Abstract
The gut-brain axis plays a critical role in the maintenance of the gastrointestinal tract homeostasis. Several enteric pathogens have developed strategies to sense neurochemical molecules to regulate their virulence in the gut. Additionally, there is growing evidence that gut dysbiosis can strongly affect host brain responses. Here we review different mechanisms that have been proposed to mediate gut-brain axis communication using Citrobacter rodentium, a natural murine enteric pathogen and one of the most widely used small animal models for studying host-microbe interactions. We highlight studies that have identified specific pathways used by C. rodentium to sense host neurochemicals during colonization as well as behavioral responses and brain pathologies affected by pathogen colonization of the gut.
Keywords: Citrobacter rodentium, gut-brain axis, neurochemicals, behavior
Introduction
Many microbial-host interactions have been described across the years; however, recently, the bidirectional crosstalk between the intestine and brain, referred to as “gut-brain axis”, has received increase attention. Enteric pathogens are exposed to several neurochemicals released in the gut and many of them can influence the pathogenesis of infectious diseases [1,2]. There is also a growing appreciation on how enteric infections can impact brain function and disease [3–6].
Citrobacter rodentium (CR) is the etiological agent of transmissible murine colonic hyperplasia, and has been extensively used to study many aspects of enteric infections and host-pathogen interactions [7,8]. Importantly, CR is also a surrogate murine model for enteropathogenic (EPEC) and enterohemorrhagic Escherichia coli (EHEC), two human enteropathogens that poorly infect mice [9,10]. Similar to EPEC and EHEC, CR harbors the locus of enterocyte effacement (LEE) pathogenicity island, which contains the genes necessary for these pathogens to form attaching-and-effacing (AE) lesions on the gut epithelium, a crucial process for colonization [11–13]. The LEE encodes a type three secretion system that injects a plethora of effectors into the host cell, which act in a coordinate manner to establish a replicative niche [14].
During colonization, CR can regulate its virulence program by sensing a diverse milieu of microbiota and host-derived signals in the gut [1,7]. In this review, we focus specifically in neurochemicals that directly modulate CR virulence and pathogenesis, while also being cognizant that other signals can indirectly influence the host response to this enteropathogen [15,16]. We discuss the current literature that evaluate CR infection, brain function and neurological diseases at the gut-brain axis interface.
Role of neurochemicals on C. rodentium virulence and pathogenesis
Epinephrine/Norepinephrine
The catecholamines epinephrine (Epi) and norepinephrine (NE) play a central role in stress responses in mammals, and directly act as neurotransmitters in both the central (CNS) and peripheral nervous systems [17]. Epi and NE also play important roles in the gut physiology and homeostasis. Whereas NE is locally produced by adrenergic neurons in the enteric nervous system (ENS), Epi is mostly synthesized in the adrenal medulla but can reach the gut through the bloodstream [18].
The presence of Epi/NE in the gut can also affect the physiology and virulence of different pathogens [19,20], many of which use the bacterial adrenergic receptors QseC and QseE to sense and respond to these neurotransmitters [21,22] (Fig. 1A). QseC and QseE are membrane-bound histidine sensor kinases. Upon sensing Epi/NE, QseC and QseE are phosphorylated and initiate a signaling cascade activating the expression of flagellar and virulence genes [23]. Interestingly, the blockade of the QseC receptor by the antagonist molecule LED209 reduces virulence of different bacterial pathogens, indicating that this could be a promising anti-virulence approach [24–26].
Figure 1:
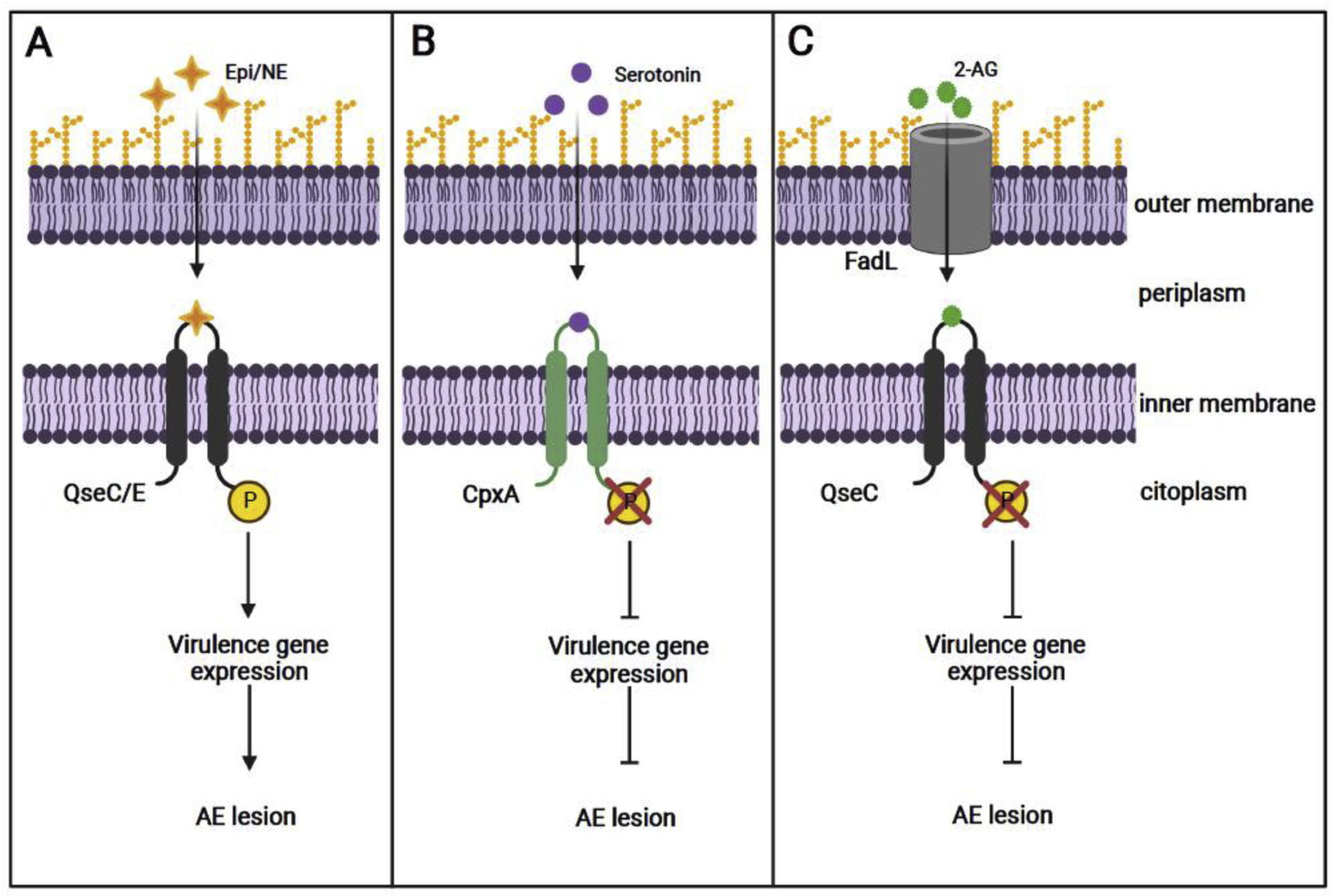
Citrobacter rodentium can sense host neurochemical signals to regulate virulence. A) Epinephrine (Epi) and norepinephrine (NE) are sensed by QseC and QseE which lead to receptor autophosphorylation and subsequent expression of virulence genes. B) Serotonin induces dephosphorylation of the bacteria CpxA receptor, which, in turn, inactivates transcriptional factors required for virulence gene expression. C) The endocannabinoid 2-AG functions as a competitive antagonist of the QseC receptor, blocking Epi/NE interaction, and consequently, reducing CR virulence expression.
A study conducted by Moreira and colleagues [27] showed that Epi and NE promote CR virulence. Mice lacking the dopamine-beta-hydroxylase (Dbh−/−) enzyme, which do not produce either NE or Epi [18], depicted decreased CR-dependent gut colonization and pathology, as well as reduced virulence gene expression. Furthermore, this study demonstrated that both adrenergic sensors, QseC and QseE, are required by CR to sense and respond to these neurotransmitters, since qseC and qseE mutants were attenuated for murine infections. Although some studies suggest that catecholamines influence growth of certain bacteria, including EHEC [19], such phenotype has not been observed in CR, suggesting that the increased CR infection phenotype induced by Epi and NE is mediated by an upregulation of virulence. Recently, it has also been shown that NE can increase a non-flagellar surface motility process in CR in a QseC-dependent manner [28]; however, it is still unclear whether this new non-flagellar motility plays any role during murine infection.
Serotonin
Serotonin is a neurotransmitter derived from the amino acid tryptophan, and plays an important role in the regulation of numerous physiological processes, including gut secretion and peristalsis, behavior and neurological functions [29]. Over 90% of serotonin in the body is synthesized in the gut, mostly by enterochromaffin (EC) cells, and different gut microbes can modulate the production of this neurotransmitter [30–32].
Recently, Kumar, Russell and colleagues [33] showed that serotonin decreases virulence gene expression in EHEC and CR, an effect mediated through the bacterial membrane-bound sensor CpxA (Fig. 1B). This study also showed that increasing intestinal serotonin levels, through genetic and pharmacological approaches, decreased LEE expression and reduced CR loads in infected animals. Conversely, the inhibition of serotonin synthesis in the gut resulted in a more severe murine infection. Of note, it has been previously reported that CR infection resulted in increased levels of released serotonin in the gut [34], which could represent a host response to this enteropathogen. It is also noteworthy that indole, another tryptophan derivative that is very abundant in the gut, decreases CR virulence and pathogenesis in a CpxA-dependent manner [35]. Taken together, these studies demonstrate the relevance of the host-derived serotonin and the microbial-derived indole converging in modulating CR pathogenesis.
Endocannabinoids
The endocannabinoid system is involved in several physiological processes, including the regulation of brain activity and immune function [36,37]. This system is composed by the cannabinoid receptors (CB1 and CB2), their lipid-based endogenous ligands (called endocannabinoids, such as anandamide and 2-arachidonoylglycerol [2-AG]), and the enzymes involved in the ligands’ synthesis and degradation [38]. Endocannabinoids exert profound effects on gut physiology and immunity, showing anti-inflammatory activity in different models of intestinal inflammation [38].
The impact of endocannabinoids on host susceptibility to enteric infections has been poorly understood. However, a recent study by Ellermann and colleagues [39] demonstrated that the endocannabinoid 2-AG directly inhibits virulence gene expression in EHEC and CR, both in vitro and in vivo. By using murine models, the authors demonstrated that elevated intestinal levels of 2-AG attenuate disease when the animals were infected with CR. This study also showed that 2-AG can cross the bacterial outer membrane through FadL, involved in the transport of long-chain fatty acids [40], and inhibit the activation of QseC, preventing the expression of the virulence genes in CR (Fig. 1C). These data propose the modulation of the endocannabinoid’s levels in the gut as a promising therapeutic approach for the treatment of enteric diseases due to the anti-virulence and anti-inflammatory activities of these endogenous molecules.
C. rodentium infection, brain function and behavior
Stress effects on microbiota composition and C. rodentium infection
Psychological stress has been shown to strongly affect gut physiology and composition both, in human and mouse [41]. Furthermore, a high comorbidity between stress-related psychiatric symptoms and gastrointestinal disorders has been reported [42–46]. This comorbidity suggests a bidirectional interaction between stressful situations, gut physiology and microbial composition.
In the past years, a series of studies have shown that different stressors can modify the severity of CR infection. By using prolonged restraint stress (RST) conditions, Bailey and colleagues [47] found that, while stress itself does not alter cytokine gene expression in the colon, it renders the mice more susceptible to CR enteric infection. Similar results were observed when mice were exposed to CR concurrently with the RST conditions [48,49]. Regarding the potential mechanisms mediating this stress-enteric pathogen infection interaction, the authors found that RST can significantly alter microbiota composition, reducing the abundance of immunomodulatory bacteria, including members from the Lactobacillus and Bifidobacterium genus, that could predispose to an increased inflammatory response to a pathogen challenge [47,49,50]. Furthermore, alterations in microbial-produced short chain fatty acids (SCFA), and their receptors were also observed, suggesting a possible role of these molecules in mice susceptibility to CR infection [49].
In line with the effects of RST, another more ethologically relevant stress, social stress, also increases the severity of CR-induced colitis. More specifically, mice challenged with CR while undergoing social disruption (SD), a mouse model of social stress, showed a sustained increase in pathogen loads and intestinal pathology [51–53]. Once again, these changes would be mediated by an interaction between SD-induced microbial modifications and alterations in SCFA levels and their receptors in the gut [51,53]. Furthermore, by using KO mice for CCL2, a monocyte recruiting chemokine, the authors demonstrated that this signal is required to the exacerbation in CR infectious colitis in SD-exposed mice [52]. Interestingly, CCL2 mRNA levels in the colon of stressed mice can be reduced by treatment with Lactobacillus reuteri, which attenuates the infection phenotype [52].
All together, these findings would support the idea of a stress-induced compositional change in microbiota driving the enhancement in CR-induced pathology. However, other factors seem to be involved and further studies are needed to really elucidate the mechanism mediating this synergistic effect that stress has on enteric pathogen vulnerability.
Effects of C. rodentium infection on host behavioral responses and brain pathologies
Gut dysbiosis and certain gut microbes have been associated with alterations in behavioral responses and with different brain pathologies [54,55]. However, there is no much information regarding the consequences that an infection with CR has for normal and pathological brain responses. Among the scarce publication in this regard, Lyte and colleagues [56] showed that soon after CR infection (7–8 hours after oral gavage), mice showed an increase in anxiety-like behaviors that is independent of the induction of circulating proinflammatory cytokines. While not specific mechanisms were dissected, the authors observed c-Fos protein activation in vagal ganglia neurons of CR-infected mice, that would suggest a sensory vagal neuron-mediated pathway [56]. In contrast with these short-term effects of CR infection in anxiety, no changes were observed after 10- or 30-days post pathogen administration [57]. However, previously infected animals showed an increased susceptibility to the detrimental effects of a subsequent acute stress exposure, showing worsened memory and learning dysfunctions, both 10 and 30 days after CR gavage, compared to non-infected mice [57]. Brain-derived neurotrophic factor (BDNF) expression was lower in the hippocampus of CR-exposed mice, pointing this growth factor reduction as a plausible cause mediating memory impairment. All of these changes were associated with alterations in microbiota composition and, in fact, were reversed by treatment with the probiotic L. reuteri [57]. In line with this idea of gut microbes affecting memory formation, the authors reported strong deficits as well as low BDNF hippocampi levels in germ-free mice when compared to specific pathogen free (SPF) animals [57].
More recently, CR infection was shown to exacerbate ongoing brain pathology observed after traumatic brain injury (TBI) in mice [58]. Infection with CR 28 days after a TBI episode significantly increases the volume of the brain lesion, the peri-lesion microglial/macrophage activation and the astrocyte reactivity and glial scar formation [58], suggesting that and enteric infection, secondary to brain injury, could exacerbates cortical tissue loss and neuroinflammation.
Finally, it has been recently demonstrated that, in genetically susceptible (Pink1−/−) mice, a CR infection can trigger Parkinson’s disease-like symptoms [59]. PINK1 is a kinase involved in initiating mitophagy, the process of degradation of mitochondria by autophagy. In these knockout mice, CR infection induces the formation of anti-mitochondrial CD8+ T cells that can be detected in the central nervus system. Importantly, these cells have the capacity of kill dopaminergic neurons in vitro. After repeated CR exposure, KO mice show a sharp decrease in the density of dopaminergic axonal varicosities in the striatum and were affected by motor impairment, phenotypes that are not observed in non-infected Pink1−/− mice or in their WT littermates [59]. While some differences among groups in SCFA were detected, no significant alterations in microbiota diversity or composition were observed between WT and Pink1−/− mice before or after CR infection [60] which support an immune-mediated mechanism as the principal component triggering the emergence of the disease related symptoms.
Overall, these evidences point microbial composition and inflammation processes as the main components involved in CR-induced changes in host brain and behavior. However, there is still a percent of uncertainty in these bacterial-host interactions. The challenge for future research would be to identify inflammation-independent processes and/or signals involved in this gut-brain communication.
Concluding remarks and future considerations
Citrobacter rodentium (CR) is one of the most widely used surrogate for studying EHEC- and EPEC-induced diseases. Furthermore, its utilization has allowed the identification of crucial host-microbial interactions involved in pathogen colonization. More recently, CR infection has also become a valuable tool in gut-brain axis studies. The fact that CR senses and responds to several different neurochemicals in the gut (i.e., catecholamines, serotonin and endocannabinoids) suggests that the modulation of intestinal levels of these molecules, as well as its bacterial sensors could be promising anti-virulence approaches for treatment of enteric infections. On the other hand, rising amount of evidence is showing that CR infection can also modulate brain function and host behavior by altering microbiota composition and gut inflammation (Fig. 2). Clearly, much remains to be explored in this field, and the challenge for future investigations should be the identification of new signals mediating gut-brain communication and unraveling molecular mechanisms by which enteric infections can affect neurological disorders and host behavior.
Figure 2:
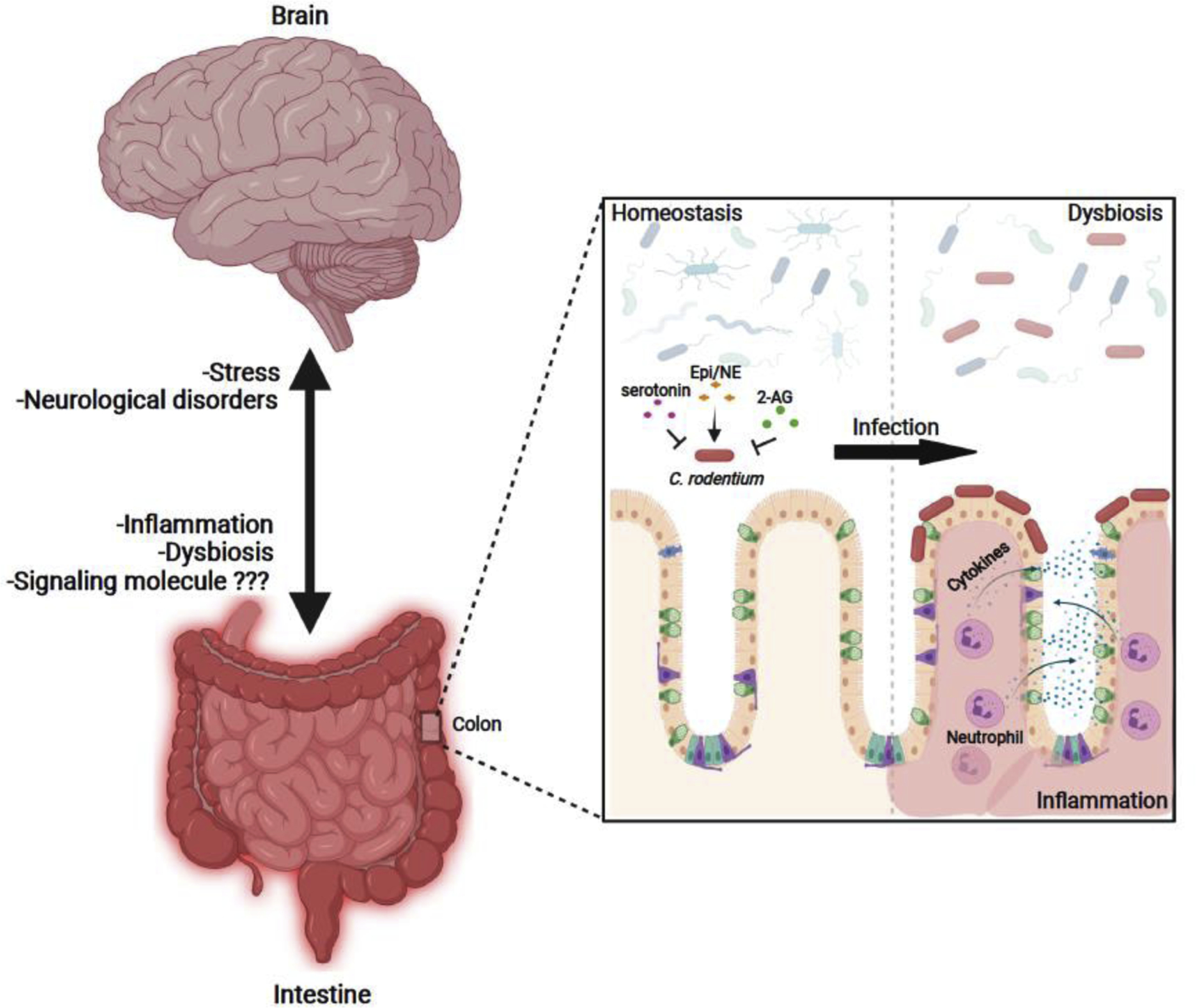
Gut-brain axis interactions observed in Citrobacter rodentium-infected mice. During infection, CR can sense different host neurochemical signals (epinephrine [Epi]/norepinephrine [NE], serotonin and 2-arachidonoylglycerol [2-AG]) to regulate virulence gene expression and colonization of the gut. CR infection alters microbial composition and increases gut inflammation inducing gut dysbiosis. Alterations in inflammation and microbial composition have been related to changes in behavioral responses and brain pathologies. At the same time, stressful situations and brain disorders have been related to changes in gut physiology and microbiota composition which, in turn, affect CR colonization and disease.
Highlights.
Gut-brain crosstalk regulates pathogen colonization and host behavioral responses
Citrobacter rodentium senses gut neurochemical signals to regulate virulence
C. rodentium infection-induced dysbiosis affects host behavioral responses and brain disorders
Animal models of stress are more susceptible to C. rodentium infection and disease
Acknowledgements
This study was supported by National Institute Health (NIH) grants AI053067, AI154597 and AI155398.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lustri BC, Sperandio V, Moreira CG: Bacterial Chat: Intestinal Metabolites and Signals in Host-Microbiota-Pathogen Interactions. Infect Immun 2017, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward SE, Krekhno Z, Finlay BB: Here, there, and everywhere: How pathogenic Escherichia coli sense and respond to gastrointestinal biogeography. Cell Microbiol 2019, 21:e13107. [DOI] [PubMed] [Google Scholar]
- 3.Giacomin PR, Kraeuter AK, Albornoz EA, Jin S, Bengtsson M, Gordon R, Woodruff TM, Urich T, Sarnyai Z, Soares Magalhães RJ: Chronic Helminth Infection Perturbs the Gut-Brain Axis, Promotes Neuropathology, and Alters Behavior. J Infect Dis 2018, 218:1511–1516. [DOI] [PubMed] [Google Scholar]
- 4.Nerius M, Doblhammer G, Tamgüney G: GI infections are associated with an increased risk of Parkinson’s disease. Gut 2020, 69:1154–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennessey C, Keogh CE, Barboza M, Brust-Mascher I, Knotts TA, Sladek JA, Pusceddu MM, Stokes P, Rabasa G, Honeycutt M, et al. : Neonatal enteropathogenic Escherichia coli infection disrupts microbiota-gut-brain axis signaling. Infect Immun 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franceschi F, Ojetti V, Candelli M, Covino M, Cardone S, Potenza A, Simeoni B, Gabrielli M, Sabia L, Gasbarrini G, et al. : Microbes and Alzheimer’ disease: lessons from H. pylori and GUT microbiota. Eur Rev Med Pharmacol Sci 2019, 23:426–430. [DOI] [PubMed] [Google Scholar]
- 7.Mullineaux-Sanders C, Sanchez-Garrido J, Hopkins EGD, Shenoy AR, Barry R, Frankel G: Citrobacter rodentium-host-microbiota interactions: immunity, bioenergetics and metabolism. Nat Rev Microbiol 2019, 17:701–715. [DOI] [PubMed] [Google Scholar]
- 8.Crepin VF, Collins JW, Habibzay M, Frankel G: Citrobacter rodentium mouse model of bacterial infection. Nat Protoc 2016, 11:1851–1876. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Tauschek M, Hart E, Hartland EL, Robins-Browne RM: Virulence regulation in Citrobacter rodentium: the art of timing. Microb Biotechnol 2010, 3:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schauer DB, Falkow S: Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun 1993, 61:2486–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vázquez A, Barba J, Ibarra JA, O’Donnell P, Metalnikov P, et al. : Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci U S A 2004, 101:3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB: A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci U S A 1995, 92:1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvis KG, Girón JA, Jerse AE, McDaniel TK, Donnenberg MS, Kaper JB: Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci U S A 1995, 92:7996–8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruano-Gallego D, Sanchez-Garrido J, Kozik Z, Núñez-Berrueco E, Cepeda-Molero M, Mullineaux-Sanders C, Naemi-Baghshomali Clark J, Slater SL, Wagner N, Glegola-Madejska I, et al. : Type III secretion system effectors form robust and flexible intracellular virulence networks. Science 2021, 371. [DOI] [PubMed] [Google Scholar]
- 15.Ren W, Liao Y, Ding X, Jiang Y, Yan J, Xia Y, Tan B, Lin Z, Duan J, Jia X, et al. : Slc6a13 deficiency promotes Th17 responses during intestinal bacterial infection. Mucosal Immunol 2019, 12:531–544. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez VT, Godinez DR, Brust-Mascher I, Nonnecke EB, Castillo PA, Gardner MB, Tu D, Sladek JA, Miller EN, Lebrilla CB, et al. : T-cell derived acetylcholine aids host defenses during enteric bacterial infection with Citrobacter rodentium. PLoS Pathog 2019, 15:e1007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega VA, Mercer EM, Giesbrecht GF, Arrieta MC: Evolutionary Significance of the Neuroendocrine Stress Axis on Vertebrate Immunity and the Influence of the Microbiome on Early-Life Stress Regulation and Health Outcomes. Front Microbiol 2021, 12:634539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhofer G, Kopin IJ, Goldstein DS: Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 2004, 56:331–349. [DOI] [PubMed] [Google Scholar]
- 19.Bearson BL: Molecular Profiling: Catecholamine Modulation of Gene Expression in Escherichia coli O157:H7 and Salmonella enterica Serovar Typhimurium. Adv Exp Med Biol 2016, 874:167–182. [DOI] [PubMed] [Google Scholar]
- 20.Sarkodie EK, Zhou S, Baidoo SA, Chu W: Influences of stress hormones on microbial infections. Microb Pathog 2019, 131:270–276. [DOI] [PubMed] [Google Scholar]
- 21.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V: The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A 2006, 103:10420–10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reading NC, Rasko DA, Torres AG, Sperandio V: The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc Natl Acad Sci U S A 2009, 106:5889–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weigel WA, Demuth DR: QseBC, a two-component bacterial adrenergic receptor and global regulator of virulence in Enterobacteriaceae and Pasteurellaceae. Mol Oral Microbiol 2016, 31:379–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q, Zou P, Cao Z, Wang Q, Fu S, Xie G, Huang J: QseC Inhibition as a Novel Antivirulence Strategy for the Prevention of Acute Hepatopancreatic Necrosis Disease (AHPND)-Causing Vibrio parahaemolyticus. Front Cell Infect Microbiol 2020, 10:594652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis MM, Russell R, Moreira CG, Adebesin AM, Wang C, Williams NS, Taussig R, Stewart D, Zimmern P, Lu B, et al. : QseC inhibitors as an antivirulence approach for Gram-negative pathogens. mBio 2014, 5:e02165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasko DA, Moreira CG, Li de R, Reading NC, Ritchie JM, Waldor MK, Williams N, Taussig R, Wei S, Roth M, et al. : Targeting QseC signaling and virulence for antibiotic development. Science 2008, 321:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreira CG, Russell R, Mishra AA, Narayanan S, Ritchie JM, Waldor MK, Curtis MM, Winter SE, Weinshenker D, Sperandio V: Bacterial Adrenergic Sensors Regulate Virulence of Enteric Pathogens in the Gut. mBio 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study is the first report of the role of catecholamines during the infection of the GI tract by a noninvasive pathogen. The study demonstrates that epinephrine and norepinephrine are sensed by C. rodentium through the bacterial adrenergic receptors QseC and QseE, and are required for full virulence activation during murine infections.
- 28.Melchior K, Moreira CG: Novel non-flagellated surface motility mediated by chemical signaling in Citrobacter rodentium. Braz J Microbiol 2019, 50:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones LA, Sun EW, Martin AM, Keating DJ: The ever-changing roles of serotonin. Int J Biochem Cell Biol 2020, 125:105776. [DOI] [PubMed] [Google Scholar]
- 30.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY: Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engevik MA, Luck B, Visuthranukul C, Ihekweazu FD, Engevik AC, Shi Z, Danhof HA, Chang-Graham AL, Hall A, Endres BT, et al. : Human-Derived Bifidobacterium dentium Modulates the Mammalian Serotonergic System and Gut-Brain Axis. Cell Mol Gastroenterol Hepatol 2021, 11:221–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, Vavilina A, McGinn J, Rendon T, Forrest LR, et al. : Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol 2019, 4:2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Russell RM, Pifer R, Menezes-Garcia Z, Cuesta S, Narayanan S, MacMillan JB, Sperandio V: The Serotonin Neurotransmitter Modulates Virulence of Enteric Pathogens. Cell Host Microbe 2020, 28:41–53.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study demonstrates that serotonin is sensed by EHEC and C. rodentium through CpxA and decreases virulence gene expression. Elevated intestinal levels of serotonin correlate with an attenuated CR colonization in mice.
- 34.O’Hara JR, Skinn AC, MacNaughton WK, Sherman PM, Sharkey KA: Consequences of Citrobacter rodentium infection on enteroendocrine cells and the enteric nervous system in the mouse colon. Cell Microbiol 2006, 8:646–660. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Sperandio V: Indole Signaling at the Host-Microbiota-Pathogen Interface. mBio 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma DS, Paddibhatla I, Raghuwanshi S, Malleswarapu M, Sangeeth A, Kovuru N, Dahariya S, Gautam DK, Pallepati A, Gutti RK: Endocannabinoid system: Role in blood cell development, neuroimmune interactions and associated disorders. J Neuroimmunol 2021, 353:577501. [DOI] [PubMed] [Google Scholar]
- 37.Chevalier G, Siopi E, Guenin-Macé L, Pascal M, Laval T, Rifflet A, Boneca IG, Demangel C, Colsch B, Pruvost A, et al. : Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun 2020, 11:6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharkey KA, Wiley JW: The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology 2016, 151:252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellermann M, Pacheco AR, Jimenez AG, Russell RM, Cuesta S, Kumar A, Zhu W, Vale G, Martin SA, Raj P, et al. : Endocannabinoids Inhibit the Induction of Virulence in Enteric Pathogens. Cell 2020, 183:650–665.e615. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This is the first report of the modulation of virulence in enteric pathogens by endocannabinoids. The study demonstrates that the endocannabinoid 2-AG antagonizes QseC to decrease virulence, and mice showing elevated intestinal levels of 2-AG are protected from C. rodentium infection.
- 40.Ellermann M, Jimenez AG, Pifer R, Ruiz N, Sperandio V: The Canonical Long-Chain Fatty Acid Sensing Machinery Processes Arachidonic Acid To Inhibit Virulence in Enterohemorrhagic Escherichia coli. mBio 2021, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doney E, Cadoret A, Dion-Albert L, Lebel M, Menard C: Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur J Neurosci 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walter JG, Kahn SA, Noe JD, Schurman JV, Miller SA, Greenley RN: Feeling Fine: Anxiety and Depressive Symptoms in Youth with Established IBD. Inflamm Bowel Dis 2016, 22:402–408. [DOI] [PubMed] [Google Scholar]
- 43.Rubin GP, Hungin AP, Chinn DJ, Dwarakanath D: Quality of life in patients with established inflammatory bowel disease: a UK general practice survey. Aliment Pharmacol Ther 2004, 19:529–535. [DOI] [PubMed] [Google Scholar]
- 44.Casellas F, Lopez-Vivancos J, Badia X, Vilaseca J, Malagelada JR: Influence of inflammatory bowel disease on different dimensions of quality of life. Eur J Gastroenterol Hepatol 2001, 13:567–572. [DOI] [PubMed] [Google Scholar]
- 45.Mackner LM, Crandall WV: Psychological factors affecting pediatric inflammatory bowel disease. Curr Opin Pediatr 2007, 19:548–552. [DOI] [PubMed] [Google Scholar]
- 46.Mardini HE, Kip KE, Wilson JW: Crohn’s disease: a two-year prospective study of the association between psychological distress and disease activity. Dig Dis Sci 2004, 49:492–497. [DOI] [PubMed] [Google Scholar]
- 47.Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M: Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun 2010, 78:1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mackos AR, Eubank TD, Parry NM, Bailey MT: Probiotic Lactobacillus reuteri attenuates the stressor-enhanced severity of Citrobacter rodentium infection. Infect Immun 2013, 81:32533263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maltz RM, Keirsey J, Kim SC, Mackos AR, Gharaibeh RZ, Moore CC, Xu J, Bakthavatchalu V, Somogyi A, Bailey MT: Prolonged restraint stressor exposure in outbred CD-1 mice impacts microbiota, colonic inflammation, and short chain fatty acids. PLoS One 2018, 13:e0196961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galley JD, Parry NM, Ahmer BMM, Fox JG, Bailey MT: The commensal microbiota exacerbate infectious colitis in stressor-exposed mice. Brain Behav Immun 2017, 60:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galley JD, Mackos AR, Varaljay VA, Bailey MT: Stressor exposure has prolonged effects on colonic microbial community structure in Citrobacter rodentium-challenged mice. Sci Rep 2017, 7:45012.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackos AR, Galley JD, Eubank TD, Easterling RS, Parry NM, Fox JG, Lyte M, Bailey MT: Social stress-enhanced severity of Citrobacter rodentium-induced colitis is CCL2-dependent and attenuated by probiotic Lactobacillus reuteri. Mucosal Immunol 2016, 9:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maltz RM, Keirsey J, Kim SC, Mackos AR, Gharaibeh RZ, Moore CC, Xu J, Somogyi A, Bailey MT: Social Stress Affects Colonic Inflammation, the Gut Microbiome, and Short-chain Fatty Acid Levels and Receptors. J Pediatr Gastroenterol Nutr 2019, 68:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Needham BD, Kaddurah-Daouk R, Mazmanian SK: Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci 2020, 21:717–731. [DOI] [PubMed] [Google Scholar]
- 55.Vuong HE, Yano JM, Fung TC, Hsiao EY: The Microbiome and Host Behavior. Annu Rev Neurosci 2017, 40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyte M, Li W, Opitz N, Gaykema RP, Goehler LE: Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia Citrobacter rodentium. Physiol Behav 2006, 89:350–357. [DOI] [PubMed] [Google Scholar]
- 57.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM: Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60:307317. [DOI] [PubMed] [Google Scholar]
- 58.Ma EL, Smith AD, Desai N, Cheung L, Hanscom M, Stoica BA, Loane DJ, Shea-Donohue T, Faden AI: Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav Immun 2017, 66:56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matheoud D, Cannon T, Voisin A, Penttinen AM, Ramet L, Fahmy AM, Ducrot C, Laplante A, Bourque MJ, Zhu L, et al. : Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1(−/−) mice. Nature 2019, 571:565–569. [DOI] [PubMed] [Google Scholar]; **This study demonstrated the mechanism by which an enteric infection with CR triggers Parkinson’s disease-like symptoms. Using a genetically susceptible mice model, the authors showed CR infection can activate an intestinal autoimmune response that, in turn, can affect brain physiology accelerating the development of behavioral and neuronal symptoms observed in Parkinson’s disease.
- 60.Cannon T, Sinha A, Trudeau LE, Maurice CF, Gruenheid S: Characterization of the intestinal microbiota during Citrobacter rodentium infection in a mouse model of infection-triggered Parkinson’s disease. Gut Microbes 2020, 12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


