Abstract
Since COVID-19 occurrence in late 2019, intense research efforts on an unprecedented scale have focused on the study of named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry mechanisms and clinical presentations. As for other coronaviruses, SARS-CoV-2 presents with extrarespiratory clinical manifestations such as diarrhea, nausea, vomiting, and abdominal pain which highlight that the gastrointestinal (GI) system as another viral target along with the typical presentations of COVID-19 which is characterized primarily by respiratory symptoms. The digestive system is involved in many systemic functions through the gut–brain axis and systemic immunity modulation. Therefore, the GI system plays an important role in the presentation of the disease, pathogenesis, and possibly treatment outcomes. This minireview summarizes recent work to study SARS-CoV-2 infection as it relates to comorbidities, GI symptoms. This will help to strategize the priorities in understanding the impact of the virus on outcomes in various aspects.
Introduction
Origin of the pandemic
Coronaviruses are part of an Orthocoronavirinae subfamily within the Coronaviridae [1]. They are positive sense single-stranded RNA viruses [1]. In December 2019, several patients with atypical pneumonia with no known origin were diagnosed in Wuhan, Hubei Province, China. After genomic analysis, it was established that this pneumonia was caused by a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2, 3∗∗]. The associated disease was named COVID-19. As of May 10, 2021, more than 157 million positive confirmed cases and more than 3.2 million deaths attributed to the infection have been reported worldwide according to the Center for Systems Science and Engineering at John Hopkins University (Johns Hopkins University 2020). The World Health Organization declared that COVID-19 was a public health emergency and on March 11 of 2020 declared it a pandemic [4]. At that time, there were no vaccines against SARS-CoV-2 or any specific treatment to fight the infection. Although some information was available from previous coronaviruses’ outbreaks (SARS-CoV-1) and Middle East Respiratory Syndrome (MERS), the extent of SARS-CoV-2 dissemination, its high rate of transmission, the variety of associated symptoms, and the numbers of deaths caught clinicians, scientists, and the general population off guard.
Clinical presentations of COVID-19
There were two prior similar short-lived pandemics namely the 2002 SARS-CoV-1 and the 2012 MERS. SARS-CoV-1 originated from and spread quickly in China with hundreds of deaths with a lethality rate of 11% (total reported cases: 8422 and 916 deaths) (Chan-Yeung and Xu 2003). MERS emerged in 2012 in the Middle East, Saudi Arabia; its mortality rate was high with around 37% [5]. Both viruses’ origins were traced back to bats and both associated with overlapping similar symptoms such as fever, cough, dyspnea, and atypical pneumonia. Although both of these viruses affected the gastrointestinal (GI) tract with symptoms such as diarrhea, patients with MERS had a higher prevalence of GI symptoms [6], a higher mortality rate, and more than 50% of the patients needed extreme treatment measures such as mechanical ventilation (in contrast to only 20% with SARS-CoV-1) [7]. Because these two previous pandemics were short lived and geographically confined, not much attention was given to extrapulmonary symptoms such as the ones reported in the GI system that can be responsible for some levels of infection dissemination through fecal–oral transmission.
How SARS-CoV-2 causes COVID-19 disease?
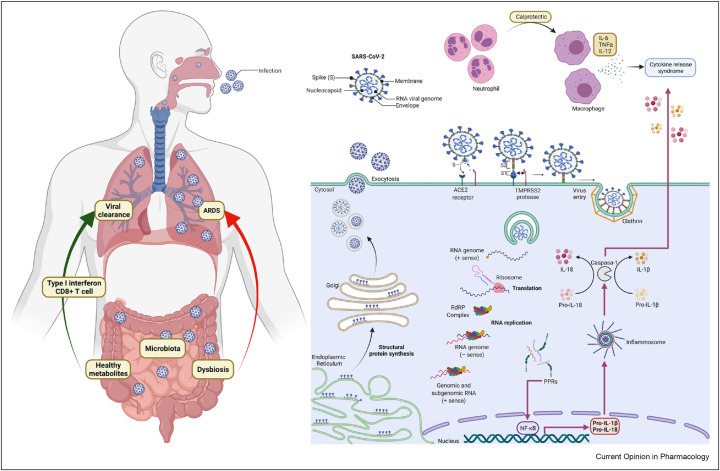
Coronavirus entry is mediated by the viral spike (S) glycoprotein (Figure 1 ) [8]. The spike in the coronaviruses is composed of a transmembrane trimetric glycoprotein protruding from the viral surface, which determines the diversity of coronaviruses and host tropism. The spike comprises two functional subunits: S1 responsible for binding to the host cell receptor and S2 for the fusion of the viral and cellular membranes. Angiotensin-converting enzyme 2 (ACE2) was identified as a functional receptor for SARS-CoV.
Figure 1.
Schematic SARS-CoV-2 infection and entry to the cells. Viral particle entry was mediated by ACE2 receptor and TMPRSS2 in the gut. Viral genome is released and then translated, replicated and transcribed so it can infected other cells and be excreted via exocytosis and stimulate the production of proinflammatory cytokines which will influence the immune response both in the gut and in the lung via the gut–lung axis. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane serine protease 2.
Most of the symptoms of the coronaviruses are primarily respiratory. The virus invades and infects the respiratory tract via the ACE2 receptor [9]. It is very important to remark that ACE2 receptors are highly expressed along the GI tract. The viral protein spike binds to the cellular surface of ACE2 and is cleaved by the transmembrane serine protease 2 (TMPRSS2) to allow the entry and subsequent propagation of the virus [10]. Both ACE2 and TMPRSS2 are expressed not only in the lung alveoli and esophageal cells, but also in the absorptive enterocytes of the ileum and colon. The enterocytes of the small bowel are the cells that contain the highest amount of ACE2 receptors in the human body [11]. The host response to infection to SARC-CoV-2 varies from mild to severe. Inflammatory response (involving dendritic cells and macrophages) is the first immune response for gaining adaptive immunity in alveola [12].
A recent study showed that the absence of a proinflammatory response in the GI system translates into downregulation of key inflammatory genes (e.g. IFNG, CXCL8, CXCL2, and IL1B) and reduction of proinflammatory dendritic cells compared with control individuals. Mortality rates in patients with COVID-19 with GI symptoms were also reported to be lower than in patients without such symptoms, which prompted Livanos et al. [13] to hypothesize that the GI system might be acting as a buffer in patients with COVID-19, and GI symptoms are likely a way to attenuate COVID-19 impact in the host [14]. Another recent international study by Vanella et al. [15] revealed major GI mucosal damage in patients with COVID-19 undergoing endoscopy, although this did not lead to prolonged hospitalization. In their cohort of 106 patients from the first wave of COVID-19 (February–May 2020), they found that almost half of them showed acute mucosal damage (found early on admission) and more than one-third had features of ischemic colitis; still, the author recognizes their findings are from a highly selected cohort of less than 1% of admitted patients with COVID-19 and did not confirm an association between SARS-CoV-2 infection and GI ischemia. As such, it is very important to further dissect the short- and long-term effects of COVID-19 on the GI system from the occurrence of GI symptoms to effects on GI organs and systemic effects, especially when the gut barrier integrity is breached. In fact, these observations become more significant in light of a recent study in nonhuman primates that showed that intragastric SARS-CoV-2 injection led to the same systemic symptoms, including respiratory system, as a pulmonary SARS-CoV-2 infection, pointing to the GI system as another potential point of entry of the virus to the host systems (Jiao et al., 2021).
GI symptoms and comorbidities in COVID-19 outcomes
Multiple studies have reported the presence of the viral RNA in fecal samples or rectal swabs in patients with COVID-19. According to a study in China, 55 of 93 patients had a positive PCR for SARS-CoV-2 RNA in the stool (59%) with a median duration of this positive PCR detection of 22 days [16]. Generally, the viral loads from stool samples of patients with COVID-19 tend to peak 2–3 weeks after the symptoms’ onset and most of the time stay positive for longer periods even after respiratory symptoms subside and respiratory specimens are negative for the viral RNA [17]. Still one has to consider the presence of opportunistic bacterial pathogens and infectivity of the virus in both aerosol particles and stools [27]. An in vitro study showed that human duodenum enteroids excreted the virus from the apical side to the lumen of the gut, suggesting the possibility of excreting the virus via the feces in patients with active disease [18]. Another aspect to consider in respect of fecal–oral transmission of the virus is that the lag time of the virus SARS-CoV-2 remains viable in the feces. Although several studies have been made for aerosol transmission, there is only simulations in laboratories where the virus particles could live from 4 h to 72 h depending on surfaces, but still more investigation needs to be carried out to describe the transmissibility of the virus in the feces [26].
There have been several studies that have addressed GI manifestations’ prevalence in patients with COVID-19. A meta-analysis [19] reported that, in 10,676 patients with COVID-19, diarrhea had a prevalence of 7.7%, abdominal pain 3.6%, and nausea and vomiting 7.8%, and in 3 of 4 studies, viral shedding in stools was reported. It is important to note that this meta-analysis included patients mostly from China and the USA. In comparison, a large dataset from Chile [20] reported that diarrhea was present in 7.3% and abdominal pain in 3.7% in a cohort of 7016 patients. In the US, which is the country with the most positive confirmed cases in the world, Ashktorab et al. [21] reported that, in 447 patients, with a majority of them being African Americans, diarrhea was predominant in 19.45% and abdominal pain was predominant in 15.8% of patients. African Americans had the highest diarrhea prevalence of 22.4%. At least 24% of the total cohort had one or more GI symptoms.
As for Latin America, currently the epicenter of the pandemic, Ashktorab et al. [22] reported that diarrhea, nausea, vomiting, and abdominal pain were highly prevalent but not associated with higher mortality. In another study on an Iranian cohort, Mokarram et al. [23] reported nausea in 42.8%, diarrhea in 31.8%, vomiting in 26.8%, and abdominal pain in 12% of patients. This cohort of patients had an elevated liver enzyme profile as well (albumin, ALP, AST, ALT) in 26.3% of patients. Studies from China [24] reported low prevalence of diarrhea with only 8.1%, anorexia in 4.7%, and nausea and vomiting in 4.3%. These differences in GI symptom prevalence highlight the presence of regional differences that need to be further characterized for an efficient management of the patients at local levels; perhaps, these differences may be justified by the presence of different spike protein mutations in the different strains of SARS-CoV-2 that are emerging currently; unfortunately, there are not many research studies in respect of this issue.
It is important to remark that GI manifestation was present not only in the adult population but also in children infected by COVID-19. The study by Chiappini et al. [29] compared different patient pools from different regions of the world in a meta-analysis for more than 1200 patients: vomiting was present in 9.3% and diarrhea was present in 8.8% of the pediatric population.
Microbiota in COVID-19
Beyond the presence of ACE2 and TMPRSS2 markers in the GI system, it is very likely that part of the GI symptoms that a sizable portion of patients with COVID-19 experience occur through a disturbance of the gut microbiome in these patients. Indeed, several studies reported a major dysbiosis (persisted imbalance of gut microbiome) in these patients as a result of COVID-19 [25]. This dysbiosis persisted weeks after discharge from hospital and respiratory clearance of the virus. Such dysbiosis is likely to continue exerting some low-grade inflammation that is thought to be associated with persistence, recurrence, and, in some cases, new symptoms in these patients. Recently, Yeoh et al.’s [28] study shows how the gut microbiota composition of patients with COVID-19 during hospitalization is correlated with plasma concentrations of several cytokines, chemokines, and inflammation markers, suggesting that the gut microbiota could play a role in modulating host immune response and potentially influence disease severity and outcomes. This opens to a potential role played by the gut microbiome in COVID-19 that could allow the use of a gut microbiome-based risk profile to identify individuals at risk of severe disease or downstream inflammatory symptoms.
Conclusion
The COVID-19 scenario is very complex in terms of characterization of the disease for prevention, diagnostics, and treatment. As of now, different variants have been discovered since the beginning of the pandemic all around the world, and each one discovered in different regions makes the panorama different in each area, which leads up to a tropism in the presentation of patients. Overall, the presentation of patients from the gastroenterological standpoint is the typical picture for diarrhea, nausea, and vomiting for both adults and children; but one has to consider the presence of pre-existing conditions in those patients (especially GI diseases) and how the gut microbiome axis influences the immune system in the setting of SARS-CoV-2 infection because it can be correlated to both treatments and outcomes.
If we take the Russian influenza pandemic which occurred in 1899 as an example, we are likely to be dealing with COVID-19–associated symptoms for years to come. How would the current vaccination campaign affect if the course of the pandemic is still unknown? Would there be different trajectories of the pandemic depending on the type of vaccines is also an open question? Do we need to supplement some probiotics with current vaccines or to patients who do not have access to vaccines, is also an area that will need to be further explored to mitigate COVID-19 systemic effects in general and the GI ones in particular.
Conflict of interest statement
Nothing declared.
Acknowledgements
Julio Y Anaya-Covarrubias, from Universidad de Guadalajara, is acknowledged for support. This project was supported (in part) by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007597.
This review comes from a themed issue on Anti-infectives (2022)
Edited by Nora A. Fierro, Santiago Mirazo and Jesus Torres-Flores
References
- 1.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020;9:1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors acknowledge the importance of knowing previous coronavirus epidemics in the past but also emphasize different characteristics that make SARS-CoV-2 unique and the lack of information at the beginning of the pandemic.
- 2.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K., Lau E., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study from the first datasets of patients in China, where the pandemic started, describes the epidemiology of the virus, how the virus disseminates from person to person esspecially via respiratory droplets but also opens the discussion for more ways to help in the transmission of the disease.
- Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study emphasizes the ways the SARS-Cov-2 attach to the ACE receptors in the lung but also on the gut which opens a new way for fecal-oral transmission, digestive disease and and extended description on the pathophysiology of COVID-19 with potential sites for treatment and vaccines.
- 4.World Health Organization . 2020. WHO Director General opening remarks at the media briefing on COVID-19.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Retrieved from. [Google Scholar]; With more than 118,000 cases, the director of the WHO officially declared the COVID-19 pandemic.
- 5.Al Sulayyim H.J., Khorshid S.M., Al Moummar S.H. Demographic, clinical, and outcomes of confirmed cases of Middle East Respiratory Syndrome coronavirus (MERS-CoV) in Najran, Kingdom of Saudi Arabia (KSA); A retrospective record based study. J Infect Publ Health. 2020;13:1342–1346. doi: 10.1016/j.jiph.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; The author of this investigation reports different clinical presentations of other coronaviruses such as MERS-CoV and the frequent gastrointestinal effects of patients, 13.7% of the overall cohort had diarrhea and 7.8% had abdominal pain.
- 6.Aleebrahim-Dehkordi E., Soveyzi F., Deravi N., Rabbani Z., Saghazadeh A., Rezaei N. Human coronaviruses SARS-CoV, MERS-CoV, and SARS-CoV-2 in children. J Pediatr Nurs. 2021;56:70–79. doi: 10.1016/j.pedn.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; Overall, children, as like in the SARS-CoV and MERS-CoV epidemic, were affected less than adults when the same scenario has been presented with SARS-CoV-2.
- 7.Al-Dorzi H.M., Alsolamy S., Arabi Y.M. Critically ill patients with Middle East respiratory syndrome coronavirus infection. Crit Care. 2016;20:65. doi: 10.1186/s13054-016-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interestingly this study reports that of critically MERS-CoV patients, 32% had gastrointestinal symptoms.
- 8.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Coronaviruses enters via the viral spike (S) glycoprotein; of this protein the HR2 helices is a potential site for a strong inhibitor entry of the virus.
- 9.Li Y., Zhang Z., Yang L., Lian X., Xie Y., Li S., Xin S., Cao P., Lu J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;23:101160. doi: 10.1016/j.isci.2020.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]; SARS-CoV-2 attaches with high affinity to DPP4 receptor. DPP4 has a potential utilization as a binding target for the virus and offers the ability to bridge between therapeutics and infection.
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; The virus uses the ACE2 receptor and the serine protease TMPRSS2 to mediate its entry to the cell, potential inhibitors of the TMPRSS2 are proposed as a target for antiviral intervention.
- 11.Zhang H., Li H.B., Lyu J.R., Lei X.M., Li W., Wu G., Lyu J., Dai Z.M. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis : Int J Infect Dis. 2020;96:19–24. doi: 10.1016/j.ijid.2020.04.027. official publication of the International Society for Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]; The intestine is one of the richest organs in the human body with ACE2 receptors.
- Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; Macrophages and Dendritic cells play a huge role in the pathogenesis and response to the SARS-CoV-2 infection. They prime naive T cells and phagocytosis. Other key molecules are IL-6 and IL-8.
- 13.Livanos A.E., Jha D., Cossarini F., Gonzalez-Reiche A.S., Tokuyama M., Aydillo T., Parigi T.L., Ramos I., Dunleavy K., Lee B., Dixon R., Chen S.T., Martinez-Delgado G., Nagula S., Ko H.M., Reidy J., Naymagon S., Grinspan A., Ahmad J., Tankelevich M., Mehandru S. Gastrointestinal involvement attenuates COVID-19 severity and mortality. medRxiv. 2020 doi: 10.1101/2020.09.07.20187666. the preprint server for health sciences, 2020.09.07.20187666. [DOI] [Google Scholar]; Overall patients with gastrointestinal manifestations tend to have a milder clinical course due to the reduced levels of key inflammatory proteins such as IL-6, CXCL8, IL-17, and CCL28.
- 14.Livanos A.E., Jha D., Cossarini F., Gonzalez-Reiche A.S., Tokuyama M., Aydillo T., Parigi T.L., Ladinsky M.S., Ramos I., Dunleavy K., Lee B., Dixon R.E., Chen S.T., Martinez-Delgado G., Nagula S., Bruce E.A., Ko H.M., Glicksberg B.S., Nadkarni G., Pujadas E., Mehandru S. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160:2435–2450.e34. doi: 10.1053/j.gastro.2021.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]; Data found highlight that the absence of a general pro-inflammatory state is frequently seen in patients with gastrointestinal symptoms.
- Vanella G., Capurso G., Burti C., Fanti L., Ricciardiello L., Souza Lino A., Boskoski I., Bronswijk M., Tyberg A., Krishna Kumar Nair G., Angeletti S., Mauro A., Zingone F., Oppong K.W., de la Iglesia-Garcia D., Pouillon L., Papanikolaou I.S., Fracasso P., Ciceri F., Rovere-Querini P., Arcidiacono P.G. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2020-000578. [DOI] [PMC free article] [PubMed] [Google Scholar]; Patients with COVID-19 showed different patterns of mucosal damage on endoscopy, specially those with D-dimers above 1850 ng/ml.
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ (Clin Res ed.) 2020;369 doi: 10.1136/bmj.m1443. m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]; The median duration of SARS-CoV-2 in stool was longer than urine or respiratory samples; this opens a constellation on the management and control of the pandemic.
- 17.Walsh K.A., Jordan K., Clyne B., Rohde D., Drummond L., Byrne P., Ahern S., Carty P.G., O'Brien K.K., O'Murchu E., O'Neill M., Smith S.M., Ryan M., Harrington P. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]; Although the duration of stool SARS-CoV-2 is longer than respiratory samples, it is now well known the significancy and the infectivity of it.
- 18.Cong Y., Ren X. Coronavirus entry and release in polarized epithelial cells: a review. Rev Med Virol. 2014;24:308–315. doi: 10.1002/rmv.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]; Coronaviruses use different ways to exit the infected cell (apical side, basolateral side, or both), this helps in understanding the spread of the infection within the host.
- 19.Sultan S., Altayar O., Siddique S.M., Davitkov P., Feuerstein J.D., Lim J.K., Falck-Ytter Y., El-Serag H.B., AGA Institute. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gastrointestinal symptoms are present in less patients than COVID-19 affected patients.
- 20.Díaz L.A., García-Salum T., Fuentes-López E., Ferrés M., Medina R.A., Riquelme A. Symptom profiles and risk factors for hospitalization in patients with SARS-CoV-2 and COVID-19: a large cohort from South America. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; In South America, the overall prevalence of gastrointestinal symptoms in COVID-19 patients was 17.6%.
- Ashktorab H., Pizuorno A., Aduli F., Laiyemo A.O., Oskrochi G., Brim H. Elevated liver enzymes, ferritin, C-reactive protein, D-dimer, and age are predictive markers of outcomes among african American and hispanic patients with coronavirus disease 2019. Gastroenterology. 2021;S0016–5085:570–579. doi: 10.1053/j.gastro.2021.03.043. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gastrointestinal symptoms on this cohort were present in 19.4% of the patients. Overall, 41% of the total patient population had abnormal liver function tests.
- 22.Ashktorab H., Pizuorno A., Oskrochi G., Fierro N.A., Sherif Z.A., Brim H. COVID-19 in Latin America: symptoms, morbidities, and gastrointestinal manifestations. Gastroenterology. 2021;160:938–940. doi: 10.1053/j.gastro.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; In Latin America, the most common COVID-19 symptoms were cough, fatigue, sore throat, and fever. Gastrointestinal symptoms were not associated with mortality.
- 23.Mokarram P., Dalivand M.M., Pizuorno A., Aligolighasemabadi F., Sadeghdoust M., Sadeghdoust E., Aduli F., Oskrochi G., Brim H., Ashktorab H. Clinical characteristics, gastrointestinal manifestations and outcomes of COVID-19 patients in Iran; does the location matters? World J Gastroenterol. 2021;27 doi: 10.12998/wjcc.v9.i18.4654. 2021. 0-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nausea, diarrhea, and elevated liver enzymes were the most common gastrointestinal symptoms in Iran.
- 24.Zheng T., Yang C., Wang H.Y., Chen X., Yu L., Wu Z.L., Sun H. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J Med Virol. 2020;92:2735–2741. doi: 10.1002/jmv.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]; In China, the most common gastrointestinal symptoms were diarrhea, anorexia, and nausea and vomiting. Gastrointestinal symptoms areindependent risk factors for clinical worsening in this cohort.
- Segal J.P., Mak J., Mullish B.H., Alexander J.L., Ng S.C., Marchesi J.R. The gut microbiome: an under-recognised contributor to the COVID-19 pandemic? Therapeut Adv Gastroenterol. 2020;13 doi: 10.1177/1756284820974914. 1756284820974914. [DOI] [PMC free article] [PubMed] [Google Scholar]; The number of total T cells CD4 and CD8 T cells are reduced in patients with COVID-19. It is known that gut microbes are the key regulators of these cells viashort chain fatty acids pathway.
- 26.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study consisted of 10 experimental conditions involving SARS-CoV-2 and SARS-CoV-1 in five environmental conditions (aerosols, plastic, stainless steel, copper, and cardboard). SARS-CoV-2 can remain viable and infectious in aerosols for hours and on surfaces up to days (depending on the inoculum shed).
- 27.Zuo T., Liu Q., Zhang F., Lui G.C., Tso E.Y., Yeoh Y.K., Chen Z., Boon S.S., Chan F.K., Chan P.K., Ng S.C. Depicting SARS-CoV-2 faecal viral activity in association with gut microbiota composition in patients with COVID-19. Gut. 2021;70:276–284. doi: 10.1136/gutjnl-2020-322294. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors found evidence for active and prolonged 'quiescent' GI infection even in the absence of GI manifestations and after recovery from respiratory infection of SARS-CoV-2. Gut microbiota of patients with active SARS-CoV-2 GI infection were characterized by the enrichment of opportunistic pathogens, loss of salutary bacteria, increased functional capacity for nucleotide, amino acid biosynthesis, and carbohydrate metabolism.
- 28.Yeoh Y.K., Zuo T., Lui G.C., Zhang F., Liu Q., Li A.Y., Chung A.C., Cheung C.P., Tso E.Y., Fung K.S., Chan V., Ling L., Joynt G., Hui D.S., Chow K.M., Ng S., Li T.C., Ng R.W., Yip T.C., Wong G.L., Ng S.C. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698–706. doi: 10.1136/gutjnl-2020-323020. [DOI] [PMC free article] [PubMed] [Google Scholar]; The gut microbiota dysbiosis after disease resolution could contribute to persistent symptoms, highlighting a need to understand how gut microorganisms are involved in inflammation and COVID-19.
- 29.Chiappini E., Licari A., Motisi M.A., Manti S., Marseglia G.L., Galli L., Lionetti P. Gastrointestinal involvement in children with SARS-COV-2 infection: an overview for the pediatrician. Pediatr Allergy Immunol. 2020;31(Suppl 26):92–95. doi: 10.1111/pai.13373. official publication of the European Society of Pediatric Allergy and Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]; Diarrhea and vomiting have been reported in about 8%–9% of cases, reaching more than 20% in some studies. Fecal shedding in children has been reported in 20%–30% of children and has been observed in both those with andwithout overt gastrointestinal involvement.