Graphical abstract

Keywords: Irritable bowel syndrome, Vardenafil, Oral medicated jellies, Pediatrics, Cyclicguanosine Mono Phosphate
Abstract
Irritable bowel syndrome (IBS); a widespread disorder in gastrointestinal tract especially in children, burdens their healthcare systems and upsets families. Great attention was paid to understand the pathophysiological cause of disorder. However, developing a convenient treatment especially for children remains a challenge. Phosphodiesterase inhibitors were recently introduced for IBS management. Vardenafil (VDF), a phosphodiesterase-5 inhibitor, exhibiting limited bioavailability when taken orally due to extensive first-pass effect, was the choice for study. This study aimed to formulate VDF jellies as a buccal dosage form to improve pediatric compliance and achieve maximum drug efficacy. VDF oral jellies were prepared by heat and congeal method, and were evaluated for their pH, content uniformity, physical stability, general appearance, and in-vitro drug release. VDF jellies (F1), with satisfactory organoleptic properties and highest percent of drug released compared to other formulations was selected as a master formula for further study to ensure in-vivo efficacy. cyclic Guanosine Mono Phosphate (cGMP), used as indicator of VDF concentration in blood, was highly increased after administration of VDF jellies (F1), compared to oral VDF suspension. Increased defecation with improved fecal consistency strongly favored oral jellies as a potential alternative route for VDF for IBS management with high pediatric acceptance.
1. Introduction
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders with a global prevalence estimated between 2% and 24% in pediatrics (Devanarayana et al. 2015). Children suffering from IBS have altered bowel activity and severe disabling upper and lower gastrointestinal symptoms (Devanarayana and Rajindrajith, 2018). This negatively affects their performance and quality of life and would consequently represent a burden on their normal daily activities (Rajindrajith et al. 2017). Unfortunately; researches in the field of pediatric neuro-gastroenterology couldn't yet reveal IBS pathophysiology, despite considering diet, genetics, and the disruption in mutual communication between brain and gut leading to visceral hypersensitivity, the most recognized risk factors (Black and Ford, 2020, Oświęcimska et al., 2017, Sinagra et al., 2016). To date, developing safe and effective treatments for IBS remains a challenge as no specific treatment is documented. Diet and stress management are recognized non-pharmacological approaches in controlling IBS but may be limited by issues of long-term patient adherence and potential hazard of nutritional insufficiencies (Foxx-Orenstein, 2016). Pharmacological remedies including bulking agents, laxatives and probiotics may help normalizing alterations in bowel habits, while tricyclic antidepressants, acid suppressing agents and antispasmodics may help to minimize visceral pain accompanying the disease, however, the overall efficacy of pharmacological agents in IBS is low (Devanarayana and Rajindrajith, 2018, Halland and Saito, 2015).
Irritable bowel syndrome (IBS) is one of the most common gastrointestinal disorders with a global prevalence estimated between 2% and 24% in pediatrics (Devanarayana et al. 2015). Children suffering from IBS have altered bowel activity and severe disabling upper and lower gastrointestinal symptoms (Devanarayana and Rajindrajith, 2018). This negatively affects their performance and quality of life and would consequently represent a burden on their normal daily activities (Rajindrajith et al. 2017). Unfortunately; researches in the field of pediatric neuro-gastroenterology couldn't yet reveal IBS pathophysiology, despite considering diet, genetics, and the disruption in mutual communication between brain and gut leading to visceral hypersensitivity, the most recognized risk factors (Black and Ford, 2020, Oświęcimska et al., 2017, Sinagra et al., 2016). To date, developing safe and effective treatments for IBS remains a challenge as no specific treatment is documented. Diet and stress management are recognized non-pharmacological approaches in controlling IBS but may be limited by issues of long-term patient adherence and potential hazard of nutritional insufficiencies (Foxx-Orenstein, 2016). Pharmacological remedies including bulking agents, laxatives and probiotics may help normalizing alterations in bowel habits, while tricyclic antidepressants, acid suppressing agents and antispasmodics may help to minimize visceral pain accompanying the disease, however, the overall efficacy of pharmacological agents in IBS is low (Devanarayana and Rajindrajith, 2018, Halland and Saito, 2015).
The role of intestinal Guanylate Cyclase-C (GC-C) and extracellular cyclic Guanosine Mono Phosphate (cGMP) in maintaining the gastrointestinal tract normal physiological function has recently been recognized and so disruption of their pathways would be linked to numerous gastrointestinal disorders including inflammatory bowel diseases and colorectal cancers. In 2012, linaclotide; GC-C agonist, gained FDA approval for the treatment of constipation-predominant IBS (Eswaran et al., 2014, Love et al., 2014). However, the use of GC-C agonists in pediatrics was limited as they may cause severe diarrhea (Sedky and Magdy, 2020). cGMP-elevating agents such as phosphodiesterase 5 (PDE-5) inhibitors are recently reported as a promising medication in pediatrics (Eswaran et al. 2014).
Vardenafil (VDF) is a selective inhibitor for PDE-5 used in management of impotence. Nonetheless, recent reports have emphasized the potential use of PDE-5 inhibitors; Sildenafil and Tadalafil, for treatment of IBS (Sedky and Magdy, 2020, Sharman et al., 2017). VDF acts by inhibiting PDE-5 thus protecting cGMP from hydrolysis, and consequently, the increment in serum cGMP levels, which in turn, can lead to smooth muscle vasodilation and thus could be beneficious to treat constipation-predominant IBS in children.
VDF suffers from low oral bioavailability (~15%) owing to hepatic metabolism besides its low aqueous solubility (Fahmy, 2015). This elicited the need to develop an alternative dosage form to bypass first pass effect and improve its in vivo fate.
Currently, VDF is an effective therapy for erectile dysfunction. Nonetheless, many reports have emphasized the effect of phosphodiesterase-5 inhibitors on IBS by only clinical trials. Unfortunately, no studies were focused to develop a formulation and study its in-vitro/in-vivo correlation to confirm the clinical findings for more prospective benefits gained by patients and consequently opens new avenues for pharmaceutical companies.
The drug is marketed only as conventional tablets, fast disintegrating tablets, or even oral jellies designed to be completely ingested and were intended for sexual impotence. Herein, the present work is addressed for pediatric, or even dysphagic patients, by exclusively developing more patient-friendly dosage form of VDF.
Pharmaceutical industry paid attention to formulate more palatable and elegant dosage forms, to achieve convenient administration and more compliance for pediatric patients. Oral Medicated Jellies (OMJs) are considered useful alternatives for wide segment of populations especially children and dysphagic patients and have been listed as a type of dosage form in the Japanese Pharmacopeia, 16th Edition, enforced in 2011 in Japan, till now (Kakino et al. 2017). Jellies are dulcet solid dosage forms for administration into the buccal cavity, designed to dissolve in either mouth or pharynx for local or systemic effect. They are described as “transparent or translucent semisolid formulations with non-greasy nature used for either lubrication or medication” (Kadhim and Ali, 2019, Sarojini et al., 2018). Numerous studies on OMJs have been made to solve bioavailability problems and improve patient compliance (Patel et al. 2020).
In this study, OMJs with VDF were prepared as demulcent jellies using gelling polymers of certain viscosities aimed to be sucked in the mouth for buccal absorption of the drug with the hope to help those having difficulty in swallowing. The formulation is beneficious for better bioavailability of VDF through by passing enterohepatic circulation faced by the marketed oral tablets and ingested jellies. Moreover, it would overcome the problem of dosage wastage, improper measurement, dose dumping, and improve patient compliance to more palatable and elegant dosage forms more than other marketed VDF products. They were evaluated for its content uniformity, physical stability, general appearance, and in-vitro release. Additionally, measurement of cGMP serum level was measured for the in-vivo evaluation. Intestinal transit, fecal output and consistency were also tracked and evaluated. Results revealed that OMJs improved the absorption of VDF through buccal mucosa and consequently succeeded to enhance its bioavailability with very promising clinical results.
2. Materials and methods
2.1. Materials
VDF was a gift from G.N.P Company (6th of October City, Giza, Egypt). Gelatin, sodium alginate, carbopol 974, gum arabic and pectin were kindly supplied by EIPICO Pharmaceuticals Chemicals Co., Egypt. Gum tragacanth, citric acid, sodium benzoate were purchased from Al - Gomhoria Company for medicines and medical supplies (Cairo, Egypt).
2.2. Preparation of oral jellies:
Various polymers were examined to formulate jellies loaded with VDF by heating and congealing technique. Polymers were optimized at a concentration that succeeded to formulate jellies of optimized consistency as illustrated in Table 1.
Table 1.
Composition of different VDF oral jellies (5 g).
| % w/w of ingredients | ||||||
|---|---|---|---|---|---|---|
| Ingredient | F1 | F2 | F3 | F4 | F5 | F6 |
| Gelatin | 8 | --- | --- | --- | --- | --- |
| Sodium alginate | --- | 3 | --- | --- | --- | --- |
| Carbopol 974 | --- | --- | 3 | --- | --- | --- |
| Gum arabic | --- | --- | --- | 20 | --- | --- |
| Gum tragacanth | --- | --- | --- | --- | 15 | --- |
| Pectin | --- | --- | --- | --- | --- | 5 |
| Glycerin | 2 | ---- | ----- | ----- | ---- | ----- |
| Triethanolamine | --- | --- | 1–2 drops | --- | --- | --- |
| Citric acid | 1 | 1 | 1 | 1 | 1 | 1 |
| Sugar syrup | 70 | 70 | 70 | 70 | 70 | 70 |
| Water | 18.6 | 25.6 | 25.6 | 8.6 | 13.6 | 23.6 |
| Sodium benzoate | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| VDF | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
Jellies were manufactured using a protocol modified from Sarojini et al. (2018). Briefly sucrose syrup was prepared in a beaker by dissolving 67 g sucrose in water to a final volume of 100 mL by heating to 80 °C and stirring continuously. The gelling agent (Gelatin Na alginate Carbopol 974 Gum Arabic Gum tragacanth or Pectin) was added to 10 mL warmed water under constant stirring along with the sucrose solution. To a gelling agent-sucrose solution 20 mg of VDF was added along with the calculated amounts of citric acid and sodium benzoate under continuous stirring. The prepared solutions were transferred in to single moulded units settle undisturbed and left to cool. After the jellies were set they were wrapped individually into butter paper and stored in dry place till further testing (Prakash et al., 2014, Jadhav et al., 2017, Taranum and Mittapally, 2018). All ingredients were calculated based on % w/w.
2.3. Characterization of OMJs
The prepared OMJs were characterized and evaluated as per the standard procedures reported in the literature (Patel et al., 2020), and outlined below.
2.3.1. Syneresis
All jellies were noticed at ambient room temperature for syneresis over a 24 h period, and those showing any sign of syneresis were rejected and not considered for further studies.
2.3.1.1. Physical appearance
The formulated jellies were inspected visually for their organoleptic properties; clarity, color and presence of any particle aggregation. The test is important regarding patient compliance.
2.3.1.2. Measurement of pH
The value of pH would influence the stability and the acceptance of the formulation taste as well (Jadhav et al. 2017). The pH of all prepared jellies was determined using digital pH meter, by dispersing exactly 0.5 g of formulation in 50 mL of distilled water and pH values were recorded.
2.3.1.3. Stickiness and grittiness
Texture of OMJs was visually inspected after mildly rubbing the sample by two fingers to feel any stickiness or grittiness.
2.3.1.4. Rheological properties
A ViscoStar-R FUNGILAB viscometer, spindle (R6) and speed of 10 rpm at 25 °C, was used in rheological study. The values of viscosity were displayed in centipoise.
2.3.1.5. Drug content
Jellies selected from each batch were individually dissolved in exactly 50 mL of Sörensens phosphate buffer of pH 6.8 which was then filtered diluted and analyzed spectrophotometrically at 270 nm using UV–visible spectrophotometer. All the formulations should be within the standard USP limits
2.3.1.6. Stability study of oral soft jellies
A freshly prepared formulation should serve as a reference standard for subjective evaluation. Samples of the optimized formula (F1) were kept at room temperature and in refrigerator for one month and were observed for syneresis, appearance, pH, viscosity, and noticed at the interval of one month.
2.3.1.7. In vitro release studies
The in vitro study for VDF release from medicated formulations was processed using a modified diffusion cell and cellophane membrane. Each jelly (5 g) was placed on cellophane membrane which was then fixed on to diffusion cell. The cellophane membrane was placed in Sörensen's phosphate buffer solution of pH 6.8 (37 ± 0.5 °C, stirring rate 100 rpm), as a receptor medium for this investigation. A sample of 2 mL was withdrawn from reservoir compartment at 10, 20, 30, 40, 50 and 60 min and spectrophotometric measurements were made at 270 nm (Cardoz and Ravikumar, 2017).
2.3.1.8. Kinetics analysis of the in-vitro release data
The data of in vitro release of VDF from the tested formulations was fitted into zero-order, first-order, Higuchi kinetic models, where the model with the highest correlation coefficient best describes the drug release (Abou El Ela et al. 2016).
2.4. In vivo study
2.4.1. Animals
Adult albino male rats, approximately 0.20–0.25 kg weight, from the animal center, Faculty of Pharmacy, Zagazig University, Egypt, were used for in vivo study. The procedures were adapted to Ethical Committee of animal handling in Zagazig University ECAHZU (Approval number: ZU-IACUC/3/F/80/2020), They were housed in clean cages in well-ventilated animal house for the period of experiment, maintained on sawdust bedding at 25 ± 5 °C and 50–80% relative humidity.
2.4.1.1. In-vivo experimental design
The rats were divided into four groups, 6 rats each, according to experimental design illustrated in Table (2). Rats in the first group served as a control group (received only distilled water). Oral administration of 0.5 mL of loperamide (3 mg/kg body weight daily for successive 3 days) was sufficient to induce constipation in the other groups (Wintola et al. 2010); and was noticed on the fourth day by putting reduced fecal pellets with stiff nature. After constipation was induced, rats in group 3 received a suspension of an equivalent amount of 20 mg VDF/ Kg of body weight via gastric gavage (Matsumoto et al. 2008). For administration of VDF oral jellies; rats in the fourth group were anaesthetized by a 25 mg/kg dose of pentobarbital (IV injection) to ensure OMJs existence in buccal cavity without escaping down to the gut (Reimer et al., 2020). Rats were placed on a table with the lower jaw horizontally fixed for insertion of the selected formula (F1) on the rat's tongue Table2.
Table 2.
Distribution of rat for in vivo study.
| Group 1 (control) | Untreated rats, received distilled water orally. |
| Group 2 (constipated control) | Constipated rats |
| Group 3 | Constipated rats, received 1 mL of VDF suspension (equivalent to calculated animal dose) via gastric gavage. |
| Group 4 | Constipated rats, received oral VDF jellies |
2.4.1.2. Measurement of intestinal transit
Three rats of each group were separated for measurement of the intestinal transit and cGMP level. According to the literature the intestinal transit was measured by administration of powdered charcoal via gastric gavage (Sagar et al., 2005, Sedky and Magdy, 2020, Sharman et al., 2017). After one hour; they were sacrificed and the small and large intestines were isolated. The percentage of length of small intestine containing the carbon to total bowel length gives an indication for intestinal transit rate × 100%.
2.4.1.3. Measurement of cGMP serum level
Blood samples collected from sacrificed rats were left at room temperature for 30 min, then centrifuged at 5000 rpm at 4 °C for 15 min to obtain serum (Gomaa et al., 2018, Abu Lila et al., 2020). cGMP Direct Immunoassay Kit (Abcam, MA, USA) according to general principle of ELISA technique and the manufacturer's recommended instructions was used for quantitative measurement of cGMP (Itoh et al. 2004). The percentage of increase in cGMP level was calculated as follows: (average cGMP of test group- average cGMP of control group)/ average cGMP of the control group X 100%.
2.4.1.4. Measurement of fecal output (number and water content%)
The other three rats of each group were evaluated for their fecal output. Rats were separated in individual cages and fecal pellets of each rat were collected for 6 h and counted. For determination of fecal water content, the weight of the collected wet fecal samples was measured and then redetermined after being allowed to dry in air. The fecal water content was calculated as follows: (the wet weight of fecal pellets – the dry weight of fecal pellets)/ the wet weight of fecal pellets X 100 % (Wu et al. 2017).
2.5. Statistical analysis
All values are expressed as the mean ± S.D. Statistical analysis was performed using one-way ANOVA using GraphPad InStat software (GraphPad Software, CA, USA). The level of significance was set at p < 0.05.
3. Results and discussion
Due to their ease of preparation and administration, OMJs have become very popular pharmaceutical dosage forms. Their properties have made them suited especially to pediatric settings since children have become more exposed to these dosage forms. Six different jelly formulations containing VDF were developed and evaluated their physical properties and drug release characteristics in vitro and in vivo as well. Additives were incorporated to best alleviate the formulation. For example, citric acid was added to adjust pH of the formulae, sugar syrup was applied as a bulking agent to provide body of the jelly, sodium benzoate was also incorporated as a preservative to prevent deterioration that may develop by high-water content in the prepared formulations, and some other additives may be used as stabilizers to prevent dryness of formulation or improving the mouth feel.
3.1. Characterization of OMJs
Different polymers were tested for their success in formulating jelly formulations and judged for syneresis, and those showed shrinkage upon storage or break-up of water from the jelly were excluded from further study. The selected six formulae were developed using different concentrations of gelling polymers to incorporate VDF. All six formulations showed subtle variations in their color from white to a pale yellow as denoted by the gelling agent (Table 3). Formulations F1, F2, F3 and F6 proved to be translucent while F4 and F5 being opaque in style. The opacity of F4 and F5 was likely to be as a result of incorporating gum in the formulations which proved to be stickier and grittier than the other formulations. However, no sugar crystallization and no drug precipitation were noticed in the prepared formulations.
Table 3.
Physical parameters, pH and rheological properties for the prepared jellies.
| Formulation | Appearance | Color | Grittiness & Stickiness | pH | Viscosity (cps) |
|---|---|---|---|---|---|
| F1 | Translucent | Pale Yellow | None | 7.3 ± 0.1 | 10500 ± 100 |
| F2 | Translucent | Off-white | None | 7.12 ± 0.2 | 12000 ± 100 |
| F3 | Translucent | White | None | 7.26 ± 0.2 | 11200 ± 100 |
| F4 | Opaque | Yellow | Sticky and gritty | 7.28 ± 0.3 | 14400 ± 160 |
| F5 | Opaque | Yellow | High stickiness and grittiness | 7.2 ± 0.1 | 15000 ± 150 |
| F6 | Translucent | Pale Yellow | None | 7.25 ± 0.2 | 12200 ± 100 |
A more or less neutral pH would be ideal due to avoid irritation of the mucosa (Abu Lila et al. 2020). All formulations showed a pH value ranged between 7.12 ± 0.2 to 7.3 ± 0.1 by being near to neutral providing acceptability among the patients.
The viscosity was found in the range of 10500 ± 100 to 15000 ± 150 cps. The drug content of F1 to F6 formulations was within the specified range of 95–105%. All formulations showed no syneresis at room temperature after incubation period of a 24 h. Physically stable OMJs had retained their color, clarity, odor and viscosity throughout shelf-life.
3.2. In vitro study of drug release
Gelatin, Na alginate, carbopol 974, gum arabic, gum tragacanth and pectin are gelling agents that have been appropriately utilized to form gel-like matrix (Kadhim and Ali, 2019, Taranum and Mittapally, 2018). The in vitro release results for all medicated jellies were demonstrated in Fig. 1. After one-hour release period, the highest release (52%) was observed for F1 prepared with gelatin, followed by F6, F2 and F3. This could be attributed to the low viscosity of F1 compared to other formulations. Similar results were obtained by Kadam et al., who showed that incorporating gelatin showed the highest release in the prepared formulations. Jellies prepared using gum tragacanth (F5) exhibited slower release (≤30%). This could be attributed to the stickiness of the formulated jelly and high viscosity.
Fig. 1.
Cumulative percent release of VDF from the formulations.
The respective rate constants of zero-order, first order and Higuchi kinetics equations were compared to best describe the drug release from OMJs. Table 4. showed the kinetics data of the release of VDF oral jellies in pH 6.8 and it was a diffusion model.
Table 4.
Kinetics data of the release of the VDF from soft lozenges containing VDF.
| Formulation | Zero-order (R2) | Diffusion (R2) | First-order (R2) | Observed order |
|---|---|---|---|---|
| F1 | 0.7004 | 0.7908 | 0.7672 | Diffusion |
| F2 | 0.6278 | 0.7334 | 0.6703 | Diffusion |
| F3 | 0.8262 | 0.9014 | 0.8468 | Diffusion |
| F4 | 0.7446 | 0.8429 | 0.7739 | Diffusion |
| F5 | 0.7060 | 0.8039 | 0.7300 | Diffusion |
| F6 | 0.7947 | 0.8812 | 0.8422 | Diffusion |
Formulations F1, F2, F3 and F6 all showed some suitability for further consideration, however, F1 was chosen due to its organoleptic properties and in vitro performance compared to other formulae. Short-term stability studies were additionally performed for the optimized formula (F1) and showed no significant changes in pH, viscosity, appearance and syneresis at room temperature and 4 °C, ensured its suitability for further studies.
3.3. In vivo results
From (Fig. 3, Fig. 4, Fig. 5); it was clear that induction of IBS was capable significantly (P < .05) of increasing intestinal transit time recorded as a reduced percentage of the distance travelled, compared to control group. The increase in intestinal transit time was significantly reversed by oral VDF and a greater improvement was seen with VDF oral jellies that was noticed by the highest percent of travelled distance.
Fig. 3.
Serum cGMP level for rat groups.
Fig. 4.
Number of fecal pellets for rat groups.
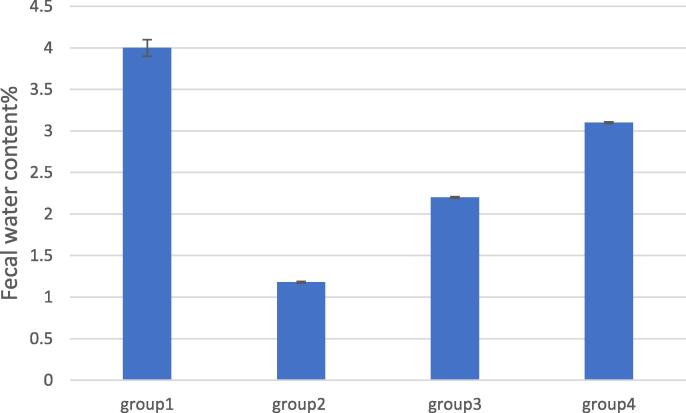
Fig. 5.
Fecal water content% for rat groups.
cGMP; a relevant biomarker of VDF level in blood, was used as indicator for therapeutic effect of F1. Rats were either exposed to VDF via F1, via oral suspension or were left untreated and their cGMP levels were measured (Fig. 2). After measurements, the VDF oral suspension raised cGMP in serum by 55.8% over the control. This was expected because of its pharmacological activity and results were in agreement with Wang et al, who found that VDF was able to increase cGMP and alter homeostasis in intestinal epithelium of mice (Wang et al. 2014). After absorption of VDF through the oral mucosa, cGMP showed a marked elevation by 289.6% after application of (F1). This came in agreement with the pharmacokinetics of drug and provided an evidence that the buccal route offers an efficient approach to bypass liver metabolism of drugs via direct penetration to blood via buccal mucosa.
Fig. 2.
Intestinal transit for rat groups (as a percentage distance travelled).
Induction of IBS- predominant constipation, decreased defecation expressed by count of fecal pellets compared to control group. This was reversed by VDF administration, either oral suspension or OMJs, by significant increase in fecal pellets (P < .05), relative to IBS untreated group. A greater improvement was noticed in the fourth group received VDF oral jellies, and this could be explained by the capability of buccal route to serve VDF from degradation by hepatic metabolism
On the same pattern; induction of constipation significantly decreased water content in fecal samples compared to control group. That was significantly improved for the third and fourth groups relative to IBS untreated group. A greater improvement was recorded for oral jellies. These results proved the superior therapeutic benefits of VDF oral jellies compared to oral solution and is considered a more convenient dosage form, especially for children who find difficulty in swallowing solid oral dosage forms.
4. Conclusion
In the current study, medicated soft jellies of VDF were designed for absorption through buccal mucosa to bypass enterohepatic circulation and consequently improving the drug bioavailability. The physicochemical parameters such as pH, appearance and viscosity showed all formulation were within the standard USP limits. Jelly F1 was promising by the highest in vitro release of VDF, moreover it showed an improvement in the fecal output characteristics and intestinal transit. A marked increase in cGMP, a marker for successful PDE-5 inhibition, was achieved compared to oral administration of VDF suspension when tested in vivo. All that have exclusively suggested VDF oral jellies as an effective approach for the management of pediatric IBS.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Eman Gomaa: Formal analysis, Writing - review & editing. Margrit M. Ayoub: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors greatly appreciated Dr. Edward J. Sayers and Dr. Noura G. Eissa for critical comments that greatly assisted the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Eman Gomaa, Email: eman_pharmaceutics@yahoo.com.
Margrit M. Ayoub, Email: margritayoub1@gmail.com.
References
- Abou el Ela A.E.S.F., Allam A.A., Ibrahim E.H. Pharmacokinetics and anti-hypertensive effect of metoprolol tartrate rectal delivery system. Drug Delivery. 2016;23(1):69–78. doi: 10.3109/10717544.2014.904021. [DOI] [PubMed] [Google Scholar]
- Abu Lila A.S., Gomaa E., Ghazy F.E.S., Hasan A.A. Treatment of pulmonary arterial hypertension by vardenafil-solid dispersion lozenges as a potential alternative drug delivery system. J. Drug Delivery Sci. Technol. 2020;55:101444. doi: 10.1016/j.jddst.2019.101444. [DOI] [Google Scholar]
- Black C.J., Ford A.C. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020;17(8):473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- Cardoz M.R., Ravikumar P. Design. Development and evaluation of novel oral medicated jellies, IAJPS. 2017;4:1746–1754. doi: 10.5281/zenodo.823242. [DOI] [Google Scholar]
- Devanarayana N.M., Rajindrajith S. Irritable bowel syndrome in children: Current knowledge, challenges and opportunities. World J. Gastroenterol. 2018;24(21):2211–2235. doi: 10.3748/wjg.v24.i21.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanarayana N.M., Rajindrajith S., Pathmeswaran A., Abegunasekara C., Gunawardena N.K., Benninga M.A. Epidemiology of irritable bowel syndrome in children and adolescents in Asia. J. Pediatr. Gastroenterol. Nutr. 2015;60:792–798. doi: 10.1097/MPG.0000000000000714. [DOI] [PubMed] [Google Scholar]
- Eswaran S., Guentner A., Chey W.D. Emerging pharmacologic therapies for constipation-predominant irritable bowel syndrome and chronic constipation. Journal of Neurogastroenterology and Motility. 2014;20(2):141–151. doi: 10.5056/jnm.2014.20.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy U.A. Nanoethosomal transdermal delivery of vardenafil for treatment of erectile dysfunction: Optimization, characterization, and in vivo evaluation. Drug Design, Development and Therapy. 2015;9:6129–6137. doi: 10.2147/DDDT.S94615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxx-Orenstein A.E. New and emerging therapies for the treatment of irritable bowel syndrome: An update for gastroenterologists. Therapeutic Advances in Gastroenterology. 2016;9(3):354–375. doi: 10.1177/1756283X16633050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa E., Abu Lila A.S., Hasan A.A., Ghazy F.E.S. Preparation and characterization of intravaginal vardenafil suppositories targeting a complementary treatment to boost in vitro fertilization process. Eur. J. Pharm. Sci. 2018;111:113–120. doi: 10.1016/j.ejps.2017.09.044. [DOI] [PubMed] [Google Scholar]
- Halland M., Saito Y.A., 2015. Irritable bowel syndrome: New and emerging treatments, BMJ (Online). 350, 1–14. https://doi.org/10.1136/bmj.h1622. [DOI] [PubMed]
- Itoh T., Nagaya N., Fujii T., Iwase T., Nakanishi N., Hamada K., Kangawa K., Kimura H. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2004;169(1):34–38. doi: 10.1164/rccm.200303-346OC. [DOI] [PubMed] [Google Scholar]
- Jadhav S.B., Bharkad V.B., Shinde M.K., Kadam V.S., Katkam P. Development and Evaluation of Oral Medicated Jelly of Ondansetron Hydrochloride. World Journal of Pharmacy and Pharmaceutical Sciences. 2017;6:1537–1549. doi: 10.20959/wjpps20179-10082. [DOI] [Google Scholar]
- Kadam V.S., Kendre J., Shendarkar G.R. and Kadam S.S., 2020. Formulation and evaluation of medicated oral jelly of Trazadone Hydrochloride. IJPSR, 2020; Vol. 11(12): 6251-6259. http://dx.doi.org/10.13040/IJPSR.0975-8232.11(12).6251-59
- Kadhim Z.M., and Ali W.K., 2019., Utilization of Natural Polymer in the Preparation of Oral Jelly of Granisetron. Al Mustansiriyah Journal of Pharmaceutical Sciences, 2019, Vol.19, No.2. https://doi.org/10.32947/ajps.19.02.00398
- Kakino Y., Hishikawa Y., Onodera R., Tahara K., Takeuchi H. Gelation factors of pectin for development of a powder form of gel, dry jelly, as a novel dosage form. Chem. Pharm. Bull. 2017;65(11):1035–1044. doi: 10.1248/cpb.c17-00447. [DOI] [PubMed] [Google Scholar]
- Sinagra E., Pompei G., Tomasello G., Cappello F., Morreale G.C., Amvrosiadis G., Rossi F., Monte A.I.L., Rizzo A.G., Raimondo D. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J. Gastroenterol. 2016;22(7):2242–2255. doi: 10.3748/wjg.v22.i7.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love B.L., Johnson A., Smith L.S. Linaclotide: A novel agent for chronic constipation and irritable bowel syndrome. American Journal of Health-System Pharmacy. 2014;71:1081–1091. doi: 10.2146/ajhp130575. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Hanai T., Uemura H., Levin R.M. Effects of chronic treatment with vardenafil, a phosphodiesterase 5 inhibitor, on female rat bladder in a partial bladder outlet obstruction model. BJU International. 2008;103:987–990. doi: 10.1111/j.1464-410X.2008.08185.x. [DOI] [PubMed] [Google Scholar]
- Oświęcimska J., Szymlak A., Roczniak W., Girczys-Połedniok K., Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Advances in Medical Sciences. 2017;62:17–30. doi: 10.1016/j.advms.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Patel S., Scott N., Patel K., Mohylyuk V., McAuley W.J., and Liu F.: Easy to Swallow “Instant” Jelly Formulations for Sustained Release Gliclazide Delivery. Journal of Pharmaceutical Sciences. Volume 109, Issue 8, August 2020, Pages 2474-2484. https://doi.org/10.1016/j.xphs.2020.04.018 [DOI] [PubMed]
- Prakash K., Satyanarayana V.M., Nagiat H.T., Fathi A.H., Shanta A.K., Prameela A.R., 2014. Formulation development and evaluation of novel oral jellies of carbamazepine using pectin, guar gum, and gellan gum, Asian Journal of Pharmaceutics. 8, 241–249. https://doi.org/10.4103/0973-8398.143937.
- Rajindrajith S., Devanarayana N.M., Benninga M.A. Constipation and Constipation-predominant Irritable Bowel Syndrome: A Comparative Study Using Rome III Criteria. J. Pediatr. Gastroenterol. Nutr. 2017;64:679–684. doi: 10.1097/MPG.0000000000001332. [DOI] [PubMed] [Google Scholar]
- Reimer J.N., Schuster C.J., Knight C.G., Pang D.S.J. and Leung V.S.Y.: Intraperitoneal injection of sodium pentobarbital has the potential to elicit pain in adult rats (Rattus norvegicus). PLOSONE. 2020. https://doi.org/10.1371/journal.pone.0238123 [DOI] [PMC free article] [PubMed]
- Sagar Lenika, Sehgal Rajesh, Ojha Sudarshan. Evaluation of antimotility effect of Lantana camara L. var. acuelata constituents on neostigmine induced gastrointestinal transit in mice. BMC Complementary and Alternative Medicine. 2005;5(1) doi: 10.1186/1472-6882-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarojini S., Anusha K., Maneesha C., Mufaquam M.A., Deepika B., Krishna Y. Oral Medicated Jellies – a Review, World Journal of. Pharm. Res. 2018;7:352–365. doi: 10.20959/wjpr20186-11502. [DOI] [Google Scholar]
- Sedky A.A., Magdy Y. Tadalafil versus linaclotide in gastrointestinal dysfunction and depressive behavior in constipation–predominant irritable bowel syndrome. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117960. [DOI] [PubMed] [Google Scholar]
- Sharman Sarah K., Islam Bianca N., Hou Yali, Usry Margaux, Bridges Allison, Singh Nagendra, Sridhar Subbaramiah, Rao Satish, Browning Darren D., Green John. Sildenafil normalizes bowel transit in preclinical models of constipation. PLoS ONE. 2017;12(4):e0176673. doi: 10.1371/journal.pone.0176673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranum R., Mittapally S. Soft Chewable Drug Delivery System: Oral Medicated Jelly and Soft Chew. Journal of Drug Delivery and Therapeutics. 2018;8:65–72. doi: 10.22270/jddt.v8i4.1784. [DOI] [Google Scholar]
- Wang R, Kwon I-K, Singh N, Islam B, Liu K, Sridhar S, Hofmann F, Browning D D. Type 2 cGMP-dependent protein kinase regulates homeostasis by blocking c-Jun N-terminal kinase in the colon epithelium. Cell Death Differ. 2014;21(3):427–437. doi: 10.1038/cdd.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintola O.A., Sunmonu T., Afolayan A.J.2010. Toxicological evaluation of aqueous extract of Aloe ferox Mill in Loperamide - induced constipated rats. Human & Experimental Toxicology. 30(5):425-31. http://dx.doi.org/10.1177/0960327110372647 [DOI] [PubMed]
- Wu Jie, Cheng Yan, Zhang Rong, Liu Dong, Luo Yu-Mei, Chen Kun-Lun, Ren Song, Zhang Jun. P2Y1R is involved in visceral hypersensitivity in rats with experimental irritable bowel syndrome. World J. Gastroenterol. 2017;23(34):6339. doi: 10.3748/wjg.v23.i34.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]