Abstract
Objective
In this pilot clinical study, we report the beneficial effects of beta glucans derived from two strains AFO-202 and N-163 of a black yeast Aureobasidium pullulans on the biomarkers for cytokine storm and coagulopathy in COVID-19 patients.
Methods
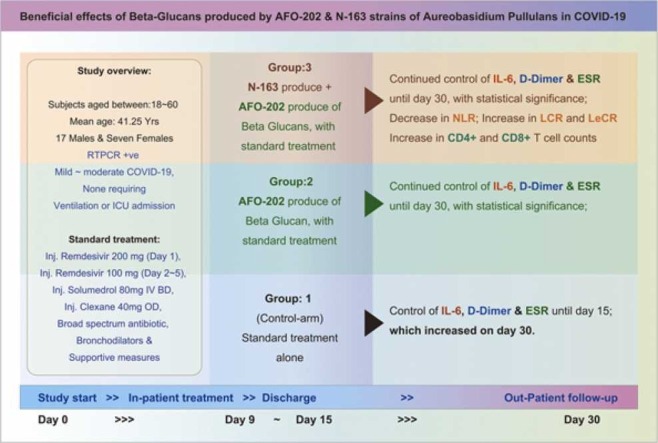
A total of 24 RT-PCR positive COVID-19 patients were recruited and randomly divided into three groups (Gr): Gr. 1 control (n = 8) – Standard treatment; Gr. 2: Standard treatment + AFO-202 beta glucan (n = 8); and Gr. 3, Standard treatment + combination of AFO-202 and N-163 beta glucans (n = 8) for 30 days.
Results
There was no mortality or requirement of ventilation of the subjects in any of the groups. There was a decrease in D-Dimer values (751 ng/ml to 143.89 ng/ml) and IL-6 values (7.395–3.16 pg/ml) in Gr. 1 in 15 days but the levels increased to abnormal levels on day 30 (D-Dimer: 202.5 ng/ml; IL-6 55.37 pg/ml); which steadily decreased up to day 30 in groups 2 (D-dimer: 560.99 ng/dl to 79.615; IL-6: 26.18–3.41 pg/ml) and 3 (D-dimer: 1614 ng/dl to 164.25 ng/dl; IL-6: 6.25–0.5 pg/ml). The same trend was observed with ESR. LCR and LeCR increased while NLR decreased significantly in Gr. 3. CD4 + and CD8 + T cell count showed relatively higher increase in Gr.3. There was no difference in CRP within the groups.
Conclusion
As these beta glucans are well known food supplements with a track record for safety, larger multi-centric clinical studies are recommended to validate their use as an adjunct in the management of COVID-19 and the ensuing long COVID-19 syndrome.
Keywords: COVID-19, IL-6, D-dimer, Cytokine storm, Coagulopathy, Immuno-modulation, Beta glucans, Adjunct treatment
Graphical Abstract
1. Introduction
COVID-19 is caused by the novel strain of enveloped RNA virus, SARS-CoV-2 [4] with symptoms including fever, cough, shortness of breath, fatigue, body aches, headache, loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting and diarrhoea. The COVID-19 is an ongoing pandemic with nearly 200 million people affected worldwide and more than four million deaths to date [31]. Many patients are hospitalized 10–12 days after a positive RT-PCR test [30]. Recovery occurs within 14 days in most of the affected patients, but it may take more than 25 days in some patients [24]. Even patients with mild symptoms may progress to requiring oxygen therapy and mechanical ventilation and/or leading to death. The progression to severe disease is attributed to the hyperactive inflammatory response leading to a cytokine storm, thereby causing organ damage [27]. A dysfunctional immune-coagulation response activating the coagulation cascade leading to a hypercoagulable state may cause adverse clinical outcomes including death. Patients with co-morbidities such as diabetes, dyslipidemia and risk of coagulation are prone to a rapid progression of the disease and an immune-inflammatory-coagulation dysregulation- related adverse outcome [27]. Several interventions are part of the standard treatment regimen, including pharmacological agents such as anti-virals, IL6 inhibitors, anti-coagulants, steroids and supplements such as vitamins or zinc (Solidarity clinical trial) [28]. The solidarity trial, an international clinical trial to help find an effective treatment for COVID-19 continues to generate evidence for the use of these interventions, and the search for effective COVID-19 therapeutics continues (Solidarity clinical trial) [28]. Many of the pharmacological interventions have associated side effects, and in regard to the supplements, zinc or ascorbic acid have not been able to significantly decrease the duration of symptoms compared with standard care [30].
Given this background, the biological-response modifier glucans (BRMG) such as the Aureobasidium pullulans produced beta glucans have been suggested as an alternative adjunct treatment based on their potential to activate both the innate and adaptive arms of the host immune response against COVID-19 [14], [23]. This BRMG has been reported to reduce the levels of IL-1β, IL-2, IL-4, IL-6, IL-12, TNF-α, IFN-γ, and sFasL while increasing IL8 and sFAS, thereby exerting an effective optimal defence against viral infection without hyperinflammation [11]. The metabolic effects of the A. pullulans beta glucan in normalizing blood glucose and lipid levels add to their benefits for use in COVID-19 as fasting blood glucose is an independent outcome of COVID-19 severity and mortality [12]. The potential of this BRMG in acting as a prophylactic supplement to help combat coagulopathy associated with COVID-19 has also been described [13].
In an animal study using healthy SD rats, AFO-202 beta glucan was beneficial in decreasing neutrophil to lymphocyte ratio (NLR) while increasing lymphocyte to CRP ratio (LCR) and the leukocyte-CRP ratio (LeCR) in 15 days [15], whereas in a non-alcoholic steatohepatitis (NASH) disease animal model, it has been demonstrated that N-163 beta glucan has the potential for anti-inflammatory, anti-fibrotic immune modulation [16]. Similarly, in healthy human volunteers, the reported decrease in pro-inflammatory markers and increase in anti-inflammatory markers in an advantageous manner with the same beta glucans [16], thus supporting their ability to aid recovery in COVID-19 was considered worth studying. In this report, we hereby present the evaluation of the beneficial outcome of beta glucans produced by A. pullulans (AFO-202 and N-163 strains) in patients with COVID-19.
2. Methods
The present study was an open label, prospective, randomized, comparative, multiple arm pilot clinical study to evaluate the immune enhancement and immunomodulatory efficacy of supplementation with A. pullulans’ novel strains produced beta glucans compared to those who undergo a conventional therapeutic regimen alone in adult subjects with COVID-19 caused by SARS-CoV2(B-CoV).
Adult subjects between 18 and 65 years (both ages and sexes inclusive) who were confirmed to be positive for SARS-CoV2 by way of RT-PCR with or without co-morbidities, and with mild to moderate COVID-19 symptoms (ICMR COVID-19 management protocol) [10] but who required hospitalization were included in the study. Severe COVID-19-affected patients requiring intensive care, children and pregnant women were excluded.
There were three groups (Gr.) comprised of eight subjects each (Total n = 24).
Gr. 1 (n = 8): The control group receiving the standard treatment, which consisted of Inj. Remdesivir 200 mg (Day 1), Inj. Remdesivir 100 mg (Day 2 to Day 5) Inj. Solumedrol 80 mg IV BD Inj. Clexane 40 mg OD, broad spectrum antibiotic bronchodilators and supportive measures.
Gr. 2 (n = 8): The intervention was standard treatment along with AFO-202 beta glucan supplement at 3 gm per day (1.0 gm granule in a sachet containing 42 mg active ingredient of ß-1,3–1,6 Glucan, with each meal).
Gr. 3 (n = 8): The intervention was AFO-202 beta glucan at 3 gm per day (1.0 gm granule with each meal) in combination with N-163 beta glucan at 10 gm per day (15 gm gel in a sachet with 90mg of ß-1,3-1,6 Glucan, with one of the meals every day).
The primary outcome was improvement in the clinical symptoms of COVID-19: time taken for improvement, complete recovery, recurrence in typical symptoms from baseline.
The secondary outcome was evaluation for required hospitalization, mortality, progression to critical care admission, oxygen/life-support and tests for biochemical parameters such as D-Dimer, IL6, ESR, CRP, NLR, LCR and LeCR.
Data were analysed using Microsoft Excel and Origin 2021b statistical software. Results are presented as mean or mean ± standard deviation for the continuous normal variables. Independent sample T-test and Kruskal-Wallis Test was used for comparison between the groups. A p-value < 0.05 was considered significant.
3. Results
The baseline characteristics of the patients are presented in Table 1. The age of the subjects in Gr. 1 Control was 33–59 years (Mean = 47.62 years); in Gr. 2, AFO-202, 18–50 years (Mean = 36.25 years); and in Gr. 3, AFO 202 +N163, 26–60 years (Mean= 39.87 years). There were 17 males and seven females recruited, and therefore the distribution was not equal across the groups. There were no significant differences among the groups in terms of body weight, BMI and blood pressure at the time of admission. All enrolled patients had mild to moderate COVID-19 (ICMR COVID-19 management protocol) and none required ventilation or ICU admission. The average duration of stay in the hospital was 4.25 days in Gr. 1, 4.75 days in Gr. 2 and 4.125 days in Gr. 3. Two to four litres of oxygen were administered in two patients in Gr. 2 and in one patient in Gr. 1 from admission up to four or five days during the hospital stay. There was no statistically significant difference among the groups in terms of duration of hospitalization or the resolution of symptoms. There was no mortality in any of the groups. There was one serious adverse effect (SAE) in Gr. 1 (Control). The subject's ECG on Visit 2 showed abnormalities after which an angiogram was taken which showed spontaneous coronary artery dissection in the left anterior descending portion of the right coronary artery. The subject was managed as per standard care under the supervision of a cardiologist.
Table 1.
Baseline characteristics of study subjects.
| Groups | Subject | Age range (years) | Gender | Body weight (Kgs) | Height (Cm) | BMI | Duration of hospital stay until recovery (No. of days) | BP (mm/Hg) |
|---|---|---|---|---|---|---|---|---|
| Gr.1 - Control | 1 | 51–55 | Male | 80 | 175 | 26.1 | 3 | 130/80 |
| 2 | 41–45 | Female | 65 | 162 | 24.8 | 3 | 110/90 | |
| 3 | 51–55 | Female | 75 | 170 | 26 | 5 | 100/70 | |
| 4 | 51–55 | Female | 62 | 170 | 21.5 | 6 | 110/ 70 | |
| 5 | 51–55 | Male | 78 | 175 | 25.5 | 4 | 130/90 | |
| 6 | 46–50 | Female | 78 | 168 | 27.6 | 4 | 120/90 | |
| 7 | 36–40 | Female | 70 | 152 | 30.3 | 5 | 120/80 | |
| 8 | 31–35 | Female | 53 | 152 | 22.9 | 4 | 130/90 | |
| Gr. 2- AFO-202 | 9 | 26–30 | Male | 60 | 156 | 24.7 | 6 | 120/80 |
| 10 | 26–30 | Male | 82 | 167 | 29.4 | 6 | 110/90 | |
| 11 | 16–20 | Male | 81 | 180 | 25 | 6 | 110/90 | |
| 12 | 46–50 | Male | 71 | 156 | 29.2 | 5 | 130/80 | |
| 13 | 46–50 | Male | 80 | 170 | 27.7 | 3 | 120/90 | |
| 14 | 41–45 | Male | 75 | 168 | 26.6 | 2 | 110/70 | |
| 15 | 46–50 | Male | 68 | 168 | 24.1 | 4 | 120/90 | |
| 16 | 31–35 | Male | 56 | 158 | 22.4 | 6 | 130/90 | |
| Gr. 3- AFO-202 + N-163 | 17 | 26–30 | Male | 72 | 170 | 24.9 | 4 | 110/90 |
| 18 | 31–35 | Male | 55 | 150 | 24.4 | 5 | 120/90 | |
| 19 | 61–65 | Male | 75 | 176 | 24.2 | 4 | 110/70 | |
| 20 | 26–30 | Male | 80 | 172 | 27 | 3 | 130/90 | |
| 21 | 36–40 | Male | 76 | 174 | 25.1 | 3 | 130/90 | |
| 22 | 26–30 | Male | 79 | 174 | 26.1 | 3 | 120/90 | |
| 23 | 41–45 | Female | 79 | 160 | 30.9 | 2 | 120/90 | |
| 24 | 51–55 | Male | 84 | 166 | 30.5 | 9 | 110/70 |
The drop-outs were high in the 30-day follow-up (11 subjects lost to follow up: three in Gr. 1 and four in groups 2 and 3 each) due to a reluctance in the subjects to come to the hospital for follow-up as most of them were worried about re-infection and psychological factors on returning to the hospital from where they were discharged.
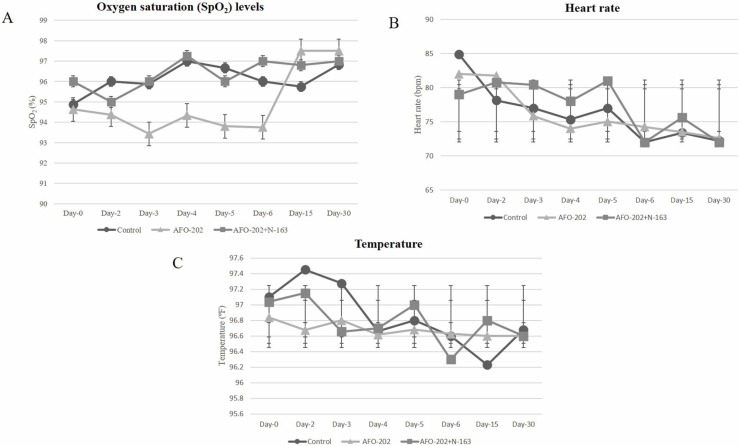
The oxygen saturation increased in all the groups in the 15- and 30-day follow-up but the difference was not significant. The temperature and heart rate also showed no significant difference among the groups ( Fig. 1).
Fig. 1.
A. Oxygen saturation (SpO2) (%) B. Heart rate (bpm) and C. Temperature (ºF) and at baseline (Day 0), day 2, day 3, day-4, day-5, day-6, day 15 and day 30 in the different treatment arms of COVID-19 subjects. CONSORT flow diagram of the trial.
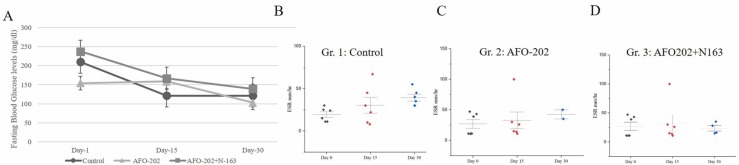
In the control group, the mean fasting blood glucose (FBG) levels on day 0 were 210.14 mg/dl which decreased to 121.5 mg/dl on day 30. In Gr. 2, the mean FBG decreased from 154.14 mg/dl to 103 mg/dl in 30 days. In Gr. 3, it decreased from 238 mg/dl to 140 mg/dl in 30 days ( Fig. 2A). The difference among the groups was not significant. ESR in Gr. 1 decreased when measured on day 15 but increased on day 30 in Gr. 1. In Gr. 2 and 3 the ESR values were lower than in control patients on day 15 and day 30, although this was not statistically significant (Fig. 2B–D).
Fig. 2.
A. Fasting blood glucose levels at baseline (Day 0), day 15 and day 30 in the different treatment arms of COVID-19 subjects showing decrease in the all groups; B-D: Erythrocyte sedimentation rate (ESR) values in subjects with COVID-19 B. Gr. 1: Control (Standard treatment), C. Gr. 2: (Standard treatment + AFO-202 beta glucan supplementation); and D. Gr. 3: (Standard treatment + AFO-202 and N-163 beta glucan supplementation) at baseline (day 0), day 15 and day 30.
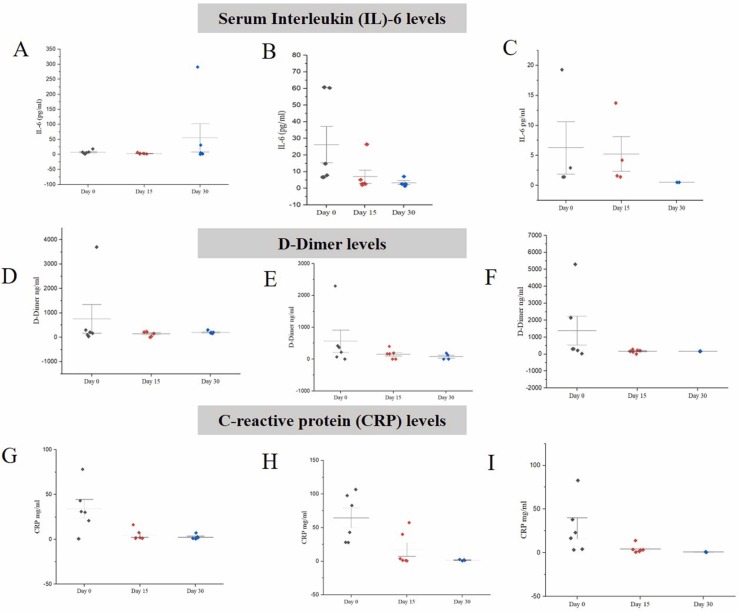
In the control group, IL6 values decreased from an average of 7.395–3.16 pg/ml in day 15 but again increased to 55.37 pg/ml at day 30. However, in Gr. 2 (AFO-202), IL6 levels steadily decreased from a mean of 26.18–6.94 pg/ml in 15 days and to 3.41 pg/ml in 30 days, while in Gr. 3 (AFO202 +N163) it decreased from 6.25 pg/ml to 5.22 pg/ml in 15 days and to 0.5 pg/ml in 30 days. The results were statistically significant (p value =0.0214) ( Fig. 3A–C).
Fig. 3.
A-C; Serum Interleukin (IL)− 6 levels in subjects with COVID-19 A. Gr. 1: Control (Standard treatment); B. Gr. 2: (Standard treatment + AFO-202 beta glucan supplementation); and C. Gr. 3: (Standard treatment + AFO-202 and N-163 beta glucan supplementation) at baseline (day 1), day 15 and day 30 wherein the decrease was statistically significant in Gr.2 and 3 (p < 0.05). D-F: D-Dimer Levels in subjects with COVID-19 D. Gr. 1: Control (Standard treatment); E. Gr. 2: (Standard treatment + AFO-202 beta glucan supplementation); and F. Gr. 3: (Standard treatment + AFO-202 and N-163 beta glucan supplementation) at baseline (day 0), day 15 and day 30 wherein the decrease was statistically significant in Gr.2 and 3 (p < 0.05); G-I: C-reactive protein (CRP) Levels in subjects with COVID-19 G. Gr. 1: Control (Standard treatment); H. Gr. 2: (Standard treatment + AFO-202 beta glucan supplementation) and I. Gr. 3: (Standard treatment + AFO-202 and N-163 beta glucan supplementation) at baseline (day 0), day 15 and day 30.
In Gr. 1 patients, levels of D-Dimer decreased until day 15 but then increased on day 30 (mean = 751 ng/dl to 143.89 ng/dl on day 15 and 202.5 ng/dl on day 30). In contrast, in groups 2 and 3, the levels of D-Dimer steadily decreased until day 30. In Gr. 2 on Day 0, was 560.99 ng/dl, while on Day 30, it was 79.615 ng/dl. In Gr. 3 on day 0, it was 1614 ng/dl to 164.25 ng/dl on day 30. This was statistically significant (p value = 0.013) (Fig. 3D-F).
CRP showed a steady decrease from day 0 to day 15 and day 30 in all the groups (Fig. 3G–I).
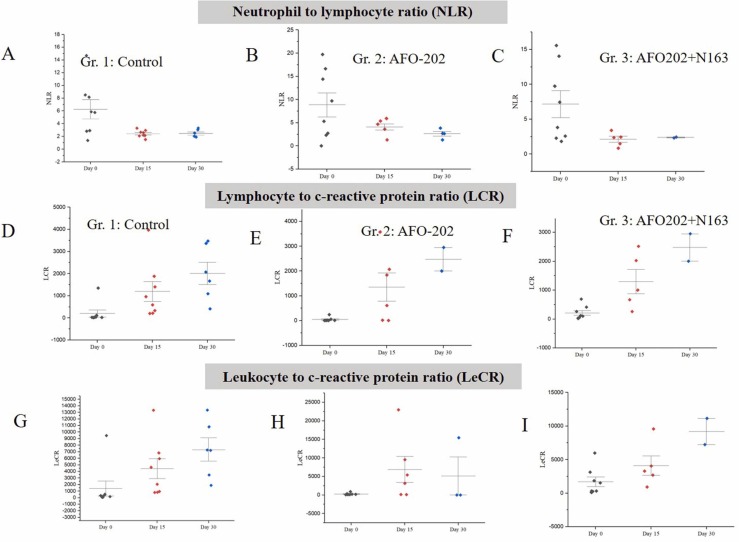
The decrease in NLR ( Fig. 4A–C) and the increase in LCR and LeCR (Fig. 4D–I) was greater in Gr. 3 at both 15 days and 30 days compared to the other groups.
Fig. 4.
A-C: Decrease in neutrophil to lymphocyte ratio (NLR) values in subjects with COVID-19 A. Gr. 1: Control (Standard treatment), B. Gr. 2: (Standard treatment + AFO-202 beta glucan supplementation) and C. Gr. 3: (Standard treatment + AFO-202 and N-163 beta glucan supplementation) at baseline (day 0), day 15 and day 30. D-F: Increase in Lymphocyte to c-reactive protein (LCR) values in subjects with COVID-19 in D. Gr. 1: Control (Standard treatment); E. Gr. 2: (Standard treatment + AFO-202 beta glucan supplementation); F. Gr. 3: (Standard treatment + AFO-202 and N-163 beta glucan supplementation);G-I: Leukocyte to c-reactive protein (LeCR) values in subjects with COVID-19 G. Gr. 1: Control (Standard treatment); H. Gr. 2: (Standard treatment + AFO-202 beta glucan supplementation); and I. Gr. 3: (Standard treatment + AFO-202 and N-163 beta glucan supplementation) at baseline (day 0), day 15 and day 30.
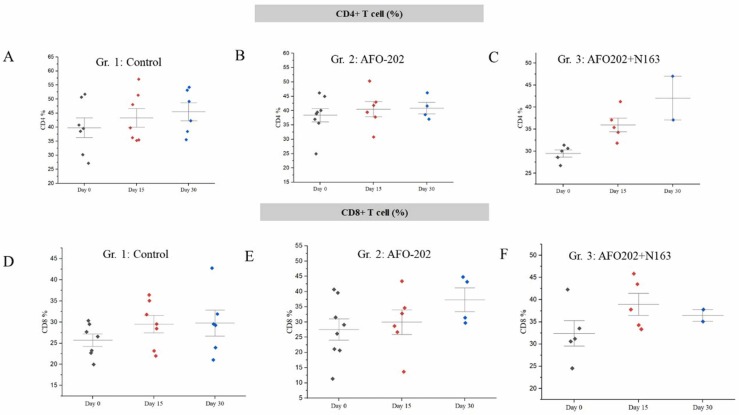
Immunological parameters: CD4 + T cell count showed relatively higher increase in Gr.3 on day 15 and day 30 ( Fig. 5A). CD8 + T cell count showed relatively higher increase in Gr.2 on day 15 and day 30 (Fig. 5B).
Fig. 5.
A-C. CD4 + T cell counts, D-F: CD8 + T cell counts at baseline (Day 0), day 15 and day 30 in the different treatment arms of COVID-19 subjects showing increase in the all groups on day 15 and day 30 compared to the baseline.
4. Discussion
The immunological profile from pathogenesis to progression to a severe disease in COVID-19 leading to a cytokine storm assessed by elevation of biomarkers such as IL6 and coagulopathy assessed by the elevation of markers such as D-Dimer has been reported to be unique [35]. Though many recover after one or two weeks of illness, the second to fourth week after initial onset has been reported to be stormy as many develop immune hyper-activation leading to cytokine storm marked by elevation of markers such as NLR and CRP, decrease of LCR and LeCR [7], or coagulopathy disruption evident by elevation of markers such as D-Dimer and ferritin. Standard care of management that has been recommended by professional bodies includes steroid based immune suppression or anti-coagulants (“Solidarity” clinical trial) [28]. Even under appropriate treatment, morbidity, mortality and related correlations to the pathogenesis remain uncertain (“Solidarity” clinical trial) [28]. There have been reports of bleeding diathesis after anti-coagulants [21], and the appropriate timing for administration of these pharmacological agents are still being debated [32]. At this hour, a safer and more efficient interventional strategy to modulate the immune system to prevent or tackle the cytokine storm and to manage the coagulopathy cascade is needed.
A balanced immune enhancement with metabolism regulation potential, of AFO-202 beta glucan has been reported earlier, while an efficient immune-modulation including anti-fibrotic effects has been reported using N-163 beta glucan ([12], [23]; a- [17]). Specially, in healthy volunteers’ alleviation of glucotoxicity by balancing of blood glucose with AFO-202 and alleviation of lipotoxicity by balancing of lipid profile when AFO-202 beta glucan is combined with N-163 beta glucan has been reported [17]. Such combinations yielding beneficial effects in animal studies [16] of NASH models have proven the anti-fibrotic and anti-inflammatory immunomodulatory capabilities. The above studies in healthy animal models, human volunteers and diseased animal models have demonstrated the potentials of these beta glucans offering a balanced immunomodulation along with the necessary immune enhancement. Having used them alone or in combination, we report results herein in COVID-19 patients yielding both benefits, such as balanced modulation of inflammatory associated parameters including IL-6, NLR, LCR and LeCR. However, in addition to the beneficial effects on the immune system, the added benefits in regulating the coagulopathy evident by decrease in D-Dimer is a unique dual advantage which has never been reported with a single standalone agent or a molecule for COVID-19 in the literature, to our knowledge.
D-dimer has been reported to have the highest C-index to predict in-hospital mortality in COVID-19 patients [27]. A D-dimer level greater than 0.5 μg/ml is associated with severe infection, and a value greater than 1 μg/ml is associated with increasing odds of in-hospital death in patients with COVID-19 [36]. In the current study, the combination of AFO-202 and N-163 beta glucans were able to bring down the D-Dimer values which was greater than 1.6 μg/ml at baseline to normal values in 15 days (Fig. 3B) that continued to be maintained even until day 30 against the control group in whom it increased. Though all the participants received anti-coagulants, the normalization of D-Dimer was significant when supplemented with beta glucans as in Gr.2 and 3.
Likewise, IL-6 levels have been reported to be significantly elevated and associated with adverse clinical outcomes in COVID-19. Inhibition of IL-6 has been suggested as a novel target for managing dysregulated host responses in patients with COVID-19 [3]. In the current study, the IL-6 values were decreased from values predictive of higher mortality due to COVID-19 > 24 pg/ml [25] to normal values which were maintained in the beta glucan groups only while it increased to values predictive of higher mortality in the control groups at day 30 (Fig. 3A). The significant and steady decrease in IL6 and D-Dimer (Fig. 3) substantiate the anti-inflammatory and anti-coagulation benefits of beta glucans. The observation that patients in groups 2 and 3 had normal levels of IL-6, D-Dimer and ESR in contrast to increased levels on day 30 in the control group strongly suggests their relevance to potential management of post-COVID or long-COVID manifestations [22]. Other significant predictors of severity in COVID-19 such as NLR, LCR and LeCR were also maintained at normal range in the beta glucan groups. Lower CD8 +T and CD4 +T cell counts have been reported to be predictive of increased severity of COVID-19 and in hospital mortality [34]. In the current study, there was relatively higher increase in CD4 + and CD8 + T cell counts in the beta glucan groups from the baseline on day 15 and day 30 (Fig. 5). There are reports which indicate the development of acute respiratory distress syndrome (ARDS) during the course of the COVID-19 disease, around the eighth day of the infection [20] which corresponds to the drop in oxygen saturation from second to the sixth day after initiation of the present study (Fig. 1A). After that phase, there was an improvement in the oxygen saturation levels.
Although several other food supplements including n-3 PUFA, zinc, and vitamins C and D [19], [26], [29], [30], [33], are being investigated for their potential applications in COVID-19 the clinical trial results have largely yielded only a modest positive outcome. Probiotics have better advantages than the other dietary supplements because of their effects at the entry point of the SARS-CoV2 virus in the intestinal epithelium and the lung-gut axis [1], [37], [6] Beta glucans are beneficial in terms of the pre-biotic effects on the gut microbiota as well [2] which could play a significant role contributing to mechanisms by which they exert their influence on the mucosal and other arms of immunity including the trained immune response [18], which needs additional elaborate research.
The safety profile of both AFO-202 and N-163 beta glucans has been further corroborated by the no SAEs or mortality in groups 2 and 3. The increase in the biomarkers for inflammation (IL6, NLR, ESR, D-Dimer) in spite of standard treatment and low molecular weight heparin administration in Gr. 1 but not in groups 2 and 3 confirms the beneficial effects of the beta-glucans as advantageous adjuncts to presently available standard treatments of COVID-19. The metabolic benefits with immune enhancement of AFO-202 alone, which when combined with N-163 beta glucans yielding a stronger immunomodulation and anti-inflammatory effect has been significantly demonstrated in the current study.
The limitations of the study include the large number of drop-outs that have been attributed to the psychosocial factors of visiting the hospital for follow-up. In addition, the sample number was less, but this is only a pilot clinical study. We conducted this study at the time of a decline in the second wave in India (Covid-19: Has India's deadly second wave peaked? BBC News) [5] when infection due to the delta variant of SARS-CoV2 virus was gradually emerging as a threat (Times of India- News article) [8] with increased contagiousness (asm.org- News article) [9]. We have not studied genomic sequencing in the subjects to identify the delta variant virus, and this needs further evaluation to study the efficacy of the beta glucans against emerging variants. The significance of beta glucan groups versus control though is evident in this study, the difference in the quantum of beneficial effects between the AFO-202 (Gr.2) and that of Gr. 3 due to smaller sample numbers is not very clear, as reported in the earlier clinical study in terms of immunomodulation in healthy volunteers [17] and anti-inflammatory, anti-fibrotic effects in NASH animal models [16], warrant a larger study to confirm the same. In such larger multi-centric clinical trials, evaluations on other biochemical and immunological parameters along with radiological diagnostics can help decipher the various underlying mechanisms to further validate the advantageous effects of these safety-proven beta-glucans so that they could be recommended for continued supplemental prophylaxis as a vaccine adjuvant (a [14])and as an adjunct to the existing treatments for the management of COVID-19.
The continuous increase in ESR, CRP, IL6 and D-Dimer on day 30 in the control group may be indicative of the post-Covid sequalae or the long COVID syndrome [22]. Having documented that the levels of these biomarkers were maintained in the normal range in the beta glucan groups even until day 30, we suggest their application in the management of long-COVID after necessary validation, with a longer duration of follow-up.
5. Conclusion
Supplementation with the beta glucans produced by the AFO-202 and N-163 strains of Aureobasidium pullulans have helped to maintain the major biomarkers of clinical severity and mortality of COVID-19 viz, IL-6, D-Dimer, NLR over 15 and 30 days compared to those who underwent standard care alone in this randomized pilot clinical study. However, being a pilot study with limited number of subjects, upon validation in larger multi-centric clinical trials, these nutraceutical agents may be considered as continuous oral supplemented adjuncts for prophylaxis and management of COVID-19 or post-COVID sequalae, especially in those with co-morbid conditions, having a safe track record for the past two decades.
Ethics approval and consent to participate
The study was registered in India’s clinical trial registry CTRI, Ref no: CTRI/2021/04/032766 (http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=54786). The study was approved by the Institutional Ethics Committee (IEC) of Shanmuga Medical Research Foundation Trust-Institutional Ethics Committee, India on 29th March, 2021.
Funding
No external funding was received for the study.
CRediT authorship contribution statement
Kadalraja Raghavan: Conceptualization, Editing. Vidyasagar Devaprasad Dedeepiya: Conceptualization, Editing. Nobunao Ikewaki: Technical inputs. Vaddi Suryaprakash: Writing – review & editing. Kosagi-Sharaf Rao: Writing – review & editing. Tohru Sonoda: Writing – review & editing. Masaru Iwasaki: Writing – review & editing. Rajappa Senthilkumar - Formal analysis. Gary A. Levy: Writing – original draft. Senthilkumar Preethy: Writing – original draft. Samuel JK Abraham: Conceptualization, Writing – original draft.
Conflict of interest statement
Author Samuel Abraham is a shareholder in GN Corporation, Japan which in turn is a shareholder in the manufacturing company of the Beta Glucans described in the study.
Acknowledgements
The authors wish to acknowledge,
-
a.
The Government of Japan and the Prefectural Government of Yamanashi for a special loan for COVID-19 related implications and M/s Yamanashi Chuo Bank for processing the transactions.
-
b.
The co-investigators Dr. Vaiyali Ramu, Emergency and Critical Care Physician, Shanmuga Hospital Pvt Ltd, India, Dr. S. R. Tiruvalavan, Clinical Research Head, Shanmuga Hospital Pvt Ltd, India, and their colleagues; Medical, nursing, para-medical, data collection - clerical staff for their valuable contribution to the clinical trial amidst the ongoing pandemic.
-
c.
Mr. Takashi Onaka, Mr. Yasunori Ikeue and Dr. Mitsuru Nagataki (Sophy Inc, Kochi, Japan), for necessary technical clarifications.
-
d.
Mr. Yoshio Morozumi and Ms. Yoshiko Amikura of GN Corporation, Japan for their liaison assistance with the conduct of the study.
-
e.
Loyola-ICAM College of Engineering and Technology (LICET) for their support to our research work.
Author contribution statement
K.R, V.D and S.A. contributed to conception and design of the study. N.I helped with technical inputs. R.S helped in literature search. S.A, G.L and S.P. drafted the manuscript. V.S, K.S.R, T.S and M.I performed critical revision of the manuscript. All the authors read, and approved the submitted version.
References
- 1.Bioithas SL (2021) The intestinal microbiota as a therapeutic target in hospitalized patients with COVID-19 infection. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04390477.
- 2.Ciecierska A., Drywień M.E., Hamulka J., Sadkowski T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig. 2019;70(4):315–324. doi: 10.32394/rpzh.2019.0082. [DOI] [PubMed] [Google Scholar]
- 3.Coomes E.A., Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev. Med. Virol. 2020;30(6):1–9. doi: 10.1002/rmv.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy G.A., Talbot P.J., editors. Advances in Experimental Medicine and Biology. Springer; US: 1995. Corona- and related viruses. current concepts in molecular biology and pathogenesis. [DOI] [Google Scholar]
- 5.Covid-19: Has India's deadly second wave peaked? (August 6, 2021). https://www.bbc.com/news/world-asia-india-57225922.
- 6.Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghahramani S., Tabrizi R., Lankarani K.B., Kashani S., Rezaei S., Zeidi N., Akbari M., Heydari S.T., Akbari H., Nowrouzi-Sohrabi P., Ahmadizar F. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur. J. Med. Res. 2020;25(1):30. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.How Delta variant is causing new spikes across the world? (August 6, 2021). https://timesofindia.indiatimes.com/india/how-delta-variant-is-causing-new-spikes-across-the-world/articleshow/84824814.cms.
- 9.How Dangerous Is the Delta Variant (B.1.617.2)? (August 6, 2021). https://asm.org/Articles/2021/July/How-Dangerous-is-the-Delta-Variant-B-1–617-2.
- 10.ICMR COVID-19 Management protocol. (August 6, 2021). https://www.icmr.gov.in/pdf/covid/techdoc/COVID_Management_Algorithm_17052021.pdf.
- 11.Ikewaki N., Fujii N., Onaka T., Ikewaki S., Inoko H. Immunological actions of Sophy beta-glucan (beta-1,3-1,6 glucan), currently available commercially as a health food supplement. Microbiol. Immunol. 2007;51(9):861–873. doi: 10.1111/j.1348-0421.2007.tb03982.x. [DOI] [PubMed] [Google Scholar]
- 12.aIkewaki N., Iwasaki M., Abraham S. Biological response modifier glucan through balancing of blood glucose may have a prophylactic potential in COVID-19 patients. J. Diabetes Metab. Disord. 2020;19(2):1–4. doi: 10.1007/s40200-020-00664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.bIkewaki N., Rao K.S., Archibold A.D., Iwasaki M., Senthilkumar R., Preethy S., Katoh S., Abraham S. Coagulopathy associated with COVID-19 – perspectives & Preventive strategies using a biological response modifier Glucan!Abstract. Thromb. J. 2020;18:27. doi: 10.1186/s12959-020-00239-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.aIkewaki N., Iwasaki M., Kurosawa G., Rao K.S., Lakey-Beitia J., Preethy S., Abraham S.J. beta glucans: wide-spectrum immune-balancing food-supplement-based enteric (β-WIFE) vaccine adjuvant approach to COVID-19. Hum. Vaccin. Immunother. 2021:1–6. doi: 10.1080/21645515.2021.1880210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.bIkewaki, N. , Raghavan, K. , Dedeepiya, V.D. , Suryaprakash, V. , Iwasaki, M. , Preethy, S. , Senthilkumar, R. , Abraham, S. (2021). Beneficial immune-regulatory effects of novel strains of Aureobasidium pullulans AFO-202 and N-163 produced beta glucans in Sprague Dawley rats. 02 August 2021, PREPRINT (Version 1) Research Square; doi:10.21203/rs.3.rs-771315/v1.
- 16.cIkewaki N., Kurosawa G., Iwasaki M., Preethy S., Dedeepiya V.D., Vaddi S., Senthilkumar R., Levy G.A., Abraham S. Hepatoprotective effects of Aureobasidium pullulans derived Beta 1,3-1,6 biological response modifier glucans in a STAM- animal model of non-alcoholic steatohepatitis. bioRxiv. 2021 doi: 10.1101/2021.07.08.451700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.dIkewaki N., Sonoda T., Kurosawa G., Iwasaki M., Dedeepiya V.D., Senthilkumar R., Preethy S., Abraham S. Immune and metabolic beneficial effects of Beta 1,3-1,6 glucans produced by two novel strains of Aureobasidium pullulans in healthy middle-aged Japanese men: an exploratory study. medRxiv. 2021 doi: 10.1101/2021.08.05.21261640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.eIkewaki N., Dedeepiya V.D., Iwasaki M., Abraham S. Commentary: beyond “TRIM” benefits of β-glucan by blood glucose and lipid balancing potentials in its defense against COVID-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.620658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leaf D.E., Ginde A.A. Vitamin D3 to Treat COVID-19: different disease, same answer. JAMA. 2021;325(11):1047–1048. doi: 10.1001/jama.2020.26850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mejía F., Medina C., Cornejo E., Morello E., Vásquez S., Alave J., Schwalb A., Málaga G. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musoke N., Lo K.B., Albano J., Peterson E., Bhargav R., Gul F., DeJoy R., 3rd, Salacup G., Pelayo J., Tipparaju P., Azmaiparashvili Z., Patarroyo-Aponte G., Rangaswami J. Anticoagulation and bleeding risk in patients with COVID-19. Thromb. Res. 2020;196:227–230. doi: 10.1016/j.thromres.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pu S.L., Zhang X.Y., Liu D.S., Ye B.N., Li J.Q. Unexplained elevation of erythrocyte sedimentation rate in a patient recovering from COVID-19: A case report. World J. Clin. Cases. 2021;9(6):1394–1401. doi: 10.12998/wjcc.v9.i6.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao K.S., Suryaprakash V., Senthilkumar R., Preethy S., Katoh S., Ikewaki N., Abraham S. Role of immune dysregulation in increased mortality among a specific subset of COVID-19 patients and immune-enhancement strategies for combatting through nutritional supplements. Front. Immunol. 2020;11:1548. doi: 10.3389/fimmu.2020.01548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabico S., Enani M.A., Sheshah E., Aljohani N.J., Aldisi D.A., Alotaibi N.H., Alshingetti N., Alomar S.Y., Alnaami A.M., Amer O.E., Hussain S.D., Al-Daghri N.M. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate COVID-19: a randomized clinical trial. Nutrients. 2021 24;13(7):2170. doi: 10.3390/nu13072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabaka P., Koščálová A., Straka I., Hodosy J., Lipták R., Kmotorková B., Kachlíková M., Kušnírová A. Role of interleukin 6 as a predictive factor for a severe course of Covid-19: retrospective data analysis of patients from a long-term care facility during Covid-19 outbreak. BMC Infect. Dis. 2021;21(1):308. doi: 10.1186/s12879-021-05945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silberstein M. Vitamin D: a simpler alternative to tocilizumab for trial in COVID-19? Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soni M., Gopalakrishnan R., Vaishya R., Prabu P. D-dimer level is a useful predictor for mortality in patients with COVID-19: analysis of 483 cases. Diabetes Metab. Syndr. 2020;14(6):2245–2249. doi: 10.1016/j.dsx.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.“Solidarity” clinical trial for COVID-19 treatments (August 6, 2021). Retrieved from 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-COVID-19-treatments〉.
- 29.Stroehlein J.K., Wallqvist J., Iannizzi C., Mikolajewska A., Metzendorf M.I., Benstoem C., Meybohm P., Becker M., Skoetz N., Stegemann M., Piechotta V. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst. Rev. 2021;5(5) doi: 10.1002/14651858.CD015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., Il’Giovine Z.J., Mehra R., McWilliams C., Nissen S.E., Desai M.Y. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients With SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Coronavirus (COVID-19) Dashboard (August 6, 2021). https://covid19.who.int/.
- 32.Winthrop K.L., Mariette X. To immunosuppress: whom, when and how? That is the question with COVID-19. Ann. Rheum. Dis. 2020;79(9):1129–1131. doi: 10.1136/annrheumdis-2020-218694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weill P., Plissonneau C., Legrand P., Rioux V., Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in Covid-19 patients? Biochimie. 2020;179:275–280. doi: 10.1016/j.biochi.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen X.S., Jiang D., Gao L., Zhou J.Z., Xiao J., Cheng X.C., He B., Chen Y., Lei P., Tan X.W., Qin S., Zhang D.Y. Clinical characteristics and predictive value of lower CD4+T cell level in patients with moderate and severe COVID-19: a multicenter retrospective study. BMC Infect. Dis. 2021;21(1):57. doi: 10.1186/s12879-020-05741-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varchetta S., Mele D., Oliviero B., Mantovani S., Ludovisi S., Cerino A., Bruno R., Castelli A., Mosconi M., Vecchia M., Roda S., Sachs M., Klersy C., Mondelli M.U. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021;18(3):604–612. doi: 10.1038/s41423-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H.H., Qin C., Chen M., Wang W., Tian D.S. D-dimer level is associated with the severity of COVID-19. Thromb. Res. 2020;195:219–225. doi: 10.1016/j.thromres.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z., Cui X., Xiao J., Meng T., Zhou W., Liu J., Xu H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv. 2020 doi: 10.1101/2020.01.30.927806. [DOI] [Google Scholar]