Abstract
This study aims to examine the association of whole blood n-3 and n-6 polyunsaturated fatty acids (PUFA) with insulin resistance (IR) in children. Whole blood fatty acids were measured in 705 children aged 2–9 years of the European IDEFICS/I.Family cohort using gas chromatography in units of weight percentage of all detected fatty acids (%wt/wt). IR was determined by the Homeostasis Model Assessment for IR (HOMA). Mixed effect models were used to assess the associations between selected baseline PUFA and HOMA z-scores at baseline and after 2- and 6-year follow-ups using models with basic and additional confounder adjustment as well as stratified by sex and weight status. In the basic model, α-linolenic (β = 1.46 SD/%wt/wt, p = 0.006) and eicosapentaenoic acid (β = 1.17 SD/%wt/wt, p = 0.001) were positively associated with baseline HOMA z-score. In the stratified analyses, α-linolenic acid was positively associated with HOMA z-score in girls only (β = 1.98 SD/%wt/wt, p = 0.006) and arachidonic acid was inversely associated with baseline HOMA in thin/normal-weight children (β = − 0.13 SD/%wt/wt, p = 0.0063). In the fully adjusted model, no statistically significant associations were seen.
Conclusions: Our overall results do not indicate a protective role of higher blood n-3 PUFA or an adverse role of higher blood arachidonic acid proportion on the risk of IR.
|
What is Known: •Intervention studies reported a beneficial effect of n-3 PUFA supplementation on insulin resistance compared with placebo while observational studies in cildren are inconclusive. •Studies have shown a positive association of n-6 arachidonic acid and insulin resistance indicating an adverse role of arachidonic acid. | |
|
What is New: •Cross-sectional and longitudinal analyses based on circulating blood fatty acid concentrations in a large cohort of European children and adolescents. •Overall results do not support a protective role of n-3 PUFA or an adverse role of arachidonic acid in insulin resistance. |
Electronic supplementary material
The online version of this article (10.1007/s00431-020-03636-1) contains supplementary material, which is available to authorized users.
Keywords: Children, HOMA, Insulin resistance, n-3 fatty acids, n-6 fatty acids, Polyunsaturated fatty acids
Introduction
The worldwide obesity epidemic has resulted in a rise of insulin resistance (IR) that is already observed in childhood and adolescence. IR is often accompanied by further components of the metabolic syndrome and tends to track into adulthood [1]. Polyunsaturated fatty acids (PUFA), particularly n-3 PUFA, may beneficially influence insulin sensitivity (IS) whereas the n-6 PUFA, arachidonic acid (AA), may have an adverse effect [2, 3]. Accordingly, in adults with overweight or obesity, a higher dietary n-6:n-3 ratio has been associated with increased insulin resistance, measured as Homeostasis Model Assessment for insulin resistance (HOMA) [4]. Correspondingly, in adolescents with overweight and obesity who participated in a weight loss program, the reduction in n-6 PUFA was observed to be directly related to reduced glucose concentrations [5]. Further, AA in adipose tissue triacylglycerol was found to be positively associated with HOMA in healthy, non-obese children [2]. Interventional studies indicated that supplemental dietary n-3 PUFA improve insulin sensitivity [6, 7]. Moreover, n-3 PUFA status of children as measured in serum, [8] serum phospholipids [9], whole blood [10], and erythrocytes [11] has been shown to be inversely associated with IR, although two studies investigating n-3 PUFA in plasma phospholipids and plasma lipids did not confirm this relation [12, 13]. In particular, in studies with mostly small sample size, the n-3 PUFA α-linoleic acid (ALA) [14], eicosapentaenoic acid (EPA) [8], docosahexaenoic acid (DHA) [10], and the sum of EPA+DHA [11], as well as AA [2], have been linked to IR and HOMA. Large longitudinal studies in children with data on fatty acids and IR from biosamples are scarce. Therefore, our longitudinal study investigated the cross-sectional and longitudinal associations between the above-mentioned PUFA and HOMA for the first time in a large cohort of young children across Europe.
Materials and methods
A subsample of the IDEFICS/I.Family cohort was included. The baseline survey (T0) took place in 2007/2008 with follow-up examinations after 2 (T1) and 6 (T3) years. Ethical approval was obtained from all participating study centers. The fatty acid blood profiles of 2600 children were analyzed and included as weight percentage of all fatty acids (FA) detected (%wt/wt). All children whose FA data were available at baseline and who participated in at least one follow-up examination were included in the present analysis (N = 705; 705 T0, 571 T1, and 342 T3; Fig. 1). HOMA was calculated as fasting insulin (μǀU/ml) × fasting glucose (mmol/l)/22.5 [15]. For the description of the characteristics of the study population, children with a HOMA greater or equal to the 90th percentile (≥P90) were considered to be insulin resistant or at risk. Mixed effect models were used to assess the associations between baseline PUFA (ALA, EPA, DHA, EPA+DHA, AA) and continuous HOMA z-score measured at baseline and 2- and 6-year follow-ups. All models were run with a basic adjustment (age, sex, country of residence, and intervention vs. control region) as well as adding further covariates (fully adjusted model; see Table 1). A p value of 0.01 was considered to be statistically significant. Detailed information on the assessment methods of fatty acids, HOMA, anthropometric measures, and covariates as well as statistical analysis methods can be found in the Supplementary Material.
Fig. 1.
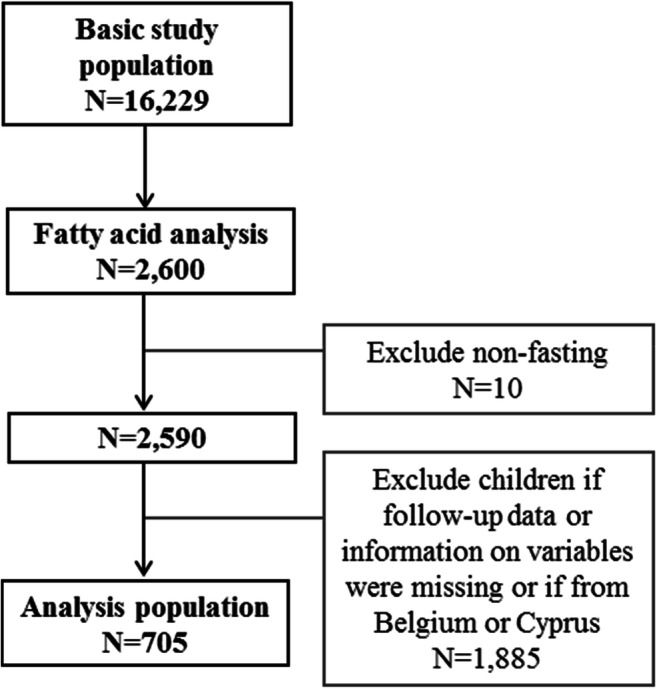
Flow chart of the inclusion and exclusion of IDEFICS/I.Family participants
Table 1.
Associations of polyunsaturated fatty acids measured at baseline with repeated measurements of HOMA z-scores at baseline and after 2 years and after 6 years of follow-up estimated on basic and fully adjusted mixed effect models
| Fatty acid | Time since baseline in years | Basic | Full adjustment† | ||
|---|---|---|---|---|---|
| β | p value | β | p value | ||
| 20:4n-6, AA | 0 | − 0.044 | 0.2477 | − 0.074 | 0.0544 |
| 2 | 0.069 | 0.0706 | 0.025 | 0.5108 | |
| 6 | 0.030 | 0.6142 | − 0.001 | 0.9879 | |
| 18:3n-3, ALA | 0 | 1.457 | 0.0062 | 0.718 | 0.1220 |
| 2 | 0.197 | 0.7537 | − 0.251 | 0.6437 | |
| 6 | − 0.235 | 0.7572 | − 0.481 | 0.4835 | |
| 20:5n-3, EPA | 0 | 1.172 | 0.0012 | 0.645 | 0.0608 |
| 2 | 0.576 | 0.1786 | 0.286 | 0.4201 | |
| 6 | − 0.816 | 0.2357 | − 1.159 | 0.0728 | |
| 22:6n-3, DHA | 0 | 0.011 | 0.9213 | 0.066 | 0.5159 |
| 2 | 0.133 | 0.2387 | 0.184 | 0.0654 | |
| 6 | − 0.007 | 0.9687 | − 0.022 | 0.8996 | |
| 20:5n-3+22:6n-3 (sum of EPA+DHA) | 0 | 0.071 | 0.4565 | 0.087 | 0.3182 |
| 2 | 0.132 | 0.1800 | 0.159 | 0.0651 | |
| 6 | − 0.055 | 0.7311 | − 0.082 | 0.6004 | |
†The basic model was adjusted for age, sex, country of residence, and control vs. intervention region. The fully adjusted model was further adjusted for birth weight, BMI z-score, pubertal status, family history of diabetes mellitus type 2, being a member of a sports club, consumption frequency of sugar/refined carbohydrates, time spent with audio-visual media, maximum ISCED level of parents, weight percentage of the sum of total SFA and total MUFA of total fatty acids
As highlighted in bold, a p value of 0.01 was used as the level of statistical significance
AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HOMA, Homeostasis Model Assessment for insulin resistance; ISCED, International Standard Classification of Education; MUFA, monounsaturated fatty acids; SFA, saturated fatty acids
Results
Supplementary Table 1 shows that HOMA of ≥P90 was more prevalent in girls and in children who had no sportsclub membership, who spent more time with audio-visual media (except for T3), who had a familial history of diabetes, and whose parents had a lower education level. Further, children with HOMA of ≥P90 were more likely to have been obese, compared with children without IR (47% vs. 12% at T0, 48% vs. 9% at T1, and 28% vs. 14% at T3). Regarding the association of different PUFA with HOMA z-scores at baseline and after 2 and 6 years of follow-up, ALA (β = 1.46, p = 0.0062, i.e., 1-unit (%wt/wt) increase of ALA was associated with a 1.46 SD higher HOMA z-score) and EPA (β = 1.17, p = 0.0012) were positively associated with HOMA at baseline in the basic model. These associations weakened over time, as reflected by lower and negative β-values for HOMA z-scores at T1 and T3 (Table 1). Similar associations were observed in the fully adjusted models. These were however not significant. In the basic model stratified by sex, ALA (β = 1.98, p = 0.0058) and EPA (β = 1.24, p = 0.0057) were positively associated with HOMA in girls but not in boys (Supplementary Table 2). When stratified by weight status in the basic model, AA was inversely associated with HOMA z-score (β = − 0.131, p = 0.0063) at baseline in thin/normal-weight children. A similar association was observed in the fully adjusted model (β = − 0.134, p = 0.0169). This was however only marginally significant (Supplementary Table 3).
Discussion
Our study was based on an exceptionally large number of measurements and provides cross-sectional and longitudinal data on the association of whole blood PUFA with HOMA in a cohort of 705 European children. In general, our findings do not indicate a role of ALA, EPA, DHA, EPA+DHA, or AA in IR. Contrary to expectations, an adverse effect of higher ALA and EPA in the total analysis sample was observed in our basic model. However, as these associations disappeared in the fully adjusted models, they may be partially explained by confounding, e.g., by covariates such as BMI or the consumption frequency of sugar and refined carbohydrates. Although IR in adolescents is influenced by various factors, obesity has the strongest effect [16]. Therefore, we considered BMI a confounder and not a mediator. Our results are in line with those of a Danish cross-sectional study including 713 children aged 8–11 years, which did not find an association between whole blood EPA and HOMA [10]. Another study including 120 adolescents with normal weight and overweight also did not find an association between n-3 and n-6 PUFA levels of plasma phospholipids and cholesterol esters and HOMA [13]. Our results in the basic model indicating an adverse effect of EPA are in line with a study involving 56 obese Mexican children, which reported significantly higher serum levels of EPA in insulin-resistant compared with non-insulin-resistant children [8]. As in our population, no association of DHA in plasma phospholipids with HOMA was observed in yet another study on 32 children with obesity [12]. In contrast, in the Danish study [10] and in a study including 10 adolescents with obesity and 15 normal-weight adolescents reporting DHA in serum phospholipids [9], DHA was inversely associated with HOMA. Concordantly, in a small Australian study including 24 children with and 24 without obesity, the sum of EPA and DHA levels of erythrocytes was moderately inversely associated with HOMA [11].
While other studies indicate an adverse role of AA, we unexpectedly observed a beneficial role of higher AA in thin/normal-weight children in our basic model. In a Spanish study with 83 healthy non-obese children, AA in adipose tissue triacylglycerols was positively associated with HOMA [2]. The reduction of plasma n-6 PUFA after a weight loss program was also reported to be associated with improved fasting glucose in adolescents [5]. Accordingly, in adult males, EPA/AA ratios of erythrocytes and EPA were negatively associated with IR in subjects with metabolic syndrome [3]. Additionally, among adults with and without diabetes, plasma AA was found to be highest among those with diabetes compared with subjects with normal glucose tolerance [17]. Besides differences in the methods applied (see Supplementary Material), a possible explanation regarding why we did not observe the expected associations in our study compared with others may be the differences in desaturase activity between study populations. In previous IDEFICS/I.Family study analyses, we observed that higher estimated delta-6 desaturase activity was associated with an increase of IR [18] and that genetic variations in the FADS1 gene, which affects delta-5 desaturase, influenced whole blood AA and EPA levels [19].
In conclusion, taking relevant confounders into account, our overall results do not point to an association between n-3 PUFA or AA and IR. Given the high prevalence of obesity in children with IR, prevention programs of IR need to focus on obesity as a known main risk factor.
Electronic supplementary material
(DOCX 88 kb)
Acknowledgments
This work was done as part of the IDEFICS study (http://www.idefics.eu) and the I.Family Study (http://www.ifamilystudy.eu/). The authors wish to thank the IDEFICS/I.Family children and their parents for participating in the extensive examinations. We are grateful for the support of school boards, head teachers and communities. The authors cordially thank Dr. Florence Samkange-Zeeb for the English language editing of the manuscript.
Abbreviations
- ALA
α-Linolenic acid
- BMI
Body mass index
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- FA
Fatty acids
- HOMA
Homeostasis Model Assessment for insulin resistance
- IDEFICS
Identification and prevention of dietary- and lifestyle-induced health effects in children and adolescents
- IR
Insulin resistance
- IS
Insulin sensitivity
- ISCED
International Standard Classification of Education
- PUFA
Polyunsaturated fatty acids
Authors’ contributions
Conceptualization, MW. Formal Analysis, CB, SM. Acquisition of data, KM, PRu, LAM, SDH, TV, DM, MT, PRi. Writing-original draft, SM. Writing-review & editing, MW, CB, KM, PRu, LAM, SDH, TV, DM, MT, PRi. All authors including SM, CB, KM, PRu, LAM, SDH, TV, DM, MT, PRi, MW have read and approved the final manuscript.
Funding information
Open access funding enabled and organized by Projekt DEAL. This study was supported by the European Commission within the Sixth RTD Framework Programme (Contract No. 016181 (FOOD)) for the IDEFICS study and within the Seventh RTD Framework Programme (Contract No. 266044) for the I.Family study.
Compliance with ethical standards
Ethical approval was obtained from all participating study centers.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Sarah Marth and Claudia Börnhorst shared first authorship.
The original online version of this article was revised: Retrospective open access.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/24/2021
A Correction to this paper has been published: 10.1007/s00431-021-04264-z
Contributor Information
Sarah Marth, Email: sarah.marth@uni-bremen.de.
Claudia Börnhorst, Email: boern@leibniz-bips.de.
Kirsten Mehlig, Email: kirsten.mehlig@gu.se.
Paola Russo, Email: prusso@isa.cnr.it.
Luis A. Moreno, Email: lmoreno@unizar.es
Stefaan De Henauw, Email: stefaan.dehenauw@ugent.be.
Toomas Veidebaum, Email: toomas.veidebaum@tai.ee.
Dénes Molnár, Email: denes.molnar@aok.pte.hu.
Michael Tornaritis, Email: tor.michael@cytanet.com.cy.
Patrizia Risé, Email: patrizia.rise@unimi.it.
Maike Wolters, Email: wolters@leibniz-bips.de.
References
- 1.Liang Y, Hou D, Zhao X, Wang L, Hu Y, Liu J, Cheng H, Yang P, Shan X, Yan Y, Cruickshank JK, Mi J. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine. 2015;50(1):87–92. doi: 10.1007/s12020-015-0560-7. [DOI] [PubMed] [Google Scholar]
- 2.Aldamiz-Echevarria L, Prieto JA, Andrade F, Elorz J, Sanjurjo P, Rodriguez SJ. Arachidonic acid content in adipose tissue is associated with insulin resistance in healthy children. J Pediatr Gastroenterol Nutr. 2007;44(1):77–83. doi: 10.1097/01.mpg.0000237931.53470.ba. [DOI] [PubMed] [Google Scholar]
- 3.Yanagisawa N, Shimada K, Miyazaki T, Kume A, Kitamura Y, Ichikawa R, Ohmura H, Kiyanagi T, Hiki M, Fukao K, Sumiyoshi K, Hirose K, Matsumori R, Takizawa H, Fujii K, Mokuno H, Inoue N, Daida H. Polyunsaturated fatty acid levels of serum and red blood cells in apparently healthy Japanese subjects living in an urban area. J Atheroscler Thromb. 2010;17(3):285–294. doi: 10.5551/jat.2618. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Castillo N, Silva-Gomez JA, Campos-Perez W, et al. High dietary omega-6:omega-3 PUFA ratio is positively associated with excessive adiposity and waist circumference. Obes Facts. 2018;11(4):344–353. doi: 10.1159/000492116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerendiain M, Montes R, Lopez-Belmonte G, et al. Changes in plasma fatty acid composition are associated with improvements in obesity and related metabolic disorders: a therapeutic approach to overweight adolescents. Clin Nutr (Edinburgh, Scotland) 2018;37(1):149–156. doi: 10.1016/j.clnu.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res (New York, NY) 2013;33(5):367–375. doi: 10.1016/j.nutres.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi E, Rafraf M, Farzadi L, Asghari-Jafarabadi M, Sabour S. Effects of omega-3 fatty acids supplementation on serum adiponectin levels and some metabolic risk factors in women with polycystic ovary syndrome. Asia Pac J Clin Nutr. 2012;21(4):511–518. [PubMed] [Google Scholar]
- 8.Sanchez Meza K, Tene Perez CE, Sanchez Ramirez CA, Muniz Valencia R, Del Toro Equihua M. Levels of eicosapentaenoic acid in obese schoolchildren with and without insulin resistance. Nutr Hosp. 2014;31(3):1102–1108. doi: 10.3305/nh.2015.31.3.8047. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson M, Marild S, Brandberg J, Lonn L, Friberg P, Strandvik B. Serum phospholipid fatty acids, adipose tissue, and metabolic markers in obese adolescents. Obesity (Silver Spring, Md) 2006;14(11):1931–1939. doi: 10.1038/oby.2006.225. [DOI] [PubMed] [Google Scholar]
- 10.Damsgaard CT, Eidner MB, Stark KD, Hjorth MF, Sjödin A, Andersen MR, Andersen R, Tetens I, Astrup A, Michaelsen KF, Lauritzen L. Eicosapentaenoic acid and docosahexaenoic acid in whole blood are differentially and sex-specifically associated with cardiometabolic risk markers in 8-11-year-old Danish children. PLoS One. 2014;9(10):e109368. doi: 10.1371/journal.pone.0109368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows T, Collins CE, Garg ML. Omega-3 index, obesity and insulin resistance in children. Int J Pediatr Obes. 2011;6(2–2):e532–e539. doi: 10.3109/17477166.2010.549489. [DOI] [PubMed] [Google Scholar]
- 12.Saito E, Okada T, Abe Y, Kuromori Y, Miyashita M, Iwata F, Hara M, Ayusawa M, Mugishima H, Kitamura Y. Docosahexaenoic acid content in plasma phospholipids and desaturase indices in obese children. J Atheroscler Thromb. 2011;18(4):345–350. doi: 10.5551/jat.6270. [DOI] [PubMed] [Google Scholar]
- 13.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005;82(6):1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu T, Yatsuya H, Toyoshima H, Sasaki S, Li Y, Otsuka R, Wada K, Hotta Y, Mitsuhashi H, Matsushita K, Murohara T, Tamakoshi K. Higher dietary intake of alpha-linolenic acid is associated with lower insulin resistance in middle-aged Japanese. Prev Med. 2010;50(5–6):272–276. doi: 10.1016/j.ypmed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419 [DOI] [PubMed]
- 16.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29(11):2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 17.Salomaa V, Ahola I, Tuomilehto J, Aro A, Pietinen P, Korhonen HJ, Penttilä I. Fatty acid composition of serum cholesterol esters in different degrees of glucose intolerance: a population-based study. Metabolism. 1990;39(12):1285–1291. doi: 10.1016/0026-0495(90)90185-F. [DOI] [PubMed] [Google Scholar]
- 18.Wolters M, Schlenz H, Börnhorst C, et al. Desaturase activity is associated with weight status and metabolic risk markers in young children. J Clin Endocrinol Metab. 2015;100(10):3760–3769. doi: 10.1210/jc.2015-2693. [DOI] [PubMed] [Google Scholar]
- 19.Wolters M, Dering C, Siani A, Russo P, Kaprio J, Risé P, Moreno LA, de Henauw S, Mehlig K, Veidebaum T, Molnár D, Tornaritis M, Iacoviello L, Pitsiladis Y, Galli C, Foraita R, Börnhorst C, IDEFICS and I. Family consortia The role of a FADS1 polymorphism in the association of fatty acid blood levels, BMI and blood pressure in young children-analyses based on path models. PLoS One. 2017;12(7):e0181485. doi: 10.1371/journal.pone.0181485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 88 kb)


