cases in Northern Sindh, Pakistan.
For the outbreak originating in Diamir in July 2019, simulations from the model similarly demonstrate a general alignment with observed cVDPV2 cases through March 2020, both in time and space (Fig. 1C). In November 2019, fewer cases were reported than expected and in Jan-Feb 2020, the model modestly underestimated the number of cases.
3.5. Simulated serotype-2 immunity
Simulated serotype-2 mucosal immunity was estimated to be low (median: 22%; IQR: 20%–23%) in March 2020 across Pakistan and Afghanistan, apart from select parts of Pakistan (i.e., provinces of GB, KP and select districts of Punjab), where mOPV2 responses were conducted between July 2019 to March 2020 (Fig. 2 A). The levels of humoral immunity were higher (68%; 57%-74%), however, mostly < 80% apart from districts with recent mOPV2 and IPV responses (Fig S5).
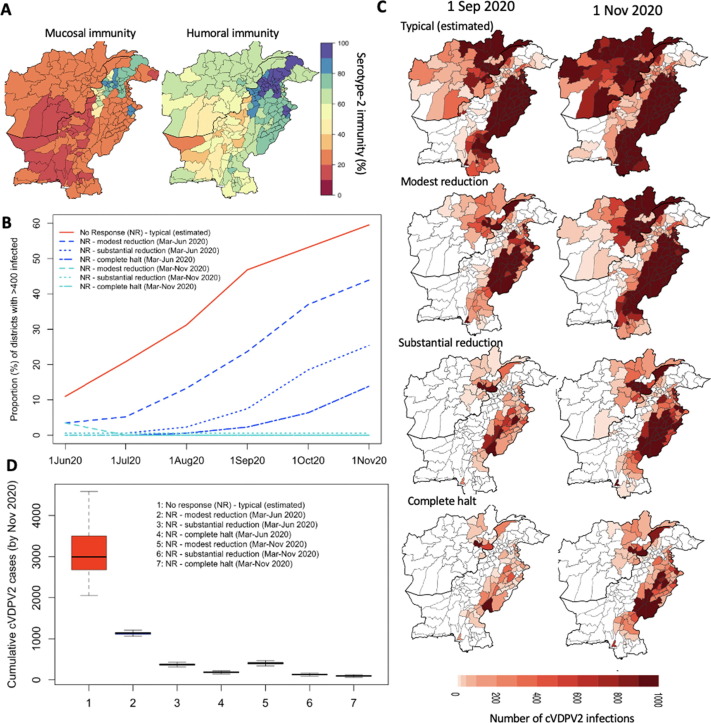
Fig. 2.
Forward simulation of cVDPV2 outbreak originating in Diamir, Pakistan, assuming no vaccination response (since March 2020) based on varying assumptions of the impact of population movement changes on poliovirus transmission due to COVID-19 lockdown. (A) Serotype-2 mucosal and humoral immunity in March 2020 based on simulations from the model. (B) Proportion of districts with > 400 cVDPV2 infections (corresponding to 1 cVDPV2 case) with no response (since March 2020) and various assumptions of poliovirus transmission from changes in transmission due to lockdown measures (typical, modest reduction, substantial reduction, complete halt) between March-June 2020 and March-November 2020). (C) Maps displaying the number of cVDPV2 infections over time under the various assumptions of movement patterns between March-June 2020. (D) Distribution of cumulative number of cVDPV2 cases between July 2019 to November 2020 across the assumptions of movement on transmission.
3.6. Simulating the spread of the 2019–20 cVDPV2 outbreak originating in Diamir, Pakistan
In the absence of vaccination activities (from March 2020 through November 2020), the cVDPV2 outbreak seeded in Diamir, Pakistan was predicted to spread rapidly during the summer months of 2020, resulting in 24% (IQR: 22–25%) and 44% (44–46%) of districts infected with > 400 infections by September and November 2020 (Fig. 2BC), respectively, corresponding to 305 (294–319) and 1,138 (1,110–1,154) cVDPV2 cumulative cases (from 1 July 2019). These results were under the assumption of modest reduction in poliovirus transmission from lockdown activities between Mar-Jun 2020 (Fig. 2D). Assuming no changes to transmission patterns instead, the outbreak was predicted to spread ∼ 3-fold faster than under the lockdown scenarios (i.e., 986 (834–1,173) and 2,992 (2,680–3,496) cases, by September and November 2020, respectively) (Fig. 2BCD). Substantially reducing transmission between Mar-Jun 2020 resulted in sustained low levels of transmission with only 7% (7–9%) and 25% (24–29%) of districts reporting > 400 infections by September and November 2020, corresponding to 137 (126–151) and 369 (354–385) cases, respectively (Fig. 2B–D). This was further reduced under the assumption of a complete halt in poliovirus transmission between Mar-Jun 2020, with 2% (2–3%) and 14% (11–17%) of districts reporting substantial numbers of infections (i.e. > 400); and 102 (93–110) and 187 (175–196) cumulative cases (Fig. 2BCD). By assuming the modest reduction, substantial reduction or complete halt in transmission remained through to the end of November 2020 (i.e. lockdown extended), the cumulative number of cases by November reduced to 405 (387–423), 128 (117–139) and 94 (86–104), for the three transmission scenarios, respectively (Fig. 2D).
Declines in RI coverage (Fig. 3 A) (i.e. 50% reduction) were predicted to substantially increase cases under settings of very high transmission, where susceptible birth cohorts accumulate rapidly (i.e. places with high birth rate) and/or in places with typically high RI coverage (Fig. 3B). This was demonstrated with 31% relative increase in median cumulative cases by November 2020 under the scenario of typical transmission) (Fig. 3CD); with 48% of this increase in case burden coming from Punjab where RI coverage, population size and birth rate are high (Fig. 3B and [18]).
3.7. Impact of outbreak response vaccination strategies
Under the assumption of modestly reduced transmission between Mar-Jun 2020, the impact of one National mOPV2 response (i.e. NID) in both countries mid-July, mid-August or mid-September, was predicted to result in an 100%, 95% and 66% relative reduction in the number of districts with substantial cVDPV2 transmission (i.e. districts with > 400 cVDPV2 infections), resulting in 222 (209–237), 393 (376–408) and 746 (718–768) cumulative cases, by November 2020 (Fig. 4 AB). The impact of one SNID along the same time schedule (i.e. mid-July, mid-August or mid-September) resulted in 10%, 3% and 1% relative reduction in districts with substantial transmission by November, if targeting only districts with > 400 cVDPV2 infections (i.e. based on districts with > 400 cVDPV2 infections 15 days prior to SNID) (i.e. 9 districts; target population:∼4.6 million) and 41%, 30% and 8% relative reduction if increasing the target population to include districts with lower levels of transmission (i.e. based on districts with > 100 cVDPV2 infections 15 days prior to SNID) (i.e. 27 districts; target population:∼11.9 million). Increasing this to 2 SNIDs in either mid-June/mid-July or mid-July/mid-August resulted in a relative reduction of 12% and 8%, respectively, by November, for targeting districts with confirmed transmission and 41% and 37% relative reduction at the larger target. A combined approach of one early SNID (i.e. mid-Jul) followed by one NID 1 or 2 months later (i.e. mid-Aug or mid-Sep) resulted in 100% and 83% relative reduction in districts with substantial transmission and 301 (285–311) and 469 (449–477) cumulative case burden by November under the more conservative target of ∼ 4.6 million. Considering the larger target population (i.e. ∼ 11.9 million) for the SNID increased this relative reduction to 100% and 100% (with 245 (232–258) and 312 (300–323) cumulative cases by November). Consistent trends were demonstrated across the varying assumptions of movement (Fig S8 and Fig. 4B), albeit with lower levels of transmission (Fig S8) and cumulative case burden (Fig. 4B).
Fig. 4.
Forward simulation of cVDPV2 outbreak originating in Diamir, Pakistan, considering impact of different vaccination strategies. (A) Impact of number, timing and geographic scope (i.e., National and Sub-National) of SIAs based on assumption of modest reduction in transmission between Mar-Jun 2020. (B) Distribution of cumulative number of cVDPV2 cases between July 2019 to November 2020 based on the different assumptions of the impact of population movement on poliovirus transmission and vaccination strategies.
The proposed strategy to implement 2 SNIDs (Jul-Aug) and 2 NIDs (Sep-Oct) in Pakistan and 2 SNIDs (Jul) and 1 NID (Sep) in Afghanistan (Fig. 5 C) predicted to result in only 2% (2–2%) of districts with substantial transmission by December 2020 and no districts reporting substantial transmission by February 2021 (Fig. 5D-E) (under the assumption of modest reduction in transmission between Mar-Jun 2020). The predicted cumulative cVDPV2 case burden for this response was 570 (550–584) and 597 (575–612) cases by November 2020 and February 2021, respectively (Fig. 5E). Reducing estimated SIA coverage (Fig. 5B) by absolute values of 10 and 20%, resulted in nearly no impact on the proportion of districts with substantial transmission by the end of 2020 (i.e. 0% and 1% relative increase). The impact of reducing or eliminating the number of NIDs on transmission and case burden was substantial. Considering no NIDs resulted in a 20% relative increase in districts with substantial transmission by the end of 2020 compared to the proposed response and a cumulative case burden of 817 (799–844) and 2334 (2301–2365) by November 2020 and February 2021, respectively. Excluding the NID in Afghanistan (September) resulted in 5% relative increase in districts with substantial transmission compared to the proposed response and increased case burden to 954 (933–989). Further excluding one of the Pakistan NIDs (October) (i.e. only one NID for Pakistan remaining), similarly resulted in 5% relative increase in districts with substantial transmission and 964 (940–988) cumulative case burden by February 2021. Finally, considering only one SNID in July with a 20% reduced SIA coverage was only modestly different from no response in terms of proportion of districts with substantial transmission. Similar trends of transmission and case burden were observed across assumptions (Fig S9 and Fig. 4B), however, decreasing the transmission substantially or completely between March-June 2020 reduced the cumulative case burden to 203 (194–216) and 124 (114–136), respectively, by November 2020; and 212 (202–223) and 127 (118–13) by February 2021, for the proposed response (see Fig. 6 ).
Fig. 6.
Risk of emergence and spread of reverted Sabin-2 from proposed SIA response (based on modest reduction in transmission between March-June 2020). (A) Proportion of districts with any (i.e. > 0) and > 100 reverted Sabin-2 infections over time under proposed response, reduced SIA coverage, and without NIDs. (B) Geographic distribution of number of reverted Sabin-2 infections over time based on proposed response with and without NIDs.
Under the proposed response, the proportion of districts with any reverted Sabin-2 infections was 12% (10–13%) in August (following 1 SNID plus initial focused SIA in Pakistan), 26% (24–28%) in October (following 2 SNIDs and 1 Pakistan NID) and 6% (5–7%) in December (following all responses) (Fig. 4A) (assuming modest reduction in transmission between Mar-Jun 2020). By February 2021, there were no districts predicted to contain reverted Sabin-2 infection. Between July-December 2020, low levels of risk (1–9 reverted Sabin-2 infections) were reported across a wide geography, including most of Punjab, parts of Sindh, Quetta (Balochistan), South-East Afghanistan and dispersed throughout various provinces of Afghanistan (Fig. 4B). Excluding NIDs from the proposed response strategy resulted in a lower proportion of districts with any reverted Sabin-2 infections in October (10% (8–12%)), but higher proportion in December (15% (11–20%)) and February (20% (13–27%)). The proportion of districts with greater levels of reverted Sabin-2 infections (i.e. > 100) was 0.6% (0.6–1.2%) in August, 1.2% (0.6–1.7%) in October and 0% (0–0.6%) in both December and February. Excluding NIDs resulted in an overall increase to 2% (1–4%) in October, 5% (4–7%) in December and 7% (5–12%) by February. The greatest risk of reverted Sabin-2 transmission was focused in Afghanistan (Kabul and surrounding provinces).
4. Discussion
GPEI paused all mass vaccination campaigns between March and June 2020 in response to the COVID-19 pandemic to adhere to national lockdowns and protect populations from SARS-CoV-2 transmission. The emergence and spread of cVDPV2 in Pakistan in late 2019 at a time when majority of children lack mucosal immunity against this serotype presents a critical challenge to interrupt transmission when opportunities to respond are limited. We developed and validated a mathematical model of poliovirus transmission to explore the risk of the current cVDPV2 outbreak in Pakistan and Afghanistan. The best-fitting model demonstrated general alignment with observed cVDPV2 cases between 2010 and 2016 and 2019–2020 in terms of geographic and temporal distribution. While the forward simulations from the model demonstrated substantial cVDPV2 transmission and case burden through 2020 (despite assuming a modest reduction in poliovirus transmission due to lockdown measures), the impact from the proposed vaccination response were promising with the potential to interrupt transmission by early 2021. This work directly informed the vaccination response strategies to the cVDPV2 outbreak in Pakistan and Afghanistan.
In the absence of vaccination response and assuming modest reduction in poliovirus transmission between Mar-Jun 2020, the cVDPV2 outbreak was predicted to spread rapidly during the summer months resulting in substantial case burden by September 2020. Without adjusting poliovirus transmission to account for lockdown in response to SARS-CoV-2, the spread of infection and resulting case burden was 3-fold higher indicating the potential indirect benefit of control strategies for COVID-19 may have poliovirus transmission. Moreover, this reduction in movement during the peak transmission in summer months shifted transmission towards the low season, likely impacting the overall magnitude of the outbreak in the absence of vaccination responses. Assuming further reductions in poliovirus transmission (i.e., strictly upheld lockdown measures) resulted in more dramatic impacts on polio case burden; however, this degree of lockdown was unlikely in Pakistan and Afghanistan [28].
RI uptake may be affected by the pandemic with declines already documented in Pakistan [29]. We simulated the effect of declines in RI in administration of IPV on the cVDPV2 outbreak but this was predicted to play a less pronounced role in altering case burden. This is partly owing to the fact that IPV in RI only induces humoral immunity and has no impact on person-to-person transmission of cVDPV2 and that RI coverage is suboptimal in many areas of Pakistan and Afghanistan. The greatest impact of reduction in RI coverage was in Punjab where RI coverage is typically high as is the birth rate; that coupled with the high population size in Punjab and fully susceptible population (in terms of mucosal immunity) also put it most at risk of cVDPV2 transmission. In Afghanistan, most of the areas typically at highest risk for cVDPV2 have very low RI coverage (i.e., South-East provinces) and therefore the impact was not pronounced.
Large scale vaccination responses (i.e. NIDs) were predicted to substantially reduce transmission and case burden, with a greater impact from earlier responses (i.e. mid-July). These findings corroborate previous findings that responding sooner to outbreaks has greater impact [16]. Targeting only districts with confirmed local transmission, resulted in only a modest impact on geographic distribution of substantial transmission and overall case burden and the larger the response, the greater the impact. Targeting districts twice with the same geographic scope resulted in only a very modest impact on overall transmission (unless the scope is large), as virus easily escapes the response zone due to the highly mobile nature of the population [30]. Following up the SNID with an NID within 1–2 months results in a similar impact compared to an early NID. Given the challenges of operationalizing a National SIA in July, this is reassuring that a focused response in areas with substantial transmission followed by an NID when possible was an impactful strategy. The proposed strategy to implement 2 SNIDs (Jul-Aug) and 2 NIDs (Sep-Oct) in Pakistan and 2 SNIDs (Jul) and 1 NID (Sep) in Afghanistan was predicted to interrupt transmission by early 2021. Declines in SIA coverage by 10–20% resulted in only modest increase in transmission compared to typical coverage, likely due to the relatively high efficacy of OPV2 (in contrast to type-1 containing vaccines against WPV1). Without NIDs, large-scale transmission was predicted to persist through 2020 and into 2021. Moreover, only 1 SNID in July at 20% reduced coverage, resulted in only modest decline in overall transmission compared to no response. This is concerning given the challenges of predicting the quality and feasibility of conducting these SIAs in the current climate.
Model simulations highlight that while the greatest risk of seeding reverted Sabin-2 is in areas targeted with OPV2, the greatest risk of subsequent spread occurs in areas not targeted with OPV2 or those with a delayed response. This risk of spread is predominantly in Afghanistan since apart from three provinces in the North (Nangarhar, Kunar and Laghman) no other areas are planned to be targeted until a potential NID in September (not yet confirmed). Across all simulations, Kabul results in greatest risk of reverted Sabin-2 infections; second greatest risk is Nangarhar. In Pakistan, the greatest risk of seeding was in South and Central Punjab, Sindh (including Karachi) and Quetta; however, the risk was minimal and did not result in sustained transmission. If not considering NIDs, while the immediate risk of reverted Sabin-2 infections is lower (due to less use of OPV2), the risk of sustained transmission is much higher, particularly in Afghanistan. In Afghanistan, while Kabul and surrounding provinces are predicted to be at the greatest risk of spread of reverted Sabin-2, the South-Eastern provinces have historically been the hotspot for cVDPV2 outbreaks due to large pockets of under-vaccinated children and substantial potential for seeding from OPV2 use (which is difficult to capture with the model given that it assumes homogeneity at the province level) and therefore may pose a risk if an NID is conducted in September 2020. Novel OPV2 (nOPV2) with increased stability (i.e., decreased risk of reversion) has been developed [31] and is expected to be available for initial emergency authorization use in late 2020 [32]; however, use of nOPV2 in Pakistan and Afghanistan will likely not be an option in 2020 or early 2021.
There are some limitations to our findings. Firstly, our model contains the simplifying assumptions of restricting the population to < 36 months of age [14], with an exponentially distributed duration of time within the cohort, an instantaneous reversion probability to account for the reversion of OPV2 virus (as the loss of key attenuating mutations may occur almost instantaneously leading to accelerated evolution in the first few days after administration [33], [34]), and a meta-population structure of spatial spread. These seemed reasonable assumptions given that the best-fit model was able to reasonably reproduce cVDPV2 case incidence between 2010 and 2016 in Pakistan and Afghanistan (in both space and time). While cVDPV2 data from Environmental Surveillance (ES) could help inform transmission dynamics (and scope of vaccination response), the heterogenous coverage of sampling sites across the two countries limits its use in a geographically structured model. We assumed the rate of within-district transmission is the same across all districts. The initial reported cases of the Diamir outbreak occurred in a different geography to historic cVDPV2 cases and this could explain the difference in the reproduction number between 2010 and 2016 and 2019–2020. Diamir is sparsely populated and experiences colder temperatures than the rest of Pakistan. However even with a slightly lower reproduction number, model simulations show an urgent need for widescale outbreak response activities to stop transmission. Furthermore, the reproduction number may be particularly different in Punjab, where sanitation has reported to be higher [35] and overall poverty lower [18]; however, due to very high historic immunity and absence of cVDPV2 cases in Punjab this was not possible to evaluate. Given the high population size, birth rate and movement patterns in Punjab (that result in a substantial amount of simulated cVDPV2 transmission and case burden), the overall predicted transmission and case burden may be lower. Moreover, the model is at the district-level in Pakistan and province-level in Afghanistan and therefore does not capture pockets of heterogeneity. While the lower coverage in provinces of Southern and Eastern Afghanistan reflect challenges with accessing children for vaccination, larger pockets of unreached children may be masked by overall immunity, impacting inferences on localized transmission and seeding risk. Furthermore, access challenges in Afghanistan have been exacerbated since May 2018 due to restrictions on house-to-house vaccination strategies imposed by insurgent groups in certain high risk areas (especially Southern provinces) and further deteriorated in 2019 [10]. While this work assumes consistent coverage across time periods, the exploration of reduced coverage by 10–20% in provinces with baseline coverage estimates of ∼ 35% (i.e. in Southern provinces) attempts to capture near complete inaccessibility. Additionally, while the case to infection ratio for cVDPV2 may be overestimated [21], [24], sensitivity analyses with a much lower ratio (i.e., 1:2000, S1 Text) produced similar results. Furthermore, for the forward simulations in 2020, we did not distinguish vaccination responses with tOPV and mOPV2 (the NIDs would potentially be conducted with tOPV). Data indicating whether tOPV is inferior to mOPV2 in serotype-2 seroconversion are inconclusive [36]. We make unconfirmed assumptions about reduction in movement during lockdowns due to SARS-CoV-2, will be variable between districts; we crudely assumed the same impact in all geographies. Moreover, while we assume movement is impacted until the end of June 2020 and formal lockdown has been lifted, behavioural patterns will likely be impacted throughout the COVID-19 pandemic. Finally, due to delays in reporting of cVDPV2 cases [37], exacerbated in the context of SARS-CoV-2 [38], the actual epidemiology will not be evident likely until early 2021.
In June 2020, we demonstrated that while cVDPV2 transmission spreads rapidly across Pakistan and Afghanistan, the proposed vaccination response was predicted to stop transmission by early 2021. In the end, a slightly different response was conducted due to the dynamically changing COVID-19 situation, unprecedented challenges with accessibility in Afghanistan and the availability of sufficient tOPV to replace bOPV in NIDs between Oct 2020 and Jan 2021 (Fig S16). Since February 2021, 20 cVDPV2 cases have been reported (2 in Pakistan and 18 in Afghanistan (as of 30 August 2021)), 85% of which were reported in March/April 2021. The response to stop the cVDPV2 outbreak must be considered within the ongoing risk of WPV1 and SARS-CoV-2. Due to the much lower expected magnitude of case burden for WPV1 (resulting from much higher serotype-1 immunity compared to serotype-2) and larger number of responses required for the same impact on immunity (owing to the lower efficacy of the serotype-1 component of vaccine), cVDPV2 response strategies are to be prioritised (increasing the number of cases averted per response). While cVDPV2 remains an urgent priority, WPV1 responses are still necessary to maintain low levels of, or interrupt, transmission and use of tOPV provides a viable option to address both. This modelling work was important in informing the urgency of response. If cVDPV2 transmission continues alongside WPV1 circulation, this modelling framework can be used to guide strategy for balancing these two priorities.
Data Availability
All data used in this study (including both Acute Flaccid Paralysis (AFP) and Supplementary Immunization Activity (SIA) data) are available from the WHO Institutional Data Access/Ethics Committee for researchers who meet the criteria for access to confidential data (email: polioresearch@who.int to apply).
Competing interests
The authors of this manuscript have the following competing interests: IMB and NCG acknowledge funding from the MRC Centre for Global Infectious Disease Analysis (reference MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. ASB is employed with the study funder (Bill & Melinda Gates Foundation) and was involved in reviewing and editing the report. The funder had no role in data analysis.
CRediT authorship contribution statement
Natalia A Molodecky: Conceptualization, Methodology, Software, Validation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Hamid Jafari: Conceptualization, Writing – review & editing. Rana M Safdar: Conceptualization, Writing – review & editing. Jamal A Ahmed: Conceptualization, Writing – review & editing. Abdirahman Mahamud: Conceptualization, Writing – review & editing. Ananda S Bandyopadhyay: Conceptualization, Funding acquisition, Writing – review & editing. Hemant Shukla: Conceptualization, Writing – review & editing. Arshad Quddus: Conceptualization, Writing – review & editing. Michel Zaffran: Conceptualization, Writing – review & editing. Roland W Sutter: Conceptualization, Writing – review & editing. Nicholas C Grassly: Supervision, Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Isobel M Blake: Supervision, Conceptualization, Methodology, Software, Validation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding for this work was provided by the Bill & Melinda Gates Foundation. We would like to thank members of the GPEI Pakistan and Afghanistan programmes and the Pakistan-Afghanistan Hub in Amman, Jordan for providing critical comments on this work. Moreover, we acknowledge the efforts of all persons involved in polio eradication globally, particularly in Pakistan and Afghanistan.
Footnotes
This article was published as part of a supplement supported by Centers for Disease Control and Prevention Global Immunization Division. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or World Health Organization or UNICEF or Bill and Melinda Gates Foundation. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.09.037.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.GPEI. Call to action to support COVID-19 response. Polio Oversight Board Statement. Available at: http://polioeradication.org/news-post/call-to-action-to-support-covid-19-response/.
- 2.GPEI. Circulating vaccine-derived poliovirus. Available at: http://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/.
- 3.Macklin G.R., O’Reilly K.M., Grassly N.C., Edmunds W.J., Mach O., Santhana Gopala Krishnan R., et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science. 2020;368(6489):401–405. doi: 10.1126/science.aba1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.L. V. Cooper et al., Risk factors for spread of vaccine-derived type 2 polioviruses in Africa following global withdrawal of trivalent oral poliovirus vaccine and impact of outbreak response with monovalent vaccine: a retrospective analysis of surveillance data. Lancet Infectious Diseases (in press). [DOI] [PMC free article] [PubMed]
- 5.GPEI. SOPs Responding to a poliovirus event of outbreak. March 2020. Available at: https://polioeradication.org/wp-content/uploads/2020/04/POL-SOP-V3.1-20200424.pdf.
- 6.Ahmadi A., Essar M.Y., Lin X., Adebisi Y.A., Lucero-Prisno D.E. Polio in Afghanistan: The Current Situation amid COVID-19. Am J Trop Med Hyg. 2020;103:1367–1369. doi: 10.4269/ajtmh.20-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.S. Ahmad et al., Polio Amidst COVID-19 in Pakistan: What are the Efforts Being Made and Challenges at Hand? Am J Trop Med Hyg 104, 446-448 (2020). [DOI] [PMC free article] [PubMed]
- 8.Din M., Ali H., Khan M., Waris A., Ullah S., Kashif M., et al. Impact of COVID-19 on polio vaccination in Pakistan: a concise overview. Rev Med Virol. 2021;31(4) doi: 10.1002/rmv.2190. https://10.1002/rmv.v31.410.1002/rmv.2190. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Essential polio vaccination campaigns resume after strict COVID-19 prevention measures. Available at: http://www.emro.who.int/fr/polio/polio-news/essential-polio-vaccination-campaigns-resume-under-strict-covid-19-prevention-measures.html.
- 10.Afghanistan National Emergency Action Plan for Polio Eradication. 2021. Available at: https://polioeradication.org/wp-content/uploads/2021/05/Afghanistan_NEAP_2021.pdf.
- 11.Molodecky N.A., et al. Risk factors and short-term projections for serotype-1 poliomyelitis incidence in Pakistan: A spatiotemporal analysis. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pons-Salort M., et al. Population Immunity against Serotype-2 Poliomyelitis Leading up to the Global Withdrawal of the Oral Poliovirus Vaccine: Spatio-temporal Modelling of Surveillance Data. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laboratory surveillance for wild and vaccine-derived polioviruses--worldwide, January 2007-June 2008. MMWR. Morbidity and mortality weekly report 57, 967-970 (2008). [PubMed]
- 14.Wagner B.G., Behrend M.R., Klein D.J., Upfill-Brown A.M., Eckhoff P.A., Hu H., et al. Quantifying the impact of expanded age group campaigns for polio eradication. PLoS ONE. 2014;9(12):e113538. doi: 10.1371/journal.pone.0113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassly N.C., Fraser C., Wenger J., Deshpande J.M., Sutter R.W., Heymann D.L., et al. New strategies for the elimination of polio from India. Science. 2006;314(5802):1150–1153. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 16.Blake I.M., Martin R., Goel A., Khetsuriani N., Everts J., Wolff C., et al. The role of older children and adults in wild poliovirus transmission. Proc Natl Acad Sci USA. 2014;111(29):10604–10609. doi: 10.1073/pnas.1323688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simini F., González M.C., Maritan A., Barabási A.-L. A universal model for mobility and migration patterns. Nature. 2012;484(7392):96–100. doi: 10.1038/nature10856. [DOI] [PubMed] [Google Scholar]
- 18.Habib M.A., et al. Community engagement and integrated health and polio immunisation campaigns in conflict-affected areas of Pakistan: a cluster randomised controlled trial. The Lancet. Global health. 2017;5:e593–e603. doi: 10.1016/S2214-109X(17)30184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melnick J.L., Ledinko N. Development of neutralizing antibodies against the three types of poliomyelitis virus during an epidemic period; the ratio of inapparent infection to clinical poliomyelitis. Am J Hyg. 1953;58:207–222. doi: 10.1093/oxfordjournals.aje.a119602. [DOI] [PubMed] [Google Scholar]
- 20.Penttinen K., Patiala R. The paralytic/infected ratio in a susceptible population during a polio type I epidemic. Ann Med Exp Biol Fenn. 1961;39:195–202. [PubMed] [Google Scholar]
- 21.Nathanson N., Kew O.M. From emergence to eradication: the epidemiology of poliomyelitis deconstructed. Am J Epidemiol. 2010;172(11):1213–1229. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelfand H.M., Leblanc D.R., Fox J.P., Conwell D.P. Studies on the development of natural immunity to poliomyelitis in Louisiana. II. Description and analysis of episodes of infection observed in study group households. Am J Hyg. 1957;65:367–385. doi: 10.1093/oxfordjournals.aje.a119876. [DOI] [PubMed] [Google Scholar]
- 23.Pons-Salort M., Burns C.C., Lyons H., Blake I.M., Jafari H., Oberste M.S., et al. Preventing Vaccine-Derived Poliovirus Emergence during the Polio Endgame. PLoS Pathog. 2016;12(7):e1005728. doi: 10.1371/journal.ppat.1005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wringe A., Fine P.E.M., Sutter R.W., Kew O.M., Esparza J. Estimating the extent of vaccine-derived poliovirus infection. PLoS ONE. 2008;3(10):e3433. doi: 10.1371/journal.pone.0003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.N. C. Grassly et al., Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. The Journal of infectious diseases 205, 1554-1561 (2012). [DOI] [PubMed]
- 26.VanderEnde K., Voorman A., Khan S., Anand A., Snider C.J., Goel A., et al. New analytic approaches for analyzing and presenting polio surveillance data to supplement standard performance indicators. Vaccine X. 2020;4:100059. doi: 10.1016/j.jvacx.2020.100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King A., Nguyen D., Ionides E. Statistical Inference for Partially Observed Markov Processes via the R Package pomp. J Stat Softw. 2016;69:1–43. [Google Scholar]
- 28.Google. COVID-19 Community Mobility Reports. Available at: https://www.google.com/covid19/mobility/.
- 29.S. Chandir, D. A. Siddiqi, H. Setayesh, A. J. Khan, Impact of COVID-19 lockdown on routine immunisation in Karachi, Pakistan. The Lancet. Global health, (2020). [DOI] [PMC free article] [PubMed]
- 30.Wesolowski A., Qureshi T., Boni M.F., Sundsøy P.R., Johansson M.A., Rasheed S.B., et al. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc Natl Acad Sci USA. 2015;112(38):11887–11892. doi: 10.1073/pnas.1504964112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Damme P., De Coster I., Bandyopadhyay A.S., Revets H., Withanage K., De Smedt P., et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet (London, England) 2019;394(10193):148–158. doi: 10.1016/S0140-6736(19)31279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GPEI. nOPV2 Frequently Asked Questions. July 2020. Available at: http://polioeradication.org/wp-content/uploads/2020/07/nOPV2-FAQs-REVISED-202007.pdf.
- 33.Stern A., Yeh M.T., Zinger T., Smith M., Wright C., Ling G., et al. The Evolutionary Pathway to Virulence of an RNA Virus. Cell. 2017;169(1):35–46.e19. doi: 10.1016/j.cell.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Famulare M., Selinger C., McCarthy K.A., Eckhoff P.A., Chabot-Couture G. Assessing the stability of polio eradication after the withdrawal of oral polio vaccine. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pakistan Bureau of Statistics. Government of Pakistan. Pakistan Social and Living Standards Measurement Survey 2018-19. Available at: http://www.pbs.gov.pk/content/pakistan-social-and-living-standards-measurement.
- 36.Sutter R.W., John T.J., Jain H., Agarkhedkar S., Ramanan P.V., Verma H., et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet (London, England) 2010;376(9753):1682–1688. doi: 10.1016/S0140-6736(10)61230-5. [DOI] [PubMed] [Google Scholar]
- 37.Blake I.M., Chenoweth P., Okayasu H., Donnelly C.A., Aylward R.B., Grassly N.C. Faster Detection of Poliomyelitis Outbreaks to Support Polio Eradication. Emerg Infect Dis. 2016;22(3):449–456. doi: 10.3201/eid2203.151394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.GPEI. Polio eradication in the context of the COVID-19 pandemic. Updated urgent country and regional recommendations. Available at: http://polioeradication.org/wp-content/uploads/2020/03/updated-POB-country-and-regional-recommendations-20200526.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.